Abstract
Background
Ventriculitis, a dreaded complication of brain abscess, meningitis, and various neurosurgical procedures, has attracted limited attention in the medical literature.
Methods
This is a retrospective, single-center study. We screened the medical files of all patients who had a brain imaging report that included the word “ventriculitis” during years 2005–2019. Only patients with clinical, microbiological, and imaging features of ventriculitis were included. Data were collected through a standardized questionnaire.
Results
Ninety-eight patients fulfilled inclusion criteria: 42 women and 56 men, and the median age was 60 years (interquartile range, 48–68). The primary mechanism for ventriculitis was classified as follows: brain abscess (n = 29, 29.6%), meningitis (n = 27, 27.6%), intraventricular catheter-related (n = 17, 17.3%), post-neurosurgery (n = 13, 13.3%), and hematogenous (n = 12, 12.2%). The main neuroimaging features were intraventricular pus (n = 81, 82.7%), ependymal enhancement (n = 70, 71.4%), and intraventricular loculations (n = 15, 15.3%). The main pathogens were streptococci (n = 44, 44.9%), Gram-negative bacilli (n = 27, 27.6%), and staphylococci (n = 15, 15.3%). In-hospital and 1-year mortality rates were 30.6% (n = 30) and 38.8% (n = 38), respectively. Neurological sequelae were reported in 34 of 55 (61.8%) survivors, including cognitive impairment (n = 11), gait disturbances (n = 9), paresis (n = 7), behavior disorder (n = 6), and epilepsy (n = 5). On multivariate analysis, age >65 years, Glasgow Coma Scale score <13 at initial presentation, status epilepticus, hydrocephalus, and positive cerebrospinal fluid culture were associated with 1-year mortality. We built a scoring system to stratify patients with ventriculitis into low risk (12.5%), intermediate risk (36.5%), and high risk (71.4%) of death.
Conclusions
Ventriculitis is a severe complication of brain abscess, meningitis, or neurosurgery, with an in-hospital mortality rate of 30% and neurological sequelae in 60% of survivors.
Keywords: brain abscess, central nervous system infections, meningitis, outcomes, ventriculitis
In this case series of 98 patients with ventriculitis, 1-year mortality was 38.8%, and 61.8% of survivors had neurological sequelae. Age >65 years, GCS-score <13 at initial presentation, status epilepticus, hydrocephalus, and positive CSF culture were associated with 1-year mortality.
Among central nervous system (CNS) infections, ventriculitis has attracted limited attention in the medical literature to date, most of which is restricted to healthcare-associated ventriculitis [1–4]. However, ventriculitis is a dreaded complication of meningitis, brain abscess, and various neurosurgical procedures, including ventricular catheter-related infections [5, 6]. To date, only small sample-size case series have been published [7, 8]. Most of them focused on ventriculitis as a complication of external ventricular drainage (EVD), also referred to as cerebrospinal fluid (CSF) shunt infections [9–12]. Although most authors agree on the dismal prognosis of ventriculitis as a complication of CNS infections, data on clinical, microbiological and imaging features, and prognostic factors remain scarce [5, 13]. In addition, there is no consensus on the definition of ventriculitis, which precludes the interpretation of findings from small sample-size case series with heterogeneous case definitions [14]. Thus, using a definition with composite clinical, microbiological, and radiological criteria, we aimed to characterize ventriculitis and to identify risk factors of poor outcomes, especially 1-year mortality and long-term neurological impairment, through a large retrospective case series.
METHODS
Study Population and Data Collection
We performed a retrospective study of all cases of ventriculitis hospitalized in the Rennes University Hospital, a 1500-bed tertiary care hospital, which serves as a referral center for complicated CNS infections in Western France (population catchment area, 1.5 million inhabitants). Patients with suspected ventriculitis were identified from the computerized radiology database. We systematically screened the medical files of all the patients for whom the diagnosis of “ventriculitis” was mentioned, in a brain imaging study report, during years 2005–2019. Among them, only adults (age ≥18 years) who had the combination of (1) clinical signs of ventriculitis, such as fever, neck stiffness, headache, altered mental status, seizure, focal neurological sign, photophobia, or phonophobia, (2) microbiological documentation, with positive CSF, and/or blood culture, and (3) imaging features suggestive of ventriculitis were included (Supplementary Table S1). We excluded cases of neuromeningeal tuberculosis (n = 1). All imaging features were reviewed by 2 physicians, including a neuroradiologist. Data were collected using a standardized questionnaire, including demographics, comorbidities, microbiological data, imaging features, and outcomes, namely, in-hospital mortality, 1-year mortality, and neurological impairment evaluated by modified Rankin-scale (mRS), which is a disability scale ranging from 0 (no symptoms) to 6 (death).
Statistical Analysis
We first described the characteristics of survivors and nonsurvivors. Results were expressed as mean and standard deviation (SD), or median and interquartile range (IQR) for quantitative variables, and n (%) for qualitative variables. To compare the baseline characteristics between survivors and nonsurvivors, Fisher’s exact test was used for categorical variables and Mann-Whitney test or t test was used for continuous variables, when appropriate.
To evaluate risk factors associated with 1-year mortality, we performed univariate Cox analyses. Survival analysis was assessed by a log-rank test for all identified predictive factors. Kaplan-Meier plots represented survival across strata. We built an adjusted Cox proportional hazards regression model to estimate main effects, 95% confidence intervals (CIs), and statistical significance. Stepwise backward variable selection using Akaike’s information criterion was conducted to construct the final model. Results from both Cox regressions were expressed as hazard ratios (HRs) and adjusted HRs with their 95% CI. The proportional hazard assumption was tested using Schoenfeld’s test. Finally, we used variables predictive of 1-year mortality to build a risk score. The weight of each variable in the score was determined depending on the strength of its association with mortality in survival analyses. We classified patients into 3 mortality risk groups: low, intermediate, and high. Sensitivity analyses were performed to handle missing baseline variables used to build the score. P < .05 were considered as significant. Statistical analyses were carried out using R-Studio 2015, Integrated Development for R (R-Studio, Boston, MA) and “survival,” “ggplot2,” and “survminer” packages.
