Abstract
Background
Myocardial and neuronal injury occur commonly after noncardiac surgery. We examined whether patients who had perioperative myocardial injury (PMI) also incurred neuronal injury, and whether myocardial and neuronal injury were associated with similar changes in inflammatory markers or overlapping clinical predictors.
Methods
A total of 114 individuals >65 yr old were recruited from two ongoing perioperative cohort studies (NCT02926417; NCT03124303). Plasma samples were collected before and daily after surgery to process assays for troponin I (PMI), neurofilament light (NfL; neuronal injury) and multiplexed plasma cytokines (inflammation). The primary outcome was the change in NfL in individuals with PMI (>40 pg ml−1 increase in troponin above preoperative values). We conducted logistic regression to identify if there were shared clinical predictors for myocardial and neuronal injury.
Results
Ninety-six patients had paired NfL and troponin data. Twenty-three of 94 subjects (24%) with PMI had greater increases in NfL (median [inter-quartile range, IQR]: 29 pg ml−1 [3–95 pg ml−1]; 2.8-fold increase) compared with subjects with no troponin increase (8 pg ml−1 [3–20]; 1.3-fold increase; P=0.008). PMI was associated with increased interleukin (IL)-1ra (P=0.005), IL-2 (P=0.045), IL-8 (P=0.002), and IL-10 (P<0.001). Logistic regression showed that intraoperative hypotension was associated with PMI (P=0.043). Preoperative stroke (P=0.041) and blood loss (P=0.002), but not intraoperative hypotension, were associated with increased NfL.
Conclusions
Postoperative troponin increases were associated with changes in NfL and inflammatory cytokines. Increases in troponin, but not NfL, were associated with intraoperative hypotension, suggesting differences in the mechanisms contributing to neuronal and myocardial injury.
Keywords: delirium, inflammation, injury, myocardial, neuronal, surgery, troponin
Editor's key points.
-
•
Myocardial injury after noncardiac surgery occurs commonly and appears to cluster with other organ injury, which is often subclinical.
-
•
The authors examined whether elevated biomarkers for myocardial and neuronal injury occur concomitantly and whether they were linked with acute inflammation (estimated by plasma cytokine changes).
-
•
Myocardial injury was defined by >40 pg ml−1 increase in troponin I from preoperative values; neurofilament light (NfL) served as a marker for neuronal injury.
-
•
Twenty-three of 94 (24%) subjects had myocardial injury which was accompanied by greater increases in NfL and higher levels of chiefly anti-inflammatory cytokines.
-
•
Neuronal injury appears to be more common in patients with myocardial injury.
Several studies have shown a correlation between postoperative troponin levels and mortality after cardiac and noncardiac surgeries.1, 2, 3 Although overt injury associated with clinical symptoms remains uncommon, plasma biomarkers may identify lesser injuries that do not develop clinical symptoms. Indeed, postoperative increases in cardiac troponin in the absence of electrocardiogram changes are more common than those associated with electrocardiogram changes, and yet have been associated with similar risks of postoperative mortality.1,3,4 Whether perioperative myocardial injury (PMI) is causally related to myocardial ischaemia or representative of the stress response, a more generalised predisposition to perioperative complications, or both warrants further enquiry.5,6 Similarly, neurofilament light (NfL) has been identified as a potential plasma biomarker of neuronal injury. A postoperative increase in NfL has been observed after surgery,7, 8, 9 and is associated with postoperative delirium,8 as has covert stroke diagnosed on MRI,10 which suggests that neuronal injury may be an important component of the pathogenesis of delirium.
Inflammation has been associated with both neurological and cardiac injury in the setting of sepsis or cardiac surgery.11,12 Preoperative inflammation may be associated with postoperative myocardial injury.13 Similarly, our previous research suggests that perioperative inflammation plays an important role in neuronal injury.8 If there is a common inflammatory aetiology underpinning neuronal and cardiac injury, circulating inflammatory cytokines may be associated with increases in both NfL and troponin.
Given the prognostic importance of PMI after noncardiac surgery3,5,11 and neuronal injury, we were interested in exploring whether there was common underlying aetiology. Therefore, we tested whether cardiac and neuronal injury were associated with similar changes in inflammatory markers or overlapping clinical including intraoperative hypotension.14,15 Because our previous work on NfL has focused on the postoperative change from preoperative values (to detect the changes attributable to perioperative care), we analysed whether a significant change in troponin (defined as a postoperative increase in troponin of >40 pg ml−1 from preoperative levels) was associated with an increase in NfL as our primary study outcome.
