Abstract
Background
Epstein-Barr virus (EBV) infection is a major cause of malignancy worldwide. Maternal antibody is thought to prevent EBV infection because it is uncommon in early infancy. Maternal HIV infection is associated with an increased incidence of EBV infection in exposed infants, which we hypothesized results from impaired transfer of EBV-neutralizing maternal antibodies.
Methods
Among Ugandan infants followed for EBV acquisition from birth, we measured antibody binding to EBV glycoproteins (gp350, gH/gL) involved in B-cell and epithelial-cell entry, as well as viral neutralization and antibody-dependent cellular cytotoxicity (ADCC) activity in plasma samples prior to infection. These serologic data were analyzed for differences between HIV-exposed uninfected (HEU) and HIV-unexposed (HUU) infants, and for associations with incident infant EBV infection.
Results
HEU infants had significantly higher titers than HUU infants for all EBV-binding and neutralizing antibodies measured (P < .01) but not ADCC activity, which was similar between groups. No antibody measure was associated with a decreased risk of EBV acquisition in the cohort.
Conclusions
Our findings indicate that in this cohort maternal antibody did not protect infants against EBV infection through viral neutralization. The identification of protective nonneutralizing antibody functions would be invaluable for the development of an EBV vaccine.
Keywords: Epstein-Barr virus, maternal antibody, neutralization, protection, primary infection, infant, HIV-exposed uninfected, cytotoxicity, immunoglobulin, herpesvirus
Epstein-Barr virus (EBV) is an oncogenic human herpesvirus that is transmitted through saliva, and infects approximately 95% of the world’s population [1]. EBV is responsible for approximately 200 000 cancers per year, and is the most common cause of infectious mononucleosis, a febrile syndrome responsible for substantial health care costs [2, 3]. As such, a vaccine against EBV is a high-ranking public health priority [3, 4]. Identification of an immune correlate of protection would greatly facilitate efforts to develop an effective vaccine.
It has been widely assumed that a vaccine able to confer sterilizing immunity to EBV would likely do so by inducing natural antibodies to one or more viral envelope proteins [3, 4]. EBV is primarily transmitted via saliva, and is thought to initially infect oral epithelial cells, followed by infection of B cells in the underlying oral lymphoid tissue, and results in the establishment of latency and lifelong infection [5]. Antibodies against the viral envelope glycoprotein gp350 in immune serum account for the majority of in vitro viral neutralizing activity in B cells, while gH/gL is the major target of epithelial-cell neutralizing antibodies [6]. gp350 facilitates attachment of virions to CD21+ and CD35+ cells, while gH/gL is essential for fusion of the host and viral membranes.
A protective role for maternal antibodies has long been assumed, based on observations that EBV infection is typically delayed for the first 6 months after birth, after which maternal antibody levels wane and infants begin to acquire EBV infection at high rates [7–12]. Although there have been animal studies that show the ability of neutralizing antibody to protect against EBV lymphomagenesis or the rhesus lymphocryptovirus orthologue in animal studies, it remains unknown whether neutralizing antibody can protect against human EBV acquisition [3, 4, 13, 14].
To examine the ability of maternal antibodies to protect against EBV infection, we took advantage of a longitudinal birth cohort study in which we characterized the precise timing of acquisition and risk factors for primary EBV infections in Ugandan infants [15]. In that cohort, EBV infection occurred significantly earlier among infants of human immunodeficiency virus (HIV)-infected, compared to HIV-uninfected, women. Previous studies have shown impaired transplacental antibody transfer due to maternal HIV infection [16, 17]. Thus, we hypothesized that maternal antibody can protect against EBV acquisition in infancy, and that HIV-exposed uninfected (HEU) infants become infected earlier than HIV-unexposed (HUU) infants as a result of having lower titers of EBV-specific maternal antibodies. We therefore attempted to determine whether neutralizing activity was correlated with protection against EBV infection.
METHODS
Study Cohort and Data
Biological samples were collected as part of a previously described cohort of 32 mother-infant pairs in Uganda [15]. All study procedures were approved by the relevant human subjects’ protection committees in Kampala, Uganda; Seattle, Washington; and Vancouver, Canada, and all subjects provided informed consent. Oral swab specimens were collected from the mothers and infants followed from birth every week for EBV quantitative polymerase chain reaction (qPCR) testing to determine the infants’ level of exposure and the week of acquisition, as described in [15]. Blood specimens were collected from mothers at the time of delivery, and from infants at 6 weeks of age and every 4 months thereafter for serologic testing. Only those infant samples collected prior to EBV infection were included in the analyses.
Measurement of Antibodies to Major Neutralizing EBV Antigens
Using the luciferase immunoprecipitation system assay, as previously described [6, 18], fusion proteins containing the EBV glycoproteins gp350 or gH/gL linked to Renilla luciferase gene were constructed in the mammalian expression vector pREN. 293-T cells were transfected with the vector, cell lysates were incubated with human sera, immunoprecipitated with protein A/G beads, washed, and coelenterazine substrate was added to detect luciferase activity. Light units (LU) were measured in a luminometer, which correspond to the level of EBV glycoprotein-specific antibodies [19].
