Abstract
Circumsporozoite protein (CSP) coats the Plasmodium falciparum sporozoite surface and is a major malaria subunit vaccine target. We measured epitope-specific reactivity to field-derived CSP haplotypes in serum samples from Malian adults and children on a custom peptide microarray. Compared to children, adults showed greater antibody responses and responses to more variants in regions proximal to and within the central repeat region. Children acquired short-lived immunity to an epitope proximal to the central repeat region but not to the central repeat region itself. This approach has the potential to differentiate immunodominant from protective epitope-specific responses when combined with longitudinal infection data.
Keywords: malaria, Plasmodium falciparum, peptide microarray, circumsporozoite protein
On a custom peptide microarray that measured naturally acquired, epitope-specific antibody reactivity to field-derived Plasmodium falciparum circumsporozoite protein haplotypes, Malian adult sera had immunoglobulin G (IgG) antibodies to the Region 1-NANP junction and children had short-lived IgG responses to the same region.
An effective malaria vaccine would be a pivotal advancement in malaria control, raising prospects for elimination and eradication [1]. After a Plasmodium-infected Anopheles mosquito bites a person, sporozoites travel to the liver and invade hepatocytes. During this quiescent liver phase, merozoites replicate before emerging into the circulation to invade erythrocytes, resulting in blood-stage infection, and illness in nonimmune individuals. A preerythrocytic vaccine would prevent illness and subsequent transmission. Circumsporozoite protein (CSP) coats the surface of the sporozoite and is a highly studied vaccine candidate antigen [2]. CSP epitopes include the conserved junction between Region 1 (R1) and the central repeat region (R1-NANP junction), the immunodominant central (NANP) repeat region, and the C-terminal polymorphic T- and B-cell epitopes (Figure 1A). RTS,S, the most advanced malaria vaccine candidate to date, is in pilot implementation testing in 3 African countries [3]. RTS,S is a truncated version of CSP that contains the immunodominant central repeat region and the polymorphic C-terminal region, but not the R1-NANP junction [4]. Vaccine efficacy against clinical malaria was 31% and 56% in infants 6–12 weeks old and children 5–17 months old, respectively, during the 12 months after vaccination in a phase 3 clinical trial, with efficacy waning over time [5]. RTS,S can prevent malaria morbidity; however, a more efficacious vaccine would provide increased benefit.
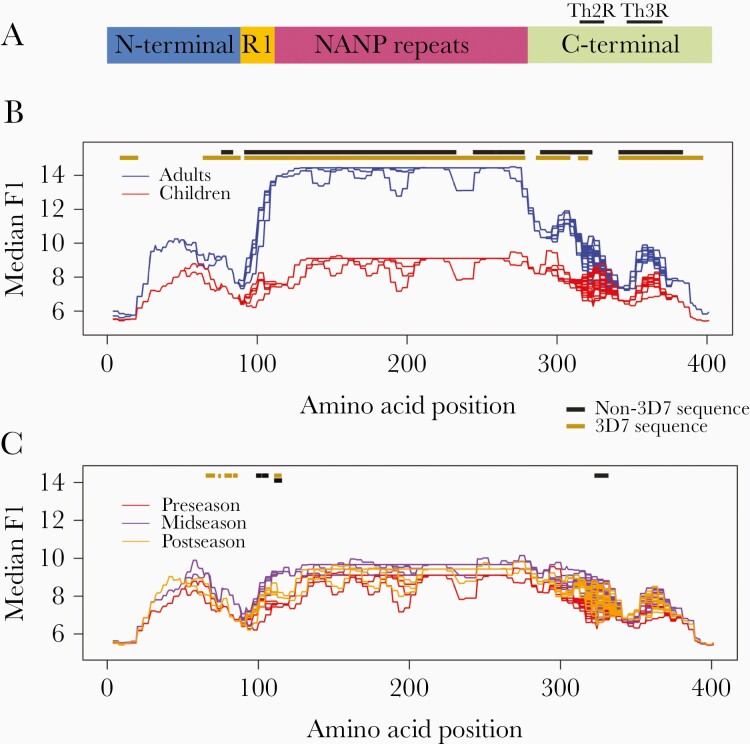
Figure 1.
