Abstract
Background:
Persistent disparities in academic performance may result from a confluence of adverse exposures accruing disproportionately to specific subpopulations.
Objective:
Our overarching objective was to investigate how multiple exposures experienced over time affect early childhood educational outcomes. We were specifically interested in whether there were: (1) racial/ethnic disparities in prevalence of adverse exposures; (2) racial/ethnic disparities in associations observed between adverse exposures and early childhood educational outcomes; and (3) interactions between exposures, suggesting that one exposure augments susceptibility to adverse effects of another exposure.
Methods:
We link geocoded North Carolina birth data for non-Hispanic white (NHW) and non-Hispanic black (NHB) children to blood lead surveillance data and 4th grade end-of-grade (EOG) standardized test scores (n = 65,151). We construct a local, spatial index of racial isolation (RI) of NHB at the block group level. We fit race-stratified multi-level models of reading and mathematics EOG scores regressed on birthweight percentile for gestational age, blood lead level, maternal smoking, economic disadvantage, and RI, adjusting for maternal- and child-level covariates and median household income.
Results:
There were marked racial/ethnic disparities in prevalence of adverse exposures. Specifically, NHB children were more likely than NHW children to be economically disadvantaged (80% vs. 40%), live in block groups with the highest quintile of RI (46% vs. 5%), have higher blood lead levels (4.6 vs. 3.7 μg/dL), and lower birthweight percentile for gestational age (mean: 39th percentile vs. 51st percentile). NHB children were less likely to have mothers who reported smoking during pregnancy (11% and 22%). We observed associations between key adverse exposures and reading and math EOG scores in 4th grade. Higher birthweight percentile for gestational age was associated with higher EOG scores, while economic disadvantage, maternal smoking, and elevated blood lead levels were associated with lower EOG scores. Associations observed for NHB and NHW children were generally not statistically different from one another, with the exception of neighborhood RI. NHB children residing in block groups in the highest RI quintile had reading and math scores 1.54 (0.74, 2.34) and 1.12 (0.38, 1.87) points lower, respectively, compared to those in the lowest RI quintile; statistically significant decrements in EOG scores associated with RI were not observed for NHW children. We did not find evidence of multiplicative interactions between exposures for NHB or NHW children.
Discussion:
Key adverse host, environmental, and social exposures accrue disproportionately to NHB children. Decrements in test scores associated with key adverse exposures were often but not always larger for NHB children, but were not significantly different from those estimated for NHW children. While we did not observe interactive effects, NHB children on average experience more deleterious combined exposures, resulting in larger decrements to test scores compared to NHW children.
Keywords: racial isolation, lead, poverty, standardized test scores, early childhood educational outcomes
INTRODUCTION
Despite considerable attention, racial/ethnic disparities in academic performance and overall educational attainment have persisted for decades (1). The “achievement gap” becomes apparent in early childhood and persists or widens with time, resulting in lower high school and college graduation rates among children belonging to racial/ethnic minority groups, particularly non-Hispanic blacks (NHB) (2). These disparities may shadow children throughout their lives, because early childhood academic outcomes are predictive of overall educational attainment (2), which is associated with health and wellbeing later in life (3). Disparities in academic performance among young children represent systemic, avoidable differences in early childhood academic achievement, as well as key targets for early intervention.
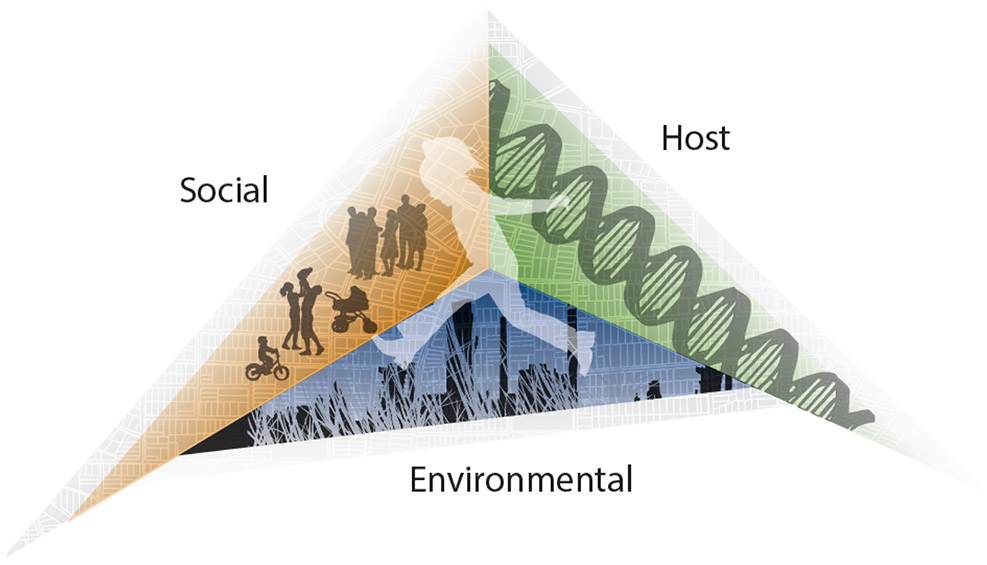
As with other disparities in health and development, the achievement gap most likely results from a confluence of forces, acting over time, that accrue disproportionately to specific subpopulations. In previous work, Miranda et al (2009) proposed a framework for understanding health disparities (Figure 1), in which environmental, social, and host factors form three sides of an integrated triangle (4). Health disparities arise when the forces exerted by the triangle’s sides are asymmetric for different population groups. Here, we use the heuristic presented in Figure 1 to consider how host, environmental, and social exposures may affect racial/ethnic disparities in early childhood academic performance. We examine the pregnancy outcome birthweight-for-gestational age (host); early childhood lead exposure and maternal smoking during pregnancy (environmental); and measures of family-level socioeconomic status (SES) and neighborhood-level racial segregation (social). For each host, environmental and social exposure, we briefly summarize evidence of racial/ethnic disparities in the exposure and associations with cognitive development.
Figure 1.
Forces shaping health and developmental outcomes. Image reproduced with permission.
Host factors: Pregnancy outcomes
One of the most persistent disparities in American health status is the pronounced difference in pregnancy outcomes between NHB and non-Hispanic white (NHW) women (5) (6) (7). Birthweight-for-gestational age is an indirect measure of fetal growth and an indicator of newborn health (7-9). Poor pregnancy outcomes, including low birthweight for gestational age, even within the normal ranges, are associated with decrements in cognitive development measures, including poorer performance on standardized tests (10, 11).
Environmental factors: Lead level and maternal smoking
A recent review of blood lead levels among children under 6 years of age concluded that, on average, NHB children had higher blood lead levels than NHW children and were more likely to have elevated blood lead levels (≥5 μg/dL) (12). Lead exposure in young children, even at low levels, is linked with learning deficits and lower scores on intelligence and standardized tests (13-21). Evidence suggests that the negative impacts of childhood lead exposure persist into adulthood, affecting intelligence and socioeconomic status (22).
Research also suggests that maternal smoking during pregnancy is associated with health and developmental issues in offspring. Children born to mothers who smoked during pregnancy are at increased risk of stillbirth (23), asthma and wheezing (24), diagnosis with attention-deficit/hyperactivity disorder (25) and impairments in cognitive development (26, 27), measured by intelligence quotient (28, 29) and standardized test scores (30).
Social factors: Family poverty and neighborhood racial residential segregation
In 2013, approximately 20% of children in the US lived in poverty. The percentage of Hispanic, NHW, and Asian children living in poverty declined between 2010 and 2013, while the percentage in poverty (38%) among NHB children held steady (31). As a result, NHB children were nearly four times as likely as NHW children to be living in poverty in 2013. Although poverty represents only one measure of a child or family’s social environment, children living in poverty fare poorly across a variety of academic measures, including lower scores on standardized tests, poorer grades in school, and lower educational attainment (32, 33).
In addition to family-level measures of social context, a growing number of studies find that neighborhood contextual factors affect children’s health and development (34). Low neighborhood SES is consistently associated with worse developmental outcomes in children (34), but there is less evidence for how other aspects of the neighborhood environment relate to child development. In particular, racial residential segregation (RRS) has been posited to be a fundamental cause of health disparities. Through the disinvestment of educational resources and employment opportunities and the concomitant concentration of multiple disadvantages, segregation fosters residential environments inimical to health and wellbeing (35).
Despite prominent conceptualizations of RRS as a key mechanism through which racial/ethnic disparities in health and development are rooted and reinforced (36, 37), few, if any, studies have examined relationships between RRS and child developmental outcomes. Thus, RRS remains an understudied but potentially important determinant of disparities in child development. Furthermore, failing to account for differences in environmental and social exposures resulting from RRS may lead to erroneous conclusions about the etiology of racial disparities in child development. That is, racial/ethnic differences in developmental outcomes that result from differential place-based exposures could be wrongly attributed to individual-level race.
Effects of multiple disadvantages on educational outcomes
To investigate how exposure to host, environmental, and social factors affects educational outcomes, we link three individual-level, geocoded datasets: statewide detailed birth records in North Carolina (NC), blood lead surveillance data, and 4th grade end-of-grade (EOG) test scores in reading and mathematics. We fit race-stratified multi-level models of EOG test scores regressed on birthweight percentile for gestational age, blood lead level, maternal smoking, family-level poverty, and a local, spatial measure of neighborhood (block group) racial isolation (RI), one dimension of RRS.
METHODS
Data sources
The analysis dataset for this study was created by linking three administrative databases for the State of North Carolina: Detailed Birth Records (NCDBR), blood lead surveillance data, and EOG educational testing data.
Detailed birth records.
The NCDBR were obtained from the North Carolina State Center for Health Statistics, Vital Statistics department. The NCDBR provides data on all documented live births occurring in NC, including information on maternal demographics, maternal and infant health, and maternal obstetrics history. In validation studies, birth certificate data have been shown to be accurate, particularly for demographic and birth outcome variables (38, 39).