Methods
Study design
The data are derived from two ongoing prospective observational cohort studies registered with ClinicalTrials.gov (NCT02926417 and NCT03124303) approved by the University of Wisconsin–Madison Institutional Review Board (2015-0960 and 2015-0374, respectively). Written informed consent was provided before surgery.
Inclusion and exclusion criteria
Adult patients scheduled for major elective non-intracranial, noncardiac surgery under general anaesthesia and requiring at least a 2 day hospital stay were eligible.8 Full inclusion and exclusion criteria for both studies are presented in Supplementary Tables S1 and S2.
Sample collection
Blood samples were taken preoperatively and for the first (up to) 4 postoperative hospital days, if possible. Plasma samples were prepared from EDTA-containing tubes preoperatively, and in the morning (06.00–10.00) of each postoperative day, spun and stored at –80°C.
Troponin assay
Samples were then sent as a batch for troponin TnI measured by MILLIPLEX® MAP Human Cardiovascular Disease Magnetic Bead single plex (Millipore Inc., Burlington, MA, USA) completed by Eve Technologies (Calgary, AB, Canada). This assay shows <10% intra-assay and <20% inter-assay coefficients of variation (precision) and 100% recovery of spiked standards (accuracy). We binarised the troponin values into positive (40 pg ml−1 increase in troponin from preoperative values) or negative.3 To fit the data as continuous variables for descriptive (non-statistical purposes), undetected values were ascribed the lowest limit of detection on the assay (0.1 ng ml−1). As the data were heavily skewed with many values under the lower limit of detection, we did not analyse troponin as continuous data variable. For our supplementary analysis, patients were designated TPP (postoperative troponin positive patient) if the absolute value of the troponin assay was >40 pg ml−1 on any of postoperative days 1–4. This increase in postoperative rise in troponin defined PMI.
Cytokine and NfL assays
A cytokine multiplex assay was completed for interleukin 1 beta (IL-1β), IL-1 receptor antagonist, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, monocyte chemoattractant protein 1 (MCP-1), and tumour necrosis factor-alpha (TNFα) (Eve Technologies). Based on running duplicates, the mean coefficient of variation was 15%, and individual cytokine data are described in Supplementary Table S3. NfL concentration was also measured using a Simoa method, as previously described in detail,8,16 which detects total NfL levels. All biomarker data were log-transformed to correct the strong skew. When the difference between preoperative and POD1 samples was calculated, we took the difference of the log-transformed data.
Assessment of delirium
Preoperatively, and twice daily postoperatively, participants underwent delirium assessments with the Confusion Assessment Method (CAM)/3D-CAM,17 or the CAM-ICU18 if the patient was intubated. Delirium severity was assessed with the validated Delirium Rating Scale-98 (DRS).19
Primary outcome
The primary outcome was the comparison of preoperative to postoperative day 1 change in NfL for PMI+ (40 pg ml−1 increase in troponin from preoperative values) and PMI– subjects.
Secondary outcome
Secondary outcomes were:
-
1.
A priori, we tested whether postoperative day 1 increases in cytokines were associated with troponin positivity. We undertook false discovery rate (FDR) correction for testing across the 10 cytokines.
-
2.
We tested whether PMI after surgery was more likely to be associated with delirium, compared with subjects in whom troponin did not increase from preoperative values.
Sensitivity analyses
We followed several leading studies in the field in designating troponin positive events based solely on postoperative troponin values (designated positive based on a value of >40 pg ml−1).3 Only one value greater than the threshold was required for this diagnosis as a troponin positive events. We also tested whether preoperative cytokine levels were associated with raised preoperative troponin values.
Statistical analysis
Mean (standard deviation) or n (%) values are presented unless otherwise stated. Multiple logistic regression was conducted to predict postoperative PMI with age, National Surgical Quality Improvement Program risk of Death (NSQIP-D), sex, hypertension treatment, coronary artery disease, prior stroke or transient ischaemic attack (TIA), vascular surgery (yes/no), intraoperative blood loss, and intraoperative hypotension (defined as the area under the curve that is more than 10% below the preoperative mean arterial pressure). The same predictors were used to identify predictors of NfL increases based on splitting the data at the median threshold. This differed slightly from our prior work,8 owing to the addition of variables associated with cardiac risk and the use of logistic regression. However, we also checked whether similar variables predicted increases in NfL as a continuous variable using linear regression. Data were analysed using R (R Foundation for Statistical Computing, Vienna, Austria). A P value <0.05 was considered statistically significant.
