Abstract
Background
Lateral lumbar interbody fusion (LLIF) affords a wide operative corridor to allow for a large interbody cage implantation for segmental reconstruction. There is a paucity of data describing segmental lordosis (SL) achieved with lordotic implants of varying angles. Here we compare changes in SL and lumbar lordosis (LL) after implantation of 6°, 10°, and 12° cages.
Methods
We retrospectively reviewed LLIF cases over a 5.5-year period. We derived SL and LL using the standard cobb angle measurement from a standing lateral radiograph. We analyzed mean changes in SL and LL over time using the linear mixed effect model to estimate these longitudinal changes.
Results
The most frequently treated level was L3–4, followed by L4–5. Significant increases in mean SL were found at each follow-up time point for all the cohorts. In an intercohort comparison, the mean changes in SL at immediate postoperative and last follow-up were significantly greater in the 10° cohort than 6° ([7.4° versus 3.1°, P = .004], [6.1° versus 2.3°, P = .025] respectively). The 12° cohort had higher mean change in SL at last follow-up than the 6° cohort (5.9° versus 2.3°, P = .022). There was no difference in mean change in SL between the 10° and 12° cohorts. No difference in overall mean LL over time was found. In terms of mean change in LL, no difference was observed except at immediate and 6-month postoperative in the 10° cohort ([9.6°, P = .001], [8.5, P = .003] respectively). By comparing mean change in LL, no difference existed except between the 10° and 6° immediately after surgery (9.6° versus 0.2°, P = .006).
Conclusions
LLIF cages significantly improve SL at the index level. However, this increase in SL is greater for 10° and 12° cages than the standard 6° cage. Use of 10° cages also resulted in overall improved LL than 6° cages.
Level of Evidence
3.
Clinical Relevance
Lateral lumbar interbody fusion.
Keywords: lateral lumbar interbody fusion, LLIF, segmental lordosis, SL, lumbar lordosis, LL, standard lordotic cages, transpsoas, cage dimension, cage angulation, 6° cage, 10°cage, 12° cage
INTRODUCTION
Minimally invasive spine surgery has gained popularity in recent years owing to the advantages of less soft-tissue trauma, less postoperative pain, shorter hospital stays, fewer complications, and more rapid return to activities of daily living. In the literature, a variety of minimally invasive spine surgery approaches have been described such as anterior, posterior, and lateral approaches; each with its own advantages and disadvantages. Lateral lumbar interbody fusion (LLIF) utilizes a retroperitoneal transpsoas corridor to gain access to the anterior and lateral spinal column and disc space via a tubular retractor under fluoroscopic guidance.1–7 With direct access to the lateral aspect of the spine, there is a relative ease of implantation of interbody cages with wide and long footprints and with varying degrees of lordosis resulting in optimal segmental height restoration and concurrent indirect decompression of neural elements.1–7
Since it was first described by Ozgur et al8 in 2006, LLIF has evolved into a common and versatile procedure in the last decade. Its application has expanded from degenerative indications to include traumatic, neoplastic, deformity, and revision surgery indications in carefully selected patients.3–7,9,10 Although the advantages include large interbody footprint, restoration of disc/foraminal height, and restoration of sagittal and coronal alignment, several drawbacks described in literature include lumbar plexus injury especially at lower lumbar disc spaces, cage migration and/or subsidence, abdominal pseudohernia, vertebral body fractures, visceral injuries, and lack of access to the L5–S1 disc space due to anatomical constraints owing to the height of the iliac crest and lumbar plexus morphology.1,3,6,7
Although a variety of cage designs are available for use during LLIF, there is a paucity of data describing the degree of lordosis achieved relative to the lordosis of the implant. While analyzing the cage dimension (width, height, and length), many assume that postoperative lordosis achieved is close to the native lordosis of the implant; however, this is not always the case. Potential reasons for less than optimal lordotic correction include graft migration and/or subsidence. To our knowledge, no studies in the literature have compared and contrasted changes in global lumbar lordosis (LL) and segmental lordosis (SL) with respect to lateral interbody cages with varying lordotic angulations. To that end, we analyzed preoperative and postoperative lateral radiographs comparing the degree of lordotic correction among patients with lumbar degenerative disc disease receiving standard 6° lordotic cage versus 10° and 12° lordotic cages. We hypothesized that the lateral lumbar interbody arthrodesis using the standard 6° lordotic cage would result in less segmental lordotic correction than using the 10° and 12° lordotic cages.
METHODS
This research was reviewed and approved by the institutional review board at our institution. By searching our institutional electronic medical record database, we conducted a retrospective chart review of all transpsoas LLIF surgeries performed by the contributing authors (R.F., R.G.F., and J.O.) from January 1, 2013 to July 1, 2019. Three cohorts were identified based on whether they received 6°, 10°, and/or 12° cages. Preoperative (preop), immediate postoperative (postop), 6-month postop, and last follow-up lateral radiographs were evaluated. The last follow-up radiographs were obtained at averages of 17.2, 16.8, and 18.1 months from the preop radiographs for the 6°, 10°, or 12° cohorts, respectively. SL, LL, and other variables
(demographic and perioperative data) were recorded. LL is defined by the Cobb angle formed between the superior endplate of S1 and L1 as depicted in Figure 1B–E. SL is defined by the Cobb angle formed by the superior endplate of the vertebra above the index level and inferior endplate of the vertebra below the index level as illustrated in Figure 1B–E. These measurements were performed on standing upright lateral films.
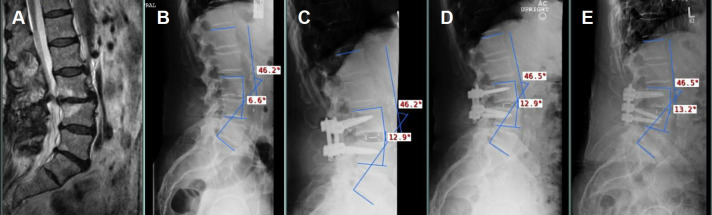
Figure 1.