Blood lead surveillance data.
Blood lead surveillance data were obtained from the state registry maintained by the Childhood Lead Poisoning Prevention Program of the Children’s Environmental Health Unit, Department of Health and Human Services in Raleigh, NC. The blood lead surveillance data include child name, birth date, test date, blood lead level, type of test (venous or capillary), and home address. The North Carolina State Laboratory for Public Health (Raleigh, NC) conducted 90% of the analyses of the blood samples. The limit of detection for lead in blood as analyzed by the State Laboratory is 1 μg/dL, but all children with blood lead level below the level of detection are assigned a value of 1 μg/dL in the state database. Blood lead levels are stored in the state database as integer values. Most of the samples were sent to the State Laboratory from private providers, indicating that the samples were collected by trained health care professionals. We used blood lead screening data from 1995-2005. In theory, all children whose parents responded “yes” or “don’t know” to any of the three questions on the CDC Lead Risk Assessment Questionnaire (40) should have been screened for lead, but it is difficult to ascertain true practice at the time. Using these dates for the blood lead screening data allows a long enough window to link these same children to their EOG scores.
End-of-grade standardized testing data.
Educational testing data were obtained from the North Carolina Education Research Data Center (NCERDC) at Duke University in Durham, NC. North Carolina children in grades 3–8 are tested in reading and mathematics at the end of the school year. These assessments are “curriculum-based multiple-choice achievement tests…specifically aligned to the North Carolina Standard Course of Study”(41). The Reading EOG test consists of multiple choice questions that cover: cognition; interpretation; critical stance; and connections (42). The Mathematics EOG consists of multiple choice questions that cover: number sense, numeration, and numerical operations; spatial sense, measurement, and geometry; patterns, relationships, and functions; and data, probability, and statistics (41).
The NCERDC maintains a database with records of all EOG test results statewide for tests from the 1995–1996 school year to the present. This database includes identifying information such as name and birth date. Additionally, the database contains data on demographics and socioeconomics, school and school district, and characteristics such as computer use in the home and English proficiency. These data can also be linked longitudinally for all years each child has taken EOG tests in North Carolina.
Methods for receiving, storing, linking, analyzing, and presenting results related to this study were all governed by a research protocol approved by the University of Notre Dame and Rice University Institutional Review Boards.
Linking datasets
Records were linked through an iterative deterministic process employing various combinations of identifying variables (first and last name, date of birth, and county of residence) and requirements on the stringency of the match strength. This process allowed for records to be linked despite misspellings, typos, and abbreviations. County of residence was only included in the first linkage iteration to allow for residential mobility. The linking schemas were designed to ensure accuracy while trying to achieve the highest number of linked records possible.
Using the NCDBR, we considered births occurring between 1997-1999 (initial n=325,610). We restricted NCDBR records in which mothers were between the ages of 15 and 44 years (excluded 1,142 NCDBR records), of NHB, NHW, or Hispanic race/ethnicity (excluded 11,795 records) and had no more than three previous live births (excluded 8,605 records). Children were singleton births (excluded 8,858 records) with no congenital anomalies (excluded 3,159 records) who weighed at least 400 g (excluded 362 records) at birth and had a gestational age at delivery between 24 and 42 weeks (excluded 883 records). Of 290,806 unique children born in North Carolina between 1 January 1997 and 31 December 1999 that met these criteria, 239,926 (82.5%) were geocoded to a parcel, building, or street, enabling them to be assigned to a 2000 census block group. Of the geocoded births, 125,508 (52.3%) were successfully matched to a 4th grade reading and math test score in 2008-2010 (encompassing three academic years: 2007/2008, 2008/2009, and 2009/2010). Most children in 4th grade between 2008-2010 would have been born between 1997-1999. Of the 125,508 children with birth and education records, 72,695 (57.9%) were linked with at least one lead test result between the ages of 9 months and 5 years.
Study sample
Of the 72,695 children linked across NCDBR (1997-1999), lead screening (1995-2005), and education datasets (2008-2010), and geocoded at time of birth, we restricted to children born to self-reported NHW and NHB mothers (excluded 5,994 records) because of small cell numbers in the Hispanic category. We also restricted to children who did not have limited English proficiency, as it can be complicated to interpret test scores among young children for whom English is their second language (excluded 940 records). We removed 603 records with missing values for key covariates (e.g., child sex; maternal educational attainment, age, marital status; and whether and how often the child used a computer at home). We removed 7 records with blood lead test results >80 μg/dL as likely outliers. Our final dataset included 65,151 children.
The characteristics of the final analysis dataset (n=65,151) differed from those of the initial births dataset consisting of 325,610 unique children born between 1997 and 1999; a comparison of the initial and final datasets is provided in the Supplemental Material (SM), Table S1. Compared to mothers in the initial dataset, mothers in the final dataset were more likely to be smokers (15% vs. 18%), less likely to be college graduates (23% vs 16%), and more likely to be unmarried at time of birth (32% vs 43%). Compared to children in the initial dataset, children in the final dataset had lower mean birthweight percentile for gestational age (48 vs. 47). The racial composition of the initial and final datasets differed: initially, 63% of mothers were NHW, 25% were NHB, and 7% were Hispanic, compared to a racial composition of 63% NHW and 37% NHB in the final dataset (Hispanics were removed due small cell numbers). The mean block-group level RI was 0.24 and 0.26 in the initial and final datasets, respectively.
Racial isolation
Because we are particularly interested in disparate outcomes for NHB, we calculated the RI index for NHB at the block group level based on 2000 Census data using a previously derived local, spatial measure of RI (43), which was in turn derived from the global spatial isolation index developed by Reardon and O’Sullivan (44). Using 2000 Census data on racial composition, we calculated block group-level RI scores by accounting for the population composition in the index block group along with adjacent block groups. The index ranges from 0 to 1, where index values close to 0 indicate that NHB in that “neighborhood environment” (i.e., the index block group and adjacent block groups) were living with nearly all non-NHB, and index values close 1 indicate that NHB in that neighborhood environment were living with nearly all NHB. We summarize the index in the Supplemental Material. Each child in the final study sample was assigned a RI index value based on the block group of maternal residence obtained from the NCDBR.
Statistical analysis
We used continuous reading and mathematics EOG test scores as dependent variables. The host exposure of interest was birthweight percentile for gestational age. Birthweight percentile for gestational age serves as a measure of fetal growth and was determined by comparing the birthweight for each birth in our study population with a national sex-specific reference distribution (45).
Environmental exposures included blood lead level and maternal smoking status during pregnancy. Blood lead level was categorized into one of four categories: 1, 2-4, 5-9, and 10+ μg/dL. Maternal smoking status was a binary variable (1=reported smoking during pregnancy).
Social exposures of interest were RI calculated at the block group level and whether the student was economically disadvantaged, available at the individual student level and indicated by participation in the free/reduced price lunch program (binary variable, 1=participation in the program). Eligibility for the free/reduced-price lunch program, which is administered by the US Department of Agriculture, is based on family income: in the 2016-2017 school year, to qualify for free/reduced price lunch, the maximum income for a household of four was $31,590 and $44,955, respectively (46). Racial isolation was categorized by quintile (higher quintiles indicate greater block group-level racial isolation [segregation] of non-Hispanics blacks).
Preliminary analysis revealed differential covariate distributions by race group (Table 1). To accommodate racial heterogeneity, we fit adjusted race-stratified models of EOG test scores regressed on exposures of interest, separately by subject matter. In addition to host, environmental, and social exposures of interest, adjusted models controlled for potentially confounding maternal and child-level factors. Maternal characteristics collected at the time of delivery included age in years, categorized as 15–19, 20–24, 25–29 (reference level), 30–34, 35–39, and 40–44; educational attainment, categorized as less than high school (<12th grade), completed high school (12th grade; reference level), and completed college (≥16 years of education); and marital status. We adjusted for child male sex, daily computer use at home, and age at which the blood lead screen occurred to control for age-dependent effects of lead exposure.
Table 1.