Sample size estimation
The sample size was determined based on prior data suggesting a 20% incidence in myocardial injury after noncardiac surgery (MINS).1,20 Although these previous studies used an absolute threshold to determine PMI status, we defined PMI as a 40 pg ml−1 increase in troponin from baseline. We estimated that there would be a 20 pg ml−1 difference in NfL levels (assuming a standard deviation of 24 pg ml−1) between individuals with, vs without, troponin increases.7 This required that we recruit 70 patients to provide 80% power (P<0.05).
Results
Subject characteristics
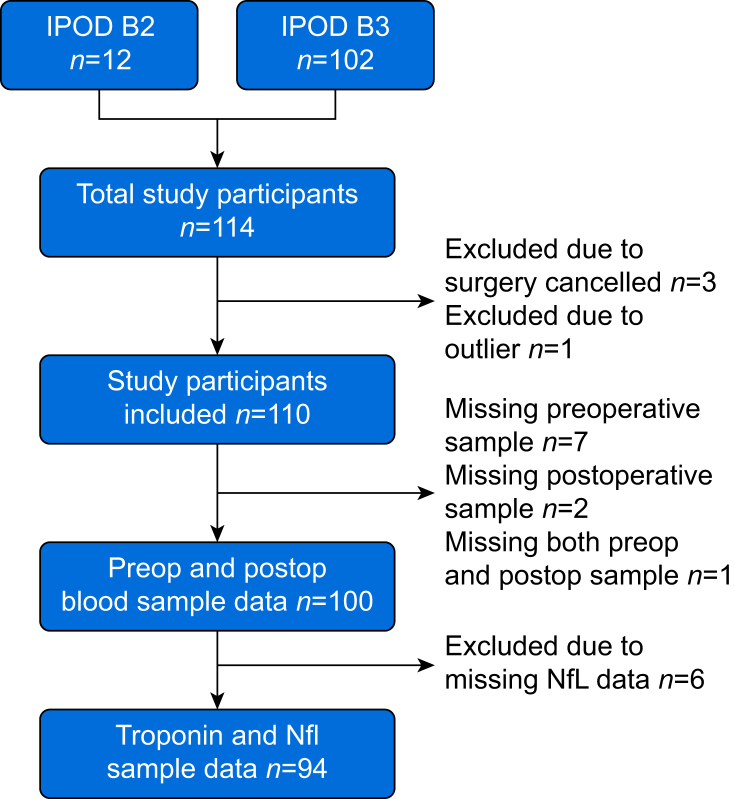
A total of 114 participants were eligible from each study. After various exclusions (Fig. 1), data from 94 subjects was available for analysis. Clinical characteristics of the subjects eligible for inclusion are shown in Table 1. Before surgery, there were 22/114 (19%) patients with detectable troponins (19%). One patient had a clinically detected postoperative non-ST elevation myocardial infarction with similar values for troponin reported by clinical (20.6 pg ml−1) and research (20.4 pg ml−1) measurements.
Fig 1.
STROBE diagram. STROBE, Strengthening The Reporting of OBservational Studies in Epidemiology. IPOD, Interventions for Postoperative Delirium - Biomarker; NfL, neurofilament light; postop, postoperative; preop, preoperative.
Table 1.
Characteristics of the patient cohort with troponin and neurofilament light data. ASA, American Society of Anesthesiologists; AUC BP <10%, area under curve for blood pressure <90% of baseline; NSQIP-D, National Surgical Quality Improvement Program Risk of Death; TIA, transient ischaemic attack; TPN, postoperative troponin negative patient; TPP, postoperative troponin positive patient.
