Abstract
Background
Collagen is the primary component in human skin. With age, there is loss of skin elasticity and collagen, resulting in wrinkle formation and reduction in skin appearance.
Aims
The objective of this randomized, triple‐blind, placebo‐controlled study was to evaluate the safety and efficacy of a hydrolyzed marine collagen (Vinh Wellness Collagen, VWC) on aspects of skin health and quality in women between 45 and 60 years of age.
Patients/Methods
Assessments of skin wrinkles, elasticity, and self‐reported appearance were conducted using the VISIA skin analysis system, Cutometer®, and Skin Quality Visual Analogue Scale. Outcomes were assessed at weeks 0 (baseline), 6, and 12.
Results
After 12 weeks, participants supplemented with VWC had a significant 35% reduction in wrinkle score (P = .035) from baseline. Participants in the VWC group showed a 24% greater reduction in wrinkles on the right side of the face than those on placebo. A planned subgroup analysis based on age showed women 45‐54 years had a significant 20% and 10% improvement in cheek skin elasticity from baseline to week 6 (P = .016) and 12 (P = .022), respectively. At week 12, participants in the VWC group reported greater percentage improvements in overall skin score (9%) and wrinkle (15%), elasticity (23%), hydration (14%), radiance (22%), and firmness (25%) scores vs placebo.
Conclusion
Supplementation with VWC was found to be safe and well‐tolerated. The results of this study support the use of fish‐derived hydrolyzed collagen for the improvement of skin health in an aging population.
Keywords: hydration, hydrolyzed collagen, skin elasticity, wrinkles
1. INTRODUCTION
Skin is composed of an outer (epidermis) and inner (dermis) layer. The nonvascular epidermis relies on the dermis to receive nutrients. This process slows with age, compromising the quality of collagen fibres. 1 Collagen, which constitutes 95% of human skin, 2 and elastin are the two primary components of the dermis. These two fibres work synergistically to form the structure of the skin. Aging damages the elastic capacity of the skin, and thus, aging skin is marked by lack of elasticity, fragmentation, and collagen bundle fragility. 3
Skin appearance is influenced by nutrition as well as endogenous and environmental factors, including the exposure to chemicals, smoking, or ultraviolet radiation. Skin aging is due to changes in the deeper layer of the skin. Oral supplements, in contrast to topical applications, represent a practical approach to the prevention of skin aging because they can be delivered to the dermis through the circulation. 4 The ability of nutritional supplements to enhance skin characteristics has received increasing attention as the North American population continues to age. 5
Collagen is the most abundant protein in mammals and is currently being promoted by nutrition, biomedicine, and cosmetics industries. 6 Gelatin, a protein which is used extensively in the food sector, is a hydrolyzed analog of collagen. 6 Subsequent enzymatic degradation of gelatin results in the generation of hydrolyzed collagen, which contains peptides ranging in molecular weights between <500 Da and 6 KDa, depending on the processing conditions. 7 Orally administered hydrolyzed collagen is absorbed in the small intestine and into the blood stream as peptides and free amino acids and distributed into the dermis for up to 14 days. 8 In the dermis, hydrolyzed collagen provides amino acids for the formation of collagen and elastin fibres, in addition to stimulating endogenous production of new collagen, elastin, and hyaluronic acid. 9
Hydrolyzed collagen is commonly derived from cow, pig, and chicken sources. In recent years, collagen derived from fish has emerged as an alternative source due to lower environmental impact and risk of disease transmission. Further, marine fish collagen and collagen peptides have a high degree of homology to human structure and bioavailability through the gastrointestinal barrier. 10 Although a number of preclinical studies provide evidence for the beneficial effect of hydrolyzed collagen on skin health, less information is known about the clinical benefits. 11 , 12 Previous studies have demonstrated improvements in skin wrinkles, elasticity, and hydration following supplementation with hydrolyzed collagen. 12 , 13 Fish collagen peptides have been shown to significantly reduce crow's feet 14 and periorbital wrinkles in women. 15 Further, hydrolyzed collagen supplementation was reported to improve skin elasticity and moisture while reducing evaporation. 16 The aim of this study was to evaluate the clinical benefits of a 12‐week supplementation of a fish‐derived collagen peptide on skin wrinkles and elasticity, and self‐reported skin appearance.
2. METHODS
2.1. Participants
This study was approved by the institutional review board (IRB Services, Aurora) on July 12, 2016 (Pro00018179), and by Health Canada on June 13, 2016 (219 935). The clinical trial was performed according to the ethical guidelines detailed in the Declaration of Helsinki (2008) and complied with the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines and Good Clinical Practice Current Step 4 Version, dated June 10, 1996. The trial was registered at Clinicaltrials.gov (NCT04449159).
The study design was a randomized, triple‐blind, placebo‐controlled, parallel study conducted at the KGK Science Inc clinic site (London, Ontario, Canada) from November 25, 2016, to July 18, 2017. Written informed consent was obtained from all participants prior to any study procedures being initiated.
Participants were included if they were females between the ages of 45‐60 with a BMI 20.0‐29.9 kg/m2 and displayed visible signs of natural and photoaging on their face, as assessed by the Fitzpatrick questionnaire at screening. All participants agreed to avoid prolonged exposure to ultraviolet (UV) radiation for the duration of the study.
Individuals were excluded if they suffered from an acute or chronic skin disease or dermatological disorder; used natural health supplements for improving the skin; were on a low protein diet; had planned or unavoidable exposure to UV radiation; had tattoos on or near the test area; used systemic corticosteroids or applied topical alpha hydroxyl acids near the test site within 4 weeks of enrolment; used topical medications near the test area within 6 weeks of enrolment; had Botulinum toxin A (Botox) treatment or filler injection (collagen, hyaluronic acid, etc) near the test sites within 2 years of enrolment; were cognitively impaired and/or unable to give informed consent; or had any other condition which in the medical investigator's opinion may adversely affect the individual's ability to complete the study or its measures or which may pose significant risk to the individual.
