Abstract
Stem cell-based therapy raises hopes for a better approach to promoting tissue repair and functional recovery. However, transplanted stem cells show a high death percentage, creating challenges to successful transplantation and prognosis. Thus, it is necessary to investigate the mechanisms underlying stem cell death, such as apoptotic cascade activation, excessive autophagy, inflammatory response, reactive oxygen species, excitotoxicity, and ischemia/hypoxia. Targeting the molecular pathways involved may be an efficient strategy to enhance stem cell viability and maximize transplantation success. Notably, a more complex network of cell death receives more attention than one crucial pathway in determining stem cell fate, highlighting the challenges in exploring mechanisms and therapeutic targets. In this review, we focus on programmed cell death in transplanted stem cells. We also discuss some promising strategies and challenges in promoting survival for further study.
Keywords: Programmed cell death, Apoptosis, Autophagy, Stem cell, Therapeutic strategies
Core Tip: The point of interest of this work is the complex mechanisms of the programmed cell death in stem cells (SCs), which suggests a series of targets as an efficient, reliable, and potential strategy to promote the SC-based therapy.
INTRODUCTION
Cell-based therapies have raised tremendous expectations and presented favorable curative effects in repairing damaged tissue and enhancing functional repair[1-3]. Stem cells (SCs) could serve as a cellular reservoir to maintain, produce, repair, and even regenerate multiple tissues with the characteristic properties of self-renewal and differentiation. Thus, SCs are developed as the preferred sources for cell-based therapies due to their ability to differentiate into a wide range of cell types and their capacity of secretion regulated by the microenvironment, also termed the “niche”[4]. Based on the stage of development, SCs can be divided into three types: Embryonic SCs (ESCs), induced pluripotent SCs (IPSCs), and adult SCs (ASCs)[5]. ESCs are derived from the inner cell mass of a blastocyst[6]. There are ethical limitations to the use of ESCs in therapy[7]. Compared with ESCs, IPSCs derived from mature body cells could be regulated to dedifferentiate into pluripotent SCs as a renewable source of alternative cells and tissues[8]. ASCs or somatic SCs (SSCs) can be found in various adult tissues, including neural SCs (NSCs), hematopoietic SCs (HSCs), mesenchymal SCs (MSCs), and epidermal SCs. Many trials have shown that ASCs can be used to treat diseases[9,10]. For example, bone marrow mononuclear cells[11], NSCs[12], and MSCs[13] are usually used to treat stroke.
SCs-based therapies are widely used in the treatment of various diseases[14-18]. Limbal stem cell therapy is used for treating burn-related corneal destruction[19], NSCs in gastrointestinal tract disorders[20], bone marrow-derived mesenchymal SCs (BM-MSCs) in diabetic cardiomyopathy[21], and MSCs in multiple sclerosis[22] and several clinical conditions. However, SC-based therapies also have limitations. Impaired cell homing regulated via various factors (such as chemokines) causes in situ tissue regeneration failure[23]. Also, a high death rate of transplanted SCs limits the therapies[24,25]. After MSC injection, over 99% of injected cells die in the left ventricular myocardium within 4 d[26].
Accumulated evidence shows a close tie between multiple types of programmed cell death (PCD) and SCs, including apoptosis, autophagy, ferroptosis, pyroptosis, and necroptosis. Studies demonstrate that p53 induces apoptosis of human ESCs (hESCs) through a mitochondrial pathway shown to be extremely sensitive to FasL-induced cell death in MSCs[27,28]. Ohgushi et al[29] observed that Rho-associated coiled-coil-containing protein kinase (ROCK)-dependent hyperactivation of myosin directly caused dissociation-induced apoptosis in hESCs and immediate activation of the Rho/ROCK/MLC2 signaling cascade. In 2010, the María group found that inhibitors of apoptosis proteins (IAPs) could promote the numbers of hematopoietic stem and progenitor cells and improve resistance to cell death[30]. Moreover, reports suggest that high levels of pro-apoptotic B-cell lymphoma 2 (Bcl-2) family members were overexpressed in hESCs[31]. Autophagy in SCs traces its history to 1980 where marrow cells revealed several abnormalities within an intrinsic myeloid precursor cell defect[32]. Lately, the role of autophagy in SC fate and aging is drawing attention due to the ability of the autophagy activator rapamycin to restore the biological properties of aged SCs by increasing their differentiation and proliferation capacity and decreasing adipogenic differentiation capacity, including the molecular mechanisms targeting 5′ AMP-activated protein kinase (AMPK) and rapamycin (mTOR)[33,34]. Research on necroptosis in SCs started relatively late but progressed rapidly to show that tumor necrosis factor α (TNF-α) could act on HSCs and progenitors for facilitating hematopoietic clearance and promoting regeneration. Furthermore, pharmaceutical inhibition of receptor-interacting protein kinase-3 (RIP3) showed a curative effect in promoting SCs, such as targeting necroptosis of intestinal SCs[35]. Some other cell death-related molecules have been increasingly recognized in SCs, such as the PI3K/AKT signaling pathway[36], MAP kinases ERK[37], JNK, and p38[38].
Some methods have been used to control programmed cell death in SCs. The concept of preconditioning was proposed by Charles E. Murry in 1986[39]. Presently, several strategies, such as using heat shock, free radical scavengers, over-expressing anti-apoptotic proteins, anti-inflammatory therapy, and co-delivery of extracellular matrix molecules, have been introduced[40-45]. Besides genetic strategies, three-dimensional culture technology and co-transplantation are novel ideas to enhance SC-based therapies.
Exploring cell death mechanisms in SCs and targeting these potential therapeutic molecules are vital to successful SC-based therapies (shown in Table 1[19-21,46-92]). In this review, we highlight the conditions or reasons leading to cell death in SC-based therapeutic approaches. Also, we demonstrate the cell death mechanism in SCs, which may provide a novel, efficient, reliable, and potential strategy in promoting SC-based therapy.
Table 1.
Summary of programmed cell deaths in stem cell-based therapy
|
Disease
|
SCs
|
Therapy models
|
Therapeutic effects
|
PCDs in SCs
|
Ref.
|
| Myocardial infarction | MSCs | Canine; porcine; mice; human | Inducing cardiac regeneration; increasing angiogenesis; repair by differentiating into cardiomyocytes | Apoptosis, autophagy, pyroptosis | [46-48] |
| iPSCs | Porcine; murine; rats; mice; non-human primates | Showing heart regeneration potential; regenerating the injured tissues; promoting a cardiomyogenic and angiogenic response | Apoptosis, autophagy, ferroptosis | [48,49] | |
| ESCs | Non-human primates | Showing heart regeneration potential; increasing angiogenic differentiation | Apoptosis, autophagy, pyroptosis | [48,50] | |
| Intracerebral hemorrhage | MSCs | Rats; primates; human | Repairing via differentiating into neurons or neuron-like cells; promoting axonal regeneration, neurogenesis, and angiogenesis | Apoptosis, autophagy, pyroptosis | [51-54] |
| NSCs | Mice, rats | Differentiating into neurons or glial cells; promoting neurogenesis and angiogenesis; promoting regeneration | Apoptosis, autophagy | [51,55-57] | |
| ESCs | Rats | Differentiating into neurons or glial cells; promoting neurogenesis and angiogenesis | Apoptosis, autophagy, pyroptosis | [51,58,59] | |
| iPSCs | Rats | Differentiating into neuroepithelium-like/neuroepithelioid SCs and neural cells; promoting neurogenesis and angiogenesis | Apoptosis, autophagy, ferroptosis | [51,60-62] | |
| Corneal reconstruction | LSCs | Human | Regenerating the corneal epithelium; differentiating into cells of the corneal epithelium | Apoptosis. | [19] |
| MSCs | Mice; rats; rabbits; human | Regenerating the corneal epithelium and corneal stroma; angiogenesis | Apoptosis, autophagy, pyroptosis | [63] | |
| Neurodegenerative disorders of the gastrointestinal tract | ESCs | Mice | Differentiating into enteric neuronal and glial cells | Apoptosis, autophagy, pyroptosis | [20,64] |
| iPSCs | Rats, mice | Differentiating into neural and glial cells | Apoptosis, autophagy, ferroptosis | [20,65] | |
| CNS-NSCs | Mice | Differentiating into neurons; regenerating and repairing ENS | Apoptosis, autophagy | [20,66,67] | |
| ENSCs | Mice; rats | Stimulating a local regenerative response; regenerating and repairing ENS; differentiating into new neurons | Apoptosis, autophagy | [20,68,69] | |
| Diabetic cardiomyopathy | MSCs | Mice; rats | Promoting angiogenesis; regenerating tissues; differentiating into cardiomyocytes and vasculature cells | Apoptosis, autophagy, pyroptosis | [21,70] |
| EPCs | Rats | Differentiating into endothelial cells to form new blood vessels and promoting neovascularization | Apoptosis | [70,71] | |
| CSCs/CPCs | Rats | Differentiating into newborn cardiomyocyte; promoting heart regeneration | Apoptosis | [70,72] | |
| iPSCs | Rats; mice | Attenuating oxidative stress and fibrosis; diminishing pro-oxidant expression and enhancing antioxidant (catalase and MnSOD) concentration; promoting heart regeneration | Apoptosis, autophagy, ferroptosis | [70,73] | |
| Diabetic retinopathy | ASCs | Rats; mice | Promoting angiogenesis; improving ischemia; offering protection against nerve damage; differentiating into photoreceptor and glial-like cells in the retina | Apoptosis | [74-77] |
| HSCs | Murine; rats | Promoting angiogenesis | Apoptosis, autophagy | [74,78] | |
| BM-MSCs | Murine; rats; mice | Differentiating into retinal glial cells; stimulating angiogenesis; promoting resident neural progenitors to regenerate neuro-retinal tissue | Apoptosis, autophagy, pyroptosis | [74,79,80] | |
| iPSCs | Rats; mice | Differentiating into cells expressing features of retinal pigment epithelial cells, retinal progenitor cells, and retinal ganglion cells, and slowing down retinal degeneration | Anti-apoptosis, autophagy, ferroptosis | [75,81] | |
| Neurological disorders | NSCs | Mice, rats, monkeys, pigs, human | Differentiating into neurons and supporting glial cells; releasing angiogenic factors to promote local tissue regeneration | Apoptosis, autophagy | [82-85] |
| HSCs | Human | Promoting cell survival; stimulating proliferation and migration of NSCs; inducing regeneration of damaged brain cells; promoting angiogenesis | Apoptosis, autophagy | [82,86] | |
| MSCs | Human | Promoting neuronal regeneration; promoting angiogenesis | Apoptosis, autophagy, pyroptosis | [82,86] | |
| Diabetes | ESCs | Mice, rats | Differentiating into cluster of insulin producing beta cells | Apoptosis, autophagy, pyroptosis | [87-89] |
| Hepatic and intestinal stem cells | Mice | Differentiating into beta cells in response to high glucose concentration | Apoptosis | [87,90] | |
| Spleen stem cells | Mice | Differentiating into insulin secreting beta cells; regenerating islet | Apoptosis | [87,91] | |
| HSCs | Mice | Differentiating into beta cells and vascular endothelial cells of the pancreas; inducing beta cell regeneration from the host cells residing in pancreas | Apoptosis, autophagy | [87,92] |
SC: Stem cell; MSCs: Mesenchymal stem cells; NSCs: Neural stem cells; ESCs: Embryonic stem cells; iPSCs: Induced pluripotent stem cells; LSCs: Limbal stem cells; CNS-NSCs: CNS-derived NSCs; ENSCs: Enteric neural stem cells; CSCs/CPC: Cardiac stem/progenitor cells; ASCs: Adipose stem cells; HSCs: Hematopoietic stem cells; BM-MSCs: Bone marrow derived mesenchymal stem cells; ENS: Enteric nervous system; EPCs: Endothelial progenitor cells; PCD: Programmed cell death.
A QUICK LOOK AT PCD
According to the death inducers, cell morphologic changes, and molecular mechanisms, cell death can be divided into two types: Non-programmed cell death caused by an external injury leading to instantaneous and irreversible cell damage[93,94], and PCD (e.g., apoptosis, autophagy, necroptosis, and pyroptosis), a common occurrence in the development of organisms without strong immune responses[95].
PCD occurs extensively during the development of pathology in various tissues. It is closely related to the therapeutic efficacy and prognosis of SC-based treatment. Robey et al[25] indicated that most cell death occurs in the first week post-transplantation. In NSC transplantation for neurological disorders in the brain, less than 4%-10% of primary NSCs survived within the first few days[96]. Similarly, Yasuda and Hayashi’s groups showed that 15% of transplanted cells survived at 1 wk and 9% at 4 wk in a rat infarction model[97]. A significantly high death rate occurred, and over 99% of MSCs died within 4 d after transplantation into the left ventricular myocardium of mice[26]. Thus, cell death may be a significant concern that needs attention.
Apoptosis
Apoptosis is the classic form of PCD without spillage of contents into the surrounding environment[98]. Apoptosis plays an important role in the orderly and efficient removal of damaged SCs to prevent cancer through two classical apoptotic pathways: The intrinsic pathway and the extrinsic pathway[99]. The intrinsic pathway, also called the mitochondrial pathway, shows a close relation with SCs[100,101]. It is closely regulated by a group of cytokines, especially the Bcl-2 family[102,103]. The extrinsic pathway is triggered by ligand-receptor binding. TNF-family receptors and cysteine-aspartic proteases, known as caspases, play a vital role in the extrinsic pathway[104].
Autophagy
Autophagy is a eukaryotic cell recycling process involving the degradation of cytoplasmic organelles, proteins, and macromolecules with the recycling of decomposition products via the mTOR/Ras-cAMP-PKA axis to maintain cellular homeostasis and enhance stem cell survival[105]. Autophagy is divided into three major types: Microautophagy, macroautophagy, and chaperone-mediated autophagy (CMA)[106]. During microautophagy, cargos are captured by lysosomal membrane invaginations or protrusions[107]. In macroautophagy, autophagosomes are regarded as typical signatures[108]. CMA focuses on molecular chaperones to identify cargo proteins containing specific pentapeptide sequences without using membrane structures to isolate cargo[109].
Necroptosis
Necroptosis is a pro-inflammatory lytic form of PCD. Necroptosis could be induced through several innate immune signaling pathways triggered by stimulating RIG-I-like receptors, TLRs, and death receptors[110,111]. Receptor-interacting serine-threonine kinases 1 and 3 (RIPK1 and 3) are phosphorylated and activated through these signaling pathways[112]. Subsequently, mixed lineage kinase domain-like (MLKL) could be activated[113].
Others
Pyroptosis, dependent on multiple molecules, such as caspase-1 and caspase-11, is widely believed to play an important role in resisting the invasion of pathogens[114]. Ferroptosis, an iron-dependent form of regulated cell death (RCD), is induced through an excessive accumulation (e.g., ROS and lipid peroxidation products) characterized by mitochondria shrinkage or dysmorphic small mitochondria[115,116]. Moreover, other types of cell death are also crucial during a series of events, such as failures in SC-based therapies. The biological correlations between the different PCD pathways are complex, where it is especially significant as a network among these pathways regarding PCD of transplanted SCs[117,118].
PCD AND ITS KEY MOLECULES IN STEM CELLS FOR TRANSPLANTATION THERAPY
PCD of SCs is usually caused by a hostile pathological environment created due to multiple conditions, including apoptotic cascade activation, excessive autophagy, inflammatory response, ROS, excitotoxicity, and ischemia/hypoxia[39]. This section systematically reviews the molecular mechanisms involved in cell death pathways and we also summarize these key molecules in Table 2[35,38,119-134].
Table 2.
