Abstract
Individuals infected with human immunodeficiency virus (HIV) 1 have increased inflammation, which has been associated with age-associated diseases. Plasma markers, cell-associated virus levels, and ability to stimulate RNA transcription in latently infected cell lines was examined in younger and older HIV-1–infected individuals with suppressed virus. Cell-associated RNA, but not intact provirus level, had positive correlation with plasma D-dimer levels. Compared with the younger group, the older group had higher D-dimer levels and a trend toward more cell-associated RNA but similar levels of intact proviruses. Even though all measured inflammatory markers were relatively higher in the older group, this greater inflammation did not induce more HIV-1 transcription in latently infected cell lines. Inflammation and HIV-1 RNA expression increase with age despite similar levels of intact infectious HIV DNA. While plasma inflammation is correlated with HIV-1 RNA expression in peripheral blood mononuclear cells, it does not induce HIV-1 transcription in latently infected cell lines.
Keywords: HIV-1 latency, accelerated aging, cell-associated HIV, inflammation, viral transcription
Plasma inflammation increases with aging in treated people with human immunodeficiency virus (HIV), is associated with intracellular HIV-1 RNA levels but not with the number of intact infectious proviruses, and does not stimulate greater HIV-1 RNA transcription in cell lines.
Although combination antiretroviral therapy (cART) routinely reduces human immunodeficiency virus (HIV) 1 replication below levels detectable by conventional clinical assays, treatment does not eliminate infection. Infection persists in latently infected cells, and virus can reemerge if cART is interrupted [1]. Furthermore, despite the effectiveness of cART in preventing viremia, low-level expression of HIV-1 cell-associated RNA (ca-RNA) remains present [2]. People with HIV (PWH) who are not receiving cART have high levels of systemic immune activation [3]. This inflammation, estimated by means of plasma cytokine/chemokine levels and cellular activation markers, decreases with cART-induced virus suppression, but it does not normalize to preinfection levels [4]. Thus, PWH who have suppressed plasma viremia experience chronic systemic inflammation compared with age-matched HIV-1–uninfected individuals.
Among HIV-1–uninfected individuals, there is a large body of literature supporting the notion that chronic systemic inflammation increases the risk for atherothrombosis, cancer, and other diseases associated with aging [5, 6]. In the current cART era, HIV-associated non-AIDS diseases, such as atherothrombosis, neurocognitive decline, and cancer, account for most of the disease and deaths in PWH with suppressed virus levels [3]. Although this may be expected because PWH are surviving longer and aging, some but not all studies suggest that optimally treated HIV-1–infected individuals have 5–10 years shorter life expectancy and increased medical burden compared with risk-adjusted uninfected counterparts [7, 8]. Similar to findings in HIV-1–uninfected individuals, these noncommunicable HIV-associated non-AIDS diseases have been associated with elevated biomarkers, such as D-dimer, C-reactive protein, soluble CD14 (sCD14), and interleukin 6 [9–12].
There are potentially multiple causes of the persistent chronic inflammation observed in virus-suppressed PWH, including the latently HIV-1–infected cells, other pre-existing coinfections, such as cytomegalovirus, and the irreversible gastrointestinal tract damage that occurs before cART initiation [3]. Previous studies have yielded conflicting conclusions about the impact of the persistently HIV-1–infected cells on systemic inflammation. Some studies, but not all, have shown a correlation between the markers of immune activation and the levels of residual DNA and ca-RNA [13–18].
To date, however, no study has examined the association between markers of inflammation and the number of intact, presumably infectious, HIV-1 proviruses. In PWH with sustained virus suppression, the majority of infected cells contain defective proviruses with deletions or hypermutations [19, 20]. The intact and defective proviruses can yield virus RNA and proteins [21, 22], which potentially allows the host to recognize the provirus harboring cell. This immune response may account for a possible association between chronic inflammation and intact infectious provirus levels. Until recently, polymerase chain reaction (PCR)–based assays that quantified the level of residual HIV-1 DNA measured both intact and mutated sequences. In the current study, we evaluated the association between age, chronic inflammation, and levels of intact proviral DNA and total ca-RNA. We also examined whether plasma with varying levels of inflammation can differentially induce HIV-1 RNA expression in latently infected cells.
MATERIALS AND METHODS
Study Population
We used samples from an existing cohort aimed at examining the interaction between HIV and aging [23]. This cohort prospectively recruited PWH in 2 age-stratified groups, a younger group (aged 18–35 years) and an older group (aged ≥50 years). No participants aged 35–50 years were enrolled. All participants had had HIV-1 infection for an undetermined duration but had been receiving cART for ≥6 months, with plasma HIV-1 RNA levels <50 copies/mL, as assessed by commercial assays. Excluded were those with active hepatitis B or C, ≥5 viral blips since cART initiation, or recent immunomodulatory therapy (oral or injected corticosteroids, plaquenil, azathioprine, methotrexate, biologic therapies, systemic interferon, local interferon, or chemotherapy and receipt of an HIV vaccine). Plasma and peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll-Hypaque centrifugation techniques, aliquoted, and stored at −80°C until future use.