2.2. Investigational product
The investigational product was Vinh Wellness Collagen (VWC), a hydrolyzed collagen powder derived from Pangasius hypophthalmus, a tropical and sustainable freshwater fish (Vinh Hoan Corporation). The placebo was matched to the investigational product using maltodextrin powder (Qinhuangdao Lihua Starch Co., Ltd). Participants were instructed to consume 10 g of hydrolyzed collagen or placebo powder daily, in the morning, on an empty stomach for 12 weeks. It was required that the product was dissolved it in at least 100 mL of water. In the event a dose was missed participants were to consume it as soon as they remembered and were instructed not to exceed 10 g of the investigational product or placebo per day.
2.3. Randomization and blinding
Eligible participants were assigned a randomization number by a blinded investigator per the order of the randomization list (www.randomization.com). Each randomization code represented an allocation to a dosing arm of the study. Following randomization, participants were identified by their initials and date of birth and were assigned a participant number at their screening visit.
The investigational product and placebo were sealed in sachets that were identical in appearance and labelled per the ICH‐GCP requirements and applicable local regulatory guidelines. Unblinded personnel who were not involved in any study assessments labelled the investigational product. The investigators, statistician, other site personnel, and participants were blinded to the products.
2.4. Compliance
Product compliance was assessed by counting the returned unused product at week 6 and week 12 and determined by the number of dosage units taken divided by the number expected, multiplied by 100. In the event of a discrepancy between the information in the study diary, compliance was based on the product returned unless an explanation for the loss was provided.
2.5. Outcome evaluation
The study investigated the changes in skin wrinkles and elasticity, and self‐reported skin appearance after 12 weeks of collagen or placebo supplementation. Clinical and qualitative assessments were conducted at screening, week 0 (baseline), week 6, and week 12 (end‐of‐study). At all visits, participants’ diaries were reviewed for concomitant therapies, adverse events, and study product use. Assessments including evaluation of nasolabial wrinkles and cheek elasticity and skin quality questionnaire are described below. Vital signs (blood pressure and heart rate) and anthropometric measurements (weight and BMI) were taken at each visit, while laboratory parameters for all safety endpoints were assessed at screening and week 12.
2.6. Skin wrinkle analysis
The skin wrinkle analysis was performed using the 6th Generation VISIA skin analysis system (Canfield Imaging Systems). The VISIA skin analysis system assesses the number of visible wrinkles and provides a score ranging from 0 to 100, with a lower score indicative of fewer wrinkles. The left and right sides of the face were analyzed separately.
Prior to testing, participants were provided a facial wipe and asked to remove all makeup, after which the face was dried with a lint‐free towel or allowed to air dry. Hair was clipped away from the face when applicable. The participant placed their face on the chin (resting) platform of the machine to perform the analysis. Manual masks were created for the nasolabial area of the face of each participant at baseline and were used at each subsequent visit.
The Modified Fitzpatrick Wrinkle Scale (MFWS) is a validated questionnaire evaluating skin wrinkle severity. The MFWS is composed of 3 major classes, in which definitions are based on a set of reference photographs and descriptions, as well as 3 interclasses based only on descriptions. Trained clinic staff applied this scale to participants by using standardized photographs of nasolabial wrinkles alone and assessment of wrinkle depth.
2.7. Skin elasticity analysis
Skin elasticity measurements were performed using the Cutometer® dual MPA 580 (Courage Khazaka electronic GmbH). A cheek free from skin damage or the cheek with the least damage (eg, scars, burns or cuts) was chosen at baseline and used for subsequent visits (week 6 and week 12), and a single Cutometer® measurement was performed. Participants did not wash their face within 2 hours prior to testing, and creams were not to be used prior to measurements. A higher score from the Cutometer® measurement indicated greater skin elasticity.
2.8. Skin quality VAS questionnaire
Skin quality was self‐assessed by using a Visual Analogue Scale (VAS) questionnaire adapted from Gold et al 2013. 17 Using a scale from 0 (no improvement) to 100 (great improvement), participants were asked to report their skin health based on skin elasticity, hydration, radiance, firmness, wrinkles, and overall feel.
2.9. Laboratory analyses
Safety parameters were analyzed by LifeLabs using standardized procedures. Analysis of hemoglobin, hematocrit, platelet count, red blood cell count (RBC), red blood cell indices, red cell distribution width (RDW), white blood cell count (WBC) and differentials (neutrophils, lymphocytes, monocytes, eosinophils, basophils), liver function tests (AST, ALT, bilirubin), and kidney function tests (Na, K, Cl, creatinine, eGFR) were performed. Urine pregnancy tests were conducted at the KGK Clinic for participants of childbearing potential at screening and baseline visits.
2.10. Adverse events
Participants recorded any adverse events (AEs) in their daily diary. AEs were documented in the study record and were classified based on the description, duration, intensity, frequency, and outcome. The Qualified Investigator assessed all AEs and determined causality and intensity. The Medical Dictionary for Regulatory Activities terminology (MEDRA) version 17.0 was used for coding.
2.11. Statistical analyses
The sample size calculation was based on the primary efficacy objective of the comparison of 12‐week skin elasticity changes between the collagen and placebo groups. With an estimated 80% power, 5% significance and 20% attrition, 50 participants were required (n = 25 per group). This calculation assumed between group variability of 0.080 R2 parameter units based on an earlier clinical trial. 12 As standard deviations of intervention outcomes tend to increase with time and the proposed study was longer than Proksch et al, 2014, the sample size was conservatively increased by 25%.
