Abstract.
Clonorchis sinensis, a trematode prevalent in East Asia, causes hepatobiliary infection. Exposure typically occurs through ingestion of raw or undercooked fish containing the encysted larval form of the parasite. Extrahepatobiliary disease has not commonly been described. In this case report, we describe an unusual case of C. sinensis infection associated with eosinophilic pneumonia. A middle-aged man from China presented with subacute cough and was found to have a bilateral diffuse eosinophilic pneumonia with associated peripheral eosinophilia. Stool microscopy revealed C. sinensis eggs, and the patient improved after treatment with prednisone and praziquantel. Pulmonary clonorchiasis should be considered in patients with eosinophilic pneumonia from areas highly endemic for this pathogen.
CASE PRESENTATION
An estimated 15 million people are infected with the trematode Clonorchis sinensis worldwide, the majority in East Asia.1,2 Whereas chronic infection with C. sinensis is associated with hepatobiliary disease, reports of extrahepatobiliary manifestations are rare. Here we describe an unusual case of C. sinensis infection associated with eosinophilic pneumonia.
A 63-year-old man from China presented with 1 month of worsening cough and weight loss. The cough was dry with scant production of white sputum, without hemoptysis. His past medical history included hypertension, type II diabetes mellitus, and stroke. He reported similar symptoms approximately 20 years prior while living in China; he was diagnosed with pulmonary tuberculosis though did not recall specific treatment. He was born in the Guangxi Province of China and emigrated to the United States 4 years before presentation. He last visited the rural Guangxi Province one year prior. During that trip, he had eaten raw freshwater fish.
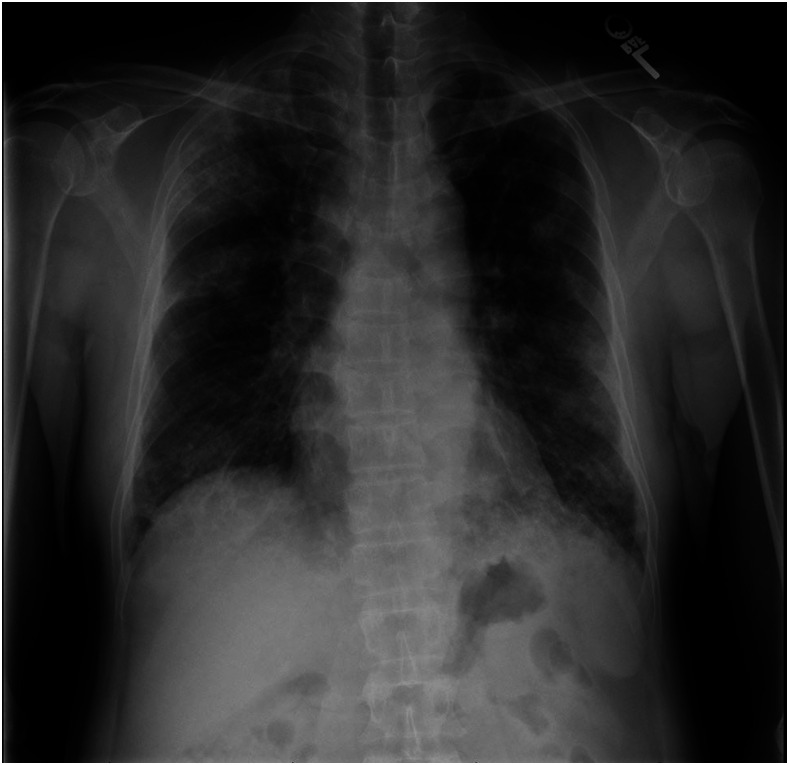