| Perioperative myocardial injury (PMI n=94) |
Postoperative troponin analysis (n=94) |
|||
|---|---|---|---|---|
| PMI+patients (n=23) | PMI–patients (n=71) | TPP (n=34) | TPN (n=60) | |
| Age (yr) | 68.4 (10.0) | 71.1 (5.1) | 69.5 (8.6) | 70.9 (5.3) |
| Female sex | 10 (43%) | 30 (42%) | 14 (41%) | 26 (43%) |
| ASA physical status >2 | 16 (70%) | 41 (63%) | 21 (62%) | 38 (63%) |
| NSQIP-D | 4.8 (7.0) | 2.1 (3.3) | 3.8 (6.0) | 2.1 (3.4) |
| Smoker | 8 (35%) | 12 (17%) | 9 (26%) | 11 (18%) |
| Chronic obstructive pulmonary disease | 5 (22%) | 7 (10%) | 6 (18%) | 6 (10%) |
| Obstructive sleep apnoea | 2 (9%) | 22 (31%) | 4 (12%) | 20 (33%) |
| BMI (kg m−2) | 29.0 (6.6) | 29.0 (5.9) | 28.9 (5.6) | 28.9 (6.2) |
| BP (systolic; mm Hg) | 134 (17) | 134 (19) | 133 (15) | 134 (19) |
| Hypertension | 18 (78%) | 48 (68%) | 27 (79%) | 39 (65%) |
| Stroke/TIA | 3 (13%) | 3 (4%) | 3 (9%) | 3 (5%) |
| Vascular surgery | 18 (78%) | 26 (37%) | 20 (59%) | 24 (40%) |
| Blood loss (ml) | 3783 (43 780) | 1176 (1866) | 2856 (3884) | 1223 (1967) |
| Operation time (min) | 458 (187) | 297 (131.8) | 384 (196) | 309 (134) |
| Intraoperative hypotension (AUC BP <90%) | 241 967 (205 820) | 127 384 (148 067) | 172 744 (187 251) | 140 298 (156 641) |
| Renal failure | 1 (4%) | 2 (3%) | 2 (6%) | 1 (2%) |
| Coronary artery disease | 3 (13%) | 9 (13%) | 4 (12%) | 8 (13%) |
Primary outcome: postoperative myocardial injury associated with neuronal injury
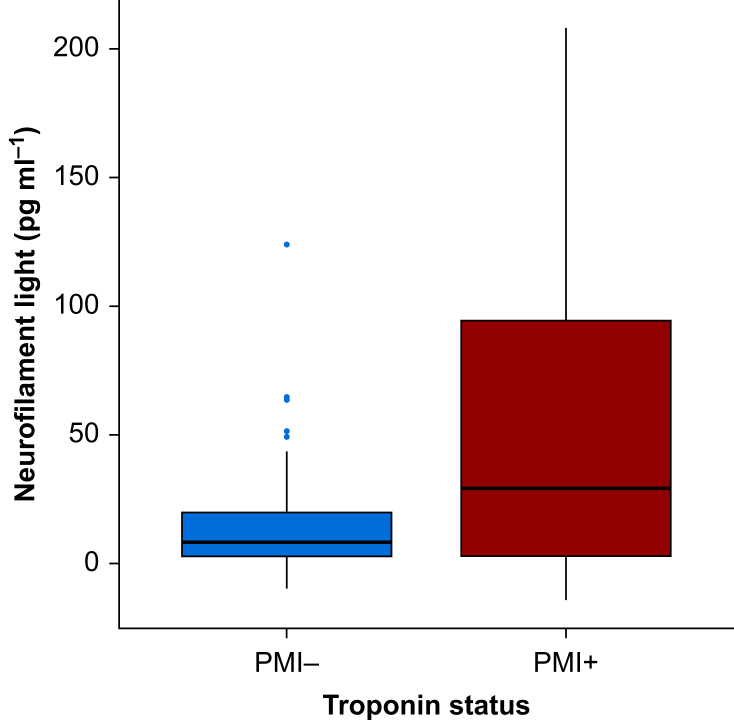
In 23 subjects (24%), the troponin increase was greater than 40 pg ml−1 from preoperative levels (PMI+). These individuals had greater increases in NfL (median [inter-quartile range, IQR]: 29 pg ml−1 [3–95]; range –14 to 208 pg ml−1) compared with individuals without troponin increase (8 pg ml−1 [3–20]; range –10 to 124 pg ml−1; P=0.008; Fig. 2). Median values for NFL corresponded to a mean 1.3-fold increase (standard error of the mean, 1.1) in individuals without troponin elevation, and a 2.8-fold increase (standard error of the mean:1.2-fold) in individuals with troponin increases >40 pg ml−1.
Fig 2.
Postoperative day 1 change in neurofilament light based on postoperative myocardial injury (PMI) status. A boxplot showing median and inter-quartile range is presented.
