Abstract
Objective:
Inspiratory holds with measures of airway pressure to estimate driving pressure (elastic work) are often limited to patients without respiratory effort. We sought to evaluate if measures of airway pressure during inspiratory holds could be used for patients with spontaneous respiratory effort during mechanical ventilation to estimate the degree of spontaneous effort and elastic work.
Design:
We compared the direction and degree of change in airway pressure during inspiratory holds versus esophageal pressure through secondary analysis of physiologic data from children with PARDS who had evidence of spontaneous respiration while on pressure control or pressure support ventilation. Children were enrolled from the intensive care units at Children’s Hospital Los Angeles.
Measurements and Main Results:
From airway pressure, we defined “plateau - peak pressure” as PMI, which was divided into three categories for analysis (< −1 (“negative”), between −1 and 1 (“neutral”), and > 1 cmH2O (“positive”). 30 children (age 36.8 (16.1–70.3) months) from 65 study days, comprising 118 inspiratory holds were included. PMI was “negative” in 29 cases, was “neutral” in 17 cases, and was “positive” in 72 cases. As PMI went from negative to neutral to positive, there was larger negative deflection in esophageal pressure −5.0 (−8.2–1.9), −5.9 (−7.6–4.3), and −10.7 (−18.1–7.9) cmH2O (p < 0.0001), respectively. There was a correlation between max negative esophageal pressure and PMI (r = −0.52), and when PMI was ≥ 7 cmH2O, the max negative esophageal pressure was >10 cmH2O. There was a stronger correlation between PMI and markers of elastic work from esophageal pressure (r = 0.84).
Conclusions:
The magnitude of plateau minus peak pressure during an inspiratory hold is correlated with the degree of inspiratory effort, particularly for those with high elastic work. It may be useful to identify patients with excessively high effort or high driving pressure.
Keywords: Inspiratory hold, plateau pressure, driving pressure, acute respiratory distress syndrome, inspiratory effort
Introduction
There is a push to preserve spontaneous breathing to prevent ventilator induced diaphragm dysfunction during mechanical ventilation (1, 2). However, when respiratory effort is strong, spontaneous breathing may exacerbate patient self-inflicted lung injury (P-SILI) (3, 4) and lead to high driving pressure. Ventilator management strategies must balance lung and diaphragm protective principles, which may include maintaining spontaneous breathing as long as respiratory effort is not excessively high. Identifying excessively high respiratory effort and high driving pressure is therefore paramount.
The airway pressure alone during mechanical ventilation does not fully reflect the pressure in the respiratory system because it ignores the reduction in pleural pressure the patient generates by contracting respiratory muscles. The accepted standard to measure respiratory effort is esophageal pressure (Pes). However, Pes measurements are not routine and have technical limitations (5–7). Some methods including Pocc (Occlusion pressure) and P0.1 (The airway occlusion pressure 0.1s) have shown a strong correlation with esophageal pressure and can be used to identify adults with strong respiratory effort (8, 9).
However, pressure generated by the patient (and the ventilator) is a function of both resistive and elastic components, derived from the equation of motion of the respiratory system. Hence, a patient can have high effort or require high pressures on the ventilator related to increases in airway resistance, or impairments in respiratory system compliance. High effort from increased airway resistance can influence diaphragm function, but will have limited impact P-SILI or VILI (ventilator-induced lung injury), as the driving pressure may not be elevated. Driving pressure reflects the change in pressure which is a result of tidal ventilation (elastic work) (10–12) and is measured by performing an inspiratory hold.
Most studies have evaluated driving pressure for patients without spontaneous breathing (10). More recently, it has been suggested that driving pressure can be estimated from the plateau pressure during an inspiratory hold for patients undergoing pressure support ventilation (13–16), if the patient becomes passive during the inspiratory hold. When spontaneous effort is high, the plateau pressure is higher than the peak pressure, and this difference is reflective of the patient’s elastic workload (PMI: Pmusc, index). Driving pressure estimated using this method has been associated with mortality in adult patients undergoing pressure-support ventilation (16).
This method to estimate patient effort and measure driving pressure during an inspiratory hold has had limited application during assisted ventilation (ie. pressure control or volume control with spontaneous effort), and has not been applied to children. We sought to evaluate the potential applicability of using an inspiratory hold to estimate patient effort and driving pressure in mechanically ventilated children with PARDS by comparing the direction and degree of change in airway pressure during an inspiratory hold with the change in Pes. This non-invasive measure can help clinicians identify mechanically ventilated patients at high risk for P-SILI.
Methods
We performed secondary analysis of physiologic data from children on pressure control or pressure support ventilation, enrolled in an ongoing randomized clinical trial testing a lung and diaphragm protective ventilation strategy (REDvent, R01HL124666) (Clinical Trials.gov NCT03266016) (17) at Children’s Hospital Los Angeles.
Patients
All patients were enrolled in the parent REDvent study, which included children between 1 month and 18 years of age who are ventilated with pediatric ARDS, with no contra-indications to implementation of a lung and diaphragm protective ventilation strategy (i.e. intracranial hypertension, Do Not Resuscitate, severe lower airway obstruction). Full inclusion and exclusion criteria can be found in the supplement.
Ventilator Management
Patients were ventilated with Servo I (Maquet, Solna, Sweden), Hamilton G5 (Hamilton Medical, Bonaduz, Switzerland), or AVEA (CareFusion, Yorba Linda, CA, USA) ventilators. However in the process of data analysis, we identified that the AVEA ventilator does not permit airway pressure to climb above the set peak inspiratory pressure during an inspiratory hold, and adjusts flow to maintain pressure. As such, we excluded all measurements from the AVEA. Synchronized intermittent mandatory ventilation (SIMV) Pressure control (PC)/Pressure support (PS) and PS/Continuous Positive Airway Pressure were the standard ventilation modes used.
Protocol for monitoring
Physiologic waveforms of Pes, flow, and airway pressure were recorded daily, during both acute and weaning phases. All patients were intubated using cuffed endotracheal tubes, as is the standard in the ICU for children with PARDS, and the cuff was inflated prior to measurements to prevent air leak. A series of 3 inspiratory and expiratory holds were performed on each patient, each day. We selected two inspiratory holds with clean waveforms and a clear plateau to use for analysis. Airway pressure was measured with a proximal sampling line placed just after the endotracheal tube, along with a self-calibrating pneumotachometer (Viasys Variflex 51000–40094). One of 3 esophageal catheters were used, based on the size of the patient (Carefusion, Avea SmartCath 6, 7, or 8Fr). The amount of air inflated into the esophageal balloon was determined prior to each measurement using a previously validated calibration algorithm (22). All sensors were connected to a custom built hardware device (New Life Box, Applied Biosignals), which recorded data at a frequency of 200 Hz. Data was subsequently post-processed in a custom-built software program for analysis (designed in C#).