B-Cell Neutralization Assay
B-cell neutralization activity was measured using infection of Raji cells (B cells) as described [18]. Plasma was serially diluted in duplicate wells of 96-well round-bottom plates containing 25 µL of complete Roswell Park Memorial Institute medium (cRPMI) in duplicate. A volume of 12.5 µL of B95-8/F virus (diluted to achieve an infection frequency of 1%–5% at the final dilution) was added and incubated at 37°C for 1 hour. cRMPI, 12.5 µL containing 4 × 106 Raji cells/mL, was added to each well and incubated for another hour at 37°C. The cells were then pelleted, washed once with cRPMI, and resuspended in cRMPI. Antibody concentration or serum dilution is reported relative to the final infection volume (50 µL). After 3 days at 37°C, cells were fixed in 2% paraformaldehyde. The percentage of green fluorescent protein (GFP)-positive Raji cells was determined on a BD LSRII cytometer. To account for any false-positive cells due to autofluorescence in the GFP channel, the average % GFP-positive cells in negative control wells (n = 5–10) was subtracted from each well. % neutralization in each well was defined as: (% GFP-positive cells in the positive control wells containing virus alone [n = 5 wells] − % GFP-positive cells in the antibody containing well) / % GFP-positive cells in the positive control wells × 100. The percent neutralization for each well was plotted as a function of the log10 of the monoclonal antibody (mAb) concentration. The neutralization curve was fit using the log (inhibitor) versus response-variable slope (4 parameters) analysis in Prism 7.03 (GraphPad Software).
Epithelial-Cell Neutralization Assay
AGS cells (1.5 × 104 per well) were seeded into a 96-well tissue culture plate. The following day plasma was diluted 1:4 in complete F12 medium in a final volume of 20 µL in a 96-well round-bottom plate followed by the addition of 20 µL of 25 × concentrated epithelial cell-tropic M81virus that expresses a luciferase reporter gene [20, 21] and incubated for 15 minutes in triplicate. Medium was aspirated from the AGS cells and replaced by the antibody-virus mixture and incubated at 37°C. Forty-eight hours later the medium was aspirated and replaced with 100 µL of Steadyglo luciferase reagent (Promega). From each well 75 µL was transferred to an opaque white-bottom 96 well plate and the relative luciferase units (RLU) in each well were determined using a FluoroSkan Ascent luminometer (ThermoFisher). To account for any background luciferase activity, the average RLUs from negative control wells (n = 5–10) were subtracted from each well. Percent neutralization in each plasma-containing well was defined as: [RLUs in the positive control wells containing virus alone (n = 5 wells) − RLUs in the plasma containing well] / RLUs cells in the positive control wells × 100. The percent neutralization for each well was plotted as a function of the log10 mAb concentration. The neutralization curve was fit using the log(inhibitor) versus response-variable slope (4 parameters) and analyzed using GraphPad Prism 6 software.
Antibody -Dependent Cellular Cytotoxicity Assay
Ninety-six–well plates were coated with recombinant EBV gp350 or gH/gL [6, 22] at 400 ng/mL. Serial 8-fold (for gp350 antibody-dependent cellular cytotoxicity [ADCC]) and 4-fold (for gH/gL ADCC) dilutions of sera were added to the wells, incubated for 15 minutes, and 5 × 105 NK-92-CD16 cells/wells were added and incubated for 5 hours at 37°C. NK-92-CD16 cells express human CD16-176V and GFP [23]. The cells were washed with phosphate-buffered saline, stained with allophycocyanin-Cy7–conjugated anti-CD107a antibody for 30 minutes, and fixed with paraformaldehyde. The percentage of NK-92-CD16 expressing CD107a on their surface was analyzed by flow cytometry.
Antibody Subclass Binding
EBV proteins gp350, gH/gL, gp42, and gB, as well as tetanus toxoid, were each conjugated to MagPlex microspheres (beads) of different regions using an antibody coupling kit (Luminex) [24]. Antigen-bead conjugates were blocked, washed, and mixed with serially diluted serum samples. After 1-hour incubation at room temperature, the beads were washed and mixed with secondary antibody conjugated to phycoerythrin. Secondary antibodies used were specific to either IgG1, IgG2, IgG3, IgG4, or IgA. After 1-hour incubation with secondary antibody, the beads were washed and mean fluorescence intensity was measured using a Luminex LX-200 instrument. Background was set as the mean fluorescent index registered with antigen-beads incubated with secondary antibody (no sample). Background reading was subtracted from all experimental sample measurements. All samples were tested in duplicates.
Statistical Analyses
As described [15], the cumulative incidence of primary infection with EBV was calculated using Kaplan-Meier methods. Risk factors for primary infection were assessed by fitting Cox proportional hazards models, which included maternal HIV infection. Antibodies were treated as time-dependent covariates in these models; for antibodies that were measured multiple times for each infant, values were carried forward no longer than 3 months. The proportional hazards assumption was assessed by testing for an interaction between the covariate and log-transformed time. For models in which the proportional hazards assumption was violated, we provided separate estimates for 2 time periods: 0–6 months and >6 months of age. SAS version 9.4 (SAS Institute), JMP, and R statistical software were used to present the data. P values of <.05 were considered statistically significant. Given the exploratory nature of the analyses and the small sample size, hazard ratios for EBV glycoprotein-specific antibody isotypes were not adjusted for multiple comparisons. The values in the Cox model are predicted concentrations of antibody isotypes at a set serum dilution, therefore the hazard ratios are treated for a continuous variable. A post-hoc power analysis, using the Hsieh and Lavori method [25], is presented in the Supplementary Material to show the effect size required given the study sample size. Assumptions for the post-hoc power analysis were that all infants would eventually become infected with EBV and that there is a negative correlation of 50% between EBV infection and any antibody of interest, after adjusting for other covariates.