Molecular mechanisms and therapeutic targets of programmed cell deaths in stem cells
|
PCDs
|
SCs
|
Molecular pathways of PCDs
|
Therapeutic target(s)
|
Therapeutic effects
|
Ref.
|
| Apoptosis | hESCs | Mitochondrial priming and p53 signaling pathway | Bcl-2 | Preventing damaged cells from compromising the genomic integrity of the population | [119] |
| HSCs | ASPP1 stimulated p53 signaling pathway | ASPP1, RUNX1 | Preventing hematological malignancies | [120] | |
| ISCs | ARTS/XIAP/caspase 9 axis | XIAP | Controlling ISC numbers and preventing the propagation of abnormal progeny | [121] | |
| MSCs | p38 MAPK regulated early apoptosis while JNK regulated late apoptosis | p38 | Protecting MSCs from oxidative stress damage | [38] | |
| NSCs | p38 MAPK signaling | TNF-α, p38 | Impairing cell viability, decreasing therapeutic effects | [122] | |
| Autophagy | iPSCs | AMPK/mTOR/ULK1 complex/PI3K complex/conjugation cascade complexes with LC3 and Atg9 during macroautophagy;KFERQ domain/Hsc 70/LAMP2A during CMA | LC3 | Removing unnecessary or dysfunctional components | [123] |
| HSCs | type III PI3K mammalian Atg6/PIP3/(Atg12-Atg5-Atg16) or (Atg4/LC3-I/Atg7/Atg3/LC3-II/PE) axis | LC3-II | Recycling cytoplasmic constituents and restoring metabolic homeostasis, and maintaining cells survival under harsh conditions | [124] | |
| NSCs | PI3K-AKT-mTOR/ULK1/the class III PI3-kinase-Beclin1 complex/PI3/PI3P/ the complex of Atg12–Atg5–Atg16L1/LC3-I/LC3-II axis | mTOR | Being involved in modulation of the embryonic neurogenesis as well as the injury repair of adult brain | [125] | |
| MSCs | PI3K/AKT/mTOR/ULK1/the class III PI3-kinase-Beclin1 complex/PI3/PI3P/the complex of Atg12–Atg5–Atg16L1/LC3-I/LC3-II axis | AKT, mTOR | Eliminating damaged organelles and biomacromolecules to maintain cellular homeostasis | [126,127] | |
| ESCs | AMPK/ mTORC1/ULK1 axis | Atg5, Atg12 | Maintaining the undifferentiated state of ESCs in vitro | [128] | |
| Necroptosis | ISCs | ZBP1/RIP3/MLKL axis | ZBP1 | Disrupting homeostasis of the epithelial barrier and promoting bowel inflammation | [35,129] |
| SSCs | RIP1 signaling pathway | RIP1 | Using Nec-1 to target RIP1 for reducing both necroptosis and apoptosis, which benefits for recovery rate and proliferation potential | [130] | |
| NPSCs | RIPK1/RIPK3/MLKL axis | HSP90 | Protecting SCs from PCD via alleviating mitochondrial dysfunction (mitochondrial membrane potential loss and ATP depletion) and oxidative stress (production of mitochondrial ROS), cellular total ROS and MDA, and downregulation of superoxide dismutase | [131] | |
| Pyroptosis | MSCs | Exosome/circHIPK3/ FOXO3a axis | circHIPK3 | Preventing pyroptosis and repairing ischemic muscle injury through a novel exosome | [132] |
| ESCs | Caspase-1 signaling pathway | N/A | Embryonic stem cell-derived exosomes inhibit doxorubicin-induced pyroptosis | [133] | |
| Ferroptosis | NPCs and IPSCs | Ferritin/ROS/lipid peroxidation axis | NCOA4, p53 | Decreasing stem cells and triggering neuronal death | [134] |
ISCs: Intestinal stem cells; iPCs: Induced pluripotent stem cells; HSCs: Hematopoietic stem cells; ESCs: Embryonic stem cells; NSCs: Neural stem cells; MSCs: Mesenchymal stem cells; EPCs: Endothelial progenitor cells; CPCs: Cardiac progenitor cells; IPSC: Pluripotent stem cells; ZBP1: Z-DNA-binding protein 1; RIP3: Receptor-interacting serine/threonine kinase 3; MLKL: Mixed lineage kinase domain like protein; PUMA: p53 upregulated modulator of apoptosis; NOXA: Known as PMAIP1, phorbol-12-myristate-13-acetate-induced protein 1; Bax: Bcl-2 associated X protein; Bak: Bcl-2 antagonist/killer 1 protein; cyt c: Cytochrome C; Apaf-1: Apoptosis protease activating factor-1; casp: Caspase; FADD: Fas-associated death domain; Bcl-2: B-cell lymphoma 2; AMPK: AMP-activated protein kinase; mTOR: Mammalian target of rapamycin; ULK1: Unc-51-like kinase complex; ROS: Reactive oxygen species; MDA: Malondialdehyde; GPX4: Glutathione peroxidase 4; circHIPK3: One of the most abundant circRNA in muscle; FOXO3a: A transcription factor of the O subclass of the forkhead family; LncRNA: Long non-coding RNAs; KLF3-AS1: Localize at chromosome 4p14 according to the exocarta database; mTOR: Mammalian target of rapamycin; ULK1: Atg1/unc-51-like kinase; LC3: Light chain 3; PI3K: Beclin-1/class III phosphatidylinositol 3-kinase; CMA: Chaperone-mediated autophagy; Hsc 70: Heat shock cognate71 kDa protein; LAMP2A: Lysosomal-associated membrane protein type 2; Atg: Autophagyassociated gene; Atg6: Vps34/Beclin-1; PIP3: Phosphatidylinositol (3,4,5) P3; PE: Phosphatidyl ethanolamine; SSCs: Spermatogonial stem cells; Nec-1: Necrostatin-1, a necroptosis inhibitor; NPSCs: Nucleus pulposus-derived stem/progenitor cells; HSP90: Heat shock protein 90; ROS: Reactive oxygen species; PCD: Programmed cell death.
Apoptosis
Recently, an emerging body of evidence has highlighted a vital role of the apoptosis effect on several cell types, including SCs[135]. Hence, it is crucial to investigate and understand the mechanisms underlying apoptosis for analysis of SC transplantation and the development of drugs targeting specific apoptotic molecules. According to the inducing signaling, apoptosis could be divided into two types: Intrinsic pathway initiated by intracellular stresses (shown in Figure 1), and extrinsic pathway responding to extracellular cues (shown in Figure 2).
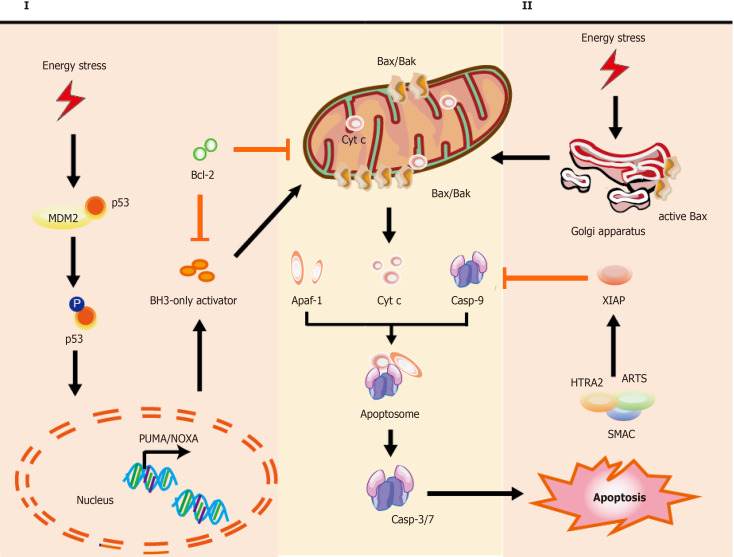
Figure 1.
Mechanisms of intrinsic apoptotic pathways in stem cells. Cell stress from various damage causes a rapid response leading to apoptosis via BH3-only activator (Way I) or active Bax directly from the Golgi (Way II) to the mitochondria, which subsequently induces a co-pathway [MOMP, cytochrome C (cyt C) releasing, etc.]. I: Stress inducers, such as DNA damage could stabilize and activate p53, which leads to p53 nuclear translocation. Subsequently, p53 exerts an impact on transcription of apoptotic genes via DNA-binding activity and its transcriptional activity (e.g., PUMA, NOXA, and Bax); II: Bax, which is monomeric in the cytoplasm, could be activated via stabilized p53 and active-Bax translocates from the Golgi to the mitochondrion. Once instigated with the apoptotic signals, active-Bax could lead to the alteration of MOMP, which undergoes dimerization and transfers to the OMM, so that relevant proteins (such as cyt C) are released into the cytosol usually confined in the intermembrane space. The released cyt C is involved in apoptosome formation via binding to the cytosolic Apaf-1. This complex recruits and activates initiator pro-casp-9, and then act-casp-9 activates downstream executor casp-3/-6/-7, leading to apoptotic cell death. In the cytoplasm, IAP antagonists (e.g., SMAC, ARTS, and HTRA2) could bind and suppress XIAP, causing the activation of casp-9 for the apoptotic pathway. The T-shaped lines indicate inhibitory interactions involved in this pathway, while the solid arrows indicate activating interactions. Bax: Apoptosis regulator Bcl-2 associated X protein; OMM: Outer membrane permeabilization; MOMP: Mitochondrial outer membrane permeabilization, cyt C: Cytochrome C; PUMA: p53 upregulated modulator of apoptosis, NOXA: Pro-apoptotic BH3-only protein, also known as PMAIP1, phorbol-12-myristate-13-acetate-induced protein 1; Apaf-1: Apoptosis protease activating factor-1; IAP: Inhibitor of apoptosis; SMAC: Second mitochondria-derived activator of caspase; ARTS: Apoptosis-related protein in the transforming growth factor-β signaling pathway; HTRA2: High-temperature-required protein A2.
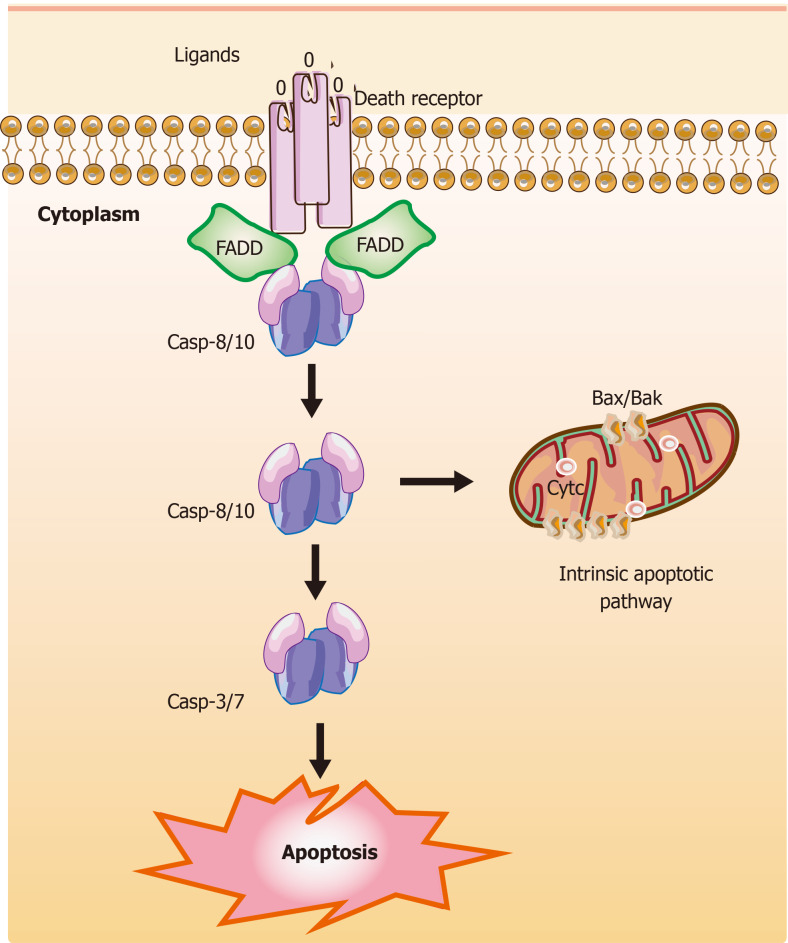
Figure 2.
Mechanisms of extrinsic apoptotic pathways in stem cells. The extrinsic apoptotic pathway (also known as the death receptor-dependent pathway) is induced by the connection between death receptors exposed on the cell surface [tumor necrosis factor (TNF) receptor] and the specific TNF family ligands. Subsequently, this signaling causes a conformational change leading to the recruitment of Fas-associated death domain (FADD) and allows interactions between FADD and casp-8 and/or the casp-10, resulting in the cleavage and activation of casp-3 and casp-7 through their death domain. Finally, the active and cleaved casp-3 induces changes in phosphatidylserine exposure, DNA fragmentation, and the formation of apoptotic bodies. Also, casp-8 can target the BH3-only protein Bid and cleave Bid to a truncated fragment t-Bid, which could connect to the extrinsic apoptotic pathways. The T-shaped lines indicate inhibitory interactions involved in this pathway, while the solid arrows indicate activating interactions. FADD: Fas-associated death domain.
The intrinsic pathway of apoptosis: In the intrinsic pathway, the initiators (e.g., ROS and radiation induced DNA damage) cause various cascade reactions resulting in the release of cytochrome C (cyt C), p53, and mitochondrial outer membrane permeabilization (MOMP). For example, hematopoietic stem and progenitor cells (HSPCs) are used for treating acquired and primary immunodeficiencies, thalassemia, and sickle cell disease. However, the presence of intrinsic apoptosis is shown in HSPC-based therapy in which excess DNA damage can trigger cumulative p53 pathway, constraining proliferation, yield, and engraftment of HSPCs, while moderate damage can lead to reversible function impairment by transient p53 inhibition[136]. According to the downstream activators of p53, two main pathways could be described: BH3-only activator (Way I shown in the left part of Figure 1) and active BAX from the Golgi (Way II shown in the right part of Figure 1) to the mitochondria.
Part I during the intrinsic pathway: During the intrinsic pathways, DNA damage, as a significant inducer, can stabilize and activate p53 by phosphorylation (for example, the phosphorylation of p53 at Ser46 can induce the p53-dependent apoptotic pathway caused by DNA damage[137]), leading to p53 nuclear translocation[119]. Subsequently, p53 exerts an impact on transcription of apoptotic proteins (namely, the related proteins) via DNA-binding activity and its transcriptional activity, such as the pro-apoptotic proteins p53 upregulated modulator of apoptosis (PUMA), NOXA (the pro-apoptotic BH3-only proteins, also known as PMAIP1 [phorbol-12-myristate-13-acetate-induced protein 1]), and apoptosis regulator Bcl-2 associated X protein (Bax)[138,139].
PUMA and NOXA can bind and activate Bax and Bcl-2 antagonist/killer-1 protein (Bak) in the cytoplasm, resulting in MOMP and release of cyt C[140]. Further, p53 can directly interact with Bax and Bak to modulate MOMP[141,142]. Of note, in the absence of cellular stress, p53 could rapidly produce and degrade in human pluripotent SCs (hPSCs), and the stabilization of p53 occurred upon DNA damage or via inhibition of MDM2 (the E3 ubiquitin ligase mouse double minute 2 homolog, which maintains low p53 levels through triggering p53 degradation)[143,144]. Interestingly, the activation of p53 is also involved in other types of cell death, such as ferroptosis[134].
Part II during the intrinsic pathway: Typically, Bax is monomeric in the cytoplasm. Studies show that active Bax localized to the Golgi held away from the mitochondrion in some hPSC lines, whereas active BAX could transform the mitochondria after cell stress as DNA damage via a rapid p53-dependent pathway during apoptosis[145]. Once instigated with the apoptotic signals, Bax could undergo dimerization and transfer to the outer membrane of mitochondria, leading to the alteration of MOMP[146], so that relevant proteins (such as cyt C) were released into the cytosol usually confined in the intermembrane space[147]. The released cyt C is involved in apoptosome formation via binding to the cytosolic apoptosis protease activating factor-1 (Apaf-1)[148]. This complex recruits and activates initiator pro-caspase-9, and then act-caspase-9 activates downstream executor caspases-3/-6/-7, leading to apoptotic cell death[148,149]. In the cytoplasm, the inhibitor of apoptosis (IAP) antagonists could bind and suppress XIAP (X-linked inhibitor of apoptosis, E3 ubiquitin-protein ligase), causing the activation of caspase-9 for the apoptotic pathway[121]. These IAP antagonists include second mitochondria-derived activator of caspase (SMAC), apoptosis-related protein in the transforming growth factor-β signaling pathway (ARTS), and mitochondrial serine protease high-temperature-required protein A2 (HTRA2)[121,148]. Koren et al[121] found highly expressed ARTS in cells comprising the intestinal SC niche, which protects Paneth cells from undergoing apoptosis.
The extrinsic pathway of apoptosis: The extrinsic apoptotic pathway is also known as the death receptor-dependent pathway induced via the connection between death receptors exposed on the cell surface (one of the numbers in the tumor necrosis factor receptor (TNFR) family) and the specific TNF family ligands mentioned above[150]. Previous research reported the effect of TNFα on the development of human hematopoietic progenitors in vitro within the role of inhibition[151] or promotion[152]. These TNFα-driven mechanisms play a vital role in HSC response to inflammatory stress for removing damaged cells and activating SCs[153]. Recently, HSC transplantation for malignancy has shown anti-tumor activity via TNFα-driven pathways[153,154]. Death receptors and their ligands cause a conformational change, which leads to the recruitment of Fas-associated death domain (FADD)[155] and allows interactions between FADD and caspase-8 and/or the caspase-10, resulting in the cleavage and activation of caspase-3 and caspase-7 through interactions between their death domain (DD)[156]. Finally, the active and cleaved caspase-3 induces changes in phosphatidylserine exposure, DNA fragmentation, and the formation of apoptotic bodies. However, reports suggest that caspase-3 activity could be elevated in nonapoptotic pathways in neural SCs[157].