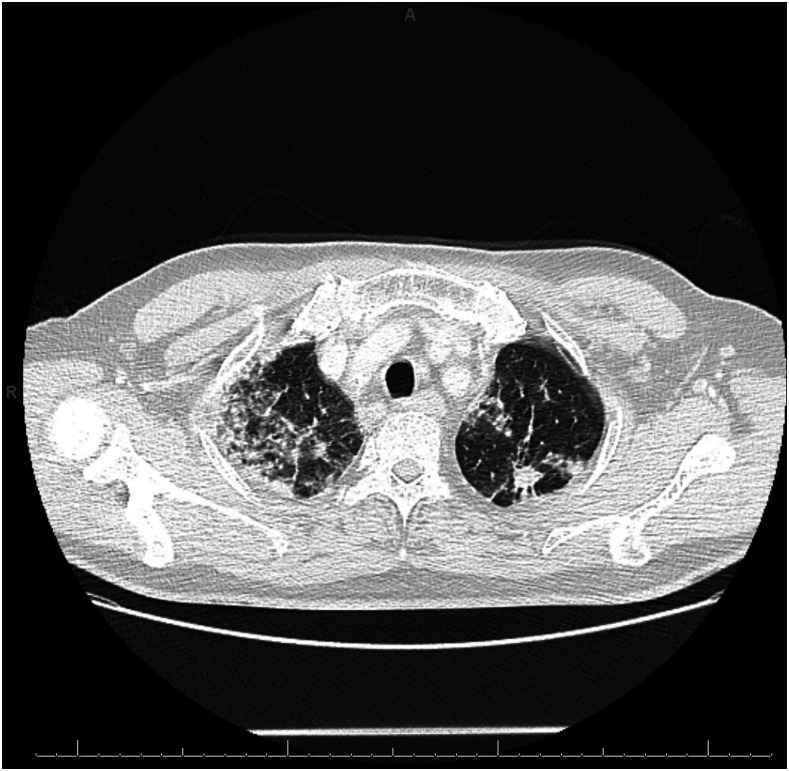
On presentation, the patient was in no acute distress. He was afebrile. Oxygen saturation was 94% on room air. Pulmonary examination was normal. His laboratory findings revealed a white blood cell (WBC) count of 11.5 cells × 109/L with 1.7% eosinophils (absolute eosinophil count of 0.2 cells × 109/L). Liver enzymes were normal. Chest X-ray showed bilateral airspace opacities, with focal consolidation in the right upper lobe and at the bases (Figure 1). Computed tomography of the chest with contrast showed multifocal reticular and ground-glass opacities, an 11-mm spiculated nodular mass within the left apex, and associated bulky mediastinal and hilar lymphadenopathy (Figure 2).
Figure 1.
Chest X-ray. Initial chest X-ray showed bilateral airspace opacities with focal consolidation in the right upper lobe and at lung bases.
Figure 2.
Computed tomography of the chest. Initial Computed tomography of the chest with contrast showed multifocal reticular and ground-glass opacities, an 11-mm spiculated nodular mass within the left apex, and associated bulky mediastinal and hilar lymphadenopathy.
Serial acid-fast smears, tuberculosis PCR, and acid-fast bacilli (AFB) culture were negative. Over the next week, his WBC count increased up to 21.2 cells × 109/L with 24.6% eosinophils (absolute eosinophil count of 5.2 cells × 109/L). He developed respiratory distress requiring 6 L of oxygen via the nasal cannula. Further evaluation was initiated for parasitic infections. Serologic testing was negative for Strongyloides stercoralis, Echinococcus spp., Trichinella spp., and lymphatic filariasis. Schistosoma mansoni antibody by ELISA, expected to be cross-reactive with other Schistosoma species, was equivocal. Thin and thick peripheral blood smear examinations were negative for blood parasites.