The safety population consists of all participants who received any amount of either study product and on whom any postrandomization safety information was available. The per‐protocol (PP) population consists of all participants who consumed at least 80% of either study product doses, did not have any major protocol violations, and completed all study visits and procedures connected with measurement of the primary variable. Only observed values were used for the analysis of the PP population. No imputation was performed for missing values of variables.
For continuous endpoints measured on multiple study visits (week 0, week 6, and week 12), descriptive statistics were presented for each study day and for the changes from baseline to each study day. Within‐group changes from baseline were assessed using the Wilcoxon signed rank test. Possible differences between groups at baseline were assessed by analysis of variance (ANOVA) with group as a fixed effect. A repeated‐measures analysis of covariance (ANCOVA) modeling was employed to assess the changes from baseline between groups. The model included baseline value as a covariate with fixed effects for group, study day, and group by study day interaction. Linear contrast statements from this model were constructed to provide between group p‐values at each time point.
A planned subgroup analysis was carried out based on age. This subgroup was created from the PP population, and all analyses were conducted in the same manner as the whole set.
Probabilities ≤.05 were considered statistically significant. All statistical analyses were completed using SAS version 9.3.1 (Cary, NC) for Microsoft Windows.
3. RESULTS
A total of 85 volunteers were screened and 50 eligible participants were enrolled, with 25 participants randomized into each study group (Figure 1). Forty‐five participants completed the study. Four participants in the VWC group and 1 participant in the placebo group withdrew consent. The PP population consisted of all participants who consumed at least 80% of their assigned product dose, did not have any major protocol violations, and who completed all study visits and procedures related to measurements of cheek skin elasticity. Participant demographic and lifestyle characteristics are presented in Table 1. Participant demographics were similar between both groups.
Figure 1.
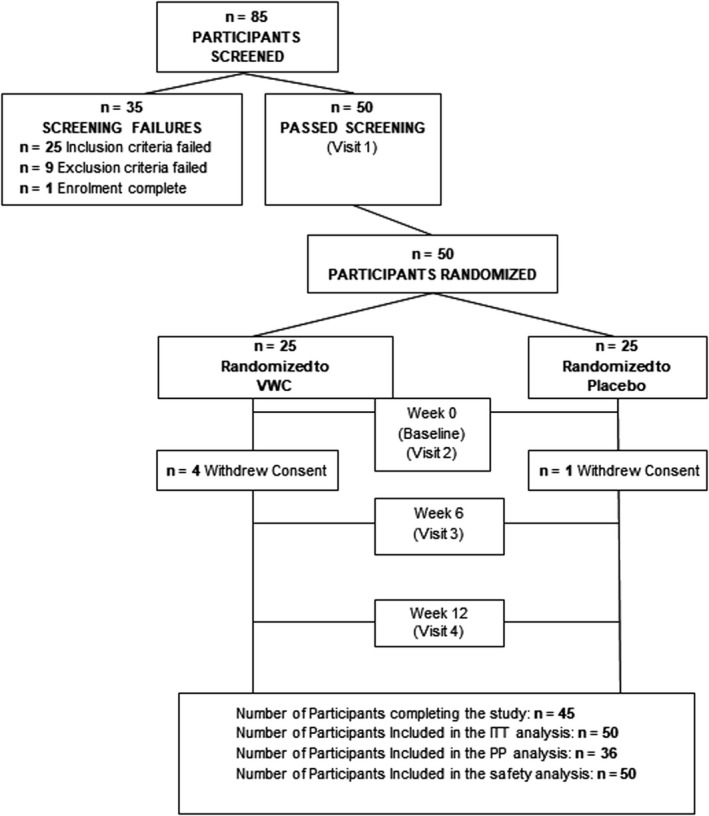
Disposition of participants in the trial
Table 1.
Demographics for participants in the per‐protocol population
|
VWC (n = 17) |
Placebo (n = 19) |
Between‐Group P‐Value b |
|
|---|---|---|---|
| Age (years) [Mean ± SD] | 53.2 ± 3.6 | 55.5 ± 3.2 | 0.054 a |
| Gender [n (%)] | |||
| Male | 0 (0%) | 0 (0%) | 1.000 |
| Female | 17 (100%) | 19 (100%) | |
| Smoking Status [n (%)] | |||
| Ex‐Smoker | 3 (18%) | 4 (21%) | 1.000 |
| No | 14 (82%) | 15 (79%) | |
| Engagement in Regular Exercise [n (%)] | |||
| No | 3 (18%) | 3 (16%) | 1.000 |
| Yes | 14 (82%) | 16 (84%) | |
| Ethnicity [n (%)] | |||
| Hispanic or Latino | 0 (0%) | 1 (5%) | 1.000 |
| Not Hispanic or Latino | 17 (100%) | 18 (95%) | |
| Race [n (%)] | |||
| East Asian | 1 (6%) | 0 (0%) | .457 |
| Eastern European White | 1 (6%) | 0 (0%) | |
| Middle Eastern | 0 (0%) | 1 (5%) | |
| Western European White | 15 (88%) | 18 (95%) | |
Probability values P ≤ .05 are statistically significant.
Abbreviations: %, percentage; n, number; SD, standard deviation.
Between‐group comparisons were made using the independent Student's t test.
Between‐group comparisons were made using the Fisher's exact test.