Summary statistics of North Carolina fourth graders from 2008–2010 linked to birth certificate data and blood lead screening records, by race/ethnicity
| NHB (n=24,197) N (%)a |
NHW (n=40,954) N (%)a |
p-valueb | |
|---|---|---|---|
| Child characteristics | |||
| Reading EOG test score, mean (SD) | 341.3 (8.5) | 347.9 (9.1) | <0.0001 |
| Math EOG test score, mean (SD) | 346.7 (7.8) | 353.1 (8.4) | <0.0001 |
| Gestational age, weeks, mean (SD) | 38.6 (2.3) | 39.9 (1.9) | <0.0001 |
| Birthweight percentile for gestational age, mean (SD) | 39.2 (27.4) | 51.4 (28.6) | <0.0001 |
| Blood lead test result (μg/dL), mean (SD) | 4.60 (3.15) | 3.65 (2.50) | <0.0001 |
| Blood lead test result (μg/dL) | |||
| 1 | 1,387 (5.73) | 4867 (11.9) | <0.0001 |
| 2-4 | 12,859 (53.1) | 25,648 (62.6) | |
| 5-9 | 8,954 (37.0) | 9,692 (23.7) | |
| ≥10 | 997 (4.12) | 747 (1.82) | |
| Age at time of lead testing (years) | 1.88 (1.22) | 1.68 (1.21) | <0.0001 |
| Male sex | 12,075 (50.0) | 21,016 (51.3) | 0.02 |
| Computer use (1=daily use) | 2,060 (8.51) | 1,553 (3.79) | <0.0001 |
| Economically disadvantaged | 19,434 (80.3) | 16,314 (39.8) | <0.0001 |
| Maternal characteristicsc | |||
| Reported smoking during pregnancy | 2,705 (11.2) | 8,993 (22.0) | <0.0001 |
| Age at time of child’s birth (years) | |||
| 15-19 | 6,170 (25.5) | 5,779 (14.1) | <0.0001 |
| 20-24 | 8,788 (36.3) | 10,898 (26.6) | |
| 25-29 | 4,935 (20.4) | 12,066 (29.5) | |
| 30-34 | 2,821 (11.7) | 8,284 (20.2) | |
| 35-39 | 1,264 (5.22) | 3,403 (8.31) | |
| 40-44 | 219 (0.91) | 524 (1.28) | |
| Educational attainment | |||
| Less than high school | 6,911 (28.6) | 8,254 (20.2) | <0.0001 |
| High school diploma | 15,382 (63.6) | 24,037 (59.7) | |
| College diploma or higher | 1,904 (7.87) | 8,663 (21.2) | |
| Unmarried at time of birth | 18,014 (74.4) | 10,110 (24.7) | <0.0001 |
| Neighborhood characteristics | |||
| Mean racial isolation (Census 2000) | 0.43 (0.23 | 0.16 (0.13) | <0.0001 |
| Racial isolation percentile based on block group of residence at time of birth (Census 2000) | |||
| ≤20th percentile (lowest RI) | 477 (1.97) | 12,559 (30.7) | <0.0001 |
| 21-40th | 2,014 (8.32) | 11,020 (26.9) | |
| 41-60th | 3,885 (16.1) | 9,153 (22.3) | |
| 61-80th | 6,819 (28.2) | 6,200 (15.1) | |
| >80th (highest RI) | 11,002 (45.5) | 2,022 (4.94) | |
| Median household income ($) | 30,720 (12,403) | 40,787 (13,539) | <0.0001 |
The cell count and percent are presented except in the case of continuous test scores, blood lead test results, and birth outcomes, where the mean and standard deviation are given as indicated next to the variable name.
Chisquare test was used to test for differences by race group for categorical variables. ANOVA was used for continuous EOG test scores which were approximately normally distributed. For other continuous variables, the Kruskal–Wallis test was used to test for differences by race group.
Maternal variables are based on reported maternal characteristics at time of the child’s birth.
Additionally, we included median household income at the block group level as a measure of neighborhood-level SES. Individuals from higher-poverty neighborhoods fare worse than those residing in low poverty neighborhoods with respect to a range of outcomes relating to health (47), education (48), and SES, with recent research providing strong evidence of causal effects of childhood neighborhood poverty levels and income as an adult (49). School and school district ID were included as random effects to account for potential unobserved heterogeneity in school and district-level quality and socioeconomic context.
To investigate whether the relationship between EOG test score and birthweight percentile for gestational age differed by blood lead level, we considered a model specification that included an interaction term between birthweight percentile for gestational age and blood lead level. We also considered a second specification that included an interaction term between blood lead level and neighborhood RI. All analyses were conducted in R, version 3.6.2 (50).
RESULTS
Sample characteristics
The mean birthweight percentile for gestational age was 51 (sd=29) for NHW children compared with 39 (sd=27) for NHB children (Table 1). The mean blood lead level among NHB children (4.60 μg/dL) was higher than that for NHW children (3.66 μg/dL), and 41% of NHB children had blood lead levels ≥ 5 μg/dL, compared to 25% of NHW children. Among NHB children, 45% lived in the highest RI quintile (neighborhoods that are mostly NHB), compared to 5% of NHW children; 2% of NHB children lived in the lowest RI quintile (neighborhoods that are mostly non-NHB), compared to 31% of NHW children. Compared with NHW children, NHB children had lower mean scores in reading (difference ~ 6.6) and mathematics (difference ~ 6.4). The interquartile range for reading scores was 12 points for both NHB and NHW; for math, the interquartile range was 11 points for NHW children and 10 for NHB children.
Distribution of exposures
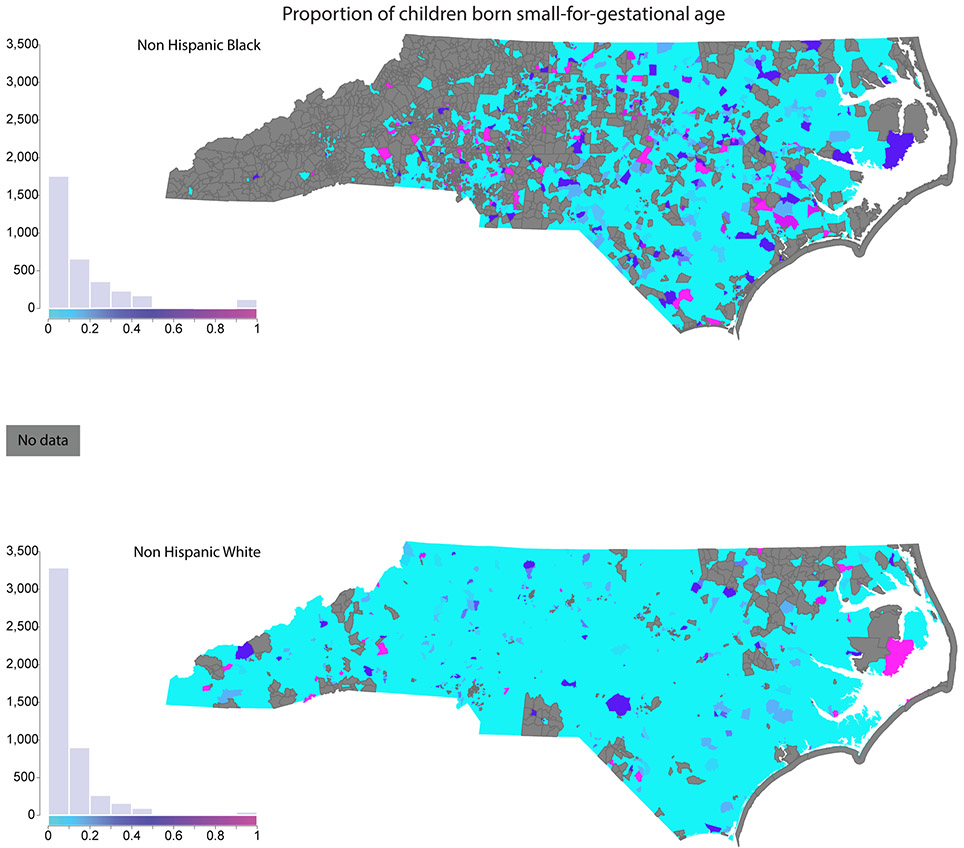
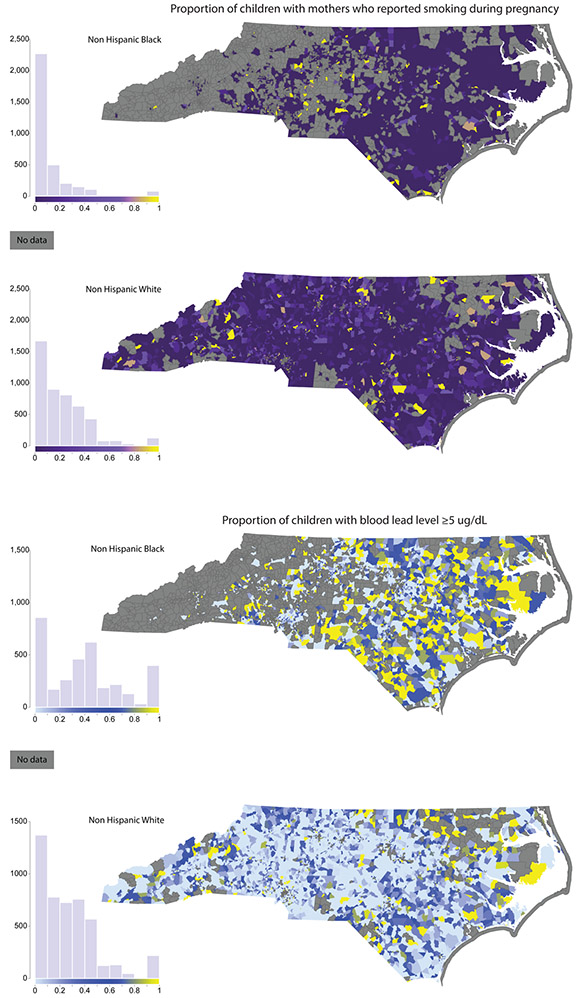
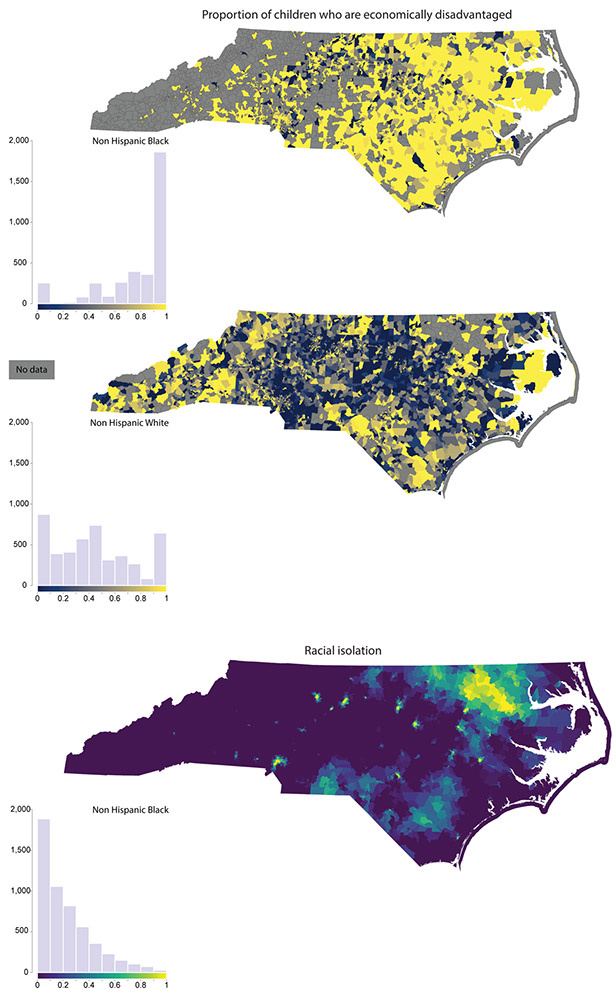
To provide a sense of the geographic distribution of key exposures, we map host, environmental, and social exposures for NHW and NHB children at the Census block group level. Figure 2 shows the proportion of infants that were born small for gestational age (<10th percentile birthweight for gestational age); Figure 3 maps the proportion of mothers who reported smoking during pregnancy (Figure 3, Panel A) and the proportion of children with blood lead levels ≥ 5 μg/dL (Figure 3, Panel B); and Figure 4 maps the proportion of children who are economically disadvantaged (Figure 4, Panel A) and RI of NHB (Figure 4, Panel B). Each figure includes a histogram showing the distribution of the variable in the NHB and NHW study population, as well.
