Abstract
Background:
The dynamin 1-like gene (DNM1L) encodes a GTPase that mediates mitochondrial and peroxisomal fission and fusion. We report a new clinical presentation associated with a DNM1L pathogenic variant and review the literature.
Results:
A 13-year-old boy with mild developmental delays and paroxysmal dystonia presented acutely with multifocal myoclonic super-refractory status epilepticus. Despite sustained and aggressive treatment, seizures persisted and care was ultimately withdrawn in the context of extensive global cortical atrophy. Rapid trio-whole exome sequencing revealed a de novo heterozygous c.1207C>T (p.R403C) pathogenic variant in DNM1L. Immunofluorescence staining of fibroblast mitochondria revealed abnormally elongated and tubular morphology.
Conclusions:
This case highlights the diagnostic importance of rapid whole exome sequencing within a critical care setting and reveals the expanding phenotypic spectrum associated with DNM1L variants. This now includes progressive paroxysmal dystonia and adolescent-onset super-refractory myoclonic status epilepticus contributing to strikingly rapid and progressive cortical atrophy and death.
Introduction:
Mitochondria are dynamic organelles responsible for cellular energy production, and they rely on a complex network of proteins for movement, fusion-fission dynamics, and replication. Defects in mitochondrial-dynamics proteins can cause abnormal fusion and fission resulting in clinical disease.1–3 One important protein in fission of both mitochondria and peroxisomes is an 80-kDa mechano-chemical GTPase of the dynamin superfamily known as dynamin 1-like protein (DNM1L)2,4, also known as dynamin-related protein 1 (DRP1)1,5,6 or dynamin-like protein 1 (DLP1).3,6 This protein is encoded by the dynamin 1-like gene (DNM1L) (OMIM #603850).
Previous case reports (see Table) have demonstrated the aggressive nature of DNM1L variants which can cause lethal neonatal-onset encephalopathy.3–10 The clinical phenotype appears dependent on the specific causative DNM1L variant, but in general, patients have experienced varying degrees of hypotonia, cognitive impairment and refractory epilepsy. Once seizures arise, recurrent episodes of focal or generalized status epilepticus often trigger cognitive regression, and death in early childhood is common.
Table:
Variable clinical characteristics in children with DNM1L variants.
| Published Study (author) | Age at Onset | Age at Reporting | Symptoms | Microscopy / Mitochondrial Analysis | Gene variants | |
|---|---|---|---|---|---|---|
| 1 | Nasca et al, 2016 5 Sibling 1 | 1 year | Alive at age 16 years | Developmental delay, strabismus, ataxia, spasticity, dysarthria. | Elongated fibroblast mitochondrial morphology with a filamentous network. Peroxisomes were larger and less uniformly distributed in the cytoplasm. | c.106A>G (p.Ser36Gly) c.346_347delGA (p.Glu116Lysfs*6) compound heterozygous recessive (parents were unaffected heterozygous carriers) |
| 2 | Nasca et al, 2016 5 Sibling 2 | 1 year | Alive at age 3 years | Developmental delay, strabismus, ataxia, spasticity, dysarthria. | No pathology reported. | c.106A>G (p.Ser36Gly) c.346_347delGA (p.Glu116Lysfs*6) compound heterozygous recessive (parents were unaffected heterozygous carriers) |
| 3 | Yoon et al, 20167 Sibling 1 | Birth | Died at 3 weeks | Hypotonia and apnea at birth. | Giant mitochondria in neurons with reduced myelination. Reduced staining of DNM1L protein in sural nerves. | c.261dup (p.Trp88Metfs*), c.385_386del (p.Glu129Lys*6) compound heterozygous recessive (parents were unaffected heterozygous carriers) |