3.1. Efficacy of VWC supplementation on skin quality
There was no significant difference in skin quality VAS scores between participants supplemented with VWC or placebo. Notably, at baseline, participants in the VWC group reported lower VAS scores compared to those in placebo (78% lower for VAS overall, 85% for elasticity, 82% for hydration, 69% for radiance, 64% for firmness, and 80% for wrinkle). However, at week 12, participants supplemented with VWC reported higher scores in all VAS parameters vs participants on the placebo (Table 2). Although not significant, participants taking VWC showed improvements from baseline to week 12 over the placebo in the VAS overall score (9%), particularly in the elasticity (23%), hydration (14%), radiance (22%), firmness (25%), and wrinkle (15%) scores (Figure 2).
Table 2.
Average skin quality scores for the per protocol population as assessed by the skin quality VAS questionnaire
|
VWC Mean ± SD (n) |
Placebo Mean ± SD (n) |
Baseline between‐Group P‐value a |
|
|---|---|---|---|
| Overall Score | |||
| Baseline | 0.59 ± 1.97 (17) | 2.63 ± 11.47 (19) | 0.538 (r) |
| Week 6 | 46.41 ± 29.40 (17) | 38.42 ± 30.63 (19) | |
| Week 12 | 54.94 ± 31.82 (17) | 52.42 ± 34.28 (19) | |
| Elasticity Score | |||
| Baseline | 0.41 ± 1.28 (17) | 2.63 ± 11.47 (19) | 0.538 (r) |
| Week 6 | 38.71 ± 30.76 (17) | 31.26 ± 28.22 (19) | |
| Week 12 | 50.35 ± 30.38 (17) | 43.26 ± 37.10 (19) | |
| Hydration Score | |||
| Baseline | 0.47 ± 1.50 (17) | 2.63 ± 11.47 (19) | 0.538 (r) |
| Week 6 | 41.35 ± 30.12 (17) | 37.32 ± 31.31 (19) | |
| Week 12 | 50.24 ± 30.65 (17) | 46.37 ± 34.75 (19) | |
| Radiance Score | |||
| Baseline | 0.41 ± 1.28 (17) | 1.32 ± 5.74 (19) | 0.538 (r) |
| Week 6 | 42.06 ± 30.15 (17) | 35.47 ± 31.31 (19) | |
| Week 12 | 49.35 ± 29.43 (17) | 41.47 ± 31.19 (19) | |
| Firmness Score | |||
| Baseline | 0.47 ± 1.50 (17) | 1.32 ± 5.74 (19) | 0.538 (r) |
| Week 6 | 41.76 ± 31.91 (17) | 31.58 ± 32.65 (19) | |
| Week 12 | 48.18 ± 31.01 (17) | 39.37 ± 32.87 (19) | |
| Wrinkle Score | |||
| Baseline | 0.53 ± 1.74 (17) | 2.63 ± 11.47 (19) | 0.538 (r) |
| Week 6 | 39.24 ± 26.04 (17) | 28.21 ± 27.46 (19) | |
| Week 12 | 46.41 ± 32.27 (17) | 42.53 ± 36.57 (19) | |
Probability values P ≤ .05 are statistically significant
Abbreviations: n, number; SD, standard deviation.
For Baseline (Day 0), between group p‐value generated by ANOVA with Group as a fixed effect (r) indicates values were ranked prior to generating ANOVA or ANCOVA
Figure 2.
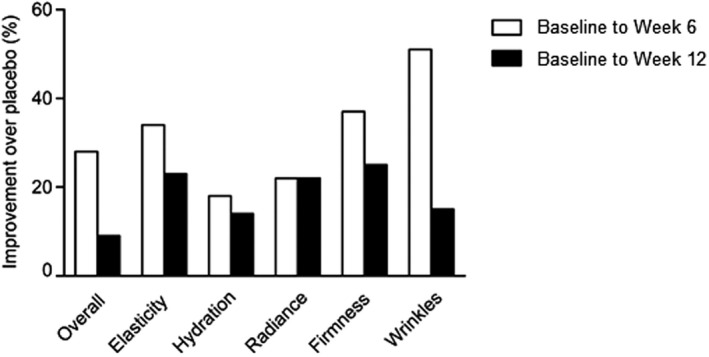
Percentage improvement from baseline (week 0) to week 6 and week 12 in skin quality VAS scores for participants taking VWC over participants taking placebo
3.2. Efficacy of VWC supplementation on wrinkle count and appearance
A typical image taken by the VISIA to determine nasolabial wrinkle count and score is shown in Figure 3A. From baseline to week 12, there were significant reductions in wrinkle score on both the left (17%, P = .009) and right (35%, P = .005) sides of the face for participants supplemented with VWC (Figure 3B and C). After 12 weeks, there was a 24% reduction in wrinkle score on the right side of the face for participants supplemented with VWC compared to placebo (P = .035) (Figure 3C).
Figure 3.
