Abstract
BACKGROUND
Hypoxic-ischemic encephalopathy (HIE) is one of the leading causes of death and long-term neurological impairment in the pediatric population. Despite a limited number of treatments to cure HIE, stem cell therapies appear to be a potential treatment option for brain injury resulting from HIE.
AIM
To investigate the efficacy and safety of stem cell-based therapies in pediatric patients with HIE.
METHODS
The study inclusion criteria were determined as the presence of substantial deficit and disability caused by HIE. Wharton’s jelly-derived mesenchymal stem cells (WJ-MSCs) were intrathecally (IT), intramuscularly (IM), and intravenously administered to participants at a dose of 1 × 106/kg for each administration route twice monthly for 2 mo. In different follow-up durations, the effect of WJ-MSCs administration on HIE, the quality of life, prognosis of patients, and side effects were investigated, and patients were evaluated for neurological, cognitive functions, and spasticity using the Wee Functional Independence Measure (Wee FIM) Scale and Modified Ashworth (MA) Scale.
RESULTS
For all participants (n = 6), the mean duration of exposure to hypoxia was 39.17 + 18.82 min, the mean time interval after HIE was 21.83 ± 26.60 mo, the mean baseline Wee FIM scale score was 13.5 ± 0.55, and the mean baseline MA scale score was 35 ± 9.08. Three patients developed only early complications such as low-grade fever, mild headache associated with IT injection, and muscle pain associated with IM injection, all of which were transient and disappeared within 24 h. The treatment was evaluated to be safe and effective as demonstrated by magnetic resonance imaging examinations, electroencephalographies, laboratory tests, and neurological and functional scores of patients. Patients exhibited significant improvements in all neurological functions through a 12-mo follow-up. The mean Wee FIM scale score of participants increased from 13.5 ± 0.55 to 15.17 ± 1.6 points (mean ± SD) at 1 mo (z = - 1.826, P = 0.068) and to 23.5 ± 3.39 points at 12 mo (z = -2.207, P = 0.027) post-treatment. The percentage of patients who achieved an excellent functional improvement (Wee FIM scale total score = 126) increased from 10.71% (at baseline) to 12.03% at 1 mo and to 18.65% at 12 mo post-treatment.
CONCLUSION
Both the triple-route and multiple WJ-MSC implantations were safe and effective in pediatric patients with HIE with significant neurological and functional improvements. The results of this study support conducting further randomized, placebo-controlled studies on this treatment in the pediatric population.
Keywords: Hypoxic-ischemic encephalopathy, Pediatric, Stem cell, Wharton jelly
Core Tip: Hypoxic-ischemic encephalopathy (HIE) emerges as one of the leading causes of morbidity and mortality in children. There are a limited number of options for treating HIE. Recently, stem cell and cellular therapies appear to be a potential treatment option for ischemic brain injury caused by HIE. The aim of this phase I open-label clinical study is to investigate the efficacy and safety of one of these stem cell-based therapies in a group of pediatric patients with HIE. Both the triple-route and multiple Wharton`s jelly-derived mesenchymal stem cells administrations were safe and effective in pediatric patients with HIE with significant neurological and functional improvements.
INTRODUCTION
Hypoxic-ischemic encephalopathy (HIE) is a type of ischemic brain injury especially in pediatric population. It is caused by a lack of oxygen supply to the brain, resulting in oxygen deprivation. HIE has high morbidity and mortality rates[1]. Today, there is a limited number of treatment options for HIE e.g., cooling[2]. Different therapeutic approaches have been used to treat and improve functional and neurological outcomes of HIE patients. Among these approaches, stem cell therapies combined with new protocols are an adopted method to prevent ischemic brain injury caused by HIE[1,2]. Bone marrow (BM) is used as the most common source to derive mesenchymal stem cells (MSCs). Yet, taking MSCs from BM requires a highly invasive procedure, and the age of the donor is an effective factor for the maximal life span of obtained cells. Nowadays, Wharton's jelly (WJ), an umbilical cord (UC) tissue, comes to the fore as a potential source of stem cells since this tissue is discarded at birth, providing an opportunity for the isolation of MSCs. With their immune-privileged status, high proliferation capacity, and absence of ethical issues, UC-MSCs appear to be an optimal therapeutic tool[3].
The issue of selecting the most appropriate route for MSC implantation is of critical importance and needs to be discussed to successfully treat HIE. Each strategy has its advantages and disadvantages, for example, the intravenous (IV) route of transplanting MSC might provide diffuse implementation while avoiding adverse reactions associated with invasive approaches. Notwithstanding, when systemically transplanted, MSCs are able to cross the blood-brain barrier; however, they can also reach to other organs such as the liver, lungs, kidneys, and spleen and be retained by them[4]. For this reason, carrying out transplantation through multiple routes can be more effective than the use of a single route. Patients tolerated the IV and intrathecally (IT) administrations well with no adverse reactions or side effects in 24 wk after treatment[5]. In our previous study, we evaluated the safety, efficacy, and practicability of both the triple-route and multiple administrations of WJ-MSCs with this treatment approach in a patient with HIE, traumatic brain injury, and cerebral palsy[6-8]. As further studies have been conducted on this subject, it is now possible to use WJ-MSCs for the clinical treatment of HIE.
The present study was designed as a phase I clinical trial to investigate the effects of both triple-route and multiple administrations of WJ-MSC. The study population was selected as pediatric HIE patients with significant functional impairments who have a limited number of treatment options. The primary outcome of the study was to investigate the safety of this treatment with magnetic resonance imaging examinations, electroencephalographies, laboratory tests, and neurological and functional scores of patients. The efficacy of this treatment was also studied.
MATERIALS AND METHODS
Study design
The present study was designed as a phase I open-label, multi-center study. The aim of the study was to assess the safety and efficacy of both triple-route and multiple administrations of WJ-MSCs. The study inclusion criteria are given in Table 1. Pediatric patients with radiologically confirmed HIE and significant functional and cognitive impairments were included in the study. Participants were followed up for a period of 1 year following the administration of WJ-MSCs. Participants were not restricted in terms of receiving any kind of medical therapy or treatment (occupational, physical, or speech therapy) during their follow-ups. The legal representatives of participants were informed about the procedure and gave written informed consent in accordance with the principles of the Helsinki Declaration. The study protocol was approved by the Turkish Ministry of Health, General Directorate of Health Services, Department of Tissue, Organ Transplantation and Dialysis Services, Scientific Committee with the protocol number of 56733164- 203-E.2351. The data of patients are given in detail in Table 2.
Table 1.