A, Schematic of the circumsporozoite protein (CSP). CSP is comprised of 4 regions: the amino- or N-terminus; Region I; the central repeat or NANP repeat region; and the carboxy- or C-terminus. For 3D7, the N-terminus spans from amino acids 1 to 93, Region I and the R1-central repeat junction span from amino acids 94 to 116, the central repeat region spans from amino acids 117 to 272, and the C-terminus spans from amino acids 273 to 397 (with Th2R at 310–327 and Th3R at 345–366) (positions based on 3D7 sequence) [7, 14, 15]. B, Seroreactivity in Malian adults vs children: The x-axis represents the amino acid position along the protein. Median smoothed log2 fluorescence intensity (FI) is displayed on the y-axis. Line graph shows the median smoothed log2 FI of adults (blue) compared to children (red) to 73 CSP haplotypes. Each line is the median smoothed log2 FI to a single CSP haplotype on the array, with overlap of lines in areas of sequence conservation. The gold line above the line graph shows where seroreactivity to the 3D7 variant was significantly greater in adults compared to children, and the black line represents areas to which seroreactivity to non-3D7 variants was significantly greater in adults compared to children (P < .05 with Benjamini–Hochberg [BH] correction). At no position along the protein did children have significantly greater seroreactivity than adults. C, Seroreactivity over the course of a malaria season in Malian children: The x-axis represents the amino acid position along the protein. Median smoothed log2 FI is displayed on the y-axis. Line graph shows the median smoothed log2 FI of children preseason (red) compared to mid-season (purple) and postseason (yellow) to 73 CSP haplotypes. Each line is the median smoothed log2 FI to a single CSP haplotype on the array, with overlap of lines in areas of sequence conservation. The gold line above the line graph shows where seroreactivity to the 3D7 variant was significantly greater in children midseason compared to preseason and the black line represents areas to which seroreactivity to the non-3D7 variants was significantly greater midseason compared to preseason (P < .05 with BH correction). No significant differences were found when comparing seroreactivity postseason to midseason or postseason to preseason.
The relatively conserved R1-NANP junction appears to elicit protective antibody responses. Recently, monoclonal antibodies (mAbs) were generated from volunteers immunized with an irradiated sporozoite vaccine and protected from controlled human malaria infection [6, 7]. In 1 study, the 5 most potent neutralizing mAbs of 9 tested in a humanized mouse model bound both the R1-NANP junction and the central repeat region, suggesting that binding at both sites is important for neutralization [6]. Another group identified a mAb that protected mice from challenge with a transgenic Plasmodium berghei strain expressing Plasmodium falciparum CSP and in a humanized mouse model challenged with P. falciparum [7]. This mAb potently binds 2 epitopes in the R1-NANP junction [7]. Given the similar binding sites of these independently generated mAbs, the R1-NANP junction represents a promising CSP epitope for further vaccine development, but little is known about naturally acquired immunity to this region of the protein.
To address gaps in knowledge regarding naturally acquired humoral immunity to diverse and specific epitopes of CSP, we used a novel, diversity-reflecting peptide microarray to compare serological responses to small CSP peptides in adults and children before the start of a malaria season, and examined changes in children’s serological responses to CSP peptides over the course of a malaria season.
METHODS
Study Participants
Serum samples used in this study originated from clinical trials of malaria vaccine candidate FMP2.1/AS02A in Bandiagara, Mali [8, 9], an area of intense seasonal malaria transmission [10]. Subjects included in the current study were randomly selected from subgroups who received control (rabies) vaccine. For 10 children who had at least 1 malaria infection during the transmission season after vaccination, peptide-specific antibody responses were measured in sera from the day of enrollment (preseason, June 2007), 90 days after vaccination (midseason, September 2007), and 240 days after vaccination (postseason, February 2008). Sera from 10 Malian adults collected at the start of the malaria transmission season, none of whom had febrile malaria illness during follow-up, and 5 North American, malaria-naive adults from University of California, Irvine, were also tested on the peptide array. Subject characteristics are detailed in Supplementary Table 1. Research protocols were approved by the institutional review boards at the University of Sciences, Techniques and Technologies of Bamako, in Mali; the University of Maryland, Baltimore; the Walter Reed Army Institute of Research; the United States Army Surgeon General; and the University of California, Irvine, where applicable. Appropriate informed consent was obtained from study participants.
Diversity-Reflecting Peptide Microarray Design
The diversity-reflecting high-density peptide array was designed using publicly available sequences and sequences derived from field samples from Mali and Southeast Asia (manuscript in review). CSP sequences were represented as 16 amino acid peptides, overlapping by 12 amino acids, printed in triplicate. From >200 available sequences, 73 unique CSP sequence haplotypes were identified, resulting in 251 unique peptides. Details of in situ peptide synthesis, serum probing, and slide imaging can be found in the Supplementary Materials. Sequence haplotypes were aligned using Clustal Omega with default settings, resulting in 401 amino acid positions (matching the longest haplotype).
