Abstract.
Based on a previous study and by incorporating new knowledge, the goal of our study was to understand more fully the pathogenesis of hemorrhagic pneumonia of severe human leptospirosis, highlighting the onset of capillary lesions by Leptospira itself and/or its antigenic/toxic products acting on the endothelium and binding to cadherins. Both events lead to loss of endothelial integrity, alter permeability, cause rupture, and open intercellular junctions, contributing to the hemorrhagic phenomena associated with severe leptospirosis.
Leptospirosis is a worldwide zoonotic infection caused by pathogenic spirochetes of the genus Leptospira. Rodents play an important role in the epidemiology of the disease as chronic carriers, persistently harboring Leptospira in their proximal kidney tubules and excreting live bacteria into the environment, thus contaminating water and soil. When humans come into contact with contaminated water or soil, these highly motile bacteria can penetrate injured skin or mucous membranes and, after complement evasion,1 disseminate through the hematogenic route causing systemic infection.
In Brazil, leptospirosis is endemic and becomes epidemic during rainy periods, mainly affecting low-income metropolitan areas of capital cities as a result of flooding, inadequate sanitation, and high infestation of infected rodents. According to the Brazilian Health Ministry, there were 3,368 confirmed cases and 280 deaths in 2019, and a total of 48,670 confirmed cases and 4,287 deaths from 2007 to 2019.2