Enrollment criteria
|
No.
|
Inclusion criteria
|
| 1 | Age < 18 |
| 2 | HIE radiologically confirmed at initial diagnosis and at study enrollment |
| 3 | The patients who does not have any chronic illness (cancer, kidney, heart/hepatic failure etc.) other than HIE. Adequate systemic organ function confirmed by normal ranged laboratory values |
| 4 | Life expectancy > 12 mo |
| 5 | No substiantial improvement despite of a treatment in neurological/functional status for the 3 mo before study enrollment |
| 6 | Severe disability defined as subject confined to a wheelchair/required to have home nursing care/needing assistance with activities of daily living |
| 7 | Expectation that the patient will receive standard post-treatment care and attend all visits |
| 8 | Signing in the written informed consent form for confirming to that know the treatment to be applied and to be willing by their parents/a surrogate |
| Exclusion criteria | |
| 1 | Presence of any other clinically significant medical/psychiatric condition, or laboratory abnormality, for which study participation would pose a safety risk in the judgment of the investigator/sponsor or history within the past year of drug/alcohol abuse |
| 2 | Recently diagnosed severe infection (meningitis, etc.)/development of liver, kidney/heart failure/sepsis or skin infection at the i.v. infusion site or positive for hepatitis B, C/HIV |
| 3 | History of uncontrolled seizure disorder |
| 4 | History of cerebral neoplasm, or cancer within the past 5 yr, with the exception of localized basal or squamous cell carcinoma |
| 5 | Having clinic symptoms that formation of white sphere number ≥ 15000/μL or platelet count ≤ 100.000/μL |
| 6 | Serum aspartate aminotransferase and serum alanine aminotransferase > 3× upper limit of normal/creatinine > 1.5× upper limit of normal |
| 7 | Participation in an another investigational stem cell study before treatment |
| 8 | The patient/parents decides to abandon the treatment or the patient death |
HIE: Hypoxic-ischemic encephalopathy; HIV: Human immunodeficiency virus.
Table 2.
Study population
|
|
Frequency
|
Percent
|
|
| Age | 1.00 | 1 | 16.7 |
| 6.00 | 2 | 33.3 | |
| 7.00 | 1 | 16.7 | |
| 9.00 | 1 | 16.7 | |
| 12.00 | 1 | 16.7 | |
| Sex | F | 4 | 66.7 |
| M | 2 | 33.3 | |
| Cause of hypoxia | Acute meningitis | 1 | 16.7 |
| Cardiac arrest after an orthopedic surgery | 1 | 16.7 | |
| Cardiac arrest due to long QT syndrome | 1 | 16.7 | |
| Cardiac arrest, unknown etiology | 1 | 16.7 | |
| Drowning in water | 1 | 16.7 | |
| Foreign body aspiration | 1 | 16.7 | |
| Duration of Hypoxia | 25.00 | 1 | 16.7 |
| 30.00 | 3 | 50.0 | |
| 45.00 | 1 | 16.7 | |
| 75.00 | 1 | 16.7 | |
| Comorbidity | Long QT syndrome | 1 | 16.7 |
| no | 4 | 66.7 | |
| Osteogenesis imperfecta | 1 | 16.7 | |
| Duration Between Hypoxia & First SCT | 6.00 | 3 | 50.0 |
| 9.00 | 1 | 16.7 | |
| 32.00 | 1 | 16.7 | |
| 72.00 | 1 | 16.7 | |
SCT: Stem cell therapy.
Procedure
Ethical considerations and consent: UCs were supplied from the Good Manufacturing Practice facility of LivMedCell (Istanbul, Turkey). In line with the approval of an institutional regulatory board (LivMedCell), various donors donated the UCs after they were informed about the purpose of the study and gave written informed consent. Postnatal UCs were obtained from full-term pregnant women who donated UCs[6-8].
UC processing and quality control
Phosphate-buffered saline (Invitrogen/Gibco, Paisley, United Kingdom) was used to wash the UCs. Tissue samples were cut into pieces of 5-10 mm3 in the form of explants following the removal of blood vessels. The explants were placed into dishes and cultured under humanized culture conditions at 37 °C with 5% CO2 until the migration of cells. When the resulting cells reached 70% to 80% confluency, they were collected and subjected to characterization tests at passage 3. The standards of the Turkish Medicines and Medical Devices Agency were followed to carry out quality control and quality assurance to produce these cells[6-8].
Characterization of WJ-MSCs by flow cytometry
Expressed surface antigens were analyzed by flow cytometry, which revealed that the cells were consistently positive for CD44, CD73, CD90, and CD105 and negative for the hematopoietic lineage markers of CD34, CD45, and Human Leukocyte class II DR antigens. The telomerase activities of WJ-MSCs were found to remain stable during the culture process with a large and flat cellular morphology[6-8].
Cell differentiation and karyotying procedure
Some stem cell expressions and the differentiation markers of TERT, SOX2, POU5F1, CD44, ZFP42, VIM, ICAM1, THY1, VCAM1, BMP2, RUNX-1, and NES were identified. These cells were confirmed to have trilineage (chondrocytes, osteoblasts, and adipocytes) differentiation capacity by differentiation analyses. Karyotyping studies showed no numerical or structural chromosomal abnormalities for these cells[6-8].
Pre-transplantation process: The final WJ-MSC preparations to be used for implantation were collected from passage 3 of cultures and kept in normal saline at final densities of 1 × 106 in 3 mL, 1 × 106 in 20 mL, 1 × 106 in 30 mL[6-8].
WJ-MSC transplantation and surgical procedures: Before initiating treatment, patients were examined by a physician team consisting of a pediatrician and a pediatric neurologist, as well as experts in neurosurgery, anesthesia and reanimation, and physical therapy and rehabilitation. Before the implantation procedure of WJ-MSCs, patients were evaluated for contraindications to sedoanalgesia or general anesthesia as well as severe infectious diseases like sepsis, and the procedure was then performed when they were stable[6-8].
In the procedure, allogeneic WJ-MSCs were administered IT, intramuscularly (IM), and IV, respectively, in the operating room by the same physician team (Kabatas S, Kaplan N, Can H, and Genç A), following the standard protocol of the MSC treatment trial (Table 3). IT administration of WJ-MSCs was performed through a lumbar puncture as described by previous studies[9]. IM administration of WJ-MSCs was performed under the guidance of ultrasound for each muscle, while IV infusion was slowly administered in 30 min. Following the completion of the procedure, patients were transferred to intensive care unit (1st level). A day later, patients were transferred to Neurosurgery Department for follow-up and initiated on physical therapy and rehabilitation. Patients did not perform exercises during the days of stem cell administration. The same protocol was followed before and after every administration.