Definition of variables
The elements of esophageal and airway pressure used for analysis are described in Figure 1 and Table 1. The pressure just before the inspiratory hold was the peak pressure (Ppeak), the pressure that reached a plateau after the inspiratory hold was the plateau pressure (Pplat), and the difference between Pplat and Ppeak was Pmusc index (PMI) (14), which was our main variable of interest. The value obtained by subtracting Positive end-expiratory pressure (PEEP) from Pplat was driving pressure (ΔP: the pressure required to expand the volume during ventilation), and the value obtained by subtracting PEEP from Ppeak was dynamic ΔP (dynΔP: the pressure provided by the ventilator which includes both resistive and elastic components).
Fig. 1.
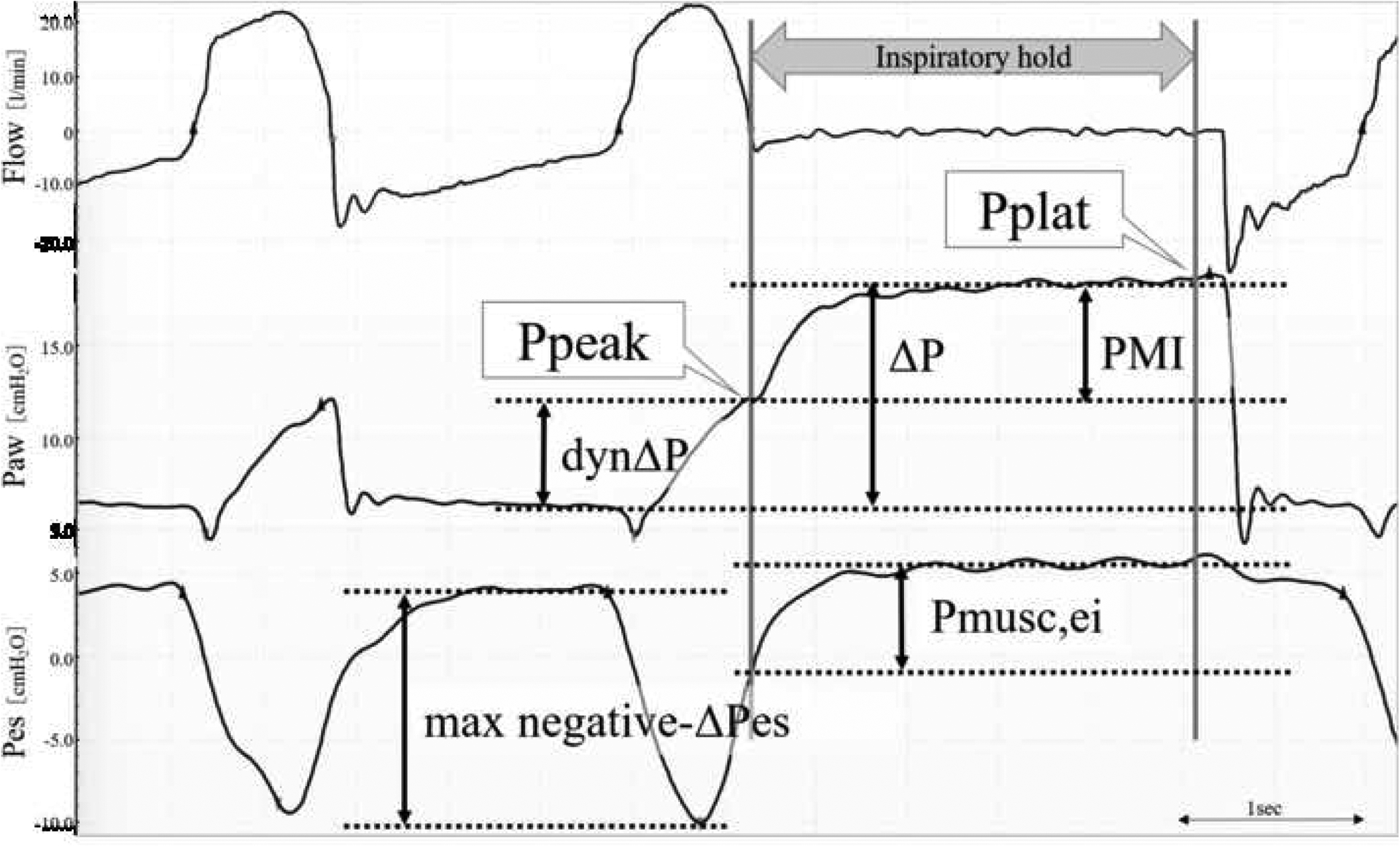
Physiologic waveforms of flow, airway pressure, and esophageal pressure (Pes) during the inspiratory hold.
Ppeak: Peak Pressure, Pplat: Plateau Pressure, PMI: Pmusc, index, dynΔP: Dynamic driving Pressure (Ppeak-PEEP), ΔP: Driving Pressure (Pplat-PEEP), max negative-ΔPes: Maximum negative esophageal pressure (resistive and elastic), Pmusc,ei: Changes in esophageal pressure during the inspiratory hold (elastic work)
Table 1.