| 4 | Yoon et al, 20167 Sibling 2 | Birth | Died at 8 days | Hypotonia and apnea at birth. | Giant mitochondria in neurons with reduced myelination. Reduced staining of DNM1L protein in sural nerves. | c.261dup (p.Trp88Metfs*), c.385_386del (p.Glu129Lys*6) compound heterozygous recessive (parents were unaffected heterozygous carriers) |
| 5 | Chao et al, 20169 Case 1 | 5 months | Died at 5 years | Developmental delay, refractory epilepsy, status epilepticus. | Muscle biopsy had reduced activity in complexes I, III, IV, and had mitochondrial pleomorphism. | c.1048G>A (p.G350R), de novo dominant |
| 6 | Sheffer et al, 20168 | Birth | Alive at 2 years | Microcephaly, development delay, insensitivity to pain. | Decreased activity of complex IV. Elongated mitochondria, normal peroxisomes. | c.1084G>A, (p.Gly362Ser) de novo dominant |
| 7 | Vanstone et al, 20154 | 6 months | Alive at 7 years | Developmental delay, refractory epilepsy. | Hyperfused mitochondrial network morphology. | c.1085G→A (p.Gly362Asp) de novo dominant |
| 8 | Chao et al, 20169 Case 2 | Birth | Died at 10 months | Hypotonia and apnea at birth, microcephaly, absence of corpus callosum, hydrocephalus, persistently elevated lactate. | Biopsy declined. | c.1135G>A, (p.E379K) de novo dominant |
| 9 | Waterham et al., 20073 | 6 days | Died at 37 days | Microcephaly, elevated lactate and very long chain fatty acids, optic atrophy. | Elongated, tubular mitochondria. | c.1184C→A (Ala395Asp) de novo dominant |
| 10 | Fahrner et al, 20166 Case 1 | 4 years | Alive at age 8 years | Focal status into generalized status epilepticus, refractory epilepsy, cognitive/motor delays. | Expression of mutant allele in mouse cells resulted in elongated tubular mitochondrial morphology. | c.1207C>T (p.R403C), de novo dominant |
| 11 | Fahrner et al, 20166 Case 2 | 5 years | Alive at age 6 years | Focal status into generalized status epilepticus, now neurological devastated with myoclonus. | No pathology reported. | c.1207C>T (p.R403C) de novo dominant |
| 12 | Zaha et al, 201610 | 6 months | Died at 18 months | Severe hypotonia, development delay, elevated CSF lactate, infantile spasms and suppression-burst EEG. | Elongation of peroxisomes and mitochondria of patient’s fibroblasts. | c.1217T>C (p.Leu406Ser) de novo dominant |
| 13 | Our patient described herein | 18 months | Died at 13 years | Developmental delay, paroxysmal dystonia, super refractory myoclonic status epilepticus. | Elongated, tubular mitochondria. | c.1207C>T (p.R403C), de novo dominant |
We present a case of a 13-year-old boy with longstanding mild global developmental delays and late childhood onset paroxysmal dystonia, who developed lethal super-refractory myoclonic status epilepticus attributed to p.R403C in DNM1L. The pathogenicity of this variant was further confirmed with immunofluorescence staining of his fibroblast mitochondria.
Clinical presentation:
The patient was the first child of his non-consanguineous Caucasian parents, both of whom had no medical conditions. Pregnancy and delivery were unremarkable and he passed the newborn screen. By 18 months, mild developmental delays were apparent, most prominently affecting his expressive language and fine motor skills. Over the years, he remained about one year behind his peers academically. At 10 years of age, the Wechsler Individual Achievement Test (3rd edition) was performed and his full scale IQ was 68 (2nd percentile) suggesting mild intellectual disability. While he was described as clumsy, his gross motor skills were normal enough to participate in a variety of athletics without difficulty. He continued to have fine motor difficulties including with handwriting, tying shoes, and buttoning clothing. He had chronic anxiety, excessive worrying, nervous habits, perseveration, inattentiveness, and poor organizational skills. He had a long-standing history of night terrors, parasomnias, and scary dreams.