Figure 2.
Host exposure: Proportion of children born small-for-gestational age (<10th percentile of birthweight percentile for gestational age). The small for gestational age variable, which is derived from the detailed birth records, is shown based on the child’s block group of residence at time of birth.
Figure 3.
Environmental exposures: Proportion of children with mothers who reported smoking during pregnancy (Panel A); and proportion of children with blood lead level ≥5 μg/dL (Panel B). The maternal smoking during pregnancy variable, which is derived from the detailed birth records, is shown based on the child’s block group of residence at time of birth. The blood lead level variable, obtained from the blood lead screening data, is shown based on the child’s residence at time of lead screening. Approximately 10% of children in the final analysis dataset were missing residential address at time of lead screening. These children are not included in the proportion of children with lead levels ≥5 μg/dL.
Figure 4.
Social exposures: proportion of children who are economically disadvantaged (Panel A) and neighborhood racial isolation of NHB (Panel B). The economic disadvantage variable, obtained from the EOG dataset, is shown based on the child’s block group of residence in 4th grade, at time of testing. Approximately 10% of children in the final analysis dataset were missing addresses at time of EOG testing. These children are not included in the proportion calculated for the economic disadvantage map. RI of NHB values are shown for all block groups with non-zero population in the neighborhood environment (i.e., the index block group and adjacent block groups).
Multiple exposures
Using individual-level information on key exposures, we identified the most common exposure combinations, considering individual-level economic disadvantage (indicated by receipt of free or reduced price lunch), maternal smoking during pregnancy, small-for-gestational age at birth (≤10th percentile birthweight for gestational age), and a blood lead level ≥ 5 μg/dL, the CDC reference threshold (51) (Table S2). For both NHB and NHW, the most common single exposure was economic disadvantage (80.3% and 39.8%, respectively), followed by elevated lead exposure (41.1% and 25.5% with blood lead levels ≥ 5 μg/dL, respectively). The most common exposure combinations were economic disadvantage and elevated lead levels, a combination experienced by 35.0% of NHB children and 12.9% of NHW children.
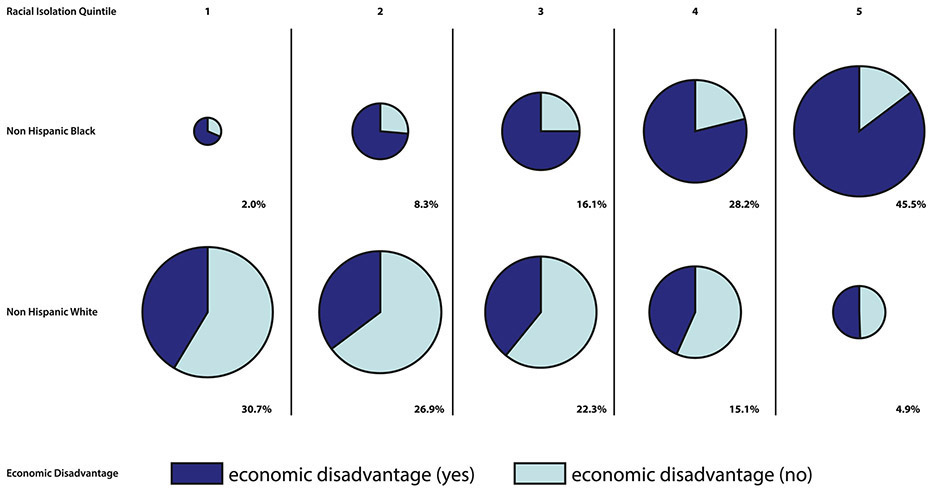
In contrast to other exposures, RI of NHB is measured at the block group (neighborhood) level, rather than the individual level, and does not have a specific threshold value to distinguish between “exposed” versus “unexposed” children. However, key individual-level adverse exposures were often more common in children residing in block groups belonging to the highest RI quintiles. To illustrate, the proportion of the study population experiencing economic disadvantage is shown by RI quintile for NHB and NHW children in Figure 5. The size of each “pie” in Figure 5 is proportional to the percentage of the NHB population (top row) and NHW population (bottom row) residing in each RI quintile.
Figure 5.
Individual-level economic disadvantage by race and neighborhood (block group) RI quintile
Very few NHB reside in the lowest RI quintile (RI quintile 1 – low racial isolation of NHB), while nearly one third of NHW children reside in this RI quintile. The majority of NHB children in this quintile experience economic disadvantage, which is not true of NHW children. Moving across the pie charts from left to right in the first row (corresponding to NHB children), a growing slice of each pie belongs to NHB children experiencing economic disadvantage. Nearly three-quarters (73.7%) of NHB children reside in the highest two RI quintiles; of those residing in these quintiles, the majority (82.6%) experience economic disadvantage. By comparison, 20.0% of NHW children reside in the highest two RI quintiles, and of these children, fewer than half experience economic disadvantage (44.9%).
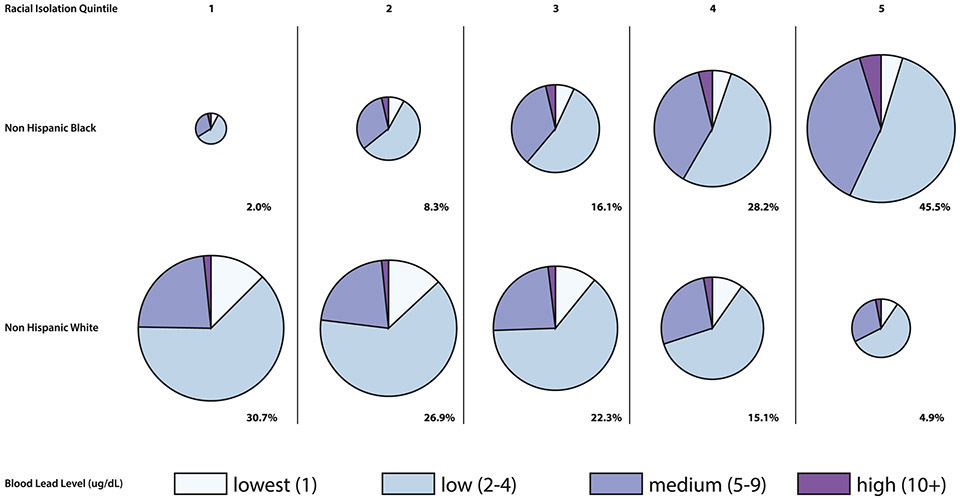
In Figure 6, the size of each pie is proportional to the percentage of the population residing in each RI quintile. Examining the first row, NHB children in the highest two RI quintiles are more likely to have elevated blood lead test results (e.g., ≥5 μg/dL) compared to children residing in the lowest two RI quintiles (42.4% vs. 35.3%, respectively). Similarly, NHW children in the highest two RI quintiles are also more likely to have elevated blood lead levels compared to NHW children in the lowest two RI quintiles (30.4% vs 23.8%, respectively). However, in any given RI quintile, NHW children are less likely to have elevated blood lead levels than NHB children in the same quintile. Overall, 31.3% of NHB children in the study sample reside in the two highest RI quintiles and have lead levels ≥ 5 μg/dL, compared to 6.1% of NHW children.
Figure 6.
Individual-level blood lead level by race and neighborhood (block group) RI quintile
Statistical Analysis
Box plots of reading and mathematics EOG scores versus blood lead level suggest a negative relationship between EOG scores and blood lead levels among both NHB and NHW (SM, Figure S1). Plots of reading and math EOG scores versus birthweight percentile are suggestive of a positive relationship between EOG scores and birthweight percentile (SM, Figure S2).
Reading and math scores among children born to mothers who smoked during pregnancy suggest a negative relationship between prenatal smoking and EOG scores. Mean reading and math scores were 3.6 and 3.3 points lower, respectively, among children born to NHW mothers who reported smoking during pregnancy compared to children born to NHW mothers who did not report smoking during pregnancy (Table 2). Among children born to NHB mothers who reported smoking during pregnancy, reading and math scores were 1.5 and 1.4 points lower, respectively, than scores of children born to mothers who did not report smoking during pregnancy.
Table 2.
Average reading and mathematics End-of-Grade test scores in children of mothers that reported smoking during pregnancy
| Reading EOG scores Mean (standard deviation) |
Mathematics EOG scores Mean (standard deviation) |
|||
|---|---|---|---|---|
| NHB mothers | NHW mothers | NHB mothers | NHW mothers | |
| Smokers | 339.9 (8.4) | 345.1 (8.8) | 345.4 (7.5) | 350.5 (8.0) |
| Non-smokers | 341.4 (8.5) | 348.7 (9.0) | 346.8 (7.8) | 353.8 (8.3) |
Results from four statistical models (test scores in 2 subjects × 2 race groups) with blood lead level as a categorical variable (1, 2-4, 5-9, ≥10 μg/dL) are presented in Tables 3 and 4. Results from equivalent models in which blood lead level was represented continuously are provided in the SM, Tables S3 and S4.