Definition of variables
| Definition | Physiologic Concepts | |
|---|---|---|
| Peak Pressure (Ppeak) | Airway pressure just before the inspiratory hold | Set airway pressure during PC/PS ventilation |
| Plateau Pressure (Pplat) | Pressure when a flat plateau is reached during an inspiratory hold | In the absence of flow and during patient’s muscle relaxation. Elastic recoil pressure of the respiratory system |
| Pmusc, index (PMI) | Changes in airway pressure during the inspiratory hold. Pplat - Ppeak |
PMI is based on the concept that the difference between plateau pressure registered by an inspiratory hold (Pplat) and pressure applied by the ventilator (PEEP + PSV or PC), represents an index of patient elastic workload. |
| Dynamic driving Pressure (dynΔP) | Ppeak - PEEP | Set amount of pressure control or pressure support by the ventilator. This pressure includes both resistive and elastic components. |
| Driving Pressure (ΔP) | Pplat - PEEP | Pressure required to expand the volume (tidal volume) during ventilation, reflective of elastic components. |
| Maximum negative pressure of esophageal pressure (max negative-ΔPes) | Difference from end-expiratory esophageal pressure to maximum negative esophageal pressure | Magnitude of patient inspiratory effort, including both resistive and elastic components. |
| Changes in esophageal pressure during the inspiratory hold (Pmusc,ei) | Difference between the relaxed plateau esophageal pressure during the inspiratory hold (plateau Pes) and the esophageal pressure at the time of inspiratory hold begins (Pes at inspiratory hold timing) | An estimate of the pressure generated by the inspiratory muscles at the end of inspiration, the patient’s elastic workload. |
Selection of data to use for analysis
This study was focused on patients who had spontaneous effort on the ventilator, but patients needed to become passive during the inspiratory hold to measure the PMI. Several exclusion criteria were therefore applied on the waveform level. All tracings were primarily reviewed by one author (MK), and waveforms with any ambiguity for calculations or exclusion criteria were discussed with two other authors to arrive at consensus (JH, RGK). These exclusion criteria were (Table S1):
Air leak > 20% (Inspiratory - Expiratory VT)/Expiratory VT
- Lack of a true plateau on airway pressure:
-
2-a.Continued decrease in airway pressure: Possible air leak (Fig. S1)
-
2-b.Plateau too short: Inspiratory hold < 1.5 seconds. (Fig. S2)
-
2-c.Expiratory effort during the inspiratory hold: Continued rise in both airway and esophageal pressure. (Fig. S3)
-
2-d.Inspiratory effort during the inspiratory hold: Abrupt drop in airway and esophageal pressure during the hold. (Fig. S4)
-
2-a.
Asynchrony: Negative Pes due to reverse triggering as patients were often not passive at the time the inspiratory hold began (Fig. S5)
Significant artifact in any of the necessary respiratory signals: (Fig. S6)
Expiratory push: Increased Pes at the end of expiration which yielded an end-expiratory Pes higher than the true value, which in turn overestimated inspiratory effort. (Fig. S7)
Statistical analysis
Data are expressed as median (inter quartile range: IQR). A p value of less than 0.05 was considered statistically significant with Mann-Whitney or Kruskall Wallis ANOVA analyses. Analyses were performed using R (R Core Team, Vienna, Austria).
The primary analysis sought to understand if the direction of change in PMI (Pplat-Ppeak) during an inspiratory hold was associated with a certain magnitude of change in Pes. PMI was calculated during each inspiratory hold, and was divided into one of three categories for analysis (PMI < −1 (plateau lower than peak, “negative”), PMI between −1 and 1 (plateau and peak similar, “neutral”), and PMI > 1 cmH2O (plateau greater than peak, “positive”). Groups were compared with a Kruskal-Wallis test, with post-hoc comparison of multiple groups. We then compared changes in Pes within these 3 groups, including max negative-ΔPes which reflects the resistive and elastic work done by the patient and Pmusc,ei which represents the elastic work done by the patient (Fig. 1, Table 1, Supplement: Equation of motion of the respiratory system). We explored whether a higher PMI was predictive of a specific negative Pes using receiver operating characteristic (ROC) analysis. Finally, we sought to determine whether there was a linear relationship between PMI and Pes derived variables. Repeated measurement correlation was performed because multiple measurements were taken on individual patients (23). Subgroup analysis was performed based on ventilator mode (SIMV-PC versus PS). Due to the ongoing nature of the parent clinical trial, outcomes such as mortality and length of ventilation are not reported.
Results
The parent REDvent trial is ongoing and has enrolled approximately 115 of 276 expected patients to date. This analysis focused on the first 68 patients from the REDvent study. 15 of these 68 were excluded from this analysis because they did not have spontaneous breathing during any of the recordings. 17 were excluded because they were ventilated with an AVEA ventilator. In general, these patients were similar to the rest of the cohort (Table S2). This left 36 patients with 191 inspiratory holds. However, 73 holds were excluded due to an inappropriate inspiratory hold waveform (Table S1), mostly from the patient not becoming passive during the hold. Ventilator settings and demographic data were similar between holds which were included versus excluded (Table S2) with the exception of slightly higher PEEP (8 vs 7 cmH2O) and slightly higher peak inspiratory pressure (22 versus 20 cmH2O) in the inspiratory holds which were excluded from analysis.
A total of 30 patients, from 65 study days, comprising 118 inspiratory holds were included in the final analysis (Fig. S8). All inspiratory holds lasted between 1.5–3 seconds. The median age was 36.8 (16.1–70.3) months, height was 94.0 (77.5–109.0) cm, weight was 14.7 (10.2–24.5) kg, 17 females (Table S2).
Comparison of three groups categorized by changes in airway pressure
During the inspiratory hold, PMI was “negative” in 29 cases, was “neutral” in 17 cases, and was “positive” in 72 cases. The difference between the Pplat-Ppeak in the negative, neutral, and positive PMI groups were −2.3 (−3.2–1.6), −0.3 (−0.5–0.7), and 4.2 (3.1–6.0) cmH2O (p < 0.0001) respectively (Table 2). As PMI went from negative to neutral to positive, DynΔP (Peak inspiratory pressure - PEEP) was lower 15.4 (13.6–17.2), 12.0 (8.5–17.3), 8.9 (7.3–10.5) cmH2O (p < 0.0001) (Fig. S9), with a larger negative deflection in max negative Pes (ΔPes) −5.0 (−8.2– 1.9), −5.9 (−7.6–4.3), and −10.7 (−18.1–7.9) cmH2O (p < 0.0001), respectively. In fact, the driving pressure (ΔP = Pplat-PEEP) was similar 12.8 (10.5–14.3), 12.5 (9.3–16.3), 13.3(11.6–15.8) cmH2O (p = 0.66) between groups. Furthermore Pmusc,ei, calculated from esophageal pressure and reflective of the patient’s elastic work, also went up as PMI went from negative to neutral to positive (Table 2).
Table 2.