RESULTS
Study Subjects and Samples
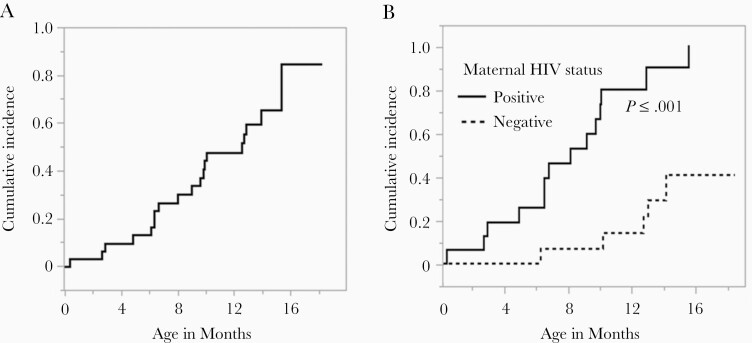
Thirty-two women and their full-term newborn infants were followed, and weekly oral swabs were tested by EBV qPCR to determine the time of infant EBV infection and exposure to viral shedding [15]. Seventeen of the women were HIV infected but none of the infants acquired HIV during the study. CD4+ T-cell counts were available for 9 (53%) of the women at enrollment (median, 441 cells/mm3; range, 385–885 cells/mm3). All women received antiretroviral prophylaxis for prevention of mother-to-child transmission of HIV, in accordance with governmental recommendations at the time (see [15] for additional details). As shown in Figure 1A, the cumulative incidence of infant EBV infection was 12.9% (95% confidence interval [CI], 5.1%–30.9%) at 6 months and 47.4% (95% CI, 31.3%–66.6%) at 12 months. As seen in Figure 1B, while no EBV infections occurred among HUU infants in the first 6 months, HEU infants began acquiring EBV infection within the first month; maternal HIV infection showed a hazard ratio (HR) of 7.2 (95% CI, 2.4–22.2; P < .001) after adjusting for the intensity of shedding exposure (the quantity of EBV detected in saliva of the infants’ household contacts) [15]. Breastfeeding was not associated with the risk of infant EBV infection in models that adjusted for potential confounders [15]. Plasma samples obtained from study infants at 6 weeks of life and every 4 months thereafter, and from mothers at the time of delivery, were used for subsequent EBV-specific antibody assays.
Figure 1.
Cumulative incidence of primary Epstein-Barr virus infection in infants. A, Primary postnatal infections occurring in the first 18 months of life in the infant cohort. B, Stratified data showing infants born to human immunodeficiency virus type 1 (HIV-1)–uninfected women (lower curve) and HIV-1–infected women (upper curve). Kaplan-Meier methodology used to estimate cumulative incidence of infection and log-rank test used to compare curves.
Antibody Binding to Gp350 and gH/gL
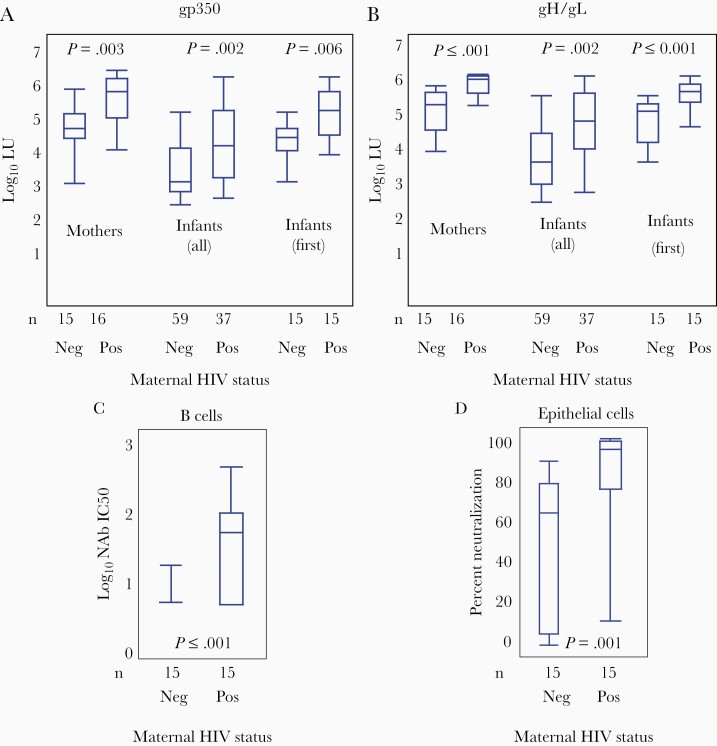
Binding antibodies to the major targets of B-cell and epithelial-cell neutralization (gp350 and gH/gL, respectively) were significantly higher in HEU compared to HUU infants, and in HIV-infected compared to HIV-uninfected mothers (Figure 2). Median log10 antibody levels (measured in LU) to gp350 in HEU infants were 5.1 (interquartile range [IQR], 3.9–5.9) in the first (6-week) infant sample, 4.1 (IQR, 2.7–5.9) in all HEU infant samples, and 5.6 (IQR, 4.0–6.1) in HIV-infected mothers. Median log10 antibody levels to gp350 in HUU infants were 4.3 (IQR, 3.2–5.0) in the first infant sample, 3.2 (IQR, 2.6–5.0) in all HUU infant samples, and 4.6 (IQR, 3.1–5.6) in HIV-uninfected mothers (Figure 2A). Median log10 antibody levels to gH/gL in HEU infants were 5.6 (IQR, 4.6–6.0) in the first infant sample, 4.8 (IQR, 2.9–6.0) in all HEU infant samples, and 5.9 (IQR, 5.2–6.0) in HIV-infected mothers. Median log10 antibody levels to gH/gL in HUU infants were 5.0 (IQR, 3.7–5.4) in the first infant sample, 3.7 (IQR, 2.6–5.4) in all HUU infant samples, and 5.2 (IQR, 4.0–5.7) in HIV-uninfected mothers (Figure 2B). Using Cox regression, even with adjustment for maternal HIV status, there was no evidence of protection by EBV-binding antibodies (Table 1).
