Abstract.
Crimean-Congo hemorrhagic fever (CCHF) is endemic in Africa, but the epidemiology remains to be defined. Using a broad database search, we reviewed the literature to better define CCHF evidence in Africa. We used a One Health approach to define the impact of CCHF by reviewing case reports, human and animal serology, and records of CCHF virus (CCHFV) isolations (1956–mid-2020). In addition, published and unpublished collection data were used to estimate the geographic distribution of Hyalomma ticks and infection vectors. We implemented a previously proposed classification scheme for organizing countries into five categories by the level of evidence. From January 1, 1956 to July 25, 2020, 494 CCHF cases (115 lethal) were reported in Africa. Since 2000, nine countries (Kenya, Mali, Mozambique, Nigeria, Senegal, Sierra Leone, South Sudan, Sudan, and Tunisia) have reported their first CCHF cases. Nineteen countries reported CCHF cases and were assigned level 1 or level 2 based on maturity of their surveillance system. Thirty countries with evidence of CCHFV circulation in the absence of CCHF cases were assigned level 3 or level 4. Twelve countries for which no data were available were assigned level 5. The goal of this review is to inform international organizations, local governments, and healthcare professionals about shortcomings in CCHF surveillance in Africa to assist in a movement toward strengthening policy to improve CCHF surveillance.
INTRODUCTION
Crimean-Congo hemorrhagic fever (CCHF) is a severe tick-borne zoonosis caused by Crimean-Congo hemorrhagic fever virus (CCHFV; Bunyavirales: Nairoviridae: Orthonairovirus).1 CCHF was first described during an outbreak among Soviet military personnel stationed in Crimea in 1944–1945.2–4 CCHF is broadly endemic in both Africa and Eurasia, with more than 30 countries having reported cases since the first cases emerged in Crimea.1,5–7 CCHF epidemiology in Africa is not well described. However, there has been a global increase in the number of reported CCHF cases since 2000, with nine countries in Africa (Table 1) and 11 countries in Asia7 reporting their first confirmed human CCHF cases.
Table 1.
Total confirmed CCHF cases in Africa by country from 1956 to July 2020
| Country (current designation) | Total confirmed cases | Total deaths | Year(s) | References |
|---|---|---|---|---|
| Burkina Faso | 1 | 0 | 1983 | 80 |
| Central African Republic | 1 | 0 | 1976 | 5 |
| Democratic Republic of the Congo | 3 | 1 | 1956, 2008 | 43,46 |
| Egypt | 4 | 1 | 1981, 2012 | 68,75 |
| Kenya | 6 | 1 | 2000, 2010, 2020 | 25,88,89 |
| Mali | 40 | 7 | 2009–2013, 2017, 2020 | 90–92 |
| Mauritania | 50 | 12 | 1983, 1988, 2003, 2007, 2012, 2017, 2018, 2019 | 57,81–86,93 |
| Mozambique | 8 | 0 | 2015 | 94 |
| Namibia | 20 | 10 | 1986–2001, 1987, 2002, 2010, 2014, 2017–2019 | 57,95–104 |
| Nigeria | 50 | 0 | 2010–2014 | 34 |
| Senegal | 8 | 1 | 2003, 2004, 2015, 2017, 2020 | 57,105–109 |
| Sierra Leone | 1 | 0 | 2016 | 110 |
| South Africa | 215 | 52 | 1981–2018, 2018, 2019 | 58–62 |
| South Sudan | 1 | 0 | 2013 | 111 |
| Sudan | 34 | 16 | 2008, 2009, 2010, 2013, 2014, 2015–2016, 2018 | 29,57,69–73 |
| Tanzania | 1 | 0 | 1986 | 112 |
| Tunisia | 5 | 0 | 2014 | 74 |
| Uganda | 45 | 13 | 1958–1965, 1963–1964, 1967, 1972, 1978, 2013, 2015, 2017, 2018, 2019, 2020 | 5,43,67,113–128 |
| Zimbabwe | 1 | 1 | 1997 | 129 |
CCHF = Crimean-Congo hemorrhagic fever. Total deaths are those among confirmed cases only. Therefore, lethality was not calculated. We were limited to available data, but this approach likely underestimates the true CCHF burden.
CCHFV virus is transmitted to humans via tick bites or through direct contact with infected animals or infected people. In the CCHFV life cycle, ticks (mainly of the species Hyalomma marginatum and Hyalomma rufipes) are both reservoirs and vectors.5,8 Many mammals (including cattle, dromedaries, goats, and sheep), reptiles, and some birds (in particular ostriches) can be infected with the virus and remain asymptomatic while the virus amplifies. As a result, livestock, animal herders, slaughterhouse workers, and healthcare workers in endemic areas are at high risk of acquiring infection.8–11
Most CCHF cases are mild or asymptomatic. Mild cases may present with nonspecific symptoms or clinical signs, such as headache, myalgia, joint pain, fever, nausea, and vomiting. A small proportion of cases are severe with sudden onset, quickly developing bruising, and severe hemorrhage. Death may occur within days of disease onset.3,12–18 CCHF lethality (5–80%) has been associated with different factors, such as access to medical care, age, virus strain, preexisting medical conditions, and route of transmission.19–22
Most reports on first autochthonous CCHF cases in individual countries were preceded by epidemiologic surveys or CCHFV isolation from ticks that provided evidence of local CCHFV circulation. For example, in Kenya (then British Kenya), evidence of CCHFV infection may have been described in as early as 1961,23 and serological evidence of human CCHFV infection was first obtained in the early 1980s.24 CCHFV was first isolated from ticks in Kenya in 1975.5 Yet, it was not until 2000 that the first Kenyan human case was encountered.25 In Sudan, serologic evidence of CCHFV in animals was obtained in 198626 and in humans in 1989.27 CCHFV was identified in ticks collected during an outbreak in 1995.28 Yet, it was not until 2008 that the first human case was described.29 In Nigeria, serologic evidence of CCHFV was first obtained in animals in the early 1960s30 and in humans in 1973.31 CCHFV was identified in Nigerian ticks in 1964.32,33 Yet, the first human case was identified in 2010.34
Thus, little is known about CCHF epidemiology in Africa, and published epidemiologic/epizootiologic data are mostly from outbreak investigations and rarely systematic. In a multistage analysis, a cohesive framework was developed to assess substantial pandemic potential for endemic high-consequence infectious disease in Africa, and CCHFV was identified with substantial index case, outbreak, epidemic, and pandemic potential compared with Ebola and Marburg virus disease and Lassa fever.35 A predictive model of CCHF distribution in Africa also pinpointed large areas in Southern, Eastern, Central, and Western Africa as being suitable for CCHFV transmission.36 The absence of additional epidemiological data is the consequence of only a few African countries entertaining active CCHF (V) surveillance systems.37
This article focuses on Africa to recognize highly CCHF-affected countries with an emphasis on regions that may not have been (known to be) CCHFV endemic in the past. We used a One Health approach to integrate vector, animal, human, and virus data to define disease status in each country. This article is in follow-up of our previous publication on Southern and Western Asia7 and is part of a series of publications mapping CCHF in the world.
METHODS
We searched PubMed, GenBank, GIDEON, Google Scholar, Scopus, ProMED, and Web of Science, among other more minor databases, for records indexed from the original descriptions of CCHF in 1944 through July 25, 2020 to identify and review the scientific literature on reported CCHF cases from all countries in Africa as defined by the UN Geoscheme (Eastern Africa: British Indian Ocean Territory; Burundi; Comoros; Djibouti; Eritrea; Ethiopia; French Southern Territories; Kenya; Madagascar; Malawi; Mauritius; Mayotte; Mozambique; Réunion; Rwanda; Seychelles; Somalia; South Sudan; Uganda; United Republic of Tanzania; Zambia; and Zimbabwe; Middle Africa: Angola; Cameroon; Central African Republic; Chad; Democratic Republic of the Congo [COD]; Equatorial Guinea; Gabon; Republic of the Congo; and São Tomé and Príncipe; Northern Africa: Algeria; Egypt; Libya; Morocco; Sudan; Tunisia; and Western Sahara; Southern Africa: Botswana; Eswatini; Lesotho; Namibia; South Africa; Western Africa: Benin; Burkina Faso; Cabo Verde; Côte d’Ivoire; Gambia; Ghana; Guinea; Guinea-Bissau; Liberia; Mali; Mauritania; Niger; Nigeria; Saint Helena, Ascension, and Tristan da Cunha; Senegal; Sierra Leone; and Togo).38
Our review included articles published in any language. We also collected information from conference presentations (if indexed in the listed electronic databases) and unpublished data/reports through personal communications. We accessed available online government reports (the National Institute for Communicable Diseases [NICD] in South Africa) and communicated with government officials (Ministries of Health in South Africa, Uganda, Zimbabwe, Kenya, and Mozambique) for confirmation of unpublished data. In addition, we accessed and reviewed published animal and human serology data, CCHFV detection or isolation data from ticks or vertebrates, and information regarding CCHFV surveillance systems. We also reviewed the U.S. National Tick Collection database39 for data that indicated the presence of CCHFV vectors in any geographic area of interest. We limited our search and consideration to H. marginatum and H. rufipes ticks because of their undisputed capacity to transmit CCHFV transstadially, transovarially, and to animals.40,41 We used Boolean combinations of search terms, including “Hyalomma,” “CCHFV,” “CCHF,” “CHF,” “Crimean,” “Crimean-Congo,” “Congo-Crimean,” “Congo virus”; “Crimean hemorrhagic fever”; “nairovirus,” and “orthonairovirus” and the names of each of the countries (or their predecessor names).
One Health country-level classification scheme.
We used a classification scheme developed by our team and previously published in an article that focused on epidemiology of CCHF in Southern and Western Asia.7 This classification system integrated vector, animal, and human data to identify CCHFV circulation in an area of interest. Countries were classified as follows: level 1 CCHF cases reported annually through established surveillance; level 2 CCHF cases reported intermittently in absence of robust surveillance; level 3 no CCHF cases reported and no robust surveillance established, but available data point toward the possibility of undetected/unreported CCHF cases (animal/human serology, CCHFV detected in Hyalomma ticks); level 4 no CCHF cases reported and no robust surveillance or epidemiologic/epizootiologic studies, but Hyalomma ticks are present; and level 5 no available data.7
RESULTS
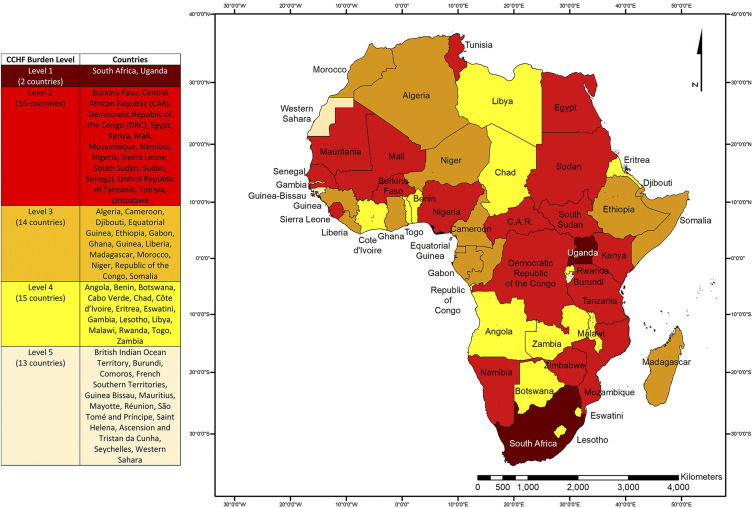
From January 1, 1956 to July 25, 2020, 19 African countries reported a total of at least 494 human CCHF cases (115 lethal), with the first case being described in COD. The regional distribution of the countries was as follows: six were in Eastern Africa, two in Middle Africa, three in Northern Africa, two in Southern Africa, and six in Western Africa (Tables 2–5). Most cases were reported from Mali, Mauritania, Namibia, Nigeria, South Africa, Sudan, and Uganda. Most countries reported cases randomly or through outbreak investigations, but South Africa and Uganda used established surveillance systems. All countries were organized into five categories by the level of evidence (Figure 1). Since 2000, nine countries (Kenya, Mali, Mozambique, Nigeria, Senegal, Sierra Leone, South Sudan, Sudan, and Tunisia) have reported their first cases (Table 5), most of which were identified via random detection in people with acute febrile disease or through outbreak investigations.
Table 2.