A 79-year-old male with prior right L3-4 microdiscectomy presents with persistent right L3 radiculopathy; the condition is due to severe spondylosis at L3-4 with retrolisthesis, disc collapse with modic endplate changes, and severe foraminal stenosis compressing right L3 nerve root. The patient underwent L3-4 interbody arthrodesis with a L50 × W22 × H13-mm 12° lateral lumbar interbody fusion cage. (A) Preop magnetic resonance image showing the aforementioned findings. (B) Preop lateral upright radiograph showing initial SL = 6.6° at L3-4 level and LL = 46.2°. (C–E) Postop lateral upright radiographs obtained after surgery (SL = 12.9°, LL = 46.2°), at the 6-month follow-up (SL = 12.9°, LL = 46.5°), and the last follow-up (SL = 13.2°, LL = 46.5°), respectively.
Inclusion criteria included patients with ages ranging from 18 to 90 years old with degenerative disc disease and/or sagittal malalignment/coronal deformity that underwent 1 or more level LLIF with or without posterior spinal instrumented fusion using 1 or more 6°, 10°, or 12° cages or any combination of the cages with a minimum follow-up of 6 months. Exclusion criteria included individuals with infection or tumors, individuals with absent or poor preop or postop radiographs, or those with inadequate follow-ups as well as those receiving nonlordotic cages. Also excluded were individuals who underwent anterior column releases or levels that received hyperlordotic cages (>12°) and/or non-LLIF cages. Sixty-five patients met the inclusion criteria and were included for analyses.
Surgical Technique
LLIF was generally performed as previously described.1–4,6,7,9,11–15 The patient was positioned in the lateral decubitus position on the operating table with a gentle flexion at the table “break” to open the flank for access to between the lower ribs and iliac crest.
Neurophysiologic monitoring was established with somatosensory and motor evoked potentials as well as free-running and evoked electromyography. Choice of interbody cage size and lordosis was surgeon-dependent based on patient anatomy and the objectives of surgery. The interbody cages were packed with various forms of synthetic allograft. The patient was then turned prone on a Jackson table to complete percutaneous posterior instrumentation with pedicle screws and rods via image-guided computer-assisted navigation or fluoroscopic guidance depending on the surgeon preference.
Statistical Analysis
Numeric data are presented as mean ± standard deviation and range when deemed necessary. The statistical analyses of the longitudinal data were performed with the help of our Biostatistics Core group using the SAS 9.4 software. Linear mixed effect model was performed as previously described.16–20 Using the linear mixed effect model, we performed intracohort and intercohort analyses of SL and LL by cohort and time (preop, immediate postop, 6-month postop, and final follow-up). The main effects for time estimated the mean change in SL and LL from preop to immediate postop, 6-month postop, and last follow-up. An interaction variable of cohort*time was included to measure the difference in change by cohort. The mixed effect model included a random intercept for a participant allowing us to account for person-to-person variability. Because the estimates were calculated by maximum likelihoods, this allowed all observed data (either unequal or equal) in each cohort to be used, so that each subject did not have to have all 4 observations (ie, data at preop, immediate postop, 6-month postop, and last follow-up). The linear mixed effect model was then used to estimate the mean SL and LL at each time point by cohort. P value < .05 was deemed statistically significant.
We used time plots to depict the mean SL and LL at preop, immediate postop, 6-month postop, and final follow-up. We plotted estimated values from the model output (as denoted by the dots) with straight lines connecting them to help visualize the trend over time. The error bars illustrate 95% confidence interval (CI) around each estimate. We used a bar graph to illustrate the estimated mean changes in SL and LL at preop to immediate postop, 6-month postop, and last follow-up between the cohorts.
RESULTS
The average ages for the 6°, 10°, and 12° cohorts were 65.6, 67.5, and 64.8 years, respectively. The most treated level was L3–4, followed by L4–5 and L2–3. The rest of the demographic and perioperative data are summarized in Table 1. Out of a total of 32 patients (52 levels) who predominantly received 6° cage LLIF, 14, 15, and 3 patients underwent 1-, 2-, and 3-level LLIF, respectively (Table 1). Also, 18 patients in this cohort underwent concurrent percutaneous instrumentation. In addition to LLIF with 6° cages, 1 patient underwent concurrent L5–S1 anterior lumbar interbody fusion (ALIF), 2 patients underwent concurrent L4–S1 ALIF, and 1 patient underwent concurrent L5–S1 transforaminal lumbar interbody fusion (TLIF) (all these levels that received non-LLIF cages were excluded from analyses). Mean preop SL was observed to be 6.2°. The estimated mean immediate postop SL was 9.3° with 3.1° increase (95% CI: 1.3°–4.9°; P = .001) from preop. At 6-month follow-up, estimated mean SL was 10.3° with 4.1° increase (95% CI: 2.3°–5.9°; P = <.0001) from preop (Tables 2 and 3). At last follow-up, SL was 8.5° with 2.3° increase (95% CI: 0.4°–4.2°; P = .021) from preop (Tables 2 and 3). There was no statistical difference in the value of LL over time. LL was observed to be 40.3° at preop, 40.5° at immediate postop (0.2° increase from preop, 95% CI: −3.3° to 3.8°; P = .900), 42.6° at 6-month follow-up (2.3° increase from preop, 95% CI: −1.4° to 6.0°; P = .218), and 43.4° at last follow-up (3.1° increase from preop, 95% CI: −0.8° to 7.2°; P = .116) (Tables 4 and 5).
Table 1.