Patient Consent Statement
The design of the work has been approved by the Ethics Committee of Rennes University Hospital (approval number 20.115). Patients were informed about the study and could refuse to be enrolled. Written informed consent was waived.
RESULTS
Baseline Characteristics
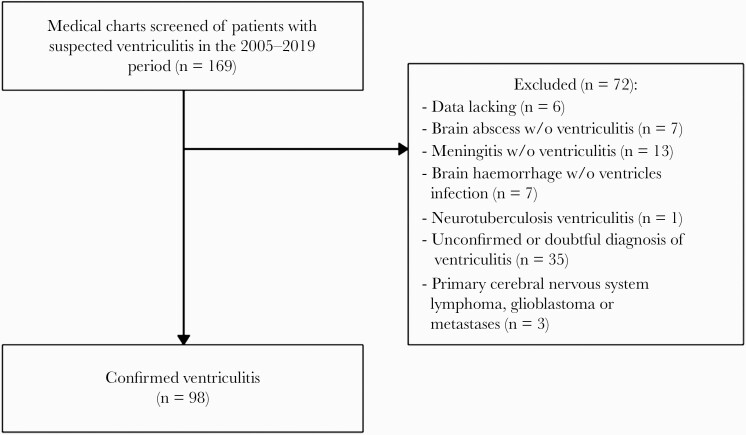
Ninety-eight patients were included in the analysis (Figure 1). The median age was 60 years (IQR, 48–68), 70.4% (n = 69) of patients were men, and 37.8% (n = 37) had a previous CNS disease. Twenty-six patients (26.5%) underwent neurosurgery within the year preceding the ventriculitis, and 20 (20.4%) had an intracranial foreign device, mainly external-ventricular drain (n = 12), and ventriculoperitoneal shunt (n = 6). A CSF leak was reported in 12 cases (12.2%). Other main comorbidities included hypertension (n = 30, 30.6%), immunosuppression (n = 30, 30.6%), diabetes mellitus (n = 19, 19.4%), and malignancy (n = 14, 14.3%).
Figure 1.
Study flowchart.
Etiologies of ventriculitis were as follows: brain abscess (29.6%), primary bacterial meningitis (27.5%), catheter-related ventricular infection (17.3%), neurosurgical site infection (13.3%), or bloodstream infection (12.2%). Ventriculitis was classified as healthcare-associated in 30 cases (30.6%).
Most patients had fever (92.2%) at initial presentation. Other common symptoms were headache (74.6%), neck stiffness (58.4%), photophobia/phonophobia (40%), and nausea (27.2%). The median Glasgow Coma Scale (GCS) score was 11 (IQR, 8–14), and 21.7% of patients (20 of 92) had a GCS score <8. Altered mental status was noted in 81 patients (82.7%). The complete triad of fever, altered mental status, and neck stiffness was found in less than one third of cases (n = 31, 31.6%). Focal neurological signs and seizures were reported in 30 (31.0%) and 18 (18.4%) patients, respectively.
Investigations
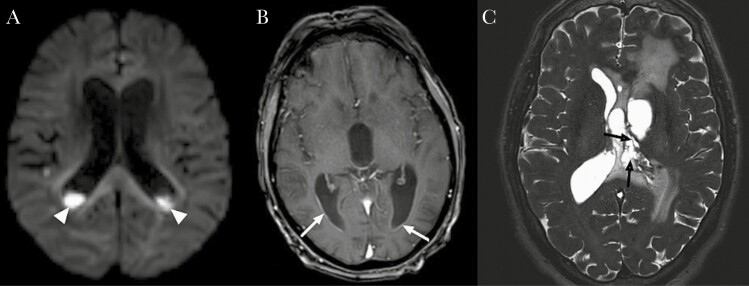
Median CSF white cell count was 2650 cell/mm3 (IQR, 651–6725), and median CSF glucose was 0.7 mmol/L (IQR, 0.1–2.2). Main pathogens were streptococci (n = 44, 44.9%), Gram-negative bacilli (n = 27, 27.6%), and staphylococci (n = 15, 15.3%). Main bacteria involved in ventriculitis were Streptococcus pneumoniae (n = 22), followed by milleri group streptococci (n = 13) and Staphylococcus aureus (n = 9). Gram-negative bacilli were common, especially Enterobacteriales (n = 15) and Pseudomonas aeruginosa (n = 4). Most common abnormalities on brain imaging were intraventricular pus (n = 81, 82.7%), ependymal enhancement (n = 70, 71.4%), and intraventricular loculations (n = 15, 15.3%) (Figure 2). Other common imaging features were brain abscess (n = 34, 34.7%), stroke (n = 24, 24.5%), vasculitis (n = 11, 11.2%), cerebral thrombophlebitis (n = 7, 7.1%), and empyema (n = 6, 6.1%).
Figure 2.
Brain imaging features of ventriculitis. (A) Diffusion-weighted image showing signal restriction (head narrows) in the occipital horn of lateral ventricles. (B) Axial gadolinium-enhanced T1-weighted: ependymal enhancement (white arrows). (C) Axial high-resolution T2-weighted: intraventricular loculations (black arrows).
