Abstract.
Strongyloides stercoralis is a soil-transmitted nematode that can cause life-threatening conditions in immunocompromised persons. In the United States, strongyloidiasis should be considered mainly in immigrants, refugees, or travelers. The confirmatory laboratory diagnosis is usually performed by detecting larvae from the stool, duodenal material, and sputum. In persons who are immunocompromised with severe strongyloidiasis, adult worms and eggs can be detected from duodenal material. For serological diagnosis, most assays use crude antigens to detect anti-S. stercoralis IgG. Recently, recombinant proteins such as rSs-NIE-1 and rSs-IR have been used to detect IgG antibodies. We used rSs-NIE-1 and rSs-IR recombinant antigens to develop a biplex Western blot assay to detect the IgG4 antibody in individuals with strongyloidiasis. The sensitivities of rSs-NIE-1 and rSs-IR were 97.4% and 90.8%, respectively, whereas the specificities were 97.6% and 98%, respectively. In conclusion, the biplex rSs-NIE-1 and rSs-IR immunoblot performs well in detecting IgG4 antibody in S. stercoralis-infected persons.
Strongyloides stercoralis is the main etiological agent of strongyloidiasis in humans. It is a soil-transmitted nematode, and its prevalence is not well documented.1 Cases in the United States mainly occur in returning travelers from endemic countries, recent immigrants, and refugees.1,2 The definitive diagnosis of S. stercoralis infection is by the identification of S. stercoralis larvae in the stool and, possibly, in sputum by microscopy. Other parasitic forms, such as the parthenogenetic female and eggs, can be found in duodenal fluid and/or biopsy specimens.3 These other forms can be found in severe strongyloidiasis, which is potentially life-threatening and can occur in immunocompromised individuals, such as Human T-cell Lymphotropic Virus Type 1-infected subjects, alcoholics, and organ transplant recipients.4 Because the larval output is minimal and happens intermittently in uncomplicated infections, microscopic examination of the stool has low sensitivity. Larvae are best visualized following recovery by the Baermann funnel sedimentation technique or after culture using Koga agar plate, charcoal culture, or the Harada-Mori filter paper technique. The Koga agar plate culture method is recognized in most studies as the most sensitive coprological method for detecting Strongyloides larvae.3,5,6
Immunodiagnosis tests for strongyloidiasis are indicated when an infection is suspected, and repeated stool examinations do not detect the parasite. However, negative serological results cannot exclude strongyloidiasis definitively. Positive results may occur as a result of residual antibody long after successful treatment in some patients or as a result of cross-reactions such as occur in hookworm infestation. Many S. stercoralis serological assays rely on native antigen sources, which need to be obtained from many infected humans’ larvae, which are difficult to find and have a high risk of laboratory contamination during the isolation process. Because of these problems, several laboratories use antigens from related species, mainly from Strongyloides ratti and Strongyloides venezuelensis, that require a complex laboratorial structure, with experimental animals, to maintain its production, turning it into a complicated and relatively expensive process.7 Despite this limitation, depending on the antigen and test platform used, serological diagnosis remains the most easily performed and sensitive test for the laboratory detection of strongyloidiasis. Both commercial and noncommercial assays have been produced using various antigens and test platforms, each differing in sensitivity and specificity. As there is no universally recognized reference standard by which to compare each of these tests’ performance, it is difficult to determine the optimal serological assay for use in diagnostic laboratories.
The serological test currently used by the Parasitic Disease Reference Diagnostic Laboratory (PDRDL) at the Centers for Disease Control and Prevention (CDC) is an enzyme-linked immunosorbent assay (ELISA) to detect IgG antibodies directed against antigens derived from S. stercoralis filariform larvae.6,8 The test has a sensitivity and specificity of 96% and 98%, respectively. This specificity was reduced to 72% when samples from patients with other infections (ascariasis, cysticercosis, echinococcosis, fascioliasis, filariasis, hookworm, paragonimiasis, and toxocariasis) were included in the calculation. To improve the assay’s specificity, we developed an ELISA using a recombinant antigen, rSs-NIE-1, to detect the IgG4 antibody. The assay specificity improved from 72–93%, without losing sensitivity.9 In our work, we sought to improve the specificity and sensitivity of S. stercoralis immunodiagnosis by using a combination of rSs-NIE-1 and rSs-IR (an immune-reactive antigen; GenBank: ABY51618.1)10 in an immunoblot format to detect S. stercoralis-specific IgG4 antibodies because an increased specificity was observed when the assay measured IgG4 rather than total IgG.9,11
We used several sets of human sera to test a standard Western blot: 1) samples collected from patients in Brazil positive for S. stercoralis in Koga agar plate culture (N = 76), 2) presumed negative samples from U.S. residents with no history of foreign travel (N = 154), and 3) a convenience panel of samples from patients with various parasitic diseases other than strongyloidiasis (N = 98) (these samples were the CDC biorepository samples from clinical specimens diagnosed at the PDRDL laboratory and found positive in their respective reference assays, but were not tested for strongyloidiasis). All clinical samples used in this study were collected after receiving written informed consent under protocols approved by the CDC Institutional Review Board (CDC Study Protocol no. 6756). Participants provided specific permission for future use of stored samples.