Summarized baseline demographic data and surgical data of all analyzed LLIF cases.
|
Items |
6° Cage (n = 32) |
10° Cage (n = 13) |
12° Cage (n = 20) |
Pa |
| 1. Sex, F:M | 14:18 | 6:7 | 9:11 | .988 |
| 2. Age, mean ± SD (range), y | 65.5 ± 8.5 (49–81) | 67.5 ± 12.0 (39–88) | 64.8 ± 11.2 (44–79) | .735 |
| 3. Operation time, mean ± SD (range), min | 245.0 ± 109.7 (86–499) | 323.9 ± 147.7 (140–494) | 202.3 ± 81.8 (73–360) | .012b |
| 4. EBL, mean ± SD (range), mL | 89.1 ± 100.3 (10–450) | 173.5 ± 180.4 (30–600) | 83.8 ± 75.0 (5–300) | .117 |
| 5. LOS, mean ± SD (range), h | 85.6 ± 54.2 (24–216) | 178.8 ± 204.2 (48–812) | 104.7 ± 63.2 (23–216) | .164 |
| 6. Preoperative diagnosis, n (% of patients) | P | |||
| Spondylolisthesis | 15 (45.5) | 5 (38.5) | 9 (45) | .991 |
| Scoliosis | 8 (24.2) | 5 (38.5) | 6 (30) | |
| DDD, foraminal stenosis | 6 (18.2) | 2 (15.4) | 3 (15) | |
| Revision surgeryc | 3 (9.1) | 1 (7.7) | 2 (6.7) | |
| 7. Levels treated, n (of levels) (% of patients) | Pa | |||
| T12–L1 | 0 | 3 (10.0) (23.1) | 0 | .007b |
| L1–2 | 0 | 5 (16.7) (38.5) | 0 | .000b |
| L2–3 | 11 (21.2) (33.3) | 7 (23.3) (53.8) | 9 (27.3) (45.0) | .423 |
| L3–4 | 24 (46.2) (72.7) | 6 (20.0) (46.2) | 11 (33.3) (55.0) | .128 |
| L4–5 | 17 (32.7) (51.5) | 9 (30.0) (69.2) | 13 (39.4) (65.0) | .602 |
| 8. Levels treated per case, n (% of patient) | P | |||
| 1 | 14 (42.4) | 4 (30.8) | 10 (50.0) | .008b |
| 2 | 15 (45.5) | 2 (15.4) | 6 (30.0) | |
| 3 | 3 (9.1) | 2 (15.4) | 4 (20.0) | |
| 4 | 0 | 4 (30.8) | 0 | |
| 5 | 0 | 1 (7.7) | 0 |
Abbreviations: DDD, degenerative disc disease; EBL, estimated blood loss; F, female; LLIF, lateral lumbar interbody fusion; LOS, length of stay; M, male; N, number.
P values obtained from testing overall difference in the groups (items 1–5 and 7 are different because the rows are not mutually exclusive; therefore, each variable/subvariable will have its own P value).
Statistical significance at P < .05.
Revision surgery includes those with pseudoarthrosis, failed decompression, cage migration from prior interbody fusion, and adjacent level disease.
Table 2.
Comparison of SL between the cages: estimated mean SL° (95% CI).
|
6° Cage |
10° Cage |
12° Cage |
|
| Preop | 6.2 (4.0–8.5) | 7.8 (4.8–10.7) | 12.8 (10.0–15.6) |
| Immediate postop | 9.3 (7.0–11.5) | 15.2 (12.2–18.2) | 17.6 (14.8–20.4) |
| 6-month postop | 10.3 (8.0–12.6) | 13.4 (10.4–16.4) | 17.7 (14.8–20.5) |
| Last follow-up | 8.5 (6.1–10.9) | 13.9 (10.6–17.1) | 18.7 (15.8–21.7) |
Abbreviations: CI, confidence interval; postop, postoperative; preop, preoperative; SL, segmental lordosis.
Table 3.
Comparison of SL between the cages: estimated mean change in SL° from preop.
|
Parameter |
6° Cage |
10° Cage |
12° Cage |
p1 |
p2 |
p3 |
| Δ1 | .004b | .236 | .115 | |||
| Mean (95% CI) | 3.1 (1.3–4.9) | 7.4 (5.1–9.8) | 4.8 (2.6–7.1) | |||
| Pa | .001b | <.0001b | <.0001b | |||
| Δ2 | .314 | .618 | .632 | |||
| Mean (95% CI) | 4.1 (2.3–5.9) | 5.6 (3.3–8.0) | 4.8 (2.6–7.1) | |||
| Pa | <.0001b | <.0001b | <.0001b | |||
| Δ3 | .025b | .022b | .632 | |||
| Mean (95% CI) | 2.3 (0.4–4.2) | 6.1 (3.4–8.8) | 5.9 (3.5–8.3) | |||
| Pa | .021b | <.0001b | <.0001b |
Abbreviations: CI, confidence interval; Δ1, mean change at immediate postop; Δ2, mean change at 6-month postop; Δ3, mean change at last follow-up; postop, postoperative; preop, preoperative; SL, segmental lordosis.
P = P value (versus preop SL); p1, P value (6° versus 10°); p2, P value (6° versus 12°); p3, P value (10° versus 12°).
Statistical significance at P < .05.
Table 4.
Comparison of LL between the cages: estimated mean LL° (95% CI).
|
Characteristics |
6° Cage |
10° Cage |
12° Cage |
| Preop | 40.3 (35.8–44.9) | 39.7 (32.5–46.8) | 46.7 (40.9–52.4) |
| Immediate postop | 40.5 (36.0–45.1) | 49.3 (42.1–56.4) | 51.0 (45.2–56.8) |
| 6-month postop | 42.6 (38.0–47.2) | 48.2 (41.0–55.3) | 49.4 (43.6–55.1) |
| Last follow-up | 43.4 (38.6–48.2) | 45.7 (37.7–53.6) | 49.0 (42.9–55.1) |
Abbreviations: CI, confidence interval; LL, lumbar lordosis; postop, postoperative; preop, preoperative.
Table 5.