Management
Neurosurgery was required for 60 patients (61.2%), including external ventricular drain insertion (n = 33, 55%), surgical drainage of collection—abscess or empyema (n = 16, 26.7%), neurosurgical device removal (n = 14, 23.3%), stereotactic drainage of brain abscess (n = 9, 15%), laminectomy (n = 4, 6.7%), surgery for CSF fistula (n = 2, 3.3%), and ventriculostomy (n = 1, 1.7%). The median duration of antibacterial treatment was 21 days (IQR, 14–42).
Outcome
In-hospital mortality was 30.6%, and 1-year mortality 38.8%. The comparison of characteristics between survivors and nonsurvivors is summarized in Tables 1 and 2. Survivors were more likely to present with neck stiffness (P = .01), whereas nonsurvivors had a lower GCS score on admission (P < .0001). Median CSF protein level was higher in nonsurvivors than in survivors (4.2 vs 2.9 g/L, P = .006). Uremia and plasma C-reactive protein were higher in nonsurvivors, whereas prothrombin ratio was lower. Microbiological parameters associated with death were (1) positive CSF culture and (2) P aeruginosa ventriculitis (Table 1). Comparison of management, imaging features, complications, and outcomes are presented in Table 2. Vasculitis and status epilepticus were more common among nonsurvivors (Table 2).
Table 1.
Baseline Characteristics and Predictors for One-Year Mortality in 98 Patients With Ventriculitis
| Characteristics | Total, n = 98 (%) | Survivor, n = 60 (%) | Nonsurvivor, n = 38 (%) | P Value |
|---|---|---|---|---|
| Baseline Characteristics Before Diagnosis of Ventriculitis | ||||
| Age, years | 60 [48–68] | 58 [47–66] | 65 [54–74] | .01 |
| Male gender | 69 (70.4) | 38 (63.3) | 31 (81.6) | .07 |
| Neurological disease | 37 (37.8) | 24 (40) | 13 (34.2) | .67 |
| Neurosurgery ≥1 year before ventriculitis | 5 (5.1) | 3 (5) | 2 (5.3) | 1 |
| Neurosurgery <1 year | 26 (26.5) | 15 (25) | 11 (28.9) | .81 |
| Neurosurgical device | 20 (20.4) | 12 (20) | 8 (21.1) | 1 |
| Ventriculoperitoneal shunt | 6 (6.1) | 5 (8.3) | 1 (2.6) | .40 |
| EVD | 12 (12.2) | 5 (8.3) | 7 (18.4) | .21 |
| EVD duration, days | 11 [9–15] | 13 [10–14] | 10 [9–15.5] | .75 |
| Craniotomia | 12 (12.2) | 8 (13.3) | 4 (10.5) | .76 |
| Head trauma | 7 (7.1) | 4 (6.7) | 3 (7.9) | 1 |
| Brain hemorrhage | 16 (16.3) | 9 (15) | 7 (18.4) | .81 |
| CSF leak | 12 (12.2) | 10 (16.7) | 2 (5.3) | .12 |
| Comorbidities | ||||
| Diabetes mellitus | 19 (19.4) | 10 (16.7) | 9 (23.7) | .44 |
| Immunosuppression | 18 (18.4) | 9 (15) | 9 (23.7) | .3 |
| Malignancy | 14 (14.3) | 5 (8.3) | 8 (21.1) | .12 |
| Alcohol abuse | 21 (21.4) | 10 (16.7) | 11 (28.9) | .21 |
| Cirrhosis | 6 (6.1) | 3 (5) | 3 (7.9) | .67 |
| Hypertension | 30 (30.6) | 15 (25) | 15 (39.5) | .18 |
| Chronic Treatment | ||||
| Corticosteroids | 10 (10.2) | 6 (10) | 4 (10.5) | 1 |
| Nonsteroidal anti-inflammatory drugs | 5 (5.1) | 4 (6.7) | 1 (2.6) | .65 |
| Symptoms at Presentation | ||||
| Fever | 83/90 (92.2) | 52/57 (91.2) | 31/33 (94) | 1 |
| Body temperature, °C | 39.0 [38.6–39.5] | 39 [38.6–39.4] | 39.1 [38.6–39.5] | .73 |
| Neck stiffness | 45/77 (58.4) | 35/51 (68.6) | 10/26 (38.5) | .01 |
| Photophobia/phonophobia | 26/65 (40) | 21/44 (47.7) | 5/21 (23.8) | .10 |
| Nausea | 18/66 (27.2) | 13/42 (31) | 5/24 (20.8) | .57 |
| Headache | 50/67 (74.6) | 36/46 (78.2) | 14/21 (66.7) | .37 |
| Glasgow Coma Scale score | 11 [8–14] | 14 [10–14] | 9 [7–13] | <.001 |
| Seizures | 18/97 (18.6) | 7/59 (11.9) | 11 (28.9) | .06 |
| Focal neurological signs | 30/95 (31.6) | 17/57 (29.8) | 13 (34.2) | .66 |
| Etiologies | ||||