Current evidence for CCHFV circulation in Eastern Africa
| Country (current designation) | CCHF cases reported | Human serology | Animal serology | Hyalomma ticks | CCHFV detected in ticks | Level of evidence |
|---|---|---|---|---|---|---|
| British Indian Ocean Territory | No | No | No | No | No | 5 |
| Burundi | No | No | No | No | No | 5 |
| Comoros | No | No | No | No | No | 5 |
| Djibouti | No | No | 1992130 | Yes131 | 2010132 | 3 |
| Eritrea | No | No | No | Yes39 | No | 4 |
| Ethiopia | No | No | No | Yes133 | 1975134 | 3 |
| French Southern Territories | No | No | No | No | No | 5 |
| Kenya | 2000, 2010, 202025,88,89 | 1980, 1983, 1987, 2010, 2009–2012, 202024,88,89,135–137 | 1961, 1972, 1974, 19865,26,44,138 | Yes39 | 1975,20085,139 | 2 |
| Madagascar | No | 1989, 2008140,141 | No | No142 | 1985143 | 3 |
| Malawi | No | No | No | Yes131,144 | No | 4 |
| Mauritius | No | No | No | No | No | 5 |
| Mayotte | No | No | No | No | No | 5 |
| Mozambique | 201594 | No | No | Yes39,145 | No | 2 |
| Réunion | No | No | No | No | No | 5 |
| Rwanda | No | No | No | Yes131 | No | 4 |
| Seychelles | No | No | No | No | No | 5 |
| Somalia | No | No | 1993, 1994, 1996146–148 | Yes39 | 1994, 1996, 2009147–149 | 3 |
| South Sudan | 2013111 | No | No | Yes150 | No | 2 |
| Uganda | 1958–1965,1963–1964,1967,1972,1978,2013,2015,2017,2018,2019,20205,43,67,113–128 | 1984, 2006151,152 | 1970, 1972138,153 | Yes154 | 1970, 1978, 1981, 20155,67,155,156 | 1 |
| United Republic of Tanzania | 1986112 | No | 19745 | Yes39,157 | No | 2 |
| Zambia | No | No | No | Yes39 | No | 4 |
| Zimbabwe | 1997129 | 1980158 | 1964–1985, 1973–1978112,159 | Yes39 | No | 2 |
CCHF = Crimean-Congo hemorrhagic fever; CCHFV = Crimean-Congo hemorrhagic fever virus. Years are listed if there is evidence of anti-CCHFV antibodies in humans or animals, CCHFV vector endemicity, or CCHFV antigen or genome detection.
Table 5.
Since 2000, nine countries have reported their first autochthonous CCHF cases in Africa
Figure 1.
Evidence of CCHF in Africa using a One Health approach. The following level classification is applied: level 1, CCHF cases reported annually through established surveillance; level 2, CCHF cases reported intermittently in absence of robust surveillance; level 3, no CCHF cases reported and no robust surveillance established, but available data point toward the possibility of undetected/unreported CCHF cases (animal/human serology, CCHFV detected in Hyalomma ticks); level 4, no CCHF cases reported and no robust surveillance or epidemiologic/epizootiologic studies, but Hyalomma ticks present; and level 5, no available data. Classification at the country level was performed for policy implications. Country boundaries do not necessarily reflect the geographic area at risk and are not necessarily endorsed by the authors. The map was created using ArcGIS Release 10.61. Source: Database of Global Administrative Areas. This figure appears in color at www.ajtmh.org.
Table 3.
Current evidence for CCHFV circulation in Middle, Northern, and Southern Africa
| Country (current designation) | CCHF cases reported | Human serology | Animal serology | Hyalomma ticks | CCHFV detected in ticks | Level of evidence |
|---|---|---|---|---|---|---|
| Middle Africa | ||||||
| Angola | No | No | No | Yes160 | No | 4 |
| Cameroon | No | 1985, 2005–2012161,162 | 2013163 | Yes39 | 2015163 | 3 |
| Central African Republic | 19765 | 1966–1979, 1979, 1979–1982, 1984 1980–1985, 199349–52,164,165 | 1979–1982, 1983, 1988, 199250,51,53,54 | Yes39 | 1972–1979, 1973, 19755,55,56 | 2 |
| Chad | No | No | No | Yes39,166 | No | 4 |
| Democratic Republic of the Congo | 1956, 200843,46 | No | 2013163,167 | Yes168,169 | No | 2 |
| Equatorial Guinea | No | 1985161 | No | No | No | 3 |
| Gabon | No | No | 2005, 2008, 2009170 | No | No | 3 |
| Republic of the Congo | No | 1985161 | No | No | No | 3 |
| São Tomé and Príncipe | No | No | No | No | No | 5 |
| Northern Africa | ||||||
| Algeria | No | No | No | Yes39,171 | 2009172 | 3 |
| Egypt | 1981, 201268,75 | 1976173 | 1976, 1986, 2004, 200926,76–78,173 | Yes39 | 2009149 | 2 |
| Libya | No | No | No | Yes39 | No | 4 |
| Morocco | No | No | No | Yes39 | 2011174 | 3 |
| Sudan | 2008, 2009, 2010, 2013, 2014, 2015–2016, 201829,57,69–73 | 1989, 1998, 2012, 2015–201627,69,175,176 | 1986–1987, 1994, 2013, 2014, 201526,177–180 | Yes39 | 1995, 2009, 201728,149,181 | 2 |
| Tunisia | 201474 | 201474 | No | Yes39 | No | 2 |
| Western Sahara | No | No | No | No | No | 5 |
| Southern Africa | ||||||
| Botswana | No | No | No | Yes39,64 | No | 4 |
| Eswatini | No | No | No | Yes65 | No | 4 |
| Lesotho | No | No | No | Yes66 | No | 4 |
| Namibia | 1986–2001, 1987 2002, 2010, 2014, 2017–201957,95–104 | 1984182 | No | Yes183 | No | 2 |
| South Africa | 1981–2018, 2018, 201958–62 | 1981, 1983, 1984–1988, 2016184–188 | 1964–1985, 1981, 1983, 1984, 1984–1988, 1993, 200210,112,159,184,186,187,189–194 | Yes39 | 1981104,184 | 1 |
CCHF = Crimean-Congo hemorrhagic fever; CCHFV = Crimean-Congo hemorrhagic fever virus. Years are listed if there is evidence of anti-CCHFV antibodies in humans or animals, CCHFV vector endemicity, or CCHFV antigen or genome detection.
Table 4.
Current evidence for CCHFV circulation in Western Africa
| Country (current designation) | CCHF cases reported | Human serology | Animal serology | Hyalomma ticks | Virus detected in ticks | Level of evidence |
|---|---|---|---|---|---|---|
| Benin | No | 1981–1983195 | No | Yes39,196 | No | 3 |
| Burkina Faso | 198380 | 198380,197 | No | Yes198,199 | No | 2 |
| Cabo Verde | No | No | No | Yes200 | No | 4 |
| Côte d'Ivoire | No | No | No | Yes201,202 | No | 4 |
| Gambia | No | No | No | Yes203 | No | 4 |
| Ghana | No | 201119 | 2009170 | Yes39,204 | 201119 | 3 |
| Guinea | No | No | No | Yes205,206 | 1978–1991207 | 3 |
| Guinea-Bissau | No | No | No | No | No | 5 |
| Liberia | No | 1981208 | No | No | No | 3 |
| Mali | 2009–2013, 2017, 202090–92 | 1991, 2009–201390,209 | 2005–2014, 2013163,210 | Yes211,212 | 2011213 | 2 |
| Mauritania | 1983, 1988, 2003, 2007, 2012, 2017, 2018, 201957,81–86,93 | 1983, 1984, 1985, 1988, 200385,86,197,214–218 | 1983, 1984, 1988, 2003, 201385,86,163,197,214,215 | Yes215 | 1983, 1984,200385,197,214,215 | 2 |
| Niger | No | No | 1984–1988219,220 | Yes221,222 | No | 3 |
| Nigeria | 2010–201434 | 1973, 1988, 2011, 2010–201431,34,223,224 | 1964–1968, 1976, 1983, 1984–1988, 201532,219,225–227 | Yes32,228 | 1964, 1964–1968, 1966, 19765,32,227,229 | 2 |
| Saint Helena, Ascension and Tristan da Cunha | No | No | No | No | No | 5 |
| Senegal | 2003, 2004, 2015, 2017, 202057,105–109 | 1986, 1989, 1987–1995230–233 | 1969, 1972, 1983, 1986, 1987–1995, 1989–1992, 1991, 20145,197,230,232,234–238 | Yes239,240 | 1963–1974, 1969–1991, 1969, 1970–1974, 1983, 1985, 1987, 1990, 1989–1992, 1991–1992, 19925,41,197,230,232,235–237,241–244 | 2 |
| Sierra Leone | 2016110 | 2007–2014245 | No | Yes239 | No | 2 |
| Togo | No | No | No | Yes246 | No | 4 |
CCHF = Crimean-Congo hemorrhagic fever; CCHFV = Crimean-Congo hemorrhagic fever virus. Years are listed if there is peer-reviewed evidence of anti-CCHFV antibodies in humans or animals, CCHFV vector endemicity, or CCHFV antigen or genome detection.
DISCUSSION
CCHFV is considered a significant threat to human health in endemic areas, including Africa. The World Health Organization (WHO) included CCHF in its blueprint of priority diseases, which lists emerging diseases that are understudied.42 CCHF was first described in 1956 in Africa,43–45 although evidence of this disease in Africa has not been clearly described. Recently, the number of CCHF cases has increased on the continent, with some countries reporting their first cases. We collected available evidence of CCHF epidemiology and classified each country based on the level of evidence. One caveat is that we were not able to establish a category for studies reporting negative data on CCHFV animal or human serology and virus isolation in ticks because of the extremely low number of such publications/reports and their limited scopes.
There are significant variability or gaps in surveillance activities and capabilities between countries. The knowledge gap resulting from the likely lack of active zoonosis surveillance, including CCHF, in resource-limited African countries can impair their awareness of, preparedness for, decision-making during, and countering emerging zoonotic infections that may cause large disease outbreaks on the continent.37
Most countries in Middle Africa do not have the diagnostic capability to detect CCHF cases, although there is a significant level of evidence that CCHFV is actively circulating. Lack of diagnostic capability is likely the major factor contributing to underestimation of CCHF endemicity in Africa compared with other endemic regions, such as the Mediterranean Basin. For example, COD is the second largest country in Africa and reported Africa’s first CCHF case in 1956,43–45 but no subsequent cases were identified until 2008.46 Animal serology yielded positive results in 2013.47 CCHFV likely has been circulating in COD for years. COD has been mired in conflict for decades and has the largest number of internally displaced people in Africa. As another example, the Central African Republic reported only one human CCHF case in 1976,48 whereas positive human and animal serology49–54 and virus isolation from ticks5,55,56 strongly support virus circulation in the area. Civil wars in the region, especially in COD and Central African Republic, have weakened the local healthcare infrastructures. Both countries would benefit from assistance by international organizations, cross-governmental support, and multi-institutional collaborations to build diagnostic capabilities by establishing, developing, or strengthening human and ecologic CCHF surveillance networks and systems.
Among countries in Southern Africa, South Africa reported 215 CCHF cases (1981–2019), the highest number of cases on the continent.57–62 The annual number of cases is available through the National Institute for Communicable Diseases (NICD) in Sandringham, Johannesburg. South Africa has a well-established surveillance system, implementing national guidelines for recognition and management of viral hemorrhagic fevers such as CCHF, and NICD’s Special Pathogen Unit is capable of diagnosing these diseases.63 However, some countries that have a border with South Africa (i.e., Botswana, Eswatini, Lesotho, and Mozambique) have not reported any cases despite the prevalence of Hyalomma ticks,39,64–66 supporting the notion that CCHFV may be circulating but has not been detected due to limited diagnostic capabilities. These countries would benefit from testing of Hyalomma ticks for CCHFV and seroprevalence studies in humans and animals.
In Eastern Africa, Uganda reported 44 cases (Table 2). There is an appreciable gap in CCHF case detection from 1978 to 2013, which could have been due to lower CCHFV activity during this period. The increased detection of cases since 2013 could have been due to any biological vector or human risk factors but is more likely due to maturity of Uganda’s surveillance system and improved diagnostic capabilities. Since 2010, to rapidly identify infectious disease outbreaks, including Ebola and Marburg virus diseases, the Uganda Virus Research Institute (UVRI) has cultivated relationships with multiple international partners and established a surveillance system in collaboration with the Ugandan Ministry of Health.67 Most newly diagnosed CCHF cases in Uganda have been confirmed through this surveillance system. Kenya, South Sudan, Tanzania, and Zimbabwe have each reported at least one case of CCHF; if they had the same diagnostic capabilities as Uganda, it is likely that more cases would have been detected.