Comparison of LL between the cages: estimated mean change in LL° from preop.
|
Parameter |
6° Cage |
10° Cage |
12° Cage |
p1 |
p2 |
p3 |
| Δ1 | .006b | .159 | .148 | |||
| Mean (95% CI) | 0.2 (−3.3–3.8) | 9.6 (4.0–15.1) | 4.3 (−0.2–8.8) | |||
| Pa | .900 | .001b | .059 | |||
| Δ2 | .068 | .886 | .112 | |||
| Mean (95% CI) | 2.3 (−1.4–6.0) | 8.5 (2.9–14.0) | 2.7 (−1.8–7.2) | |||
| Pa | .218 | .003b | .234 | |||
| Δ3 | .456 | .803 | .379 | |||
| Mean (95% CI) | 3.1 (−0.8–7.2) | 6.0 (−0.6–12.6) | 2.3 (−2.7–7.2) | |||
| Pa | .116 | .075 | .361 |
Abbreviations: CI, confidence interval; Δ1, mean change at immediate postop; Δ2, mean change at 6-month postop; Δ3, mean change at last follow-up; LL, lumbar lordosis; postop, postoperative; preop, preoperative.
P = P value (versus preop LL); p1, P value (6° versus 10°); p2, P value (6° versus 12°); p3, P value (10° versus 12°).
Statistical significance at P < .05.
The cohort receiving the 10° cage consisted of 13 patients (30 levels) in Table 1. Four, two, two, four, and one patient(s) received 1-, 2-, 3-, 4-, and 5-level LLIF, respectively (Table 1). Ten patients underwent concurrent percutaneous pedicle screw fixation, and 1 patient underwent laminectomy for further decompression. One patient underwent concurrent L5–S1 ALIF, 2 patients received concurrent 20° LLIF cages at L2–3, 1 patient received concurrent 20° LLIF cage at L3–4, 1 patient received concurrent 25° LLIF cage at L3–4, and 1 patient received concurrent 20° LLIF cage at L4–5 (all these levels were excluded from the analysis). Mean SL at preop, immediate, 6-month, and last postop follow-ups were recorded as 7.8°, 15.2°, 13.4°, and 13.9°, respectively, as illustrated in Table 2. From preop, there were 7.4° increase (95% CI: 5.1°–9.8°; P = <.0001) in SL at immediate postop, 5.6° increase (95% CI: 3.3°–8.0°; P = <.0001) at 6-month follow-up, and 6.1° increase (95% CI: 3.4°–8.8°; P = <.0001) at last follow-up (Table 3). Although there was significant change in LL from preop (39.7°) to immediate postop (49.3°, 9.6° increase, 95% CI: 4.0°–15.1°; P = .001) and from preop to 6-month postop (48.2°, 8.49° increase, 95% CI: 2.9°–14.0°; P = .068), no statistically significant change in LL was detected from preop to last follow-up (45.7°, 6.0° increase, 95% CI: −0.6° to 12.6°; P = .075) (Tables 4 and 5).
Furthermore, the 12° cage was used in 20 patients (33 levels) as illustrated in Table 1. Ten, six, and four patients underwent a 1-, 2-, and 3-level LLIF, respectively (Table 1). Ten patients received concurrent posterior pedicle screw fixation. Two patients received L4–S1 ALIF cages in addition to 12° LLIF cages at the index levels (these levels were excluded from analyses). In this cohort, the mean preop, immediate postop, 6-month postop, and last follow-up SLs were recorded as 12.8°, 17.6°, 17.7°, and 18.7°, respectively (Table 2). From preop, there were 4.8° increase (95% CI: 2.6°–7.1°; P = <.0001) in SL at immediate postop, 4.8° increase (95% CI: 2.6°–7.1°; P = <.0001) at 6-month postop, and 5.9° increase (95% CI: 3.5°–8.3°; P = <.0001) at last follow-up (Table 3). Like the 6° group, there was no statistically significant difference in estimated LL over time. LL was 46.7° at preop, 51° at immediately postop (4.3° increase, 95% CI: −0.2° to 8.8°; P = .059), 49.4° at 6-month postop (2.7° increase, 95% CI: −1.8° to 7.2°; P = .234), and 49.0° at last follow-up (2.3° increase, 95% CI: −2.7° to 7.2°; P = .361) (Tables 4 and 5).
The model also provided an intercohort comparison at the specific study time points. The mean change in SL in the 10° group was significantly larger than that of the 6° cohort at immediate postop and last follow-up ([7.4 versus 3.1, P = .004], [6.1 versus 2.3, P = .025], respectively) (Table 3). The 12° cohort only showed significantly greater mean change in SL compared with the 6° cohort at last follow-up (5.9 versus 2.3, P = .022) (Tables 3). However, there was no statistically significant difference in the mean change in SL between the 10° and 12° cohorts at all time points (P = .115, .632, .917, respectively) (Table 3). When compared with the 6° cohort, the 10° cohort did show statistically larger estimated mean change in LL at immediate postop (P = .006), which we believe was probably skewed by the concurrent nonlordotic cages or hyperlordotic cages (20° and 25°) (Table 5). Otherwise, no significant difference was detected between the cohorts at any other time points (Table 5).
Three patients received concurrent 6° and 12° LLIF cages. One patient with prior L4–5 TLIF underwent removal of migrated TLIF cage followed by a L4–5 LLIF with a 6° cage with favorable outcome. One patient with prior L4–5 LLIF (6° cage) suffered from persistent severe low back pain secondary to pseudoarthrosis at L4–5 subsequently underwent removal of L4–5 LLIF cage and placement of L4–5 ALIF. Three patients with prior fusion and persistent severe back pain secondary to scoliosis and spondylolisthesis failed nonoperative management and subsequently underwent LLIFs with favorable outcome.
DISCUSSION
Lumbar interbody fusion techniques have become a mainstay in the operative treatment of lumbar degenerative disease. Numerous comparative studies have analyzed the differences between lateral, posterior, transforaminal, and anterior interbody fusions in terms of technique and outcomes.7,21–25 However, formulating an optimal surgical strategy to treat specific lumbar conditions depends on many important factors, including sagittal realignment segmentally, regionally, and in some cases globally. In this study, we investigated the extent of SL gain using LLIF cages with varying lordotic angulation. Our study shows that cages with higher lordotic angulation (10° and 12°) demonstrated superior SL gain that was maintained over the follow-up time period compared with standard 6° lordotic cages. Due to the heterogeneity of surgical procedures and cage types used within any given case, we did not detect a significant difference in LL gain using cages with higher lordotic angle (see Figures 4 and 5).