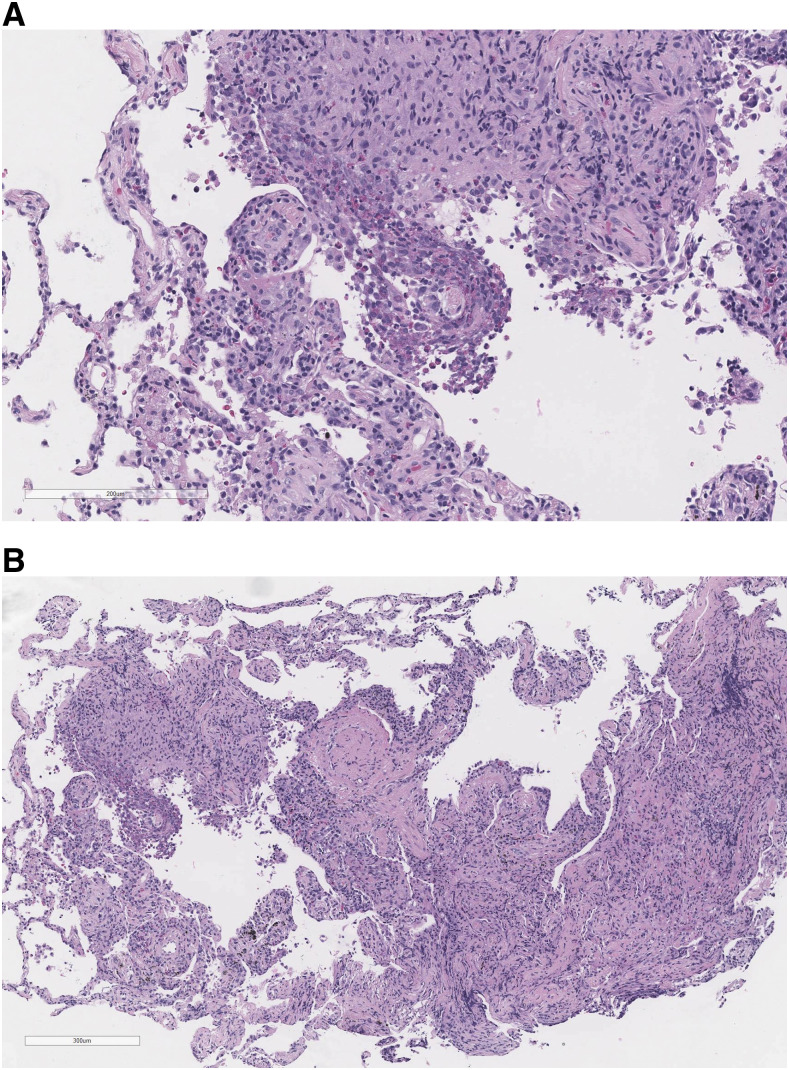
The patient underwent bronchoscopy and ultrasound-guided transbronchial needle aspiration. Bronchoalveolar lavage (BAL) microscopy for parasites was negative. Lung biopsy with hematoxylin and eosin staining revealed focal organizing pneumonia and diffuse chronic inflammation with prominent eosinophilic infiltration, characteristic of nonspecific interstitial pneumonitis or hypersensitivity pneumonitis (Figure 3). Acid-fast bacilli and Gomori methenamine-silver stains were negative. Given the concern for eosinophilic pneumonia, prednisone 60 mg daily was initiated with rapid symptomatic improvement.
Figure 3.
Pathology findings. Left lower lung tissue biopsy with hematoxylin and eosin staining revealed alveoli with focal organizing pneumonia and diffuse chronic inflammation with prominent eosinophilic infiltration, characteristic of nonspecific interstitial pneumonitis or hypersensitivity pneumonitis. This figure appears in color at www.ajtmh.org.
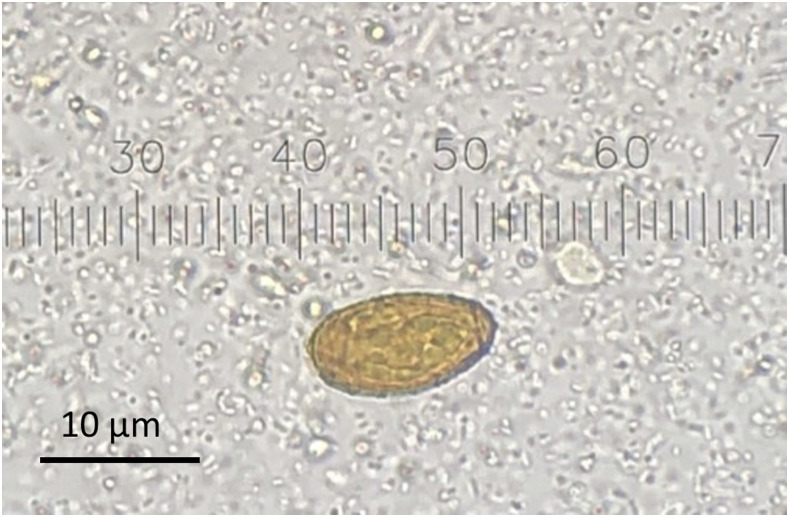
Stool microscopy for parasites revealed C. sinensis eggs in three samples (Figure 4). Stool microscopy was performed with concentrated wet mount, using the Apacor “Midi Parasep SF” fecal parasite concentrator system, and unconcentrated trichrome stain. The patient completed six doses of praziquantel 1800 mg every 5 hours, which he tolerated well. An abdominal ultrasound showed mild common bile duct wall thickening, but no evidence of obstruction or ductal dilation. Chest X-ray performed 3 days after initiating prednisone showed mild improvement in airspace opacities. On hospital day 21, he was discharged home without supplemental oxygen. Prednisone was continued with plans to taper.