Table 3.
Reading EOG scores among 4th grade children
| NHB | NHW | |
|---|---|---|
| Intercept | 347.4 (346.4, 348.3) | 349.9 (349.5, 350.4) |
| Child characteristics | ||
| Birthweight percentile for gestational age | 0.25 (0.14, 0.35) | 0.22 (0.14, 0.31) |
| Blood lead test result (μg/dL) | ||
| 1 | Reference | Reference |
| 2-4 | −0.57 (−1.02, −0.12) | −0.55 (−0.81, −0.30) |
| 5-9 | −1.08 (−1.54, −0.62) | −0.79 (−1.08, −0.50) |
| ≥10 | −1.82 (−2.49, −1.16) | −1.38 (−2.02, −0.74) |
| Age at time of lead testing (linear, continuous term) | −0.16 (−0.24, −0.073) | −0.16 (−0.23, −0.091) |
| Male sex (1=male) | −1.71 (−1.92, −1.51) | −0.84 (−1.00, −0.68) |
| Computer use (1=daily) | −3.07 (−3.43, −2.70) | −2.59 (−3.02, −2.18) |
| Economically disadvantaged (1= yes) | −2.23 (−2.52, −1.94) | −2.61 (−2.80, −2.41) |
| Maternal characteristics | ||
| Reported smoking during pregnancy (1=smoker) | −0.24 (−0.57, 0.10) | −0.63 (−0.85, −0.42) |
| Age at birth (years) | ||
| 15-19 | 1.07 (0.72, 1.42) | 0.72 (0.41, 1.03) |
| 20-24 | 0.11 (−0.18, 0.40) | −0.41 (−0.64, −0.19) |
| 25-29 | Reference | Reference |
| 30-34 | 0.39 (0.018, 0.77) | 0.54 (0.30, 0.77) |
| 35-39 | 0.58 (0.079, 1.08) | 0.93 (0.61, 1.25) |
| 40-44 | −0.33 (−1.43, 0.76) | 0.83 (0.10, 1.55) |
| Educational attainment | ||
| Less than high school | −1.93 (−2.20, −1.66) | −2.44 (−2.69, −2.20) |
| High school diploma | Reference | Reference |
| College diploma or higher | 2.85 (2.43, 3.27) | 4.09 (3.86, 4.32) |
| Unmarried at time of birth (1=unmarried) | −0.84 (−1.12, −0.57) | −0.49 (−0.71, −0.27) |
| Neighborhood characteristics | ||
| Racial isolation in block group of residence at time of birth (Census 2000) | ||
| ≤20th percentile (lowest RI) | Reference | Reference |
| 21-40th | −1.10 (−1.91, −0.28) | 0.19 (−0.045, 0.42) |
| 41-60th | −1.07 (−1.86, −0.28) | 0.22 (−0.051, 0.49) |
| 61-80th | −1.15 (−1.93, −0.36) | −0.12 (−0.43, 0.19) |
| >80th (highest RI) | −1.54 (−2.34, −0.74) | 0.12 (−0.33, 0.56) |
| Median household income (Census 2000) | 0.42 (0.28, 0.57) | 0.30 (0.19, 0.40) |
The interquartile range for reading scores was 12 for both NHB and NHW.
Table 4.
Mathematics EOG scores among 4th grade children
| NHB | NHW | |
|---|---|---|
| Intercept | 351.0 (350.1, 351.9) | 353.9 (353.4, 354.4) |
| Child characteristics | ||
| Birthweight percentile for gestational age | 0.37 (0.27, 0.47) | 0.35 (0.27, 0.42) |
| Blood lead test result (μg/dL) | ||
| 1 | Reference | Reference |
| 2-4 | −0.30 (−0.71, 0.12) | −0.39 (−0.62, −0.15) |
| 5-9 | −0.82 (−1.25, −0.40) | −0.61 (−0.87, −0.34) |
| ≥10 | −1.05 (−1.66, −0.44) | −1.13 (−1.71, −0.54) |
| Age at time of lead testing (linear, continuous term) | −0.098 (−0.18, −0.019) | −0.13 (−0.19, −0.070) |
| Male sex (1=male) | −0.51 (−0.70, −0.32) | 0.46 (0.32, 0.61) |
| Computer use (1=daily) | −2.41 (−2.75, −2.01) | −1.91 (−2.29, −1.53) |
| Economically disadvantaged (1= yes) | −1.77 (−2.03, −1.50) | −2.41 (−2.59, −2.23) |
| Maternal characteristics | ||
| Reported smoking during pregnancy (1=smoker) | −0.24 (−0.55, 0.075) | −0.47 (−0.67, −0.28) |
| Age at birth (years) | ||
| 15-19 | 1.01 (0.68, 1.33) | 0.62 (0.34, 0.90) |
| 20-24 | 0.18 (−0.09, 0.44) | −0.32 (−0.52, −0.11) |
| 25-29 | Reference | Reference |
| 30-34 | 0.31 (−0.035, 0.66) | 0.40 (0.18, 0.61) |
| 35-39 | 0.22 (−0.25, 0.68) | 0.59 (0.30, .88) |
| 40-44 | 0.031 (−0.98, 1.04) | 0.50 (−0.16, 1.16) |
| Educational attainment | ||
| Less than high school | −1.879 (−2.04, −1.54) | −2.28 (−2.50, −2.06) |
| High school diploma | Reference | Reference |
| College diploma or higher | 2.80 (2.44, 3.32) | 4.03 (3.81, 4.24) |
| Unmarried at time of birth (1=unmarried) | −0.78 (−1.03, −0.52) | −0.69 (−0.89, −0.49) |
| Neighborhood characteristics | ||
| Racial isolation in block group of residence at time of birth (Census 2000) | ||
| ≤20th percentile (lowest RI) | Reference | Reference |
| 21-40th | −0.67 (−1.42, 0.87) | 0.29 (0.077, 0.51) |
| 41-60th | −0.77 (−1.50, −0.03) | 0.40 (0.15, 0.65) |
| 61-80th | −0.91 (−1. 64, −0.17) | 0.14 (−0.15, 0.43) |
| >80th (highest RI) | −1.12 (−1.87, −0.38) | 0.074 (−0.33, 0.48) |
| Median household income (Census 2000) | 0.39 (0.27, 0.54) | 0.37 (0.28, 0.47) |
The interquartile range for math scores was 11 and 10 for NHW and NHB children, respectively.
Reading scores
Birthweight percentile for gestational age was positively associated with reading scores among both NHW and NHB, indicating that children who weighed less at birth, controlling for length of pregnancy (including infants born within the term range, ≥37 weeks), had lower test scores compared to their higher percentile counterparts born at the same gestational age. A one standard deviation increase in birthweight percentile (sd=29 for NHW and sd=27 for NHB) was associated with an increase of 0.22 (95% confidence interval: 0.14, 0.31) and 0.25 (0.14, 0.35) points in reading scores among NHW and NHB, respectively (Table 3).
Higher lead levels were associated with worse reading test scores among both NHB and NHW, and decrements in reading scores associated with higher lead levels were larger for NHB than NHW. Specifically, NHW and NHB children with blood lead levels of 2-4 μg/dL had reading scores that were 0.55 (0.30, 0.81) and 0.57 (0.12, 1.02) points lower, respectively, compared to the reference group (blood lead level =1 μg/dL). For NHW and NHB children with blood lead levels of 5-9 μg/dL, reading scores were 0.79 (0.50, 1.08) and 1.08 (0.62, 1.54) points lower. For context, the 1.08 decrement in reading scores represents 9% of the interquartile range of 12. NHW and NHB children with blood lead levels ≥10 μg/dL had reading scores that were 1.38 (0.74, 2.02) and 1.82 (1.16, 2.49) points lower, respectively, compared to the reference group. Over 40% of NHB children and approximately 25% of NHW children had blood lead levels ≥5 μg/dL.
Maternal smoking was associated with lower reading test scores among NHW, but not NHB. Specifically, NHW children whose mothers smoked during pregnancy had reading scores that were 0.63 (0.42, 0.85) points lower compared to those whose mothers did not smoke. The association between maternal smoking and reading test scores was not significant among NHB, perhaps related to the lower rates of smoking during pregnancy among NHB in this study population.
Among NHW, neighborhood RI was not associated with reading scores, which should not surprise us as RI is defined here relative to NHB. Among NHB, children in higher RI quintiles had larger decrements in reading scores, and the magnitude of the coefficients increases in a notable fashion. For example, NHB children born in a block group belonging to the highest RI quintile would have reading scores approximately 1.54 (0.74, 2.34) points lower than a child born in a block group belonging to the lowest RI quintile.
In addition to the results presented in Table 3, we also examined race-stratified adjusted models of reading EOG scores that included an interaction term between birthweight percentile for gestational age and blood lead level, and a second model that include an interaction term between blood lead level and neighborhood RI. Neither of the interaction terms were statistically significant, so we report results from the adjusted model without any interactions.
Mathematics scores
A one standard deviation increase in birthweight percentile for gestational age was associated with an increase of 0.33 (0.25, 0.41) and 0.38 (0.28, 0.48) points in math EOG scores among NHW and NHB, respectively (Table 4).