Among Northern African countries, Egypt, Sudan, and Tunisia have reported CCHF cases. Egypt was the first country in the region to experience CCHF in 1981.68 Sudan detected its first case in 200829 and has been reporting cases annually since.29,59,69–73 Tunisia’s first case dates to 2014.74 Although Egypt only recognized CCHF in 1981 and 2012,68,75 positive animal serology and CCHFV detection in ticks have been reported intermittently, supporting the notion that CCHFV is circulating in the region.26,76–78 Although Sudan has reported 34 CCHF cases since 2008, most of these cases have been diagnosed in the setting of outbreak investigations. Libya, which borders Egypt, Sudan, and Tunisia, has not reported any CCHF cases. Among Northern African countries, Egypt has a unique infrastructure and a long-standing history of partnership with the U.S. Naval Medical Research Unit Three (NAMRU-3) as long as it was situated in Cairo Egypt. NAMRU-3 worked closely with Egypt’s Ministry of Health and Population, the U.S. National Institutes of Health (NIH), the WHO, the U.S. Agency for International Development, and the U.S. Centers for Disease Control and Prevention (CDC).79 NAMRU-3 was recently relocated to Italy, but a similar entity with a similar infrastructure associated with international collaboration in partnership with local governments could be developed to (re)establish a CCHF diagnostic surveillance system in the region.
In Western Africa, Burkina Faso and Mauritania reported their first CCHF cases in 1983.80 Burkina Faso has not reported any cases since then, whereas Mauritania has been reporting cases intermittently through outbreak investigations.59,81–86 Mali, Nigeria, Senegal, and Sierra Leone have each reported at least one case since 2003. Although not bordering other countries that have reported CCHF cases, Nigeria has reported the highest number of cases (50) in Western Africa since 2014.34 Niger, located between Nigeria and Mali, may therefore be affected by numerous undiagnosed CCHF cases.
Diagnosis of new CCHF cases in eight African countries (Table 1; mainly in Eastern Northern and Western Africa) since 2000 is most likely primarily due to improvement in diagnostic capabilities rather than ecological changes related to vector and human behavior. These countries still lack established surveillance systems and would benefit significantly from collaboration with within and outside their regions to diagnose additional cases and better estimate the epidemiology of CCHF on the continent.
The current (2017) WHO map outlining CCHF endemicity in Africa87 is based on reports of cases, human serology, and presence of CCHFV tick vectors. However, most countries in Africa do not have CCHF diagnostic capability, likely leading to underestimation of cases. Our One Health approach to country classification more comprehensively integrates additional factors, such as animal serology and inclusion of more granular data on vector presence. This approach revealed evidence of CCHFV circulation in Northern and Middle African regions that were not considered high risk in a previous CCHFV distribution modeling effort.36 Based on our examination of CCHFV epidemiology in Africa, we recommend the following:
-
1.
Egypt, South Africa, and Uganda, that is, countries with diagnostic capabilities, should provide rapid diagnostic support during CCHF outbreaks. Furthermore, we propose that these countries collaborate to establish a network and act as regional diagnostic centers to define a realistic epidemiology of CCHF for the entire continent. To be most effective, this collaboration effort should include active CCHF surveillance, testing of Hyalomma ticks for CCHFV, animal and human serology testing, improved healthcare infrastructure, community education about CCHFV transmission, and providing personal protective equipment (PPE).
-
2.
Burkina Faso, Central African Republic, COD, Kenya, Sierra Leone, South Sudan, Tanzania, and Zimbabwe, which have rarely reported cases despite serological and tick surveillance data being in support of virus circulation (level 2), would benefit most from establishing active CCHF surveillance systems.
-
3.
Hyalomma ticks are present in most African countries, but information about CCHFV circulation is limited. Countries assigned level 4 (Angola, Benin, Botswana, Cabo Verde, Chad, Côte d’lvoire, Eritrea, Gambia, Lesotho, Libya, Malawi, Rwanda, Swaziland, Togo, and Zambia) will benefit from testing of Hyalomma ticks for CCHFV and from animal/human seroprevalence studies in collaboration.
-
4.
Countries with limited evidence of Hyalomma tick presence, assigned level 5 (Burundi, Comoros, Guinea Bissau, Mauritius, Mayotte, Réunion, São Tomé and Príncipe, Saint Helena, Ascension, and Tristan da Cunha, Seychelles, and Western Sahara), would benefit from tick surveillance studies.
-
5.
Most African countries also lack healthcare infrastructures to support outbreak investigations, early case detection, patient isolation, and patient medical care. Multi-institutional collaboration should focus on raising awareness about routes of CCHFV transmission and providing PPE to prevent community and nosocomial outbreaks.
Our study has important limitations. First, we were dependent on publications and reports indexed in public databases or referenced in works indexed in databases. Consequently, we may have missed important datasets that were never officially published or circulate only within governments or institutions as internal reports. Second, we had to assume that all studies within a category (virus isolation, human serology, and animal serology) were of the same quality and hence could be treated equally. However, studies considerably differ in approach and rigor, and hence a positive result reported in one work could have been considered a negative result using a different method or vice versa. Third, artificially created geographic borders of course do not affect virus ecology and transmission, but biotopes and ecological niches may differ considerably in adjacent areas. Thus, two countries may have been counted as CCHFV endemic despite one of them having a homogenous CCHFV distribution throughout, whereas the second country may only have a single CCHFV hotspot. We erred on the side of caution by assuming that countries adjacent to CCHF-endemic countries may also have circulating CCHFV, but, of course, this does not have to be the case. Ecologic niche modeling might be a solution for addressing both issues, but is outside the scope of this manuscript. Finally, our study does not address climate change over time, nor the increasing human activity and human-induced changes of biotopes, both of which could be associated with CCHFV vector and host migrations and therefore changes in CCHF prevalence over time. We call on international public health organizations (the WHO and the World Organization for Animal Health) to create or expand partnerships with local governments to provide support for human, tick, and animal CCHFV surveillance in Africa and to ultimately increase knowledge about CCHFV ecology and CCHF epidemiology.
Acknowledgment:
We thank Anya Crane (NIH/NIAID/DCR/Integrated Research Facility at Fort Detrick, Frederick, MD) for critically editing the manuscript.
REFERENCES
- 1.Spiropoulou CF, Bente DA, 2020. Orthohantavirus, orthonairovirus, orthobunyavirus, and phlebovirus. Howley PM, Knipe DM, Whelan SPJ, eds. Fields Virology. Philadelphia, Pennsylvania: Wolters Kluwer/Lippincott Williams & Wilkins, 750–783. [Google Scholar]
- 2.Sipovskij PV, 1944. Slučai svoeobraznogo želudočno-kišečnogo krovotečeniâ. Klin Med (Mosk) 22: 64–67. [Google Scholar]
- 3.Sokolov AE, Čumakov MP, Kolachev AA, 1945. Krymskaâ Gemorragičeskaâ Lihoradka (Ostryj Infekcionnyj Kapillârotoksikoz). Simferopol, USSR: Izdaniye Otdel'noj Primorskoj Armii. [Google Scholar]
- 4.Ioffe VÛ, 1944. Ob odnoj svoeobraznoj forme gemorragičeskogo diateza. Klin Med (Mosk) 22: 25–30. [Google Scholar]
- 5.Hoogstraal H, 1979. The epidemiology of tick-borne Crimean-Congo hemorrhagic fever in Asia, Europe, and Africa. J Med Entomol 15: 307–417. [DOI] [PubMed] [Google Scholar]
- 6.Ergonul O, Whitehouse CA, 2007. Crimean-Congo Hemorrhagic Fever: A Global Perspective. Dordrecht, Netherlands: Springer. [Google Scholar]
- 7.Blair PW, Kuhn JH, Pecor DB, Apanaskevich DA, Kortepeter MG, Cardile AP, Polanco Ramos A, Keshtkar-Jahromi M, 2019. An emerging biothreat: Crimean-Congo hemorrhagic fever virus in southern and western Asia. Am J Trop Med Hyg 100: 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spengler JR, Estrada-Peña A, Garrison AR, Schmaljohn C, Spiropoulou CF, Bergeron É, Bente DA, 2016. A chronological review of experimental infection studies of the role of wild animals and livestock in the maintenance and transmission of Crimean-Congo hemorrhagic fever virus. Antivir Res 135: 31–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mostafavi E, Chinikar S, Moradi M, Bayat N, Meshkat M, Fard MK, Ghiasi SM, 2013. A case report of Crimean Congo hemorrhagic fever in ostriches in Iran. Open Virol J 7: 81–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shepherd AJ, Swanepoel R, Leman PA, Shepherd SP, 1987. Field and laboratory investigation of Crimean-Congo haemorrhagic fever virus (Nairovirus, family Bunyaviridae) infection in birds. Trans R Soc Trop Med Hyg 81: 1004–1007. [DOI] [PubMed] [Google Scholar]
- 11.Kar S, Rodriguez SE, Akyildiz G, Cajimat MNB, Bircan R, Mears MC, Bente DA, Keles AG, 2020. Crimean-Congo hemorrhagic fever virus in tortoises and Hyalomma aegyptium ticks in east Thrace, Turkey: potential of a cryptic transmission cycle. Parasit Vectors 13: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bodur H, Akinci E, Ascioglu S, Öngürü P, Uyar Y, 2012. Subclinical infections with Crimean-Congo hemorrhagic fever virus, Turkey Emerg Infect Dis 18: 640–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwarz TF, Nsanze H, Ameen AM, 1997. Clinical features of Crimean-Congo haemorrhagic fever in the United Arab Emirates. Infection 25: 364–367. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization , 2020. Crimean-Congo Haemorrhagic Fever. Geneva, Switzerland: WHO. Available at: https://www.who.int/health-topics/crimean-congo-haemorrhagic-fever/#tab=tab_1. Accessed March 23, 2021. [Google Scholar]
- 15.al-Abri SS, et al. 2019. Clinical and molecular epidemiology of Crimean-Congo hemorrhagic fever in Oman. PLoS Negl Trop Dis 13: e0007100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ergonul O, 2007. Clinical and pathologic features of Crimean-Congo hemorrhagic fever. Ergonul O, Whitehouse CA, eds. Crimean-Congo Hemorrhagic Fever: A Global Perspective. Dordrecht, Netherlands: Springer, 207–220. [Google Scholar]
- 17.Sağmak Tartar A, Balin SÖ, Akbulut A, Demirdağ K, 2019. Türkiye’nin doğusunda Kırım Kongo kanamalı ateşi: epidemiyolojik ve klinik değerlendirme. Türk Parazitol Derg 43: 26–29. [Google Scholar]