Several pathogenic species of the genus Leptospira can cause a wide range of acute clinical manifestations, from mild flu-like illness to a severe form of disease called Weil’s syndrome, characterized by jaundice, renal failure, decreased platelet count, bleeding, hypotension, and severe pulmonary hemorrhagic syndrome, which is a major cause of death. Pulmonary involvement in leptospirosis manifests as intra-alveolar hemorrhage and acute respiratory distress syndrome that sometimes requires intubation and mechanical ventilation. Strategies such as early renal replacement therapy and lung protective ventilation using a low tidal volume, with an initial tidal volume of 6 mL/kg, a positive end expiratory pressure of 5 cm H2O, and a limited plateau pressure of ≤ 30 cm H2O can improve outcomes.3
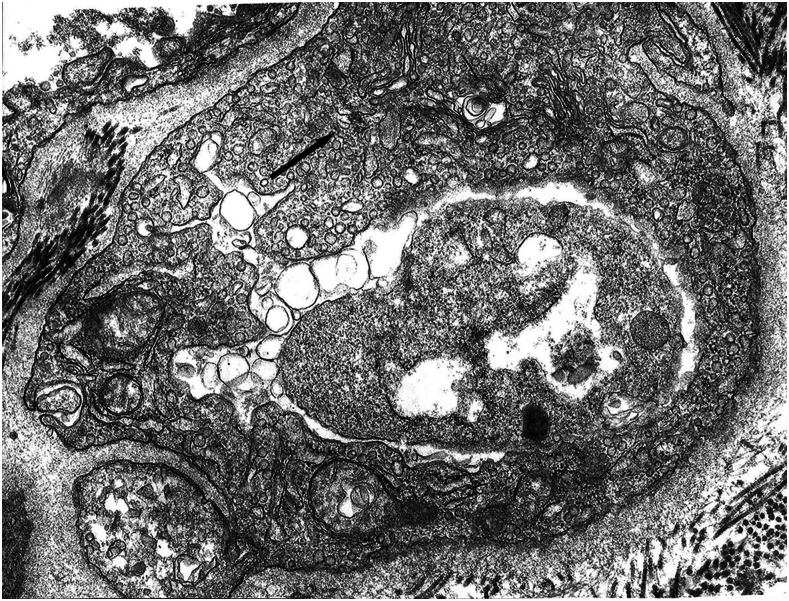
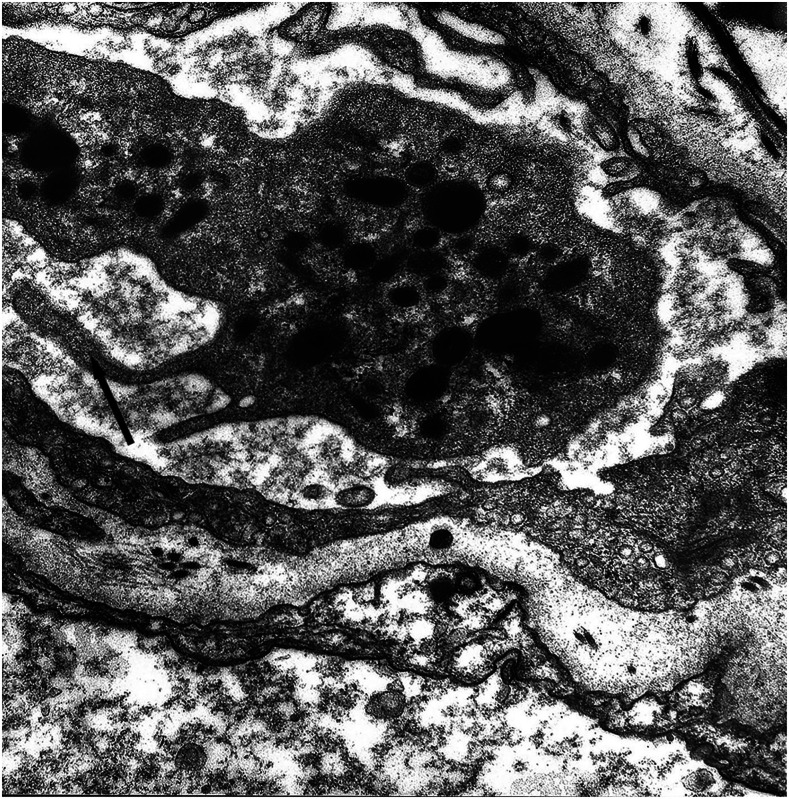
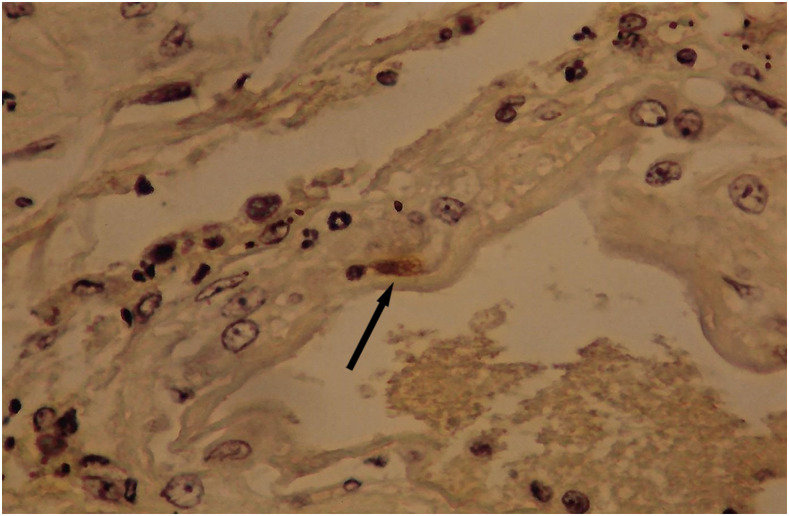
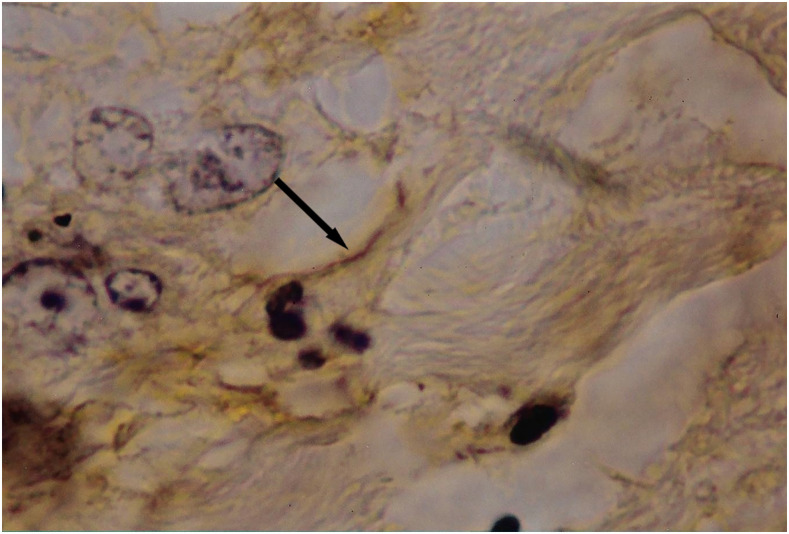
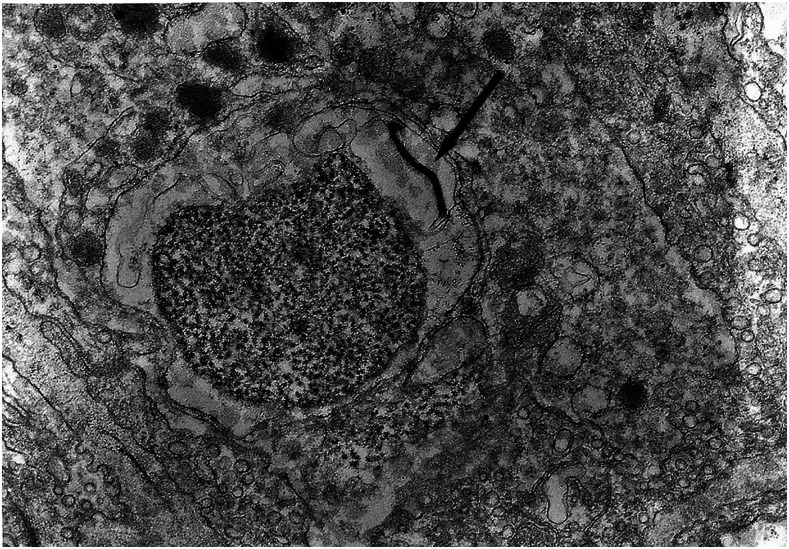
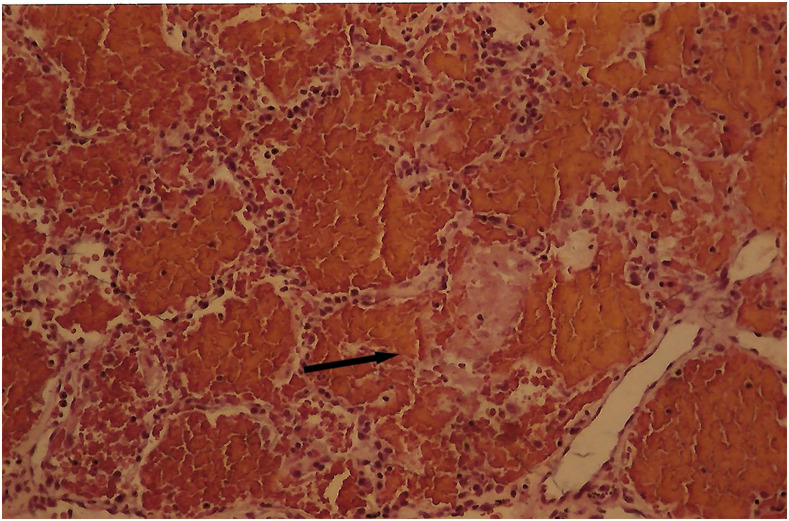
It is known that the initial process of leptospiral adhesion to biomolecules present on epithelial cells is an important step for host colonization and invasion. The interaction of Leptospira with several components of the extracellular matrix (ECM) and cell surface receptors has been investigated previously.4–6 In a study by Nicodemo et al.,7 lung fragments from 12 patients with leptospirosis were collected immediately after death, and were studied by light and electron microscopy, and by immunohistochemistry to describe the morphological and ultrastructural aspects of the lung and platelets in leptospirosis. The ultrastructural findings were uniform and constant. Capillary lesions were characterized by swelling of the endothelial cells, an increase in pinocytotic vesicles, giant dense bodies in the cytoplasm, and emission of pseudopods, showing clear signals of morphological activation of these