At 11 years of age, he presented to a neurology clinic with worsening paroxysmal dystonia exacerbated by exercise and anxiety. Dystonia started in the right leg, occurring exclusively during exercise (not with fright or startling), lasting 30 seconds, and characterized by painless sustained muscle contraction beyond his control causing his leg to turn inward, often causing him to fall, and with preserved consciousness. Over several months these dystonic episodes started occurring with lesser degrees of activity, lasting upwards of 15 minutes, occurring 2–3 days per week, sometimes clustering, and mainly affecting his legs bilaterally (again with preserved consciousness), but later affecting his hands and trunk. Extensive evaluations at that time included normal head magnetic resonance imaging (MRI), normal metabolic investigations (including serum lactate, ammonia, creatine kinase, copper, ceruloplasmin, uric acid, serum amino acids, urine organic acids, urine creatine disorders panel, and urine purine & pyrimidine panel), and normal testing for two genes associated with episodic kinesigenic dyskinesias - PRRT2 and SLC2A1. No optic atrophy or other funduscopic abnormalities were seen on a non-dilated eye exam. His dystonia did not respond to a several month trial of high-dose carbamazepine. A routine electroencephalogram (EEG) identified normal background activity but a propensity towards generalized seizures, including several bursts of generalized 2–3 Hz spike and wave activity. There was no activation during sleep or with hyperventilation and photic stimulation. Carbamazepine was subsequently switched for levetiracetam without clinical improvement of his dystonic episodes. Evaluation of his cerebrospinal fluid (CSF) neurotransmitters revealed non-specific low levels of serotonin and dopamine metabolites, with normal levels of protein, glucose, nucleated cells, and amino acids. A course of carbidopa-levodopa produced resolution of these dystonic episodes for several months until increasing doses began to lose efficacy.
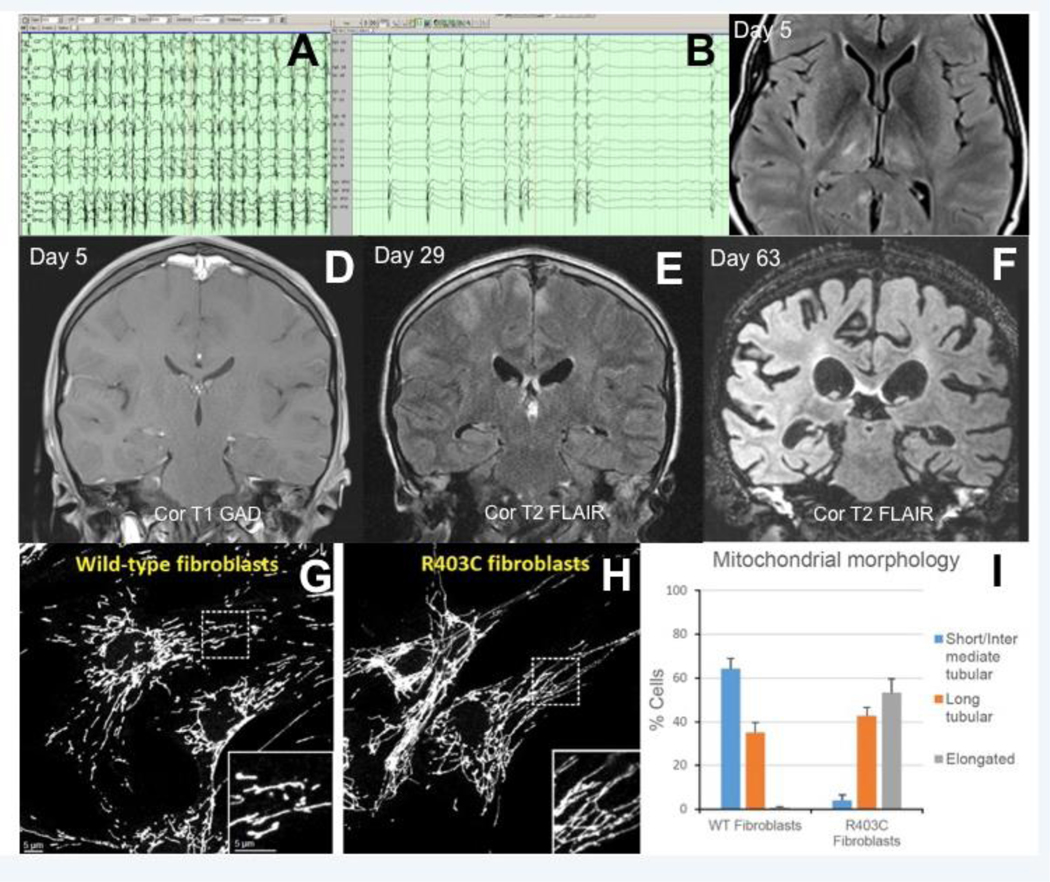
At age 13, he had progressive worsening of the dystonic episodes and new sleep difficulties, but no infections or trauma, when he presented to a local hospital after developing myoclonus involving his face, tongue, lips, and eyes. This responded to lorazepam, but he returned the next day with continued myoclonus, now also involving his limbs and torso, that affected his ability to communicate and swallow. He was transferred to our tertiary hospital for management of refractory status epilepticus which included emergence of generalized tonic-clonic seizures. Continuous video-EEG monitoring confirmed multi-focal cortical myoclonus and electrographic status epilepticus (Figure A).