- 18.Tripathi S, Bhati R, Gopalakrishnan M, Bohra GK, Tiwari S, Panda S, Sahay RR, Yadav PD, Nag VL, Garg MK, 2020. Clinical profile and outcome of patients with Crimean Congo haemorrhagic fever: a hospital based observational study from Rajasthan, India. Trans R Soc Trop Med Hyg 114: 643–649. [DOI] [PubMed] [Google Scholar]
- 19.Akuffo R, et al. 2016. Crimean-Congo hemorrhagic fever virus in livestock ticks and animal handler seroprevalence at an abattoir in Ghana. BMC Infect Dis 16: 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharifi-Mood B, Mardani M, Keshtkar-Jahromi M, Rahnavardi M, Hatami H, Metanat M, 2008. Clinical and epidemiologic features of Crimean-Congo hemorrhagic fever among children and adolescents from southeastern Iran. Pediatr Infect Dis J 27: 561–563. [DOI] [PubMed] [Google Scholar]
- 21.Tezer H, Sucaklı IA, Saylı TR, Celikel E, Yakut I, Kara A, Tunc B, Ergonul O, 2010. Crimean-Congo hemorrhagic fever in children. J Clin Virol 48: 184–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leblebicioglu H, 2010. Crimean-Congo haemorrhagic fever in Eurasia. Int J Antimicrob Agents 36 (Suppl 1): S43–S46. [DOI] [PubMed] [Google Scholar]
- 23.Nevill L, 1961. Correspondence. East Afr Med J 38: 174. [Google Scholar]
- 24.Johnson BK, et al. 1983. Viral haemorrhagic fever surveillance in Kenya, 1980–1981. Trop Geogr Med 35: 43–47. [PubMed] [Google Scholar]
- 25.Dunster L, Dunster M, Ofula V, Beti D, Kazooba-Voskamp F, Burt F, Swanepoel R, DeCock KM, 2002. First documentation of human Crimean-Congo hemorrhagic fever, Kenya. Emerg Infect Dis 8: 1005–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morrill JC, Soliman AK, Imam IZ, Botros BAM, Moussa MI, Watts DM, 1990. Serological evidence of Crimean-Congo haemorrhagic fever viral infection among camels imported into Egypt. J Trop Med Hyg 93: 201–204. [PubMed] [Google Scholar]
- 27.McCarthy MC, Haberberger RL, Salib AW, Soliman BA, el-Tigani A, Khalid IO, Watts DM, 1996. Evaluation of arthropod-borne viruses and other infectious disease pathogens as the causes of febrile illnesses in the Khartoum province of Sudan. J Med Virol 48: 141–146. [DOI] [PubMed] [Google Scholar]
- 28.Hassanein KM, el-Azazy OME, 2000. Isolation of Crimean-Congo hemorrhagic fever virus from ticks on imported Sudanese sheep in Saudi Arabia. Ann Saudi Med 20: 153–154. [DOI] [PubMed] [Google Scholar]
- 29.Aradaib IE, Erickson BR, Mustafa ME, Khristova ML, Saeed NS, Elageb RM, Nichol ST, 2010. Nosocomial outbreak of Crimean-Congo hemorrhagic fever, Sudan Emerg Infect Dis 16: 837–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Causey OR, Kemp GE, Madbouly MH, Lee VH, 1969. Arbovirus surveillance in Nigeria, 1964–1967. Bull Soc Pathol Exot Filiales 62: 249–253. [PubMed] [Google Scholar]
- 31.David-West TS, Cooke AR, David-West AS, 1974. Seroepidemiology of Congo virus (related to the virus of Crimean haemorrhagic fever) in Nigeria. Bull World Health Organ 51: 543–546. [PMC free article] [PubMed] [Google Scholar]
- 32.Causey OR, Kemp GE, Madbouly MH, David-West TS, 1970. Congo virus from domestic livestock, African hedgehog, and arthropods in Nigeria. Am J Trop Med Hyg 19: 846–850. [DOI] [PubMed] [Google Scholar]
- 33.Williams RW, Causey OR, Kemp GE, 1972. Ixodid ticks from domestic livestock in Ibadan, Nigeria as carriers of viral agents. J Med Entomol 9: 443–445. [DOI] [PubMed] [Google Scholar]
- 34.Bukbuk DN, Dowall SD, Lewandowski K, Bosworth A, Baba SS, Varghese A, Watson RJ, Bell A, Atkinson B, Hewson R, 2016. Serological and virological evidence of Crimean-Congo haemorrhagic fever virus circulation in the human population of Borno state, northeastern Nigeria. PLoS Negl Trop Dis 10: e0005126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pigott DM, et al. 2017. Local, national, and regional viral haemorrhagic fever pandemic potential in Africa: a multistage analysis. Lancet 390: 2662–2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Messina JP, et al. 2015. The global distribution of Crimean-Congo hemorrhagic fever. Trans R Soc Trop Med Hyg 109: 503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chauhan RP, Dessie ZG, Noreddin A, El Zowalaty ME, 2020. Systematic review of important viral diseases in Africa in light of the “One health” concept. Pathogens 9: 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.United Nations Statistics Division , 2020. Standard Country or Area Codes for Statistical Use (M49). Geographic Regions. Available at: https://unstats.un.org/unsd/methodology/m49/. Accessed March 23, 2021. [Google Scholar]
- 39.Georgia Southern University , 2020. United States National Tick Collection. Available at: https://cosm.georgiasouthern.edu/usntc/. Accessed March 23, 2021. [Google Scholar]
- 40.Gargili A, Estrada-Peña A, Spengler JR, Lukashev A, Nuttall PA, Bente DA, 2017. The role of ticks in the maintenance and transmission of Crimean-Congo hemorrhagic fever virus: a review of published field and laboratory studies. Antivir Res 144: 93–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeller HG, Cornet JP, Camicas JL, 1994. Experimental transmission of Crimean-Congo hemorrhagic fever virus by west African wild ground-feeding birds to Hyalomma marginatum rufipes ticks. Am J Trop Med Hyg 50: 676–681. [DOI] [PubMed] [Google Scholar]
- 42.World Health Organization , 2020. Prioritizing Diseases for Research and Development in Emergency Contexts. Geneva, Switzerland: WHO. Available at: https://www.who.int/activities/prioritizing-diseases-for-research-and-development-in-emergency-contexts. Accessed March 23, 2021. [Google Scholar]
- 43.Simpson DIH, Knight EM, Courtois G, Williams MC, Weinbren MP, Kibukamusoke JW, 1967. Congo virus: a hitherto undescribed virus occurring in Africa. Part 1-human isolations - clinical notes. East Afr Med J 44: 86–92. [PubMed] [Google Scholar]
- 44.Woodall JP, Williams MC, Simpson DIH, 1967. Congo virus: a hitherto undescribed virus occurring in Africa. Part 2-identification studies. East Afr Med J 44: 93–98. [PubMed] [Google Scholar]
- 45.Woodall JP, 2007. Personal reflections. Ergonul O, Whitehouse CA, eds. Crimean-Congo Hemorrhagic Fever: a Global Perspective. Dordrecht, Netherlands: Springer, 23–32. [Google Scholar]
- 46.Grard G, Drexler JF, Fair J, Muyembe JJ, Wolfe ND, Drosten C, Leroy EM, 2011. Re-emergence of Crimean-Congo hemorrhagic fever virus in central Africa. PLoS Negl Trop Dis 5: e1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sas MA, Mertens M, Isselmou E, Reimer N, el Mamy BO, Doumbia B, Groschup MH, 2017. Crimean-Congo hemorrhagic fever virus-specific antibody detection in cattle in Mauritania. Vector Borne Zoonotic Dis 17: 582–587. [DOI] [PubMed] [Google Scholar]
- 48.Robin Y, 1977. Centre collaborateur O.M.S. de référence et de recherche pour les arbovirus. Dakar, Senegal: Rapport de l'Institute Pasteur. [Google Scholar]
- 49.Georges AJ, Saluzzo JF, Gonzalez JP, Dussarat GV, 1980. Arboviroses en centrafrique: incidence et aspects diagnostiques chez l’homme. Méd Trop (Mars) 40: 561–568. [PubMed] [Google Scholar]
- 50.Gonzalez JP, Mc Cormick JB, Saluzzo JF, Georges AJ, 1983. Les fièvres hémorragiques africaines d’origine virale: contribution à leur étude en République Centrafricaine. Cah O.R.S.T.O.M. Entomol Méd Parasit XXI: 119–130. [Google Scholar]
- 51.Georges AJ, Gonzalez JP, 1986. Could Crimea-Congo haemorrhagic fever be a biohazard in the central African Republic? Trans R Soc Trop Med Hyg 80: 994–995. [DOI] [PubMed] [Google Scholar]
- 52.Johnson ED, Gonzalez JP, Georges A, 1993. Haemorrhagic fever virus activity in equatorial Africa: distribution and prevalence of filovirus reactive antibody in the central African Republic. Trans R Soc Trop Med Hyg 87: 530–535. [DOI] [PubMed] [Google Scholar]
- 53.Mathiot CC, Hervé VM, Georges AJ, 1990. Antibodies to haemorrhagic fever viruses and to selected arboviruses in monkeys from the central African Republic. Trans R Soc Trop Med Hyg 84: 732–733. [DOI] [PubMed] [Google Scholar]
- 54.Guilherme JM, Gonella-Legall C, Legall F, Nakoume E, Vincent J, 1996. Seroprevalence of five arboviruses in Zebu cattle in the central African Republic. Trans R Soc Trop Med Hyg 90: 31–33. [DOI] [PubMed] [Google Scholar]
- 55.Dégallier N, Cornet JP, Saluzzo JF, Germain M, Hervé JP, Camicas JL, Sureau P, 1985. Ecologie des arbovirus à tiques en République Centrafricaine. Bull Soc Pathol Exot Filiales 78: 296–310. [PubMed] [Google Scholar]
- 56.Sureau P, Cornet JP, Germain M, Camicas JL, Robin Y, 1976. Enquête sur les arbovirus transmis par les tiques en République Centrafricaine (1973–1974). Isolement des virus Dugbe, CHF/Congo, Jos et Bhanja. Bull Soc Pathol Exot Filiales 69: 28–33. [PubMed] [Google Scholar]
- 57.National Institute for Communicable Diseases , 2018. Crimean-Congo haemorrhagic fever update. Commun Dis Surv Bull 17: 2. [Google Scholar]
- 58.National Institute for Communicable Diseases , 2018. Crimean-Congo haemorrhagic fever in South Africa. Commun Dis Surv Bull 17: 3. [Google Scholar]
- 59.National Institute for Communicable Diseases , 2018. Crimean-Congo haemorrhagic fever in free state province. Commun Dis Surv Bull 17: 3. [Google Scholar]
- 60.International Society for Infectious Diseases , 2019. Crimean-Congo Hemorrhagic Fever - Africa (02): South Africa (NC). ProMED archive number 20190330.6394622. Available at: https://promedmail.org/promed-post/?id=20190330.6394622. Accessed March 23, 2021. [Google Scholar]
- 61.International Society for Infectious Diseases , 2019. Crimean-Congo Hemorrhagic Fever - Africa (06): South Africa (NW). ProMED archive number 20190511.6462345. Available at: https://promedmail.org/promed-post/?id=20190511.6462345. Accessed March 23, 2021. [Google Scholar]
- 62.National Institute for Communicable Diseases , 2019. An update on CCHF. Commun Dis Surv Bull 18: 2. [Google Scholar]
- 63.National Institute for Communicable Diseases , 2020. Surveillance. Available at: https://www.nicd.ac.za/our-services/surveillance/. Accessed March 23, 2021. [Google Scholar]
- 64.Carmichael IH, 1976. Ticks from the African buffalo (Syncerus caffer) in Ngamiland, Botswana. Onderstepoort J Vet Res 43: 27–29. [PubMed] [Google Scholar]
- 65.Theiler G, 1956. Zoological survey of the union of South Africa. Tick survey, part IX. The distribution of the three South African Hyalommas or bontpoots. Onderstepoort J Vet Res 27: 249–260. [Google Scholar]
- 66.Apanaskevich DA, Horak IG, 2008. The genus Hyalomma Koch, 1844: V. re-evaluation of the taxonomic rank of taxa comprising the H. (Euhyalomma) marginatum Koch complex of species (Acari: Ixodidae) with redescription of all parasitic stages and notes on biology. Int J Acarol 34: 13–42. [Google Scholar]
- 67.Balinandi S, et al. 2018. Investigation of an isolated case of human Crimean-Congo hemorrhagic fever in central Uganda, 2015. Int J Infect Dis 68: 88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weidmann M, et al. 2016. Biosafety standards for working with Crimean-Congo hemorrhagic fever virus. J Gen Virol 97: 2799–2808. [DOI] [PubMed] [Google Scholar]