Secondary outcomes
Troponin elevation and inflammation
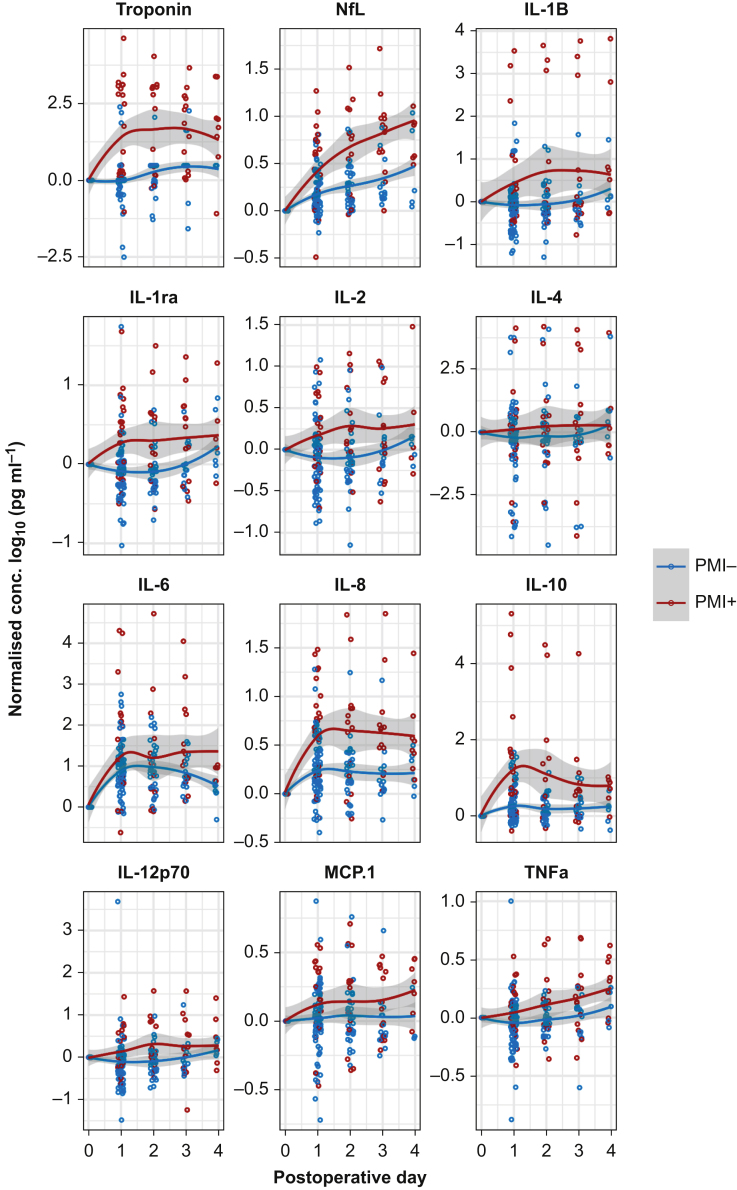
After FDR correction, PMI (>40 pg ml−1 increase from baseline) was associated with IL-1ra (P=0.005), IL-2 (P=0.045), IL-8 (P=0.002), and IL-10 (P<0.001) sampled on postoperative day 1 (Fig. 3; Supplementary Fig. S1).
Fig 3.
Time course of perioperative changes in biomarkers for patients with significant troponin changes from preoperative levels (red) or not (blue). Data are normalised to preoperative values and for the purposes of display only are shown as mean plus standard error of the mean across all panels. IL, interleukin; MCP-1, monocyte chemoattractant protein 1; NfL, neurofilament light; PMI, postoperative myocardial injury; Postop, postoperative; TNF, tumour necrosis factor.
Delirium and myocardial injury
PMI was associated with postoperative delirium (odds ratio=3.8; 95% confidence interval, 1.4–10.2); χ2 test, P=0.012) and more severe delirium (Mann–Whitney test, P=0.003).
Association between clinical features and changes in troponin and NfL
In univariate analyses, patients with PMI had a higher NSQIP-D (P=0.003), blood loss (P=0.025), intraoperative hypotension (area under the curve that is more than 10% below the preoperative mean arterial pressure, P=0.032), and were more likely to undergo vascular surgery (P<0.001). Multiple logistic regression analysis found that intraoperative hypotension was associated with PMI (Table 2). As the troponin analyses were based on a binary endpoint (positive yes/no), we binarised the NfL data based on the median of the data. Logistic regression again showed that preoperative stroke (P=0.041) and blood loss (P=0.002), but not intraoperative hypotension, were associated with higher NfL values (Table 2).
Table 2.