The expression of rSs-NIE-1 has been described previously.9 For expression of rSs-IR, after transforming an expression vector, pGs21a, containing a fusion gene of Ss-IR, Schistosoma japonicum glutathione S-transferase (GST) and 6 × histidine (HIS) gene into Escherichia coli bacteria, successful recombinant colonies were grown under the selection of 100 µg/mL ampicillin at 37°C, with shaking at 200 rpm using the MaxQ 480R HP shaker incubator (Thermofisher, Waltham, MA). When the cell density reached an optical density of 1.3 at 600 nm, the production of rSs-IR was induced with 0.5 mM isopropyl β-D-1-thiogalactopyranoside at 15°C overnight with shaking at 200 rpm. Cells were collected by centrifugation at 8,000 g at 4°C, for 20 minutes, and the wet pellets were resuspended with 120 mL 50 mM Tris-hydrogen chloride (HCl) with a pH of 8.0 + 150 mM sodium chloride (NaCl) + 10% glycerol. After sonication for 3 seconds and immersion in ice for 6 seconds, for a total of 15 minutes, at 500 watts, cell pellets were spun down at 13,000 rpm at 4°C for 20 minutes. The supernatant was loaded onto a 3-mL preequilibrated nickel affinity column. The protein was washed with 30 mL 50 mM Tris-HCl (pH, 8.0) + 150mM NaCl + 10% glycerol and then eluted with 30 mL 50 mM Tris-HCl (pH, 8.0) + 150mM NaCl + 10% glycerol supplemented gradient imidazole (20 mM/100 mM/500 mM). The eluted proteins were then pooled and dialyzed into 50 mM Tris-HCl (pH, 8.0) + 150mM NaCl + 10% glycerol using a 14-kDa cutoff dialysis membrane for 4 hours and changed to fresh buffer for an additional 16 hours at 4°C. After dialysis, the sample was filtered through a 0.22-µm membrane. The GST and HIS tags were left intact in the fusion protein. Proteins were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS‐PAGE) and Western blot using standard protocols for molecular weight and purity measurements. The primary antibody for Western blot was mouse‐anti‐GST monoclonal antibody (GenScript, catalog no. A00186, GenScript, Piscataway, NJ). The concentration was determined by Bradford protein assay with bovine serum albumin as a standard.
After titrating the optimum antigen concentration, rSs-NIE-1 and rSs-IR recombinant proteins were separated electrophoretically using Criterion TGX (Bio-Rad, catalog no. 567-1092, Bio-Rad, Hercules, CA) at 1.6 ng/mm and 6.25 ng/mm, respectively, and then transferred to nitrocellulose membranes (Whatman Protran BA83, catalog no 10541103, 0.2-μm pore size, Whatman plc, Maidstone, Kent, UK). The blots were cut into 2.5-mm strips and stored in PBS + 0.1% sodium azide at 4°C before use. The immunoblot was performed to detect IgG4-specific antibodies, as described previously.9,12,13 A serum sample was considered positive if reactivity occurred with a band at ∼50 kDa for rSs-NIE-1 and/or ∼30 kDa for rSs-IR.