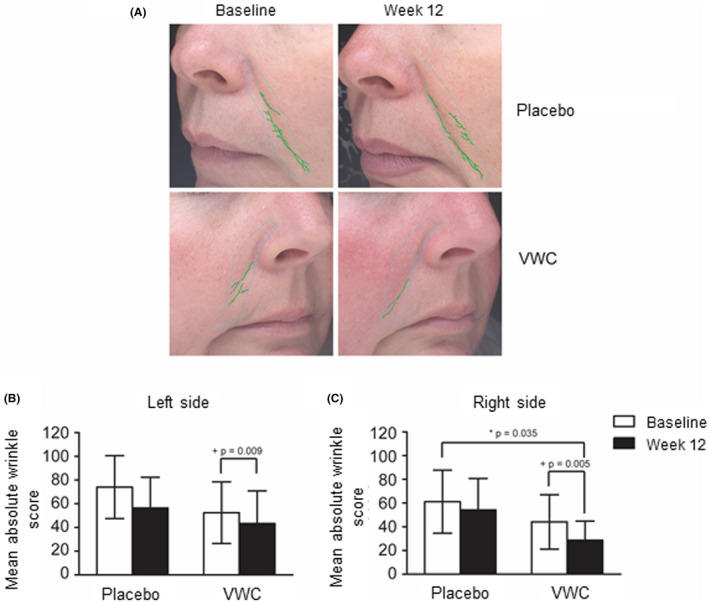
Nasolabial wrinkle analysis of participants supplemented with VWC or placebo. (A) Representative VISIA images at baseline and after 12 weeks of supplementation. (B) Change in the average absolute wrinkle score for the left side of the face. (C) Change in average absolute wrinkle score for the right side of the face. n = 11 (placebo); n = 12 (VWC). + Within group p‐values generated by the Wilcoxon signed rank test. Probability values P ≤ .05 are statistically significant
3.3. Efficacy of VWC supplementation on skin elasticity
There was no significant difference in cheek skin elasticity between VWC and placebo groups after 12 weeks. Participants supplemented with VWC experienced an 11% improvement in cheek elasticity from baseline to week 6 (P = .032). However, this was not maintained as there was a 6% reduction in elasticity from weeks 6 to 12. Participants on the placebo showed a 5% improvement from baseline to week 6 and a 3% improvement from week 6 to week 12.
Notably, in a planned subgroup analysis based on age, participants between 45 and 54 years of age in the VWC group showed a significant 20% and 10% improvement in cheek skin elasticity from baseline to week 6 (P = .032) and week 12 (P = .027), respectively (Figure 4).
Figure 4.
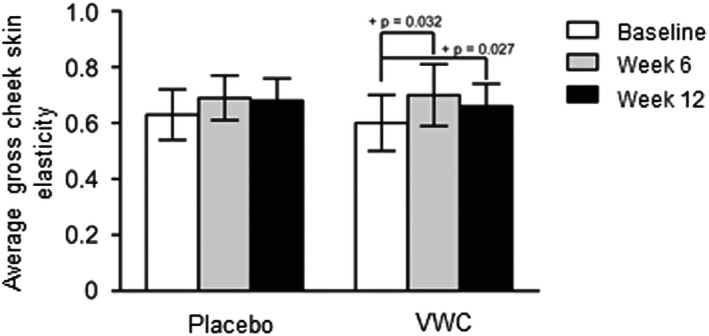
Gross cheek skin elasticity after supplementation with VWC for participants 45‐54 years of age (n = 12), assessed by the Cutometer®. Higher values indicate more elastic skin. + Within group p‐values generated by the Wilcoxon signed rank test. Probability values P ≤ .05 are statistically significant
3.4. Safety evaluation of VWC supplementation
A total of 32 AEs were recorded in this study, with 19 unique participants experiencing these events. Of these, 18 AEs were reported by participants in the VWC group, and 14 in the placebo. Of the 18 AEs in the VWC group, 17 were reported as being not related or unlikely to be related. One report of mild nausea was the only AE reported as being possibly related to the investigational product. All 14 of the AEs in the placebo were reported as being not related or unlikely to be related. There was no difference in the number of AEs reported between the groups and all were resolved by the end of the study.
There was no between‐group differences in hematological and clinical chemistry parameters at screening or after 12 weeks (Table 3), and all participants were deemed healthy. For both groups, all hematology, clinical chemistry, electrolytes, and liver and kidney function markers remained within healthy clinical reference ranges. Any changes in these safety parameters were deemed not clinically significant. There were no significant differences in vital signs or anthropometric measurements between VWC and placebo (data not shown).
Table 3.
Hematology and clinical chemistry analysis at screening and week 12 for participants in the safety population
|
VWC Mean ± SD (n) |
Placebo Mean ± SD (n) |
Between Group P‐value | |
|---|---|---|---|
| Hemoglobin Concentration (g/L) | |||
| Screening | 131.5 ± 7.4 (25) | 132.3 ± 6.9 (25) | .696 a |
| Week 12 | 129.9 ± 8.7 (23) | 131.1 ± 8.2 (24) | .636 a |
| Hematocrit (L/L) | |||
| Screening | 0.39 ± 0.02 (25) | 0.39 ± 0.02 (25) | .660 a |
| Week 12 | 0.39 ± 0.02 (23) | 0.39 ± 0.02 (24) | .486 a |
| White Blood Cell Count (×109/L) | |||
| Screening | 5.91 ± 1.50 (25) | 6.10 ± 1.91 (25) | .688 a |
| Week 12 | 5.66 ± 1.56 (23) | 6.08 ± 1.62 (24) | .373 a |
| Red Blood Cell Count (×E12/L) | |||
| Screening | 4.36 ± 0.22 (25)) | 4.45 ± 0.37 (25) | .289 a |
| Week 12 | 4.33 ± 0.29 (23) | 4.44 ± 0.37 (24) | .261 a |
| Mean Corpuscular Volume (fL) | |||
| Screening | 90.1 ± 3.3 (25) | 89.1 ± 5.8 (25) | .459 a |
| Week 12 | 90.0 ± 3.8 (23) | 89.2 ± 5.3 (24) | .560 a |
| Mean Corpuscular Hemoglobin (pg) | |||
| Screening | 30.16 ± 1.15 (25) | 29.83 ± 1.97 (25) | .466 a |
| Week 12 | 30.04 ± 1.48 (23) | 29.63 ± 2.07 (24) | .445 a |
| Mean Corpuscular Hemoglobin Concentration (g/L) | |||
| Screening | 335.1 ± 5.1 (25) | 335.1 ± 6.5 (25) | 1.000 a |
| Week 12 | 334.1 ± 5.9 (23) | 332.2 ± 7.6 (24) | .341 a |
| Red Cell Distribution Width (%) | |||
| Screening | 13.39 ± 0.54 (25) | 13.36 ± 0.64 (25) | .867 a |