Figure.
Continuous-video electroencephalography (A-B). Migratory facial and multi-limb clinical myoclonus correlating with fluctuating but continuous generalized 2–3 Hz (poly)spike-and-wave discharges (A). Clinical seizure activity resolved with high dose enteral phenobarbital, but generalized periodic discharges persisted (B). Axial T2 FLAIR magnetic resonance image on day 5 of admission revealed hyperintensities (and subtle restricted diffusion on diffusion-weighted imaging - not shown) most prominently affecting his thalami bilaterally, right caudate and lentiform nucleus, and bilateral posterior hippocampi (C). Serial brain MRIs revealed rapidly progressive and diffuse cortical atrophy (D-F). Our patient’s fibroblasts were cultured and their mitochondrial structure analyzed by immunofluorescence against the mitochondrial outer membrane protein Tom20, and compared to a normal control sample (G). The patient’s fibroblasts contained mitochondria that lack normal morphology, including a preponderance of abnormally elongated mitochondria (H). Quantified analysis of mitochondrial morphology comparing the normal control (WT fibroblasts) with our patient (p.R403C fibroblasts) (I).
Treatment:
After failing a high dose midazolam infusion (1.92 mg/kg/hr or 32 mcg/kg/min), a barbiturate coma was induced which led to a burst suppression pattern on EEG and resolution of clinical seizures (Figure B). A pentobarbital infusion was originally used but due to emergence of life-threatening acute renal failure from propylene glycol toxicity, this was switched to a high-dose enteral phenobarbital regimen with serum drug levels often exceeding 300 mcg/mL (1300 μmol/L). Unfortunately, over the next 9 weeks, recurrent attempts to wean the barbiturate coma led to clinical reemergence of aggressive multifocal myoclonus. Attempts to vary the degree of barbiturate-induced cortical suppression while adjusting anti-seizure medications were futile. While initially targeting EEG-confirmed seizure suppression and then a burst suppression interburst interval of 2–10 seconds, we gradually escalated (after repeated wean failures) to more prolonged electrographic suppression, including 4 days of complete electrographic suppression.
Treatment failures included trials of lorazepam, fosphenytoin (administered day 1 of presentation at referring hospital), levetiracetam, topiramate, pyridoxine, lacosamide, felbamate, clonazepam, piracetam, ketamine, isoflurane and allopregnanolone. Immunomodulatory trials included high dose methylprednisolone and anakinra. Due to strong concern for a mitochondrial pathology, valproic acid was never administered. After discussion of the risks and benefits, the ketogenic diet was trialed without benefit for 2 weeks with sustained beta-hydroxybutyrate levels > 4 mmol/L.
Investigations during acute presentation:
Initial diagnostic evaluation was extensive. Brain MRI obtained on day 1 of his status epilepticus was entirely normal (not shown). On day 5 of admission, repeat neuroimaging showed T2 hyperintensities and restricted diffusion most prominently affecting his thalami bilaterally, right basal ganglia, and bilateral hippocampi (Figure C). A lactate peak was detected over his thalamus on MR spectroscopy (not shown). Normal investigations included chromosomal microarray, Fragile X, comprehensive mtDNA testing, as well as skin and muscle biopsies with coenzyme Q10 and respiratory chain analysis. Muscle biopsy demonstrated nonspecific nondiagnostic findings of a vacuolar myopathy (type undetermined), probable mild lysosomal dysfunction, and mild denervation atrophy with associated mild reinnervation. Repeat dilated funduscopic examination by ophthalmology on hospital day 20 revealed no optic atrophy or other abnormalities.
Lab testing during the hospitalization was nondiagnostic. Lactate peaked at 16 mmol/L during renal failure but promptly returned to <2 mmol/L after starting dialysis. Serum testing for ammonia, creatine kinase, lactate dehydrogenase, congenital disorders of glycosylation, peroxisomal panel (very long chain fatty acids), lysosomal enzyme panel, ceramide trihexosides and sulfatides, amino acids, carnitine, acylcarnitine, comprehensive fatty acid panel, and vitamins B1, B2, B6, B12, and folate were noncontributory. Serum and CSF autoimmune epilepsy panels were normal. Cerebrospinal fluid analysis revealed normal protein and 1 nucleated cell. Urine testing was unremarkable for organic acids, amino acids, acylglycines, purine and pyrimidine panels.