Comparison of three groups categorized by changes in airway pressure
| Characteristic | total | PMI negative | PMI neutral | PMI positive | P value |
|---|---|---|---|---|---|
| N | 118 | 29 (24.6) | 17 (14.4) | 72 (61.0) | |
| Airway Pressure Terms | |||||
| Ppeak (cmH2O) | 18.0 (15.4–22.9) | 23.4 (20.9–25.7) | 20.1 (16.2–23.2)* | 16.3 (14.0–18.3)* | <0.0001 |
| Pplat (cmH2O) | 21.1 (17.8–22.9) | 21.3 (17.9–23.7) | 19.8 (16.7–22.5) | 21.0 (18.2–22.8) | 0.78 |
| PEEP (cmH2O) | 7.7 (5.9–8.5) | 8.1 (7.5–8.5) | 7.9 (5.9–8.1) | 6.7 (5.6–8.0) | 0.034 |
| PMI (cmH2O) | 2.6 (−1–4.6) | −2.3 (−3.2– −1.6) | −0.3 (−0.5–0.7) | 4.2 (3.1–6.0) | <0.0001** |
| dynΔP (cmH2O) | 10.4 (8.2–14.5) | 15.4 (13.6–17.2)* | 12.0 (8.5–17.3)* | 8.9 (7.3–10.5) | <0.0001 |
| Driving Pressure (ΔP) (cmH2O) | 13.0 (10.9–15.6) | 12.8 (10.5–14.3) | 12.5 (9.3–16.3) | 13.3 (11.6–15.8) | 0.66 |
| Esophageal Pressure Terms | |||||
| max negative-ΔPes (cmH2O) | −8.9 (−13.7– −5.6) | −5.0 (−8.2– −1.9)* | −5.9 (−7.6– −4.3)* | −10.7 (−18.1– −7.9) | <0.0001 |
| Pmusc,ei (cmH2O) | 5.0 (1.5–9.1) | 0.3 (−0.3–1.5)* | 2.6 (0.7–3.8)* | 7.3 (5.0–12.0) | <0.0001 |
Data are presented as median (Inter quartile Range) or number (%)
Significantly different from the PMI positive group.
Significant differences between all groups.
The max negative-ΔPes value which predicted a positive PMI with the highest sensitivity and specificity was −6.1 cmH2O (sensitivity = 90.3%, specificity = 63.0%), and overall higher max negative-ΔPes had excellent discrimination of positive PMI ([AUC] = 0.83, 95% CI, 0.75–0.91) (Fig. S10). In general, PMI was reproducible between breaths, with a mean difference in PMI between the two inspiratory holds used for analysis per patient per day of 1.0 ± 1.1 cmH2O (Fig. S11).
Dose response relationship between magnitude of change in airway pressure during an inspiratory hold and esophageal pressure
Figure 2 shows the repeated measures correlation between PMI (measured from airway pressure) and max negative-ΔPes (resistive and elastic work from esophageal pressure) and Pmusc,ei (elastic work from esophageal pressure). PMI was most strongly correlated with Pmusc,ei (r = 0.84, p < 0.0001), although PMI also had good correlation with max negative-ΔPes (r = −0.52, p < 0.0001). The dose-response relationship between PMI and Pes shows that when PMI is ≥ 7 cmH2O, the change in Pes is >10 cmH2O (Fig. 3).
Fig. 2.
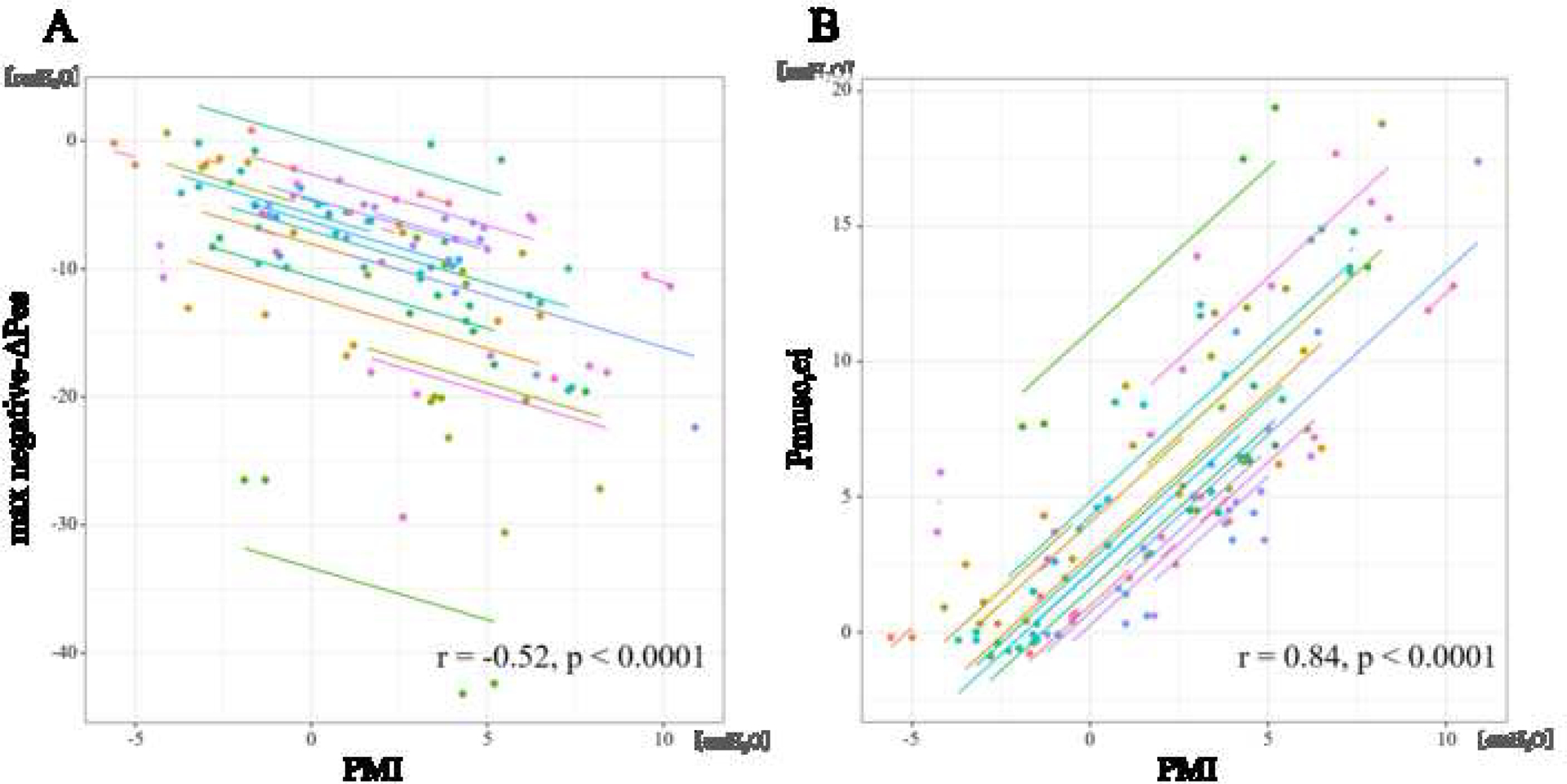
Relationship between Esophageal pressure and PMI.