Remarkably, caspase-8 can target the BH3-only protein Bid (BH3-interacting domain death agonist) and cleave Bid to a truncated fragment t-Bid[158]. Capper et al[159] and Jia et al[160] showed that decreased Bid could inhibit apoptosis, promote proliferation, and delay senescence in human periodontal ligament SCs (h-PDLSCs) via activated Yes-associated protein, and low levels of caspase-8 were detected in stem cell features through hypermethylation. Subsequently, t-Bid could directly translocate to the outer mitochondrial membrane after activating apoptotic regulator Bax and inhibiting Bcl-2, leading to co-engages between the intrinsic apoptotic pathway and the extrinsic apoptotic pathway[158]. Some evidence shows that activation of the extrinsic pathway and inhibition of caspase-8 can induce necroptosis[161,162].
Emerging findings indicate that Bcl-2 family proteins play a vital role in SCs (e.g., overexpression of Bcl-2 in MSCs[163], ESCs[164], and neuroepithelial SCs (NESCs)[165] improved their survival). The three functional groups Bak and Bax, BH3-only proteins, and Bcl-2 maintain a balance between SC survival and death. For example, high levels of Bcl-2 were measured in HFSCs for antiapoptosis in contrast to differentiated cells[166,167]. In the SCs, Bax performs as an activated conformation sequestered in the Golgi apparatus held away from the mitochondrion. Following stresses such as DNA damage, active Bax translocates to the mitochondrial outer membrane to initiate MOMP and the apoptotic cascade, which bypasses the conventional intrinsic and extrinsic apoptotic pathways[168,169]. However, the mechanism underlying the localization of active Bax at the Golgi and active Bax-induced pore formation in the Golgi stacks is unclear.
Autophagy
As a self-protective catabolic mechanism within the cells, autophagy exerts a key influence in sustaining SC homeostasis by maintaining stemness, upregulating quiescence, managing differentiation via remodeling, and self-renewal via metabolic reprogramming[170-173]. Autophagy contributes to metabolic regulation through increased glycolysis to generate ATP in the hypoxic milieu for balancing SC fate[174,175]. For example, autophagy plays a vital role in maintaining the quiescence of SCs (e.g., HSCs and muscle SCs (MuSCs)) via rejuvenating aged quiescent SCs controlled by various autophagy pathways such as the p38/mitogen-activated protein kinase (MAPK) signaling pathway[176,177]. Uncovering the autophagy mechanisms underlying SC quiescence presents novel therapeutic strategies to release the cells out of the quiescent state, promoting their proliferation and differentiation (such as induced activation of quiescent NSCs for neuron injury), or re-establishing quiescence to prevent aberrant proliferation and differentiation or premature senescence (such as anti-cancer therapeutics), which carry the risk of cancer SCs (CSCs)[178,179]. These stressors (e.g., starvation, oxidative stress, infection, and hypoxia) stimulate the cascade of autophagy as follows (shown in Figure 3)[180].
Figure 3.
Overview of the mechanisms during autophagy in stem cells. There are three types of autophagy [macroautophagy (section a), microautophagy (section b), and chaperone-mediated autophagy (section c)] based on different pathways; however, they produce the same results. Besides these proteins, key transcription factors closely related to autophagy are shown. The T-shaped lines indicate inhibitory interactions involved in this pathway, while the solid arrows indicate activating interactions. A: Typically, the mTORC1 complex functions as an inhibitor to control the initiation of autophagy. Under environmental stresses and physiological stressors, AMPK is activated to inhibit the activity of mTORC1, leading to a release of the ULK1 (Unc-51-like kinase complex, also known as ATG1) complex to induce autophagy. This initiation process is known as the phagophore assembly site (PAS) formation. Next, PI3 is phosphorylated to PI3P via the class III PI3-kinase-Beclin1 complex formed by core subunits of Beclin1 (Atg6), Atg14 L, and Vps34-Vps15, resulting in autophagosome formation. The Atg12-Atg5-Atg16L1 complex acts as a regulator for enveloping and translocating the cytoplasmic cargo to the lysosome in misfolded-protein degradation. Atg4 can cleave LC3 (Atg8) to generate cytosolic LC3-I. Atg3 (E2 enzymes) and Atg7 (E1-like enzymes) can lead the conjugation of PE to LC3-I to form lipidated LC3-II, which is combined with the autophagosome membrane to complete and elongate autophagosome formation. Finally, the autophagosome contents undergo degradation due to low lysosomal pH; B: In microautophagy, misfolded or/and toxic proteins can be directly engulfed by the lysosomal membrane and degraded in the lysosome; C: During chaperone-mediated autophagy, the heat shock cognate 70 kDa protein (HSC70) chaperones attach to the pentapeptide motif KFERQ (namely Lys-Phe-Glu-Arg-Gln) for delivery to lysosomes via a specific receptor LAMP2A. Also, some of the key transcription factors are closely linked to the stem cell state and the occurrence of autophagy (bottom). FOXO3A can enhance autophagosome formation via autophagy gene expression in hematopoietic stem cells and breast cancer stem-like cells, which is needed to mitigate an energy crisis and allow cell survival. Besides FOXO3A, other transcription factors such as SOX2, STAT3, OCT4, KLF4, and c-Myc are also vital for reprogramming in the initial creation of stem cells at the genetic level during autophagy.
During autophagy, the formation of multi-protein complexes is associated with morphologic changes (shown in Figure 3). Initiation of autophagy is controlled by nutrient sensors, namely, mTOR and AMPK[173,181]. Typically, the mTORC1 complex functions as an inhibitor for autophagy. Under environmental stresses and physiological stressors, AMPK is activated to inhibit the activity of mTORC1, leading to a release of the ULK1 (Unc-51-like kinase complex, also known as ATG1) complex to induce autophagy, which is usually inhibited by mTORC1[182]. This initiation process is known as the phagophore assembly site (PAS) formation, which is regarded as indispensable for nucleation in the next stage. Compared with somatic mouse embryonic fibroblasts, whole-cell extracts of iPSCs and ESCs express high levels of AMPK and phosphorylated AMPK[183]. Interestingly, AMPK inhibition in mouse bone marrow-derived MSCs can upregulate both autophagy and apoptosis in hypoxia and serum deprivation conditions, suggesting crosstalk between autophagy and apoptosis through AMPK-ULK1 pathways[184,185]. Mutations in mTOR lead to smaller brains in mouse cortical development, and fewer proliferating neural progenitors result from disruption of NSC self-renewal[181].
Next, PI3 is phosphorylated to PI3P via the class III PI3-kinase-Beclin1 complex formed by core subunits of Beclin1 (Atg6), Atg14 L, and Vps34-Vps15, resulting in autophagosome formation[186,187]. The Atg12-Atg5-Atg16L1 complex acts as a regulator for enveloping and translocating the cytoplasmic cargo to the lysosome within misfolded-protein degradation[188]. Atg4 can cleave LC3 (Atg8) to generate cytosolic LC3-I. Atg3 (E2 enzymes) and Atg7 (E1-like enzymes) can lead the conjugation of PE to LC3-I to form lipidated LC3-II, which is combined with the autophagosome membrane to complete and elongate autophagosome formation[189]. Finally, the autophagosome contents undergo degradation due to low lysosomal pH. Some evidence demonstrates that autophagy plays an important role in reprogramming to form iPSCs, while iPSCs colony formation shows reprogramming failure due to the lack of Atg3, Atg5, or Atg7[190,191]. Autophagy is necessary for SC survival and sustenance. It is critical for SC differentiation in which co-localized dots of Tuj1-positive and GFP-LC3-positive cells are monitored and progress increasingly during NSC differentiation[192].
In microautophagy, misfolded or/and toxic proteins can be directly engulfed by the lysosomal membrane and degraded in the lysosome[193]. During chaperone-mediated autophagy, the heat shock cognate 70 kDa protein (HSC70) chaperones attach to the pentapeptide motif KFERQ (namely Lys-Phe-Glu-Arg-Gln) for delivery to lysosomes via a specific receptor LAMP2A. Reports suggest that targeting peptide HSC70 during autophagy can dramatically decrease amyloid-β (Aβ) oligomers in iPSCs with superior neuroprotective activity[194]. However, the molecular mechanism between autophagy and SCs is still unclear and remains to be further explored.
Apart from these vital targets, key transcription factors are closely linked to the stem cell state and the occurrence of autophagy (shown in Figure 3). For example, FOXO3A can enhance autophagosome formation via autophagy gene expression in hematopoietic SCs and breast cancer stem-like cells, which is needed to mitigate an energy crisis and allow cell survival[182,195]. Moreover, an elevated level of SOX2 is detected in NSCs, which is important for self-renewal; downregulation of SOX2 is observed in differentiated neurons and glia[196]. Besides SOX2, other transcription factors such as STAT3, OCT4, KLF4, and c-Myc are also vital for reprogramming in the initial creation of iPSCs at the genetic level[197].
Necroptosis
The occurrence of necroptosis in SCs has recently been reported. Wang et al[35] found that gut stem cell necroptosis resulting from genome instability triggered bowel inflammation. Moreover, TNF-α could promote the survival and myeloid differentiation of HSC via activating a strong and specific p65-nuclear factor κB (NF-κB)-dependent gene program that prevents necroptosis rather than apoptosis to poise HSCs for myeloid cell production[153].
Others
In addition to apoptosis and autophagy (mentioned above), reports on other cell death types have led to studies exploring cell death mechanisms, such as ferroptosis and pyroptosis[35,132,198-203]. Notably, different cell death mechanisms can simultaneously occur in disease (termed as ‘PANoptosis’), suggesting a complex but practical integrated network between various cell death mechanisms in SCs[204,205].
Ferroptosis had been observed in SCs with an imbalance of iron homeostasis, a significant upregulation of cytosolic free iron content, and DNA/protein/lipid oxidative damage, leading to an obvious senescence phenotype and spontaneous death in iPSC-derived neuronal precursor cells (NPCs)[134,206]. iPSCs and gene-correction are used for treating Pelizaeus-Merzbacher disease (PMD) but subsequently undergo cell death after the pre-myelinating stage with evidence for caspase-3-dependent apoptosis in approximately 40% of cells and ferroptosis[205]. Thus, iron chelators and lipophilic antioxidants can lead to downregulation of apoptosis and ferroptosis[205]. Further, transfusional iron overload (IOL) may have clinical importance as a character close to transplant-related mortality in hematopoietic stem cell transplantation (SCT) for hematologic malignancies (HM)[198].
For pyroptosis (TLR4-NLRP3-mediated cell death pathway), a large body of evidence shows that stem cell transplantation can function as an inhibitor for pyroptosis, suggesting a novel approach called stem cell-derived exosome treatment[207,208], and numerous molecular pathways, such as exosome/LncRNA KLF3-AS1/miR-138-5p/Sirt1 axis and exosome/circHIPK3/FOXO3a axis, are presented[132,133,209].
All kinds of RCDs contribute to making a constant effort to maintain a homoeostatic balance, in which it is especially significant for the therapeutic effects of SC-based therapy. As for apoptosis in SCs, the intrinsic and extrinsic pathways play a synergistic role in ensuring the multi-cellular organisms to keep normal cells, and remove abnormally proliferating cells or other defective cells. Failure to regulate apoptosis would lead to the uncontrolled growth and division of cells during pathological process. In this regard, whether the SCs that we utilized in transplantation would be uncontrolled someday is also a potential challenge. Compared with apoptosis, autophagy could be regarded as a source of energy through digestion of cellular structures and/or organelles against multiple stresses such as nutrient deprivation (caloric restriction). These two main RCD pathways are widely studied and also some novel ways such as active-Bax in Golgi to inducing apoptosis will be further dug out. Remarkably, Bcl-2 as a co-regulator during these two pathways might be a potential target not only for apoptosis but also for autophagy. Others RCDs such as neroptosis, pyroptosis, and ferroptosis are also found in transplanted SCs, but their detail signaling and application need to keep digging. All in all, various cell death mechanisms are under investigation (apart from the cell death types described). Notably, it is necessary to focus on the overall network between different molecular cell death pathways.
STRATEGIES TO PROMOTE STEM CELL SURVIVAL FOR TRANSPLANTATION THERAPY
As mentioned above, the microenvironment exerts a vital role in the survival of SCs. Many studies have contributed to providing a wide range of strategies to enhance stem cell transplantation therapy via improving the microenvironment, including preconditioning strategy (e.g., exposure to oxidative stress, heat shock, and ischemic/hypoxic injury), pretreatment (e.g., drug treatment, cytokines, antioxidants, nitric oxide, glucose deprivation, growth factors, miRNAs, and exosomes), genetic modification, and co-transplantation of different cell types (shown in Figure 4 and Table 3)[210]-[228].
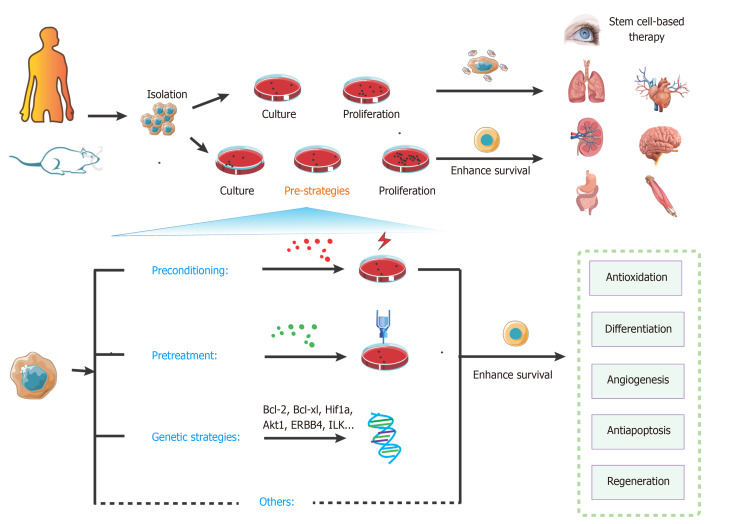
Figure 4.
Overview of key strategies to enhance stem cell transplantation therapy. The steps of stem cell-based transplantation therapy include drawing the materials, isolation, culture, proliferation, and transplantation. Compared with the classic approaches, pre-strategies could enhance survival of stem cells. These pre-strategies mainly include preconditioning, pretreatment, genetic strategies, and other methods. They can effectively activate various signaling pathways for protecting cells from injury and promoting survival.
Table 3.