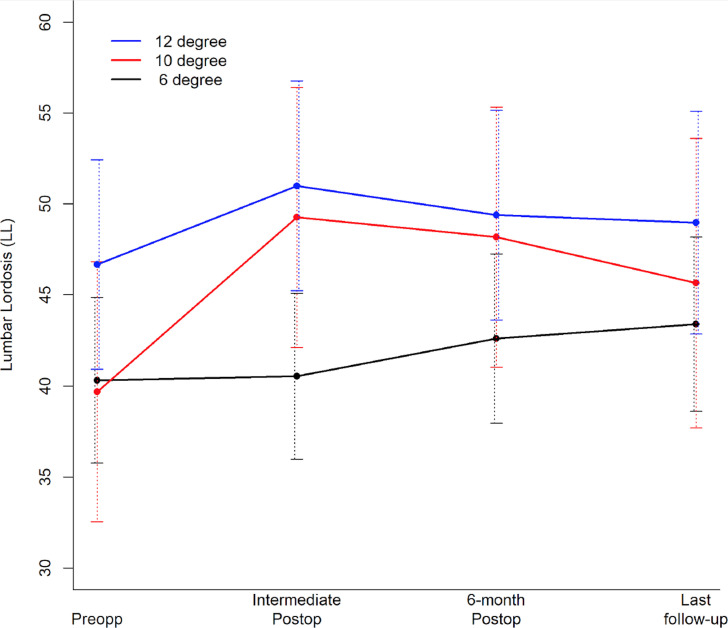
Figure 4.
Follow-up time plot depicting mean lumbar lordosis (LL). Error bars correspond to 95% CIs. No significant change in mean LL at any point of the follow-up.
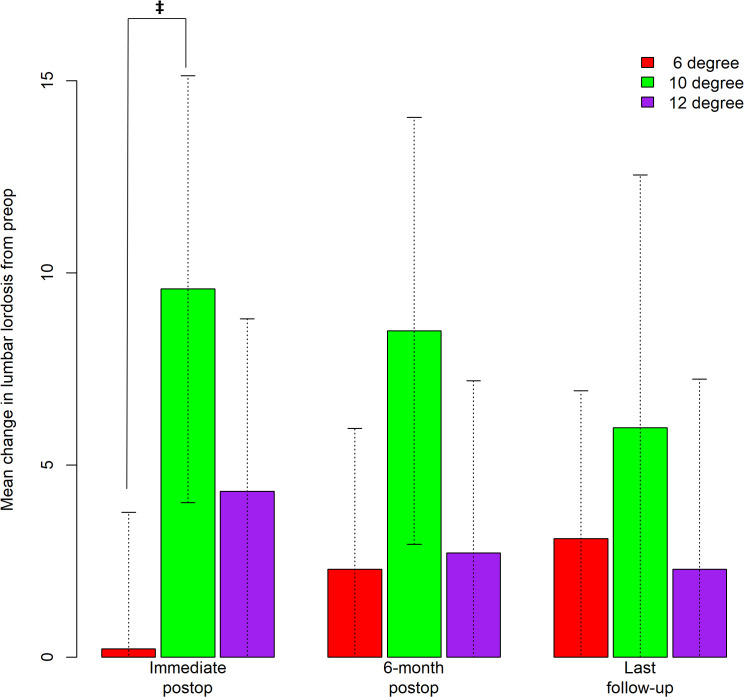
Figure 5.
Bar graph depicting predicted mean change in lumbar lordosis (LL) from preop to immediate postop, 6-month postop, and the last follow-up. Error bars correspond to 95% CIs. Only statistically significant difference exists between the 6° and 10° cohort (0.23° versus 9.58°, p = .0057) at the immediate postop period.
Although several LLIF studies in the literature have analyzed lordotic correction using different cage dimensions (ie, height, width, or length) or cage angulation, none of these studies quantify the degree of postoperative lordosis gain relative to the lordotic angle of the cage.1,7,11,15,22
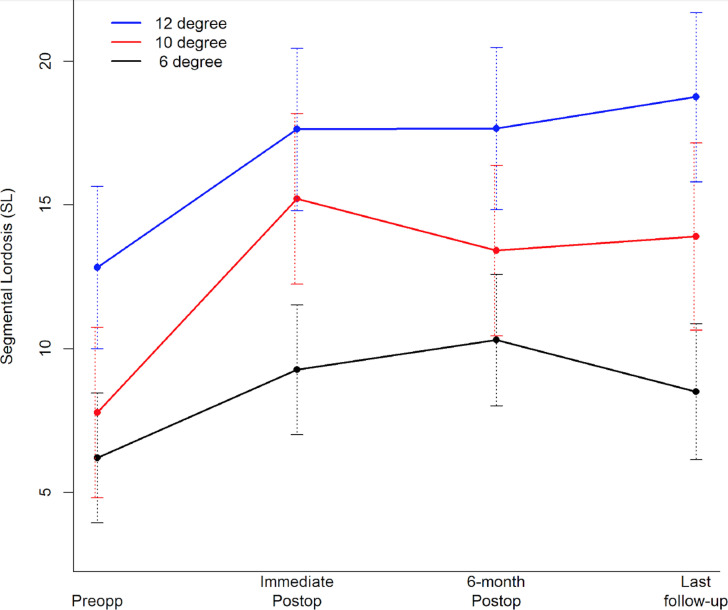
Depending on the pathology (degenerative disease, deformity, or tumor) and the magnitude of neural element compression, the chosen cage size (dimension and angle) is often patient-dependent. Because the goal of LLIF procedures is to restore normal SL, cages with varying lordotic angle are used anecdotally by different spine surgeons to achieve desirable SL. In fact, radiographic analysis comparing 10° lordotic to nonlordotic cages in LLIF in 1 study showed significantly greater increase in SL with a 10° cage at 6-weeks' postoperative time, although neither cage showed significant change in global LL.15 Overall, our results demonstrated that significant increase in mean SL may be achieved using cages with higher lordotic angle, which is consistent with several other studies in the literature irrespective of the methodologies used in measuring SL.1,2,13–15,22,24–26 We demonstrated significantly improved SL with all lordotic cages at any time point (Figure 2). The magnitude of segmental lordotic gain with using the 10° cage was significantly greater than the 6° cage at immediate postop (Figure 3). Similarly, the magnitude of segmental lordotic gain with either 10° or 12° cage was significantly greater than the 6° case at last follow-up (Figure 3).