Data were tabulated and analyzed using Microsoft Excel. Protein purity was ∼75%, as estimated by densitometry analysis of the Coomassie Blue-stained SDS-PAGE gel. The optimum concentration for detection was at 1.6 ng/mm for rSs-NIE-1 and 6.25 ng/mm for rSs-IR. At these concentrations, the performance of a combination of rSs-NIE-1 and rSs-IR demonstrated a sensitivity of 97% and a specificity of 98%. Separately, the sensitivity of rSs-NIE-1 is 97.4%, and is 90.8% for rSs-IR. The specificity of rSs-NIE-1 and rSs-IR immunoblot is 97.6 and 98.0%, respectively (Table 1). Both assays cross-reacted with one sample positive for Shistosoma mansoni. Also, it was observed cross-reacting with cysticercosis samples (Table 2), with the rSs-NIE-1 immunoblot cross-reacting with five samples (27.8%, 5 of 18), whereas the rSs-IR immunoblot cross-reacted with four samples (22.2%, 4 of 18) (Figure 1).
Table 1.
Performance of rSs-NIE-1 and rSs-IR strongyloidiasis immunoblot
| Assay characteristics | rSs-NIE-1 | 95% CI | rSs-IR | 95% CI |
|---|---|---|---|---|
| Sensitivity: N positive/N tested (%) | 74/76 (97.4) | 90.8–99.7 | 69/76 (90.8) | 81.9–96.2 |
| Specificity: N Negative/N tested (%) | 246/252 (97.6) | 94.9–99.1 | 247/252 (98.0%) | 95.4–99.4 |
Table 2.
Cross-reactivity of rSs-NIE-1 and rSs-IR strongyloidiasis immunoblot
| Conditions represented by sera | No. of sera tested | N (cross-reactivity in %) | |
|---|---|---|---|
| rSs-NIE-1 immunoblot | rSs-IR immunoblot | ||
| U.S. negatives | 154 | 0 | 0 |
| Amebiasis | 10 | 0 | 0 |
| Cysticercosis | 18 | 5 (27.8) | 4 (22.2) |
| Echinococcosis | 12 | 0 | 0 |
| Filariasis | 7 | 0 | 0 |
| Malaria falciparum | 4 | 0 | 0 |
| Schistosomiasis | 35 | 1 (2.9) | 1 (2.9) |
| Toxocariasis | 10 | 0 | 0 |
| Trichinellosis | 2 | 0 | 0 |
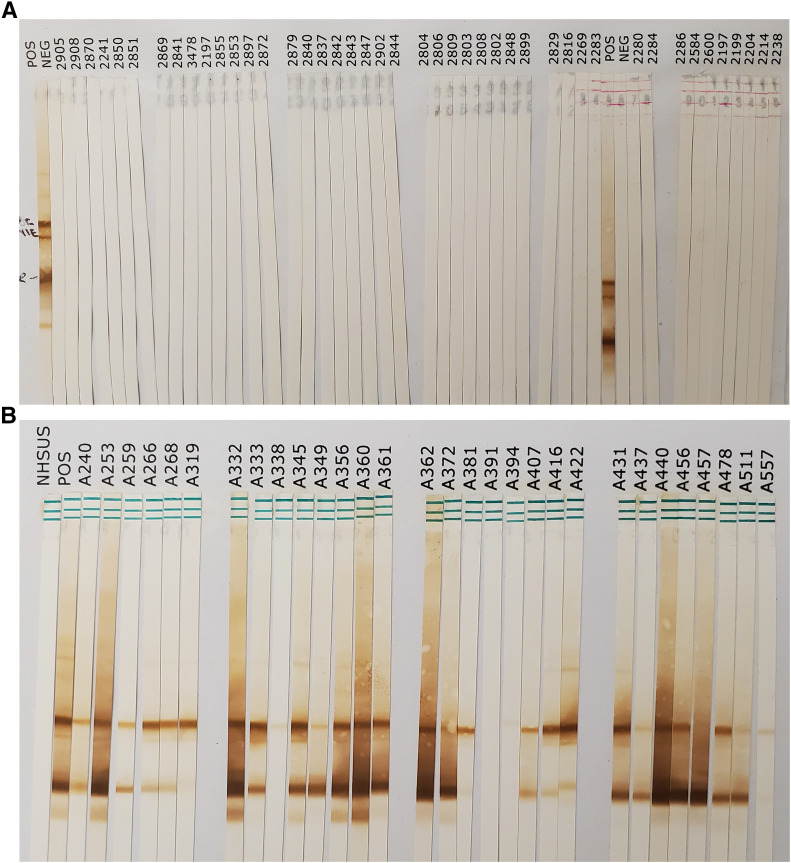
Figure 1.