Logistic regression models for perioperative myocardial injury status and median change in neurofilament light. M, male; NSQIP-D, National Surgical Quality Improvement Project risk of Death; TIA, transient ischaemic attack.
| Postoperative myocardial injury |
Neurofilament light |
|||
|---|---|---|---|---|
| Z value | P value | Z value | P value | |
| Intercept | −1.053 | 0.293 | 0.836 | 0.403 |
| Age | 0.544 | 0.586 | −0.867 | 0.386 |
| Sex (M) | −0.736 | 0.461 | −1.103 | 0.270 |
| NSQIP-D | 0.540 | 0.589 | −0.981 | 0.327 |
| Hypertension | −0.432 | 0.665 | −0.166 | 0.868 |
| Coronary artery disease | −0.309 | 0.757 | 0.228 | 0.820 |
| Stroke/TIA | 0.995 | 0.320 | 2.049 | 0.041∗ |
| Vascular surgery | 1.870 | 0.062 | −1.902 | 0.057 |
| Blood loss | 0.674 | 0.500 | 3.036 | 0.002∗ |
| Intraoperative hypotension | 2.028 | 0.043∗ | 0.410 | 0.682 |
∗p < 0.05
Sensitivity analyses for diagnosis of troponin positive status after surgery alone
Troponin positive events after surgery occurred in 34 patients, which was strongly associated with raised preoperative troponin values (P<0.001; Supplementary Fig. S2). Troponin positive events after surgery were associated with increases in NfL and IL-10 (Supplementary Fig. S3) and intraoperative hypotension, but not blood loss (Supplementary Table S4). We also tested whether any of the preoperative cytokines were associated with preoperative troponin positivity. After FDR correction, IL-2 was significantly associated with preoperative troponin positivity (P=0.049, Supplementary Fig. S4).
Discussion
We found that NfL levels increased on postoperative day 1 to a greater extent in patients with PMI. These data suggest that patients who are vulnerable to myocardial injury may also be vulnerable to neuronal injury. The perioperative change in troponin was associated with changes in IL-1ra, IL-2, IL-8, and IL-10. These data suggest that postoperative day 1 changes in inflammation may be associated with myocardial injury. Of course, these data do not imply a causal relationship but do suggest there may be some pathological overlap with neuronal injury (which we recently showed was associated with IL-1beta, IL-8, and IL-108). These data are consistent with our sampling on postoperative day 1 and recent evidence that troponin increases are evident within 6 h of surgery.5
Overall, in this cohort, myocardial injury appears to be driven by events on the day of surgery associated with the duration of intraoperative hypotension, as observed previously.15 Our data are largely coherent with recent data highlighting the role of preoperative inflammation (defined by the neutrophil/lymphocyte ratio) in predisposing to myocardial injury11 (although our data suggest that preoperative cytokines themselves may not identify that predisposition). Postoperative associations with the neutrophil chemoattractant, IL-8, and both troponin and NfL increase suggest a potential common mechanism centred around neutrophil activation. Further studies should attempt to manipulate inflammation to reduce the burden of cardiac and neuronal injury. In terms of clinical predictors, the regression models showed that PMI+ subjects were more likely to have lower intraoperative blood pressure. This represents a discordance from NfL increases, as intraoperative hypotension was not associated with neuronal injury in our analyses. Rather, NfL changes were associated with blood loss (though it is important to note that at the univariate level both blood loss and intraoperative hypotension were associated with PMI). Overall, our data suggest that patients who are vulnerable to myocardial injury may also be vulnerable to neuronal injury, but there are subtle differences in the provoking aetiology. However, convergence in the mechanisms of how inflammation may contribute to the cellular injury (in either organ) is suggested by overlapping cytokine predictors. We suspect that myocardial injury maybe driven by perioperative hypotension and autonomic stress,6 whereas neuronal injury may require a breakdown in the blood–brain barrier driven by inflammation.8 Given that inflammation is known to exacerbate ischaemic injury, our data suggest that inflammation may be a modifiable target to prevent the elaboration of perioperative cardiac and neuronal injury.