Figure 2.
Follow-up time plot depicting mean segmental lordosis (SL) preop, immediately postop, at the 6-month postop, and the last follow-up. Error bars correspond to 95% CIs.
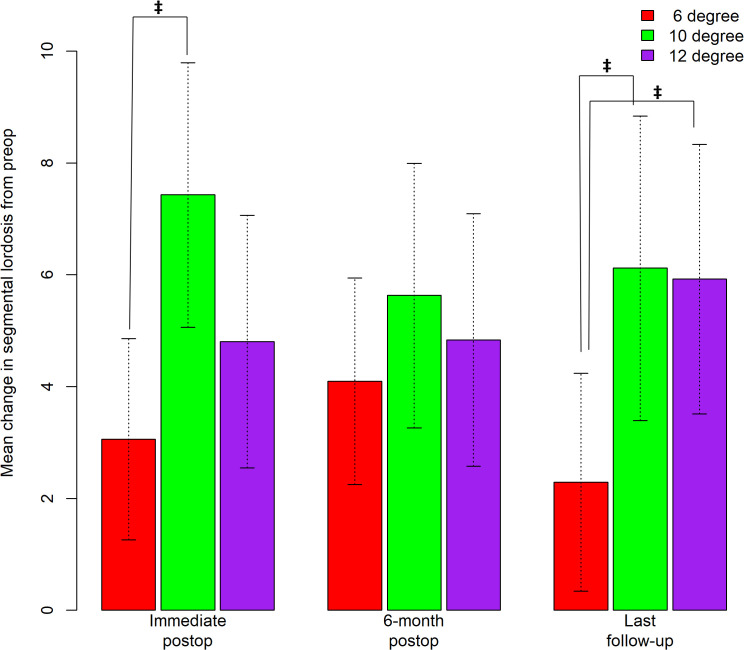
Figure 3.
Bar graph depicting mean change in segmental lordosis (SL) from preop to immediate postop, 6-month postop, and the last follow-up. Error bars correspond to 95% CIs. ‡ indicates statistical significance in increase in mean change in SL from preop to immediate postop between the 6° and 10° cage (3.06° versus 7.43°, p = .0041) and mean change in SL from preop to last follow-up between the 6° and 10° cage (2.30° versus 6.12°, p = .0253) and between the 6° and 12° cage (2.30° versus 5.93°, p = .0219).
With regards to global spine alignment, we observed a significant increase in mean LL in the 10° cohort at immediate and 6-month postop (Figure 4). Also, when compared with the 6° cohort at immediate postop, the 10° cohort did show significant higher mean change in LL from preop (Figure 4). However, these observed increases are confounded by the fact that some of the patients in 10° cohorts also received 20° or 25° cages at certain levels. Also, many of our cases were 1-level or 2-level LLIF. As such, the contributions of individual SL gain at 1 or 2 levels would not be expected to alter the overall LL. In that regard, it is difficult to derive meaningful inferences from the lack of statistically significant changes in LL irrespective of the cage implant used after surgery (Figure 4) and may also be a factor in similar results observed in several other studies in the literature.7,12,14,22,26
The lack of correlation between changes in SL and LL can also be explained in part by the fact that standard LL varies over a wide range (44°–62°) and normal SL varies by level starting at 2.6°–4° at L1–2 and increasing at each subsequent lumbar level with a maximal SL of 20.2°–24° at L5–S1.26,27 Sembrano et al23 speculated a compensatory change in which incremental gain in SL at the index level is cancelled out by loss at adjacent levels resulting in zero change in overall LL. Though a different methodology was used in calculating SL, Pesenti et al27 reported heterogeneous distribution of lordosis throughout the entire lumbar spine such that 62% of lordosis is derived from the L4–S1 levels. Because the most frequently treated level in this study was L3–4, followed by L4–5 and L2–3, the cumulative effect of SL at 1 or 2 levels had insufficient impact on the overall LL. Therefore, to significantly increase LL, we theorize that cages of higher lordotic angles (≥12°) will need to be implanted in distal lumbar segments in carefully selected patients with thorough preoperative surgical planning that should incorporate analyses of the remaining spinopelvic parameters. Also, the standard 6° cage can only restore the most cephalad lumbar level to a normal state, whereas 10° or 12° can further augment LL when used at the cephalad levels if desired.
There is an assumption that the postoperative lordotic correction achieved is equivalent to the native lordosis of the implant; however, this is not always the case. Potential reasons for less than optimal lordotic correction include graft migration and/or subsidence. Our study showed 5.76%, 6.67%, and 15.2% subsidence rates for the 6°, 10°, and 12° groups, respectively. We believe that a larger patient sample size, especially in the 10° and 12° groups, may have resulted in lower subsidence rates than reported here. Some of the factors contributing to subsidence reported in the literature included technique and experience, implant material, bone quality, cage dimension, and degree of bone remodeling with change in spine biomechanics after cage implantation and/or excessive decortication of the endplate.2,28 Because we did not stratify based on any of aforementioned factors, it is difficult to interpret subsidence rates within the context of this study. Tempel et al28 reported 8.7% subsidence rate in patients with DEXA T-score < −1.0 who underwent stand-alone LLIF, 48% of which required augmentation with posterior instrumentation. One study reported increased subsidence in stand-alone LLIF using standard (18 mm) compared with wide cage (22 mm) illustrating the importance of cage width in reducing subsidence.13 Similarly, several other studies have reported significant decrease in subsidence rate with wider cages.6,12,29 In a systematic review pooling 21 articles and a total of 1362 patients, the incidence of subsidence in LLIF was reported to be 10.3% and only 2.7% resulted in reoperation.30 Kotwal et al1 reported cage subsidence in 34 out of total 237 levels operated on. Although most of the cases of subsidence we reported in our study were radiographically subtle and asymptomatic, 1 patient with prior stand-alone LLIF suffered from severe subsidence resulting in re-stenosis that necessitated further decompression and instrumented fusion.