| Week 12 | 13.37 ± 0.50 (23) | 13.48 ± 0.67 (24) | .545 a |
| Platelet Count (×109/L) | |||
| Screening | 264 ± 54 (25) | 257 ± 46 (25) | .618 a |
| Week 12 | 257 ± 58 (23) | 256 ± 45 (24) | .956 a |
| Neutrophil Count (×109/L) | |||
| Screening | 3.41 ± 1.09 (25) | 3.49 ± 1.39 (25) | .813 a |
| Week 12 | 3.18 ± 1.03 (23) | 3.33 ± 1.09 (24) | .638 a |
| Lymphocyte Count (×109/L) | |||
| Screening | 1.81 ± 0.57 (25) | 1.92 ± 0.78 (25) | .636 a , c |
| Week 12 | 1.75 ± 0.63 (23) | 2.08 ± 0.80 (24) | .120 a , c |
| Monocyte Count (×109/L) | |||
| Screening | 0.504 ± 0.149 (25) | 0.472 ± 0.159 (25) | .466 a |
| Week 12 | 0.522 ± 0.124 (23) | 0.483 ± 0.143 (24) | .333 a |
| Eosinophil Count (×109/L) | |||
| Screening | 0.148 ± 0.145 (25) | 0.172 ± 0.209 (25) | .558 b |
| Week 12 | 0.174 ± 0.163 (23) | 0.167 ± 0.087 (24) | .443 b |
| Basophil Count (×109/L) | |||
| Screening | 0.020 ± 0.041 (25) | 0.012 ± 0.033 (25) | .454 b |
| Week 12 | 0.022 ± 0.042 (23) | 0.017 ± 0.038 (24) | .673 b |
| Creatinine Concentration (μmol/L) | |||
| Screening | 72.4 ± 12.6 (25) | 71.5 ± 10.0 (25) | .766 a |
| Week 12 | 75.5 ± 12.0 (23) | 73.2 ± 10.7 (24) | .505 a |
| Sodium Concentration (mmol/L) | |||
| Screening | 141.60 ± 2.06 (25) | 142.28 ± 2.07 (25) | .250 a |
| Week 12 | 141.39 ± 2.48 (23) | 141.88 ± 2.46 (24) | .505 a |
| Potassium Concentration (mmol/L) | |||
| Screening | 4.60 ± 0.46 (25) | 4.65 ± 0.49 (25) | .701 a |
| Week 12 | 4.66 ± 0.35 (23) | 4.76 ± 0.53 (24) | .443 a |
| Chloride Concentration (mmol/L) | |||
| Screening | 101.92 ± 2.08 (25) | 101.88 ± 2.30 (25) | .949 a |
| Week 12 | 102.22 ± 2.24 (23) | 101.92 ± 2.00 (24) | .629 a |
| Bilirubin Concentration (μmol/L) | |||
| Screening | 6.3 ± 3.1 (25) | 7.3 ± 4.1 (25) | .583 a , c |
| Week 12 | 7.04 ± 2.82 (23) | 6.62 ± 2.50 (24) | .679 a , c |
| Estimated Glomerular Filtration Rate(mL/min/1.73m2) | |||
| Screening | 83.0 ± 14.5 (25) | 82.8 ± 12.7 (25) | .975 a |
| Week 12 | 79.0 ± 14.2 (23) | 81.2 ± 14.0 (24) | .593 a |
| Aspartate Transaminase (U/L) | |||
| Screening | 19.2 ± 4.7 (25) | 20.6 ± 4.7 (25) | .315 a , c |
| Week 12 | 19.5 ± 6.0 (23) | 19.7 ± 5.0 (24) | .733 a , c |
| Alanine Transaminase (U/L) | |||
| Screening | 18.6 ± 6.4 (25) | 19.8 ± 7.1 (25) | .556 a , c |
| Week 12 | 18.9 ± 10.0 (23) | 18.6 ± 8.5 (24) | .890 a , c |
Probability values P ≤ .05 are statistically significant.
Abbreviaions: n, number; SD, standard deviation.
Between‐group comparisons were made using the independent Student's t test.
Between‐group comparisons were made using the Mann‐Whitney U test.
Logarithmic transformation was required to achieve normality.
4. DISCUSSION
This randomized, triple‐blind, placebo‐controlled study evaluated the efficacy and safety of Vinh Wellness Collagen on face wrinkles, elasticity, and self‐reported improvements in skin health. Participants in the VWC group reported greater improvements in overall in skin elasticity, hydration, radiance, firmness, and wrinkle score, suggesting that VWC supplementation had a beneficial effect on self‐perceived skin appearance. After 12 weeks, participants supplemented with VWC showed a significant 24% improvement in the absolute wrinkle score on the right side of the face compared to placebo. On both sides of the face, there was a significant decrease in the wrinkle score from baseline to week 12 for participants supplemented with VWC.
The improvements in skin health observed in the current study are consistent with previous studies examining collagen supplementation. Supplementation with 3 g of collagen peptide was associated with a higher report of participant satisfaction compared to placebo. 13 Similar improvements in facial wrinkles in this study were reported following 30 days of hydrolyzed collagen supplementation in females. 18 Bonnet al. (2017) found that following 12‐week supplementation with 10 g of fish collagen peptides, women had a significant 10% reduction in periorbital wrinkles. 19 As the improvement found by Bonnet et al (2017) was for periorbital wrinkles, a direct comparison to the current study cannot be drawn. However, one possible reason for the greater improvement in wrinkle score observed in the current study may be due to higher concentration of anti‐oxidant amino acids (glycine, proline, hydroxyproline) found in VWC.