| Community-acquired ventriculitisa | 68 (69.4) | 45 (73.3) | 24 (63.2) | .37 |
| Primary bacterial meningitis | 27 (27.5) | 17 (28.3) | 10 (26.3) | 1 |
| Community-acquired brain abscess(es) | 29 (29.6) | 18 (30) | 11 (28.9) | 1 |
| Hematogenousb | 12 (12.2) | 9 (15) | 3 (7.9) | .36 |
| Healthcare-associated ventriculitisc | 30 (30.6) | 16 (26.7) | 14 (36.8) | .37 |
| Neurosurgical site infectiond | 13 (13.3) | 8 (13.3) | 5 (13.2) | 1 |
| Ventricular catheter-related | 17 (17.3) | 9 (15) | 8 (21.1) | .58 |
| CSF Parameters | ||||
| Lumbar puncture | 87 (88.8) | 54 (90) | 33 (86.8) | .75 |
| White cells count/mm3 | 2650 [651–6725] | 2461 [687–6075] | 2850 [660–7250] | .60 |
| Neutrophils, % | 90 [82–96] | 86 [80–95] | 92 [86–97] | .08 |
| Protein, g/L | 3.6 [1.9–5.8] | 2.9 [1.5–4.8] | 4.2 [3–7.7] | .006 |
| Glucose, mmol/L | 0.7 [0.1–2.2] | 0.8 [0.3–2.4] | 0.5 [0.04–1.7] | .16 |
| Microbiology | ||||
| Positive CSF Gram stain | 41/87 (47) | 22/54 (40.7) | 19/33 (57.6) | .12 |
| Positive CSF culture | 53/87 (60.9) | 29/54 (53.7) | 24/33 (72.7) | .04 |
| Positive blood culture | 32/92 (34.8) | 23/57 (40.4) | 9/35 (25.7) | .18 |
| Identification by 16S rRNAe | 12/34 (35.3) | 6/22 (27.3) | 6/12 (50) | .27 |
| Staphylococci | 15 (15.3) | 8 (13.3) | 7 (18.4) | .57 |
| Staphylococcus aureus | 9 (9.1) | 5 (8.3) | 4 (10.5) | |
| Streptococci | 44 (44.9) | 30 (50) | 14 (36.8) | .22 |
| Streptococcus pneumoniae | 22 (22.4) | 15 (25) | 7 (18.4) | |
| milleri group | 13 (13.3) | 8 (13.3) | 5 (13.2) | |
| Gram-negative bacilli | 27 (27.6) | 14 (23.3) | 13 (34.2) | .26 |
| Pseudomonas aeruginosa | 4 (4.1) | 0 (0) | 4 (10.5) | .02 |
| Strict anaerobes | 9 (8.1) | 6 (10) | 3 (7.9) | 1 |
| Fungi | 1 (1) | 1 (1.7) | 0 (0) | 1 |
Abbreviations: CSF, cerebrospinal fluid; EVD, external ventricular drain; IQR, interquartile range; rRNA, ribosomal ribonucleic acid.
NOTE: Data are presented as median [IQR] or number (%).
aCommunity-acquired ventriculitis pathogens: Streptococcus pneumoniae (n = 22), other Streptococci (n = 9), Streptococcus intermedius (n = 6), Porphyromonas sp (n = 5), Streptococcus constellatus (n = 4), Pseudomonas aeruginosa (n = 2), Staphyloccocus aureus (n = 2), Escherichia coli (n = 2), Prevotella sp (n = 2), Fusobacterium sp (n = 2), Streptococcus anginosus (n = 1), Haemophilus influenzae (n = 1), Neisseria meningitidis (n = 1), Listeria monocytogenes (n = 1), Klebsiella sp (n = 1), Cutibacterium acnes (n = 1), Actinomyces meyeri (n = 1), Nocardia nova (n = 1), Aggregatibacter aphrophilus (n = 1), Parvimonas micra (n = 1), Bulleidia sp (n = 1), Eikenella corrodens (n = 1).
bHematogenous ventriculitis included sustained bacteremia due to infective endocarditis (n = 6), vertebral osteomyelitis without infective endocarditis (n = 4), arthritis (n = 1), and abdominal infection (n = 1).
cHealthcare-associated ventriculitis pathogens: S aureus (n = 7), coagulase-negative staphylococci (n = 6), Enterobacter sp (n = 5), Escherichia coli (n = 4), Klebsiella sp (n = 3), P aeruginosa (n = 2), S constellatus (n = 2), H influenzae (n = 1), Candida albicans (n = 1).
dIncluded nosocomial brain abscess (n = 3) and empyema (n = 2).
ePathogens detected by 16S rRNA were as follows, in survivors: S pneumoniae (n = 1), S intermedius (n = 1), Enterobabacter sp (n = 1), Porphyromonas gingivalis (n = 1), Porphyromonas endodontalis (n = 1), Fusobacterium nucleatum (n = 1); in nonsurvivors: Streptococcus intermedius (n = 1), Nocardia nova (n = 1), Cutibacterium acnes (n = 1), E coli (n = 2), P aeruginosa (n = 1).
Table 2.