Limitations
Our study is limited by the retrospective nature of the data analysis introducing inherent biases. Also, postoperative computed tomography imaging might have provided more accurate determination of degrees of subsidence; however, this imaging modality was not routinely performed on these patients at follow-ups. Variability in Cobb angle measurement could have inadvertently introduced measurement error as well. The number of levels analyzed may have limited the power of this study. Also, the last follow-up time is not uniform and may have skewed the result as well. For example, cohorts were not stratified based on stand-alone versus pedicle screw augmentation due to the limited number of patients who would need to be included in each stratum.
Another limitation of this study was overall follow-up rate of 70% owing to patient compliance, tertiary referral patterns in the Chicago area, and patients choosing to follow locally. That said, we utilized a linear mixed effects model to estimate the mean trend in this longitudinal data set with slightly skewed data. All patients have preop and immediate postop radiographs. Although patients who were missing both 6-month and last follow-up radiographs were eliminated from this study, we included patients with at least 6-month follow-up radiographs.
CONCLUSION
Overall, our study illustrates that application of 6°, 10°, or 12° cages during LLIF procedures can significantly improve SL that is maintained over time. However, the magnitude of SL achieved with the implantation 10° or 12° cage is greater than with the 6° cage. By confirming these anatomic results radiographically, these data may serve as a guide when planning lumbar interbody reconstructive procedures. Specifically, degree of cage lordosis selection likely needs to be tailored to each of the levels being treated (eg, L1–2 versus L4–5), patient-specific anatomy, and the overall goals of the surgical procedure including total amount of desired lordosis correction segmentally, regionally, and globally.
ACKNOWLEDGMENTS
The authors express their gratitude to Todd Beck, MS, a statistician from our institution Bioinformatics and Biostatistics Core, who assisted tremendously with the statistical analyses. This research was reviewed and approved by the Institutional Review Board at Rush University Medical Center.
Footnotes
Disclosures and COI: Dr Fontes is a consultant for Globus Medical. Dr Fessler is a consultant for DePuy Synthes, receives royalties from DePuy Synthes, and has co-ownership in In Queue Innovations. Dr O'Toole is a consultant for Globus Medical, receives royalties from Globus Medical, receives royalties from RTI Surgical, and has stock ownership in TheraCell, Inc. The other authors received no funding for this study and report no conflicts of interest.
REFERENCES
- 1.Kotwal S, Kawaguchi S, Lebl D, et al. Minimally invasive lateral lumbar interbody fusion: clinical and radiographic outcome at a minimum 2-year follow-up. J Spinal Disord Tech. 2015;28(4):119–125. doi: 10.1097/BSD.0b013e3182706ce7. [DOI] [PubMed] [Google Scholar]
- 2.Malham GM, Ellis NJ, Parker RM, et al. Maintenance of segmental lordosis and disk height in stand-alone and instrumented extreme lateral interbody fusion (XLIF) Clin Spine Surg. 2017;30(2):E90–E98. doi: 10.1097/BSD.0b013e3182aa4c94. [DOI] [PubMed] [Google Scholar]
- 3.McGowan JE, Kanter AS. Lateral approaches for the surgical treatment of lumbar spondylolisthesis. Neurosurg Clin N Am. 2019;30(3):313–322. doi: 10.1016/j.nec.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Park P. Three-dimensional computed tomography-based spinal navigation in minimally invasive lateral lumbar interbody fusion: feasibility, technique, and initial results. Neurosurgery. 2015;11(suppl 2):259–267. doi: 10.1227/NEU.0000000000000726. [DOI] [PubMed] [Google Scholar]
- 5.Pawar A, Hughes A, Girardi F, et al. Lateral lumbar interbody fusion. Asian Spine J. 2015;9(6):978–983. doi: 10.4184/asj.2015.9.6.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salzmann SN, Shue J, Hughes AP. Lateral lumbar interbody fusion-outcomes and complications. Curr Rev Musculoskelet Med. 2017;10(4):539–546. doi: 10.1007/s12178-017-9444-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu DS, Bach K, Uribe JS. Minimally invasive anterior and lateral transpsoas approaches for closed reduction of grade II spondylolisthesis: initial clinical and radiographic experience. Neurosurg Focus. 2018;44(1):E4. doi: 10.3171/2017.10.FOCUS17574. [DOI] [PubMed] [Google Scholar]
- 8.Ozgur BM, Aryan HE, Pimenta L, Taylor WR. Extreme lateral interbody fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J Off J North Am Spine Soc. 2006;6:435–443. doi: 10.1016/j.spinee.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Campbell PG, Nunley PD, Cavanaugh D, et al. Short-term outcomes of lateral lumbar interbody fusion without decompression for the treatment of symptomatic degenerative spondylolisthesis at L4-5. Neurosurg Focus. 2018;44(1):E6. doi: 10.3171/2017.10.FOCUS17566. [DOI] [PubMed] [Google Scholar]