Strategies to enhance stem cell transplantation therapy
|
Strategy
|
Method
|
Target
|
Effects
|
Molecular mechanisms
|
Ref.
|
| Preconditioning | Short repeated ischemia/reperfusion | ESCs | Enhancing the tolerance of subsequent prolonged lethal ischemia | Promoting the expression of trophic factors, inducing the release and activation of PKC, PKB, or Akt, NF-κB and Src protein tyrosine kinases, and subsequently upregulating COX-2, iNOS, HO-1, Mn superoxide dismutase, aldose reductase, and antiapoptotic genes | [210-212] |
| Hypoxia | MSCs | Promoting mesenchymal stem cell migration and survival | Increasing the expression of lncRNA-p21, HIF-1α, and CXCR4/7(both were chemokine SDF-1 receptors) | [213] | |
| CSCs | Promoting survival and cardiogenic differentiation | Inducing the activation of the HIF-1α/apelin/APJ axis | [214] | ||
| NSCs | Promoting survival and neuroprotective properties, and facilitating functional recovery in vivo | Upregulating HIF1-α and HIF target genes such as EPO and VEGF and neurotrophic, and growth factors | [215] | ||
| Hydrogen peroxide preconditioning | BMSCs | Improving the therapeutic potential for wound healing | Upregulating cyclin D1, SDF-1, and its receptors CXCR4/7 expression, and activating the PI3K/Akt/mTOR pathway, but inhibiting the expression of p16 and GSK-3β | [216] | |
| Nitric oxide donor preconditioning | hCSCs | Enhancing survival | Upregulating phosphorylation of NRF2, NFκB, STAT3, ERK, and AKT, as well as increasing the protein expression of HO-1 and COX2 | [217] | |
| Heat shocking | MSCs | Promoting migration | Triggering the activation of ERK and PI3K/Akt signaling pathways via HSP90 | [218] | |
| Pretreatment | Oxytocin | MSCs | Antiapoptosis and cell protection | Increasing the expression of Akt and phospho-ERK1/2 proteins, rapid calcium mobilization, and upregulation of antiapoptotic and angiogenic genes including HSP27/32/70, TIMP-1/2/3, VEGF, thrombospondin, and matrix metalloproteinase-2 | [219] |
| Minocycline | NSCs | Increasing the capacity of migration, proliferation, and differentiation to improve neurological recovery | Increasing the expression of Nrf2 | [220,221] | |
| Melatonin | MSCs | Inducing fewer fibrotic damage | Downregulating the levels of TNF-α, TGF-β, and α-SMA, and upregulating the expression of E-cadherin | [222] | |
| Extremely low-level lasers | MSCs | Enhancing the migration of MSCs; promoting the proliferation rate of SCs | Allowing the FAK and ERK1/2 pathways and increasing PDGF and HGF; inducing the up-regulation of mitochondrial ROS and NO | [223,224] | |
| Genetic strategies | Overexpressing pro-survival factors | hNSCs | Improving short- and long-term survival | Overexpression of Bcl-2, Bcl-xl, Hif1a, or/and Akt1 | [225] |
| Genetic modification | MSCs | Potentiating MSC survival | Overexpression of ERBB4 and ILK | [226] | |
| 3D technology | Hydrogels mimicking | MSCs, ESCs, EPCs | Role in stem cell differentiation, changing matrix stiffness, mechanical stress and strain, nonlinear elastic, microenvironments and viscoelastic microenvironments | N/A | [227] |
| Co-transplantation | Co-transplantation of MSCs and HSCs | MSCs HSCs | Enhancing therapeutic effects | N/A | [228] |
ESCs: Embryonic stem cells; NSCs: Neural stem cells; MSCs: Mesenchymal stem cells; HSCs: Hematopoietic stem cells; EPCs: Endothelial progenitor cells; hNSCs: Human neural stem cells; SCs: Stem cells; Hsp70/90: Heat shock protein 70/90; ERK: Extracellular regulated protein kinases; Nrf2: Nuclear factor erythroid 2; TNF: Tumor necrosis factor; TGF: Tumor growth factor; SMA: Smooth muscle actin; HGF: Hepatocyte growth factor; ROS: Reactive oxygen species; Bcl-2: B-cell lymphoma 2; ERBB4: Erb-b2 receptor tyrosine kinase 4; ILK: Integrin-linked kinase; SDF-1: Stromal-derived factor-1; EPO: Erythropoietin; VEGF: Vascular endothelial growth factor; TIMP: Tissue inhibitor of metalloproteinase; PDGF: Platelet-derived growth factor.
Preconditioning strategy
Preconditioning strategies mainly help to promote tolerance of SCs and progenitor cells derived from SCs. These triggers aim to alter cell signaling and metabolism for adaptation to appropriate and mild stress conditions and sublethal insults [e.g., ischemic preconditioning (IPC), hypoxia, anoxia, hydrogen sulfide (H2S), hydrogen dioxide (H2O2), and carbon monoxide (CO)].
In detail, IPC of SCs is considered an efficient method to promote cell survival. After a repeated short cycle of ischemic/reperfusion (I/R), some of the chemical signals (e.g., ROS, NO, and adenosine) can release and trigger cell protection via a cascade of survival factors such as the activation of protein kinase C (PKC), protective protein kinase B (PKB or Akt), nuclear factor κB (NF-κB), and Src protein tyrosine kinases, and subsequent upregulation of cyclooxygenase-2 (COX-2), inducible NO synthase (iNOS), heme oxygenase-1 [HO-1], Mn superoxide dismutase, aldose reductase, and anti-apoptotic genes (Bcl-xL, Mcl-1, c-FLIPS, and c-FLIPL)[210]. During ischemia/hypoxia or heat shock preconditioning, the level of Hsp70 and Hsp90 is upregulated. Reports suggest that Hsp70/90 can inhibit SMAC in the myocardium to prevent activation of caspase-3/9 (pathway described above)[211,212].
Similarly, hypoxia-inducible factor (HIF-1) is upregulated during hypoxia preconditioning to inhibit tumor suppressor p53, reduce oxidative phosphorylation, upregulate VEGF receptor levels, and promote the activation of Akt to target caspases and Bcl-2 for anti-apoptosis[229,230]. Recent findings reveal that OM-MSC (olfactory mucosa mesenchymal SC) with hypoxic preconditioning functions as an inhibitor for apoptosis and pyroptosis in microglial cells through activation of HIF-1α in vitro[231]. Hypoxia-preconditioned SCs can also upregulate paracrine activity, and their exosomes are also considered a novel transplantation therapy. For example, MSC-derived exosomes with hypoxia preconditioning show promising potential as an effective means for optimized bone fracture healing via exosomal miR-126 and the SPRED1/Ras/Erk signaling pathway[232].
Besides preconditioning with ischemia and hypoxia, oxidative stress and heat shocking are also the most common preconditions for SCs within a similar rationale. Chronic exposure to oxidative stress (e.g., H2O2, H2S, and CO) produces protective effects by activating mitochondrial ROS production, resulting in ERK activation and anti-apoptotic protein expression for cell proliferation, migration, anoikis, autophagy, and survival[216,233,234]. Moreover, heat shocking precondition of mesenchymal SCs can induce HSPs to activate ERK and PI3K/Akt signaling pathways, resulting in increased expression of trophic factors, proteins, and genes for cell protection[218].
Pretreatment strategy
Pretreatment is a strategy for successfully protecting transplantable SCs, using various factors before implantation, whereas preconditioning refers to providing a specific environment within sublethal insults. These factors include antioxidants, cytokines, growth factors, and drug therapy (phosphodiesterase inhibitors, glucose deprivation, pro-survival protein expression, and anti-apoptotic proteins).
To date, various drugs have been developed for the pretreatment of SCs. Pretreatment with pharmacological inhibitors can result in increased expression of survival signaling and a high Bcl-2/Bax ratio in the early phase (2 h), and activation of the JAK/STAT signaling pathway in the late phase (24 h) for cardioprotection[210]. Also, Ji group has reported the protective effect of histochrome pretreatment against oxidative stress in cardiac progenitor cells (CPCs) via upregulating Bcl-2 and Bcl-xL and downregulating Bax and H2O2-induced cleaved caspase-3[235]. Moreover, short-term incubation either with an antioxidant N-acetyl-L-cysteine (NAC) or a specific inhibitor of TNFR 1 signaling can prevent TNF-α-mediated ROS accumulation in HSCs[154]. MSC pretreatment with oxytocin (OT) [10(-10) to 10(-6) M] in response to signaling events can induce Akt and phospho-Ras-dependent extracellular signal-regulated kinase (ERK)1/2, rapid calcium mobilization, and upregulation of anti-apoptotic and angiogenic genes, including HSP27/32/70, tissue inhibitor of metalloproteinase (TIMP)-1/2/3, vascular endothelial growth factor, thrombospondin, and matrix metalloproteinase-2[219]. Minocycline preconditioning increases Nrf2 expression and neuroprotective paracrine secretion. It promotes migration, proliferation, and differentiation of NSCs to improve neurological recovery after NSC transplantation[220,221]. The molecular mechanism involves upregulation of antioxidant genes and reduced oxidative stress grafted cell death following transplantation, resulting in low-rate cell death[221]. Some studies have shown the benefits of melatonin pretreatment on MSC-based therapy with a reduction in the levels of TNF-α, TGF-β, and α-SMA, and upregulation of E-cadherin expression that induces less fibrotic damage[222].
Trophic factors and cytokines are also considered effective pretreatment approaches for regulating MSC fate. For example, SC pretreatment with IL-1β can promote migration and survival of MSCs and improve function in type 2 diabetes, acute myocardial infarction, and neural disorders via upregulating the expression of various cytokines, chemokines, and adhesion molecules [e.g., IL-6/8/23A, TNF-α, CCL5/20, CXCL1/3/5/6/10/11, VCA-1 (vascular cell adhesion molecule 1), and ICAM-1/4 (intercellular adhesion molecule 1 and 4)]. IL-1β can induce phosphorylation of NF-κB, but not PI3K/AKT and ERK1/2 pathways[236]. In the NSC pretreatment strategy, a series of experiments using IL-6 show that it can reprogram NSCs to tolerate hostile environments via activating STAT3 to increase the levels of superoxide dismutase 2 (SOD2) for anti-apoptosis against inflammatory cytokines and oxidative stress via mitochondrial-dependent apoptotic pathways[237,238]. Some other molecular targets, including Rho-associated kinase inhibition, TGF-β2 treatment, SDF-1 signaling of PI3K/Akt, and p38 MAPK inhibition via anti-apoptotic pathways, also enhanced SC survival during treatment[239].
Compared with chemical pretreatment methods discussed above, physical factors such as extremely low-level lasers, pulsed electromagnetic fields (PEMF), mechanical stretch, and nanochelating-based nanocomplexes (e.g., GFc7) are also used as pretreatment methods to enhance SC-based therapy[240-243]. For example, pretreatment with extremely low-level lasers improves the migration ability of MSCs via activation of FAK and ERK1/2 pathways and increased expression of platelet-derived growth factor (PDGF) and HGF. Furthermore, it also promotes the proliferation rate of SCs by inducing the upregulation of mitochondrial ROS and NO and enhancing the expression of the S-phase proportion in MSCs[223,224].
Genetic strategy
Genetic strategies have raised hopes for better SCs-based therapy since they were introduced more than a decade ago[244,245]. The core idea of this technology is to target key genes and the expression of factors related to the fate of SCs. Under different death stimuli, overexpression of various factors such as TNFR, Akt1, stromal cell-derived factor-1 (SDF-1), and hepatocyte growth factor (HGF) is beneficial for the repopulation of SCs[246]. Studies on modified transplanted hNSCs show improved short- and long-term survival of transplanted hNSCs via overexpression of these pro-survival factors, including Bcl-2, Bcl-xl, Hif1a, or/and Akt1[225]. Genetic modification for ERBB4 (erb-b2 receptor tyrosine kinase 4) and ILK overexpression could potentiate MSC survival[226]. In recent years, the CRISPR/Cas9 system has been widely used for genome editing applied in genetic modification of SCs for in vivo applications such as neural regeneration, bone regeneration, treatment of blood disorders, and cartilage tissue engineering[247]. Although gene modification promises to enhance tolerance to damage "at the root," there are still formidable predictability challenges and potential long-term side effects.
Others
Recently, three-dimensional culture technologies (e.g., MSC encapsulation technique) mimicking the physical environment to sustain the viability of SCs to induce multi-lineage differentiation are used to protect SCs from PCD as an innate immune system and provide favorable mediators such as cytokines and growth factors[227,248]. However, the time, cost, and labor efficiency of three-dimensional technologies for SCs may be non-negligible challenges, and a combination of biocompatible materials based on simple and easy methods is needed for SC-based therapy. Moreover, co-transplantation of different cell types offers an alternative strategy to improve outcomes of SC-based treatment. Studies show promising results with co-transplantation of human fetal mesenchymal and hematopoietic SCs in type 1 diabetes, epidermal neural crest SCs (EPI-NCSC), and olfactory ensheathing cells (OEC)[228,249]. However, the significance of co-transplantation for SC-based therapy is still unclear[250,251].
As described above, these pre-strategies could provide transplanted stem cell with a certain microenvironment to improve the survival. The core ideas of these methods are to upregulate the survival factors (e.g., Bcl-2, Akt, SMAC, mTOR, SOD2, STAT3, HSC 70, ERK, and Nrf2) and downregulate the death catalyzers (e.g., caspase, p53, TNFa, Bax, cyt C, XIAP, MAPK, and Atg) (shown in Figure 5). Bcl-2 might be regarded as a key molecule that raised tremendous expectations, which plays a vital role in both apoptotic and autophagy pathways. Given the fact that gene strategies seem to be hardly accepted in clinical trials to improve effectiveness of SC-based transplantation, preconditioning and pretreatment may provide a cost-effective and handy option. Remarkably, distinct types of transplanted cells or distinct aiming organs show noticeable differences not only in their signaling but also their response to the local area, so studies need to find a right composition as well as an effective target of any applied transplanted SC system.
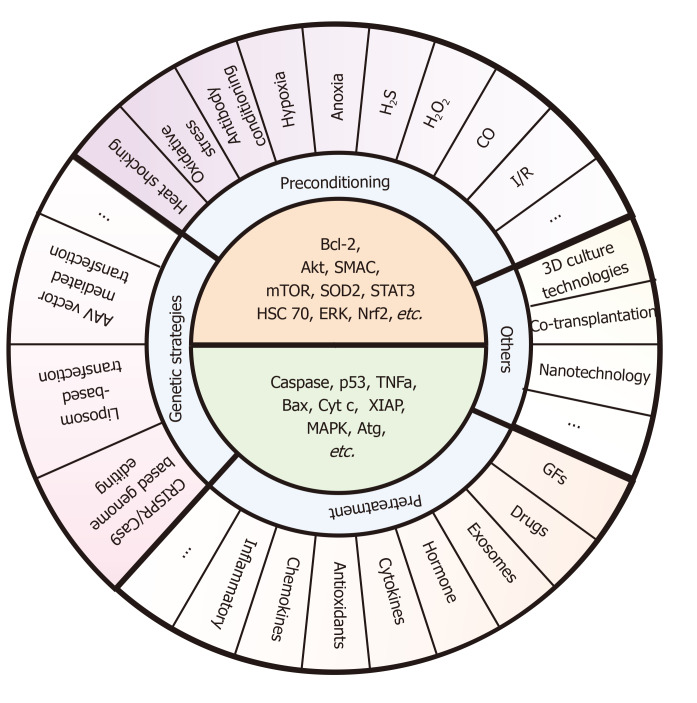
Figure 5.
Specific pre-strategies and their key molecule targets for enhancing stem cell transplantation therapy. These pre-strategies mainly include preconditioning (e.g., exposure to oxidative stress, heat shock, and ischemic/hypoxic injury), pretreatment (e.g., drug treatment, cytokines, antioxidants, nitric oxide, glucose deprivation, growth factors, miRNAs, and exosomes), genetic strategies (e.g., AAV vector mediated transfection, Liposome-based transfection, and CRISPR/Cas9-based genome editing), and other methods (e.g., 3D culture technologies, co-transplantation, and nanotechnology). The core ideas of these pre-strategies are to upregulate the survival factors (e.g., Bcl-2, Akt, SMAC, mTOR, SOD2, STAT3, HSC 70, ERK, and Nrf2) and downregulate the death catalyzers (e.g., caspase, p53, TNFa, Bax, Cyt c, XIAP, MAPK, and Atg). However, there are few methods targeting all of these molecules at the same time during the co-network. Also, studies pay more attention to certain signaling such as Bcl-2 and mTOR, and other signals such as Atg or XIAP still need further mining.
CONCLUSION
The SC pool plays a driving role in tissue homeostasis and harm repair. Lately, SC-based therapies may be regarded as a potential strategy that raised tremendous expectations and presented favorable curative effects in enhancing functional repair and repairing damaged tissue. Given the fact that a considerable number of studies on SC-based therapy verify that RCDs occur extensively during the development of the transplanted SCs, RCDs show a crucial role in the therapeutic efficacy and progression of this treatment. Also, RCD interventions may offer opportunities for a better clinical application.
Recently, there have been tremendous strides in understanding the fate of SCs post-transplantation related to self-condition and microenvironment. Along this line, targeting multiple signal transduction pathways in PCDs and survival processes would provide novel approaches for enhancing SC-based therapies. However, the interactions are complex and involve multiple networks rather than one crucial pathway (as the recent term ‘PANoptosis’), thus necessitating further research. Moreover, various factors involved in specific pathways may change during stem cell differentiation or show microenvironmental divergence in different cell types, stages of development, and stimuli.
Several approaches can prevent the loss of a vast majority of transplanted SCs, such as preconditioning, pretreatment, and genetic strategies. Important insights into the molecular pathways that control PCD of SCs may unlock novel and potential avenues for regenerative drugs and more efficient therapy. These pre-strategies provide SCs with harsh or nutrient-rich environment to improve the SCs via upregulating the survival factors and downregulating the death catalyzers. A summary diagram is shown in Figure 6. Recently, some of the novel technologies such as 3D culture technologies, co-transplantation, and nanotechnology also show promising prospects. Furthermore, safer use, better results, and highly feasible and beneficial methods are required for clinical applications.
Figure 6.
Role of regulated cell deaths in stem cell-based transplantation and therapeutic pre-strategies to improve the therapy. Stem cell-based therapy has been used in various diseases. A number of stimuli may induce regulated cell deaths (RCDs) in transplanted stem cells (SCs), which results in poorer outcomes. Different signals involved in distinct types of RCDs may provide some targets to improve SC-based transplantation. These therapeutic strategies include preconditioning, pretreatment, gene strategies, and so on. IPC: Ischemic preconditioning; PCD: Programmed cell death; MLKL: Mixed lineage kinase domain like protein; GSDME: Gasdermin E.