Table 3.
Administration schedule
|
Rounds
|
Route
|
WJ-MSC
|
| Round 1 | IT | 1 × 106/kg in 3 mL |
| IV | 1 × 106/kg in 30 mL | |
| IM | 1 × 106/kg in 20 mL | |
| Round 2 (2nd week) | IT | 1 × 106/kg in 3 mL |
| IV | 1 × 106/kg in 30 mL | |
| IM | 1 × 106/kg in 20 mL | |
| Round 3 (4th week) | IT | 1 × 106/kg in 3 mL |
| IV | 1 × 106/kg in 30 mL | |
| IM | 1 × 106/kg in 20 mL | |
| Round 4 (6th week) | IT | 1 × 106/kg in 3 mL |
| IV | 1 × 106/kg in 30 mL | |
| IM | 1 × 106/kg in 20 mL |
WJ-MSC: Wharton’s jelly-derived mesenchymal stem cells; IT: Intrathecal; IV: Intravenosus; IM: Intramuscular.
Pretreatment neurological examination
Patients were evaluated before treatment with a comprehensive examination by a physician team consisting of medical and rehabilitation doctors. Each step of the neurological and functional evaluation was documented in detail. Patients were evaluated for spasticity with the Modified Ashworth (MA) scale and for quality of life with the Wee Functional Independence Measure (Wee FIM) scale based on the statements of their parents[10].
Safety evaluation criteria
The safety criteria for the procedure were determined as follows: Any evidence of infection, headache, fever, pain, allergic reactions or shock, leukocytosis, an elevated level of C-reactive protein, and perioperative complications (wound site infections, analgesia, and anesthesia-related complications) during 7-14 d post-treatment. The safety criteria for WJ-MSC administration were determined as follows: Any evidence of infection, development of cancer, neuropathic pain, and worsening neurological status during the 1-year follow-up[6,8].
Follow-up assessment of treatment success
For follow-up assessment of treatment success, patients were evaluated neurologically and functionally in detail. They were evaluated for spasticity with the MA Scale and for quality of life with the Wee FIM Scale[11,12]. Moreover, patients were also evaluated for secondary infections, neuropathic pain, urinary tract infections, or decubitus ulcers.
Statistical analysis
The non-parametric tests of Friedman Test and Wilcoxon Signed Ranks Test were employed to measure the change in the pre-treatment and post-treatment Wee FIM and MA Scale scores of patients. As the number of data was not sufficient for parametric tests, nonparametric tests were carried out.
RESULTS
Safety of procedure and adverse events
The procedure was well tolerated by patients with no severe adverse events associated with the procedure. Three patients developed only early complications such as low-grade fever, mild headache associated with IT injection, and muscle pain associated with IM injection, all of which were transient and disappeared within 24 h (Table 4). No other adverse events or safety issues were reported during the 1-year follow-up period.
Table 4.
Early and late complications of the procedures
|
Complications
|
Patient No. 1 | Patient No. 2 | Patient No. 3 | Patient No. 4 | Patient No. 5 | Patient No. 6 | |||||||||||||||||||
|
Administration
|
Administration
|
Administration
|
Administration
|
Administration
|
Administration
|
||||||||||||||||||||
|
1st
|
2nd
|
3rd
|
4th
|
1st
|
2nd
|
3rd
|
4th
|
1st
|
2nd
|
3rd
|
4th
|
1st
|
2nd
|
3rd
|
4th
|
1st
|
2nd
|
3rd
|
4th
|
1st
|
2nd
|
3rd
|
4th
|
||
| Early | Infection | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Fever | - | - | - | - | - | - | - | - | - | + | - | - | - | + | - | - | + | + | - | - | - | - | - | - | |
| Pain | - | - | - | - | - | - | - | - | + | - | + | - | + | + | - | - | + | - | - | - | - | - | - | - | |
| Headache | - | - | - | - | - | - | - | - | - | - | + | - | + | - | - | - | - | - | - | - | - | - | - | - | |
| Increased level of C-reactive protein | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Leukocytosis | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Allergic reaction or shock | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Perioperative complications | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Late | Secondary infections | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Urinary tract infections | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Deterioration of neurological status | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Neuropathic pain | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Carcinogenesis | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
–: Not present; +: Present.
Wee FIM scale score
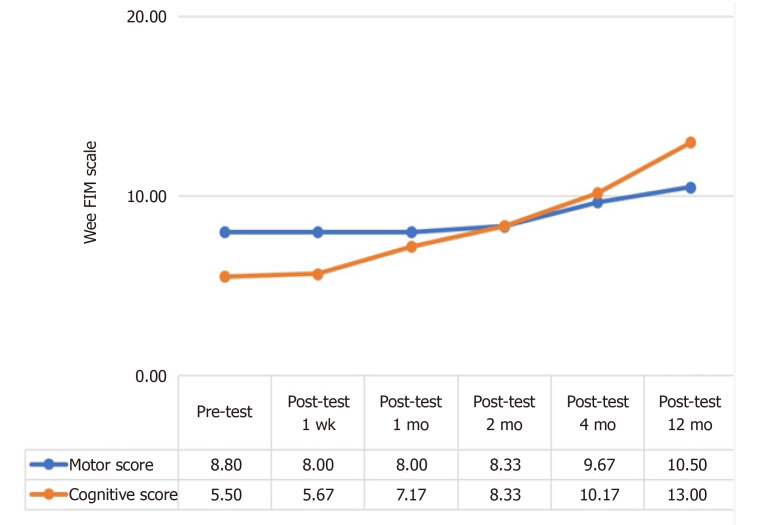
Despite a slight increase in the post-treatment 4-mo and 12-mo Wee FIM Motor scores of patients, the increase in their cognitive scores continued throughout the post-treatment follow-up period.
The analysis as shown in Table 5 revealed that participants had a statistically significant difference in their pre-treatment and post-treatment Jee FIM Motor scores (χ2 = 23.444, P < 0.001). The differences between binary measurements were determined by the Wilcoxon signed-rank test. As a result of the analysis, there was no significant difference between pretest and one-week posttest scores (z = 0.000, P > 0.05); between one-week posttest and one-month posttest scores (z = 0.000, P > 0.05); between one-month posttest and two-month posttest scores (z = -1.414, P > 0.05); between two-month posttest and four-month posttest scores (z = -2.070, P < 0.05); and between the four-month posttest and 12-mo posttest scores (z = -1.633, P > 0.05). On the other hand, when the pretest score and subsequent measurements were compared, no significant difference was observed between the pretest score and one-week posttest (z = 0.000, P > 0.05), one-month posttest (z = 0.000, P > 0.05), two-month posttest (z = -1.414, P > 0.05) 12-mo posttest (z = -2.041, P < 0.05) scores whereas there was a significant difference between the pretest score and four-month posttest (z = -2.236, P < 0.05) scores. In other words, while there was no difference in the Wee FIM Motor scores of the patients until the 4th postoperative month, a significant increase was observed in the 4th month (Figure 1 and Table 5).