cells in human leptospirosis (Figures 1 and 2). Leptospiral antigen was detected in eight cases as positive granular material on the luminal surface of the endothelium and in the cytoplasm of the endothelial cells of septal capillaries, and in the filamentous form, attached to the endothelium of the septal capillaries (Figures 3 and 4). Therefore, the pneumopathy of human leptospirosis seemed to be specific and triggered initially by Leptospira (Figure 5) and/or by their antigenic/toxic products interacting with endothelial cell surface receptors and/or the ECM. Nicodemo et al.7 concluded that lung involvement in severe human disease presents as hemorrhagic pneumopathy with septal capillary injury (Figure 6), which should be considered the most important factor in the pathogenesis of the bleeding disturbances.
Figure 1.
Septal capillary with edema and increased pinocytotic vesicles in endothelial cell, ×81.900 original magnification.
Figure 2.
Septal capillary with pseudopod emission by the endothelial cell and platelet, ×42.560 original magnification.
Figure 3.
Granular leptospiral antigen (arrow) in an endothelial cell of pulmonary capillary; avidin–biotin stained, ×600 original magnification. This figure appears in color at www.ajtmh.org.
Figure 4.
Filamentous leptospiral antigen (arrow) attached to the endothelium of a septal capillary; avidin–biotin stained, ×1,000 original magnification. This figure appears in color at www.ajtmh.org.
Figure 5.
Leptospira (arrow) next to the mononuclear cell in septal capillary; conventional electron microscopy, 2.1 × 19.500.
Figure 6.
Alveoli filled with erythrocytes and ruptured alveolar septa; hematoxylin eosin stain, ×150 magnification. This figure appears in color at www.ajtmh.org.