Higher lead levels were associated with lower math test scores. In NHW children with blood lead levels of 2-4 μg/dL, math scores were 0.39 (0.27, 0.42) points lower compared to NHW children in the reference group (blood lead level =1 μg/dL). Among NHB children with blood lead levels of 2-4 μg/dL, while the coefficient tended negative, math scores were not statistically significantly different from those in the reference group. However, NHW and NHB children with blood lead levels in the range of 5-9 μg/dL had math scores that were 0.61 (0.34, 0.87) and 0.82 (0.40, 1.25) points lower, respectively, compared to the reference group. NHW and NHB children with blood lead levels ≥10 μg/dL had reading scores that were 1.13 (0.54, 1.71) and 1.05 (0.44, 1.66) points lower, respectively, compared to the reference group.
Among NHB, higher levels of RI were generally associated with larger decrements in mathematics scores. Non-Hispanic black children born in the highest quintile of RI had math scores 1.12 (0.38, 1.87) points lower compared to NHB children born in the lowest quintile of RI. For NHB, the relationship between RI and test scores exhibits an impact on test scores that increases with each quintile of racial isolation, with the second quintile trending negative (but not statistically significant) at −0.67 (−1.42, 0.87), the third quintile significant at −0.77 (−1.50, −0.032), the fourth quintile significant and still larger in magnitude at −0.91 (−1.64, −0.17), and the fifth quintile having the largest impact at −1.12 (−1.87, −0.38).
We also examined race-stratified adjusted models of mathematics EOG scores that included an interaction term between birthweight percentile for gestational age and blood lead level, and a second model that include an interaction term between blood lead level and neighborhood RI. Neither interaction term was statistically significant, so we report results from the adjusted model without any interactions.
DISCUSSION
In a 2016 review article on neighborhood environments, Minh et al. cite a process called biological embedding (34), through which “social and environmental experiences in a child's early years are theorized to shape physiological changes that have lifelong protective or detrimental effects on children's learning, behavior, health and wellbeing.” The authors go on to emphasize the potential productiveness of “a research agenda using longitudinal population-based data linkages.”
While we are not positioned to evaluate physiological changes, we do successfully link children longitudinally across three administrative datasets (birth records, lead screening, and educational testing records), which we geographically reference based on children’s home addresses. We link the geographically referenced administrative data to multiple measures of social and environmental exposures via shared geography. The resulting spatial data architecture allows us to examine the concomitant effects of social, environmental, and host factors on educational testing outcomes.
Although the coefficients on the key social and environmental variables may appear small, they are meaningful. The interquartile range (IQR) in reading EOG scores was 12 points for both NHB and NHW; for math, the IQR was 11 and 10 for NHB and NHW, respectively. For reading EOG scores, the coefficients associated with the highest category of lead exposure (blood lead level ≥ 10 μg/dL) were 15% and 12% of the IQR for NHB and NHW, respectively. The economic disadvantage coefficients were 19% and 22% of the IQR for NHB and NHW, respectively, and neighborhood RI was 13% and <1% of the IQR for NHB and NHW, respectively. The coefficients on birthweight percentile for gestational age and maternal smoking were, for both NHB and NHW, ≤5% of the IQR.
We also consider the magnitude of coefficients on the key host, social, and environmental exposures relative to that of maternal educational attainment (comparing children born to mothers without a high school degree versus those who completed high school), widely acknowledged to be an important predictor of academic performance (52). The magnitude of the coefficient for the highest level of lead exposure (NHB: −1.82 [−2.49, −1.16]; NHW: −1.38 [−2.02, −0.74]) was comparable to maternal educational attainment for NHB (−1.93 [−2.20, −1.66]) and over half the size of maternal educational attainment for NHW (−2.44 [−2.69, −2.20]). The coefficients on economic disadvantage, as measured by free/reduced lunch, (NHB: −2.23 [−2.52, −1.94]; NHW: −2.61 [−2.80, −2.41]) were larger than those of maternal educational attainment. Neighborhood RI of NHB in the highest quintile of RI (NHB: −1.54 [−2.34, −0.74]); NHW: 0.12 [−0.33, 0.56]) was comparable to the size of maternal educational attainment for NHB, but <5% of the maternal educational attainment coefficient for NHW. Coefficients on birthweight percentile for gestational age and smoking were about one tenth the size of the maternal educational attainment, with the exception of smoking among NHW (−0.63 [−0.85, −0.42]), which was one quarter the size of maternal educational attainment.
The relevance of multiple exposures may depend, in part, on whether the etiologic mechanisms of exposure are transient or irreversible. That is, it is not known whether the combined effects of host, environmental, and social exposures are additive, or if, for example, being in a low birthweight percentile for gestational age heightens susceptibility to adverse effects of lead exposure. Because the effects of birthweight percentile for gestational age, lead exposure, and neighborhood exposures are persistent (10, 22, 48), the cumulative effect of these combined exposures is of interest. Here, we did not observe evidence of interactions between birthweight percentile for gestational age and blood lead level, or neighborhood RI and blood lead level. Nonetheless, significant effects are observed for multiple host, social, and environmental exposures, and these exposures accrue disproportionately to NHB children.
For example, consider the exposures of economic disadvantage and neighborhood RI, and their associations with reading scores. Approximately 61% of NHB children in the study sample reside in block groups in the highest two RI quintiles and experience economic disadvantage; based on the estimated regression coefficients, this group of children are expected to have decrements in test scores ranging from approximately −3.38 to −3.77 points. By comparison, only 9% of NHW children in the study sample experience this combination of exposures (i.e., residence in block groups belonging to the highest two RI quintiles and economic disadvantage), and the estimated decrement in their test scores is −2.61 points. For lead, 31.3% of NHB children reside in the highest two RI quintiles and have lead levels ≥5 μg/dL, with an estimated decrement in test scores ranging from −2.23 to −3.36 points. This compares with a decrement ranging from −0.79 to −1.38 points for the 6.10% of NHW with comparable exposure levels. Thus, even in the absence of interactive effects between adverse exposures, NHB children experience more deleterious combined exposures, resulting in larger decrements to test scores compared to NHW children.
Although standardized test scores in reading and mathematics are imperfect measures of intelligence or cognitive ability, EOG performance is associated with socioeconomic outcomes in the longer-term (53). Decrements in EOG scores associated with key exposures, such as lower birthweight percentile, higher blood lead level, or higher neighborhood RI may represent a decline of only one or two points, but individually or in combination with one another or other exposures, these decrements may have significant consequences. In particular, EOG performance is used to make decisions about children’s educational progress, as well as access to augmented resources.
For children on the low end of the test score distribution, one or two points may mean missing the cut-off for progression to the next grade. In other work, grade retention is associated with lower high school graduation rates (54), indicating the potential for long-term implications of poor school performance, even in early grades. For children at the high end of the test score distribution, one or two points may determine eligibility for advanced and intellectually gifted programs, which rely heavily on test scores to sort students. To the extent that the adverse exposures cumulate more in poor and minority communities, we are essentially shaping cultural perceptions of what gifted populations look like.
To the best of our knowledge, this is the first study to estimate associations between EOG test scores and RI, a formal measure of RRS. Racial segregation has been associated with health outcomes in children and adults, such as infant (55, 56) and adult mortality (57, 58) and cardiovascular health (59), and represents an understudied but potentially important determinant of disparities in child development. In this study sample, at time of birth, nearly half (45.5%) of NHB children resided in block groups belonging to the highest quintile of RI. A NHB child residing in a block group (at birth) belonging to the highest RI quintile is estimated to have a reading score approximately 1.54 points lower than a comparable child born in a block group belonging to the lowest RI quintile. For comparison, this is similar in magnitude to associations observed for a child born to a NHB mother who did not finish high school compared to a mother with a high school diploma or a child with a blood lead level ≥10 μg/dL compared to a child with a blood lead level = 1 μg/dL.
While formulated relative to NHB, and thus difficult to interpret in the NHW models, our local, spatial measure of RI adds an important contribution to the environmental health literature. Despite numerous policy measures and changing attitudes, on average, minorities in the United States still tend to reside in locally segregated neighborhoods. This work explicitly implicates RI in the educational performance of NHB children. We posit that this results from the accumulation of disadvantages that accrue to segregated neighborhoods. This methodological approach is meant to shift conversation from race, a non-modifiable individual-level attribute, as a driver of outcomes to the experience of minorities in segregated communities (modifiable) as a driver of outcomes. This work thus broadens the conversation on policy interventions to support the full achievement of all children in the United States.
This study has several limitations. We identified associations between exposures and educational outcomes, but we cannot infer causality from these associations. We controlled for RI at time of birth but did not consider associations between EOG scores and neighborhood RI at other points in time (e.g., time of lead screening, EOG testing). However, a study of verbal ability in African American children in Chicago concluded that the effects of neighborhood on verbal ability in children were “not instantaneous, but rather manifested several years later” (48). This finding supports our use of neighborhood RI at birth, years prior to EOG testing. Relatedly, we did not consider how moving between neighborhoods may relate to test scores, either due to residential instability or through the conditions of the different neighborhoods a child lives in. Considering residential instability and neighborhood conditions at other points in the life course is an opportunity for future work. Another limitation relates to the study sample’s representativeness of North Carolina’s population. For example, the study sample excluded children born to mothers with a residential address at the time of the child’s birth that could not be found/matched in a reference address dataset. It is possible that the individuals removed from the analysis may systematically differ with respect to characteristics affecting exposure and/or outcomes (i.e., those without geocodable addresses may be a more transient, lower SES population), which may affect the generalizability of results. As described in the Methods section, the final analysis dataset differed from the initial dataset (NCDBR) with respect to maternal- and child-level demographic characteristics. We only used one lead test result for each child, as opposed to repeated measures over time. While it would be ideal to have multiple measurements of lead for each child, such repeated measures are not available through the state’s blood lead surveillance data, as very few children had multiple lead test results. Repeated measures of lead exposure are typically only available in small studies that follow children over time. Here we attempt to leverage the more population-level data that the state’s surveillance data provides. Moreover, single measures of blood lead levels have been used in previous studies of lead exposure and developmental outcomes (17, 18, 60, 61).