Virologic Assays
Cellular DNA and RNA were obtained from the same PBMC aliquot using the All Prep DNA/RNA mini kit (Qiagen). Total HIV-1 DNA and the number of total cells were quantified as described elsewhere, with minor modifications [24]. PCR amplicons were used as templates for the quantitative standards rather than plasmid DNA. HIV-1 ca-RNA quantification was based on an in vitro transcribed RNA standard and using a 1-step reverse-transcription quantitative PCR reaction with 4X Fast Virus Master Mix (Applied Biosystems). Primer and probe sequences are available on request. All samples were assessed in triplicate, a minimum of 2 independent times. An intact proviral DNA assay was used to estimate the quantity of intact inducible HIV-1 proviruses [20]. Briefly, the HIV-1 psi region and the HIV-1 envelope were targeted with multiplex droplet digital PCR. Double and single positive droplets were deemed as having intact and defective provirus, respectively. Negative droplets contained no DNA or cellular DNA lacking HIV-1. Total cell copies and the level of DNA shearing were estimated by amplifying and quantifying the human RPP30 gene at 2 positions. The total number of intact proviruses was estimated after correcting for the estimated DNA shearing, as detailed elsewhere [20]. Droplet digital PCR was accessed through the Boston University Genome Sciences Institute.
Inflammatory Plasma Assessments
Stored frozen plasma samples were thawed and assayed for tumor necrosis factor (TNF) α, IL–6, sCD14, and soluble CD163 (sCD163) using a bead-based multiplex assay (Milliplex) and for D-dimer and C-reactive protein using an enzyme-linked immunosorbent assay (RD Systems and Abcam) kits, as described elsewhere [25, 26]. Assays were run according to manufacturer’s instructions. Quantification was performed on a Magpix (Luminex) instrument equipped with xPNENT 4.2 software and a luminometer. Values were based on a standard curve, and any reading below the standard curve was assigned the lowest quantifiable value. Plasma measurements were not available for all participants because of limited sample quantities and degradation.
RNA Expression Among Latently Infected Cell Lines
HIV-1 latently infected cell lines (J-Lat 6.3 and ACH-2) were obtained from the National Institutes of Health AIDS Research Reagent and Reference Program. J-Lat 6.3 and ACH-2 are T-cell lines carrying a single defective and infectious HIV-1 proviral copy respectively [27, 28]. These cells were cultured and passaged in Roswell Park Memorial Institute (RPMI) 1640 medium containing 10% fetal bovine serum and supplemented with 100 U/mL penicillin/streptomycin (RPMI 1640 complete medium) [29]. Approximately 105 latently infected cells were incubated in 200 µL of RPMI 1640 complete medium or RPMI 1640 medium supplemented with 10% plasma from PWH and 100 U/mL penicillin/streptomycin in a 96-well plate. After 24 hours, cells were stimulated with either TNF-α (5 ng/mL) or no cytokines. At 24 hours after incubation, RNA and DNA were isolated from the cultured cells. All incubation conditions were examined in duplicate independent wells and pooled for nucleic acid extraction. Although ACH-2 harbor an infectious provirus, HIV-1 replication could not be measured in our assay, because antiretroviral medications were present in all the patient plasma.
Statistical Analysis
All HIV-1 RNA and DNA levels and plasma cytokine concentrations were log10-transformed to yield normalized distributions. Comparisons among groups were done using the following tests: Fisher exact, χ 2, t (normal distributions), and Mann-Whitney (nonparametric distributions). Associations were examined using Pearson and Spearman correlations for normally distributed and nonparametric data, respectively. In the multivariable linear regression analysis, the dependent variable was plasma D-dimer, ca-RNA, or intact proviral DNA levels. Covariates included in the initial model were age, sex, ethnicity/race, pretherapy plasma virus level, nadir and enrollment absolute CD4 T-cell count, years on therapy, and the antiretroviral anchor. Covariates were removed from this multivariable model in a stepwise backward fashion if the P value was >.10, starting with the weakest predictor. All P values are based on 2-sided tests. Benjamini-Hochberg (BH) and Bonferroni corrections were used to account for multiple comparisons. All tests were done using GraphPad Prism 8.2.1 software.
Study Approval
All studies were approved by the Boston University, Brigham and Women’s Hospital, and Massachusetts General Hospital institutional review boards. Written informed consent was received from participants before inclusion in the study.
RESULTS
Study Population
We examined 57 HIV-1 cART-treated and virologically suppressed individuals who were aged 18–35 (n = 23) or ≥50 (n = 34) years (Table 1). Women were part of the older but not the younger group. The younger group included a significantly higher proportion of nonwhite, nonblack participants (Hispanic or other). The younger group had higher pre-cART plasma HIV-1 levels and recorded nadir and enrollment absolute CD4 T-cell counts, although the differences were not statistically significant. The younger group had been receiving cART for a significantly shorter duration. The antiretroviral agent anchoring the cART regimen did not differ among the groups. As expected, more individuals in the older group had pre-existing conditions.