Although not significant, the improvement in cheek skin elasticity over the 12‐week VWC supplementation period is consistent with previously published research that utilized similar doses and study populations. Korean females and males experienced a significant improvement in elasticity following 12‐week supplementation with 3 g of collagen peptide and vitamin C 13 or 3 g hydrolyzed fish collagen combined with astaxanthin. 20 Supplementation with 10 g of collagen peptides combined with vitamins A, C, and E and zinc significantly improved gross cheek elasticity in females aged 40‐60 years after 90 days. 21 This suggests collagen peptides may act synergistically with other nutrients to improve skin elasticity. Future studies should consider investigating the potential synergistic effects of VWC with other skin enhancing nutrients.
This is the first study to report a differential response between right and left facial areas with collagen supplementation. As the absolute wrinkle score reflects several factors, both photoaging and melanoma are generally more prevalent on the left side of the face due to sun exposure while driving. 22 Sleeping habits causing micro‐pressure over an extended time have also been suggested to contribute to aging lines and faster aging on the left side of the face. 23 It may be that the left cheek was more photoaged and more affected by sleep lines than the right, making the determination of product efficacy challenging. This is one possible explanation for the significant results between VWC and placebo for the right cheek, but only significant changes from baseline to week 12 for the VWC group for the left. This suggests that longer usage of the product may result in significant differences between VWC and placebo on the left side of the face; however, this warrants further investigation in future studies.
A planned subgroup analysis based on age found that females between 45 and 54 years supplemented with VWC had significant improvements in skin elasticity at week 12. Compared to baseline, these women had 20% and 10% improvements in the cheek skin gross elasticity at weeks 6 and 12, respectively. There were no significant differences observed in women between the ages of 55 and 60 years. These results are in contrast to a previous study demonstrating improvements in skin elasticity were more pronounced in women over the age of 50 compared to women under 50 years old. 12 However, these conflicting results may be due to differences in the location of measurement, dosage used, and the population studied. One possible explanation to glean from this observation is that oral collagen supplementation may affect distinct areas of the body differently and could have greater efficacy on certain body regions. A larger sample size powered based on the findings of this study, particularly within the 45‐54 age group, should be considered in future studies.
A limitation of measurements and specifically re‐measurements of any viscoelastic property is the phenomenon of hysteresis. As such, repeated measurements of viscoelastic material are discouraged as they cannot account for the inability of the property (ie, skin) to return to its prestrained state prior to the next measurement cycle and the impression is then carried forward into subsequent measurements. In the current study, 9 Cutometer® measurements for gross elasticity were either out of range or missing. Unfortunately, these measurements could not be repeated without introducing the confounding factor of hysteresis. The test‐re‐rest reliability of skin elasticity measurements while avoiding cofounding has been described in a previous study. 24 Given this information, a potential solution would be to perform baseline measurements of cheek skin elasticity on both cheeks, such that any measurement out of range can be performed on the other cheek without hindering the statistical significance of the results. Recognizing that it may not be possible to use the values from one cheek to replace another, previous studies have included a rest period of 45 minutes between repeated measurements in order to avoid consequences of hysteresis. 24 Further, repeated measurements, up to three times, 25 would provide a mean score and potentially accommodate out of range or missing values. Future work should incorporate these methods to reduce missing data.
5. CONCLUSION
In conclusion, participants supplemented for 12 weeks with Vinh Wellness Collagen showed improvements in wrinkle scores on both sides of the face, cheek skin hydration and self‐reported elasticity, hydration, radiance, firmness, and wrinkle scores. Overall, the current study demonstrates that VWC was safe and well tolerated in a healthy female population as a similar number of adverse events were reported between groups; none were classified as probably related or related to VWC and all blood safety parameters for the complete blood count, electrolytes; and liver and kidney markers were within normal clinical ranges. Future studies are needed to examine potential synergistic effects of VWC with other skin enhancing nutrients and the mechanism of action associated with collagen supplementation and improvements of skin health. The results of this study support the use of fish‐derived hydrolyzed collagen for the improvement of skin health in an aging population.