Footnotes
Conflict-of-interest statement: Xiong K has received research funding from the National Natural Science Foundation of China.
Manuscript source: Invited manuscript
Peer-review started: February 26, 2021
First decision: April 20, 2021
Article in press: May 7, 2021
Specialty type: Biochemistry and molecular biology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ariga K, Tanabe S S-Editor: Gong ZM L-Editor: Wang TQ P-Editor: Xing YX
Contributor Information
Xi-Min Hu, Department of Anatomy and Neurobiology, School of Basic Medical Sciences, Central South University, Changsha 410013, Hunan Province, China; Department of Dermatology, Xiangya Hospital, Central South University, Changsha 410013, Hunan Province, China.
Qi Zhang, Department of Anatomy and Neurobiology, School of Basic Medical Sciences, Central South University, Changsha 410013, Hunan Province, China.
Rui-Xin Zhou, Department of Anatomy and Neurobiology, School of Basic Medical Sciences, Central South University, Changsha 410013, Hunan Province, China.
Yan-Lin Wu, Department of Anatomy and Neurobiology, School of Basic Medical Sciences, Central South University, Changsha 410013, Hunan Province, China.
Zhi-Xin Li, Department of Anatomy and Neurobiology, School of Basic Medical Sciences, Central South University, Changsha 410013, Hunan Province, China.
Dan-Yi Zhang, Department of Anatomy and Neurobiology, School of Basic Medical Sciences, Central South University, Changsha 410013, Hunan Province, China.
Yi-Chao Yang, Department of Anatomy and Neurobiology, School of Basic Medical Sciences, Central South University, Changsha 410013, Hunan Province, China.
Rong-Hua Yang, Department of Burns, Fo Shan Hospital of Sun Yat-Sen University, Foshan 528000, Guangdong Province, China.
Yong-Jun Hu, Department of Cardiovascular Medicine, Hunan People's Hospital (the First Affiliated Hospital of Hunan Normal University, Changsha 410005, Hunan Province, China.
Kun Xiong, Department of Anatomy and Neurobiology, School of Basic Medical Sciences, Central South University, Changsha 410013, Hunan Province, China. xiongkun2001@163.com.
References
- 1.Kimbrel EA, Lanza R. Next-generation stem cells - ushering in a new era of cell-based therapies. Nat Rev Drug Discov. 2020;19:463–479. doi: 10.1038/s41573-020-0064-x. [DOI] [PubMed] [Google Scholar]
- 2.Raffin C, Vo LT, Bluestone JA. Treg cell-based therapies: challenges and perspectives. Nat Rev Immunol. 2020;20:158–172. doi: 10.1038/s41577-019-0232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellis C, Ramzy A, Kieffer TJ. Regenerative medicine and cell-based approaches to restore pancreatic function. Nat Rev Gastroenterol Hepatol. 2017;14:612–628. doi: 10.1038/nrgastro.2017.93. [DOI] [PubMed] [Google Scholar]
- 4.Clevers H, Loh KM, Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346:1248012. doi: 10.1126/science.1248012. [DOI] [PubMed] [Google Scholar]
- 5.Kolios G, Moodley Y. Introduction to stem cells and regenerative medicine. Respiration. 2013;85:3–10. doi: 10.1159/000345615. [DOI] [PubMed] [Google Scholar]
- 6.Vazin T, Freed WJ. Human embryonic stem cells: derivation, culture, and differentiation: a review. Restor Neurol Neurosci. 2010;28:589–603. doi: 10.3233/RNN-2010-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zarzeczny A, Caulfield T. Emerging ethical, legal and social issues associated with stem cell research & and the current role of the moral status of the embryo. Stem Cell Rev Rep. 2009;5:96–101. doi: 10.1007/s12015-009-9062-4. [DOI] [PubMed] [Google Scholar]
- 8.Glicksman MA. Induced Pluripotent Stem Cells: The Most Versatile Source for Stem Cell Therapy. Clin Ther. 2018;40:1060–1065. doi: 10.1016/j.clinthera.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Gurusamy N, Alsayari A, Rajasingh S, Rajasingh J. Adult Stem Cells for Regenerative Therapy. Prog Mol Biol Transl Sci. 2018;160:1–22. doi: 10.1016/bs.pmbts.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Poiana G, Gioia R, Sineri S, Cardarelli S, Lupo G, Cacci E. Transcriptional regulation of adult neural stem/progenitor cells: tales from the subventricular zone. Neural Regen Res. 2020;15:1773–1783. doi: 10.4103/1673-5374.280301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prasad K, Sharma A, Garg A, Mohanty S, Bhatnagar S, Johri S, Singh KK, Nair V, Sarkar RS, Gorthi SP, Hassan KM, Prabhakar S, Marwaha N, Khandelwal N, Misra UK, Kalita J, Nityanand S InveST Study Group. Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke: a multicentric, randomized trial. Stroke. 2014;45:3618–3624. doi: 10.1161/STROKEAHA.114.007028. [DOI] [PubMed] [Google Scholar]
- 12.Baker EW, Kinder HA, West FD. Neural stem cell therapy for stroke: A multimechanistic approach to restoring neurological function. Brain Behav. 2019;9:e01214. doi: 10.1002/brb3.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY STARTING collaborators. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010;28:1099–1106. doi: 10.1002/stem.430. [DOI] [PubMed] [Google Scholar]
- 14.Trounson A, McDonald C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell. 2015;17:11–22. doi: 10.1016/j.stem.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Yang R, Yang S, Zhao J, Hu X, Chen X, Wang J, Xie J, Xiong K. Progress in studies of epidermal stem cells and their application in skin tissue engineering. Stem Cell Res Ther. 2020;11:303. doi: 10.1186/s13287-020-01796-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang R, Liu F, Wang J, Chen X, Xie J, Xiong K. Epidermal stem cells in wound healing and their clinical applications. Stem Cell Res Ther. 2019;10:229. doi: 10.1186/s13287-019-1312-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, Zhang CG, Jia YL, Hu L. Tissue Inhibitor of Metalloprotease-1 (TIMP-1) Regulates Adipogenesis of Adipose-derived Stem Cells (ASCs) via the Wnt Signaling Pathway in an MMP-independent Manner. Curr Med Sci. 2020;40:989–996. doi: 10.1007/s11596-020-2265-2. [DOI] [PubMed] [Google Scholar]
- 18.Stoddard-Bennett T, Pera RR. Stem cell therapy for Parkinson's disease: safety and modeling. Neural Regen Res. 2020;15:36–40. doi: 10.4103/1673-5374.264446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363:147–155. doi: 10.1056/NEJMoa0905955. [DOI] [PubMed] [Google Scholar]
- 20.Kulkarni S, Becker L, Pasricha PJ. Stem cell transplantation in neurodegenerative disorders of the gastrointestinal tract: future or fiction? Gut. 2012;61:613–621. doi: 10.1136/gut.2010.235614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ammar HI, Shamseldeen AM, Shoukry HS, Ashour H, Kamar SS, Rashed LA, Fadel M, Srivastava A, Dhingra S. Metformin impairs homing ability and efficacy of mesenchymal stem cells for cardiac repair in streptozotocin-induced diabetic cardiomyopathy in rats. Am J Physiol Heart Circ Physiol. 2021;320:H1290–H1302. doi: 10.1152/ajpheart.00317.2020. [DOI] [PubMed] [Google Scholar]
- 22.Martino G, Franklin RJ, Baron Van Evercooren A, Kerr DA Stem Cells in Multiple Sclerosis (STEMS) Consensus Group. Stem cell transplantation in multiple sclerosis: current status and future prospects. Nat Rev Neurol. 2010;6:247–255. doi: 10.1038/nrneurol.2010.35. [DOI] [PubMed] [Google Scholar]
- 23.Li X, He XT, Yin Y, Wu RX, Tian BM, Chen FM. Administration of signalling molecules dictates stem cell homing for in situ regeneration. J Cell Mol Med. 2017;21:3162–3177. doi: 10.1111/jcmm.13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mastri M, Lin H, Lee T. Enhancing the efficacy of mesenchymal stem cell therapy. World J Stem Cells. 2014;6:82–93. doi: 10.4252/wjsc.v6.i2.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robey TE, Saiget MK, Reinecke H, Murry CE. Systems approaches to preventing transplanted cell death in cardiac repair. J Mol Cell Cardiol. 2008;45:567–581. doi: 10.1016/j.yjmcc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geng YJ. Molecular mechanisms for cardiovascular stem cell apoptosis and growth in the hearts with atherosclerotic coronary disease and ischemic heart failure. Ann N Y Acad Sci. 2003;1010:687–697. doi: 10.1196/annals.1299.126. [DOI] [PubMed] [Google Scholar]
- 27.Qin H, Yu T, Qing T, Liu Y, Zhao Y, Cai J, Li J, Song Z, Qu X, Zhou P, Wu J, Ding M, Deng H. Regulation of apoptosis and differentiation by p53 in human embryonic stem cells. J Biol Chem. 2007;282:5842–5852. doi: 10.1074/jbc.M610464200. [DOI] [PubMed] [Google Scholar]
- 28.Fan VH, Tamama K, Au A, Littrell R, Richardson LB, Wright JW, Wells A, Griffith LG. Tethered epidermal growth factor provides a survival advantage to mesenchymal stem cells. Stem Cells. 2007;25:1241–1251. doi: 10.1634/stemcells.2006-0320. [DOI] [PubMed] [Google Scholar]
- 29.Ohgushi M, Matsumura M, Eiraku M, Murakami K, Aramaki T, Nishiyama A, Muguruma K, Nakano T, Suga H, Ueno M, Ishizaki T, Suemori H, Narumiya S, Niwa H, Sasai Y. Molecular pathway and cell state responsible for dissociation-induced apoptosis in human pluripotent stem cells. Cell Stem Cell. 2010;7:225–239. doi: 10.1016/j.stem.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 30.García-Fernández M, Kissel H, Brown S, Gorenc T, Schile AJ, Rafii S, Larisch S, Steller H. Sept4/ARTS is required for stem cell apoptosis and tumor suppression. Genes Dev. 2010;24:2282–2293. doi: 10.1101/gad.1970110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madden DT, Davila-Kruger D, Melov S, Bredesen DE. Human embryonic stem cells express elevated levels of multiple pro-apoptotic BCL-2 family members. PLoS One. 2011;6:e28530. doi: 10.1371/journal.pone.0028530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parmley RT, Crist WM, Ragab AH, Boxer LA, Malluh A, Lui VK, Darby CP. Congenital dysgranulopoietic neutropenia: clinical, serologic, ultrastructural, and in vitro proliferative characteristics. Blood. 1980;56:465–475. [PubMed] [Google Scholar]
- 33.Ho TT, Warr MR, Adelman ER, Lansinger OM, Flach J, Verovskaya EV, Figueroa ME, Passegué E. Autophagy maintains the metabolism and function of young and old stem cells. Nature. 2017;543:205–210. doi: 10.1038/nature21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma Y, Qi M, An Y, Zhang L, Yang R, Doro DH, Liu W, Jin Y. Autophagy controls mesenchymal stem cell properties and senescence during bone aging. Aging Cell. 2018;17 doi: 10.1111/acel.12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang R, Li H, Wu J, Cai ZY, Li B, Ni H, Qiu X, Chen H, Liu W, Yang ZH, Liu M, Hu J, Liang Y, Lan P, Han J, Mo W. Gut stem cell necroptosis by genome instability triggers bowel inflammation. Nature. 2020;580:386–390. doi: 10.1038/s41586-020-2127-x. [DOI] [PubMed] [Google Scholar]
- 36.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 37.Dang LT, Feric NT, Laschinger C, Chang WY, Zhang B, Wood GA, Stanford WL, Radisic M. Inhibition of apoptosis in human induced pluripotent stem cells during expansion in a defined culture using angiopoietin-1 derived peptide QHREDGS. Biomaterials. 2014;35:7786–7799. doi: 10.1016/j.biomaterials.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei H, Li Z, Hu S, Chen X, Cong X. Apoptosis of mesenchymal stem cells induced by hydrogen peroxide concerns both endoplasmic reticulum stress and mitochondrial death pathway through regulation of caspases, p38 and JNK. J Cell Biochem. 2010;111:967–978. doi: 10.1002/jcb.22785. [DOI] [PubMed] [Google Scholar]
- 39.Yu SP, Wei Z, Wei L. Preconditioning strategy in stem cell transplantation therapy. Transl Stroke Res. 2013;4:76–88. doi: 10.1007/s12975-012-0251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drowley L, Okada M, Beckman S, Vella J, Keller B, Tobita K, Huard J. Cellular antioxidant levels influence muscle stem cell therapy. Mol Ther. 2010;18:1865–1873. doi: 10.1038/mt.2010.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haider HKh, Ashraf M. Strategies to promote donor cell survival: combining preconditioning approach with stem cell transplantation. J Mol Cell Cardiol. 2008;45:554–566. doi: 10.1016/j.yjmcc.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O'Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 43.Noort WA, Feye D, Van Den Akker F, Stecher D, Chamuleau SA, Sluijter JP, Doevendans PA. Mesenchymal stromal cells to treat cardiovascular disease: strategies to improve survival and therapeutic results. Panminerva Med. 2010;52:27–40. [PubMed] [Google Scholar]
- 44.Lu G, Haider HK, Jiang S, Ashraf M. Sca-1+ stem cell survival and engraftment in the infarcted heart: dual role for preconditioning-induced connexin-43. Circulation. 2009;119:2587–2596. doi: 10.1161/CIRCULATIONAHA.108.827691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tilkorn DJ, Davies EM, Keramidaris E, Dingle AM, Gerrand YW, Taylor CJ, Han XL, Palmer JA, Penington AJ, Mitchell CA, Morrison WA, Dusting GJ, Mitchell GM. The in vitro preconditioning of myoblasts to enhance subsequent survival in an in vivo tissue engineering chamber model. Biomaterials. 2012;33:3868–3879. doi: 10.1016/j.biomaterials.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 46.Shafei AE, Ali MA, Ghanem HG, Shehata AI, Abdelgawad AA, Handal HR, Talaat KA, Ashaal AE, El-Shal AS. Mesenchymal stem cell therapy: A promising cell-based therapy for treatment of myocardial infarction. J Gene Med. 2017;19 doi: 10.1002/jgm.2995. [DOI] [PubMed] [Google Scholar]
- 47.Abdelwahid E, Kalvelyte A, Stulpinas A, de Carvalho KA, Guarita-Souza LC, Foldes G. Stem cell death and survival in heart regeneration and repair. Apoptosis. 2016;21:252–268. doi: 10.1007/s10495-015-1203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khodayari S, Khodayari H, Amiri AZ, Eslami M, Farhud D, Hescheler J, Nayernia K. Inflammatory Microenvironment of Acute Myocardial Infarction Prevents Regeneration of Heart with Stem Cells Therapy. Cell Physiol Biochem. 2019;53:887–909. doi: 10.33594/000000180. [DOI] [PubMed] [Google Scholar]
- 49.Templin C, Zweigerdt R, Schwanke K, Olmer R, Ghadri JR, Emmert MY, Müller E, Küest SM, Cohrs S, Schibli R, Kronen P, Hilbe M, Reinisch A, Strunk D, Haverich A, Hoerstrup S, Lüscher TF, Kaufmann PA, Landmesser U, Martin U. Transplantation and tracking of human-induced pluripotent stem cells in a pig model of myocardial infarction: assessment of cell survival, engraftment, and distribution by hybrid single photon emission computed tomography/computed tomography of sodium iodide symporter transgene expression. Circulation. 2012;126:430–439. doi: 10.1161/CIRCULATIONAHA.111.087684. [DOI] [PubMed] [Google Scholar]