One month after admission, rapid trio-based whole exome sequencing (WES), chosen for its fast turnaround time in our patient’s critical situation, but at a higher cost, was performed clinically at Baylor Genetics. Baylor Genetics requires samples from patient and parents for their rapid test, and performs WES on an Illumia HiSeq Instrument with 100 base paired-end reads. The quality control metrics of the sequencing data are generally achieved: >70% of reads aligned to target, >95% of target bases covered at >20X, >85% of target bases covered at >40X, mean coverage of the target bases is >100X. The rapid WES results were available 13 days after the test was ordered and demonstrated a de novo heterozygous c.1207C>T (p.R403C) pathogenic variant in DNM1L.
His brain MRI was repeated on day 63 of admission and showed a drastic amount of global cerebral volume loss (Figure D–F). Perfusion imaging (arterial spin labeling) revealed limited-to-no cerebral perfusion of his cortex (not shown). Care was withdrawn on day 66 of hospitalization. Autopsy revealed an enlarged liver and spleen, in addition to diffuse cerebral atrophy, gliosis, and degeneration.
Mitochondrial pathology:
Fibroblasts from our patient were analyzed for mitochondrial morphology (performed by RL and DCC). Immunofluorescence against the mitochondrial outer membrane protein Tom20 demonstrated a paucity of structurally normal mitochondria. Instead mitochondria were substantially elongated and tubular when compared to wild type control samples (Figure G–I).
Discussion:
In our patient, WES identified a heterozygous de novo pathogenic variant [c.1207C>T (p.R403C)] in the dynamin 1-like gene (DNM1L), encoding the dynamin 1-like protein (DNM1L), involved in mitochondrial and peroxisomal fission and fusion, that led to progressive neurological dysfunction and ultimately death. Evaluation of our patient’s fibroblast mitochondria further confirmed the pathogenicity of this variant.
Expanding phenotype of DNM1L variants
Patients with DNM1L variants previously reported are summarized below (see Table). Our patient presented with mild global developmental delays in infancy - mainly expressive language and fine motor domains - followed by late childhood progressive paroxysmal dystonia triggered by exercise and anxiety that responded temporarily to dopamine supplementation. He had concomitant low CSF serotonin and dopamine and an EEG suggesting a propensity towards generalized seizures. In early adolescence, he presented with bilateral multifocal myoclonus, progressing rapidly to super-refractory status epilepticus.
Two patients with the same p.R403C DNM1L variant were recently reported in the literature.6 These patients exhibited normal neurodevelopment until ages 4 and 5 years, when they developed focal clonic seizures evolving into refractory status epilepticus with subsequent moderate-to-severe cognitive regression and refractory epilepsy.6 Interestingly, these patients both had unilateral thalamic lesions that were T2 hyperintense and demonstrated restricted diffusion acutely.6 Our patient had similar, but bilateral, thalamic lesions with additional involvement of his basal ganglia and hippocampi (Figure C). His bilateral hippocampal changes likely reflect, to some extent, persistent seizure activity.
Prolonged critical care admission for refractory status epilepticus is associated at times with mild global or focal brain atrophy, reflecting some combination of extremely high seizure burden, and the medications used to treat these persistent seizures (i.e. barbiturates). However, in our patient the brain atrophy was severe and to a much greater extent than expected from the refractory status epilepticus and treatments alone, suggesting the underlying mitochondrial etiology had contributed to this devastatingly rapid global brain atrophy. It also suggests an unrelenting increased energy imbalance between ongoing seizures and mitochondria that were unable to adequately meet these increased energy demands, consequently leading to the diffuse neuronal and glial cell death observed over 9 weeks (Figure D–F).
Previous functional characterization of the p.R403C DNM1L variant in mouse embryonic fibroblasts revealed a dominant negative mechanism that impaired mitochondrial fission.6 Cell lines cultured from this transgenic p.R403C murine model showed abnormally elongated and tubular mitochondria, demonstrating clearly the pathogenicity of this variant.6 We confirmed this observation in humans by culturing our patient’s fibroblasts and demonstrating a lack of normal mitochondria with predominant elongated and tubular morphology.