Evans M, Lewis ED, Zakaria N, Pelipyagina T, Guthrie N. A randomized, triple‐blind, placebo‐controlled, parallel study to evaluate the efficacy of a freshwater marine collagen on skin wrinkles and elasticity. J Cosmet Dermatol. 2020;20:825–834. 10.1111/jocd.13676
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
REFERENCES
- 1. Bonta M, Daina L, Mutiu G. The process of ageing reflected by histological changes in the skin. Rom J Morphol Embryol. 2013;54(3 suppl):797‐804. [PubMed] [Google Scholar]
- 2. Gelse K, Poschl E, Aigner T. Collagens–structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55(12):1531‐1546. [DOI] [PubMed] [Google Scholar]
- 3. Baumann L. Skin ageing and its treatment. J Pathol. 2007;211(2):241‐251. [DOI] [PubMed] [Google Scholar]
- 4. Calleja‐Agius J, Brincat M, Borg M. Skin connective tissue and ageing. Best Pract Res Clin Obstet Gynaecol. 2013;27(5):727‐740. [DOI] [PubMed] [Google Scholar]
- 5. Skovgaard GRL, Jensen AS, Sigler ML. Effect of a novel dietary supplement on skin aging in post‐menopausal women. Eur J Clin Nutr. 2006;60(10):1201‐1206. [DOI] [PubMed] [Google Scholar]
- 6. Zague V. A new view concerning the effects of collagen hydrolysate intake on skin properties. Arch Dermatol Res. 2008;300(9):479‐483. [DOI] [PubMed] [Google Scholar]
- 7. Djagny VB, Wang Z, Xu S. Gelatin: a valuable protein for food and pharmaceutical industries: review. Crit Rev Food Sci Nutr. 2001;41(6):481‐492. [DOI] [PubMed] [Google Scholar]
- 8. Watanabe‐Kamiyama M, Shimizu M, Kamiyama S, et al. Absorption and effectiveness of orally administered low molecular weight collagen hydrolysate in rats. J Agricultural Food Chem. 2010;58(2):835‐841. [DOI] [PubMed] [Google Scholar]
- 9. Sibilla S, Godfrey M, Brewer S, Budh‐Raja A, Genovese L. An overview of the beneficial effects of hydrolysed collagen as a nutraceutical on skin properties: scientific background and clinical studies. Open Nutraceuticals J. 2015;8:29‐42. [Google Scholar]
- 10. De Luca C, Mikhal’chik EV, Suprun MV, Papacharalambous M, Truhanov AI, Korkina LG. Skin antiageing and systemic redox effects of supplementation with marine collagen peptides and plant‐derived antioxidants: a single‐blind case‐control clinical study. Oxid Med Cell Longev. 2016;2016:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tanaka M, Yamamoto Y, Misawa E, et al. Effects of aloe sterol supplementation on skin elasticity, hydration, and collagen score: a 12‐week double‐blind, randomized controlled trial. Skin Pharmacol Physiol. 2016;29(6):309‐317. [DOI] [PubMed] [Google Scholar]
- 12. Proksch E, Schunck M, Zague V, Segger D, Degwert J, Oesser S. Oral intake of specific bioactive collagen peptides reduces skin wrinkles and increases dermal matrix synthesis. Skin Pharmacol Physiol. 2014;27(3):113‐119. [DOI] [PubMed] [Google Scholar]
- 13. Choi SY, Ko EJ, Lee YH, et al. Effects of collagen tripeptide supplement on skin properties: A prospective, randomized, controlled study. J Cosmet Laser Ther. 2014;16(3):132‐137. [DOI] [PubMed] [Google Scholar]
- 14. Luc Duteil CQR, Bruno‐Bonnet C, Lacour JP. Effect of low dose type I fish collagen peptides combined or not with silicon on skin aging signs in mature women. JOJ Case Stud. 2018;6(4):001–005. [Google Scholar]
- 15. Koizumi S, Inoue N, Shimizu M, Kwon C‐J, Kim H‐Y, Park KS. Effects of Dietary Supplementation with Fish Scales‐Derived Collagen Peptides on Skin Parameters and Condition: A Randomized, Placebo‐Controlled, Double‐Blind Study. Int J Peptide Res Ther. 2018;24(3):397‐402. [Google Scholar]
- 16. Sumida E, Hiorata A, Kuwaba K. The Effect of Oral Ingestion of Collagen Peptide on Skin Hydration and Biochemical Data of Blood. J Nutr Food. 2004;7:45‐52. [Google Scholar]
- 17. Gold MH, Biron JA. Safety and Cosmetic Effects of Photodynamic Therapy using Hexyl Aminolevulinate and Intense Pulsed Light: A Pilot Study Conducted in Subjects with Mild‐to‐moderate Facial Photodamage. J Clin Aesthet Dermatol. 2013;6(10):27‐31. [PMC free article] [PubMed] [Google Scholar]
- 18. Zhou S‐L, Wang H‐Y, Yue D‐X. Clinical Effects and Safety of Oral Treatment with Low‐Molecular Fish Collagen Hydrolysate on Female Facial Skin Properties. J Pract Dermatol. 2011;4(3). [Google Scholar]
- 19. Bonnet D.Introduction to Naticol® marine collagen peptides for anti‐aging and overview of clinical studies. https://pdfs.semanticscholar.org/fed1/fa27de3676b4626343d35ea1d1567ca3f216.pdf Accessed October 5 2017.
- 20. Yoon HS, Cho HH, Cho S, Lee SR, Shin MH, Chung JH. Supplementating with dietary astaxanthin combined with collagen hydrolysate improves facial elasticity and decreases matrix metalloproteinase‐1 and ‐12 expression: a comparative study with placebo. J Medicinal Food. 2014;17(7):810‐816. [DOI] [PubMed] [Google Scholar]
- 21. Campos P, Melo M, Calixto L, Fossa M. An oral supplementation based on hydrolyzed collagen and vitamins improves skin elasticity and dermis echogenicity: a clinical placebo‐controlled study. Clinical Pharmacology & Biopharmaceutics. 2015;04(03):1–6. [Google Scholar]
- 22. Paulson KG, Iyer JG, Nghiem P. Asymmetric lateral distribution of melanoma and Merkel cell carcinoma in the United States. J Am Acad Dermatol. 2011;65(1):35‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Poljsak B, Godic A, Lampe T, Dahmane R. The influence of the sleeping on the formation of facial wrinkles. J Cosmet Laser Ther. 2012;14(3):133‐138. [DOI] [PubMed] [Google Scholar]
- 24. Peperkamp K, Verhulst AC, Tielemans HJP, Winters H, van Dalen D, Ulrich DJO. The inter‐rater and test‐retest reliability of skin thickness and skin elasticity measurements by the DermaLab Combo in healthy participants. Skin Res Technol. 2019;25(6):787‐792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pmc MBG, Meloo M, Calixto L, Fossa M. An oral supplementation based on hydrolyzed collagen and vitamins improves skin elasticity and dermis echogenicity: a clinical placebo‐controlled study. Clin Pharmacol Biopharm. 2015;4(142):2. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from the authors.