- 50.Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, Gantz JA, Fugate JA, Muskheli V, Gough GM, Vogel KW, Astley CA, Hotchkiss CE, Baldessari A, Pabon L, Reinecke H, Gill EA, Nelson V, Kiem HP, Laflamme MA, Murry CE. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273–277. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao L, Xu W, Li T, Chen J, Shao A, Yan F, Chen G. Stem Cell Therapy: A Promising Therapeutic Method for Intracerebral Hemorrhage. Cell Transplant. 2018;27:1809–1824. doi: 10.1177/0963689718773363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cui J, Cui C, Cui Y, Li R, Sheng H, Jiang X, Tian Y, Wang K, Gao J. Bone Marrow Mesenchymal Stem Cell Transplantation Increases GAP-43 Expression via ERK1/2 and PI3K/Akt Pathways in Intracerebral Hemorrhage. Cell Physiol Biochem. 2017;42:137–144. doi: 10.1159/000477122. [DOI] [PubMed] [Google Scholar]
- 53.Feng M, Zhu H, Zhu Z, Wei J, Lu S, Li Q, Zhang N, Li G, Li F, Ma W, An Y, Zhao RC, Qin C, Wang R. Serial 18F-FDG PET demonstrates benefit of human mesenchymal stem cells in treatment of intracerebral hematoma: a translational study in a primate model. J Nucl Med. 2011;52:90–97. doi: 10.2967/jnumed.110.080325. [DOI] [PubMed] [Google Scholar]
- 54.Yang C, Zhou L, Gao X, Chen B, Tu J, Sun H, Liu X, He J, Liu J, Yuan Q. Neuroprotective effects of bone marrow stem cells overexpressing glial cell line-derived neurotrophic factor on rats with intracerebral hemorrhage and neurons exposed to hypoxia/reoxygenation. Neurosurgery. 2011;68:691–704. doi: 10.1227/NEU.0b013e3182098a8a. [DOI] [PubMed] [Google Scholar]
- 55.Wang Z, Cui C, Li Q, Zhou S, Fu J, Wang X, Zhuge Q. Intracerebral transplantation of foetal neural stem cells improves brain dysfunction induced by intracerebral haemorrhage stroke in mice. J Cell Mol Med. 2011;15:2624–2633. doi: 10.1111/j.1582-4934.2011.01259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee HJ, Kim KS, Kim EJ, Choi HB, Lee KH, Park IH, Ko Y, Jeong SW, Kim SU. Brain transplantation of immortalized human neural stem cells promotes functional recovery in mouse intracerebral hemorrhage stroke model. Stem Cells. 2007;25:1204–1212. doi: 10.1634/stemcells.2006-0409. [DOI] [PubMed] [Google Scholar]
- 57.Tang T, Li XQ, Wu H, Luo JK, Zhang HX, Luo TL. Activation of endogenous neural stem cells in experimental intracerebral hemorrhagic rat brains. Chin Med J (Engl) 2004;117:1342–1347. [PubMed] [Google Scholar]
- 58.Nonaka M, Yoshikawa M, Nishimura F, Yokota H, Kimura H, Hirabayashi H, Nakase H, Ishizaka S, Wanaka A, Sakaki T. Intraventricular transplantation of embryonic stem cell-derived neural stem cells in intracerebral hemorrhage rats. Neurol Res. 2004;26:265–272. doi: 10.1179/016164104225014049. [DOI] [PubMed] [Google Scholar]
- 59.Bain G, Kitchens D, Yao M, Huettner JE, Gottlieb DI. Embryonic stem cells express neuronal properties in vitro. Dev Biol. 1995;168:342–357. doi: 10.1006/dbio.1995.1085. [DOI] [PubMed] [Google Scholar]
- 60.Qin J, Ma X, Qi H, Song B, Wang Y, Wen X, Wang QM, Sun S, Li Y, Zhang R, Liu X, Hou H, Gong G, Xu Y. Transplantation of Induced Pluripotent Stem Cells Alleviates Cerebral Inflammation and Neural Damage in Hemorrhagic Stroke. PLoS One. 2015;10:e0129881. doi: 10.1371/journal.pone.0129881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qin J, Gong G, Sun S, Qi J, Zhang H, Wang Y, Wang N, Wang QM, Ji Y, Gao Y, Shi C, Yang B, Zhang Y, Song B, Xu Y. Functional recovery after transplantation of induced pluripotent stem cells in a rat hemorrhagic stroke model. Neurosci Lett. 2013;554:70–75. doi: 10.1016/j.neulet.2013.08.047. [DOI] [PubMed] [Google Scholar]
- 62.Qin J, Song B, Zhang H, Wang Y, Wang N, Ji Y, Qi J, Chandra A, Yang B, Zhang Y, Gong G, Xu Y. Transplantation of human neuro-epithelial-like stem cells derived from induced pluripotent stem cells improves neurological function in rats with experimental intracerebral hemorrhage. Neurosci Lett. 2013;548:95–100. doi: 10.1016/j.neulet.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 63.Mansoor H, Ong HS, Riau AK, Stanzel TP, Mehta JS, Yam GH. Current Trends and Future Perspective of Mesenchymal Stem Cells and Exosomes in Corneal Diseases. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20122853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kawaguchi J, Nichols J, Gierl MS, Faial T, Smith A. Isolation and propagation of enteric neural crest progenitor cells from mouse embryonic stem cells and embryos. Development. 2010;137:693–704. doi: 10.1242/dev.046896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ben-David U, Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat Rev Cancer. 2011;11:268–277. doi: 10.1038/nrc3034. [DOI] [PubMed] [Google Scholar]
- 66.Micci MA, Kahrig KM, Simmons RS, Sarna SK, Espejo-Navarro MR, Pasricha PJ. Neural stem cell transplantation in the stomach rescues gastric function in neuronal nitric oxide synthase-deficient mice. Gastroenterology. 2005;129:1817–1824. doi: 10.1053/j.gastro.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 67.Micci MA, Pasricha PJ. Neural stem cells for the treatment of disorders of the enteric nervous system: strategies and challenges. Dev Dyn. 2007;236:33–43. doi: 10.1002/dvdy.20975. [DOI] [PubMed] [Google Scholar]
- 68.Tsai YH, Murakami N, Gariepy CE. Postnatal intestinal engraftment of prospectively selected enteric neural crest stem cells in a rat model of Hirschsprung disease. Neurogastroenterol Motil. 2011;23:362–369. doi: 10.1111/j.1365-2982.2010.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lindley RM, Hawcutt DB, Connell MG, Edgar DH, Kenny SE. Properties of secondary and tertiary human enteric nervous system neurospheres. J Pediatr Surg. 2009;44:1249–55; discussion 1255. doi: 10.1016/j.jpedsurg.2009.02.048. [DOI] [PubMed] [Google Scholar]
- 70.Liu M, Chen H, Jiang J, Zhang Z, Wang C, Zhang N, Dong L, Hu X, Zhu W, Yu H, Wang J. Stem cells and diabetic cardiomyopathy: from pathology to therapy. Heart Fail Rev. 2016;21:723–736. doi: 10.1007/s10741-016-9565-4. [DOI] [PubMed] [Google Scholar]
- 71.Cheng Y, Guo S, Liu G, Feng Y, Yan B, Yu J, Feng K, Li Z. Transplantation of bone marrow-derived endothelial progenitor cells attenuates myocardial interstitial fibrosis and cardiac dysfunction in streptozotocin-induced diabetic rats. Int J Mol Med. 2012;30:870–876. doi: 10.3892/ijmm.2012.1083. [DOI] [PubMed] [Google Scholar]
- 72.Delucchi F, Berni R, Frati C, Cavalli S, Graiani G, Sala R, Chaponnier C, Gabbiani G, Calani L, Del Rio D, Bocchi L, Lagrasta C, Quaini F, Stilli D. Resveratrol treatment reduces cardiac progenitor cell dysfunction and prevents morpho-functional ventricular remodeling in type-1 diabetic rats. PLoS One. 2012;7:e39836. doi: 10.1371/journal.pone.0039836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yan B, Singla DK. Transplanted induced pluripotent stem cells mitigate oxidative stress and improve cardiac function through the Akt cell survival pathway in diabetic cardiomyopathy. Mol Pharm. 2013;10:3425–3432. doi: 10.1021/mp400258d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gaddam S, Periasamy R, Gangaraju R. Adult Stem Cell Therapeutics in Diabetic Retinopathy. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20194876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kramerov AA, Ljubimov AV. Stem cell therapies in the treatment of diabetic retinopathy and keratopathy. Exp Biol Med (Maywood) 2016;241:559–568. doi: 10.1177/1535370215609692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ezquer M, Urzua CA, Montecino S, Leal K, Conget P, Ezquer F. Intravitreal administration of multipotent mesenchymal stromal cells triggers a cytoprotective microenvironment in the retina of diabetic mice. Stem Cell Res Ther. 2016;7:42. doi: 10.1186/s13287-016-0299-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rajashekhar G, Ramadan A, Abburi C, Callaghan B, Traktuev DO, Evans-Molina C, Maturi R, Harris A, Kern TS, March KL. Regenerative therapeutic potential of adipose stromal cells in early stage diabetic retinopathy. PLoS One. 2014;9:e84671. doi: 10.1371/journal.pone.0084671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bhatwadekar AD, Duan Y, Korah M, Thinschmidt JS, Hu P, Leley SP, Caballero S, Shaw L, Busik J, Grant MB. Hematopoietic stem/progenitor involvement in retinal microvascular repair during diabetes: Implications for bone marrow rejuvenation. Vision Res. 2017;139:211–220. doi: 10.1016/j.visres.2017.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tzameret A, Sher I, Belkin M, Treves AJ, Meir A, Nagler A, Levkovitch-Verbin H, Barshack I, Rosner M, Rotenstreich Y. Transplantation of human bone marrow mesenchymal stem cells as a thin subretinal layer ameliorates retinal degeneration in a rat model of retinal dystrophy. Exp Eye Res. 2014;118:135–144. doi: 10.1016/j.exer.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 80.Moisseiev E, Smit-McBride Z, Oltjen S, Zhang P, Zawadzki RJ, Motta M, Murphy CJ, Cary W, Annett G, Nolta JA, Park SS. Intravitreal Administration of Human Bone Marrow CD34+ Stem Cells in a Murine Model of Retinal Degeneration. Invest Ophthalmol Vis Sci. 2016;57:4125–4135. doi: 10.1167/iovs.16-19252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Park TS, Bhutto I, Zimmerlin L, Huo JS, Nagaria P, Miller D, Rufaihah AJ, Talbot C, Aguilar J, Grebe R, Merges C, Reijo-Pera R, Feldman RA, Rassool F, Cooke J, Lutty G, Zambidis ET. Vascular progenitors from cord blood-derived induced pluripotent stem cells possess augmented capacity for regenerating ischemic retinal vasculature. Circulation. 2014;129:359–372. doi: 10.1161/CIRCULATIONAHA.113.003000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alessandrini M, Preynat-Seauve O, De Bruin K, Pepper MS. Stem cell therapy for neurological disorders. S Afr Med J. 2019;109:70–77. doi: 10.7196/SAMJ.2019.v109i8b.14009. [DOI] [PubMed] [Google Scholar]
- 83.Stoddard-Bennett T, Reijo Pera R. Treatment of Parkinson's Disease through Personalized Medicine and Induced Pluripotent Stem Cells. Cells. 2019;8 doi: 10.3390/cells8010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tang Y, Wang J, Lin X, Wang L, Shao B, Jin K, Wang Y, Yang GY. Neural stem cell protects aged rat brain from ischemia-reperfusion injury through neurogenesis and angiogenesis. J Cereb Blood Flow Metab. 2014;34:1138–1147. doi: 10.1038/jcbfm.2014.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, Yang L, Beal MF, Surmeier DJ, Kordower JH, Tabar V, Studer L. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Min K, Song J, Kang JY, Ko J, Ryu JS, Kang MS, Jang SJ, Kim SH, Oh D, Kim MK, Kim SS, Kim M. Umbilical cord blood therapy potentiated with erythropoietin for children with cerebral palsy: a double-blind, randomized, placebo-controlled trial. Stem Cells. 2013;31:581–591. doi: 10.1002/stem.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Saleem M, Sabir S, Akhtar MF, Zahid S, Niazi SG, Naeem M, Saleem U, Saleem A. Stem Cell Therapy for Diabetes Mellitus: Recent Progress and Hurdles. Crit Rev Eukaryot Gene Expr. 2019;29:471–482. doi: 10.1615/CritRevEukaryotGeneExpr.2019025723. [DOI] [PubMed] [Google Scholar]
- 88.Schroeder IS, Rolletschek A, Blyszczuk P, Kania G, Wobus AM. Differentiation of mouse embryonic stem cells to insulin-producing cells. Nat Protoc. 2006;1:495–507. doi: 10.1038/nprot.2006.71. [DOI] [PubMed] [Google Scholar]
- 89.Lester LB, Kuo HC, Andrews L, Nauert B, Wolf DP. Directed differentiation of rhesus monkey ES cells into pancreatic cell phenotypes. Reprod Biol Endocrinol. 2004;2:42. doi: 10.1186/1477-7827-2-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tang DQ, Lu S, Sun YP, Rodrigues E, Chou W, Yang C, Cao LZ, Chang LJ, Yang LJ. Reprogramming liver-stem WB cells into functional insulin-producing cells by persistent expression of Pdx1- and Pdx1-VP16 mediated by lentiviral vectors. Lab Invest. 2006;86:83–93. doi: 10.1038/labinvest.3700368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kodama S, Kühtreiber W, Fujimura S, Dale EA, Faustman DL. Islet regeneration during the reversal of autoimmune diabetes in NOD mice. Science. 2003;302:1223–1227. doi: 10.1126/science.1088949. [DOI] [PubMed] [Google Scholar]
- 92.Tian C, Bagley J, Cretin N, Seth N, Wucherpfennig KW, Iacomini J. Prevention of type 1 diabetes by gene therapy. J Clin Invest. 2004;114:969–978. doi: 10.1172/JCI22103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol. 2014;15:135–147. doi: 10.1038/nrm3737. [DOI] [PubMed] [Google Scholar]
- 94.Galluzzi L, Bravo-San Pedro JM, Vitale I, Aaronson SA, Abrams JM, Adam D, Alnemri ES, Altucci L, Andrews D, Annicchiarico-Petruzzelli M, Baehrecke EH, Bazan NG, Bertrand MJ, Bianchi K, Blagosklonny MV, Blomgren K, Borner C, Bredesen DE, Brenner C, Campanella M, Candi E, Cecconi F, Chan FK, Chandel NS, Cheng EH, Chipuk JE, Cidlowski JA, Ciechanover A, Dawson TM, Dawson VL, De Laurenzi V, De Maria R, Debatin KM, Di Daniele N, Dixit VM, Dynlacht BD, El-Deiry WS, Fimia GM, Flavell RA, Fulda S, Garrido C, Gougeon ML, Green DR, Gronemeyer H, Hajnoczky G, Hardwick JM, Hengartner MO, Ichijo H, Joseph B, Jost PJ, Kaufmann T, Kepp O, Klionsky DJ, Knight RA, Kumar S, Lemasters JJ, Levine B, Linkermann A, Lipton SA, Lockshin RA, López-Otín C, Lugli E, Madeo F, Malorni W, Marine JC, Martin SJ, Martinou JC, Medema JP, Meier P, Melino S, Mizushima N, Moll U, Muñoz-Pinedo C, Nuñez G, Oberst A, Panaretakis T, Penninger JM, Peter ME, Piacentini M, Pinton P, Prehn JH, Puthalakath H, Rabinovich GA, Ravichandran KS, Rizzuto R, Rodrigues CM, Rubinsztein DC, Rudel T, Shi Y, Simon HU, Stockwell BR, Szabadkai G, Tait SW, Tang HL, Tavernarakis N, Tsujimoto Y, Vanden Berghe T, Vandenabeele P, Villunger A, Wagner EF, Walczak H, White E, Wood WG, Yuan J, Zakeri Z, Zhivotovsky B, Melino G, Kroemer G. Essential vs accessory aspects of cell death: recommendations of the NCCD 2015. Cell Death Differ. 2015;22:58–73. doi: 10.1038/cdd.2014.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 96.Mooney R, Majid AA, Mota D, He A, Aramburo S, Flores L, Covello-Batalla J, Machado D, Gonzaga J, Aboody KS. Bcl-2 Overexpression Improves Survival and Efficacy of Neural Stem Cell-Mediated Enzyme Prodrug Therapy. Stem Cells Int. 2018;2018:7047496. doi: 10.1155/2018/7047496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hayashi M, Li TS, Ito H, Mikamo A, Hamano K. Comparison of intramyocardial and intravenous routes of delivering bone marrow cells for the treatment of ischemic heart disease: an experimental study. Cell Transplant. 2004;13:639–647. doi: 10.3727/000000004783983558. [DOI] [PubMed] [Google Scholar]
- 98.Van Opdenbosch N, Lamkanfi M. Caspases in Cell Death, Inflammation, and Disease. Immunity. 2019;50:1352–1364. doi: 10.1016/j.immuni.2019.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer. 2002;2:277–288. doi: 10.1038/nrc776. [DOI] [PubMed] [Google Scholar]