Table 1.
Study Population and Demographic Characteristics by Age Group
| Characteristic | All PWH (n = 57) | Younger PWH (n = 23) | Older PWH (n = 34) |
P Value a |
|---|---|---|---|---|
| Age years, median (range) | 52 (22–76) | 29 (22–35) | 57 (50–76) | … |
| Female sex, no. (%) | 4 (7.0) | 0 (0) | 4 (11.8) | .14 |
| Ethnicity/race, no. (%) | ||||
| White non-Hispanic | 34 (59.6) | 11 (47.8) | 23 (67.6) | .02b |
| Black non-Hispanic | 18 (31.6) | 7 (30.4) | 11 (32.3) | |
| Hispanic or other | 5 (8.8) | 5 (21.7) | 0 (0) | |
| Last pretherapy plasma HIV-1 RNA level, median (range), log10 copies/mL [no. missing data] |
4.72 (1.92–7.82) [n = 6] | 5.00 (3.17–7.82) [n = 2] | 4.64 (1.92–7.66) [n = 4] | .11c |
| CD4 T-cell count, median (range) cells/µL [no. missing data] |
||||
| Nadir count | 282 (9–1028) [n = 1] | 337 (16–1028) [n = 0] | 262 (9–803) [n = 1] |
.20c |
| Count at enrollment |
308.5 (9–1028) [n = 1] | 369 (25–1028) [n = 0] | 301 (9–921) [n = 1] |
.27c |
| Duration of therapy, median (range), y | 3.33 (0.69–22.57) | 2.19 (0.69–6.74) | 4.72 (0.86–22.57) | <.001c |
| Antiretrovirals | ||||
| NNRTI | 38 (67) | 15 (65) | 23 (68) | .92b |
| Protease inhibitor | 8 (14) | 3 (13) | 5 (15) | |
| Integrase inhibitor | 11 (19) | 5 (22) | 6 (18) | |
| Comorbid conditions | ||||
| Coronary artery disease | 3 (5) | 0 (0) | 3 (9) | .27 |
| Hypertension | 11 (19) | 2 (7) | 9 (26) | .17 |
| Diabetes mellitus | 8 (14) | 1 (4) | 7 (21) | .13 |
| Hyperlipidemia | 14 (25) | 1 (4) | 13 (38) | .004 |
| Chronic kidney disease | 4 (7) | 1 (4) | 3 (9) | .64 |
| Pulmonary diseased | 8 (14) | 3 (13) | 5 (15) | .99 |
| Cancer | 6 (11) | 1 (4) | 5 (15) | .38 |
| Gastrointestinale | 10 (18) | 1 (4) | 9 (26) | .04 |
| Neurologicf | 6 (11) | 0 (0) | 6 (18) | .07 |
| Psychiatricg | 20 (35) | 7 (30) | 13 (38) | .58 |
Abbreviations: HIV-1, human immunodeficiency virus type 1; NNRTI, nonnucleoside reverse-transcriptase inhibitor; PWH, people with HIV.
a P value for comparison between age groups, based on Fisher exact test unless otherwise specified.
b P value based on χ 2 test.
c P value based on Mann-Whitney U test.
dPulmonary diseases included chronic obstructive pulmonary disease, asthma, history of pneumonia, and latent tuberculosis.
eGastrointestinal conditions included Barrett esophagus, colonic polyps, diverticulitis, diverticulosis, gastritis, acid reflux, Lynch syndrome, and irritable bowel syndrome.
fNeurologic conditions included history of meningitis, tremor, neuropathy, seizure disorder, Parkinson disease, tardive dyskinesia, and traumatic brain injury.
gPsychiatric conditions included depression, anxiety, posttraumatic stress disorder, bipolar disease, episode of psychosis, and Asperger syndrome.
Correlation of Plasma Inflammation With HIV-1 ca-RNA but Not Intact Proviral DNA Levels
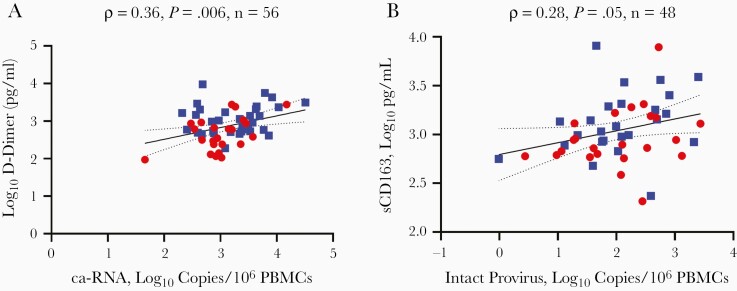
We measured 6 plasma inflammatory markers. Plasma D-dimer levels showed a significant correlation with HIV-1 ca-RNA levels (ρ = 0.36; P = .006) (Figure 1A). This D-dimer association remained statistically significant after adjustment for multiple comparisons (6 independent tests; BH P = .04 or, for the Bonferroni method, any unadjusted P value <.008). Although there were no significant associations with the other plasma markers, there was a statistical trend with sCD14 (ρ = 0.23; P = .09) and sCD163 (ρ = 0.25; P = .09); however, sCD163 data were not available from 9 participants.