Researchers have been trying to understand more fully the mechanisms by which pathogenic Leptospira interact with and alter the endothelium. One such mechanism of adhesion to host cells is the capacity of attachment to the cadherins, which are a family of calcium-dependent transmembrane adhesion proteins that function to maintain cell–cell integrity and serve as receptors for Leptospira interrogans. Leptospira bind to vascular endothelial cadherin located at the intercellular junctions and to neural cadherin distributed primarily across the cell surface. Both events can lead to a loss in endothelial integrity, altered permeability, endothelial disruption, opening of intercellular junctions, and vascular leakage, contributing to the pathogenesis of hemorrhagic syndrome.8–11
Multiple Leptospira adhesins have been reported to bind cells via cadherins or the ECM molecules fibronectin, collagen, laminin, elastin, and plasminogen.12 According to Kochi et al.,11 the recombinant proteins coded by the genes LIC 11711 and LIC 12587 of L. interrogans serovar Copenhageni are conserved among pathogenic strains and are probably surface-exposed in the bacterial surface, and were characterized as novel E-cadherin ligands.
Bacterial attachment to ECM components is an important step for the possible role of this specificity for ECM molecules; some are exclusive laminin-binding proteins whereas others have broader spectrum-binding profiles (Lig B, Lsa 21, Lip L53). These proteins also may play a role in the colonization of host tissues.3–12
From published research, severe pneumopathy of human leptospirosis appears to be specific and triggered directly by Leptospira and/or their antigenic/toxic products acting on endothelial cell surface receptors or even interacting with components of the ECM. Deposits of leptospiral antigen on host cell membranes and alteration of cadherin expression lead to loss of vascular integrity, vascular leakage, invasion, dissemination, and involvement of multiple organs and tissues, causing severe illness.
Further studies are needed to clarify the importance of the various proteins expressed by pathogenic Leptospira during infection and their relation to virulence and pathogenicity to improve our understanding of the pathogenesis of human leptospirosis.
Acknowledgment:
We thank Dr. Fatuma Catherine Atieno Odongo for her assistance in the preparation of this manuscript.
REFERENCES
- 1.Fraga TR, Isaac L, Barbosa AS, 2016. Complement evasion by pathogenic Leptospira. Front Immunol 21: 623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ministério da Saúde , 2020. Available at: https://antigo.saude.gov.br/images/pdf/2020/fevereiro/07/obito-lepto-2007-2019.pdf and https://antigo.saude.gov.br/images/pdf/2020/fevereiro/07/conf-mes-lepto-2019.pdf.
- 3.Smith S, Liu Y-H, Carter A, Kennedy BJ, Dermedgoglou A, Poulgrain SS, Paavola M P, Minto TL, Luc M, Hanson J, 2019. Severe leptospirosis in tropical Australia: optimising intensive care unit management to reduce mortality. PLoS Negl Trop Dis 13: e0007929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evangelista KV, Coburn J, 2010. Leptospira as an emerging pathogen: a review of its biology, pathogenesis and host immune responses. Future Microbiol 5: 1413–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyahara S, Saito M, Kanemaru T, Villanueva SY, Gloriani NG, Yoshida S, 2014. Destruction of the hepatocyte junction by intercellular invasion of Leptospira causes jaundice in a hamster model of Weil’s disease. Int J Exp Pathol 95: 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sato H, Coburn J, 2017. Leptospira interrogans causes quantitative and morphological disturbances in adherens junctions and other biological groups of proteins in human endothelial cells. PLoS Negl Trop Dis 27: e0005830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicodemo AC, Duarte MI, Alves VA, Takakura CF, Santos RT, Nicodemo EL, 1997. Lung lesions in human leptospirosis: microscopic, immunohistochemical, and ultrastructural features related to thrombocytopenia. Am J Trop Med Hyg 56: 181–187. [DOI] [PubMed] [Google Scholar]
- 8.Del Carlo Bernardi F, Ctenas B, da Silva LF, Nicodemo AC, Saldiva PH, Dolhnikoff M, Mauad T, 2012. Immune receptors and adhesion molecules in human pulmonary leptospirosis. Hum Pathol 43: 1601–1610. [DOI] [PubMed] [Google Scholar]
- 9.Evangelista K, Franco R, Schwab A, Coburn J, 2014. Leptospira interrogans binds to cadherins. PLoS Negl Trop Dis 8: e2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Brito T, Silva AMGD, Abreu PAE, 2018. Pathology and pathogenesis of human leptospirosis: a commented review. Rev Inst Med Trop São Paulo 60: e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kochi LT, Fernandes LGV, Souza GO, Vasconcellos SA, Heinemann MB, Romero EC, Kirchgatter K, Nascimento ALTO, 2019. The interaction of two novel putative proteins of Leptospira interrogans with E-cadherin, plasminogen and complement components with potential role in bacterial infection. Virulence 10: 734–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vieira ML, Fernandes LG, Domingos RF, Oliveira R, Siqueira GH, Souza NM, Teixeira AR, Atzingen MV, Nascimento AL, 2014. Leptospiral extracellular matrix adhesins as mediators of pathogen–host interactions. FEMS Microbiol Lett 352: 129–139. [DOI] [PubMed] [Google Scholar]