- 100.Tang Y, Luo B, Deng Z, Wang B, Liu F, Li J, Shi W, Xie H, Hu X. Mitochondrial aerobic respiration is activated during hair follicle stem cell differentiation, and its dysfunction retards hair regeneration. PeerJ. 2016;4:e1821. doi: 10.7717/peerj.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li J, Jiang TX, Hughes MW, Wu P, Yu J, Widelitz RB, Fan G, Chuong CM. Progressive alopecia reveals decreasing stem cell activation probability during aging of mice with epidermal deletion of DNA methyltransferase 1. J Invest Dermatol. 2012;132:2681–2690. doi: 10.1038/jid.2012.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Singh R, Letai A, Sarosiek K. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol. 2019;20:175–193. doi: 10.1038/s41580-018-0089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rasmussen ML, Gama V. A connection in life and death: The BCL-2 family coordinates mitochondrial network dynamics and stem cell fate. Int Rev Cell Mol Biol. 2020;353:255–284. doi: 10.1016/bs.ircmb.2019.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bossen C, Ingold K, Tardivel A, Bodmer JL, Gaide O, Hertig S, Ambrose C, Tschopp J, Schneider P. Interactions of tumor necrosis factor (TNF) and TNF receptor family members in the mouse and human. J Biol Chem. 2006;281:13964–13971. doi: 10.1074/jbc.M601553200. [DOI] [PubMed] [Google Scholar]
- 105.Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306:990–995. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Parzych KR, Klionsky DJ. An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal. 2014;20:460–473. doi: 10.1089/ars.2013.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mijaljica D, Prescott M, Devenish RJ. Microautophagy in mammalian cells: revisiting a 40-year-old conundrum. Autophagy. 2011;7:673–682. doi: 10.4161/auto.7.7.14733. [DOI] [PubMed] [Google Scholar]
- 108.Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ. 2005;12 Suppl 2:1542–1552. doi: 10.1038/sj.cdd.4401765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang Q, Wang R, Zhu L. Chaperone-Mediated Autophagy. Adv Exp Med Biol. 2019;1206:435–452. doi: 10.1007/978-981-15-0602-4_20. [DOI] [PubMed] [Google Scholar]
- 110.Newton K, Manning G. Necroptosis and Inflammation. Annu Rev Biochem. 2016;85:743–763. doi: 10.1146/annurev-biochem-060815-014830. [DOI] [PubMed] [Google Scholar]
- 111.Ruan ZH, Xu ZX, Zhou XY, Zhang X, Shang L. Implications of Necroptosis for Cardiovascular Diseases. Curr Med Sci. 2019;39:513–522. doi: 10.1007/s11596-019-2067-6. [DOI] [PubMed] [Google Scholar]
- 112.Newton K. RIPK1 and RIPK3: critical regulators of inflammation and cell death. Trends Cell Biol. 2015;25:347–353. doi: 10.1016/j.tcb.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 113.Khan N, Lawlor KE, Murphy JM, Vince JE. More to life than death: molecular determinants of necroptotic and non-necroptotic RIP3 kinase signaling. Curr Opin Immunol. 2014;26:76–89. doi: 10.1016/j.coi.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 114.Brennan MA, Cookson BT. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol Microbiol. 2000;38:31–40. doi: 10.1046/j.1365-2958.2000.02103.x. [DOI] [PubMed] [Google Scholar]
- 115.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B 3rd, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen J, Wang Y, Wu J, Yang J, Li M, Chen Q. The Potential Value of Targeting Ferroptosis in Early Brain Injury After Acute CNS Disease. Front Mol Neurosci. 2020;13:110. doi: 10.3389/fnmol.2020.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nikoletopoulou V, Markaki M, Palikaras K, Tavernarakis N. Crosstalk between apoptosis, necrosis and autophagy. Biochim Biophys Acta. 2013;1833:3448–3459. doi: 10.1016/j.bbamcr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 118.Bedoui S, Herold MJ, Strasser A. Emerging connectivity of programmed cell death pathways and its physiological implications. Nat Rev Mol Cell Biol. 2020;21:678–695. doi: 10.1038/s41580-020-0270-8. [DOI] [PubMed] [Google Scholar]
- 119.Liu JC, Lerou PH, Lahav G. Stem cells: balancing resistance and sensitivity to DNA damage. Trends Cell Biol. 2014;24:268–274. doi: 10.1016/j.tcb.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yamashita M, Nitta E, Suda T. Regulation of hematopoietic stem cell integrity through p53 and its related factors. Ann N Y Acad Sci. 2016;1370:45–54. doi: 10.1111/nyas.12986. [DOI] [PubMed] [Google Scholar]
- 121.Koren E, Yosefzon Y, Ankawa R, Soteriou D, Jacob A, Nevelsky A, Ben-Yosef R, Bar-Sela G, Fuchs Y. ARTS mediates apoptosis and regeneration of the intestinal stem cell niche. Nat Commun. 2018;9:4582. doi: 10.1038/s41467-018-06941-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen NN, Wei F, Wang L, Cui S, Wan Y, Liu S. Tumor Necrosis Factor Alpha Induces Neural Stem Cell Apoptosis Through Activating p38 MAPK Pathway. Neurochem Res. 2016;41:3052–3062. doi: 10.1007/s11064-016-2024-8. [DOI] [PubMed] [Google Scholar]
- 123.Kolahdouzmohammadi M, Totonchi M, Pahlavan S. The Role of iPSC Modeling Toward Projection of Autophagy Pathway in Disease Pathogenesis: Leader or Follower. Stem Cell Rev Rep. 2021;17:539–561. doi: 10.1007/s12015-020-10077-8. [DOI] [PubMed] [Google Scholar]
- 124.You L, Jin S, Zhu L, Qian W. Autophagy, autophagy-associated adaptive immune responses and its role in hematologic malignancies. Oncotarget. 2017;8:12374–12388. doi: 10.18632/oncotarget.13583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang M, Liang X, Cheng M, Yang L, Liu H, Wang X, Sai N, Zhang X. Homocysteine enhances neural stem cell autophagy in in vivo and in vitro model of ischemic stroke. Cell Death Dis. 2019;10:561. doi: 10.1038/s41419-019-1798-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xiang J, Jiang T, Zhang W, Xie W, Tang X, Zhang J. Human umbilical cord-derived mesenchymal stem cells enhanced HK-2 cell autophagy through MicroRNA-145 by inhibiting the PI3K/AKT/mTOR signaling pathway. Exp Cell Res. 2019;378:198–205. doi: 10.1016/j.yexcr.2019.03.019. [DOI] [PubMed] [Google Scholar]
- 127.Yang B, Cai Z, Zhang W, Yin D, Zhao W, Yang M. Autophagy alleviates the decrease in proliferation of amyloid β142treated bone marrow mesenchymal stem cells via the AKT/mTOR signaling pathway. Mol Med Rep. 2019;19:4091–4100. doi: 10.3892/mmr.2019.10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Suvorova II, Knyazeva AR, Petukhov AV, Aksenov ND, Pospelov VA. Resveratrol enhances pluripotency of mouse embryonic stem cells by activating AMPK/Ulk1 pathway. Cell Death Discov. 2019;5:61. doi: 10.1038/s41420-019-0137-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu Y, Liu T, Lei T, Zhang D, Du S, Girani L, Qi D, Lin C, Tong R, Wang Y. RIP1/RIP3-regulated necroptosis as a target for multifaceted disease therapy (Review) Int J Mol Med. 2019;44:771–786. doi: 10.3892/ijmm.2019.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhao Y, Zhu X, Zhang L, Ferguson CM, Song T, Jiang K, Conley SM, Krier JD, Tang H, Saadiq I, Jordan KL, Lerman A, Lerman LO. Mesenchymal Stem/Stromal Cells and their Extracellular Vesicle Progeny Decrease Injury in Poststenotic Swine Kidney Through Different Mechanisms. Stem Cells Dev. 2020;29:1190–1200. doi: 10.1089/scd.2020.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hu B, Zhang S, Liu W, Wang P, Chen S, Lv X, Shi D, Ma K, Wang B, Wu Y, Shao Z. Inhibiting Heat Shock Protein 90 Protects Nucleus Pulposus-Derived Stem/Progenitor Cells From Compression-Induced Necroptosis and Apoptosis. Front Cell Dev Biol. 2020;8:685. doi: 10.3389/fcell.2020.00685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yan B, Zhang Y, Liang C, Liu B, Ding F, Wang Y, Zhu B, Zhao R, Yu XY, Li Y. Stem cell-derived exosomes prevent pyroptosis and repair ischemic muscle injury through a novel exosome/circHIPK3/ FOXO3a pathway. Theranostics. 2020;10:6728–6742. doi: 10.7150/thno.42259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tavakoli Dargani Z, Singla DK. Embryonic stem cell-derived exosomes inhibit doxorubicin-induced TLR4-NLRP3-mediated cell death-pyroptosis. Am J Physiol Heart Circ Physiol. 2019;317:H460–H471. doi: 10.1152/ajpheart.00056.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cozzi A, Orellana DI, Santambrogio P, Rubio A, Cancellieri C, Giannelli S, Ripamonti M, Taverna S, Di Lullo G, Rovida E, Ferrari M, Forni GL, Fiorillo C, Broccoli V, Levi S. Stem Cell Modeling of Neuroferritinopathy Reveals Iron as a Determinant of Senescence and Ferroptosis during Neuronal Aging. Stem Cell Reports. 2019;13:832–846. doi: 10.1016/j.stemcr.2019.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yang ZY, Zhou L, Meng Q, Shi H, Li YH. An appropriate level of autophagy reduces emulsified isoflurane-induced apoptosis in fetal neural stem cells. Neural Regen Res. 2020;15:2278–2285. doi: 10.4103/1673-5374.285004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schiroli G, Conti A, Ferrari S, Della Volpe L, Jacob A, Albano L, Beretta S, Calabria A, Vavassori V, Gasparini P, Salataj E, Ndiaye-Lobry D, Brombin C, Chaumeil J, Montini E, Merelli I, Genovese P, Naldini L, Di Micco R. Precise Gene Editing Preserves Hematopoietic Stem Cell Function following Transient p53-Mediated DNA Damage Response. Cell Stem Cell 2019; 24: 551-565. :e8. doi: 10.1016/j.stem.2019.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Feng L, Hollstein M, Xu Y. Ser46 phosphorylation regulates p53-dependent apoptosis and replicative senescence. Cell Cycle. 2006;5:2812–2819. doi: 10.4161/cc.5.23.3526. [DOI] [PubMed] [Google Scholar]
- 138.Menendez D, Inga A, Resnick MA. The expanding universe of p53 targets. Nat Rev Cancer. 2009;9:724–737. doi: 10.1038/nrc2730. [DOI] [PubMed] [Google Scholar]
- 139.Blanpain C, Mohrin M, Sotiropoulou PA, Passegué E. DNA-damage response in tissue-specific and cancer stem cells. Cell Stem Cell. 2011;8:16–29. doi: 10.1016/j.stem.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 140.Leibowitz BJ, Yang L, Wei L, Buchanan ME, Rachid M, Parise RA, Beumer JH, Eiseman JL, Schoen RE, Zhang L, Yu J. Targeting p53-dependent stem cell loss for intestinal chemoprotection. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aam7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- 142.Pietsch EC, Perchiniak E, Canutescu AA, Wang G, Dunbrack RL, Murphy ME. Oligomerization of BAK by p53 utilizes conserved residues of the p53 DNA binding domain. J Biol Chem. 2008;283:21294–21304. doi: 10.1074/jbc.M710539200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang ZN, Chung SK, Xu Z, Xu Y. Oct4 maintains the pluripotency of human embryonic stem cells by inactivating p53 through Sirt1-mediated deacetylation. Stem Cells. 2014;32:157–165. doi: 10.1002/stem.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xu C, Fan CD, Wang X. Regulation of Mdm2 protein stability and the p53 response by NEDD4-1 E3 ligase. Oncogene. 2015;34:281–289. doi: 10.1038/onc.2013.557. [DOI] [PubMed] [Google Scholar]
- 145.TeSlaa T, Setoguchi K, Teitell MA. Mitochondria in human pluripotent stem cell apoptosis. Semin Cell Dev Biol. 2016;52:76–83. doi: 10.1016/j.semcdb.2016.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zaltsman Y, Shachnai L, Yivgi-Ohana N, Schwarz M, Maryanovich M, Houtkooper RH, Vaz FM, De Leonardis F, Fiermonte G, Palmieri F, Gillissen B, Daniel PT, Jimenez E, Walsh S, Koehler CM, Roy SS, Walter L, Hajnóczky G, Gross A. MTCH2/MIMP is a major facilitator of tBID recruitment to mitochondria. Nat Cell Biol. 2010;12:553–562. doi: 10.1038/ncb2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Morales-Cruz M, Figueroa CM, González-Robles T, Delgado Y, Molina A, Méndez J, Morales M, Griebenow K. Activation of caspase-dependent apoptosis by intracellular delivery of Cytochrome c-based nanoparticles. J Nanobiotechnology. 2014;12:33. doi: 10.1186/s12951-014-0033-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bratton SB, Salvesen GS. Regulation of the Apaf-1-caspase-9 apoptosome. J Cell Sci. 2010;123:3209–3214. doi: 10.1242/jcs.073643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell. 2011;21:92–101. doi: 10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wu X, Hu X, Zhang Q, Liu F, Xiong K. Regulatory Role Of Chinese Herbal Medicine In Regulated Neuronal Death. CNS Neurol Disord Drug Targets. 2020 doi: 10.2174/1871527319666200730165011. [DOI] [PubMed] [Google Scholar]
- 151.Broxmeyer HE, Williams DE, Lu L, Cooper S, Anderson SL, Beyer GS, Hoffman R, Rubin BY. The suppressive influences of human tumor necrosis factors on bone marrow hematopoietic progenitor cells from normal donors and patients with leukemia: synergism of tumor necrosis factor and interferon-gamma. J Immunol. 1986;136:4487–4495. [PubMed] [Google Scholar]
- 152.Caux C, Saeland S, Favre C, Duvert V, Mannoni P, Banchereau J. Tumor necrosis factor-alpha strongly potentiates interleukin-3 and granulocyte-macrophage colony-stimulating factor-induced proliferation of human CD34+ hematopoietic progenitor cells. Blood. 1990;75:2292–2298. [PubMed] [Google Scholar]
- 153.Yamashita M, Passegué E. TNF-α Coordinates Hematopoietic Stem Cell Survival and Myeloid Regeneration. Cell Stem Cell 2019; 25: 357-372. :e7. doi: 10.1016/j.stem.2019.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ishida T, Suzuki S, Lai CY, Yamazaki S, Kakuta S, Iwakura Y, Nojima M, Takeuchi Y, Higashihara M, Nakauchi H, Otsu M. Pre-Transplantation Blockade of TNF-α-Mediated Oxygen Species Accumulation Protects Hematopoietic Stem Cells. Stem Cells. 2017;35:989–1002. doi: 10.1002/stem.2524. [DOI] [PubMed] [Google Scholar]
- 155.Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11:621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 156.Salvesen GS, Walsh CM. Functions of caspase 8: the identified and the mysterious. Semin Immunol. 2014;26:246–252. doi: 10.1016/j.smim.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fernando P, Brunette S, Megeney LA. Neural stem cell differentiation is dependent upon endogenous caspase 3 activity. FASEB J. 2005;19:1671–1673. doi: 10.1096/fj.04-2981fje. [DOI] [PubMed] [Google Scholar]
- 158.Delbridge AR, Grabow S, Strasser A, Vaux DL. Thirty years of BCL-2: translating cell death discoveries into novel cancer therapies. Nat Rev Cancer. 2016;16:99–109. doi: 10.1038/nrc.2015.17. [DOI] [PubMed] [Google Scholar]
- 159.Capper D, Gaiser T, Hartmann C, Habel A, Mueller W, Herold-Mende C, von Deimling A, Siegelin MD. Stem-cell-like glioma cells are resistant to TRAIL/Apo2L and exhibit down-regulation of caspase-8 by promoter methylation. Acta Neuropathol. 2009;117:445–456. doi: 10.1007/s00401-009-0494-3. [DOI] [PubMed] [Google Scholar]
- 160.Jia L, Gu W, Zhang Y, Jiang B, Qiao X, Wen Y. Activated Yes-Associated Protein Accelerates Cell Cycle, Inhibits Apoptosis, and Delays Senescence in Human Periodontal Ligament Stem Cells. Int J Med Sci. 2018;15:1241–1250. doi: 10.7150/ijms.25115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lichý M, Szobi A, Hrdlička J, Horváth C, Kormanová V, Rajtík T, Neckář J, Kolář F, Adameová A. Different signalling in infarcted and non-infarcted areas of rat failing hearts: A role of necroptosis and inflammation. J Cell Mol Med. 2019;23:6429–6441. doi: 10.1111/jcmm.14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tang Q, Li W, Dai N, Gao Y, Han Y, Cheng G, Gu C. The Role of Necroptosis, Apoptosis, and Inflammation in Fowl Cholera-Associated Liver Injury in a Chicken Model. Avian Dis. 2017;61:491–502. doi: 10.1637/11732-073017-Reg.1. [DOI] [PubMed] [Google Scholar]