Scatter plots for the repeated measures correlations (RMCORR) between esophageal pressure derived parameters max negative-ΔPes (resistive and elastic work) (A) and Pmusc,ei (elastic work) (B) and airway pressure derived PMI. Correlation coefficients and adjusted P values are shown in each comparison. For comparison, data from individuals are colored differently, with a single color for all time points from the same individual. The dots represent data for each patient and corresponding lines represent linear relationship for each patient. This technique is needed because repeated measures per patient are possible.
Fig. 3.
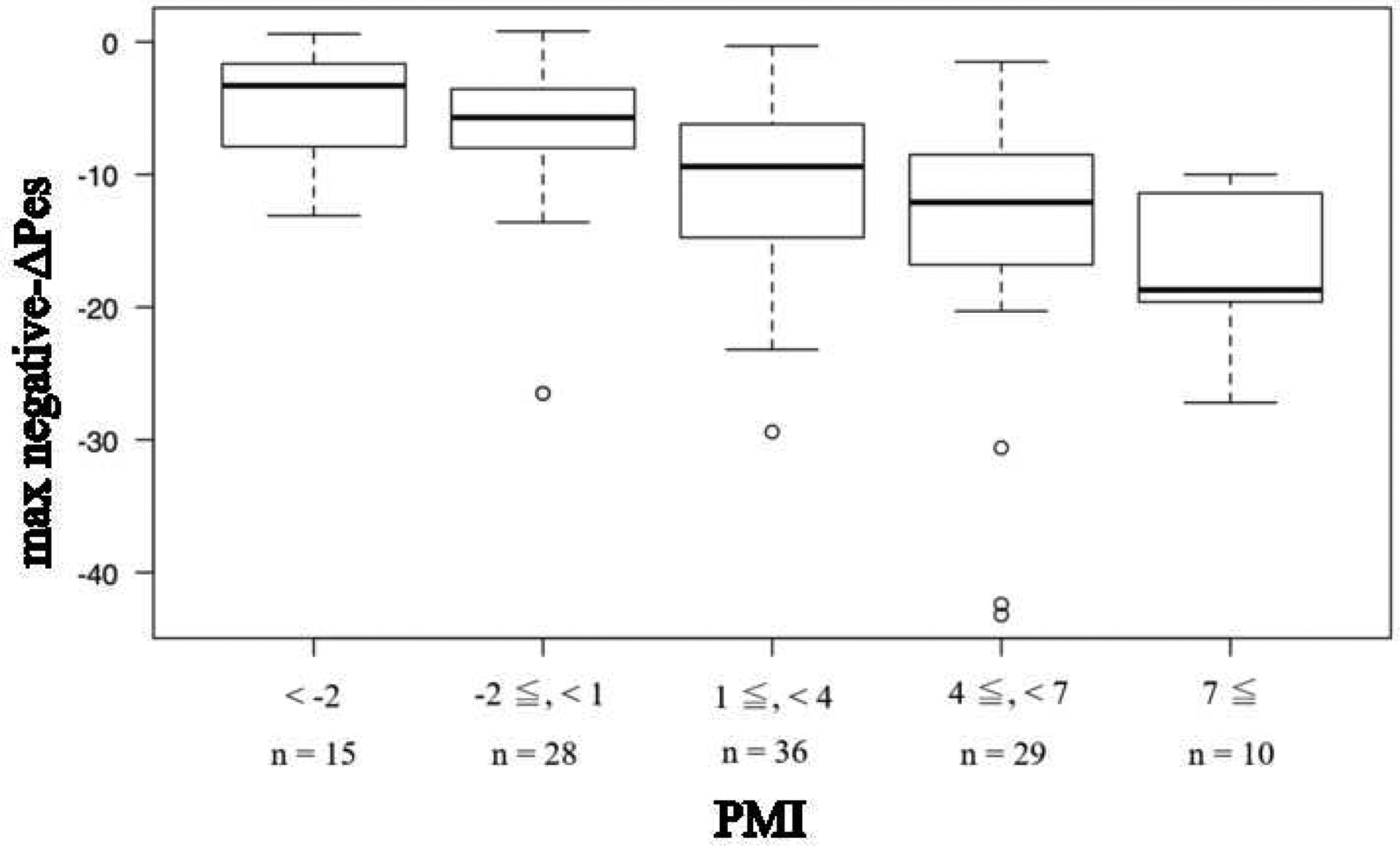
The dose-response relationship between PMI and max negative-ΔPes.
When the PMI exceeds 7 cmH2O, the change of Pes is shown to exceed 10 cmH2O.
Bar is median, box is interquartile range, and whisker is nonoutlier range.
Subgroup analysis: mode of ventilation
Of the 118 inspiratory holds on 30 patients, 81 holds were during SIMV-PC (during a time cycled-breath) and 37 holds were during PS (Fig. S8). PMI was positive in 44 (54.3%) cases during SIMV-PC and 28 (75.7%) cases during PS (Table S3). While there were differences in dynΔP and max negative-ΔPes between PC and PS, overall ΔP was similar 13.0 (10.5–15.9) vs. 13.0 (11.4–15.8) cmH2O (p = 0.75) between PC and PS (Fig. S12). Importantly, mode of ventilation did not appear to affect the correlation between PMI and max negative-ΔPes and Pmusc,ei (Fig. S13).
Discussion
We have found in a cohort of patients with PARDS, that when Pplat was higher than Ppeak (positive PMI) during an inspiratory hold, the patient had at least a 6 cmH2O spontaneous reduction in pleural pressure during inspiration. Furthermore, the larger the PMI, the more elastic work the patient is doing to move the tidal volume (Pmusc,ei), which should factor into calculations of driving pressure. As such, reducing the applied inspiratory pressure during mechanical ventilation (i.e. pressure control or delta P) may not reduce driving pressure if the patient has respiratory effort. This technique appears to work during both PC and PS modes of ventilation, but it is crucial to ensure that there is a true plateau in airway pressure (between 1.5–3 seconds) for valid interpretation.