Furthermore, our findings may be biased as a result of unmeasured confounders, i.e., factors that relate to both a child’s performance on tests and the key exposures of interest. We attempted to mitigate potential bias by controlling for maternal- and child-level covariates, such as maternal education and computer usage, and included random effects at the school and school district levels. We did not consider potential for mediation relationships, but consider this a possible avenue for future research. Finally, the prenatal smoking variable obtained from the NCDBR is self-reported and may not accurately represent smoking prevalence during pregnancy. However, maternal smoking during pregnancy is likely to be underreported, which should bias the association between maternal smoking and EOG scores towards the null. It is also possible that the associations for maternal prenatal smoking estimated in this analysis reflect childhood exposure to tobacco smoke (62), particularly if women who reported smoking during pregnancy continue to smoke after giving birth.
This study has important strengths as well. To the best of our knowledge, it is one of the first studies to examine both lead exposure and pregnancy outcomes with respect to standardized test scores and the first study to examine associations between neighborhood RI and standardized test scores. We consider RI as a social exposure, considering it as a possible proxy for the cumulation of social and environmental dis-amenities that accrue in predominantly minority communities.
Consideration of pregnancy outcomes is important, as research has demonstrated that outcomes such as low birthweight are associated with cognitive development (63), but most studies of lead and cognition do not have information about the child’s birth. Such studies are precluded from investigating, for example, whether adverse birth outcomes interact with lead exposure to negatively affect test scores. To the best of our knowledge, only one other study has considered the effect of lead exposure on 3rd grade standardized test scores while adjusting for adverse pregnancy outcomes (61). Evens et al (2015), concluded that lead exposure was associated with decrements in test scores, even after adjustment for individual-level confounders, including birth outcomes. However, this study was based in an exclusively urban, predominantly NHB (64%) and Hispanic (28%) student population in Chicago, Illinois, and the analysis adjusted only for individual-level confounders, despite evidence linking neighborhood conditions – primarily measures of poverty – with childhood health and developmental outcomes (34, 64).
Our study is responsive to a recent call for the use of population-level databases linking data on place and childhood developmental outcomes to advance a research agenda that prioritizes policy-relevant questions about how, where, and for whom neighborhoods are important (34).
The relatively large sample size allowed us to evaluate associations between multiple exposures of interest and EOG test scores, separately among NHB and NHW; such an analysis would not be feasible in a dataset of a few hundred or even a few thousand children. Finally, the study sample represents a relatively diverse population of NHW and NHB children residing in communities with differing degrees of urbanicity, racial/ethnic composition, and wealth.
In this work, we find disparities in exposure. For example, NHW children are more likely to be exposed to maternal smoking compared to NHB children; and NHB children are more likely to be exposed to lead and at higher levels than NHW children. We also find disparities in effect. For example, high maternal educational attainment has a larger association with child testing outcomes for NHW compared to NHB; the impact of lead exposure in the same ranges is associated with a greater decrement in test scores among NHB children compared to NHW children; and statistically significant decrements in test scores are associated with neighborhood RI for NHB children but not NHW children.
Increasingly, scientific evidence indicates that adult health disparities are rooted in adverse exposures sustained in utero and during infancy and early childhood (65). Thus, to fully eradicate health disparities, we must consider not only those adverse exposures that occur in close temporal proximity to the outcome, but also the fundamental causes of disparities. These may include socially and spatially patterned physical, environmental, and socioeconomic exposures occurring at different stages of the life course (66). Although evaluating the health effects of multiple adverse exposures sustained across space and time is a challenge, requiring longitudinal and spatial datasets, improved understanding of the cumulative effect of multiple adverse exposures, particularly those sustained in early life, will inform approaches to achieving health equity and fostering healthy communities.
Supplementary Material
HIGHLIGHTS.
Key exposures accrue disproportionately to non-Hispanic Black (NHB) children
Economic disadvantage in 4th grade was associated with lower 4th grade test scores
Higher blood lead levels were associated with lower 4th grade test scores
Neighborhood racial isolation at birth was associated with lower test scores for NHB children only
Acknowledgements:
We thank Claire Osgood for data management expertise and Joshua Tootoo for preparation of graphics.
Funding:
Research reported in this publication was supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Number R00MD011304 (Dr. Bravo) and by the National Institute of Environmental Health Sciences under Award Number R01ES028819 (Dr. Miranda). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Research reported in this publication was also supported by the Robert Wood Johnson Foundation New Connections Program under Award Number 73828 (Dr. Bravo). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Robert Wood Johnson Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Competing Interests: The authors declare they have no actual or potential competing financial interests.
REFERENCES
- 1.Norman O, Ault CR Jr, Bentz B, Meskimen L. The black–white “achievement gap” as a perennial challenge of urban science education: A sociocultural and historical overview with implications for research and practice. The Journal of Research in Science and Teaching. 2001;38(10):1101–14. [Google Scholar]
- 2.Finn JD, Gerber SB, Boyd-Zaharias J. Small Classes in the Early Grades, Academic Achievement, and Graduating From High School. Journal of Educational Pyschology. 2005;97(2):214–23. [Google Scholar]
- 3.Pampel FC, Krueger PM, Denney JT. Socioeconomic Disparities in Health Behaviors. Annual Reviews in Sociology. 2010;2010(36):349–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miranda ML, Kim D, Reiter J, Overstreet Galeano MA, Maxson P. Environmental contributors to the achievement gap. Neurotoxicology. 2009;30(6):1019–24. doi: S0161-813X(09)00166-1 [pii]; 10.1016/j.neuro.2009.07.012 [doi]; PMCID: PMC2789840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Vital Statistics Data Available Online,. In: Prevention CfDCa, editor. Atlanta, GA: 2006. [Google Scholar]
- 6.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Drake P. Births: Final Data for 2016. National Vital Statistics Reports. 2018;61(1). [PubMed] [Google Scholar]
- 7.Kramer MS, Ananth CV, Platte RW, Joseph KS. US Black vs White disparities in foetal growth: physiological or pathological? . International Journal of Epidemiology. 2006;35(5):1187–95. [DOI] [PubMed] [Google Scholar]
- 8.Hay WW, Catz CS, Grave GD, Yaffe SJ. Workshop summary: fetal growth: its regulation and disorders. . Pediatrics 1997;99(585-591). [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Physical status: the use and interpretation of anthropometry. Geneva, Switzerland: 1995. [Google Scholar]
- 10.Anthopolos R, Edwards SE, Miranda ML. Effects of maternal prenatal smoking and birth outcomes extending into the normal range on academic performance in fourth grade in North Carolina, USA. Paediatric and Perinatal Epidemiology. 2013;27(6):564–74. [DOI] [PubMed] [Google Scholar]
- 11.Kirkegaard I, Obel C, Hedegaard M, Henriksen TB. Gestational age and birth weight in relation to school performance of 10-year-old children: a follow-up study of children born after 32 completed weeks. Pediatrics. 2006;118:1600–6. [DOI] [PubMed] [Google Scholar]
- 12.White BM, Bonilha HS, Ellis C Jr. Racial/Ethnic Differences in Childhood Blood Lead Levels Among Children <72 Months of Age in the United States: a Systematic Review of the Literature. Journal of Racial and Ethnic Health Disparities. 2016;3(1):145–53. [DOI] [PubMed] [Google Scholar]
- 13.Canfield RL, Henderson CR Jr., Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. NEnglJMed. 2003;348(16):1517–26. doi: 10.1056/NEJMoa022848 [doi];348/16/1517 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.B DC. Very low lead exposures and children’s neurodevelopment. . Current Opinion in Pediatrics. 2008;20:172–7. [DOI] [PubMed] [Google Scholar]
- 15.Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, Canfield RL, Dietrich KN, Bornschein R, Greene T, Rothenberg SJ, Needleman HL, Schnaas L, Wasserman G, Graziano J, Roberts R. Low-level environmental lead exposure and children's intellectual function: an international pooled analysis. EnvironHealthPerspect. 2005;113(7):894–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tellez-Rojo MM, Bellinger DC, Arroyo-Quiroz C, Lamadrid-Figueroa H, Mercado-Garcia A, Schnaas-Arrieta L, Wright RO, Hernandez-Avila M, Hu H. Longitudinal associations between blood lead concentrations lower than 10 microg/dL and neurobehavioral development in environmentally exposed children in Mexico City. Pediatrics. 2006;118(2):e323–e30. [DOI] [PubMed] [Google Scholar]
- 17.Miranda ML, Kim D, Galeano MA, Paul CJ, Hull AP, Morgan SP. The relationship between early childhood blood lead levels and performance on end-of-grade tests. EnvironHealthPerspect. 2007;115(8):1242–7. doi: 10.1289/ehp.9994 [doi]; PMCID: PMC1940087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miranda ML, Maxson P, Kim D. Early childhood lead exposure and exceptionality designations for students. IntJChild Health HumDev. 2010;3(1):77–84; PMCID: PMC3082958. [PMC free article] [PubMed] [Google Scholar]