Figure 4.
Stool microscopy for parasites. Clonorchis sinensis egg. This figure appears in color at www.ajtmh.org.
DISCUSSION AND REVIEW OF LITERATURE
Clonorchis sinensis is not traditionally thought to be associated with pulmonary disease, although case reports have been described previously.3–8 A comprehensive search was performed using the PubMed database from inception until February 2019. Search terms included “C. sinensis,” “Clonorchis,” and “clonorchiasis,” combined with the terms “eosinophilic pneumonia” and “pulmonary eosinophilia.” References in each manuscript were reviewed to identify additional cases.
In addition to the present case, we encountered six other case reports and series in the literature, describing a total of 14 cases of pulmonary disease associated with C. sinensis (Table 1).3–8 The first recorded case was published in 1949.3 The patients ranged in age from 23 months to 54 years. The descriptions were from China, Hong Kong, and Korea. Most patients had pulmonary symptoms, lasting from days to 1 month before presentation. Two patients had no reported pulmonary symptoms; one presented with epigastric discomfort and fever, and the other with rash on the extremities.5,7 All had transient pulmonary infiltrates on imaging. All were diagnosed with C. sinensis infection, 13 of the 14 cases by visualization of eggs on stool microscopy, but in one case by serum antibody testing.8 Two patients had transbronchial lung biopsy showing eosinophilic pneumonia.6,7 Treatment varied; many received antiparasitic therapy, usually praziquantel, although nine patients in one case series received no therapy.4 Only one patient received glucocorticoids.6 All patients improved over time, ranging from days to months.
Table 1.
Characteristics of cases reported in the literature of pulmonary disease attributed to C. sinensis
| Author, year of publication | Patient age, gender | Geographic location | Exposure history | Symptoms | Maximum reported peripheral eosinophilia (%) | Imaging findings | Method of diagnosis of Clonorchis infection | Treatment |
|---|---|---|---|---|---|---|---|---|
| Cartwright, 19493 | 21, male | Shanghai, China | Consumption of “poorly cooked native fish” | Fever, chills, productive cough | 74 | Transient bilateral pulmonary infiltrates | Clonorchis ova identified in stool | Mapharsen therapy |
| Engel, 19674 | 20–50 (9 patients), male | Hong Kong | Unknown | Transitory hemoptysis | Unknown | Transitory infiltration (seen in two of nine cases) | Clonorchis ova identified in stool | No treatment |
| Mo, 19845 | 36, male | China | Ingestion of raw freshwater fish and cooked crabs | Epigastric discomfort, fever, diaphoresis | 76 | Transient bilateral pulmonary infiltrates | Clonorchis ova identified in stool and bile | Praziquantel |
| Lee, 19986 | 37, male | Korea | Unknown | Dyspnea, cough | 71.4 | Nodular pulmonary parenchymal infiltrates | Clonorchis ova identified in stool | Praziquantel, corticosteroids |
| Lee, 20037 | 54, male | Korea | Ingestion of raw freshwater fish | Rash on extremities | 35 | Migrating pulmonary nodules and patchy densities | Clonorchis ova identified in stool, positive skin test for C. sinensis | Praziquantel |
| Sheng, 20178 | 23 months, female | China | Ingestion of raw freshwater crayfish | Cough, wheezing | 9.7 | Bilateral ground-glass attenuation and reticular opacities | Identification of C. sinensis–specific IgG in serum | Praziquantel |