Smoothed Fluorescence Intensity Determination
Quantity of bound immunoglobulin G (IgG) antibodies was approximated by the measured fluorescence intensity (FI) for each peptide. Mean log2 FI was calculated for replicate peptides. Because overlapping peptides can contain the same epitope, a sliding window-based average smoothing procedure was used to increase the signal-to-noise ratio by leveraging information between neighboring peptides [11]. Each peptide along the CSP sequence was represented by its midpoint (end position minus beginning position divided by 2). Each amino acid position along the protein sequence was represented by a smoothed log2 FI equal to the average FI for all peptides with a midpoint 6 positions before or after the given amino acid position. For example, the amino acid at position 100 had a corresponding smoothed log2 FI equal to the average of all peptides with midpoint positions at 94 through 106. Each smoothed log2 FI corresponding to each amino acid position included the log2 FI of at least 3 peptides. Overall, CSP sequences had a maximum of 5 amino acid substitutions per position along the protein. When considering flanking amino acids at each substitution, each amino acid position had 1–29 unique smoothed log2 FI, which we termed variant amino acids (mean, 4; total unique variant amino acids, 1682). Serorecognition was a dichotomous variable, defined as positive if the smoothed log2 FI at an amino acid position was >2.5 standard deviations above the mean smoothed log2 FI of the North American malaria-naive adults.
Statistical Analyses
The Fisher exact test was used for serorecognition comparisons between adults and children. Changes in serorecognition over a malaria transmission season were analyzed using the binomial exact test. The Wilcoxon-rank sum test and the Wilcoxon signed-rank test were used for group and matched seroreactivity comparisons, respectively. For seroreactivity comparisons, only amino acids categorized as serorecognized in at least 1 of the groups were compared. Pearson product moment correlation was used to examine the correlation between seroreactivity and the number of infections in a malaria season. Adjustment for multiple comparisons was performed using the Benjamini–Hochberg procedure. Potential epitopes were defined as contiguous amino acid sequences >3 amino acids in length where the seroreactivity was significantly increased by Wilcoxon rank-sum test or Wilcoxon signed-rank test for at least 80% of the contiguous amino acids (P < .05 after Benjamini–Hochberg correction). Statistical analyses were performed in R software, version 3.6.1.
RESULTS
Serorecognition and Seroreactivity in Malian Adults and Children at the Start of the Malaria Season
Adult sera recognized more variant amino acids of CSP than children (median for adults, 1044 variant amino acids [range, 564–1476]; median for children, 160 variant amino acids [range, 22–799]; total variant amino acids, 1682; P < .0001). At 313 of the 401 amino acid positions within CSP, adults recognized more variants than children (P < .05 for all), including variant amino acids in the R1-NANP junction, the central repeat region, and the Th2R and Th3R regions (Supplementary Figure 1).
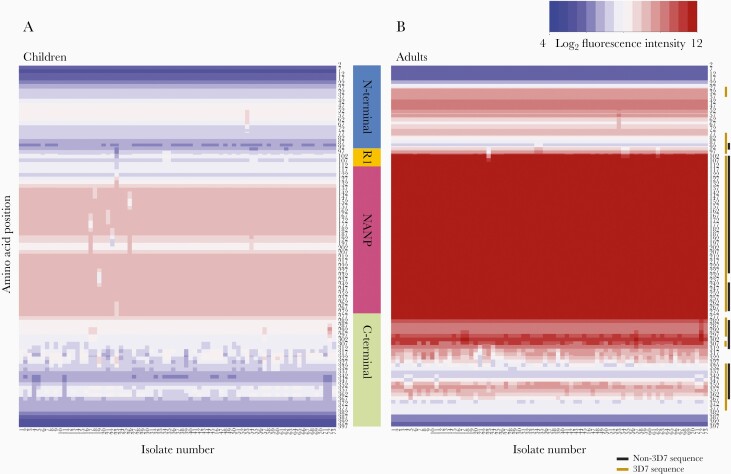
Adults had greater seroreactivity than children to variant amino acids within known epitopes, including the R1-NANP junction (P = .0003–.002) and central repeat region (P = .0004–.0005), but not a large portion of the Th2R 3D7 sequence. (Figure 1B, Supplementary Table 2). Heatmaps of the median preseason seroreactivity of adults and children to each of the 73 CSP haplotypes on the peptide array visually demonstrate these differences in seroreactivity (Figure 2).