- 19.Bellinger DC, Needleman HL. Intellectual impairment and blood lead levels. New England Journal of Medicine. 2003;349(5):500–2. [DOI] [PubMed] [Google Scholar]
- 20.Canfield RL, Henderson CRJ, Cory-Slechta DA, Cox C, Jusko TA. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. New England Journal of Medicine. 2003;348(16):1517–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Needleman HL, Leviton A, Bellinger D. Lead-associated intellectual deficit. New England Journal of Medicine. 1982;306(6):367. [DOI] [PubMed] [Google Scholar]
- 22.Reuben A, Caspi A, Belsky DW, Broadbent J, Harrington H, Sugden K, Houts RM, Ramrakha S, Poulton R, Moffitt TE. Association of Childhood Blood Lead Levels With Cognitive Function and Socioeconomic Status at Age 38 Years and With IQ Change and Socioeconomic Mobility Between Childhood and Adulthood. Journal of the American Medical Association. 2017;317(12):1244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salihu HM, Sharma PP, Getahun D, Hedayatzadeh M, Peters S, Kirby RS, Alio AP, Gaafer-Ahmed H. Prenatal tobacco use and risk of stillbirth: A case-control and bidirectional case-crossover study. Nicotine and Tobacco Research. 2008;10:159–66. [DOI] [PubMed] [Google Scholar]
- 24.Gilliland FD, Li Y, Peters JM. Effects of maternal smoking during pregnancy and environmental tobacco smoke on asthma and wheezing in children. American Journal of Respiratory and Critical Care Medicine. 2001;163:429–36. [DOI] [PubMed] [Google Scholar]
- 25.Huang L, Wang Y, Zhang L, Zheng Z, Zhu T, Qu Y, Mu D. Maternal Smoking and Attention-Deficit/Hyperactivity Disorder in Offspring: A Meta-analysis. Pediatrics. 2018;141(1). [DOI] [PubMed] [Google Scholar]
- 26.Chen C, Clifford A, Lang L, Anstey KJ. Is exposure to secondhand smoke associated with cognitive parameters of children and adolescents? A systematic literature review. Annals of Epidemiology. 2013;23(10):652–61. [DOI] [PubMed] [Google Scholar]
- 27.DiFranza JR, Aligne CA, Weitzman M. Prenatal and postnatal environmental tobacco smoke exposure and children’s health. Pediatrics. 2004;113:1007–15. [PubMed] [Google Scholar]
- 28.Mortensen EL, Michaelsen KF, Sanders SA, Reinisch JM. A dose-response relationship between maternal smoking during late pregnancy and adult intelligence in male offspring. Paediatric and Perinatal Epidemiology. 2005;19:4–11. [DOI] [PubMed] [Google Scholar]
- 29.Rahu K, Rahu M, Pullman H, Allik J. Effect of birth weight, maternal education and prenatal smoking on offspring intelligence at school age. Early Human Development. 2010;86:493–7. [DOI] [PubMed] [Google Scholar]
- 30.Anthopolos R, Edwards SE, Miranda ML. Effects of maternal prenatal smoking and birth outcomes extending into the normal range on academic performance in fourth grade in North Carolina, USA. Paediatr and Perinatal Epidemiology. 2013;27(6):564–74. [DOI] [PubMed] [Google Scholar]
- 31.Pattien E, Krogstad JM. Black child poverty rate holds steady, even as other groups see declines: Pew Research Center; 2015. [cited 2018 July 8]. Available from: http://www.pewresearch.org/fact-tank/2015/07/14/black-child-poverty-rate-holds-steady-even-as-other-groups-see-declines/. [Google Scholar]
- 32.Duncan GJ, Brooks-Gunn J. Consequences of Growing Up Poor. New York, NY: Russell Sage Foundation; 1997. [Google Scholar]
- 33.Hair NL, Hanson JL, Wolfe BL, Pollak SD. Association of Child Poverty, Brain Development, and Academic Achievement. Journal of the American Medical Association Pediatrics. 2015;169(9):822–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Minh A, Muhajarine N, Janus M, Brownell M, Guhn M. A review of neighborhood effects and early child development: How, where, and for whom, do neighborhoods matter? Health and Place. 2017;46:155–74. [DOI] [PubMed] [Google Scholar]
- 35.Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Reports. 2001;116:404–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson WJ, 1987. . The truly disadvantaged: the inner city, the underclass, and public policy. Chicago, IL: The University of Chicago; 1987. [Google Scholar]
- 37.Charles CZ. The dynamcis of racial residential segregation. Annual Reviews in Sociology. 2003;29:167–207. [Google Scholar]
- 38.Buescher P Smoking in pregnancy in North Carolina. North Carolina Medical Journal 1997;58:356–60. [PubMed] [Google Scholar]
- 39.Buescher PA, Taylor KP, Davis MH, Bowling JM. The quality of the new birth certificate data: a validation study in North Carolina. American journal of public health. 1993;83(8):1163–5. Epub 1993/08/01. PubMed PMID: 8342728; PMCID: PMC1695166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.CDC. Screening Young Children for Lead Poisoning: Guidance for State and Local Public Health Officials. Atlanta, Georgia: Centers for Disease Control and Prevention., 1997. [Google Scholar]
- 41.North Carolina Public Schools. Understanding North Carolina end-of-grade testing. Raleigh, NC: 2004. [Google Scholar]
- 42.North Carolina Public Schools Accountability Services Division. The North Carolina testing program, 2006-2007. Raleigh, NC: 2006. [Google Scholar]
- 43.Anthopolos R, James SA, Gelfand AE, Miranda ML. A spatial measure of neighborhood level racial isolation applied to low birthweight, preterm birth, and birthweight in North Carolina. SpatSpatiotemporalEpidemiol. 2011;2(4):235–46. doi: S1877-5845(11)00023-2 [pii]; 10.1016/j.sste.2011.06.002 [doi]; PMCID: NIH Public Access Policy does not apply. [DOI] [PubMed] [Google Scholar]
- 44.Reardon SF, O'Sullivan D. Measures of spatial segregation. Sociological methodology. 2004;34(1):121–62. [Google Scholar]
- 45.Miranda ML, Messer LC, Kroeger GL. Associations between the quality of the residential built environment and pregnancy outcomes among women in North Carolina. EnvironHealth Perspect. 2012;120(3):471–7. doi: 10.1289/ehp.1103578 [doi]; PMCID: PMC3295337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.North Carolina State Board of Education. Eligibility for free or reduced price meals in the National School Lunch Program announced 2016. [cited 2018 July 8]. Available from: http://www.ncpublicschools.org/newsroom/news/2017-18/20170829-01.
- 47.Diez Roux AV, Mair C. Neighborhoods and health. Annals of the New York Academy of Sciences. 2010;1186:125–45. [DOI] [PubMed] [Google Scholar]
- 48.Sampson R, Sharkey P, Raudenbush SW. Durable Effects of Concentrated Disadvantage on Verbal Ability among African-American Children. Proceedings of the National Academy of Sciences 2008;105:845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chetty R, Hendren N, Katz L. The Effects of Exposure to Better Neighborhoods on Children: New Evidence from the Moving to Opportunity Project. American Economic Review. 2016;106(4). [DOI] [PubMed] [Google Scholar]
- 50.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria: 2019. [Google Scholar]
- 51.CDC. Blood Lead Reference Value Atlanta, GA; 2020. Available from: https://www.cdc.gov/nceh/lead/data/blood-lead-reference-value.htm. [Google Scholar]
- 52.Harding JF, Morris PA, Hughes D. The Relationship between Maternal Education and Children's Academic Outcomes: A Theoretical Framework. Journal of Marriage and Family. 2015;77:60–76. [Google Scholar]
- 53.Currie J, Thomas D. Early test scores, school quality, and SES: Long run effects on wage and employment outcomes. Research in Labor Economics. 2001;20:103–32. [Google Scholar]
- 54.Jimerson SR. Meta-analysis of grade retention research: implications for practice in the 21st century. School Psychology Review. 2001;30:420–37. [Google Scholar]
- 55.Hearst MO, Oakes JM, Johnson PJ. The effect of racial residential segregation on black infant mortality. American Journal of Epidemiology. 2008;168(11):1247–54. [DOI] [PubMed] [Google Scholar]
- 56.Polednak AP. Trends in US urban black infant mortality, by degree of residential segregation. American journal of public health. 1996;86(5):723–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hart KD, Kunitz SJ, Sell RR, Mukamel DB. Metropolitan governance, residential segregation, and mortality among African Americans. American journal of public health. 1998;88(3):434–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jackson SA, Anderson RT, Johnson NJ, Sorlie PD. The Relation of Residential Segregation to All-Cause Mortality: A Study in Black and White. American journal of public health. 2000;90:615–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kershaw KN, Albrecht SS. Racial/ethnic residential segregation and cardiovascular disease risk. Current Cardiovascular Risk Reports. 2015;9(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Delgado CF, Ullery MA, Jordan M, Duclos C, Rajagopalan S, Scott K. Lead Exposure and Developmental Disabilities in Preschool-Aged Children. Journal of Public Health Management and Practice. 2018;24(2):e10–e7. [DOI] [PubMed] [Google Scholar]
- 61.Evens A, Hyryhorczuk D, Lanphear BP, Rankin KM, Lewis DA, Forst L, Rosenberg D. The impact of low-level lead toxicity on school performance among children in the Chicago Public Schools: A population-based retrospective cohort study. Environmental Health. 2015;14(21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yolton K, Dietrich K, Auinger P, Lanphear BP, Hornung R. Exposure to environmental tobacco smoke and cognitive abilities among U.S. children and adolescents. Environmental Health Perspectives. 2005;113:98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aarnoudse-Moens C, Weisglas-Kuperus N, van Goudoeverver B, O. J Meta–analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124:717–28. [DOI] [PubMed] [Google Scholar]
- 64.Morrissey TW, Vinopal KM. Neighborhood Poverty and Children's Academic Skills and Behavior in Early Elementary School. Journal of Marriage and Family 2017;80:182–97. [Google Scholar]
- 65.Shonkoff JP, Garner AS, The Committee on Psychosocial Aspects of Child and Family Health, The Committe on Early Childhood Adoption and Dependent Care, The Section on Developmental and Behavioral Pediatrics. The Lifelong Effects of Early Childhood Adversity and Toxic Stress. Pediatrics. 2012;129(1):e232–e46. doi: doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- 66.Jones NL, Gilman SE, Cheng TL, Drury SS, Hill CV, Geronimous AT. Life Course Approaches to the Causes of Health Disparities. American journal of public health. 2019;109(Suppl 1):S48–S55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.