- 10.Cole CD, McCall TD, Schmidt MH, Dailey AT. Comparison of low back fusion techniques: transforaminal lumbar interbody fusion (TLIF) or posterior lumbar interbody fusion (PLIF) approaches. Curr Rev Musculoskelet Med. 2009;2(2):118–126. doi: 10.1007/s12178-009-9053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joseph JR, Smith BW, Patel RD, Park P. Use of 3D CT-based navigation in minimally invasive lateral lumbar interbody fusion. J Neurosurg Spine. 2016;25(3):339–344. doi: 10.3171/2016.2.SPINE151295. [DOI] [PubMed] [Google Scholar]
- 12.Kim SJ, Lee YS, Kim YB, et al. Clinical and radiological outcomes of a new cage for direct lateral lumbar interbody fusion. Korean J Spine. 2014;11(3):145–151. doi: 10.14245/kjs.2014.11.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marchi L, Abdala N, Oliveira L, et al. Radiographic and clinical evaluation of cage subsidence after stand-alone lateral interbody fusion. J Neurosurg Spine. 2013;19(1):110–11. doi: 10.3171/2013.4.SPINE12319. 8. [DOI] [PubMed] [Google Scholar]
- 14.Scherman DB, Rao PJ, Phan K, et al. Outcomes of direct lateral interbody fusion (DLIF) in an Australian cohort. J Spine Surg. 2019;5(1):1–12. doi: 10.21037/jss.2019.01.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sembrano JN, Horazdovsky RD, Sharma AK, et al. Do lordotic cages provide better segmental lordosis versus nonlordotic cages in lateral lumbar interbody fusion (LLIF)? Clin Spine Surg. 2017;30(4):E338–E343. doi: 10.1097/BSD.0000000000000114. [DOI] [PubMed] [Google Scholar]
- 16.Chang Y, Singer DE, Wu YA, Keller RB, Atlas SJ. The effect of surgical and nonsurgical treatment on longitudinal outcomes of lumbar spinal stenosis over 10 years. J Am Geriatr Soc. 2005;53(5):785–792. doi: 10.1111/j.1532-5415.2005.53254.x. [DOI] [PubMed] [Google Scholar]
- 17.Choi YH, Kwon SW, Moon JH, et al. Lateral lumbar interbody fusion and in situ screw fixation for rostral adjacent segment stenosis of the lumbar spine. J Korean Neurosurg Soc. 2017;60(6):755–762. doi: 10.3340/jkns.2017.0606.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delitto A, Piva SR, Moore CG, et al. Surgery versus nonsurgical treatment of lumbar spinal stenosis: a randomized trial. Ann Intern Med. 2015;162(7):465–473. doi: 10.7326/M14-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Panchal R, Denhaese R, Hill C, et al. Anterior and lateral lumbar interbody fusion with supplemental interspinous process fixation: outcomes from a multicenter, prospective, randomized, controlled study. Int J Spine Surg. 2018;12(2):172–184. doi: 10.14444/5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pretz CR, Graham JE, Middleton A, Karmarkar AM, Ottenbacher KJ. Longitudinal investigation of rehospitalization patterns in spinal cord injury and traumatic brain injury among Medicare beneficiaries. Arch Phys Med Rehabil. 2017;98(5):997–1003. doi: 10.1016/j.apmr.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Deng H, Long X, et al. A comparative study of perioperative complications between transforaminal versus posterior lumbar interbody fusion in degenerative lumbar spondylolisthesis. Eur Spine J. 2016;25(5):1575–1580. doi: 10.1007/s00586-015-4086-8. [DOI] [PubMed] [Google Scholar]
- 22.Saadeh YS, Joseph JR, Smith BW, et al. Comparison of segmental lordosis and global spinopelvic alignment after single-level lateral lumbar interbody fusion or transforaminal lumbar interbody fusion. World Neurosurg. 2019;126:e1374–e1378. doi: 10.1016/j.wneu.2019.03.106. [DOI] [PubMed] [Google Scholar]
- 23.Sembrano JN, Tohmeh A, Isaacs R. SOLAS. Degenerative Study Group two-year comparative outcomes of mis lateral and mis transforaminal interbody fusion in the treatment of degenerative spondylolisthesis: part I: clinical findings. Spine. 2016;41(suppl 8):S123–S132. doi: 10.1097/BRS.0000000000001471. [DOI] [PubMed] [Google Scholar]
- 24.Teng I, Han J, Phan K, Mobbs R. A meta-analysis comparing ALIF, PLIF, TLIF and LLIF. J Clin Neurosci. 2017;44:11–17. doi: 10.1016/j.jocn.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 25.Yan DL, Pei FX, Li J, Soo CL. Comparative study of PILF and TLIF treatment in adult degenerative spondylolisthesis. Eur Spine J. 2008;17(10):1311–1316. doi: 10.1007/s00586-008-0739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le TV, Vivas AC, Dakwar E, et al. The effect of the retroperitoneal transpsoas minimally invasive lateral interbody fusion on segmental and regional lumbar lordosis. ScientificWorldJournal. 2012;2012:516706. doi: 10.1100/2012/516706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pesenti S, Lafage R, Stein D, et al. The amount of proximal lumbar lordosis is related to pelvic incidence. Clin Orthop Relat Res. 2018;476(8):1603–1611. doi: 10.1097/CORR.0000000000000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tempel ZJ, Gandhoke GS, Okonkwo DO, Kanter AS. Impaired bone mineral density as a predictor of graft subsidence following minimally invasive transpsoas lateral lumbar interbody fusion. Eur Spine J. 2015;24(suppl 3):414–419. doi: 10.1007/s00586-015-3844-y. [DOI] [PubMed] [Google Scholar]
- 29.Le TV, Baaj AA, Dakwar E, et al. Subsidence of polyetheretherketone intervertebral cages in minimally invasive lateral retroperitoneal transpsoas lumbar interbody fusion. Spine. 2012;37:1268–1273. doi: 10.1097/BRS.0b013e3182458b2f. [DOI] [PubMed] [Google Scholar]
- 30.Macki M, Anand SK, Surapaneni A, et al. Subsidence rates after lateral lumbar interbody fusion: a systematic review. World Neurosurg. 2019;122:599–606. doi: 10.1016/j.wneu.2018.11.121. [DOI] [PubMed] [Google Scholar]