Table 5.
Friedman test results regarding the change in the Wee Functional Independence Measure Motor scores of the patients before and after the operation
|
|
n
|
Mean
|
SD
|
Mean rank
|
χ 2 |
df
|
P
value
|
| Pre-test | 6 | 8.00 | 0.00 | 2.50 | 23.444 | 5 | 0.000 |
| Post-test 1 wk | 6 | 8.00 | 0.00 | 2.50 | |||
| Post-test 1 mo | 6 | 8.00 | 0.00 | 2.50 | |||
| Post-test 2 mo | 6 | 8.33 | 0.52 | 3.17 | |||
| Post-test 4 mo | 6 | 9.67 | 0.82 | 4.92 | |||
| Post-test 12 mo | 6 | 10.50 | 1.52 | 5.42 |
SD: Standard deviation.
Figure 1.
Change in the mean pretest and posttest the Wee Functional Independence Measure Motor and cognitive scores of the patients. Wee FIM: Wee Functional Independence Measure.
The analysis as shown in Table 6 revealed that participants had a statistically significant difference in their pre-treatment and post-treatment Wee FIM cognitive scores (χ2 = 28.255, P < 0.001). The differences between binary measurements were determined by the Wilcoxon signed-rank test. As a result of the analysis, no significant difference was observed between pretest and one-week posttest scores (z = -1.000, P > 0.05); between one-week posttest and one-month posttest scores (z = -1.841, P > 0.05); between two-month posttest and four-month posttest scores (z = -2.041, P < 0.05); and between the four-month posttest and 12-mo posttest scores (z = -2.264, P < 0.05), whereas the difference between the one-month posttest and two-month posttest scores was significant (z = -2.070, P < 0.05). On the other hand, when the pretest score and subsequent measurements were compared, no significant difference was observed between the pretest score and one-week posttest (z = -1.000, P > 0.05), one-month posttest (z = -1.826, P > 0.05) scores, whereas there was a significant difference between the pretest score and two-month (z = -2.023, P < 0.05), four-month (z = -2.207, P < 0.05) and 12-mo posttest (z = -2.201, P < 0.05) posttest scores. While there was no difference in the Wee FIM Cognitive scores of the patients until the second postoperative month, a significant increase was observed in the second month (Figure 1 and Table 6).
Table 6.
Friedman test results regarding the change in the Wee Functional Independence Measure cognitive scores of the patients before and after the operation
|
|
n
|
Mean
|
SD
|
Mean rank
|
χ 2 |
df
|
P
value
|
| Pre-test | 6 | 5.50 | 0.55 | 1.67 | 28.255 | 5 | 0.000 |
| Post-test 1 wk | 6 | 5.67 | 0.82 | 1.83 | |||
| Post-test 1 mo | 6 | 7.17 | 1.60 | 2.75 | |||
| Post-test 2 mo | 6 | 8.33 | 2.25 | 3.83 | |||
| Post-test 4 mo | 6 | 10.17 | 3.06 | 4.92 | |||
| Post-test 12 mo | 6 | 13.00 | 2.83 | 6.00 |
SD: Standard deviation.
MA scale score
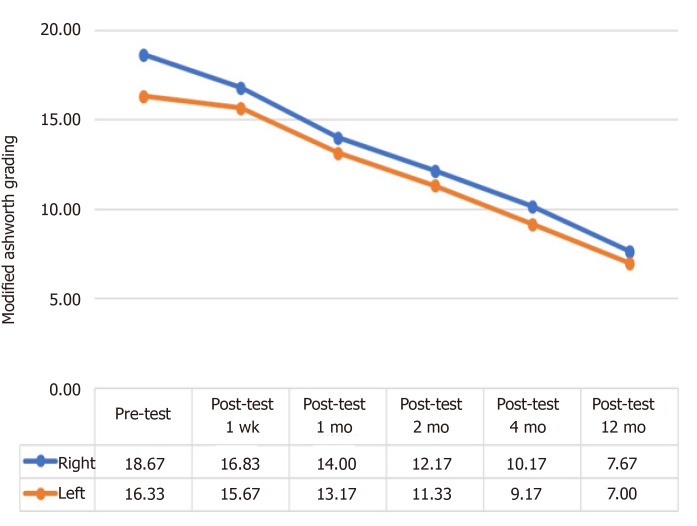
Patients had a continuous decrease in their MA scale right and left scores throughout the follow-up period, which was indicative of improvement.
The analysis as shown in Table 7 revealed that patients had a statistically significant difference in their pre-treatment and post-treatment macrophage activation syndrome (MAS) right scores (χ2 = 29.439, P < 0.001). The differences between binary measurements were determined by the Wilcoxon signed-rank test. As a result of the analysis, no significant difference was observed between pretest and one-week posttest scores (z = -1.841, P > 0.05) and between two-month posttest and four-month posttest scores (z = -1.857, P > 0.05), whereas there were significant differences between the one-week posttest and one-month posttest scores (z = -2.214, P < 0.05); between the one-month posttest and two-month posttest scores (z = - 2.041, P < 0.05); and between four-month posttest and 12-mo posttest scores (z = -2.264, P < 0.05). In other words, while there was no significant difference in patients' MAS Right values in the first post-treatment week, the values started to decrease significantly in parallel with the recovery after the first week (Figure 2 and Table 7).
Table 7.
Friedman test results regarding the change in the macrophage activation syndrome right scores of the patients before and after the operation
|
|
n
|
Mean
|
SD
|
Mean rank
|
χ 2 |
df
|
P
value
|
| Pre-test | 6 | 18.67 | 4.72 | 5.83 | 29.439 | 5 | 0.000 |
| Post-test 1 wk | 6 | 16.83 | 6.15 | 5.17 | |||
| Post-test 1 mo | 6 | 14.00 | 7.62 | 3.92 | |||
| Post-test 2 mo | 6 | 12.17 | 6.94 | 2.92 | |||
| Post-test 4 mo | 6 | 10.17 | 6.65 | 2.17 | |||
| Post-test 12 mo | 6 | 7.67 | 6.12 | 1.00 |
SD: Standard deviation.