Management and Outcomes of the 98 Patients With Ventriculitis
| Total, n = 98 | Survivor, n = 60 | Nonsurvivor, n = 38 | P Value | |
|---|---|---|---|---|
| Management | ||||
| Neurosurgery (%) | 60 (61.2) | 34 (56.7) | 26 (68.4) | .29 |
| Time from symptoms onset to anti-infective treatment start, days | 1 [0–4] | 1 [0–4] | 1 [0–4] | .77 |
| Initial intravenous anti-infective treatment | 95 (97) | 57 (95) | 38 (100) | .28 |
| Duration of anti-infective treatment, days | 21 [14–42] | 28 [18–42] | 16 [14–29] | .007 |
| Brain Imaging Features and Complications | ||||
| CT Scan | 90 (91.8) | 53 (88.3) | 37 (97.4) | .15 |
| MRI | 85 (86.7) | 55 (91.7) | 30 (78.9) | .12 |
| Intraventricular pus | 81 (82.7) | 49 (81.7) | 32 (84.2) | .79 |
| Ependymal enhancement | 70 (71.4) | 40 (66.7) | 30 (78.9) | .25 |
| Intraventricular loculations | 15 (15.3) | 10 (16.7) | 5 (13.2) | .77 |
| Brain abscess(es) | 34 (34.7) | 22 (36.7) | 12 (32.6) | .67 |
| Ischemia | 24 (24.5) | 11 (18.3) | 13 (34.2) | .09 |
| Empyema | 6 (6.1) | 2 (3.3) | 4 (10.5) | .20 |
| Acute hydrocephalus | 44 (44.9) | 22 (36.7) | 22 (57.9) | .06 |
| Intracranial hypertension/mass effect | 34 (34.7) | 18 (30) | 16 (42.1) | .28 |
| Brain herniation | 10 (10.2) | 5 (8.3) | 5 (13.2) | .50 |
| Vasculitis | 11 (11.2) | 2 (3.3) | 9 (23.7) | .003 |
| Cerebral thrombophlebitis | 7 (7.1) | 4 (6.7) | 3 (7.9) | 1 |
| Status epilepticus | 11 (11.2) | 2 (3.3) | 9 (23.7) | .003 |
| Outcomes | ||||
| In-hospital mortality | 30 (30.6) | 0 (0) | 30 (78.9) | - |
| One-year mortality | 38/93 (40.8) | 0 (0) | 38 (100) | - |
| ICU admission | 72 (73.5) | 38 (63.3) | 34 (89.5) | .005 |
| Mechanical ventilation | 59 (60.6) | 27 (45.0) | 32 (84.2) | <.001 |
| Vasopressive agents | 20/94 (21.3) | 7/58 (12.1) | 13/36 (36.1) | .009 |
| Length of hospital stay, days | 25 [16–47] | 25 [16–54] | 24 [16–45] | .86 |
Abbreviations: CT, computed tomography; ICU, intensive care unit; IQR, interquartile range; MRI, magnetic resonance imaging.
NOTE: Data are presented as median [IQR] or number (%).
At last follow-up, the mRS was 3 (IQR, 1–6) overall and 1 (IQR, 1–3) for survivors. Long-term neurological impairment was documented in 61.8% of survivors (n = 34 of 55). Main sequelae were cognitive impairment (n = 11 of 55, 20%), gait disturbances (n = 9 of 55, 16.4%), paresis (n = 7 of 55, 12.7%), behavior disorder (n = 6 of 55, 10.9%), fatigue (n = 5 of 55, 9.1%), epilepsy (n = 5 of 55, 9.1%), memory disorder (n = 5 of 55, 9.1%), hearing loss (n = 4 of 55, 7.3%), headache (n = 2 of 55, 3.6%), swallowing disorder (n = 2 of 55, 3.6%), and diplopia (n = 2 of 55, 3.6%). In addition, 18.2% of survivors (n = 10 of 55) required ventriculoperitoneal shunt due to chronic hydrocephalus postventriculitis. Imaging follow-up was performed in 69.6% of survivors (n = 39 of 56), and two thirds had improvement or normalization of imaging features (n = 26 of 39, 66.7%).
Survival Analysis and Predictive Factor of Mortality
The global 1-year survival is plotted into a Kaplan-Meier curve (Supplementary Figure S1). In univariate analysis, hazard ratios for overall mortality at 1 year were higher in patients more than 65 years old, with GCS score <13 at initial presentation, cerebral vasculitis, status epilepticus, hydrocephalus, positive CSF culture, CSF protein level >3 g/L, and P aeruginosa ventriculitis (Table 3). There was no difference in mortality according to the aetiology of ventriculitis.
Table 3.
Multivariate Analysis of Predictive Factors of Mortality
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Characteristics | HR (95% CI) | P Value | Adjusted HR (95% CI) | P Value |
| Comorbidities | ||||
| Age >65 years | 2.4 (1.25–4.50) | .008a | 3.2 (1.39–7.18) | .006 |
| Male sex | 2.1 (0.93–4.81) | .07a | ||
| CSF leak | 0.4 (0.09–1.48) | .16 | ||
| Malignancy | 2.1 (0.94–4.50) | .07 | ||
| Alcohol abuse | 1.8 (0.91–3.68) | .09 | ||
| Hypertension | 1.6 (0.85–3.12) | .14 | ||
| Diabetes | 1.4 (0.66–2.96) | .38 | ||
| Neurological Complications | ||||
| Initial GCS score <13 | 3.1 (1.48–6.45) | .003a | 2.7 (1.13–6.53) | .02 |
| Acute hydrocephalus | 2.0 (1.04–3.78) | .04a | 2.4 (1.1–5.43) | .03 |
| Empyema | 2.8 (1.01–8.02) | .05a | ||
| Stroke | 2.2 (1.10–4.3) | .03a | ||
| Cerebral vasculitis | 6.8 (3.13–14.77) | <.0001a | ||
| Seizures at presentation | 2.0 (0.97–3.96) | .06 | ||
| Status epilepticus | 4.3 (2.02–9.19) | .0002a | 3.9 (1.49–10.04) | .005 |
| Microbiology and CSF Parameters | ||||
| Positive CSF direct examination | 2.0 (0.99–4.11) | .05 | ||
| Positive CSF culture | 2.5 (1.11–5.5) | .03a | 2.5 (1.06–5.89) | .04 |
| CSF protein >3 g/L | 3.1 (1.33–7.12) | .009a | ||
| Gram-negative bacilli | 1.6 (0.80–3.08) | .19 | ||
| Pseudomonas aeruginosa | 3.6 (1.25–10.35) | .02a | ||
Abbreviations: CI, confidence interval; CSF, cerebrospinal fluid; GCS, Glasgow coma scale; HR, hazard ratio.
aVariables included in the backward stepwise selection.
We included 98 patients, 93 of whom with a follow-up over 1 year in the multivariable Cox regression model. Five patients were lost to follow-up before 1 year and data were censored at time of last follow-up. Risk factors for death in the multivariable Cox regression model were as follows: age >65 years, GCS score <13 at initial presentation, status epilepticus, hydrocephalus, and positive CSF culture. The C-index of the final model was 0.788. There was no time-dependent effect of these factors, using a global Schoenfeld test (P = .17). For each predictive factor, a Kaplan-Meier plot was generated (Supplementary Figure S1).