Figure 2.
Heatmaps of the median seroreactivity of Malian adults and children before the start of a malaria season. Each column (x-axis) represents the median seroreactivity of all children (A) or adults (B) for each of the 73 CSP haplotypes; each row (y-axis) represents a position along the protein sequence. Adults showed greater median seroreactivity than children, especially to R1 and the central repeat region (P < .05 with BH correction). The gold line next to the heat map shows where seroreactivity to the 3D7 sequence was significantly higher in adults compared to children, and the black line represents areas where seroreactivity to non-3D7 sequences was significantly higher in adults compared to children.
Serorecognition and Seroreactivity in Children Over the Course of a Malaria Season
Sera from children recognized a median of 160 (range, 22–799), 363 (range, 66–691), and 186 (range, 93–767) variant amino acids pre-, mid-, and postseason, respectively (pre- to midseason comparison, P = .05; mid- to postseason comparison, P = .11; and post- to preseason comparison, P = .15). The median number of variants serorecognized by children at each CSP amino acid position did not differ significantly in comparisons between time points (P = .6–1.0) (Supplementary Figure 2).
Median seroreactivity to CSP variant amino acids peaked in children at midseason and remained elevated postseason compared to preseason (P < .0001 for all pairwise comparisons). This is visualized in the heatmaps of median seroreactivity of the children pre-, mid-, and postseason to each of the 73 CSP haplotypes on the peptide array (Supplementary Figure 3). When comparing the median seroreactivity for each serorecognized variant amino acid, children at midseason had greater seroreactivity to a few potential epitopes in the N-terminus, R1-NANP junction, and 1 non-3D7 Th2R sequence than children preseason (P = .04; Figure 1C, Supplementary Table 3). However, no significant differences in FI for each serorecognized variant amino acid were observed when comparing post- to pre- or midseason samples, which indicates that the responses gained during the season waned by the end of the season.
Association of Seroreactivity With Number of Infections in a Season
Children experienced 1–5 malaria infections (symptomatic or asymptomatic) over a single malaria season. Seroreactivity to CSP variant amino acids before the start of the malaria season did not correlate with the number of infections during the subsequent season (r = –0.63 to 0.71; P = 1.0 for all).
Discussion
A better understanding of epitope specificity in protective humoral responses would inform next generation preerythrocytic vaccine development. In this study, we examined humoral responses to small and diverse epitopes of CSP to identify specific CSP epitopes with differential serological responses between adults and children and in children over a malaria season in Bandiagara, Mali. Adults responded to more variant amino acids and had greater antibody responses to the R1-NANP junction and the central repeat region, but not to the Th2R 3D7 variant sequence included in the RTS,S vaccine. Children displayed some short-lived antibody responses to the R1-NANP junction and to a portion of the Th2R epitope during the malaria season but did not develop significant serorecognition of the central repeat region.
Our finding that naturally acquired antibody responses to the R1-NANP junction are greater in semi-immune adults than in susceptible children is consistent with recent studies that identified the R1-NANP junction as a potential site of protective antibodies induced by an irradiated sporozoite vaccine [6, 7]. The CSP haplotypes included on the array had minimal variation in the R1-NANP junction with only 5 variants of the KQPADGNPDPNANPNVD peptide identified as a binding site for protective antibodies by Tan et al [6] and Kisalu et al [7] and 2 variants at the NPDPNANPNVDPNAN secondary binding site for antibodies identified by Kisalu et al [7]. Therefore, if antibodies targeting the R1-NANP junction are protective, lack of sequence diversity at this region would be advantageous for subunit vaccine development. A limited number of naturally occurring variants could be included in a multipeptide CSP-based vaccine. The lack of sequence diversity at these antibody binding sites may be a consequence of their essential role in protein function. Region I of the N-terminal domain is important for sporozoite attachment to and invasion of hepatocytes [12]. Cleavage of CSP at Region I triggers hepatocyte invasion, and cleavage inhibitors prevent hepatocyte invasion both in vivo and in vitro [13]. Therefore, extensive variation that disrupts hepatocyte attachment and invasion would likely result in a fitness cost to the parasite.