Figure 2.
Change in the mean pretest and posttest macrophage activation syndrome right and left scores of the patients.
The analysis as shown in Table 8 revealed that patients had a statistically significant difference in their pre-treatment and post-treatment MAS left scores (z = 29.000, P < 0.001). Wilcoxon Signed Ranks Test was performed between the binary measurements to identify the differences between variables. As a result of the analysis, no significant difference was observed between pretest and one-week posttest scores (z = -1.342, P > 0.05) and between two-month posttest and four-month posttest scores (z = -1.841, P > 0.05), whereas there were significant differences between the one-week posttest and one-month posttest scores (z = -2.041, P < 0.05); between the one-month posttest and two-month posttest scores (z = - 2.032, P < 0.05); and between four-month posttest and 12-mo posttest scores (z = -2.264, P < 0.05). In other words, while there was no significant difference in patients’ MAS Left values in the first post-treatment week, the values started to decrease significantly in parallel with the recovery after the first week (Figure 2 and Table 8).
Table 8.
Friedman test results regarding the change in the macrophage activation syndrome left scores of the patients before and after the operation
|
|
n
|
Mean
|
SD
|
Mean rank
|
χ
2
|
df
|
P
value
|
| Pre-test | 6 | 16.33 | 5.13 | 5.58 | 29.000 | 5 | 0.000 |
| Post-test 1 wk | 6 | 15.67 | 5.89 | 5.25 | |||
| Post-test 1 mo | 6 | 13.17 | 7.33 | 4.08 | |||
| Post-test 2 mo | 6 | 11.33 | 6.31 | 2.92 | |||
| Post-test 4 mo | 6 | 9.17 | 5.74 | 2.17 | |||
| Post-test 12 mo | 6 | 7.00 | 5.33 | 1.00 |
SD: Standard deviation.
DISCUSSION
HIE is one of the leading causes of death and long-term neurological impairment in the pediatric population. Today, there is a limited number of treatment options for HIE, e.g., cooling. Different therapeutic approaches have been used to treat and improve functional and neurological outcomes of HIE patients. Among these approaches, MSCs come to the fore as a potential treatment option for ischemic brain injury caused by HIE[13]. MSCs are the most frequently used regenerative cells in clinical trials since they have a relatively safe profile, ease of isolation, and ability to reduce cell apoptosis, ameliorate oxidative stress and inflammation, and recover energy failure[14]. Results of a meta-analysis of preclinical studies on HIE showed the potential of treatment with mesenchymal stromal cells for improving neurological functions[15].
Furthermore, although regenerative cells are characterized by a low level of immunogenicity, autologous transplantation probably appears to be linked with a lower risk of immune rejection and infection development[16]. Allogeneic stem cell transplantation might provide significant advantages in terms of practicability[17]. In recent years, the UC comes to the fore as the most commonly used tissue to harvest regenerative[18,19]. In preclinical studies, UC-MSCs administration has been suggested to enhance axonal regeneration and nerve fiber remyelination and promote sensorimotor functions with better long-term neurological outcomes[20-23]. Stem cell transplantation for ischaemic stroke, a Cochrane review, evaluated three small trials conducted on adults[24]. There is one Cochrane review on MSC-based therapies for the prevention and treatment of bronchopulmonary dysplasia in preterm infants[25]. There are also early phase trials on the use of cord blood cells and MSCs or (or the combination of both) for severe intraventricular hemorrhage (NCT02274428), bronchopulmonary dysplasia, and HIE[26,27].
Despite the promising potential of stem cells and progenitor cells for HIE in experimental and clinical pilot studies, cell therapy in humans still remains in the initial stage[28]. The study by Miao et al[9] reported that 47 patients (47%), including 12 patients with spinal cord injury, 11 patients with cerebral palsy, 9 patients with post-brain infarction syndrome, 9 patients with post-traumatic brain syndrome, 3 patients with motor neuron disease, and 3 patients with spinocerebellar ataxia had an improvement in their functional indices a year after the intrathecal administration of UC-MSCs[9]. Preclinical studies have shown substantial favorable effects of intraarterial, intracisternal, intratracheal, intravenous, or intracerebral administrations of allogeneic WJ-MSCs[29]. The present study demonstrated that both triple-route and multiple administrations of allogeneic WJ-MSCs were safe and improved the functional status of patients.
The current study is the largest trial of both triple route and multiple implantations of allogeneic WJ-MSC in pediatric patients with HIE and the first to evaluate allogeneic WJ-MSC therapy in this population regardless of our previous pilot study[6]. With a dose of 1 × 106/kg for each route, patients developed mild adverse reactions, all of which were transient and disappeared with 24 h. Three patients developed only early complications such as low-grade fever, mild headache associated with IT injection, and muscle pain associated with IM injection, all of which were transient and disappeared within 24 h.
Patients with chronic HIE usually exhibit functional deterioration but pediatric patients included in this study exhibited a constant functional improvement through the 12-mo follow-up. HIE-related impairments usually show a bimodal recovery pattern. The majority of HIE survivors first exhibit a little spontaneous recovery, for instance, improvement in the motor system during the first months. However, they experience a significant deterioration in functional status a year after the onset of HIE. This is of note as there is a limited number of treatment options for patients with chronic HIE.
In the current study, functional gains were seen, though were modest in magnitude. Although moderate, patients included in the present study showed functional improvements. Despite that, a 2.5-point increase in the Wee FIM Scale motor scores of chronic HIE patients are of great value (Table 5). Although patients had a low increase in their Wee FIM motor scores, they achieved an increase of 7.5 points in their post-treatment cognitive scores (Table 6). This continuous improvement through the 12-mo follow-up indicates the broad effects of MSC on brain functions. There is a need for larger, placebo-controlled studies to verify these results; however, these results also support that this treatment can be used as a promising approach to improve the functions of patients with chronic HIE. Treatment-specific outcome measures may be the subject of future studies to obtain more detailed estimates of behavioral improvements in the neural systems of patients. In the present study, the percentage of patients who achieved an excellent functional improvement (Wee FIM scale total score = 126) increased from 10.71% (at baseline) to 12.03% at 1 mo and to 18.65% at 12 mo post-treatment (Tables 5 and 6). Preclinical studies conducted with animal models of HIE have shown a significant improvement in the functions of treatment groups with MSCs. The potential of MSC therapies to treat neurologic conditions is associated with their ability to restore energy failure, inhibit the inflammatory response, and enhance neurogenesis as well as angiogenesis in the hypoxic brain area. Our study is in line with preclinical studies on HIE in terms of continuous improvement in functions through the 12-mo follow-up[15].