Immunoblot of rSs-NIE-1 and rSs-IR recombinant proteins. Two recombinant antigens, rSs-NIE-1 at 1.6 ng/mm and rSs-IR at 6.25 ng/mm, were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis and the proteins were transferred to a nitrocellulose membrane (0.45 µm). The nitrocellulose membrane was cut into 2.5-mm strips. The strips were probed with 500 µL of (A) negative human serum samples and (B) positive Strongyloides stercoralis human serum diluted 1:100 in phosphate-buffered saline (PBS) + 0.3% Tween + 5% milk overnight at 4°C. The strips were washed four times with PBS/0.3% Tween at 5-minute intervals. Then, 500 μL of the conjugate (mouse antihuman IgG4–horse radish peroxidase labeled, diluted 1:1000 in PBS/0.3% Tween-20) was added. After incubation for 1 hour at room temperature, the strips were washed twice with PBS/0.3% Tween and then two times with PBS only. All washes were done at 5-minute intervals. After the final wash, strips were exposed to 500 µL diaminobenzidine in PBS + 10 µL of 30% hydrogen peroxide for 10 minutes at room temperature. The reactions were stopped by quick washing (in seconds) 10 times in deionized water. Interpretation of positivity and negativity was based on bands at 50 kDa for rSs-NIE-1 and/or 30 kDa for rSs-IR. This figure appears in color at www.ajtmh.org.
The sensitivity of rSs-NIE-1 and rSs-IR biplex immunoblot reported here is comparable to the previously reported assays using both recombinant antigens. In a multiassay comparison study, Bisoffi et al.14 reported that the NIE luciferase immunoprecipitation system (LIPS) detected 85% agar culture-positive S. stercoralis with 95% specificity. Simultaneously, in the format of ELISA, the NIE-ELISA has a sensitivity of 75% and a specificity of 89%. In a review of the assay performance, the group reported that NIE-ELISA has sensitivities and specificities within the range of 71–97% and 91–100%, respectively14,15; NIE-LIPS has a sensitivity and specificity of 97 and 100%.10–12,16 The rSs-IR has not been used extensively in immunoassays. In previous studies, rSs-IR IgG4 ELISA had a sensitivity of 45% and a specificity of 100%, while a rSsIR-LIPS assay had a sensitivity and specificity of 97%.10 In the immunoblot format, the SsIR presented lower sensitivity compared with SsIR-LIPS.
In this work, the reported cross-reactivities were against samples from subjects with cysticercosis and schistosomiasis. The cysticercosis sera originated from Peru whereas the schistosomiasis samples were received from Brazil. There is a possibility that these cross-reactivities are coinfected with S. stercoralis, because both are from endemic areas in Peru and Brazil. Even if this is a true cross-reactivity, the biplex immunoblot assay has a much lower cross-reactivity against sera from the subject with schistosomiasis than other immunodiagnostic platforms.17,18 This assay’s high specificity has the potential for screening immigrants and refugees where co-infection is common.19 A limitation of our study is the use of defined strongyloidiasis based on agar plate culture. Although we collected three samples from each patient to increase the sensitivity of the assay, it is still possible that the assay does not detect samples that are positive only by molecular detection, such as by polymerase chain reaction (PCR). In the future, the use of more defined positive samples based on agar plate culture and/or PCR will give a better estimate of the true sensitivity of the assay.
To summarize, a high-performing assay for detecting S. stercoralis-specific antibodies has been developed. Because this assay uses recombinant proteins, thus negating the need for native parasite materials, it can be adopted more readily for public health laboratories for diagnosing refugees and travelers with suspected strongyloidiasis returning from tropical countries.