C. sinensis = Clonorchis sinensis.
Clonorchis sinensis is a trematode prevalent in East Asia that infects the hepatobiliary system. There are an estimated 15 million people infected worldwide, with approximately 13 million in China.1,2 Most infected persons are asymptomatic.2,9 When present, most of the signs and symptoms are related to worm burden and associated with inflammation and obstruction of the biliary system.1 When left untreated, infection may persist for up to 30 years, and can lead to hepatomegaly, cirrhosis, and cholangiocarcinoma.9 Most infections occur in persons resident in, or who have travelled to, endemic areas. The main risk factor for infection is consumption of raw or undercooked fish.9
The life cycle of C. sinensis begins with freshwater snails, the first intermediate host, which ingest the embryonated eggs of C. sinensis.9 Within the snail, eggs release miracidia which develop into sporocysts, rediae, and finally cercariae.9 Cercariae are released from the snail, and enter freshwater fish or shrimps, the second intermediate hosts, and encyst, becoming metacercariae.9 Definitive hosts such as humans and other carnivores including cats and dogs become infected by eating raw or undercooked fish containing these metacercariae.2 After ingestion, metacercariae excyst in the duodenum, and then migrate to the hepatobiliary system, where they mature.10 The mature trematodes release eggs which are passed from the bile into the stool of infected persons.9 Excreted embryonated eggs are ingested by snails, resuming the life cycle.
We propose three potential pathophysiologic mechanisms by which C. sinensis could contribute to pulmonary disease. The first, and the likely mechanism in this case, is pulmonary eosinophilia resulting from an immunological hypersensitivity reaction. This mechanism has been well described in tropical pulmonary eosinophilia, a hypersensitivity reaction to lymphatic filarial parasites, which can manifest years after leaving the endemic area.11 The robust immune response in tropical pulmonary eosinophilia is, in part, mediated by an expansion of IL-4 and IL-5 producing cells, promoting polyclonal expansion of eosinophils.12 Although liver flukes are not typically thought to cause pulmonary disease, there have been reports of Fasciola, another liver trematode, causing lung involvement by this mechanism, lending biologic plausibility to this idea.13,14 It is likely that C. sinensis infection similarly contributed to an immunological hypersensitivity reaction, resulting in pulmonary manifestations in the case described here.
A second less likely putative mechanism for pulmonary manifestations with C. sinensis is by direct pulmonary migration of the adult or larval parasite itself. Excluding the lung fluke Paragonimus which directly invades the pulmonary parenchyma, Schistosoma is one trematode known to migrate through the lung in the larval form.11 Schistosoma and other helminths which transiently migrate in this way typically present with Loeffler syndrome, characterized by transient, migratory pulmonary infiltrates with pulmonary and peripheral eosinophilia.15,16 A similar mechanism was considered with C. sinensis, although deemed very unlikely; if this mechanism were plausible, larval stages would be expected to migrate through the lungs early after infection; however, this patient had last visited China with potential exposure to C. sinensis 1 year prior to presentation. Moreover, neither BAL microscopy nor lung pathology showed C. sinensis larvae. A third mechanism was considered, similar to schistosomiasis causing portal hypertension and pulmonary vascular disease via migration of eggs from the portal to pulmonary vasculature, but was deemed highly unlikely because of the absence of Clonorchis eggs on lung pathology, as well as the presentation of eosinophilic pneumonia rather than liver fibrosis with pulmonary hypertension.17,18
Clonorchis sinensis infection is typically diagnosed by microscopic identification of eggs in stool specimens. Eggs of C. sinensis are difficult to distinguish morphologically from those of Opisthorchis species, and generally, these infections are differentiated by epidemiological clues when possible.9 The geographic origin of this patient’s infection in China allows the diagnosis of clonorchiasis. In addition, determining whether C. sinensis eggs in stool represent the causative pathogen versus asymptomatic shedding in the setting of an alternative disease can be difficult. However, in this case, no alternative cause of pulmonary symptoms was found.
Treatment of C. sinensis infection involves praziquantel or albendazole, which are the antiparasitic therapies of choice.9 On the other hand, treatment of eosinophilic pneumonia includes systemic glucocorticoid therapy, generally tapered over several weeks to months.19 Of the 14 cases of this entity in the literature, only one patient received glucocorticoid therapy,6 and most patients, although not all, received antiparasitic therapy. However, all were reported to show clinical improvement over time. The optimal therapy for eosinophilic pneumonia associated with C. sinensis infection is unclear based on the limited data; however, we suggest antiparasitic therapy with consideration of systemic glucocorticoids based on the severity of respiratory symptoms.
Acknowledgments:
We would like to acknowledge the contribution of the DPDx and epidemiology teams from the Division of Parasitic Diseases and Malaria at the Centers for Disease Control and Prevention for their assistance in clinical diagnosis of this case and identifying additional published cases. We would also like to thank Dr. Janet Yang for her translation of the medical journals written in the Korean language.