The present study has several strengths. The study population consisted of chronic HIE patients with substantial functional impairments, who have a limited number of treatment options. However, optimization of regenerative cells requires considering several factors. Some examples of these factors are the source to derive stem cells, processing of cells, number of passage, frequency, dose, timing, and administration route, all of which have an effect on treatment efficacy[30]. Moreover, various laboratory processing techniques, including cell expansion medium, oxygen tension, number of passage, the use of cryopreserved or fresh cells, also have an effect on the therapeutic potential of regenerative cells[31-33]. Since the use of multiple passages may damage cellular functions, fewer passages should be preferred as much as possible[34,35]. In the present study, the cells used in infusion were allogeneic so the requirement for immunosuppressive therapy was excluded when compared with autologous cell therapies. This comparatively immune-privileged feature of MSC makes it possible to use this approach in a large pediatric population with HIE. The safety of the treatment was evaluated by including both triple-route and multiple administrations of WJ-MSC in the study protocol. The limit of cell culture was set at 3 passages, which provided an important advantage considering that the use of multiple passages may damage cellular functions of MSCs such as differentiation, proliferation, viability, and homing. The safety evaluation also included comprehensive laboratory tests through a 1-year follow-up.
The study also has several limitations, which are not including a control group to compare behavioral gains since the study was designed as a safety study, and not studying the mechanism of action. Stem cell therapies to improve outcomes of patients with chronic HIE are likely to act through multiple mechanisms, including the release of growth factors and anti-inflammatory effects, and probably exosomes. Future studies can focus on this. Patients with HIE can benefit from restorative therapies to a maximum extent when they combine the treatment with training, which was not given to patients included in this study. The present study showed that both triple-route and multiple administrations of WJ-MSC were safe in the pediatric HIE population suffering from substantial functional impairments. The results of this study also demonstrate the functional benefit of WJ-MSC therapy, which should be verified in controlled studies. Collectively, the results of this study support future studies to evaluate both triple-route and multiple administrations of WJ-MSC in pediatric HIE with its mechanism of action.
CONCLUSION
Stem cell therapies appear to be a potential treatment option for brain injury resulting from HIE. In recent years, stem cell therapies, especially WJ-MSCs therapy, have led to the development of novel treatment protocols for ischemic brain injury. However, many unanswered questions on stem cell therapies still remain. There is a need for much effort to devote to thoroughly elucidating how stem cell therapy works, what paracrine mediators are important, when and what type of therapy should be used, and which patients are eligible candidates for this treatment. Therefore, there is a need for further preclinical studies to optimize the treatment protocol as well as multicenter clinical trials to confirm safety and efficacy.
ARTICLE HIGHLIGHTS
Research background
Hypoxic-ischemic encephalopathy (HIE) is one of the leading causes of death and long-term neurological impairment in the pediatric population.
Research motivation
Despite a limited number of treatments to cure HIE, stem cell therapies appear to be a potential treatment option for brain injury resulting from HIE.
Research objectives
The present study investigated the efficacy and safety of stem cell-based therapies in pediatric patients with HIE.
Research methods
Wharton’s jelly-derived mesenchymal stem cells (WJ-MSCs) were intrathecally, intramuscularly, and intravenously administered to participants at a dose of 1 × 106/kg for each administration route twice monthly for 2 mo. In different follow-up durations, the effect of WJ-MSCs administration on HIE as well as the quality of life and prognosis of patients was investigated, and patients were evaluated for neurological, cognitive functions, and spasticity using the Wee Functional Independence Measure Scale and Modified Ashworth Scale to determine the associated adverse reactions.
Research results
Three patients developed only early complications which were transient and disappeared within 24 h. The treatment was evaluated to be safe and effective as demonstrated by magnetic resonance imaging examinations, electroencephalographies, laboratory tests, and neurological and functional scores of patients. Patients exhibited significant improvements in all neurological functions during the 12-mo follow-up period.
Research conclusions
Multiple triple-route WJ-MSC administrations were found to be safe for pediatric HIE patients, indicating neurological and functional improvement.
Research perspectives
More comprehensive randomized and placebo-controlled studies can be conducted to further support the results of this study.
Footnotes
Institutional review board statement: Ethical approval to report this case was obtained from the IRB of Turkish Ministry of Health, Department of Organ, Tissue Transplant and Dialysis Services’ Scientific Committee, Ankara, Turkey, No. 56733164-203-E. 2351.
Informed consent statement: There is human subject in this article and written informed consents were obtained from the patient for their anonymized information to be published in this article and before the stem cell therapies.
Conflict-of-interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Manuscript source: Invited manuscript
Peer-review started: December 24, 2020
First decision: February 14, 2021
Article in press: April 22, 2021
Specialty type: Cell and tissue engineering
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cazorla E S-Editor: Fan JR L-Editor: A P-Editor: Xing YX
Contributor Information
Serdar Kabatas, Department of Neurosurgery, University of Health Sciences, Gaziosmanpaşa Training and Research Hospital, Istanbul 34255, Turkey; Pediatric Allergy-Immunology, Marmara University, Institute of Health Sciences, Istanbul 34854, Turkey; Center for Stem Cell & Gene Therapy Research and Practice, University of Health Sciences, Istanbul 34255, Turkey. kabatasserdar@hotmail.com.
Erdinç Civelek, Department of Neurosurgery, University of Health Sciences, Gaziosmanpaşa Training and Research Hospital, Istanbul 34255, Turkey; Pediatric Allergy-Immunology, Marmara University, Institute of Health Sciences, Istanbul 34854, Turkey.
Eyüp Can Savrunlu, Department of Neurosurgery, University of Health Sciences, Gaziosmanpaşa Training and Research Hospital, Istanbul 34255, Turkey.
Necati Kaplan, Department of Neurosurgery, Istanbul Rumeli University, Çorlu Reyap Hospital, Tekirdağ 59860, Turkey.
Osman Boyalı, Department of Neurosurgery, University of Health Sciences, Gaziosmanpaşa Training and Research Hospital, Istanbul 34255, Turkey.
Furkan Diren, Department of Neurosurgery, University of Health Sciences, Gaziosmanpaşa Training and Research Hospital, Istanbul 34255, Turkey.
Halil Can, Department of Neurosurgery, Istanbul Biruni University, Faculty of Medicine, Istanbul 34010, Turkey; Department of Neurosurgery, Istanbul Medicine Hospital, Istanbul 34203, Turkey.