These findings echo previous studies conducted on PS ventilation in adults (14, 15, 24–26) and are congruent with the concept that driving pressure calculated using an inspiratory hold in this manner is similar when patients are spontaneously breathing compared to when they have no inspiratory effort during controlled mechanical ventilation, if they have the same tidal volume (15).
To aid with clinical interpretation, we elected to divide the change in airway pressure during an inspiratory hold into 3 groups: negative, neutral, and positive PMI. While we did not specifically control tidal volume, we found that amongst these three groups, as PMI increased, the dynΔP (PIP-PEEP) set on the ventilator was lower, but there was no difference in Pplat or driving pressure (ΔP). This re-inforces that reducing the level of assistance provided by the ventilator may have no effect on the driving pressure in the lung when the patient is breathing spontaneously. By performing an inspiratory hold, we can estimate the PMI (the pressure generated by the patients respiratory muscles which are responsible for movement of tidal volume), and calculate the driving pressure. This has important clinical implications when considering the risks of VILI and P-SILI.
Previous studies have limited estimation of PMI to PS ventilation (14, 15, 25). Our study evaluated both PC and PS ventilation. This is important because the end of inspiration differs between time cycled breaths in PC and flow cycled breaths in PS. It is possible that during time cycled breaths, inspiratory muscles may not be fully relaxed at the end of inspiration. This may impact the estimate of Pmusc,ei, which is meant to reflect the pressures generated at the very end of inspiration. Theoretically this may be less true in PS ventilation, since the cycle terminates when flow has decelerated (standard flow cycle termination is 20–30% in this study) and the patient wishes to cease inspiration, although flow is not zero. We found that the correlation between PMI and max negative-ΔPes and Pmusc,ei were similar if not stronger during PC ventilation compared to PS ventilation. This may relate to the higher delta Pes in general in PS compared to PC ventilation, and that higher inspiratory effort leads to higher flow which may increase resistive work. It may also be an issue of smaller sample size.
While we have demonstrated that it is possible to use an inspiratory hold to calculate PMI to estimate patient effort and driving pressure, there are limitations. First, this technique is dependent upon patients remaining passive at the end of inspiration to tolerate the inspiratory hold. As was clear from our data, many patients (approximatley 1/3 of all holds) did not tolerate an inspiratory hold to generate a reliable plateau. Approximately half of the excluded cases were because we were unable to measure Pplat during the hold due to inspiratory or expiratory efforts. It is possible that the technique could be accomplished using shorter plateaus, but in most of these excluded patients, no plateau was seen at all. Interestingly we found slightly higher peak pressure and PEEP in patients who had inspiratory hold excluded (Table S2), with no difference in the max negative esophageal pressure. We would have thought that these patients in which inspiratory holds could not be performed would have stronger respiratory effort. However, this does not appear to be the case.
Second, while we have shown that high PMI implies high respiratory effort, it is important to remember that PMI may not be elevated if the patient has high effort from increases in airway resistance or ineffective triggers, as PMI is most reflective of elastic work and provides a method to estimate driving pressure. Third, we excluded patients with reverse triggers (patient effort which is initiated during a mandatory time-cycled breath) because they were not passive at the time the inspiratory hold began, but interestingly we often saw a similar positive PMI (Figure S5), which does highlight the important impact that reverse triggering can have on driving and plateau pressure. This should be an area of future investigation. Fourth, some ventilators do not permit application of this technique, either because inspiratory holds are not permitted on PS ventilation, or because the pressure is not permitted to rise above the set pressure. To that end, care must be taken to ensure that the upper alarm limits for pressure are not exceeded, as these will also under-estimate the PMI as the pressure may not be allowed to exceed a certain value.
Despite these limitations, we have shown that when the plateau pressure is higher than peak pressure (positive PMI) during an inspiratory hold, patients have at least modest inspiratory effort (> 6 cmH2O), and when the PMI climbs to 7 or higher, the patient likely has high respiratory effort (at least 10 cmH2O). This may signal the need to provide more assistance to the patient. Importantly, PMI most closely correlates to the elastic work the patient performs for tidal ventilation, and is therefore crucial for calculations of driving pressure. In circumstances when this driving pressure is high, interventions may need to target reducing respiratory drive to prevent VILI and P-SILI, as simply reducing the applied pressure control or delta pressure on the ventilator will likely result in higher patient effort, without a reduction in driving pressure.
Future study is needed to understand whether this estimate of driving pressure in spontaneously breathing patients has the same clinical relevance and association with outcome as driving pressure obtained for passive patients during controlled ventilation.
Supplementary Material
Fig. S1 Continued decrease in airway pressure:
Even after adjusting the cuff pressure of the endotracheal tube, measuring the tidal volume, and excluding those with large leaks, in some cases the airway pressure continuously dropped during the inspiratory hold, and a plateau was not reached.
Fig. S2 Plateau too short:
If the hold time was short, it was excluded because it could not be determined whether the plateau was reached. The minimum time required for the inspiratory hold was defined as 1.5 seconds.
Fig. S3 Expiratory effort during the inspiratory hold:
Excluded cases in which esophageal pressure or/and airway pressure increased due to expiratory effort during the inspiratory hold and passive conditions were not achieved.
Fig. S4 Inspiratory effort during the inspiratory hold:
Excluded cases in which esophageal pressure or/and airway pressure decreased due to inspiratory effort during the inspiratory hold, preventing 1.5 second inspiratory hold and plateau.
Fig. S5 Asynchrony:
For example, negative esophageal pressure due to reverse triggering, was excluded because the patients were often not passive at the time the inspiratory hold began.
Fig. S6 Significant artifact in any of the necessary respiratory signals:
The unpredictable movement of esophageal pressure during the inspiratory hold.
Fig. S7 Expiratory push:
Children may have an abdominal push at the end of expiration (arrow). In these cases, the end-expiratory esophageal pressure may be overestimating the true value, which will overestimate the inspiratory effort.
Table S1. Exclusion criteria of a waveform
Fig. S8 Consolidated Standards of Reporting diagram showing eligible, included, and excluded data.
Fig. S9 Comparison of three groups: negative, neutral, and positive PMI.