Figure 2.
Binding antibody titers against gp350 and gH/gL and neutralization levels of Epstein-Barr virus (EBV) infection in B and epithelial cells. Distribution of log10 light units (LU) for gp350 (A) and gH/gL (B), a measure of the antibody titer, by maternal human immunodeficiency virus (HIV) status, positive (Pos) or negative (Neg). Data are shown for first maternal samples at birth, all infant samples prior to EBV infection, and first infant sample (6 weeks). Neutralizing antibody levels in B cells (C) and epithelial cells (D) in infants based on the HIV status of their mothers. Boxes represent the interquartile range, whiskers represent the minimum and maximum values, and horizontal bars show the median values. Exact 2-sample Wilcoxon test was used to compare both maternal data and data from first sample per infant by maternal serostatus. Generalized estimated equations were used to compare all infant pre-EBV infection samples against gp350 and gH/gL between HIV exposed and unexposed infants. P < .05 was considered significant.
Table 1.
Unadjusted and Adjusted Cox Model Estimates for Risk of Epstein-Barr Virus Acquisition
| Covariate | Unadjusted HR (95% CI) | P Value | Adjusted HRa (95% CI) | P Value |
|---|---|---|---|---|
| gp350 binding antibody per log10 increase | 1.4 (.6–3.2) | .417 | 1.0 (.4–2.5) | .945 |
| gH/gL binding antibody per log10 increase | 2.0 (.7–5.7) | .182 | 1.2 (.4–3.5) | .704 |
| B-cell neutralization IC50 per log10 increase | 3.0 (1.3–7.0) | .012 | 1.2 (.4–3.2) | .734 |
| Epithelial-cell neutralization per log10 increase | 1.02 (1.00–1.04) | .019 | 1.02 (1.00–1.03) | .100 |
| gp350 ADCC CD107a+ % per 1-unit increase | ||||
| 0–6 mo | 0.03 (.0–6.9) | .209 | 0.1 (.0–8.6) | .279 |
| >6 mo | 1.3 (1.1–1.6) | .004 | 1.2 (1.0–1.4) | .086 |
| gH/gL ADCC CD107a+ % per 1-unit increase | ||||
| 0–6 mo | 0.5 (.0–6.6) | .596 | 0.3 (.0–8.2) | .488 |
| >6 mo | 1.9 (.8–4.5) | .158 | 2.4 (.9–6.7) | .096 |
Abbreviations: ADCC, antibody-dependent cellular cytotoxicity; CI, confidence interval; HR, hazard ratio; IC50, 50% inhibitory concentration.
aMultivariate analysis adjusted for maternal HIV status.
Neutralizing Antibodies
Although antibody binding measures to gp350 and gH/gL have been shown to correlate well with neutralizing activity [18, 19], we speculated that perhaps this may not hold true in HIV infection. As such, we assessed neutralizing activity using a functional assay in which antibody-mediated inhibition of infection of either B cells or epithelial cells by a recombinant GPF-EBV is measured by flow cytometry (Figure 2C and 2D). Using exact 2-sample Wilcoxon test, log10 of the 50% inhibitory concentration (IC50) of neutralizing antibody in B cells was significantly higher in HEU infants (1.8; IQR, 0.7–2.6) compared to HUU infants (0.7; IQR, 0.7–1.2; P < .001). Similarly, percent neutralization in epithelial cells was significantly higher in HEU infants (94.4%; IQR, 11.7%–99.5%) than HUU infants (64.4%; IQR, 0%–89.5%; P = .001). Among all infants in the cohort, neutralizing antibody titers were positively correlated with risk of EBV acquisition in univariate analysis, but this association was no longer statistically significant after adjustment for maternal HIV status (Table 1).