- 69.Bower H, et al. 2019. Detection of Crimean-Congo haemorrhagic fever cases in a severe undifferentiated febrile illness outbreak in the Federal Republic of Sudan: a retrospective epidemiological and diagnostic cohort study. PLoS Negl Trop Dis 13: e0007571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kohl C, Eldegail M, Mahmoud I, Schrick L, Radonic A, Emmerich P, Rieger T, Gunther S, Nitsche A, Osman AA, 2016. Crimean Congo hemorrhagic fever, 2013 and 2014 Sudan. Int J Infect Dis 53S: 4–163. [Google Scholar]
- 71.Abdelhakam HAA, Taha MA, 2014. Crimean-Congo hemorrhagic fever (CCHF) in southern Kordofan. Sudan J Paediatr 14: 81–84. [PMC free article] [PubMed] [Google Scholar]
- 72.Elata AT, Karsany MS, Elageb RM, Hussain MA, Eltom KH, Elbashir MI, Aradaib IE, 2011. A nosocomial transmission of Crimean-Congo hemorrhagic fever to an attending physician in north Kordufan, Sudan. Virol J 8: 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aradaib IE, Erickson BR, Karsany MS, Khristova ML, Elageb RM, Mohamed MEH, Nichol ST, 2011. Multiple Crimean-Congo hemorrhagic fever virus strains are associated with disease outbreaks in Sudan, 2008–2009. PLoS Negl Trop Dis 5: e1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wasfi F, et al. 2016. Sero-epidemiological survey of Crimean-Congo hemorrhagic fever virus in Tunisia. Parasite 23: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.El-Bahnasawy MM, Sabah AAA, Saleh HAA, Morsy TA, 2012. The tick-borne Crimean-Congo hemorrhagic fever in Africa, Asia, Europe, and America: what about Egypt? J Egypt Soc Parasitol 42: 373–384. [DOI] [PubMed] [Google Scholar]
- 76.Darwish MA, Imam IZE, Omar FM, Hoogstraal H, 1978. Results of a preliminary seroepidemiological survey for Crimean-Congo hemorrhagic fever virus in Egypt. Acta Virol 22: 77. [PubMed] [Google Scholar]
- 77.Mohamed M, Said AR, Murad A, Graham R, 2008. A serological survey of Crimean-Congo haemorrhagic fever in animals in the Sharkia Governorate of Egypt. Vet Ital 44: 513–517. [PubMed] [Google Scholar]
- 78.Horton KC, Wasfy M, Samaha H, Abdel-Rahman B, Safwat S, Abdel Fadeel M, Mohareb E, Dueger E, 2014. Serosurvey for zoonotic viral and bacterial pathogens among slaughtered livestock in Egypt. Vector Borne Zoonotic Dis 14: 633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Naval Medical Research and Development , 2020. Naval Medical Research Unit - No. 3. Available at: https://www.med.navy.mil/sites/nmrc/Cairo/Pages/HomeCairo.aspx. Accessed March 23, 2021. [Google Scholar]
- 80.Saluzzo JF, Digoutte JP, Cornet M, Baudon D, Roux J, Robert V, 1984. Isolation of Crimean-Congo haemorrhagic fever and Rift valley fever viruses in upper volta. Lancet 1: 1179. [DOI] [PubMed] [Google Scholar]
- 81.Kebede S, Duales S, Yokouide A, Alemu W, 2010. Trends of major disease outbreaks in the African region, 2003–2007. East Afr J Public Health 7: 20–29. [DOI] [PubMed] [Google Scholar]
- 82.International Society for Infectious Diseases , 2018. Crimean-Congo Hemorrhagic Fever - Mauritania: (GD). ProMED archive number 20180502.5778839. Available at: https://promedmail.org/promed-post/?id=20180502.5778839. Accessed March 23, 2021. [Google Scholar]
- 83.Kleib AS, Salihy SM, Ghaber SM, Sidiel BW, Sidiya KC, Bettar ES, 2016. Crimean-Congo hemorrhagic fever with acute subdural hematoma, Mauritania, 2012. Emerg Infect Dis 22: 1305–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Saluzzo JF, Aubry P, McCormick J, Digoutte JP, 1985. Haemorrhagic fever caused by Crimean Congo haemorrhagic fever virus in Mauritania. Trans R Soc Trop Med Hyg 79: 268. [DOI] [PubMed] [Google Scholar]
- 85.Nabeth P, et al. 2004. Crimean-Congo hemorrhagic fever, Mauritania. Emerg Infect Dis 10: 2143–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gonzalez JP, LeGuenno B, Guillaud M, Wilson ML, 1990. A fatal case of Crimean-Congo haemorrhagic fever in Mauritania: virological and serological evidence suggesting epidemic transmission. Trans R Soc Trop Med Hyg 84: 573–576. [DOI] [PubMed] [Google Scholar]
- 87.World Health Organization , 2020. Geographic Distribution of Crimean-Congo Haemorrhagic Fever. Geneva, Switzerland: WHO. Available at: https://www.who.int/images/default-source/health-topics/crimean-congo-haemorrhagic-fever/global-cchfrisk-2017.png?sfvrsn=4b961c4c_6. Accessed March 23, 2021. [Google Scholar]
- 88.Lwande OW, Irura Z, Tigoi C, Chepkorir E, Orindi B, Musila L, Venter M, Fischer A, Sang R, 2012. Seroprevalence of Crimean Congo hemorrhagic fever virus in Ijara district, Kenya. Vector Borne Zoonotic Dis 12: 727–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nyataya J, Maraka M, Lemtudo A, Masakhwe C, Mutai B, Njaanake K, Estambale BB, Nyakoe N, Siangla J, Waitumbi JN, 2020. Serological evidence of yersiniosis, tick-borne encephalitis, west Nile, hepatitis E, Crimean-Congo hemorrhagic fever, Lyme borreliosis, and brucellosis in febrile patients presenting at diverse hospitals in Kenya. Vector Borne Zoonotic Dis 20: 348–357. [DOI] [PubMed] [Google Scholar]
- 90.Safronetz D, et al. 2016. Vectorborne infections, Mali Emerg Infect Dis 22: 340–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Baumann J, Knüpfer M, Ouedraogo J, Traoré BY, Heitzer A, Kané B, Maiga B, Sylla M, Kouriba B, Wölfel R, 2019. Lassa and Crimean-Congo hemorrhagic fever viruses, Mali Emerg Infect Dis 25: 999–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.International Society for Infectious Diseases , 2020. Crimean-Congo Hemorrhagic Fever - Africa (03): Mali (Mopti) fatal. ProMED archive number 20200207.6963297. Available at: https://promedmail.org/promed-post/?id=20200207.6963297. Accessed March 23, 2021. [Google Scholar]
- 93.Boushab BM, Kelly M, Kébé H, Bollahi MA, Basco LK, 2020. Crimean-Congo hemorrhagic fever, Mauritania. Emerg Infect Dis 26: 817–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Muianga AF, et al. 2017. First serological evidence of Crimean-Congo haemorrhagic fever in febrile patients in Mozambique. Int J Infect Dis 62: 119–123. [DOI] [PubMed] [Google Scholar]
- 95.International Society for Infectious Diseases , 2019. Crimean-Congo Hemorrhagic Fever - Africa (09): Namibia, Update. ProMED archive number 20190524.6482638. Available at: https://promedmail.org/promed-post/?id=20190524.6482638. Accessed March 23, 2021. [Google Scholar]
- 96.International Society for Infectious Diseases , 2018. Crimean-Congo Hemorrhagic Gever - Namibia (03): (KH). ProMED archive number 20180407.5730960. Available at: https://promedmail.org/promed-post/?id=20180407.5730960. Accessed March 23, 2021. [Google Scholar]
- 97.International Society for Infectious Diseases , 2018. Crimean-Congo Hemorrhagic Fever - Namibia (02): (OS). ProMED archive number 20180305.5665590. Available at: https://promedmail.org/promed-post/?id=20180305.5665590. Accessed March 23, 2021. [Google Scholar]
- 98.International Society for Infectious Diseases , 2018. Crimean-Congo Hemorrhagic Fever - Namibia: (OH). ProMED archive number 20180209.5615643. Available at: https://promedmail.org/promed-post/?id=20180209.5615643. Accessed March 23, 2021. [Google Scholar]
- 99.International Society for Infectious Diseases , 2017. Crimean-Congo Hemorrhagic Fever - Namibia (04): (OS) fatal, contacts. ProMED archive number 20170812.5244149. Available at: https://promedmail.org/promed-post/?id=20170812.5244149. Accessed March 23, 2021. [Google Scholar]
- 100.International Society for Infectious Diseases , 2017. Crimean-Congo Hemorrhagic Fever - Namibia (03): (OH). ProMED archive number 20170324.4923675. Available at: https://promedmail.org/promed-post/?id=20170324.4923675. Accessed March 23, 2021. [Google Scholar]
- 101.International Society for Infectious Diseases , 2017. Crimean-Congo Hemorrhagic Fever - Namibia: (OH) fatal, quarantine. ProMED archive number 20170227.4866654. Available at: https://promedmail.org/promed-post/?id=20170227.4866654. Accessed March 23, 2021. [Google Scholar]
- 102.International Society for Infectious Diseases , 2014. Crimean-Congo Hemorrhagic Fever - South Africa Ex Namibia. ProMED archive number 20140919.2788764. Available at: https://promedmail.org/promed-post/?id=20140919.2788764. Accessed March 23, 2021. [Google Scholar]
- 103.Burt FJ, Swanepoel R, 2005. Molecular epidemiology of African and Asian Crimean-Congo haemorrhagic fever isolates. Epidemiol Infect 133: 659–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zivcec M, Albarino CG, Guerrero LIW, Ksiazek TG, Nichol ST, Swanepoel R, Rollin PE, Spiropoulou CF, 2017. Genome sequences of Crimean-Congo hemorrhagic fever virus strains isolated in South Africa, Namibia, and Turkey. Genome Announc 5: e01060-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.International Society for Infectious Diseases , 2015. Crimean-Congo hemorrhagic fever - Senegal (SL). ProMED archive number 20151111.3784725. Available at: https://promedmail.org/promed-post/?id=20151111.3784725. Accessed March 23, 2021. [Google Scholar]
- 106.Tall A, et al. 2009. Deux cas de fièvre hémorragique de Crimée-Congo. (FHCC) contractée au Sénégal, en 2004, par des résidentes temporaires. Bull Soc Pathol Exot 102: 159–161. [PubMed] [Google Scholar]
- 107.Nabeth P, Thior M, Faye O, Simon F, 2004. Human Crimean-Congo hemorrhagic fever, Sénégal. Emerg Infect Dis 10: 1881–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.International Society for Infectious Diseases , 2019. Crimean-Congo Hemorrhagic Fever - Africa (12): Senegal. ProMED archive number 20191127.6797965. Available at: https://promedmail.org/promed-post/?id=20191127.6797965. Accessed March 23, 2021. [Google Scholar]
- 109.Jauréguiberry S, Tattevin P, Tarantola A, Legay F, Tall A, Nabeth P, Zeller H, Michelet C, 2005. Imported Crimean-Congo hemorrhagic fever. J Clin Microbiol 43: 4905–4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang Y, et al. 2019. Next-generation sequencing study of pathogens in serum from patients with febrile jaundice in Sierra Leone. Biomed Environ Sci 32: 363–370. [DOI] [PubMed] [Google Scholar]
- 111.International Society for Infectious Diseases , 2013. Crimean-Congo Hemorrhagic Fever - Uganda (04): clarification. ProMED archive number 20130826.1903826. Available at: https://promedmail.org/promed-post/?id=20130826.1903826. Accessed March 23, 2021. [Google Scholar]
- 112.Swanepoel R, Shepherd AJ, Leman PA, Shepherd SP, McGillivray GM, Erasmus MJ, Searle LA, Gill DE, 1987. Epidemiologic and clinical features of Crimean-Congo hemorrhagic fever in Southern Africa. Am J Trop Med Hyg 36: 120–132. [DOI] [PubMed] [Google Scholar]
- 113.Kalunda M, Mukwaya LG, 1978. Isolation of Congo Virus from Man and Ticks in Uganda. International Virology IV: Abstracts of the Fourth International Congress for Virology. The Hague, Netherlands: Section on Virology of the International Association of Microbiological Societies, 290. [Google Scholar]