Overall, we described responses to CSP epitopes, including field-derived variants, and identified peptides that may represent potential markers of protective immunity. Although this initial investigation included a limited sample size, results achieved statistical significance and provide justification for additional testing in similar populations with longitudinal infection surveillance data and in persons who participate in CSP-based P. falciparum vaccine trials with controlled human malaria infection. The research here reports novel findings of naturally acquired humoral immune responses to small and diverse peptides of CSP, including variants derived from parasites circulating in malaria-endemic areas. Our approach has the potential to differentiate immunodominant from protective responses to field variants, and findings are highly relevant to preerythrocytic vaccine development.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We acknowledge all of our collaborators for this project and the study participants and their families.
Disclaimer. The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID) at the NIH (grant numbers R21AI119733 and K23AI125720 to A. A. B.); NIH grant T32 AI007524 for salary support for D. J. F.-K; the Burroughs Wellcome Fund/American Society of Tropical Medicine and Hygiene Postdoctoral Fellowship in Tropical Infectious Disease to D. J. F.-K.; and a Passano Foundation Clinician-Investigator Award to A. A. B. Support for training for A. O. was provided by the National Heart, Lung and Blood Institute (award numbers 1K01HL140285-01A1 and U19 AI110820). Biological samples were collected during clinical trials funded by the NIAID (contract number N01AI85346 and cooperative agreement number U19AI065683); the Fogarty International Center, NIH (grant number D43TW001589); and the United States Department of Defense and the United States Agency for International Development (contract number W81XWH-06-1-0427).
Potential conflicts of interest. J. C. T. is an employee of Nimble Therapeutics, a company spun out from Roche Sequencing Solutions to commercialize the peptide array technology. J. J. P. is an employee, officer, and board member of Nimble Therapeutics. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: American Society of Tropical Medicine and Hygiene Annual Meeting, National Harbor, Maryland, 21 November 2019.
References
- 1.Plowe CV, Alonso P, Hoffman SL. The potential role of vaccines in the elimination of falciparum malaria and the eventual eradication of malaria. J Infect Dis 2009; 200:1646–9. [DOI] [PubMed] [Google Scholar]
- 2.Laurens MB. The promise of a malaria vaccine—are we closer? Annu Rev Microbiol 2018; 72:273–92. [DOI] [PubMed] [Google Scholar]
- 3.Adepoju P. RTS,S malaria vaccine pilots in three African countries. Lancet 2019; 393:1685. [DOI] [PubMed] [Google Scholar]
- 4.Casares S, Brumeanu TD, Richie TL. The RTS,S malaria vaccine. Vaccine 2010; 28:4880–94. [DOI] [PubMed] [Google Scholar]
- 5.RTS,S Clinical Trials Partnership. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet 2015; 6736:31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan J, Sack BK, Oyen D, et al. . A public antibody lineage that potently inhibits malaria infection through dual binding to the circumsporozoite protein. Nat Med 2018; 24:401–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kisalu NK, Idris AH, Weidle C, et al. . A human monoclonal antibody prevents malaria infection by targeting a new site of vulnerability on the parasite. Nat Med 2018; 24:408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thera MA, Doumbo OK, Coulibaly D, et al. . Safety and immunogenicity of an AMA-1 malaria vaccine in Malian adults: results of a phase 1 randomized controlled trial. PLoS One 2008; 3:e1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thera MA, Doumbo OK, Coulibaly D, et al. . A field trial to assess a blood-stage malaria vaccine. N Engl J Med 2011; 365:1004–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coulibaly D, Travassos MA, Kone AK, et al. . Stable malaria incidence despite scaling up control strategies in a malaria vaccine-testing site in Mali. Malar J 2014; 13:374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imholte GC, Sauteraud R, Korber B, et al. . A computational framework for the analysis of peptide microarray antibody binding data with application to HIV vaccine profiling. J Immunol Methods 2013; 395:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rathore D, Sacci JB, de la Vega P, McCutchan TF. Binding and invasion of liver cells by Plasmodium falciparum sporozoites. Essential involvement of the amino terminus of circumsporozoite protein. J Biol Chem 2002; 277:7092–8. [DOI] [PubMed] [Google Scholar]
- 13.Coppi A, Pinzon-Ortiz C, Hutter C, Sinnis P. The Plasmodium circumsporozoite protein is proteolytically processed during cell invasion. J Exp Med 2005; 201:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doud MB, Koksal AC, Mi LZ, Song G, Lu C, Springer TA. Unexpected fold in the circumsporozoite protein target of malaria vaccines. Proc Natl Acad Sci U S A 2012; 109:7817–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Julien JP, Wardemann H. Antibodies against Plasmodium falciparum malaria at the molecular level. Nat Rev Immunol 2019; 19:761–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.