Antibody-Dependent Cellular Cytotoxicity
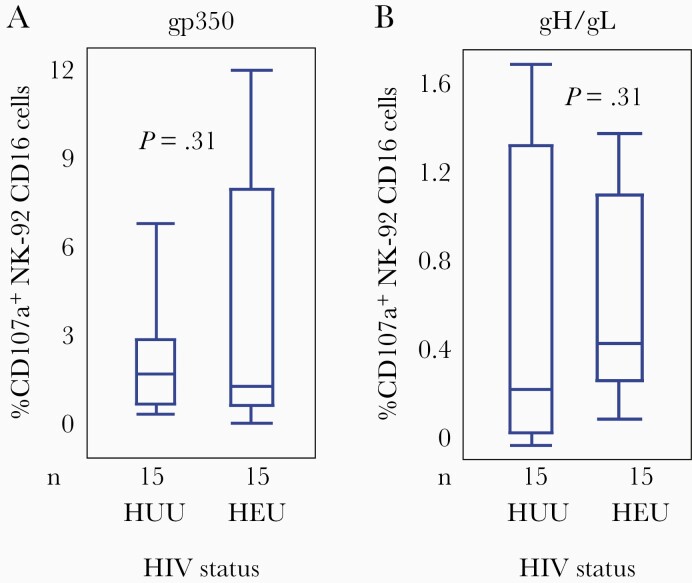
Natural killer (NK) cells mediate classical ADCC. Activation of NK for cytotoxicity results in expression of CD017a on their surface, which is a marker for degranulation of the cells. Levels of NK cell activation from antibody bound to gp350 or gH/gL were not significantly different between HEU and HUU infants, unlike neutralization and binding antibody titers (Figure 3). Median levels of NK cell activation (% CD107a+) by gp350 binding were 1.9% (IQR, 1.2%–12.0%) in HEU infants and 2.3% (IQR, 1.3%–6.5%) in HUU infants (P = .31). Median levels by gH/gL-binding were 0.43% (IQR, 0.11%–1.33%) in HEU infants and 0.29% (IQR, 0.05%–1.70%) in HUU infants (P = .31). Because the proportional hazards assumption was violated for these models, we presented separate estimates for 0–6 month of age and >6 months of age. When adjusted for maternal HIV status, neither gp350 nor gH/gL ADCC levels were associated with the risk of EBV infection in infants, in either time period (Table 1).
Figure 3.
Antigen-dependent cellular cytotoxicity (ADCC) levels against gp350 and gH/gL. The percentage of NK-92 CD16 cells that expressed CD107a on their surface in response to gp350 (A) and gH/gL (B) is shown for human immunodeficiency virus (HIV)-exposed uninfected (HEU) and HIV-unexposed uninfected (HUU) infants. Data are from the first samples obtained 6 weeks after birth, pre–Epstein-Barr virus infection. Boxes represent the interquartile range, whiskers represent the minimum and maximum values, and horizontal bars show the median values. Exact 2-sample Wilcoxon test used for comparing groups. P < .05 was considered significant.
IgG Subclass-Specific Binding to EBV Envelope Glycoproteins
Immunoglobulin isotypes against EBV glycoprotein gp350, gH/gL, gp42, and gB, as well as tetanus toxoid, were measured by the Luminex method in infant blood at 6 weeks of age. As expected [16, 17], levels of IgG subclasses against tetanus toxoid tended to be lower among HEU than HUU infants (Table 2), and levels of IgA, which does not readily cross the placenta, were negligible (data not shown). In contrast, but consistent with other findings from this study, the opposite trend of higher titers among HEU infants was seen for several EBV-specific subclasses of IgG (Table 2). Using Cox regression, higher IgG2 to gH/gL was associated with an increased risk of EBV infection; none of the antibodies measured showed evidence of a protective effect against EBV acquisition.
Table 2.