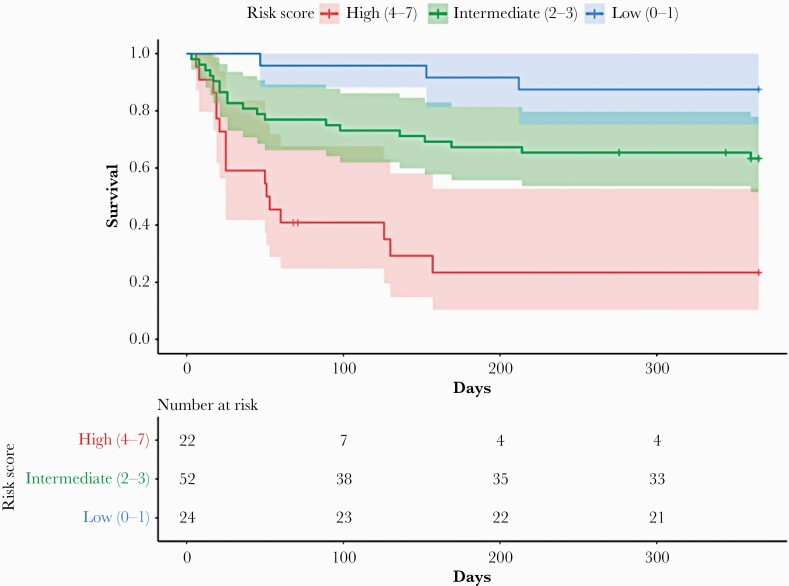
We used the identified predictive factors of 1-year mortality to create a risk score (Table 4). Patients were stratified into 3 categories: low risk (score 0–1), intermediate risk (2–3), and high risk (4–7). One-year mortality was 12.5% in the low-risk group, 36.5% in the intermediate-risk group, and 71.4% in the high-risk group (Figure 3). Using the low-risk group as the baseline comparator, 1-year mortality was increased in the intermediate-risk group (hazard ratio, 3.6; 95% CI, 1.05–12.05; P = .04) and in the high-risk-group (hazard ratio, 12.0; 95% CI, 3.40–42.53; P < .001).
Table 4.
Score System to Predict One-Year Mortality
| Parameter | Score |
|---|---|
| Age, Years | |
| >65 | 2 |
| <65 | 0 |
| Status Epilepticus | |
| Yes | 2 |
| No | 0 |
| Hydrocephalus | |
| Yes | 1 |
| No | 0 |
| CSF Culture | |
| Positive | 1 |
| Negative | 0 |
| Initial GCS Score | |
| <13 | 1 |
| ≥13 | 0 |
| Risk Group | |
| - Low | 0–1 |
| - Intermediate | 2–3 |
| - High | 4–7 |
In bold: total sum score.
Abbreviations: CSF, cerebrospinal fluid; GCS, Glasgow coma scale.
Figure 3.
Kaplan-Meier survival estimates using 1-year mortality risk score.
DISCUSSION
The primary findings of this case series of ventriculitis are as follows: (1) most cases are associated with brain abscess (32.7%), bacterial meningitis (27.6%), or intraventricular catheter (17.3%); (2) characteristic brain imaging features include intraventricular pus (82.7%), ependymal enhancement (71.4%), and intraventricular loculations (15.3%); (3) main pathogens are streptococci (44.9%), Gram-negative bacilli (27.6%), and staphylococci (15.3%); (4) the prognosis is poor, with in-hospital mortality rates of 30.6%, whereas 61.8% of survivors suffer from long-term neurological sequelae.
To the best of our knowledge, this is by far the largest case series on ventriculitis in the medical literature to date. Although ventriculitis is mentioned as a dreaded complication of various categories of CNS infections, it has been quite neglected thus far, with no consensual diagnostic criteria [15]. Lyke et al [7] defined nosocomial ventriculitis as a positive culture of a CSF obtained from an intraventricular catheter at least 2 days after it was inserted. Lozier et al defined [16] ventriculitis in patients who underwent ventriculostomy as the association of clinical and biological signs of meningitis, whatever the results of CSF culture and brain neuroimaging. However, advances in brain imaging, particularly magnetic resonance imaging (MRI), has dramatically improved our ability to diagnose ventriculitis.
Previous studies on EVD-related ventriculitis identified EVD duration >11 days, iterative CSF sampling, and intraventricular hemorrhage as the 3 main risk factors for ventriculitis in patients with EVD [9]. In our study, the proportion of ventriculitis classified as EVD-related was low (12.2%), but this may differ from one site to another, due to case mix. Indeed, the risk of ventriculitis after EVD implantation was recently estimated between 4.8 to 12.7 cases per 1000 EVD-days [17]. Regarding microbiology, previous studies identified Gram-negative bacilli and staphylococci as the 2 main categories of pathogens responsible for ventriculitis [14, 18, 19], but these studies were all of limited sample size (n < 20). In our study, streptococci were the leading causative species (44.9%), with S pneumoniae as the leading cause of ventriculitis in patients with bacterial meningitis and milleri group streptococci as the a major cause of ventriculitis related to brain abscesses [20, 21]. A recent review of primary bacterial ventriculitis complicating community-acquired meningitis also identified S pneumoniae as the primary pathogen. Of note, the authors only found 7 cases in the medical literature [22], whereas our single-center case series identified 27 well documented cases, which outlines that ventriculitis has been dramatically neglected thus far.