Our study had both strengths and limitations. We strictly adhered to multiple comparison correction, and it is worth noting that with a larger sample size we may have observed significant associations of additional cytokines with PMI status. Furthermore, the discordance of univariate and multivariate analyses makes us less confident that the clinical predictors of PMI and NfL increases do not overlap. Larger studies are required to test this more thoroughly. However, our data were primarily limited by our focus on postoperative sampling. More frequent collection on the day of surgery will be necessary to clarify whether shorter-term inflammatory effects are important in the pathogenesis of early myocardial injury.5 Also, it is important to note that, myocardial injury may evolve over time, at least in some subjects (Fig. 3). It is possible that this may reflect ongoing injury, or a secondary hit occurs in the postoperative period in certain vulnerable individuals. Future research should address whether postoperative factors exacerbate the injury incurred on postoperative day 1. With only one myocardial infarction in this cohort, we are limited in our ability to comment further on the relationship of MINS and perioperative myocardial infarction. It is important to reflect that the single myocardial infarction occurred on postoperative day 2 (not day 1). In this subject, the research troponin value on postoperative day 1 was 1100 pg ml−1, indicating the presence of myocardial injury (>40 pg ml−1) before the clinical non-ST elevation myocardial infarction. This patient appeared to progress from myocardial injury to non-ST elevation myocardial infarction. Future studies should identify factors that affect the transition from MINS to major adverse cardiac events and whether ongoing inflammation may be a modifiable risk factor for this transition. Another limitation is that although troponin has a diagnostic value for binarizing a myocardial injury diagnosis, NfL does not yet have a similar, clinically defined, important threshold. Yet, we found similar results for linear regression and logistic regression using a binarised threshold for NfL changes. This reassures us that the differences observed were not just attributable to differences in the analytic approach. Finally, NfL may be released after damage from non-intracranial insults including peripheral nerve injury. Future work should validate these findings with cerebrospinal fluid biomarkers and imaging biomarkers of cerebral injury.
Despite the intense research into PMI, our data question whether focus only on postoperative troponin values is sufficient for understanding modifiable risk factors in perioperative care. Our data highlight that preoperative testing is key for identification of subjects on a raised ‘troponin trajectory’. Indeed, identification of the modifiable factors for PMI may require that we examine the change from preoperative levels (as per our primary outcome). Nonetheless it is reassuring that in extensive supplementary analyses focused only on diagnosis of myocardial injury using only postoperative data, the results were consistent with the primary outcome.
In summary, our data suggest that patients who are vulnerable to PMI may also be vulnerable to neuronal injury. Furthermore, the differing associations with intraoperative hypotension suggest that there may be important differences in the underlying aetiologies of cardiac and neuronal injury, although inflammation may play an important role in both pathologies.
Authors' contributions
Design of the research: RDS, with input from RL and RAP
Data collection: RDS, LC, CC, MW, MP, AB, TB
Data analysis: RDS, CC, HZ, KB, TB
All authors contributed to interpretation and writing of the article.
Declarations of interest
HZ has served at scientific advisory boards for Denali, Roche Diagnostics, Wave, Samumed, Siemens Healthineers, Pinteon Therapeutics and CogRx, has given lectures in symposia sponsored by Fujirebio, Alzecure and Biogen, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). KB has served as a consultant, at advisory boards, or at data monitoring committees for Abcam, Axon, Biogen, JOMDD/Shimadzu. Julius Clinical, Lilly, MagQu, Novartis, Roche Diagnostics, and Siemens Healthineers, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. The other authors declare that they have no conflicts of interest.
Funding
RDS, RL, and RAP are supported by US National Institutes of Health grant R01 AG063849-01. RDS is supported by US National Institutes of Health grant 1R01NS117901-01. HZ is a Wallenberg Scholar supported by grants from the Swedish Research Council (#2018-02532), the European Research Council (#681712), Swedish State Support for Clinical Research (#ALFGBG-720931), the Alzheimer's Drug Discovery Foundation (ADDF), USA (#201809-2016862), and the UK Dementia Research Institute at UCL. KB is supported by the Swedish Research Council (#2017-00915), the Alzheimer's Drug Discovery Foundation (ADDF), USA (#RDAPB-201809-2016615), the Swedish Alzheimer Foundation (#AF-742881), Hjärnfonden, Sweden (#FO2017-0243), the Swedish state under the agreement between the Swedish government and the County Councils, the ALF-agreement (#ALFGBG-715986), and European Union Joint Program for Neurodegenerative Disorders (JPND2019-466-236).
Handling editor: Gareth Ackland
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2020.10.012.