REFERENCES
- 1.Qian MB, Utzinger J, Keiser J, Zhou XN, 2016. Clonorchiasis. Lancet 387: 800–810. [DOI] [PubMed] [Google Scholar]
- 2.Tang ZL, Huang Y, Yu XB, 2016. Current status and perspectives of Clonorchis sinensis and clonorchiasis: epidemiology, pathogenesis, omics, prevention and control. Infect Dis Poverty 5: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cartwright GE, 1949. An unusual case of clonorchiasis with marked eosinophilia and pulmonary infiltrations. Am J Med 6: 259–266. [DOI] [PubMed] [Google Scholar]
- 4.Engel D, 1967. Haemoptysis and transitory lung-infiltrations associated with Clonorchis sinensis. Beitr Klin Erforsch Tuberk Lungenkr 134: 259–264. [DOI] [PubMed] [Google Scholar]
- 5.Mo LR, Chen CY, Luh KT, Hsieh WC, 1984. Clonorchiasis with clinical presentation of Löffler’s syndrome. A case report. Taiwan Yi Xue Hui Za Zhi 83: 960–965. [PubMed] [Google Scholar]
- 6.Lee DY, Kim SJ, Lee JH, Kim DW, Lee JK, 1998. A case of clonorchiasis with clinical presentation of eosinophilic pneumonia. Tuberc Respir Dis 46: 643–648. [Google Scholar]
- 7.Lee HK, Jin SL, Lee HP, Choi SJ, Yum HK, 2003. Loffler’s syndrome associated with Clonorchis sinensis infestation. Korean J Intern Med 18: 255–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheng YJ, Xu D, Wu L, Chen ZM, 2017. Clonorchiasis complicated with diffuse parenchymal lung disease in children. Chin Med J 130: 2895–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CDC , 2018. CDC - Clonorchis. Available at: https://www.cdc.gov/parasites/clonorchis/index.html. Accessed February 10, 2019. [Google Scholar]
- 10.Chong , 2010. Clonorchis Sinensis in Raw Freshwater Fish. Available at: https://www.cfs.gov.hk/english/multimedia/multimedia_pub/multimedia_pub_fsf_52_01.html. Accessed February 10, 2019. [Google Scholar]
- 11.Kunst H, Mack D, Kon OM, Banerjee AK, Chiodini P, Grant A, 2011. Parasitic infections of the lung: a guide for the respiratory physician. Thorax 66: 528–536. [DOI] [PubMed] [Google Scholar]
- 12.O’Bryan L, Pinkston P, Kumaraswami V, Vijayan V, Yenokida G, Rosenberg HF, Crystal R, Ottesen EA, Nutman TB, 2003. Localized eosinophil degranulation mediates disease in tropical pulmonary eosinophilia. Infect Immun 71: 1337–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arjona R, Riancho JA, Aguado JM, Salesa R, González-Macías J, 1995. Fascioliasis in developed countries: a review of classic and aberrant forms of the disease. Medicine (Baltimore) 74: 13–23. [DOI] [PubMed] [Google Scholar]
- 14.Krsak M, Patel NU, Poeschla EM, 2019. Case report: hepatic fascioliasis in a young Afghani woman with severe wheezing, high-grade peripheral eosinophilia, and liver lesions: a brief literature review. Am J Trop Med Hyg 100: 588–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weissler JC, 2017. Eosinophilic lung disease. Am J Med Sci 354: 339–349. [DOI] [PubMed] [Google Scholar]
- 16.Crofton JW, Livingstone JL, Oswald NC, Roberts ATM, 1952. Pulmonary eosinophilia. Thorax 7: 1–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolosionek E, Graham BB, Tuder RM, Butrous G, 2011. Pulmonary vascular disease associated with parasitic infection--the role of schistosomiasis. Clin Microbiol Infect 17: 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham BB, Bandeira AP, Morrell NW, Butrous G, Tuder RM, 2010. Schistosomiasis-associated pulmonary hypertension: pulmonary vascular disease: the global perspective. Chest 137: 20S–29S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, 2012. Harrison’s Principles of Internal Medicine, 18th Edition. New York, NY: McGraw Hill Medical. [Google Scholar]