- 114.International Society for Infectious Diseases , 2019. Crimean-Congo hemorrhagic fever - Africa: Uganda (MZ). ProMED archive number 20190106.6244399. Available at: https://promedmail.org/promed-post/?id=20190106.6244399. Accessed March 23, 2021. [Google Scholar]
- 115.International Society for Infectious Diseases , 2018. Crimean-Congo Hemorrhagic Fever - East Africa (11): Uganda (RK). ProMED archive number 20181116.6145864. Available at: https://promedmail.org/promed-post/?id=20181116.6145864. [Google Scholar]
- 116.International Society for Infectious Diseases , 2018. Crimean-Congo Hemorrhagic Fever - East Africa (10): Uganda (BR). ProMED archive number 20181102.6123834. Available at: https://promedmail.org/promed-post/?id=20181102.6123834. Accessed March 23, 2021. [Google Scholar]
- 117.International Society for Infectious Diseases , 2018. Crimean-Congo Hemorrhagic Fever - East Africa (09): Uganda (KM). ProMED archive number 20180826.5988686. Available at: https://promedmail.org/promed-post/?id=20180826.5988686. Accessed March 23, 2021. [Google Scholar]
- 118.International Society for Infectious Diseases , 2018. Crimean-Congo Hemorrhagic Fever - East Africa (08): Uganda, WHO. ProMED archive number 20180806.5949636. Available at: https://promedmail.org/promed-post/?id=20180806.5949636. Accessed March 23, 2021. [Google Scholar]
- 119.International Society for Infectious Diseases , 2018. Crimean-Congo Hemorrhagic Fever - East Africa (05): Uganda (MD) alert. ProMED archive number 20180614.5852928. Available at: https://promedmail.org/promed-post/?id=20180614.5852928. Accessed March 23, 2021.. [Google Scholar]
- 120.International Society for Infectious Diseases , 2018. Crimean-Congo Hemorrhagic Fever - East Africa (03): Uganda (NK). ProMED archive number 20180201.5598684. Available at: https://promedmail.org/promed-post/?id=20180201.5598684. Accessed March 23, 2021. [Google Scholar]
- 121.International Society for Infectious Diseases , 2018. Crimean-Congo Hemorrhagic Fever - East Africa: Uganda (LW). ProMED archive number 20180106.5541551. Available at: https://promedmail.org/promed-post/?id=20180106.5541551. Accessed March 23, 2021. [Google Scholar]
- 122.International Society for Infectious Diseases , 2019. Crimean-Congo Hemorrhagic Fever - Africa (10): Uganda (LY) Fatal. ProMED archive number 20190805.6605945. Available at: https://promedmail.org/promed-post/?id=20190805.6605945. Accessed March 23, 2021. [Google Scholar]
- 123.International Society for Infectious Diseases , 2019. Crimean-Congo Hemorrhagic Fever - Africa (04): Uganda (JI) Fatal. Update. ProMED archive number 20190424.6436525. Available at: https://promedmail.org/promed-post/?id=20190424.6436525. Accessed March 23, 2021. [Google Scholar]
- 124.International Society for Infectious Diseases , 2018. Crimean-Congo Hemorrhagic Fever - East Africa (04): Uganda. ProMED archive number 20180528.5821815. Available at: https://promedmail.org/promed-post/?id=20180528.5821815. [Google Scholar]
- 125.Switkes J, Nannyonga B, Mugisha JYT, Nakakawa J, 2016. A mathematical model for Crimean-Congo haemorrhagic fever: tick-borne dynamics with conferred host immunity. J Biol Dyn 10: 59–70. [DOI] [PubMed] [Google Scholar]
- 126.Munube GMR, Yazarna GW, Nsubuga E, 1972. The out-patient dispensary. East Afr Virus Res Inst Rep 22: 6–7. [Google Scholar]
- 127.Knight EM, Henderson BE, Tukei PM, Lule M, West R, 1968. Case histories with arbovirus isolations. East Afr Virus Res Inst Rep 17: 14–15. [Google Scholar]
- 128.International Society for Infectious Diseases , 2020. Crimean-Congo Hemorrhagic Fever - Africa (02): Uganda (Kagadi). ProMED archive number 20200128.6924924. Available at: https://promedmail.org/promed-post/?id=20200128.6924924. Accessed March 23, 2021. [Google Scholar]
- 129.Stuart J, 1998. Suspected case of Crimean/Congo haemorrhagic fever in British traveller returning from Zimbabwe. Euro Surveill 2. Available at: https://www.eurosurveillance.org/content/10.2807/esw.02.08.01256-en#html_fulltext. [Google Scholar]
- 130.Chantal J, Dorchies P, Legueno B, 1994. A study on some zoonoses in Djibouti Republic. 1. Ruminants from Djibouti slaughterhouse. Rev Méd Vét (Toulouse) 145: 633–640. [Google Scholar]
- 131.Walker A, Bouattour A, Camicas JL, Estrada-Peña A, Horak I, Latif A, Pegram R, Preston P, 2014. Ticks of Domestic Animals in Africa: a Guide to Identification of Species. Edinburgh, Scotland, UK: Bioscience Reports, 114–121. [Google Scholar]
- 132.Horton KC, Fahmy NT, Watany N, Zayed A, Mohamed A, Ahmed AA, Rollin PE, Dueger EL, 2016. Crimean Congo hemorrhagic fever virus and Alkhurma (Alkhumra) virus in ticks in Djibouti. Vector Borne Zoonotic Dis 16: 680–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kumsa B, Socolovschi C, Almeras L, Raoult D, Parola P, 2015. Occurrence and genotyping of Coxiella burnetii in ixodid ticks in Oromia, Ethiopia. Am J Trop Med Hyg 93: 1074–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wood OL, Lee VH, Ash JS, Casals J, 1978. Crimean-Congo hemorrhagic fever, Thogoto, Dugbe, and Jos viruses isolated from ixodid ticks in Ethiopia. Am J Trop Med Hyg 27: 600–604. [DOI] [PubMed] [Google Scholar]
- 135.Tigoi C, Lwande O, Orindi B, Irura Z, Ongus J, Sang R, 2015. Seroepidemiology of selected arboviruses in febrile patients visiting selected health facilities in the lake/river basin areas of lake Baringo, lake Naivasha, and Tana River, Kenya. Vector Borne Zoonotic Dis 15: 124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Morrill JC, Johnson BK, Hyams C, Okoth F, Tukei PM, Mugambi M, Woody J, 1991. Serological evidence of arboviral infections among humans of coastal Kenya. J Trop Med Hyg 94: 166–168. [PubMed] [Google Scholar]
- 137.Johnson BK, Ocheng D, Gichogo A, Okiro M, Libondo D, Tukei PM, Ho M, Mugambi M, Timms GL, French M, 1983. Antibodies against haemorrhagic fever viruses in Kenya populations. Trans R Soc Trop Med Hyg 77: 731–733. [DOI] [PubMed] [Google Scholar]
- 138.Čumakov MP, Smirnova SE, 1972. Obnaruženie antitel k virusu krymskoj gemorragičekoj lihoradki v syvortkah krovi dikih i domašnih životnyh iz Irana i Afriki. Čumakov MP, ed. Aktual’nye Problemy Virusologii I Profilaktiki Virusnyh Zabolevanij - Tezisy XVII Naučnoj Sessii Instituta, Posvâŝënnoj Aktual’nym Problemam Virusologii I Profilaktiki Virusnyh Zabolevanij. Moscow, USSR: Akademii medicinskih nauk SSSR, Institut poliomielita i virusnyh èncefalitov, 367–368. [Google Scholar]
- 139.Sang R, et al. 2011. Crimean-Congo hemorrhagic fever virus in hyalommid ticks, northeastern Kenya. Emerg Infect Dis 17: 1502–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mathiot CC, Fontenille D, Georges AJ, Coulanges P, 1989. Antibodies to haemorrhagic fever viruses in Madagascar populations. Trans R Soc Trop Med Hyg 83: 407–409. [DOI] [PubMed] [Google Scholar]
- 141.Andriamandimby SF, Marianneau P, Rafisandratantsoa JT, Rollin PE, Heraud JM, Tordo N, Reynes JM, 2011. Crimean-Congo hemorrhagic fever serosurvey in at-risk professionals, Madagascar, 2008 and 2009. J Clin Virol 52: 370–372. [DOI] [PubMed] [Google Scholar]
- 142.Uilenberg G, Hoogstraal H, Klein JM, 1979. Les tiques (Ixodoidea) de Madagascar et leur rôle vecteur. Tananarive, Madagascar: Arch Inst Pasteur Madagascar Numéro Spécial, 1–153. [Google Scholar]
- 143.Mathiot CC, Fontenille D, Digoutte JP, Coulanges P, 1988. First isolation of Congo-Crimean haemorrhagic fever virus in Madagascar. Ann Inst Pasteur Virol 139: 239–241. [DOI] [PubMed] [Google Scholar]
- 144.Berggren SA, 1978. Cattle ticks in Malawi. Vet Parasitol 4: 289–297. [Google Scholar]
- 145.Horak IG, Nyangiwe N, de Matos C, Neves L, 2009. Species composition and geographic distribution of ticks infesting cattle, goats and dogs in a temperate and in a subtropical region of south-east Africa. Onderstepoort J Vet Res 76: 263–276. [DOI] [PubMed] [Google Scholar]
- 146.Laughlin LW, Legters LJ, 1993. Disease threats in Somalia. Am J Trop Med Hyg 48: vi–x. [PubMed] [Google Scholar]
- 147.Williams RJ, al-Busaidy S, Mehta FR, Maupin GO, Wagoner KD, al-Awaidy S, Suleiman AJM, Khan AS, Peters CJ, Ksiazek TG, 2000. Crimean-Congo haemorrhagic fever: a seroepidemiological and tick survey in the Sultanate of Oman. Trop Med Int Health 5: 99–106. [DOI] [PubMed] [Google Scholar]
- 148.Khan AS, et al. 1997. An outbreak of Crimean-Congo hemorrhagic fever in the United Arab Emirates, 1994–1995. Am J Trop Med Hyg 57: 519–525. [DOI] [PubMed] [Google Scholar]
- 149.Chisholm K, Dueger E, Fahmy NT, Samaha HA, Zayed A, Abdel-Dayem M, Villinski JT, 2012. Crimean-Congo hemorrhagic fever virus in ticks from imported livestock, Egypt Emerg Infect Dis 18: 181–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Salih DA, Julla II, Hassan SM, el Hussein AM, Jongejan F, 2008. Reliminary survey of ticks (Acari: Ixodidae) on cattle in central Equatoria state, southern Sudan. Onderstepoort J Vet Res 75: 47–53. [DOI] [PubMed] [Google Scholar]
- 151.Rodhain F, Gonzalez JP, Mercier E, Helynck B, Larouze B, Hannoun C, 1989. Arbovirus infections and viral haemorrhagic fevers in Uganda: a serological survey in Karamoja district, 1984. Trans R Soc Trop Med Hyg 83: 851–854. [DOI] [PubMed] [Google Scholar]
- 152.Clements TL, Rossi CA, Irish AK, Kibuuka H, Eller LA, Robb ML, Kataaha P, Michael NL, Hensley LE, Schoepp RJ, 2019. Chikungunya and o’nyong-nyong viruses in Uganda: implications for diagnostics. Open Forum Infect Dis 6: ofz001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kirâ D, Semënov BF, Tret’âkov FI, Gromaševskij VL, Madžomba E, 1972. Predvaritel’noe soobŝenie ob issledovanii syvorotok životnyh iz vostočnoj Afriki na antitela k virusu Kongo metodom diffuzionnoj precipitacii v agare. Čumakov MP, ed. Aktual’nye Problemy Virusologii I Profilaktiki Virusnyh Zabolevanij - Tezisy XVII Naučnoj Sessii Instituta, Posvâŝënnoj Aktual’nym Problemam Virusologii I Profilaktiki Virusnyh Zabolevanij. Moscow, USSR: Akademii Medicinskih nauk SSSR, Institut Poliomielita i Virusnyh èncefalitov, 368–369. [Google Scholar]
- 154.Kaiser MN, Sutherst RW, Bourne AS, 1982. Relationship between ticks and Zebu cattle in southern Uganda. Trop Anim Health Prod 14: 63–74. [DOI] [PubMed] [Google Scholar]
- 155.Kirâ D, 1972. Novye dannye o viruse Kongo v Vostočnoj Afrike: vydelenie virusa Kongo iz kleŝej Amblyomma variegatum. Čumakov MP, ed. Aktual’nye Problemy Virusologii I Profilaktiki Virusnyh Zabolevanij - Tezisy XVII Naučnoj Sessii Instituta, Posvâŝënnoj Aktual’nym Problemam Virusologii I Profilaktiki Virusnyh Zabolevanij. Moscow, USSR: Akademii Medicinskih nauk SSSR, Institut Poliomielita i Virusnyh èncefalitov, 348. [Google Scholar]