Figure 1.
Correlation between virus measurements and plasma inflammatory markers. Association between intracellular human immunodeficiency virus type 1 (HIV-1) cell associated RNA (ca-RNA) and D-dimer (A) and between intact proviral DNA and soluble CD163 (sCD163) (B). Data from younger (gray circles) and older (black squares) participants are shown, along with ρ values, P values for Pearson correlation, and the number of data points. Black and dotted lines represent linear regression and 95% confidence interval, respectively. Abbreviation: PBMCs, peripheral blood mononuclear cells.
Multivariable linear regression analysis was used to account for the baseline demographic differences between the older and younger groups (Table 1). In the final model, ca-RNA level, age, pretherapy log10 plasma HIV-1 copies, and ethnicity/race were predictive of plasma D-dimer levels (Table 2). Specifically, for every 10-fold increase in ca-RNA, there was a 0.23-log10 increase in plasma D-dimer level.
Table 2.
Predictors of Plasma D-Dimer Levels in Multivariable Linear Regression Analysisa
| Predictor | Estimate, β Value (95% CI) |
P Value |
|---|---|---|
| Intercept | .67 (− .24 to 1.58) | .14 |
| Cell-associated RNA | .23 (.02–.43) | .03 |
| Age | .02 (.008–.02) | <.001 |
| pre-cART HIV-1 RNA | .14 (.03–.24) | .01 |
| Race/ethnicity: Black | .21 (−.03 to .45) | .08 |
| Race/ethnicity: Hispanic/other | .41 (.03–.79) | .03 |
Abbreviations: cART, combination antiretroviral therapy; CI, confidence interval; HIV-1, human immunodeficiency virus type 1.
aCovariates included in the initial model were age, sex, ethnicity/race, pretherapy plasma virus level, nadir and enrollment absolute CD4 T-cell count, years of therapy, and antiretroviral anchor. The covariates shown exclude those with a P value >.10.
To our knowledge, no prior study has examined associations with intact proviral DNA levels and markers of inflammation. Intact proviruses may potentially induce greater host immune responses because of low-level spreading infection or higher expression of structurally intact virus proteins. We assayed intact proviral DNA in a median of 96 674 PBMCs (range, 22 469–3 483 606). There was no significant difference between the numbers of cells examined in the older (median, 87 124; range, 22 469–412 421) and younger group (107 645; 29 568–3 483 606) (P = .23) groups. Intact proviral DNA levels in bulk PBMCs (median, 2.1; range, −0.01 to 3.4 log10 per 106 PBMCs) were higher in our cohort than previously documented in bulk PBMCs from another cohort of virus-suppressed PWH [30]. This observed variation could be attributed to differences in the duration of suppressive cART (median, 3.3 vs 9 years) among the cohorts because intact proviruses may decay with prolonged cART [20]. There was a statistical trend in the association between the number of intact proviral DNA and sCD163 (ρ= 0.28; P = .05) (Figure 1B), but this was not adjusted for multiple comparisons. No associations were observed with the other plasma inflammatory markers.
Association of Aging With Inflammation and Nonsignificantly Higher HIV-1 ca-RNA Levels
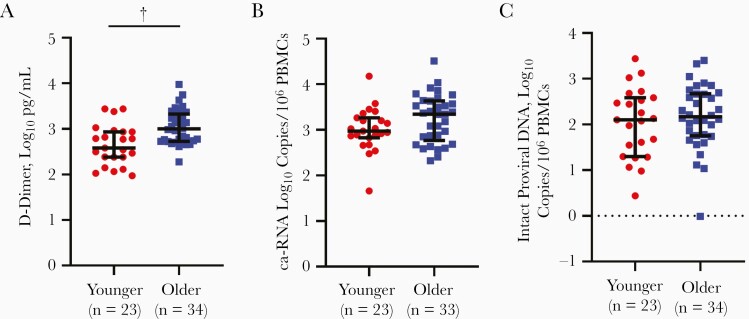
Higher plasma inflammatory markers have been observed with increasing age [31, 32]. The multivariable linear regression analysis also suggested that with every year of age, there was 0.02-log10 increase in plasma D-dimer level (Table 2). The median of all 6 measured plasma inflammatory markers was higher in the older than in the younger group (Table 3), although after correction for multiple comparisons, only the D-dimer difference was statistically significant (unadjusted P < .001; BH P = .004) (Figure 2A).
Table 3.