To the best of our knowledge, the largest study on imaging features of pyogenic ventriculitis to date included 17 patients [23]. Intraventricular pus, visible in restricted water diffusion, was the most frequent sign, followed by ependymal enhancement and hydrocephalus. Intraventricular loculations were rarely observed, whereas a high proportion of patients presented with stroke and/or vasculitis. The sensitivity and specificity of tomography and brain MRI may be suboptimal [24]: indeed, ventricular pus may be misinterpreted as intraventricular hemorrhage, especially in the absence of ependymal enhancement. However, progress in brain imaging, especially MRI techniques, have improved our ability to identify various complications of meningitis, including ventriculitis [25, 26].
A major finding of our study is the poor prognosis of ventriculitis, with 1-year mortality rate close to 40%, compared to 10%–20% in contemporary cohorts of bacterial meningitis and brain abscess [27–30]. In addition, 60% of patients who survived after ventriculitis presented with long-term neurological sequelae. A recent study of meningitis/ventriculitis due to Acinetobacter sp after neurosurgery found similar mortality rate (40%). Glasgow Coma Scale <8 on admission, age >40 years, EVD, comorbidities, and CSF white cell count >200/mm3 were associated with increased risk of death [31]. In our final Cox model, we found that factors associated with mortality were as follows: (1) age >65 years, (2) GCS score <13 on admission, (3) status epilepticus, (4) hydrocephalus, and (5) positive CSF culture. These prognostic factors are in line with those that have been associated with other CNS infections [3, 32, 33]. We found that CSF culture yielding P aeruginosa was a risk factor for death in univariate, but not in our multivariate analysis. One study found that CSF culture positive for P aeruginosa was associated with higher mortality in EVD infections [34]. We built a risk-score taking into account the main risk factors identified in multivariate analysis. Although this score would require external validation before large application, it may be of interest to identify very high-risk patients in whom closer monitoring and/or additional interventions may be warranted.
This study has limitations. First, as a single-center study, our findings may not be generalizable: For example, the proportion and characteristics of healthcare-associated ventriculitis may differ according to case mix. Second, this study suffers from potential biases inherent to its observational retrospective design, although the proportion of missing data remains low. Third, due to the absence of written protocol, and the long study period (2005–2019), the management of ventriculitis was not standardized. As a consequence, our study was not powered to analyze the impact of different antibacterial treatment regimens or the benefit of their intraventricular administration [35]. Fourth, risk scores never perform as well as in the derivation cohort. Hence, its validation in a different cohort is necessary.
However, our study has strength, including the use of a stringent definition, combining clinical, microbiological, and neuroimaging criteria, which ensures that all patients included indeed had infectious ventriculitis. In addition, because patients were identified through systematic screening of all brain imaging studies performed during the study period, this study provides a comprehensive description of contemporary ventriculitis, whatever the mechanisms, with a sample size much larger than previous studies.
CONCLUSIONS
In conclusion, ventriculitis is a severe complication of brain abscess, meningitis, or neurosurgery, with an in-hospital mortality rate of 30% and neurological sequelae in 60% of survivors. The advent in brain imaging studies with expanded access to brain MRI has improved our ability to diagnose this neglected disease. Prospective multicenter studies are requested to better define optimal management of this dreaded complication of CNS infection.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Tunkel AR, Hasbun R, Bhimraj A, et al. 2017 Infectious Diseases Society of America’s Clinical Practice Guidelines for Healthcare-Associated Ventriculitis and Meningitis. Clin Infect Dis 2017; 64:e34–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Durand M, Calderwood MS, Weber DJ, et al. Acute bacterial meningitis in adults. N Engl J Med 1973; 328:21–8. [DOI] [PubMed] [Google Scholar]
- 3. Bijlsma MW, Brouwer MC, Kasanmoentalib ES, et al. Community-acquired bacterial meningitis in adults in the Netherlands, 2006–14: a prospective cohort study. Lancet Infect Dis 2016; 16:339–47. [DOI] [PubMed] [Google Scholar]
- 4. Costerus JM, Brouwer MC, Sprengers MES, et al. Cranial computed tomography, lumbar puncture, and clinical deterioration in bacterial meningitis: a nationwide cohort study. Clin Infect Dis 2018; 67:920–6. [DOI] [PubMed] [Google Scholar]
- 5. Hazany S, Go JL, Law M. Magnetic resonance imaging of infectious meningitis and ventriculitis in adults. Top Magn Reson Imaging 2014; 23:315–25. [DOI] [PubMed] [Google Scholar]
- 6. Harris L, Munakomi S. Ventriculitis. StatPearls. Treasure Island, FL: StatPearls Publishing; 2020. [Google Scholar]
- 7. Lyke KE, Obasanjo OO, Williams MA, et al. Ventriculitis complicating use of intraventricular catheters in adult neurosurgical patients. Clin Infect Dis 2001; 33:2028–33. [DOI] [PubMed] [Google Scholar]