- 156.Koehler JW, Delp KL, Kearney BJ, Conrad TA, Schoepp RJ, Garrison AR, Altimura LA, Rossi CA, Minogue TD, 2017. Crimean-Congo Hemorrhagic Fever Nairovirus Isolate UCCR4417 Segment M, Complete Sequence. Available at: https://www.ncbi.nlm.nih.gov/nuccore/KY484045.1?report=genbank&to=5365. Accessed March 23, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yeoman GH, Walker JB, Ross JPJ, Docker TM, 1967. The Ixodid Ticks of Tanzania. A Study of the Zoogeography of the Ixodidae of an East African Country. London, UK: Commonwealth Institute of Entomology. [Google Scholar]
- 158.Blackburn NK, Searle L, Taylor P, 1982. Viral haemorrhagic fever antibodies in Zimbabwe schoolchildren. Trans R Soc Trop Med Hyg 76: 803–805. [DOI] [PubMed] [Google Scholar]
- 159.Shepherd AJ, Swanepoel R, Shepherd SP, McGillivray GM, Searle LA, 1987. Antibody to Crimean-Congo hemorrhagic fever virus in wild mammals from Southern Africa. Am J Trop Med Hyg 36: 133–142. [DOI] [PubMed] [Google Scholar]
- 160.Gomes AF, Pombal AM, Jr., Venturi L, 1994. Observations on cattle ticks in Huila province (Angola). Vet Parasitol 51: 333–336. [DOI] [PubMed] [Google Scholar]
- 161.Gonzalez JP, et al. 1989. Antibody prevalence against haemorrhagic fever viruses in randomized representative central African populations. Res Virol 140: 319–331. [DOI] [PubMed] [Google Scholar]
- 162.Sadeuh-Mba SA, Yonga Wansi GMY, Demanou M, Gessain A, Njouom R, 2018. Serological evidence of Rift Valley fever phlebovirus and Crimean-Congo hemorrhagic fever orthonairovirus infections among pygmies in the east region of Cameroon. Virol J 15: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sas MA, 2016. Novel Serological and Molecular Assays for Crimean-Congo Hemorrhagic Fever Virus Infections and Their Application in Prevalence Studies on Sub-Saharan African Countries. DVM dissertation, University of Veterinary Medicine, Hannover, Germany. [Google Scholar]
- 164.Saluzzo JF, Gonzalez JP, Hervé JP, Georges AJ, 1981. Enquête sérologique sur la prévalence de certains arbovirus dans la population humaine du sud-est de la République Centrafricaine en 1979. Bull Soc Pathol Exot Filiales 74: 490–499. [PubMed] [Google Scholar]
- 165.Meunier DMY, Johnson ED, Gonzalez JP, Georges-Courbot MC, Madelon MC, Georges AJ, 1987. Données sérologiques actuelles sur les fièvres hémorragiques virales en République centrafricaine. Bull Soc Pathol Exot Filiales 80: 51–61. [PubMed] [Google Scholar]
- 166.Mura A, Socolovschi C, Ginesta J, Lafrance B, Magnan S, Rolain JM, Davoust B, Raoult D, Parola P, 2008. Molecular detection of spotted fever group rickettsiae in ticks from Ethiopia and Chad. Trans R Soc Trop Med Hyg 102: 945–949. [DOI] [PubMed] [Google Scholar]
- 167.Sas MA, Mertens M, Kadiat JG, Schuster I, Pongombo CPS, Maloba AGK, Groschup MH, 2017. Serosurvey for Crimean-Congo hemorrhagic fever virus infections in ruminants in Katanga province, Democratic Republic of the Congo. Ticks Tick Borne Dis 8: 858–861. [DOI] [PubMed] [Google Scholar]
- 168.Theiler G, 1962. The Ixodoidea parasites of vertebrates in Africa south of the Sahara (Ethiopian region). Project S.9958-Report to the Director of Veterinary Services, Onderstepoort-June 1962. Onderstepoort, Pretoria, Gauteng, South Africa: Onderstepoort Veterinary Institute. [Google Scholar]
- 169.Elbl A, Anastos G, 1966. Ixodid ticks (Acarina, Ixodidae) of central Africa, volume IV. Genera Aponomma Neumann, 1899, Boophilus Curtice, 1891, Dermacentor Koch, 1844, Haemaphysalis Koch, 1844, Hyalomma Koch, 1844 and Rhipicentor Nuttall and Warburton, 1908. Lists and bibliography. Annales - Serie in 8 - Sciences Zoologiques 148. Musée Royal de l'Afrique Centrale, Tervuren, Belgique. [Google Scholar]
- 170.Müller MA, et al. 2016. Evidence for widespread infection of African bats with Crimean-Congo hemorrhagic fever-like viruses. Sci Rep 6: 26637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yousfi-Monod R, Aeschlimann A, 1986. Recherches sur les tiques (Acarina, Ixodidae), parasites de bovidés dans l’Ouest algérien. I — inventaire systématique et dynamique saisonnière. Ann Parasitol Hum Comp 61: 341–358. [DOI] [PubMed] [Google Scholar]
- 172.Kautman M, Tiar G, Papa A, Široký P, 2016. AP92-like Crimean-Congo hemorrhagic fever virus in Hyalomma aegyptium ticks, Algeria. Emerg Infect Dis 22: 354–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Darwish M, Imam IZE, Omar FM, Hoogstraal H, 1977. A seroepidemiological survey for Crimean-Congo hemorrhagic fever virus in humans and domestic animals in Egypt. J Egypt Public Health Assoc LII: 156–163. [Google Scholar]
- 174.Palomar AM, Portillo A, Santibáñez P, Mazuelas D, Arizaga J, Crespo A, Gutiérrez O, Cuadrado JF, Oteo JA, 2013. Crimean-Congo hemorrhagic fever virus in ticks from migratory birds, Morocco. Emerg Infect Dis 19: 260–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Watts DM, el-Tigani A, Botros BAM, Salib AW, Olson JG, McCarthy M, Ksiazek TG, 1994. Arthropod-borne viral infections associated with a fever outbreak in the northern province of Sudan. J Trop Med Hyg 97: 228–230. [PubMed] [Google Scholar]
- 176.Rahden P, Adam A, Mika A, Jassoy C, 2019. Elevated human Crimean-Congo hemorrhagic fever virus seroprevalence in Khashm el Girba, Eastern Sudan. Am J Trop Med Hyg 100: 1549–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hassanein KM, el-Azazy OME, Yousef HM, 1997. Detection of Crimean-Congo haemorrhagic fever virus antibodies in humans and imported livestock in Saudi Arabia. Trans R Soc Trop Med Hyg 91: 536–537. [DOI] [PubMed] [Google Scholar]
- 178.Adam IA, Mahmoud MAM, Aradaib IE, 2013. A seroepidemiological survey of Crimean Congo hemorrhagic fever among cattle in North Kordufan State, Sudan. Virol J 10: 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Suliman HM, Adam IA, Saeed SI, Abdelaziz SA, Haroun EM, Aradaib IE, 2017. Crimean Congo hemorrhagic fever among the one-humped camel (Camelus dromedaries) in Central Sudan. Virol J 14: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ibrahim AM, Adam IA, Osman BT, Aradaib IE, 2015. Epidemiological survey of Crimean Congo hemorrhagic fever virus in cattle in east Darfur state, Sudan. Ticks Tick Borne Dis 6: 439–444. [DOI] [PubMed] [Google Scholar]
- 181.Chitimia-Dobler L, et al. 2019. Crimean-Congo haemorrhagic fever virus in Hyalomma impeltatum ticks from north Kordofan, the Sudan. Int J Infect Dis 89: 81–83. [DOI] [PubMed] [Google Scholar]
- 182.Joubert JJ, van der Merwe CA, Lourens JH, Lecatsas G, Siegrühn C, 1991. Serological markers of hepatitis B virus and certain other viruses in the population of eastern Caprivi, Namibia. Trans R Soc Trop Med Hyg 85: 101–103. [DOI] [PubMed] [Google Scholar]
- 183.Biggs HC, Langenhoven JW, 1984. Seasonal prevalence of ixodid ticks on cattle in the Windhoek district of south west Africa/Namibia. Onderstepoort J Vet Res 51: 175–182. [PubMed] [Google Scholar]
- 184.Swanepoel R, Struthers JK, Shepherd AJ, McGillivray GM, Nel MJ, Jupp PG, 1983. Crimean-Congo hemorrhagic fever in South Africa. Am J Trop Med Hyg 32: 1407–1415. [DOI] [PubMed] [Google Scholar]
- 185.Centers for Disease Control , 1981. Crimean-Congo hemorrhagic fever - South Africa. MMWR Morb Mortal Wkly Rep 30: 349–351. [PubMed] [Google Scholar]
- 186.Swanepoel R, Shepherd AJ, Leman PA, Shepherd SP, 1985. Investigations following initial recognition of Crimean-Congo haemorrhagic fever in South Africa and the diagnosis of 2 further cases. S Afr Med J 68: 638–641. [PubMed] [Google Scholar]
- 187.Fisher-Hoch SP, McCormick JB, Swanepoel R, Van Middlekoop A, Harvey S, Kustner HG, 1992. Risk of human infections with Crimean-Congo hemorrhagic fever virus in a South African rural community. Am J Trop Med Hyg 47: 337–345. [DOI] [PubMed] [Google Scholar]
- 188.Vawda S, Goedhals D, Bester PA, Burt F, 2018. Seroepidemiologic survey of Crimean-Congo hemorrhagic fever virus in selected risk groups, South Africa. Emerg Infect Dis 24: 1360–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Burt FJ, Spencer DC, Leman PA, Patterson B, Swanepoel R, 1996. Investigation of tick-borne viruses as pathogens of humans in South Africa and evidence of Dugbe virus infection in a patient with prolonged thrombocytopenia. Epidemiol Infect 116: 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Shepherd AJ, Swanepoel R, Shepherd SP, Leman PA, Blackburn NK, Hallett AF, 1985. A nosocomial outbreak of Crimean-Congo haemorrhagic fever at Tygerberg Hospital. Part V. Virological and serological observations. S Afr Med J 68: 733–736. [PubMed] [Google Scholar]
- 191.Centers for Disease Control , 1984. Congo-Crimean hemorrhagic fever - Republic of South Africa. MMWR Morb Mortal Wkly Rep 33: 535–536, 541, 548. [PubMed] [Google Scholar]
- 192.Swanepoel R, Shepherd AJ, Leman PA, Shepherd SP, Miller GB, 1985. A common-source outbreak of Crimean-Congo haemorrhagic fever on a dairy farm. S Afr Med J 68: 635–637. [PubMed] [Google Scholar]
- 193.International Society for Infectious Diseases , 2013. Crimean-Congo Hemorrhagic Fever - South Africa (02): Background. ProMED archive number 20130116.1501273. Available at: https://promedmail.org/promed-post/?id=20130116.1501273. [Google Scholar]
- 194.Kemp A, Msimang V, Weyer J, Paweska J, 2014. Crimean-Congo haemorrhagic fever and tick-bite fever in South Africa, 2012–2014. Commun Dis Surv Bull 12: 59–65. [Google Scholar]
- 195.Gonzalez JP, Baudon D, McCormick JB, 1984. Premières études sérologiques dans les populations humaines de Haut-Volta et du Benin sur les fièvres hémorragiques africaines d’origine virale. OCCGE Inform 12: 113–116. [Google Scholar]
- 196.Abiola FA, Karimou M, Houeto P, 1991. Cholinesterase activity in bovine ticks and its inhibition in vitro by organophosphates acaricids. Rev Méd Vét (Toulouse) 142: 147–152. [Google Scholar]
- 197.Digoutte JP, Saluzzo JF, Adam F, 1985. Données récentes sur les fièvres hémorragiques en Afrique de l'Ouest. Bull Soc Pathol Exot Filiales 78: 874–878. [PubMed] [Google Scholar]
- 198.Kabore H, Salembere MS, Tamboura HH, 1998. Seasonal variation of ticks on cattle in Burkina Faso. Ann N Y Acad Sci 849: 398–401. [DOI] [PubMed] [Google Scholar]
- 199.Biguezoton A, Adehan S, Adakal H, Zoungrana S, Farougou S, Chevillon C, 2016. Community structure, seasonal variations and interactions between native and invasive cattle tick species in Benin and Burkina Faso. Parasit Vectors 9: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.de Meira MTV, Ruffié J, de Sousa HT, 1957. Algumas carraças das ilhas de Cabo Verde. An Inst Med Trop (Lisb) 14: 425–427. [PubMed] [Google Scholar]
- 201.Knopf L, Komoin-Oka C, Betschart B, Jongejan F, Gottstein B, Zinsstag J, 2002. Seasonal epidemiology of ticks and aspects of cowdriosis in N'Dama village cattle in the Central Guinea savannah of Côte d’Ivoire. Prev Vet Med 53: 21–30. [DOI] [PubMed] [Google Scholar]
- 202.Boka OM, Achi L, Adakal H, Azokou A, Yao P, Yapi YG, Kone M, Dagnogo K, Kaboret YY, 2017. Review of cattle ticks (Acari, Ixodida) in ivory coast and geographic distribution of Rhipicephalus (Boophilus) microplus, an emerging tick in West Africa. Exp Appl Acarol 71: 355–369. [DOI] [PubMed] [Google Scholar]