Of the twelve previously reported cases (see Table), eight had more severe disease, presenting in infancy with profound developmental delay, epilepsy, encephalopathy, and often death.3,4,7–10 The p.R403C variant appears to give the mildest clinical phenotype when compared to other de novo DNM1L variants. Histologically, p.R403C had a milder morphological abnormality compared to the c.1184C>A (p.A395D) variant.6 While the majority of the twelve cases have de novo variants in DNM1L (8/12; 66%), 4 cases (2 sets of siblings) had recessive inheritance of DNM1L variants. In one sibling pair, compound heterozygous frameshift variants were identified leading to an extremely severe phenotype with death prior to one month; however heterozygous carrier parents were unaffected. Yoon and colleagues speculated that homozygous knockout of DNM1L is likely not compatible with life, as supported by the cases in their study as well as mouse DNM1L knockout models.7,11 In contrast, the second sibling pair with compound heterozygous DNM1L variants, including a frameshifting variant (p.Glu116Lysfs*6) and a missense variant (p.Ser36Gly), had relatively mild phenotypes with developmental delays in late infancy and no catastrophic epilepsy to date, with unaffected carrier parents.5 Unlike the vast majority of previously described de novo missense variants which have been experimentally proven or speculated to be dominant negative, the missense variant, p.Ser36Gly, in the compound heterozygous siblings was shown experimentally to be a hypomorphic. Altogether, it seems as if haploinsufficiency alone is not enough to cause a phenotype, as parent carriers are unaffected and compound heterozygosity where one allele is knocked out and the other is hypomorphic may lead to a less severe phenotype as compared to the de novo, dominant negative pathogenic variation in DNM1L. While more cases are necessary to substantiate these findings, a genotype-phenotype correlation between dominant negative and loss-of-function variation in DNM1L seems to be emerging.
Even prior to the WES results, our clinical suspicion for mitochondrial dysfunction was high, and we chose to avoid valproic acid which can exacerbate injury. A ketogenic diet was cautiously introduced, as it has shown efficacy in treating seizures among children with super-refractory status epilepticus12, among children with refractory myoclonic status epilepticus13, and among patients with refractory epilepsy secondary to mitochondrial disease.14 Ketosis was maintained for a two week span without benefit.
Importance of whole exome sequencing
Despite extensive evaluation, typical abnormalities observed in mitochondrial and peroxisomal diseases were not present in our patient. This included normal serum and CSF lactate, normal very long chain fatty acids, and a muscle biopsy that revealed normal histology and normal mitochondrial respiratory chain enzyme analysis. These abnormalities were also absent in most of the other DNM1L patients previously reported (see Table). After almost one month of aggressive critical care management we had yet to identify an etiology. Rather than doing a step-wise approach with genetic testing (ie. epilepsy panel – none of which currently test for DNM1L), we immediately opted for WES. Because of his critical condition we chose rapid WES, which significantly reduced the turnaround time from 3–4 months down to 2 weeks. While the methodologies between rapid WES and standard WES are often the same, rapid WES typically has a higher cost and can require parental samples for analysis. In our case, rapid trio-based WES identified a heterozygous pathogenic variant affecting DNM1L that explained his devastating neurological condition in 13 days. In the context of a critical care situation, as with our patient, the rapid results were of great value because these findings enabled his parents to make a more informed decision to allow natural death to occur in the context of a truly grim prognosis. In addition, given that neither parent carried a pathogenic variant, it is significantly less likely that their younger daughter is similarly affected, however low level or gonadal mosaicism cannot be entirely ruled out by WES. As the turnaround time for WES improves, its use, even in the critical care setting, can lead to changes in patient care, provide knowledge of new diseases, and importantly, give answers to suffering families.