Comparison of dynΔP (Ppeak-PEEP) (A), ΔP (Driving Pressure: Pplat-PEEP) (B), max negative-ΔPes (C), and Pmusc,ei (D) in eachgroup.
The bar is the median, the outer box is the interquartile range, and the whiskers are the nonoutlier range. Significant differences are indicated with * (p < 0.01).
Fig. S10 Receiver operating characteristic (ROC) curve evaluating a positive PMI versus the value of maximum negative esophageal pressure. The optimal cutoff value for esophageal pressure was −6.1 cmH2O, sensitivity was 90.3%, and specificity was 63.0%. The area under the ROC curve was 0.826 (95% CI 0.746 – 0.907).
Figure. S11 Reproducibility of PMI and max negative-ΔPes between breaths
The Bland-Altman analysis for the agreement between two times of PMI (A) and max negative-ΔPes (B).
Solid lines, bias (A 1.0 cmH2O, B 1.4 cmH2O); broken lines, ±1.96 SD of the bias (A 3.1, −1.1 cmH2O, B 4.3, −1.5 cmH2O).
Fig. S12 Comparison of ventilation modes.
Comparison between ventilation modes for each parameter. DynΔP (A), ΔP (B), and max negative-ΔPes (C) are displayed. The bar is the median, the outer box is the interquartile range, and and whisker is nonoutlier range. Significant differences are shown with the * (p < 0.01).
Fig. S13 Relationship between esophageal pressure and PMI stratified by ventilator mode.
Scatter plot of repeated measures correlation (RMCORR) between esophageal pressure and PMI. The upper figure shows the max negative-ΔPes(A) and Pmusc,ei (B) in SIMV-PC, and the lower figure shows the max negative-ΔPes (C) and Pmusc, ei(D) in PS.
Correlation coefficients and adjusted P values are shown in each comparison. For comparison, data from individuals are colored differently, with a single color for all time points from the same individual.
Table S2. Patient Characteristics
Data are presented as median (inter quartile range: IQR).
SIMV-PC: Synchronized intermittent mandatory ventilation - Pressure control, PS: Pressure support, RR: respiratory rate, PIP: peak inspiratory pressure, PEEP: positive end-expiratory pressure, FIO2: inspired oxygen fraction, P/F ratio: PaO2 (arterial oxygen tension) / FIO2 ratio, ETCO2: end—tidal carbon dioxide tension, PBW: predicted body weight, ΔPes: Changes in esophageal pressure during mechanical ventilation. Changes in esophageal pressure during three breaths during mechanical ventilation were measured, and the average value was calculated.
Table S3. Comparison of ventilator modes
Data are presented as median (inter quartile range: IQR)
6). Financial support:
National Heart Lung and Blood Institute (NHLBI), United States National Institutes of Health (R01HL124666, PI: Khemani)
Footnotes
Conflict of Interest:
GB has received lecturing fees from Draeger Medical, Getinge, Hamilton and GE Healthcare.
RGK is a consultant for Orange Med and Hamilton Medical. The other authors disclose no conflict of interest.
References
- 1.Goligher EC, Fan E, Herridge MS, et al. : Evolution of diaphragm thickness during mechanical ventilation: Impact of inspiratory effort. Am J Respir Crit Care Med 2015; 192:1080–1088 [DOI] [PubMed] [Google Scholar]
- 2.Mechanical ventilation, diaphragm weakness and weaning: A Perspective R: Rehabilitation Perspective. Respir Physiol Neurobiol 2014; 189:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshida T, Torsani V, Gomes S, et al. : Spontaneous effort causes occult pendelluft during mechanical ventilation. Am J Respir Crit Care Med 2013; 188:1420–1427 [DOI] [PubMed] [Google Scholar]
- 4.Yoshida T, Uchiyama A, Matsuura N, et al. : The comparison of spontaneous breathing and muscle paralysis in two different severities of experimental lung injury. Crit Care Med 2013; 41:536–545 [DOI] [PubMed] [Google Scholar]
- 5.Akoumianaki E, Maggiore SM, Valenza F, et al. : The application of esophageal pressure measurement in patients with respiratory failure. Am J Respir Crit Care Med 2014; 189:520–531 [DOI] [PubMed] [Google Scholar]
- 6.Hedenstierna G: Esophageal pressure: Benefit and limitations. Minerva Anestesiol 2012; 78:959–966 [PubMed] [Google Scholar]
- 7.Brochard L: Measurement of esophageal pressure at bedside: Pros and cons. Curr Opin Crit Care 2014; 20:39–46 [DOI] [PubMed] [Google Scholar]
- 8.Alberti A, Gallo F, Fongaro A, et al. : P0.1 is a useful parameter in setting the level of pressure support ventilation. Intensive Care Med 1995; 21:547–553 [DOI] [PubMed] [Google Scholar]
- 9.Conti G, Antonelli M, Arzano S, et al. : Equipment review: Measurement of occlusion pressures in critically ill patients [Internet]. Crit Care 1997; 1:89–93Available from: http://ccforum.com/content/1/3/89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amato MBP, Meade MO, Slutsky AS, et al. : Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 2014; 372:747–755 [DOI] [PubMed] [Google Scholar]
- 11.Tonetti T, Vasques F, Rapetti F, et al. : A driving pressure and mechanical power: New targets for VILI prevention. Ann Transl Med 2017; 5:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grieco DL, Chen L, Dres M, et al. : Should we use driving pressure to set tidal volume? Curr Opin Crit Care 2017; 23:38–44 [DOI] [PubMed] [Google Scholar]
- 13.Bertoni M, Telias I, Urner M, et al. : A novel non-invasive method to detect excessively high respiratory effort and dynamic transpulmonary driving pressure during mechanical ventilation. Crit Care 2019; 23:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foti G, Cereda M, Banfi G, et al. : End-inspiratory airway occlusion: A method to assess the pressure developed by inspiratory muscles in patients with acute lung injury undergoing pressure support. Am J Respir Crit Care Med 1997; 156:1210–1216 [DOI] [PubMed] [Google Scholar]
- 15.Bellani G, Grasselli G, Teggia-Droghi M, et al. : Do spontaneous and mechanical breathing have similar effects on average transpulmonary and alveolar pressure? A clinical crossover study [Internet]. Crit Care 2016; 20:1–10Available from: 10.1186/s13054-016-1290-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bellani G, Grassi A, Sosio S, et al. : Driving Pressure Is Associated with Outcome during Assisted Ventilation in Acute Respiratory Distress Syndrome. Anesthesiology 2019; 131:594–604 [DOI] [PubMed] [Google Scholar]