Plasma Inflammatory Markers in the Younger and Older Groups
| Marker | Median Value (Range) [No. With Data] | P Valuea | ||
|---|---|---|---|---|
| Younger Group | Older Group | Unadjusted | BH | |
| sCD14, log10 ng/mL | 3.02 (2.83–3.43) [n = 23] | 3.14 (2.92–3.64) [n = 34] | .01 | .05 |
| sCD163, log10 ng/mL | 2.90 (2.32–3.90) [n = 23] | 3.08 (2.37–3.91) [n = 25] | .11 | |
| D-dimer, log10 pg/mL | 2.58 (1.97–3.44) [n = 23] | 3.00 (2.28–3.98) [n = 34] | <.001 | .04 |
| CRP, log10 ng/mL | 3.14 (1.97–4.41) [n = 23] | 3.58 (2.56–4.03) [n = 34] | .06 | |
| TNF-α, log10 pg/mL | 1.89 (1.75–2.03) [n = 23] | 1.91 (1.68–2.09) [n =34] | .12 | |
| IL-6, log10 pg/mL | 1.83 (1.66–2.02) [n = 15] | 1.94 (1.62–2.61) [n = 23] | .01 | .05 |
Abbreviations: BH, Benjamini-Hochberg; CRP, C-reactive protein; IL-6, interleukin 6; sCD14, soluble CD14; sCD163, soluble CD163; TNF, tumor necrosis factor.
a P value for comparison between age groups, based on t test, without adjustment for multiple comparisons or with BH correction for multiple comparisons.
Figure 2.
Older people with human immunodeficiency virus (PWH) have higher levels plasma inflammation than younger PWH, as shown by comparison of D-dimer (A), cell-associated RNA (ca-RNA) (B), and intact proviral DNA (C) between younger (gray circles) and older (black squares) groups. Black bars represent medians with interquartile range. †P < .001 (2-sided Student t test ). Numbers of individual data points are noted parenthetically. Abbreviation: PBMCs, peripheral blood mononuclear cells.
We hypothesized that levels of HIV-1 ca-RNA, but not intact DNA, would be higher in the older than in the younger group because ca-RNA but not intact provirus level was associated with plasma inflammation (Figure 1 and Table 2). Indeed, ca-RNA levels were higher in the older than in the younger group, although the difference only showed a statistical trend (P = .08) (Figure 2B). Compared with younger PWH, the older group had longer duration of cART therapy (Table 1), and this prolonged drug exposure may affect ca-RNA levels. In multivariable linear regression analysis, 1 year of older age was associated with 0.007-log10 higher HIV-1 ca-RNA levels (P = .11) after adjustment for duration of cART therapy. The other baseline demographic characteristics did not have a significant association with HIV-1 ca-RNA. Thus, although the difference was not statistically significant, ca-RNA trended higher in older compared with younger PWH.
On the other hand, intact proviral DNA level did not demonstrate a statistically trending difference between the 2 age-stratified groups (Figure 2C). Age (β = .43; P = .67), duration of cART therapy (β = −.31; P = .30), and absolute CD4 T-cell count at enrollment (β = −.0001; P = .74) also did not predict the intact proviral DNA levels in univariate linear regression analyses. There were also no significant associations in multivariable linear regression analysis. Although not statistically significant, the negative correlation aligns with previous observation that intact provirus levels decrease with prolonged duration of cART-mediated virus suppression [20].
Effect of Plasma Inflammation and Aging on HIV-1 RNA Production in Latently Infected Cells
The directionality of the association between systemic inflammation and HIV transcription remains uncertain. Although not mutually exclusive, an inflammatory milieu may drive HIV-1 transcription or, alternatively, intracellular HIV-1 RNA expression may induce inflammation. Our observed association between ca-RNA level and plasma inflammation (Figure 1 and Table 2) does not distinguish between these possibilities. We examined the impact of the plasma inflammatory milieu on HIV-1 transcription to further explore this relationship.
We examined 2 different latently infected cell lines (J-Lat 6.3 and ACH-2) because HIV-1 transcriptional capacity in latent cells varies depending on the integration site and other cell characteristics [30]. Thus, we expected varying results from the 2 different cell lines. Cell lines were incubated in media containing 10% plasma from PWH [27, 28]. A 10% plasma fraction was used because J-Lat 6.3 and ACH-2 cell cultures are routinely done with RPMI 1640 medium containing 10% fetal bovine syndrome. The level of HIV-1 transcription was also measured in the cell lines in the presence or absence of TNF-α, because this cytokine has been previously shown to stimulate HIV-1 RNA transcription [27, 28]. HIV-1 RNA was detectable in both cell lines over a range of TNF-α concentrations, demonstrating that HIV-1 transcription could be induced with minimal cytokine stimulation. (Supplementary Figure 1A and 1B).