Ali Genç, Department of Neurosurgery, Istanbul Asya Hospital, Istanbul 34250, Turkey.
Tunç Akkoç, Pediatric Allergy-Immunology, Marmara University, Istanbul 34899, Turkey.
Erdal Karaöz, Center for Regenerative Medicine and Stem Cell Research & Manufacturing (LivMedCell), Liv Hospital, Istanbul 34340, Turkey; Department of Histology and Embryology, Istinye University, Faculty of Medicine, Istanbul 34010, Turkey; Center for Stem Cell and Tissue Engineering Research and Practice, Istinye University, Istanbul 34340, Turkey.
Data sharing statement
No additional data are available.
References
- 1.Qin X, Cheng J, Zhong Y, Mahgoub OK, Akter F, Fan Y, Aldughaim M, Xie Q, Qin L, Gu L, Jian Z, Xiong X, Liu R. Mechanism and Treatment Related to Oxidative Stress in Neonatal Hypoxic-Ischemic Encephalopathy. Front Mol Neurosci. 2019;12:88. doi: 10.3389/fnmol.2019.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang J, Yang C, Chen J, Luo M, Qu Y, Mu D, Chen Q. Umbilical cord mesenchymal stem cells and umbilical cord blood mononuclear cells improve neonatal rat memory after hypoxia-ischemia. Behav Brain Res. 2019;362:56–63. doi: 10.1016/j.bbr.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Ohki A, Saito S, Fukuchi K. Magnetic resonance imaging of umbilical cord stem cells labeled with superparamagnetic iron oxide nanoparticles: effects of labelling and transplantation parameters. Sci Rep. 2020;10:13684. doi: 10.1038/s41598-020-70291-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park WS, Ahn SY, Sung SI, Ahn JY, Chang YS. Mesenchymal Stem Cells: The Magic Cure for Intraventricular Hemorrhage? Cell Transplant. 2017;26:439–448. doi: 10.3727/096368916X694193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joerger-Messerli MS, Marx C, Oppliger B, Mueller M, Surbek DV, Schoeberlein A. Mesenchymal Stem Cells from Wharton's Jelly and Amniotic Fluid. Best Pract Res Clin Obstet Gynaecol. 2016;31:30–44. doi: 10.1016/j.bpobgyn.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Kabataş S, Civelek E, İnci Ç, Yalçınkaya EY, Günel G, Kır G, Albayrak E, Öztürk E, Adaş G, Karaöz E. Wharton's Jelly-Derived Mesenchymal Stem Cell Transplantation in a Patient with Hypoxic-Ischemic Encephalopathy: A Pilot Study. Cell Transplant. 2018;27:1425–1433. doi: 10.1177/0963689718786692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kabatas S, Civelek E, Sezen GB, Kaplan N, Savrunlu EC, Cetin E, Diren F, Karaoz E. Functional Recovery After Wharton's Jelly-Derived Mesenchymal Stem Cell Administration in a Patient with Traumatic Brain Injury: A Pilot Study. Turk Neurosurg. 2020;30:914–922. doi: 10.5137/1019-5149.JTN.31732-20.1. [DOI] [PubMed] [Google Scholar]
- 8.Okur SÇ, Erdoğan S, Demir CS, Günel G, Karaöz E. The Effect of Umbilical Cord-derived Mesenchymal Stem Cell Transplantation in a Patient with Cerebral Palsy: A Case Report. Int J Stem Cells. 2018;11:141–147. doi: 10.15283/ijsc17077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miao X, Wu X, Shi W. Umbilical cord mesenchymal stem cells in neurological disorders: A clinical study. Indian J Biochem Biophys. 2015;52:140–146. [PubMed] [Google Scholar]
- 10.Thorpe ER, Garrett KB, Smith AM, Reneker JC, Phillips RS. Outcome Measure Scores Predict Discharge Destination in Patients With Acute and Subacute Stroke: A Systematic Review and Series of Meta-analyses. J Neurol Phys Ther. 2018;42:2–11. doi: 10.1097/NPT.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 11.Huang H, Young W, Chen L, Feng S, Zoubi ZMA, Sharma HS, Saberi H, Moviglia GA, He X, Muresanu DF, Sharma A, Otom A, Andrews RJ, Al-Zoubi A, Bryukhovetskiy AS, Chernykh ER, Domańska-Janik K, Jafar E, Johnson WE, Li Y, Li D, Luan Z, Mao G, Shetty AK, Siniscalco D, Skaper S, Sun T, Wang Y, Wiklund L, Xue Q, You SW, Zheng Z, Dimitrijevic MR, Masri WSE, Sanberg PR, Xu Q, Luan G, Chopp M, Cho KS, Zhou XF, Wu P, Liu K, Mobasheri H, Ohtori S, Tanaka H, Han F, Feng Y, Zhang S, Lu Y, Zhang Z, Rao Y, Tang Z, Xi H, Wu L, Shen S, Xue M, Xiang G, Guo X, Yang X, Hao Y, Hu Y, Li J, Ao Q, Wang B, Lu M, Li T. Clinical Cell Therapy Guidelines for Neurorestoration (IANR/CANR 2017) Cell Transplant. 2018;27:310–324. doi: 10.1177/0963689717746999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darteyre S, Renaud C, Vuillerot C, Presles E, Kossorotoff M, Dinomais M, Lazaro L, Gautheron V, Chabrier S AVCnn Group. Quality of life and functional outcome in early school-aged children after neonatal stroke: a prospective cohort study. Eur J Paediatr Neurol. 2014;18:347–353. doi: 10.1016/j.ejpn.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Nabetani M, Shintaku H, Hamazaki T. Future perspectives of cell therapy for neonatal hypoxic-ischemic encephalopathy. Pediatr Res. 2018;83:356–363. doi: 10.1038/pr.2017.260. [DOI] [PubMed] [Google Scholar]
- 14.Trounson A, McDonald C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell. 2015;17:11–22. doi: 10.1016/j.stem.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Archambault J, Moreira A, McDaniel D, Winter L, Sun L, Hornsby P. Therapeutic potential of mesenchymal stromal cells for hypoxic ischemic encephalopathy: A systematic review and meta-analysis of preclinical studies. PLoS One. 2017;12:e0189895. doi: 10.1371/journal.pone.0189895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gebler A, Zabel O, Seliger B. The immunomodulatory capacity of mesenchymal stem cells. Trends Mol Med. 2012;18:128–134. doi: 10.1016/j.molmed.