- 8. Pandey S, Li L, Deng XY, et al. Outcome following the treatment of ventriculitis caused by multi/extensive drug resistance Gram negative bacilli; Acinetobacter baumannii and Klebsiella pneumonia. Front Neurol 2019; 9:1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beer R, Lackner P, Pfausler B, Schmutzhard E. Nosocomial ventriculitis and meningitis in neurocritical care patients. J Neurol 2008; 255:1617–24. [DOI] [PubMed] [Google Scholar]
- 10. Pickering LK, Ericsson CD, Ruiz-Palacios G, et al. Intraventricular and parenteral gentamicin therapy for ventriculitis in children. Am J Dis Child 1978; 132:480–3. [DOI] [PubMed] [Google Scholar]
- 11. Dallacasa P, Dappozzo A, Galassi E, et al. Cerebrospinal fluid shunt infections in infants. Childs Nerv Syst 1995; 11:643–8; discussion 649. [DOI] [PubMed] [Google Scholar]
- 12. Srihawan C, Habib O, Salazar L, Hasbun R. Healthcare-associated meningitis or ventriculitis in older adults. J Am Geriatr Soc 2017; 65:2646–50. [DOI] [PubMed] [Google Scholar]
- 13. Buckwold FJ, Hand R, Hansebout RR. Hospital-acquired bacterial meningitis in neurosurgical patients. J Neurosurg 1977; 46:494–500. [DOI] [PubMed] [Google Scholar]
- 14. Agrawal A, Cincu R, Timothy J. Current concepts and approach to ventriculitis. Infect Dis Clin Pract 2008; 16:100–4. [Google Scholar]
- 15. Guanci MM. Ventriculitis of the central nervous system. Crit Care Nurs Clin North Am 2013; 25:399–406. [DOI] [PubMed] [Google Scholar]
- 16. Lozier AP, Sciacca RR, Romagnoli MF, Connolly ES Jr. Ventriculostomy-related infections: a critical review of the literature. Neurosurgery 2002; 51:170–81; discussion 181–2. [DOI] [PubMed] [Google Scholar]
- 17. Humphreys H, Jenks P, Wilson J, et al. ; Healthcare Infection Society Working Party on Neurosurgical Infections . Surveillance of infection associated with external ventricular drains: proposed methodology and results from a pilot study. J Hosp Infect 2017; 95:154–60. [DOI] [PubMed] [Google Scholar]
- 18. Mangi RJ, Holstein LL, Andriole VT. Treatment of Gram-negative bacillary meningitis with intrathecal gentamicin. Yale J Biol Med 1977; 50:31–41. [PMC free article] [PubMed] [Google Scholar]
- 19. Kaiser AB, McGee ZA. Aminoglycoside therapy of Gram-negative bacillary meningitis. N Engl J Med 1975; 293:1215–20. [DOI] [PubMed] [Google Scholar]
- 20. Sonneville R, Ruimy R, Benzonana N, et al. ; ESCMID Study Group for Infectious Diseases of the Brain (ESGIB) . An update on bacterial brain abscess in immunocompetent patients. Clin Microbiol Infect 2017; 23:614–20. [DOI] [PubMed] [Google Scholar]
- 21. Helweg-Larsen J, Astradsson A, Richhall H, et al. Pyogenic brain abscess, a 15 year survey. BMC Infect Dis 2012; 12:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lesourd A, Magne N, Soares A, et al. Primary bacterial ventriculitis in adults, an emergent diagnosis challenge: report of a meningoccal case and review of the literature. BMC Infect Dis 2018; 18:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fukui MB, Williams RL, Mudigonda S. CT and MR imaging features of pyogenic ventriculitis. 2001; 22:1510–6. [PMC free article] [PubMed] [Google Scholar]
- 24. Busl KM. Nosocomial infections in the neurointensive care unit. Neurosurg Clin N Am 2018; 29:299–314. [DOI] [PubMed] [Google Scholar]
- 25. Mohan S, Jain KK, Arabi M, Shah GV. Imaging of meningitis and ventriculitis. Neuroimaging Clin N Am 2012; 22:557–83. [DOI] [PubMed] [Google Scholar]
- 26. Pezzullo JA, Tung GA, Mudigonda S, Rogg JM. Diffusion-weighted MR imaging of pyogenic ventriculitis. AJR Am J Roentgenol 2003; 180:71–5. [DOI] [PubMed] [Google Scholar]
- 27. Thigpen MC, Messonnier NE, Hadler JL, Reingold A, et al. Bacterial meningitis in the United States, 1998–2007. N Engl J Med 2011; 364:2016–25. [DOI] [PubMed] [Google Scholar]
- 28. de Gans J, van de Beek D; European Dexamethasone in Adulthood Bacterial Meningitis Study Investigators . Dexamethasone in adults with bacterial meningitis. N Engl J Med 2002; 347:1549–56. [DOI] [PubMed] [Google Scholar]
- 29. Brouwer MC, Coutinho JM, van de Beek D. Clinical characteristics and outcome of brain abscess: systematic review and meta-analysis. Neurology 2014; 82:806–13. [DOI] [PubMed] [Google Scholar]
- 30. Brouwer MC, Tunkel AR, McKhann GM 2nd, van de Beek D. Brain abscess. N Engl J Med 2014; 371:447–56. [DOI] [PubMed] [Google Scholar]
- 31. Sharma R, Goda R, Borkar SA, et al. Outcome following postneurosurgical Acinetobacter meningitis: an institutional experience of 72 cases. Neurosurg Focus 2019; 47:E8. [DOI] [PubMed] [Google Scholar]
- 32. Betjemann JP, Lowenstein DH. Status epilepticus in adults. Lancet Neurol 2015; 14:615–24. [DOI] [PubMed] [Google Scholar]
- 33. Hirsch LJ, Gaspard N, van Baalen A, et al. Proposed consensus definitions for new-onset refractory status epilepticus (NORSE), febrile infection-related epilepsy syndrome (FIRES), and related conditions. Epilepsia 2018; 59: 739–44. [DOI] [PubMed] [Google Scholar]
- 34. Sam JE, Lim CL, Sharda P, Wahab NA. The organisms and factors affecting outcomes of external ventricular drainage catheter-related ventriculitis: a penang experience. Asian J Neurosurg 2018; 13:250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lewin JJ 3rd, Cook AM, Gonzales C, et al. Current practices of intraventricular antibiotic therapy in the treatment of meningitis and ventriculitis: results from a multicenter retrospective cohort study. Neurocrit Care 2019; 30:609–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.