Unadjusted and Adjusted Cox Model Estimates for Risk of EBV Acquisition Isotype-Specific Binding to EBV Glycoproteins
| Antibody Isotype Specific to EBV Glycoprotein | HEU vs HUU t (P Value) | Unadjusted HR (95% CI) | P Value | Adjusted HRa (95% CI) | P Value |
|---|---|---|---|---|---|
| IgG1 to gp350 | 0.86 (.400) | 1.00 (.99–1.00) | .178 | 1.00 (.99–1.00) | .405 |
| IgG1 to gH/gL | 3.21 (.004) | 1.00 (.99–1.01) | .231 | 1.00 (.99–1.00) | .679 |
| IgG1 to gp42 | 2.25 (.033) | 1.00 (.99–1.00) | .335 | 0.99 (.99–1.00) | .746 |
| IgG1 to gB | 0.08 (.940) | 0.99 (.99–1.00) | .927 | 0.99 (.99–1.00) | .773 |
| IgG1 to TT | −3.26 (.003) | 1.00 (.99–1.00) | .101 | 1.00 (.99–1.00) | .786 |
| IgG2 to gp350 | 2.53 (.023) | 1.01 (1.00–1.02) | .012 | 1.01 (.99–1.01) | .210 |
| IgG2 to gH/gL | −0.73 (.473) | 1.12 (.92–1.37) | .261 | 1.29 (1.03–1.60) | .024 |
| IgG2 to gp42 | 1.03 (.319) | 1.19 (.90–1.58) | .230 | 1.05 (.79–1.39) | .743 |
| IgG2 to gB | 1.57 (.137) | 1.00 (.99–1.01) | .572 | 1.00 (.99–1.01) | .665 |
| IgG2 to TT | −2.82 (.009) | 1.00 (.99–1.00) | .254 | 1.00 (.99–1.00) | .620 |
| IgG3 to gp350 | 1.75 (.100) | 1.01 (.99–1.01) | .077 | 1.00 (.99–1.00) | .367 |
| IgG3 to gH/gL | 2.27 (.036) | 1.05 (.95–1.16) | .354 | 0.98 (.88–1.09) | .679 |
| IgG3 to gp42 | 0.42 (.676) | 0.93 (.77–1.11) | .416 | 0.83 (.67–1.03) | .089 |
| IgG3 to gB | 1.57 (.137) | 1.00 (.99–1.00) | .172 | 1.00 (.99–1.00) | .651 |
| IgG3 to TT | 0.48 (.634) | 1.00 (.99–1.00) | .278 | 1.00 (.99–1.00) | .448 |
| IgG4 to gp350 | 1.72 (.096) | 1.41 (.60–3.3) | .432 | 0.84 (.35–2.01) | .694 |
| IgG4 to gH/gL | 0.57 (.576) | 1.09 (.55–2.18) | .799 | 1.09 (.55–2.17) | .803 |
| IgG4 to gp42 | 0.19 (.847) | 1.00 (.30–3.4) | .990 | 1.94 (.52–7.34) | .325 |
| IgG4 to gB | 0.75 (.460) | 1.25 (.91–1.73) | .170 | 1.27 (.91–1.78) | .155 |
| IgG4 to TT | −1.18 (.249) | 1.00 (.99–1.00) | .617 | 1.00 (.99–1.00) | .467 |
Bold values indicate statistical significance.
Abbreviations: CI, confidence interval; EBV, Epstein-Barr virus; HEU, HIV-exposed uninfected infants; HIV, human immunodeficiency virus; HR, hazard ratio; HUU, HIV-unexposed uninfected infants.
aMultivariate analysis adjusted for maternal HIV status.
DISCUSSION
An effective EBV vaccine is a priority due to its oncogenic burden on millions of children and adults in the developing world. A vaccine would also be able to decrease health care costs in the developed world by eliminating infectious mononucleosis, which has been associated with an increased risk of developing Hodgkin lymphoma and multiple sclerosis [10, 26–28]. In this study, we evaluated a large panel of potential humoral correlates of protection against primary EBV infection in a cohort of Ugandan infants, beginning at birth. Almost all infants in this region are infected with EBV by the age of 3 years [1, 9, 15]. Within this cohort, HEU infants were infected as early as 2 weeks after birth, significantly earlier than HUU infants. Because maternal antibody levels are highest in the first 6 months of infancy, and maternal HIV-1 infection impairs transplacental antibody transfer [7–9, 11, 16, 17], we hypothesized that HEU infants would have lower levels of maternal neutralizing EBV-specific antibodies.
Surprisingly, not only did we find no evidence for protection against EBV acquisition from neutralizing antibodies, but HEU infants had significantly higher titers than HUU infants. Antibody titer, including EBV-specific antibodies, in the mother is generally proportional to the level transferred to infants [29]. Higher antibody titers (including neutralizing antibodies) to herpes group viruses in HIV-infected compared to HIV-uninfected individuals has been reported, and may be a marker of increased viral replication resulting from worse immune control [30, 31]. Pathogen-specific differences in the level and type of antibody that is transferred across the placenta have been described [29, 32, 33]. With the advantage of having the strong perturbation of maternal HIV-1 infection on infant EBV acquisition risk, our results argue that neutralizing maternal antibodies are not strongly protective against EBV infection, rather than simply being unable to detect an association. Incomplete protection by humoral immunity would be consistent with EBV superinfection, which may occur in healthy individuals as with other viruses [34]. It should be noted that due to the small sample size we may not have been able to discern small protective effects, nor would we be able to assess the possible impact of combinations of modestly protective antibodies. However, we estimate reasonable power to detect an antibody measure that conferred >50% protection against EBV acquisition (Supplementary Figure 1). Other limitations of the study include the inability to test for levels of EBV-specific antibodies or other mucosal immune factors in saliva or breast milk, which might be important given the oral route of infection, and would be of interest to include in future studies.