REFERENCES
- 1.Schar F, Trostdorf U, Giardina F, Khieu V, Muth S, Marti H, Vounatsou P, Odermatt P, 2013. Strongyloides stercoralis: global distribution and risk factors. PLoS Negl Trop Dis 7: e2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russell ES, Gray EB, Marshall RE, Davis S, Beaudoin A, Handali S, McAuliffe I, Davis C, Woodhall D, 2014. Prevalence of Strongyloides stercoralis antibodies among a rural Appalachian population: Kentucky, 2013. Am J Trop Med Hyg 91: 1000–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia LS, 2016. Diagnostic Medical Parasitology. Washington, DC: ASM Press. [Google Scholar]
- 4.De Souza JN, et al. 2018. Case report: Strongyloides stercoralis hyperinfection in a patient with HTLV-1: an infection with filariform and rhabditiform larvae, eggs, and free-living adult females output. Am J Trop Med Hyg 99: 1583–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ines Ede J, Souza JN, Santos RC, Souza ES, Santos FL, Silva ML, Silva MP, Teixeira MC, Soares NM, 2011. Efficacy of parasitological methods for the diagnosis of Strongyloides stercoralis and hookworm in faecal specimens. Acta Trop 120: 206–210. [DOI] [PubMed] [Google Scholar]
- 6.Loutfy MR, Wilson M, Keystone JS, Kain KC, 2002. Serology and eosinophil count in the diagnosis and management of strongyloidiasis in a non-endemic area. Am J Trop Med Hyg 66: 749–752. [DOI] [PubMed] [Google Scholar]
- 7.De Souza JN, Cruz ADV, Araujo WAC, Sampaio LM, Allegretti SM, Teixeira MCA, Handali S, Galvao-Castro B, Soares NM, 2020. Alcohol consumption alters anti-Strongyloides stercoralis antibodies production. Immunobiology 225: 151898. [DOI] [PubMed] [Google Scholar]
- 8.Krolewiecki AJ, et al. 2010. Improved diagnosis of Strongyloides stercoralis using recombinant antigen-based serologies in a community-wide study in northern Argentina. Clin Vaccine Immunol 17: 1624–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rascoe LN, Price C, Shin SH, McAuliffe I, Priest JW, Handali S, 2015. Development of Ss-NIE-1 recombinant antigen based assays for immunodiagnosis of strongyloidiasis. PLoS Negl Trop Dis 9: e0003694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramanathan R, Burbelo PD, Groot S, Iadarola MJ, Neva FA, Nutman TB, 2008. A luciferase immunoprecipitation systems assay enhances the sensitivity and specificity of diagnosis of Strongyloides stercoralis infection. J Infect Dis 198: 444–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arifin N, Hanafiah KM, Ahmad H, Noordin R, 2019. Serodiagnosis and early detection of Strongyloides stercoralis infection. J Microbiol Immunol Infect 52: 371–378. [DOI] [PubMed] [Google Scholar]
- 12.Tsang VC, Brand JA, Boyer AE, 1989. An enzyme-linked immunoelectrotransfer blot assay and glycoprotein antigens for diagnosing human cysticercosis (Taenia solium). J Infect Dis 159: 50–59. [DOI] [PubMed] [Google Scholar]
- 13.Tsang VC, Peralta JM, Simons AR, 1983. Enzyme-linked immunoelectrotransfer blot techniques (EITB) for studying the specificities of antigens and antibodies separated by gel electrophoresis. Methods Enzymol 92: 377–391. [DOI] [PubMed] [Google Scholar]
- 14.Bisoffi Z, et al. 2014. Diagnostic accuracy of five serologic tests for Strongyloides stercoralis infection. PLoS Negl Trop Dis 8: e2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buonfrate D, Formenti F, Perandin F, Bisoffi Z, 2015. Novel approaches to the diagnosis of Strongyloides stercoralis infection. Clin Microbiol Infect 21: 543–552. [DOI] [PubMed] [Google Scholar]
- 16.Ravi V, Ramachandran S, Thompson RW, Andersen JF, Neva FA, 2002. Characterization of a recombinant immunodiagnostic antigen (NIE) from Strongyloides stercoralis L3-stage larvae. Mol Biochem Parasitol 125: 73–81. [DOI] [PubMed] [Google Scholar]
- 17.Gam AA, Neva FA, Krotoski WA, 1987. Comparative sensitivity and specificity of ELISA and IHA for serodiagnosis of strongyloidiasis with larval antigens. Am J Trop Med Hyg 37: 157–161. [DOI] [PubMed] [Google Scholar]
- 18.Ines Ede J, Silva ML, Souza JN, Teixeira MC, Soares NM, 2013. The role of glycosylated epitopes in the serodiagnosis of Strongyloides stercoralis infection. Diagn Microbiol Infect Dis 76: 31–35. [DOI] [PubMed] [Google Scholar]
- 19.Agbata EN, Bisoffi Z, et al. 2018. Effectiveness of screening and treatment approaches for schistosomiasis and strongyloidiasis in newly-arrived migrants from endemic countries in the EU/EEA: a systematic review. Int J Environ Res Public Health 16: 11. 10.3390/ijerph16010011. [DOI] [PMC free article] [PubMed] [Google Scholar]