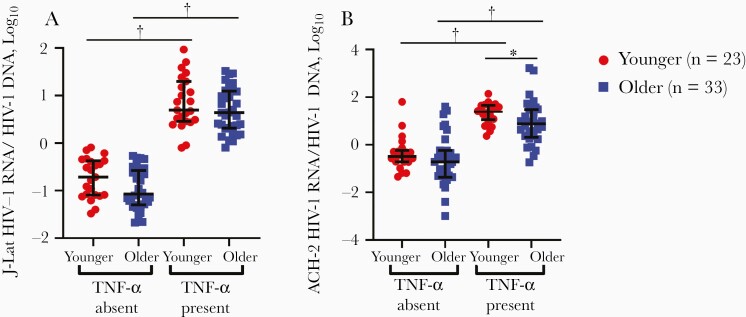
Although the younger versus older group had higher median levels of all 6 measured inflammatory markers than the younger group (Table 3), HIV-1 RNA relative to DNA levels trended higher among JLat cells incubated in plasma from the younger versus the group, but only in the absence of TNF-α (P = .05) and not in its presence (P = .23) (Figure 3A). RNA relative to DNA levels were also higher in ACH-2 cells incubated in plasma from younger versus older PWH, but only in the presence of TNF-α (P = .04), not in its absence (P = .38) (Figure 3B). As a positive control, higher HIV-1 RNA levels were observed with the presence than with the absence of TNF-α in both cell lines conditioned with participant plasma, implying that the patient plasma did not prevent HIV-1 transcription from the latent provirus (Figure 3A and 3B). There was no correlation in the observed transcription induction in the presence of participant plasma among the 2 different cell lines (data not shown). This suggests that the higher pre-existing inflammation present in plasma does not induce greater HIV-1 transcription in these 2 different cell lines.
Figure 3.
Tumor necrosis factor (TNF) α in the presence of plasma stimulates RNA transcription in latently infected cells. Levels of J-Lat (A) and ACH-2 (B) human immunodeficiency virus type 1 (HIV-1) RNA per HIV-1 DNA were measured in the presence or absence of 5-ng/mL TNF-α and plasma from younger (gray circles) or older (black squares) people with HIV. Black bars represent medians with interquartile range. *P < .05; †P < .001.
DISCUSSION
Even with suppressive cART, findings of some but not all studies suggest that PWH have shorter life expectancies than age-matched uninfected individuals [7, 8]. The increased mortality rate among PWH has been attributed to a higher incidence of age-associated morbid conditions that potentially emerge as a result of chronic inflammation [9–12]. The causes of chronic inflammation in individuals with suppressed virus level are likely to be multifactorial, and specific drivers remain uncertain.
In the current study, we showed that inflammation, as determined by plasma markers, is associated with intracellular HIV-1 RNA but not with intact proviral DNA levels. Furthermore, plasma inflammation increases with age in PWH, similar to what has been observed in HIV-1–uninfected individuals [33]. We also found that in vitro HIV-1 RNA transcription in latent cell line models do not increase in the presence of plasma from older individuals, who had higher levels of inflammatory markers. In aggregate, our observations suggest that as PWH age, they have increased systemic inflammation and potentially higher levels of HIV-1 transcription, independent of intact proviral numbers. This age-associated inflammation, however, does not induce HIV-1 RNA transcription in latently infected cell lines. Aging is also not associated with higher levels of infectious virus. Our observations suggest that aging may be associated with greater uncontrolled HIV-1 RNA transcription, and this promotes chronic inflammation in the absence of new rounds of virus replication. Larger cohorts need to be examined to confirm our findings.
Previous studies have also examined the link between cellular or plasma markers of inflammation with HIV-1 intracellular DNA and RNA levels. These investigations have yielded conflicting data, possibly owing to cohort differences, such as the duration of cART and prospective versus cross-sectional study design [13–18]. To our knowledge, previous studies have not examined intact proviral DNA but instead have measured total or integrated DNA and ca-RNA or plasma HIV-1, using ultrasensitive assays. In recent years, it has been shown that levels of intact proviral DNA, as opposed to HIV-1 ca-RNA and total HIV-1 DNA, are better correlated with inducible infectious virus among virus-suppressed PWH [20]. Similar intact provirus levels in younger and older PWH receiving long-term cART implies that there are no age difference in ongoing virus replication. Thus, low-level infectious virus production does not account for the enhanced plasma inflammation in older PWH.
In contrast to intact proviral DNA, we observed that HIV-1 ca-RNA, a measurement of transcriptional activity, showed a modest link with D-dimer. The majority of the intracellular HIV-1 RNA, as opposed to intact proviral DNA, may constitute both infectious and noninfectious virus. First, most of the persistent HIV-1 DNA in virus-suppressed PWH is defective and will not yield infectious virus [19]. The integrated defective DNA, however, can still yield HIV-1 RNA, and in some cases this RNA is translated to viral proteins [21, 22]. Second, the small amount of HIV-1 RNA that may lead to infectious virions will likely not yield new productively infected cells, because the presence of cART prevents spreading infection. It is important to note that current antiretroviral drugs prevent the generation of infectious virus and subsequent infection, but they do not inhibit HIV-1 transcription and RNA production. Thus, PWH receiving suppressive cART with no evidence of ongoing virus replication continue to have ongoing HIV-1 RNA production [2].