The temporal pattern of EBV infection during infancy, and the effect of earlier acquisition in infants of mothers with HIV or malaria infections, strongly suggests that maternal antibody provides protection [1, 9, 15]. Thus, although the ability of maternal antibody to prevent infant EBV infection has not been formally proven, our findings indicate the potential protective role of nonneutralizing antibody functions. Although, we did not detect a significant association between ADCC activity and risk of EBV acquisition, it is interesting that the levels of cytotoxicity were relatively similar between the HEU and HUU infants, in contrast to neutralizing antibodies. Of note, these assays were limited to only 2 viral antigens, which may not reflect ADCC responses against infected cells or viral particles in vivo. Of specific IgG subtypes binding EBV glycoproteins, one positive correlation between antibody level and EBV infection (IgG2 binding to gH/gL) was observed. This association is tenuous given the small sample size and large number of comparisons; however, it is conceivable that some antibody functions might increase risk of EBV acquisition, as has been noted for HIV-1 [35]. This would again be highly valuable information for EBV vaccine development.
In conclusion, although it is still unclear which maternal antibodies might provide protection against EBV infection during early infancy, our study indicates that neutralizing antibodies did not play a major role. Additional studies are needed to further characterize nonneutralizing functions of maternal antibodies that may be protective, the identification of which would be invaluable for the development of a prophylactic EBV vaccine. Importantly, our findings do not preclude the possibility that a vaccine might be able to protect against EBV infection through induction of highly neutralizing antibodies [14]. Furthermore, a vaccine that is unable to provide sterilizing immunity but that is able to modulate EBV infection to prevent disease might be equally valuable [3, 4].
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by the Canadian Institute of Health Research (grant number RN225081-324003 to S. G.); the National Institute of Allergy and Infectious Diseases (NIAID) (grant number R01AI147846 to J. I. C.); and the Intramural Research Program of the NIAID.
Potential conflicts of interest. C. C. reports grants, personal fees, and nonfinancial support from Janssen Pharmaceuticals; and grants and nonfinancial support from GSK and TempTime Corporation. S. G. reports grants from GSK, Merck, VBI, and Meridian; and consulting fees from GSK, Merck, Moderna, and Curevo. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: 42nd Annual International Herpesvirus Conference, July 2017, Ghent, Belgium; 43rd Annual International Herpesvirus Conference, July 2018, Vancouver, BC, Canada; University of Nairobi STD/AIDS Annual Collaborative Scientific Review, January 2019, Nairobi, Kenya; BC Children’s Hospital Healthy Starts Research Day, February 2019, Vancouver, BC, Canada; Keystone Symposia Molecular Approaches to Vaccines and Immune Monitoring, February 2019, Keystone, CO; and University of British Columbia Pathology Research Day, May 2019, Vancouver, BC, Canada.
References
- 1. de-The G, Day NE, Geser A, et al. Sero-epidemiology of the Epstein-Barr virus: preliminary analysis of an international study—a review. IARC Sci Publ 1975; 11:3–16. [PubMed] [Google Scholar]
- 2. Stratton K, Durch J, Lawrence R. Overview of analytic approach and results. In: Stratton K, Durch J, Lawrence R, eds. Vaccines for the 21st Century. Washington, DC: National Academies Press, 2000. http://www.nap.edu/catalog/5501. Accessed 19 October 2020. [PubMed] [Google Scholar]
- 3. Cohen JI, Fauci AS, Varmus H, Nabel GJ. Epstein-Barr virus: an important vaccine target for cancer prevention. Sci Transl Med 2011; 3:107fs7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cohen JI, Mocarski ES, Raab-Traub N, Corey L, Nabel GJ. The need and challenges for development of an Epstein-Barr virus vaccine. Vaccine 2013; 31:B194–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Odumade OA, Hogquist KA, Balfour HH Jr. Progress and problems in understanding and managing primary Epstein-Barr virus infections. Clin Microbiol Rev 2011; 24:193–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bu W, Joyce MG, Nguyen H, et al. Immunization with components of the viral fusion apparatus elicits antibodies that neutralize Epstein-Barr Virus in B cells and epithelial cells. Immunity 2019; 50:1305–16.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Slyker JA, Casper C, Tapia K, et al. Clinical and virologic manifestations of primary Epstein-Barr Virus (EBV) infection in Kenyan infants born to HIV-infected women. J Infect Dis 2013; 207:1798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Biggar RJ, Henle W, Fleisher G, Böcker J, Lennette ET, Henle G. Primary Epstein-Barr virus infections in African infants. I. Decline of maternal antibodies and time of infection. Int J Cancer 1978; 22:239–43. [DOI] [PubMed] [Google Scholar]
- 9. Piriou E, Asito AS, Sumba PO, et al. Early age at time of primary Epstein-Barr virus infection results in poorly controlled viral infection in infants from Western Kenya: clues to the etiology of endemic Burkitt lymphoma. J Infect Dis 2012; 205:906–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rickinson AB, Fox CP. Epstein–Barr virus and infectious mononucleosis: what students can teach us. J Infect Dis 2012; 207:6–8. [DOI] [PubMed] [Google Scholar]