- 17.Khemani RG, Hotz JC, Klein MJ, et al. : A Phase II randomized controlled trial for lung and diaphragm protective ventilation (Real-time Effort Driven VENTilator management) [Internet]. Contemp Clin Trials 2020; 88:105893.Available from: 10.1016/j.cct.2019.105893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Pediatric Acute Lung Injury Consensus Conference Group: Pediatric Acute Lung Injury Consensus Conference Group. Pediatr Crit Care Med 2015; 16:428–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheifetz IM: Pediatric ARDS. Respir Care 2017; 62:718–731 [DOI] [PubMed] [Google Scholar]
- 20.Khemani RG, Smith LS, Zimmerman JJ, et al. : Pediatric acute respiratory distress syndrome: Definition, incidence, and epidemiology: Proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med 2015; 16:S23–S40 [DOI] [PubMed] [Google Scholar]
- 21.Khemani RG, Smith L, Lopez-Fernandez YM, et al. : Paediatric acute respiratory distress syndrome incidence and epidemiology (PARDIE): an international, observational study. Lancet Respir Med 2019; 7:115–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hotz JC, Sodetani CT, Van Steenbergen JV., et al. : Measurements obtained from esophageal balloon catheters are affected by the esophageal balloon filling volume in children with ARDS. Respir Care 2018; 63:177–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bakdash JZ, Marusich LR: Repeated measures correlation. Front Psychol 2017; 8:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russotto V, Bellani G, Foti G: Respiratory mechanics in patients with acute respiratory distress syndrome. Ann Transl Med 2018; 6:382–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sajjad H, Schmidt GA, Brower RG, et al. : Can the plateau be higher than the peak pressure? Ann Am Thorac Soc 2018; 15:754–759 [DOI] [PubMed] [Google Scholar]
- 26.Bellani G, Grassi A, Sosio S, et al. : Plateau and driving pressure in the presence of spontaneous breathing [Internet]. Intensive Care Med 2019; 45:97–98Available from: 10.1007/s00134-018-5311-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Continued decrease in airway pressure:
Even after adjusting the cuff pressure of the endotracheal tube, measuring the tidal volume, and excluding those with large leaks, in some cases the airway pressure continuously dropped during the inspiratory hold, and a plateau was not reached.
Fig. S2 Plateau too short:
If the hold time was short, it was excluded because it could not be determined whether the plateau was reached. The minimum time required for the inspiratory hold was defined as 1.5 seconds.
Fig. S3 Expiratory effort during the inspiratory hold:
Excluded cases in which esophageal pressure or/and airway pressure increased due to expiratory effort during the inspiratory hold and passive conditions were not achieved.
Fig. S4 Inspiratory effort during the inspiratory hold:
Excluded cases in which esophageal pressure or/and airway pressure decreased due to inspiratory effort during the inspiratory hold, preventing 1.5 second inspiratory hold and plateau.
Fig. S5 Asynchrony:
For example, negative esophageal pressure due to reverse triggering, was excluded because the patients were often not passive at the time the inspiratory hold began.
Fig. S6 Significant artifact in any of the necessary respiratory signals:
The unpredictable movement of esophageal pressure during the inspiratory hold.
Fig. S7 Expiratory push:
Children may have an abdominal push at the end of expiration (arrow). In these cases, the end-expiratory esophageal pressure may be overestimating the true value, which will overestimate the inspiratory effort.
Table S1. Exclusion criteria of a waveform
Fig. S8 Consolidated Standards of Reporting diagram showing eligible, included, and excluded data.
Fig. S9 Comparison of three groups: negative, neutral, and positive PMI.
Comparison of dynΔP (Ppeak-PEEP) (A), ΔP (Driving Pressure: Pplat-PEEP) (B), max negative-ΔPes (C), and Pmusc,ei (D) in eachgroup.
The bar is the median, the outer box is the interquartile range, and the whiskers are the nonoutlier range. Significant differences are indicated with * (p < 0.01).
Fig. S10 Receiver operating characteristic (ROC) curve evaluating a positive PMI versus the value of maximum negative esophageal pressure. The optimal cutoff value for esophageal pressure was −6.1 cmH2O, sensitivity was 90.3%, and specificity was 63.0%. The area under the ROC curve was 0.826 (95% CI 0.746 – 0.907).
Figure. S11 Reproducibility of PMI and max negative-ΔPes between breaths
The Bland-Altman analysis for the agreement between two times of PMI (A) and max negative-ΔPes (B).
Solid lines, bias (A 1.0 cmH2O, B 1.4 cmH2O); broken lines, ±1.96 SD of the bias (A 3.1, −1.1 cmH2O, B 4.3, −1.5 cmH2O).
Fig. S12 Comparison of ventilation modes.
Comparison between ventilation modes for each parameter. DynΔP (A), ΔP (B), and max negative-ΔPes (C) are displayed. The bar is the median, the outer box is the interquartile range, and and whisker is nonoutlier range. Significant differences are shown with the * (p < 0.01).
Fig. S13 Relationship between esophageal pressure and PMI stratified by ventilator mode.
Scatter plot of repeated measures correlation (RMCORR) between esophageal pressure and PMI. The upper figure shows the max negative-ΔPes(A) and Pmusc,ei (B) in SIMV-PC, and the lower figure shows the max negative-ΔPes (C) and Pmusc, ei(D) in PS.
Correlation coefficients and adjusted P values are shown in each comparison. For comparison, data from individuals are colored differently, with a single color for all time points from the same individual.
Table S2. Patient Characteristics
Data are presented as median (inter quartile range: IQR).
SIMV-PC: Synchronized intermittent mandatory ventilation - Pressure control, PS: Pressure support, RR: respiratory rate, PIP: peak inspiratory pressure, PEEP: positive end-expiratory pressure, FIO2: inspired oxygen fraction, P/F ratio: PaO2 (arterial oxygen tension) / FIO2 ratio, ETCO2: end—tidal carbon dioxide tension, PBW: predicted body weight, ΔPes: Changes in esophageal pressure during mechanical ventilation. Changes in esophageal pressure during three breaths during mechanical ventilation were measured, and the average value was calculated.
Table S3. Comparison of ventilator modes
Data are presented as median (inter quartile range: IQR)