Bedside-to-bench research connects the clinical presentation to the cellular abnormality
Identification of the DNM1L variant led to targeted assessment of our patient’s fibroblast mitochondrial morphology. This provided histologic proof that the p.R403C variant was associated with abnormally elongated and tubular mitochondrial morphology (Figure G–I) and confirmed the causative nature of his DNM1L variant. Morphological analysis from mitochondria and peroxisomes from patients affected by different DNM1L variants (see Table), have shown similar elongated and tubular morphology.3,5,10
Conclusion:
The integration of bedside-to-bench collaboration in a critical care setting allowed for quick characterization and confirmation of genotype pathogenicity of our patient’s de novo p.R403C DNM1L variant. We further expand the clinical presentation of DNM1L variants to include late childhood onset progressive paroxysmal dystonia and adolescent-onset super-refractory myoclonic status epilepticus contributing to rapidly progressive global cerebral atrophy and death. DNM1L variants should be considered in children across the pediatric age spectrum with cognitive or developmental impairment, especially when paroxysmal dystonia and refractory epilepsy with recurrent episodes of status epilepticus are present. As rapid whole exome sequencing becomes more readily available and affordable, its relevance in the acute pediatric critical care setting will certainly improve.
Acknowledgement:
We thank our patient’s family for allowing us to share his story and the innumerable health providers who contributed to his care.
Footnotes
Disclosures:
The authors do not have any potential conflicts of interest.
The authors have no potential conflicts of interests and no financial support was obtained to produce this manuscript.
References:
- 1.Chen H, Chan DC. Mitochondrial dynamics--fusion, fission, movement, and mitophagy--in neurodegenerative diseases. Hum Mol Genet 2009;18:R169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frohlich C, Grabiger S, Schwefel D, et al. Structural insights into oligomerization and mitochondrial remodelling of dynamin 1-like protein. EMBO J 2013;32:1280–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waterham HR, Koster J, van Roermund CW, Mooyer PA, Wanders RJ, Leonard JV. A lethal defect of mitochondrial and peroxisomal fission. N Engl J Med 2007;356:1736–41. [DOI] [PubMed] [Google Scholar]
- 4.Vanstone JR, Smith AM, McBride S, et al. DNM1L-related mitochondrial fission defect presenting as refractory epilepsy. Eur J Hum Genet 2016;24:1084–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nasca A, Legati A, Baruffini E, et al. Biallelic Mutations in DNM1L are Associated with a Slowly Progressive Infantile Encephalopathy. Hum Mutat 2016;37:898–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fahrner JA, Liu R, Perry MS, Klein J, Chan DC. A novel de novo dominant negative mutation in DNM1L impairs mitochondrial fission and presents as childhood epileptic encephalopathy. Am J Med Genet A 2016;170:2002–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoon G, Malam Z, Paton T, et al. Lethal Disorder of Mitochondrial Fission Caused by Mutations in DNM1 L. J Pediatr 2016;171:313–6 e1–2. [DOI] [PubMed] [Google Scholar]
- 8.Sheffer R, Douiev L, Edvardson S, et al. Postnatal microcephaly and pain insensitivity due to a de novo heterozygous DNM1L mutation causing impaired mitochondrial fission and function. Am J Med Genet A 2016;170:1603–7. [DOI] [PubMed] [Google Scholar]
- 9.Chao YH, Robak LA, Xia F, et al. Missense variants in the middle domain of DNM1L in cases of infantile encephalopathy alter peroxisomes and mitochondria when assayed in Drosophila. Hum Mol Genet 2016;25:1846–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaha K, Matsumoto H, Itoh M, et al. DNM1L-related encephalopathy in infancy with Leigh syndrome-like phenotype and suppression-burst. Clin Genet 2016;90:472–4. [DOI] [PubMed] [Google Scholar]
- 11.Ishihara N, Nomura M, Jofuku A, et al. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol 2009;11:958–66. [DOI] [PubMed] [Google Scholar]
- 12.Appavu B, Vanatta L, Condie J, Kerrigan JF, Jarrar R. Ketogenic diet treatment for pediatric super-refractory status epilepticus. Seizure 2016;41:62–5. [DOI] [PubMed] [Google Scholar]
- 13.Caraballo RH, Valenzuela GR, Armeno M, Fortini S, Mestre G, Cresta A. The ketogenic diet in two paediatric patients with refractory myoclonic status epilepticus. Epileptic disorders : international epilepsy journal with videotape 2015;17:491–5. [DOI] [PubMed] [Google Scholar]
- 14.Paleologou E, Ismayilova N, Kinali M. Use of the Ketogenic Diet to Treat Intractable Epilepsy in Mitochondrial Disorders. Journal of clinical medicine 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]