- 11. Chan KH, Tam JSL, Peiris JSM, Seto WH, Ng MH. Epstein–Barr virus (EBV) infection in infancy. J Clin Virol 2001; 21:57–62. [DOI] [PubMed] [Google Scholar]
- 12. Smith NA, Baresel PC, Jackson CL, et al. Differences in the Epstein-Barr virus gp350 IgA antibody response are associated with increased risk for coinfection with a second strain of Epstein-Barr virus. J Infect Dis 2019; 219:955–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sashihara J, Hoshino Y, Bowman JJ, et al. Soluble rhesus lymphocryptovirus gp350 protects against infection and reduces viral loads in animals that become infected with virus after challenge. PLoS Pathog 2011; 7:e1002308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Swati S, Homad L, Akins NR, et al. Neutralizing antibodies protect against oral transmission of lymphocryptovirus. Cell Rep Med 2020; 1:100033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gantt S, Orem J, Krantz EM, et al. Prospective characterization of the risk factors for transmission and symptoms of primary human herpesvirus infections among Ugandan infants. J Infect Dis 2016; 214:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jones CE, Naidoo S, De Beer C, Esser M, Kampmann B, Hesseling AC. Maternal HIV infection and antibody responses against vaccine-preventable diseases in uninfected infants. JAMA 2011; 305:576–84. [DOI] [PubMed] [Google Scholar]
- 17. Cumberland P, Shulman CE, Chris Maple PA, et al. Maternal HIV infection and placental malaria reduce transplacental antibody transfer and tetanus antibody levels in newborns in Kenya. J Infect Dis 2007; 196:550–7. [DOI] [PubMed] [Google Scholar]
- 18. Sashihara J, Burbelo PD, Savoldo B, Pierson TC, Cohen JI. Human antibody titers to Epstein–Barr virus (EBV) gp350 correlate with neutralization of infectivity better than antibody titers to EBV gp42 using a rapid flow cytometry-based EBV neutralization assay. Virology 2009; 391:249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bu W, Hayes GM, Liu H, et al. Kinetics of Epstein-Barr virus (EBV) neutralizing and virus-specific antibodies after primary infection with EBV. Clin Vaccine Immunol 2016; 23:363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tsai MH, Raykova A, Klinke O, et al. Spontaneous lytic replication and epitheliotropism define an Epstein-Barr virus strain found in carcinomas. Cell Rep 2013; 5:458–70. [DOI] [PubMed] [Google Scholar]
- 21. Bilger A, Plowshay J, Ma S, et al. Leflunomide/teriflunomide inhibit Epstein-Barr virus (EBV)- induced lymphoproliferative disease and lytic viral replication. Oncotarget 2017; 8:44266–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kanekiyo M, Bu W, Joyce MG, et al. Rational design of an Epstein-Barr virus vaccine targeting the receptor-binding Site. Cell 2015; 162:1090–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Binyamin L, Alpaugh RK, Hughes TL, Lutz CT, Campbell KS, Weiner LM. Blocking NK cell inhibitory self-recognition promotes antibody-dependent cellular cytotoxicity in a model of anti-lymphoma therapy. J Immunol 2008; 180:6392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Snijder J, Ortego MS, Weidle C, et al. An antibody targeting the fusion machinery neutralizes dual-tropic infection and defines a site of vulnerability on Epstein-Barr virus. Immunity 2018; 48:799–811.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hsieh FY, Lavori PW. Sample-size calculations for the Cox proportional hazards regression model with nonbinary covariates. Control Clin Trials 2000; 21:552–60. [DOI] [PubMed] [Google Scholar]
- 26. Levin LI, Munger KL, O’Reilly EJ, Falk KI, Ascherio A. Primary infection with the Epstein-Barr virus and risk of multiple sclerosis. Ann Neurol 2010; 67:824–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Handel AE, Williamson AJ, Disanto G, Handunnetthi L, Giovannoni G, Ramagopalan SV. An updated meta-analysis of risk of multiple sclerosis following infectious mononucleosis. PLoS One 2010; 5:e12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hjalgrim H, Askling J, Rostgaard K, et al. Characteristics of Hodgkin’s lymphoma after infectious mononucleosis. N Engl J Med 2003; 349:1324–32. [DOI] [PubMed] [Google Scholar]
- 29. Pou C, Nkulikiyimfura D, Henckel E, et al. The repertoire of maternal anti-viral antibodies in human newborns. Nat Med 2019; 25:591–6. [DOI] [PubMed] [Google Scholar]
- 30. Rahman MA, Kingsley LA, Breinig MK, et al. Enhanced antibody responses to Epstein-Barr virus in HIV-infected homosexual men. J Infect Dis 1989; 159:472–9. [DOI] [PubMed] [Google Scholar]
- 31. Kaye S, Miles D, Antoine P, et al. Virological and immunological correlates of mother-to-child transmission of cytomegalovirus in The Gambia. J Infect Dis 2008; 197:1307–14. [DOI] [PubMed] [Google Scholar]
- 32. Martinez DR, Fong Y, Li SH, et al. Fc characteristics mediate selective placental transfer of IgG in HIV-infected women. Cell 2019; 178:190–201.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jennewein MF, Goldfarb I, Dolatshahi S, et al. Fc glycan-mediated regulation of placental antibody transfer. Cell 2019; 178:202–15.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Walling DM, Brown AL, Etienne W, Keitel WA, Ling PD. Multiple Epstein-Barr virus infections in healthy individuals. J Virol 2003; 77:6546–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim JH, Excler J-L, Michael NL. Lessons from the RV144 Thai phase III HIV-1 vaccine trial and the search for correlates of protection. Annu Rev Med 2015; 66:423–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.