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Hare JM, DiFede DL, Rieger AC, Florea V, Landin AM, El-Khorazaty J, Khan A, Mushtaq M, Lowery MH, Byrnes JJ, Hendel RC, Cohen MG, Alfonso CE, Valasaki K, Pujol MV, Golpanian S, Ghersin E, Fishman JE, Pattany P, Gomes SA, Delgado C, Miki R, Abuzeid F, Vidro-Casiano M, Premer C, Medina A, Porras V, Hatzistergos KE, Anderson E, Mendizabal A, Mitrani R, Heldman AW. Randomized Comparison of Allogeneic Versus Autologous Mesenchymal Stem Cells for Nonischemic Dilated Cardiomyopathy: POSEIDON-DCM Trial. J Am Coll Cardiol. 2017;69:526–537. doi: 10.1016/j.jacc.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitsialis SA, Kourembanas S. Stem cell-based therapies for the newborn lung and brain: Possibilities and challenges. Semin Perinatol. 2016;40:138–151. doi: 10.1053/j.semperi.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoon D, Kim H, Lee E, Park MH, Chung S, Jeon H, Ahn CH, Lee K. Study on chemotaxis and chemokinesis of bone marrow-derived mesenchymal stem cells in hydrogel-based 3D microfluidic devices. Biomater Res. 2016;20:25. doi: 10.1186/s40824-016-0070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donega V, van Velthoven CT, Nijboer CH, Kavelaars A, Heijnen CJ. The endogenous regenerative capacity of the damaged newborn brain: boosting neurogenesis with mesenchymal stem cell treatment. J Cereb Blood Flow Metab. 2013;33:625–634. doi: 10.1038/jcbfm.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donega V, Nijboer CH, van Velthoven CT, Youssef SA, de Bruin A, van Bel F, Kavelaars A, Heijnen CJ. Assessment of long-term safety and efficacy of intranasal mesenchymal stem cell treatment for neonatal brain injury in the mouse. Pediatr Res. 2015;78:520–526. doi: 10.1038/pr.2015.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu AM, Lu G, Tsang KS, Li G, Wu Y, Huang ZS, Ng HK, Kung HF, Poon WS. Umbilical cord-derived mesenchymal stem cells with forced expression of hepatocyte growth factor enhance remyelination and functional recovery in a rat intracerebral hemorrhage model. Neurosurgery. 2010;67:357–65; discussion 365. doi: 10.1227/01.NEU.0000371983.06278.B3. [DOI] [PubMed] [Google Scholar]
- 23.Morán J, Stokowska A, Walker FR, Mallard C, Hagberg H, Pekna M. Intranasal C3a treatment ameliorates cognitive impairment in a mouse model of neonatal hypoxic-ischemic brain injury. Exp Neurol. 2017;290:74–84. doi: 10.1016/j.expneurol.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Boncoraglio GB, Bersano A, Candelise L, Reynolds BA, Parati EA. Stem cell transplantation for ischemic stroke. Cochrane Database Syst Rev. 2010:CD007231. doi: 10.1002/14651858.CD007231.pub2. [DOI] [PubMed] [Google Scholar]
- 25.Pierro M, Thébaud B, Soll R. Mesenchymal stem cells for the prevention and treatment of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst Rev. 2017;11:CD011932. doi: 10.1002/14651858.CD011932.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang KA, Lee JH, Suh YH. Therapeutic potential of human adipose-derived stem cells in neurological disorders. J Pharmacol Sci. 2014;126:293–301. doi: 10.1254/jphs.14R10CP. [DOI] [PubMed] [Google Scholar]
- 27.Cotten CM, Murtha AP, Goldberg RN, Grotegut CA, Smith PB, Goldstein RF, Fisher KA, Gustafson KE, Waters-Pick B, Swamy GK, Rattray B, Tan S, Kurtzberg J. Feasibility of autologous cord blood cells for infants with hypoxic-ischemic encephalopathy. J Pediatr 2014; 164: 973-979. :e1. doi: 10.1016/j.jpeds.2013.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhasin A, Kumaran SS, Bhatia R, Mohanty S, Srivastava MVP. Safety and Feasibility of Autologous Mesenchymal Stem Cell Transplantation in Chronic Stroke in Indian patients. A four-year follow up. J Stem Cells Regen Med. 2017;13:14–19. doi: 10.46582/jsrm.1301003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L, Li Y, Romanko M, Kramer BC, Gosiewska A, Chopp M, Hong K. Different routes of administration of human umbilical tissue-derived cells improve functional recovery in the rat after focal cerebral ischemia. Brain Res. 2012;1489:104–112. doi: 10.1016/j.brainres.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 30.Möbius MA, Thébaud B. Stem Cells and Their Mediators - Next Generation Therapy for Bronchopulmonary Dysplasia. Front Med (Lausanne) 2015;2:50. doi: 10.3389/fmed.2015.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frey NV, Lazarus HM, Goldstein SC. Has allogeneic stem cell cryopreservation been given the 'cold shoulder'? An analysis of the pros and cons of using frozen vs fresh stem cell products in allogeneic stem cell transplantation. Bone Marrow Transplant. 2006;38:399–405. doi: 10.1038/sj.bmt.1705462. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan A, Sackett K, Sumstad D, Kadidlo D, McKenna DH. Impact of starting material (fresh vs cryopreserved marrow) on mesenchymal stem cell culture. Transfusion. 2017;57:2216–2219. doi: 10.1111/trf.14192. [DOI] [PubMed] [Google Scholar]
- 33.Parody R, Caballero D, Márquez-Malaver FJ, Vázquez L, Saldaña R, Madrigal MD, Calderón C, Carrillo E, Lopez-Corral L, Espigado I, Carmona M, López-Villar O, Pérez-Simón JA. To freeze or not to freeze peripheral blood stem cells prior to allogeneic transplantation from matched related donors. Eur J Haematol. 2013;91:448–455. doi: 10.1111/ejh.12140. [DOI] [PubMed] [Google Scholar]
- 34.Bellayr IH, Catalano JG, Lababidi S, Yang AX, Lo Surdo JL, Bauer SR, Puri RK. Gene markers of cellular aging in human multipotent stromal cells in culture. Stem Cell Res Ther. 2014;5:59. doi: 10.1186/scrt448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wagner W, Horn P, Castoldi M, Diehlmann A, Bork S, Saffrich R, Benes V, Blake J, Pfister S, Eckstein V, Ho AD. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS One. 2008;3:e2213. doi: 10.1371/journal.pone.0002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.