Appendix ASupplementary data
The following is the Supplementary data to this article:
References
- 1.Puelacher C., Lurati Buse G., Seeberger D. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation. 2018;137:1221–1232. doi: 10.1161/CIRCULATIONAHA.117.030114. [DOI] [PubMed] [Google Scholar]
- 2.Croal B.L., Hillis G.S., Gibson P.H. Relationship between postoperative cardiac troponin I levels and outcome of cardiac surgery. Circulation. 2006;114:1468–1475. doi: 10.1161/CIRCULATIONAHA.105.602370. [DOI] [PubMed] [Google Scholar]
- 3.Devereaux P.J., Chan M.T., Alonso-Coello P. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2012;307:2295–2304. doi: 10.1001/jama.2012.5502. [DOI] [PubMed] [Google Scholar]
- 4.Noordzij P.G., van Geffen O., Dijkstra I.M. High-sensitive cardiac troponin T measurements in prediction of non-cardiac complications after major abdominal surgery. Br J Anaesth. 2015;114:909–918. doi: 10.1093/bja/aev027. [DOI] [PubMed] [Google Scholar]
- 5.Ackland G.L., Abbott T.E.F., Jones T.F. Early elevation in plasma high-sensitivity troponin T and morbidity after elective noncardiac surgery: prospective multicentre observational cohort study. Br J Anaesth. 2020;124:535–543. doi: 10.1016/j.bja.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.May S.M., Abbott T.E.F., Del Arroyo A.G. MicroRNA signatures of perioperative myocardial injury after elective noncardiac surgery: a prospective observational mechanistic cohort study. Br J Anaesth. 2020;125:661–671. doi: 10.1016/j.bja.2020.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evered L., Silbert B., Scott D.A., Zetterberg H., Blennow K. Association of changes in plasma neurofilament light and tau levels with anesthesia and surgery: results from the CAPACITY and ARCADIAN studies. JAMA Neurol. 2018;75:542–547. doi: 10.1001/jamaneurol.2017.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casey C.P., Lindroth H., Mohanty R. Postoperative delirium is associated with increased plasma neurofilament light. Brain. 2019;143:47–54. doi: 10.1093/brain/awz354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alifier M., Olsson B., Andreasson U. Cardiac surgery is associated with biomarker evidence of neuronal damage. J Alzheimers Dis. 2020;74:1211–1220. doi: 10.3233/JAD-191165. [DOI] [PubMed] [Google Scholar]
- 10.Neuro VISION Perioperative covert stroke in patients undergoing non-cardiac surgery (NeuroVISION): a prospective cohort study. Lancet. 2019;394:1022–1029. doi: 10.1016/S0140-6736(19)31795-7. [DOI] [PubMed] [Google Scholar]
- 11.Hunter J.D., Doddi M. Sepsis and the heart. Br J Anaesth. 2010;104:3–11. doi: 10.1093/bja/aep339. [DOI] [PubMed] [Google Scholar]
- 12.Landis R.C., Brown J.R., Fitzgerald D. Attenuating the systemic inflammatory response to adult cardiopulmonary bypass: a critical review of the evidence base. J Extra Corpor Technol. 2014;46:197–211. [PMC free article] [PubMed] [Google Scholar]
- 13.Ackland G.L., Abbott T.E.F., Cain D. Preoperative systemic inflammation and perioperative myocardial injury: prospective observational multicentre cohort study of patients undergoing non-cardiac surgery. Br J Anaesth. 2019;122:180–187. doi: 10.1016/j.bja.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Waes J.A., van Klei W.A., Wijeysundera D.N., van Wolfswinkel L., Lindsay T.F., Beattie W.S. Association between intraoperative hypotension and myocardial injury after vascular surgery. Anesthesiology. 2016;124:35–44. doi: 10.1097/ALN.0000000000000922. [DOI] [PubMed] [Google Scholar]
- 15.Abbott T.E.F., Pearse R.M., Archbold R.A. A prospective international multicentre cohort study of intraoperative heart rate and systolic blood pressure and myocardial injury after noncardiac surgery: results of the VISION study. Anesth Analg. 2018;126:1936–1945. doi: 10.1213/ANE.0000000000002560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gisslen M., Price R.W., Andreasson U. Plasma concentration of the nurofilament light protein (NFL) is a biomarker of CNS injury in HIV infection: a cross-sectional study. EBioMedicine. 2016;3:135–140. doi: 10.1016/j.ebiom.2015.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcantonio E.R., Ngo L.H., O'Connor M. 3D-CAM: derivation and validation of a 3-minute diagnostic interview for CAM-defined delirium: a cross-sectional diagnostic test study. Ann Intern Med. 2014;161:554–561. doi: 10.7326/M14-0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ely E.W., Inouye S.K., Bernard G.R. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 19.Trzepacz P.T., Mittal D., Torres R., Kanary K., Norton J., Jimerson N. Validation of the Delirium Rating Scale-revised-98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatr Clin Neurosci. 2001;13:229–242. doi: 10.1176/jnp.13.2.229. [DOI] [PubMed] [Google Scholar]
- 20.Ekeloef S., Alamili M., Devereaux P.J., Gogenur I. Troponin elevations after non-cardiac, non-vascular surgery are predictive of major adverse cardiac events and mortality: a systematic review and meta-analysis. Br J Anaesth. 2016;117:559–568. doi: 10.1093/bja/aew321. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.