- 203.Claxton J, Leperre P, 1991. Parasite burdens and host susceptibility of Zebu and N’Dama cattle in village herds in Gambia. Vet Parasitol 40: 293–304. [DOI] [PubMed] [Google Scholar]
- 204.Ammah-Attoh V, 1966. Reproduction in the tick Hyalomma marginatum rufipes Koch, 1844 under laboratory conditions, with notes on mating and insemination. Ghana J Sci 6: 9–14. [Google Scholar]
- 205.Tomassone L, Camicas JL, Pagani P, Diallo OT, Mannelli A, De Meneghi D, 2004. Monthly dynamics of ticks (Acari:Ixodida) infesting N'Dama cattle in the Republic of Guinea. Exp Appl Acarol 32: 209–218. [DOI] [PubMed] [Google Scholar]
- 206.Mediannikov O, Diatta G, Zolia Y, Balde MC, Kohar H, Trape JF, Raoult D, 2012. Tick-borne rickettsiae in Guinea and Liberia. Ticks Tick Borne Dis 3: 43–48. [DOI] [PubMed] [Google Scholar]
- 207.Butenko AM, 1996. Izučenie cirkulâcii arbovirusov v Gvinejskoj Respublike. Med Parazitol (Mosk)) 2: 40–45. [PubMed] [Google Scholar]
- 208.van der Waals FW, Pomeroy KL, Goudsmit J, Asher DM, Gajdusek DC, 1986. Hemorrhagic fever virus infections in an isolated rainforest area of central Liberia. Limitations of the indirect immunofluorescence slide test for antibody screening in Africa. Trop Geogr Med 38: 209–214. [PubMed] [Google Scholar]
- 209.Traoré A, Dao S, Jouanelle J, Bougoudogo F, Toure Y, Maiga K, 2005. A propos des premières observations sérologiques de la fièvre hémorragique de Crimée Congo au Mali. Mali Méd XX: 52–53. [PubMed] [Google Scholar]
- 210.Maiga O, et al. 2017. Serosurvey of Crimean-Congo hemorrhagic fever virus in cattle, Mali, west Africa. Am J Trop Med Hyg 96: 1341–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Teel PD, Bay DE, Ajidagba PA, 1988. Ecology, distribution and host relationships of ticks (Acari: Ixodidae) infesting livestock in Mali. Bull Entomol Res 78: 407–424. [Google Scholar]
- 212.Diarra AZ, Almeras L, Laroche M, Berenger JM, Koné AK, Bocoum Z, Dabo A, Doumbo O, Raoult D, Parola P, 2017. Molecular and MALDI-TOF identification of ticks and tick-associated bacteria in Mali. PLoS Negl Trop Dis 11: e0005762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zivcec M, Maïga O, Kelly A, Feldmann F, Sogoba N, Schwan TG, Feldmann H, Safronetz D, 2014. Unique strain of Crimean-Congo hemorrhagic fever virus, Mali Emerg Infect Dis 20: 911–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Saluzzo JF, Digoutte JP, Camicas JL, Chauvancy G, 1985. Crimean-Congo haemorrhagic fever and Rift Valley fever in south-eastern Mauritania. Lancet 1: 116. [DOI] [PubMed] [Google Scholar]
- 215.Saluzzo JF, Camicas JL, Chartier C, Martinez D, Digoutte JP, 1986. Le virus de la fièvre hémorragique de Crimée-Congo (CCHF) en Mauritanie. Cah ORSTOM Entomol Méd Parasit 24: 129–137. [Google Scholar]
- 216.Lepers JP, Billon C, Pesce JL, Rollin PE, De Saint-Martin J, 1988. Etude sero-épidémiologique en Mauritanie (1985–1986): fréquence des tréponematoses, du virus de l’hepatite B, du virus VIH et des fièvres hemorragiques virales. Bull Soc Pathol Exot Filiales 81: 24–31. [PubMed] [Google Scholar]
- 217.Faye O, et al. 2006. Successive outbreaks of viral haemorrhagic fevers (CCHF and RVF) in Mauritania, 2003. Am J Trop Med Hyg 75: 192. [Google Scholar]
- 218.Rollin PE, Lepers JP, Rodhain F, Coudrier D, Sureau P, 1987. Viral haemorrhagic fever seroepidemiology in Mauritania. Trans R Soc Trop Med Hyg 81: 520. [DOI] [PubMed] [Google Scholar]
- 219.Mariner JC, Morrill J, Ksiazek TG, 1995. Antibodies to hemorrhagic fever viruses in domestic livestock in Niger: Rift Valley fever and Crimean-Congo hemorrhagic fever. Am J Trop Med Hyg 53: 217–221. [DOI] [PubMed] [Google Scholar]
- 220.Bloch N, Diallo I, 1991. Enquête sérologique chez les petits ruminants dans quatre départements du Niger. Rev Elev Méd Vét Pays Trop 44: 397–404. [PubMed] [Google Scholar]
- 221.Hoffmann G, Lindau M, 1971. Zecken an Nutz- und Wildtieren in Niger. Z für Angew Entomologie 69: 72–82. [Google Scholar]
- 222.Parola P, Inokuma H, Camicas JL, Brouqui P, Raoult D, 2001. Detection and identification of spotted fever group Rickettsiae and Ehrlichiae in African ticks. Emerg Infect Dis 7: 1014–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Tomori O, Fabiyi A, Sorungbe A, Smith A, McCormick JB, 1988. Viral hemorrhagic fever antibodies in Nigerian populations. Am J Trop Med Hyg 38: 407–410. [DOI] [PubMed] [Google Scholar]
- 224.Bukbuk DN, et al. 2014. Development and validation of serological assays for viral hemorrhagic fevers and determination of the prevalence of Rift Valley fever in Borno state, Nigeria. Trans R Soc Trop Med Hyg 108: 768–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Umoh JU, Ezeokoli CD, Ogwu D, 1983. Prevalence of antibodies to Crimean-haemorrhagic fever-Congo virus in cattle in northern Nigeria. Int J Zoonoses 10: 151–154. [PubMed] [Google Scholar]
- 226.Oluwayelu D, et al. 2020. Prevalence of antibodies to Crimean-Congo hemorrhagic fever virus in ruminants, Nigeria, 2015. Emerg Infect Dis 26: 744–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Fabiyi A, 1978. Viral hemorrhagic fevers (Congo and Lassa) in Nigeria. Cherepanov AI, ed. Transcontinental Connections of Migratory Birds and Their Role in the Distribution of Arboviruses. Papers of a Symposium, July - August 1976. Akademgorodok, Novosibirsk Oblast, RSFSR, USSR: Nauka, 251–255. [Google Scholar]
- 228.Lorusso V, Picozzi K, de Bronsvoort BMC, Majekodunmi A, Dongkum C, Balak G, Igweh A, Welburn SC, 2013. Ixodid ticks of traditionally managed cattle in central Nigeria: where Rhipicephalus (Boophilus) microplus does not dare (yet?). Parasit Vectors 6: 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sanchez AJ, Vincent MJ, Nichol ST, 2002. Characterization of the glycoproteins of Crimean-Congo hemorrhagic fever virus. J Virol 76: 7263–7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wilson ML, Gonzalez JP, LeGuenno B, Cornet JP, Guillaud M, Calvo MA, Digoutte JP, Camicas JL, 1990. Epidemiology of Crimean-Congo hemorrhagic fever in Senegal: temporal and spatial patterns. Calisher CH, ed. Hemorrhagic Fever with Renal Syndrome, Tick- and Mosquito-Borne Viruses. Archives of Virology Supplementum, vol. 1. Vienna, Austria: Springer, 323–340. [Google Scholar]
- 231.Chapman LE, Wilson ML, Hall DB, LeGuenno B, Dykstra EA, Ba K, Fisher-Hoch SP, 1991. Risk factors for Crimean-Congo hemorrhagic fever in rural northern Senegal. J Infect Dis 164: 686–692. [DOI] [PubMed] [Google Scholar]
- 232.Sylla PM, Molez JF, Cornet JP, Camicas JL, Pourrut X, 2008. Variabilité climatique et répartition de la fièvre hémorragique de Crimée Congo et de la cowdriose, maladies à tiques au Sénégal. Acarologia XLVIII: 155–161. [Google Scholar]
- 233.Wilson ML, LeGuenno B, Guillaud M, Desoutter D, Gonzalez JP, Camicas JL, 1990. Distribution of Crimean-Congo hemorrhagic fever viral antibody in Senegal: environmental and vectorial correlates. Am J Trop Med Hyg 43: 557–566. [DOI] [PubMed] [Google Scholar]
- 234.Čunihin SP, Čumakov MP, Butenko AM, Smirnova SE, Tofflib R, Kamikas ŽL, Roben I, Kornet M, Šabon Ž, 1969. Rezul’taty obsledovaniâ na antitela k virusu krymskoj gemorragičeskoj lihoradki syvorotok krovi lûdej, domašnih i dikih životnyh v respublike Senegal (Zapadnaâ Afrika). Čumakov MP, ed. Arbovirusy (Kleŝevoj I Âponskij Èncefality, Gemorragičeskie Lihoradki I Drugie Arbovirusnye Infektsii). Materialy HVI Naučnoj Sessii Instituta Poliomielita I Virusnyh Entsefalitov SSSR. Moscow, USSR: Akademii medicinskih nauk SSSR, Institut poliomielita i virusnyh èncefalitov, 158–160. [Google Scholar]
- 235.Zeller HG, Cornet JP, Diop A, Camicas JL, 1997. Crimean-Congo hemorrhagic fever in ticks (Acari: Ixodidae) and ruminants: field observations of an epizootic in Bandia, Senegal (1989–1992). J Med Entomol 34: 511–516. [DOI] [PubMed] [Google Scholar]
- 236.Zeller HG, Cornet JP, Camicas JL, 1994. Crimean-Congo haemorrhagic fever virus infection in birds: field investigations in Senegal. Res Virol 145: 105–109. [DOI] [PubMed] [Google Scholar]
- 237.Robin Y, Camicas JL, Jan C, Heme G, Cornet M, Valade M, 1978. Ecology of tick arboviruses in arid areas of Senegal. Transcontinental Connections of Migratory Birds and Their Role in the Distribution of Arboviruses. Papers of a Symposium, July–August 1976. Akademgorodok, Novosibirsk Oblast, RSFSR, USSR: Nauka, Siberian Branch, 209–211. [Google Scholar]
- 238.Mangombi JB, Roqueplo C, Sambou M, Dahmani M, Mediannikov O, Comtet L, Davoust B, 2020. Seroprevalence of Crimean-Congo hemorrhagic fever in domesticated animals in northwestern Senegal. Vector Borne Zoonotic Dis 20: 797–799. [DOI] [PubMed] [Google Scholar]
- 239.Morel PC, 1958. Les tiques des animaux domestiques de l'afrique occidentale française. Rev Elev Méd Vét Pays Trop 11: 153–189. [Google Scholar]
- 240.Sambou M, Faye N, Bassène H, Diatta G, Raoult D, Mediannikov O, 2014. Identification of rickettsial pathogens in ixodid ticks in northern Senegal. Ticks Tick Borne Dis 5: 552–556. [DOI] [PubMed] [Google Scholar]
- 241.Camicas JL, Robin Y, le Gonidec G, Saluzzo JF, Jouan A, Cornet JP, Chauvancy G, Ba K, 1986. Etude écologique et nosologique des arbovirus transmis par les tiques au Sénégal. 3. Les vecteurs potentiels du virus de la fièvre hémorragique de Crimée-Congo (virus CCHF) au Sénégal et en Mauritanie. Cah ORSTOM Entomol Méd Parasit 24: 255–264. [Google Scholar]
- 242.Cornet JP, Zeller H, Camicas JL, 1995. Contribution à l'étude des tiques (Acarina: Ixodina) vectrices du virus de la fièvre hémorragique Crimée-Congo (CCHF) au Sénégal. 2-biologie, aux stases préimaginales, de Hyalomma marginatum rufipes. Acarologia XXXVI: 293–295. [Google Scholar]
- 243.Camicas JL, Wilson ML, Cornet JP, Digoutte JP, Calvo MA, Adam F, Gonzalez JP, 1990. Ecology of ticks as potential vectors of Crimean Congo hemorrhagic fever virus in Senegal: epidemiological implications. Calisher CH, ed. Hemorrhagic Fever with Renal Syndrome, Tick- and Mosquito-Borne Viruses. Archives of Virology Supplementum, vol. 1. Vienna, Austria: Springer, 303–322. [Google Scholar]
- 244.Cornet JP, Bâ YS, Bâ K, Chauvancy G, Zeller H, 1997. Contribution à l'étude des tiques (Acarina: Ixodida) vectrices du virus de la fièvre hémorragique Crimée-Congo (CCHF), au Sénégal. 5-Etude biologique et virologique de la faune ixodidienne des terriers. Acarologia XXXVIII: 43–45. [Google Scholar]
- 245.O’Hearn AE, Voorhees MA, Fetterer DP, Wauquier N, Coomber MR, Bangura J, Fair JN, Gonzalez JP, Schoepp RJ, 2016. Serosurveillance of viral pathogens circulating in west Africa. Virol J 13: 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Kulo AE, Assogba K, Talaki E, Poutouli W, 2012. Répartition spatiale des tiques (Ixodidae) sur un pâturage cultive à La station expérimentale agronomique de l’Ecole Supérieure d’Agronomie - campus Universitaire de Lomé au Togo. Livestock Res Rural Dev 24: 113. [Google Scholar]