Findings of earlier studies suggest that the presence of HIV-1 RNA alone in the absence of protein or infectious virus induces inflammation. These findings argue that nuclear export of HIV-1 intron containing RNA induces an innate immune response in CD4 T cells, dendritic cells, and macrophages [34, 35]. In addition to these in vitro HIV-1 studies, ex vivo and animal studies also support the notion that endogenous retroviral RNA expression can induce inflammation [36]. This endogenous retroviral expression–induced inflammation has been proposed as a contributor to age-associated inflammation, and aging has been associated with derepression of endogenous retrovirus transcription [36].
Consistent with these data, we observed that HIV-1 RNA levels trended higher in the older than in the younger PWH even though the older group had been receiving suppressive cART for a longer time. This observed difference should not have a binary interpretation just because it was not statistically significant. Indeed, higher intracellular HIV-1 RNA levels have also been observed among older compared with younger HIV-1–suppressed individuals in another study [14]. Although directionally consistent, a significant association between HIV-1 ca-RNA and D-dimer was observed only in younger PWH (ρ = 0.50; P = .01), not in older PWH (ρ = 0.16; P = .37), possibly owing to smaller sample sizes. Together, these data suggest that aging is correlated with lower HIV-1 transcriptional control, although nonvirus processes may also affect the associated higher inflammation observed in older PWH.
Our in vitro studies further show that the higher levels of inflammatory mediators present in plasma samples from older compared with younger PWH did not affect the HIV-1 transcriptional landscape in latent cells. Our observations would argue that the pre-existing inflammatory milieu present in older PWH does not induce HIV-1 transcription in latently infected cells. We acknowledge that these in vitro studies do not perfectly mimic the in vivo conditions where the majority of infected cells are present in tissues rather than peripheral blood, and where infected cell characteristics, such as integration site, affect transcriptional capacity. Collectively, our observations suggest that the enhanced inflammation observed with aging does not promote greater HIV-1 transcription, but rather that the presence of HIV-1 RNA in the absence of any new rounds of infection promotes innate immune activation [34, 35]. Our model argues that novel drugs that lower the levels of intracellular HIV-1 RNA, something current antiretrovirals cannot accomplish, may affect the chronic inflammation observed in PWH, especially as they age.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank all the participants who contributed samples for these studies. We also thank Katherine Bruner for assistance with the intact proviral DNA assay.
Author contributions. A. O., N. L., and M. S. designed the research studies and analyzed the data. A. O., C. C., J. E. S. C., and M. S. performed experiments. N. L. provided clinical samples. A. O., J. E. S. C., N. L., and M. S. provided input regarding data interpretation. M. S. wrote the manuscript with input from the other authors.
Financial support. This work was supported by the National Institutes of Health (grants AG060890 to N. L. and M. S. and AI145661 to M. S.), the Boston University Genomic Science Institute, and the Providence/Boston Center for AIDS Research (grant P30AI042853).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Siliciano JD, Siliciano RF. Assays to measure latency, reservoirs, and reactivation. Curr Top Microbiol Immunol 2018; 417:23–41. [DOI] [PubMed] [Google Scholar]
- 2. Furtado MR, Callaway DS, Phair JP, et al. Persistence of HIV-1 transcription in peripheral-blood mononuclear cells in patients receiving potent antiretroviral therapy. N Engl J Med 1999; 340:1614–22. [DOI] [PubMed] [Google Scholar]
- 3. Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity 2013; 39:633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wada NI, Jacobson LP, Margolick JB, et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS 2015; 29:463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Libby P, Kobold S. Inflammation: a common contributor to cancer, aging, and cardiovascular diseases-expanding the concept of cardio-oncology. Cardiovasc Res 2019; 115:824–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Furman D, Campisi J, Verdin E, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med 2019; 25:1822–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. May MT, Gompels M, Delpech V, et al. ; UK Collaborative HIV Cohort (UK CHIC) Study . Impact on life expectancy of HIV-1 positive individuals of CD4+ cell count and viral load response to antiretroviral therapy. AIDS 2014; 28:1193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marcus JL, Leyden WA, Alexeeff SE, et al. Comparison of overall and comorbidity-free life expectancy between insured adults with and without HIV infection, 2000–2016. JAMA Netw Open 2020; 3:e207954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kuller LH, Tracy R, Belloso W, et al. ; INSIGHT SMART Study Group . Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 2008; 5:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baker JV, Sharma S, Grund B, et al. Systemic inflammation, coagulation, and clinical risk in the START trial. Open Forum Infect Dis 2017; 4:ofx262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wada NI, Bream JH, Martínez-Maza O, et al. Inflammatory biomarkers and mortality risk among HIV-suppressed men: a multisite prospective cohort study. Clin Infect Dis 2016; 63:984–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tenorio AR, Zheng Y, Bosch RJ, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis 2014; 210:1248–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hatano H, Jain V, Hunt PW, et al. Cell-based measures of viral persistence are associated with immune activation and programmed cell death protein 1 (PD-1)-expressing CD4+ T cells. J Infect Dis 2013; 208:50–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gandhi RT, McMahon DK, Bosch RJ, et al. ; ACTG A5321 Team . Levels of HIV-1 persistence on antiretroviral therapy are not associated with markers of inflammation or activation. PLoS Pathog 2017; 13:e1006285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cockerham LR, Siliciano JD, Sinclair E, et al. CD4+ and CD8+ T cell activation are associated with HIV DNA in resting CD4+ T cells. PLoS One 2014; 9:e110731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Poizot-Martin I, Faucher O, Obry-Roguet V, et al. Lack of correlation between the size of HIV proviral DNA reservoir and the level of immune activation in HIV-infected patients with a sustained undetectable HIV viral load for 10 years. J Clin Virol 2013; 57:351–5. [DOI] [PubMed] [Google Scholar]
- 17. Gandhi RT, Zheng L, Bosch RJ, et al. The effect of raltegravir intensification on low-level residual viremia in HIV-infected patients on antiretroviral therapy: a randomized controlled trial. PLoS Med 2010; 7:e1000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Besson GJ, Lalama CM, Bosch RJ, et al. HIV-1 DNA decay dynamics in blood during more than a decade of suppressive antiretroviral therapy. Clin Infect Dis 2014; 59:1312–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bruner KM, Murray AJ, Pollack RA, et al. Defective proviruses rapidly accumulate during acute HIV-1 infection. Nat Med 2016; 22:1043–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bruner KM, Wang Z, Simonetti FR, et al. A quantitative approach for measuring the reservoir of latent HIV-1 proviruses. Nature 2019; 566:120–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Imamichi H, Dewar RL, Adelsberger JW, et al. Defective HIV-1 proviruses produce novel protein-coding RNA species in HIV-infected patients on combination antiretroviral therapy. Proc Natl Acad Sci U S A 2016; 113:8783–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pollack RA, Jones RB, Pertea M, et al. Defective HIV-1 proviruses are expressed and can be recognized by cytotoxic T lymphocytes, which shape the proviral landscape. Cell Host Microbe 2017; 21:494–506.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Scully E, Lockhart A, Huang L, et al. Elevated levels of microbial translocation markers and CCL2 among older HIV-1-infected men. J Infect Dis 2016; 213:771–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Malnati MS, Scarlatti G, Gatto F, et al. A universal real-time PCR assay for the quantification of group-M HIV-1 proviral load. Nat Protoc 2008; 3:1240–8. [DOI] [PubMed] [Google Scholar]
- 25. Belkina AC, Starchenko A, Drake KA, et al. Multivariate computational analysis of gamma delta T cell inhibitory receptor signatures reveals the divergence of healthy and ART-suppressed HIV+ aging. Front Immunol 2018; 9:2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Olson A, Ragan EJ, Nakiyingi L, et al. Brief report: pulmonary tuberculosis is associated with persistent systemic inflammation and decreased HIV-1 reservoir markers in coinfected Ugandans. J Acquir Immune Defic Syndr 2018; 79:407–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jordan A, Bisgrove D, Verdin E. HIV reproducibly establishes a latent infection after acute infection of T cells in vitro. EMBO J 2003; 22:1868–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Folks TM, Clouse KA, Justement J, et al. Tumor necrosis factor alpha induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proc Natl Acad Sci U S A 1989; 86:2365–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Etemad B, Fellows A, Kwambana B, et al. Human immunodeficiency virus type 1 V1-to-V5 envelope variants from the chronic phase of infection use CCR5 and fuse more efficiently than those from early after infection. J Virol 2009; 83:9694–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jiang C, Lian X, Gao C, et al. Distinct viral reservoirs in individuals with spontaneous control of HIV-1. Nature 2020; 585:261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell 2013; 153:1194–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci 2014; 69(suppl 1):S4–9. [DOI] [PubMed] [Google Scholar]
- 33. Furman D, Chang J, Lartigue L, et al. Expression of specific inflammasome gene modules stratifies older individuals into two extreme clinical and immunological states. Nat Med 2017; 23:174–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Akiyama H, Miller CM, Ettinger CR, Belkina AC, Snyder-Cappione JE, Gummuluru S. HIV-1 intron-containing RNA expression induces innate immune activation and T cell dysfunction. Nat Commun 2018; 9:3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McCauley SM, Kim K, Nowosielska A, et al. Intron-containing RNA from the HIV-1 provirus activates type I interferon and inflammatory cytokines. Nat Commun 2018; 9:5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. De Cecco M, Ito T, Petrashen AP, et al. L1 drives IFN in senescent cells and promotes age-associated inflammation. Nature 2019; 566:73–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.