- 163.Ni X, Ou C, Guo J, Liu B, Zhang J, Wu Z, Li H, Chen M. Lentiviral vector-mediated co-overexpression of VEGF and Bcl-2 improves mesenchymal stem cell survival and enhances paracrine effects in vitro. Int J Mol Med. 2017;40:418–426. doi: 10.3892/ijmm.2017.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Schratt G, Philippar U, Hockemeyer D, Schwarz H, Alberti S, Nordheim A. SRF regulates Bcl-2 expression and promotes cell survival during murine embryonic development. EMBO J. 2004;23:1834–1844. doi: 10.1038/sj.emboj.7600188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Liu W, Yue W, Wu R. Overexpression of Bcl-2 promotes survival and differentiation of neuroepithelial stem cells after transplantation into rat aganglionic colon. Stem Cell Res Ther. 2013;4:7. doi: 10.1186/scrt155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lagadinou ED, Sach A, Callahan K, Rossi RM, Neering SJ, Minhajuddin M, Ashton JM, Pei S, Grose V, O'Dwyer KM, Liesveld JL, Brookes PS, Becker MW, Jordan CT. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell. 2013;12:329–341. doi: 10.1016/j.stem.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sun Q, Wang Y, Desgrosellier JS. Combined Bcl-2/Src inhibition synergize to deplete stem-like breast cancer cells. Cancer Lett. 2019;457:40–46. doi: 10.1016/j.canlet.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 168.Dumitru R, Gama V, Fagan BM, Bower JJ, Swahari V, Pevny LH, Deshmukh M. Human embryonic stem cells have constitutively active Bax at the Golgi and are primed to undergo rapid apoptosis. Mol Cell. 2012;46:573–583. doi: 10.1016/j.molcel.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Czabotar PE, Westphal D, Dewson G, Ma S, Hockings C, Fairlie WD, Lee EF, Yao S, Robin AY, Smith BJ, Huang DC, Kluck RM, Adams JM, Colman PM. Bax crystal structures reveal how BH3 domains activate Bax and nucleate its oligomerization to induce apoptosis. Cell. 2013;152:519–531. doi: 10.1016/j.cell.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 170.García-Prat L, Sousa-Victor P, Muñoz-Cánoves P. Proteostatic and Metabolic Control of Stemness. Cell Stem Cell. 2017;20:593–608. doi: 10.1016/j.stem.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 171.Esteban-Martínez L, Sierra-Filardi E, Boya P. Mitophagy, metabolism, and cell fate. Mol Cell Oncol. 2017;4:e1353854. doi: 10.1080/23723556.2017.1353854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gu H, Shi X, Liu C, Wang C, Sui N, Zhao Y, Gong J, Wang F, Zhang H, Li W, Zhao T. USP8 maintains embryonic stem cell stemness via deubiquitination of EPG5. Nat Commun. 2019;10:1465. doi: 10.1038/s41467-019-09430-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Komorowska K, Doyle A, Wahlestedt M, Subramaniam A, Debnath S, Chen J, Soneji S, Van Handel B, Mikkola HKA, Miharada K, Bryder D, Larsson J, Magnusson M. Hepatic Leukemia Factor Maintains Quiescence of Hematopoietic Stem Cells and Protects the Stem Cell Pool during Regeneration. Cell Rep. 2017;21:3514–3523. doi: 10.1016/j.celrep.2017.11.084. [DOI] [PubMed] [Google Scholar]
- 174.He Q, Wang L, Zhao R, Yan F, Sha S, Cui C, Song J, Hu H, Guo X, Yang M, Cui Y, Sun Y, Sun Z, Liu F, Dong M, Hou X, Chen L. Mesenchymal stem cell-derived exosomes exert ameliorative effects in type 2 diabetes by improving hepatic glucose and lipid metabolism via enhancing autophagy. Stem Cell Res Ther. 2020;11:223. doi: 10.1186/s13287-020-01731-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 175.Shyh-Chang N, Ng HH. The metabolic programming of stem cells. Genes Dev. 2017;31:336–346. doi: 10.1101/gad.293167.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.García-Prat L, Martínez-Vicente M, Perdiguero E, Ortet L, Rodríguez-Ubreva J, Rebollo E, Ruiz-Bonilla V, Gutarra S, Ballestar E, Serrano AL, Sandri M, Muñoz-Cánoves P. Autophagy maintains stemness by preventing senescence. Nature. 2016;529:37–42. doi: 10.1038/nature16187. [DOI] [PubMed] [Google Scholar]
- 177.Boonsanay V, Zhang T, Georgieva A, Kostin S, Qi H, Yuan X, Zhou Y, Braun T. Regulation of Skeletal Muscle Stem Cell Quiescence by Suv4-20h1-Dependent Facultative Heterochromatin Formation. Cell Stem Cell. 2016;18:229–242. doi: 10.1016/j.stem.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 178.Cho IJ, Lui PP, Obajdin J, Riccio F, Stroukov W, Willis TL, Spagnoli F, Watt FM. Mechanisms, Hallmarks, and Implications of Stem Cell Quiescence. Stem Cell Reports. 2019;12:1190–1200. doi: 10.1016/j.stemcr.2019.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Li G, Tang X, Zhang S, Jin M, Wang M, Deng Z, Liu Z, Qian M, Shi W, Wang Z, Xie H, Li J, Liu B. SIRT7 activates quiescent hair follicle stem cells to ensure hair growth in mice. EMBO J. 2020;39:e104365. doi: 10.15252/embj.2019104365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Aymard E, Barruche V, Naves T, Bordes S, Closs B, Verdier M, Ratinaud MH. Autophagy in human keratinocytes: an early step of the differentiation? Exp Dermatol. 2011;20:263–268. doi: 10.1111/j.1600-0625.2010.01157.x. [DOI] [PubMed] [Google Scholar]
- 181.Ka M, Condorelli G, Woodgett JR, Kim WY. mTOR regulates brain morphogenesis by mediating GSK3 signaling. Development. 2014;141:4076–4086. doi: 10.1242/dev.108282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Warr MR, Binnewies M, Flach J, Reynaud D, Garg T, Malhotra R, Debnath J, Passegué E. FOXO3A directs a protective autophagy program in haematopoietic stem cells. Nature. 2013;494:323–327. doi: 10.1038/nature11895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gong J, Gu H, Zhao L, Wang L, Liu P, Wang F, Xu H, Zhao T. Phosphorylation of ULK1 by AMPK is essential for mouse embryonic stem cell self-renewal and pluripotency. Cell Death Dis. 2018;9:38. doi: 10.1038/s41419-017-0054-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhang Z, Yang M, Wang Y, Wang L, Jin Z, Ding L, Zhang L, Jiang W, Gao G, Yang J, Lu B, Cao F, Hu T. Autophagy regulates the apoptosis of bone marrow-derived mesenchymal stem cells under hypoxic condition via AMP-activated protein kinase/mammalian target of rapamycin pathway. Cell Biol Int. 2016;40:671–685. doi: 10.1002/cbin.10604. [DOI] [PubMed] [Google Scholar]
- 185.Jang JE, Eom JI, Jeung HK, Cheong JW, Lee JY, Kim JS, Min YH. Targeting AMPK-ULK1-mediated autophagy for combating BET inhibitor resistance in acute myeloid leukemia stem cells. Autophagy. 2017;13:761–762. doi: 10.1080/15548627.2016.1278328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 187.Chen T, Shen L, Yu J, Wan H, Guo A, Chen J, Long Y, Zhao J, Pei G. Rapamycin and other longevity-promoting compounds enhance the generation of mouse induced pluripotent stem cells. Aging Cell. 2011;10:908–911. doi: 10.1111/j.1474-9726.2011.00722.x. [DOI] [PubMed] [Google Scholar]
- 188.Wible DJ, Chao HP, Tang DG, Bratton SB. ATG5 cancer mutations and alternative mRNA splicing reveal a conjugation switch that regulates ATG12-ATG5-ATG16L1 complex assembly and autophagy. Cell Discov. 2019;5:42. doi: 10.1038/s41421-019-0110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Takahashi T, Shimizu K, Shimazaki K, Toda H, Nibuya M. Environmental enrichment enhances autophagy signaling in the rat hippocampus. Brain Res. 2014;1592:113–123. doi: 10.1016/j.brainres.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 190.He J, Kang L, Wu T, Zhang J, Wang H, Gao H, Zhang Y, Huang B, Liu W, Kou Z, Zhang H, Gao S. An elaborate regulation of Mammalian target of rapamycin activity is required for somatic cell reprogramming induced by defined transcription factors. Stem Cells Dev. 2012;21:2630–2641. doi: 10.1089/scd.2012.0015. [DOI] [PubMed] [Google Scholar]
- 191.Wang S, Xia P, Ye B, Huang G, Liu J, Fan Z. Transient activation of autophagy via Sox2-mediated suppression of mTOR is an important early step in reprogramming to pluripotency. Cell Stem Cell. 2013;13:617–625. doi: 10.1016/j.stem.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 192.Yazdankhah M, Farioli-Vecchioli S, Tonchev AB, Stoykova A, Cecconi F. The autophagy regulators Ambra1 and Beclin 1 are required for adult neurogenesis in the brain subventricular zone. Cell Death Dis. 2014;5:e1403. doi: 10.1038/cddis.2014.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Coelho BP, Fernandes CFL, Boccacino JM, Souza MCDS, Melo-Escobar MI, Alves RN, Prado MB, Iglesia RP, Cangiano G, Mazzaro GR, Lopes MH. Multifaceted WNT Signaling at the Crossroads Between Epithelial-Mesenchymal Transition and Autophagy in Glioblastoma. Front Oncol. 2020;10:597743. doi: 10.3389/fonc.2020.597743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Dou J, Su P, Xu C, Wen Z, Mao Z, Li W. Targeting Hsc70-based autophagy to eliminate amyloid β oligomers. Biochem Biophys Res Commun. 2020;524:923–928. doi: 10.1016/j.bbrc.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kim DH, Jang JH, Kwon OS, Cha HJ, Youn HJ, Chun KS, Surh YJ. Nuclear Factor Erythroid-Derived 2-Like 2-Induced Reductive Stress Favors Self-Renewal of Breast Cancer Stem-Like Cells via the FoxO3a-Bmi-1 Axis. Antioxid Redox Signal. 2020;32:1313–1329. doi: 10.1089/ars.2019.7730. [DOI] [PubMed] [Google Scholar]
- 196.Mercurio S, Serra L, Nicolis SK. More than just Stem Cells: Functional Roles of the Transcription Factor Sox2 in Differentiated Glia and Neurons. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20184540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Karagiannis P, Takahashi K, Saito M, Yoshida Y, Okita K, Watanabe A, Inoue H, Yamashita JK, Todani M, Nakagawa M, Osawa M, Yashiro Y, Yamanaka S, Osafune K. Induced Pluripotent Stem Cells and Their Use in Human Models of Disease and Development. Physiol Rev. 2019;99:79–114. doi: 10.1152/physrev.00039.2017. [DOI] [PubMed] [Google Scholar]
- 198.Leitch HA, Fibach E, Rachmilewitz E. Toxicity of iron overload and iron overload reduction in the setting of hematopoietic stem cell transplantation for hematologic malignancies. Crit Rev Oncol Hematol. 2017;113:156–170. doi: 10.1016/j.critrevonc.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 199.Huang Y, Wang S, Huang F, Zhang Q, Qin B, Liao L, Wang M, Wan H, Yan W, Chen D, Liu F, Jiang B, Ji D, Xia X, Huang J, Xiong K. c-FLIP regulates pyroptosis in retinal neurons following oxygen-glucose deprivation/recovery via a GSDMD-mediated pathway. Ann Anat. 2021;235:151672. doi: 10.1016/j.aanat.2020.151672. [DOI] [PubMed] [Google Scholar]
- 200.Chen Y, Li Y, Guo L, Hong J, Zhao W, Hu X, Chang C, Liu W, Xiong K. Bibliometric Analysis of the Inflammasome and Pyroptosis in Brain. Front Pharmacol. 2020;11:626502. doi: 10.3389/fphar.2020.626502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yan WT, Lu S, Yang YD, Ning WY, Cai Y, Hu XM, Zhang Q, Xiong K. Research trends, hot spots and prospects for necroptosis in the field of neuroscience. Neural Regen Res. 2021;16:1628–1637. doi: 10.4103/1673-5374.303032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wang M, Wan H, Wang S, Liao L, Huang Y, Guo L, Liu F, Shang L, Huang J, Ji D, Xia X, Jiang B, Chen D, Xiong K. RSK3 mediates necroptosis by regulating phosphorylation of RIP3 in rat retinal ganglion cells. J Anat. 2020;237:29–47. doi: 10.1111/joa.13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Guo LM, Wang Z, Li SP, Wang M, Yan WT, Liu FX, Wang CD, Zhang XD, Chen D, Yan J, Xiong K. RIP3/MLKL-mediated neuronal necroptosis induced by methamphetamine at 39°C. Neural Regen Res. 2020;15:865–874. doi: 10.4103/1673-5374.268902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Karki R, Sharma BR, Tuladhar S, Williams EP, Zalduondo L, Samir P, Zheng M, Sundaram B, Banoth B, Malireddi RKS, Schreiner P, Neale G, Vogel P, Webby R, Jonsson CB, Kanneganti TD. Synergism of TNF-α and IFN-γ Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell 2021; 184: 149-168. :e17. doi: 10.1016/j.cell.2020.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Nobuta H, Yang N, Ng YH, Marro SG, Sabeur K, Chavali M, Stockley JH, Killilea DW, Walter PB, Zhao C, Huie P Jr, Goldman SA, Kriegstein AR, Franklin RJM, Rowitch DH, Wernig M. Oligodendrocyte Death in Pelizaeus-Merzbacher Disease Is Rescued by Iron Chelation. Cell Stem Cell 2019; 25: 531-541. :e6. doi: 10.1016/j.stem.2019.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Orellana DI, Santambrogio P, Rubio A, Yekhlef L, Cancellieri C, Dusi S, Giannelli SG, Venco P, Mazzara PG, Cozzi A, Ferrari M, Garavaglia B, Taverna S, Tiranti V, Broccoli V, Levi S. Coenzyme A corrects pathological defects in human neurons of PANK2-associated neurodegeneration. EMBO Mol Med. 2016;8:1197–1211. doi: 10.15252/emmm.201606391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Qin B, Zhang Q, Chen D, Yu HY, Luo AX, Suo LP, Cai Y, Cai DY, Luo J, Huang JF, Xiong K. Extracellular vesicles derived from mesenchymal stem cells: A platform that can be engineered. Histol Histopathol. 2021:18297. doi: 10.14670/HH-18-297. [DOI] [PubMed] [Google Scholar]
- 208.Qin B, Zhang Q, Hu XM, Mi TY, Yu HY, Liu SS, Zhang B, Tang M, Huang JF, Xiong K. How does temperature play a role in the storage of extracellular vesicles? J Cell Physiol. 2020;235:7663–7680. doi: 10.1002/jcp.29700. [DOI] [PubMed] [Google Scholar]
- 209.Mao Q, Liang XL, Zhang CL, Pang YH, Lu YX. LncRNA KLF3-AS1 in human mesenchymal stem cell-derived exosomes ameliorates pyroptosis of cardiomyocytes and myocardial infarction through miR-138-5p/Sirt1 axis. Stem Cell Res Ther. 2019;10:393. doi: 10.1186/s13287-019-1522-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Yadav M, Kumari P, Yadav V, Kumar S. Pharmacological preconditioning with phosphodiestrase inhibitor: an answer to stem cell survival against ischemic injury through JAK/STAT signaling. Heart Fail Rev. 2020;25:355–366. doi: 10.1007/s10741-019-09822-0. [DOI] [PubMed] [Google Scholar]
- 211.Doeppner TR, Doehring M, Kaltwasser B, Majid A, Lin F, Bähr M, Kilic E, Hermann DM. Ischemic Post-Conditioning Induces Post-Stroke Neuroprotection via Hsp70-Mediated Proteasome Inhibition and Facilitates Neural Progenitor Cell Transplantation. Mol Neurobiol. 2017;54:6061–6073. doi: 10.1007/s12035-016-0137-3. [DOI] [PubMed] [Google Scholar]
- 212.Jiang B, Xiao W, Shi Y, Liu M, Xiao X. Heat shock pretreatment inhibited the release of Smac/DIABLO from mitochondria and apoptosis induced by hydrogen peroxide in cardiomyocytes and C2C12 myogenic cells. Cell Stress Chaperones. 2005;10:252–262. doi: 10.1379/CSC-124R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Meng SS, Xu XP, Chang W, Lu ZH, Huang LL, Xu JY, Liu L, Qiu HB, Yang Y, Guo FM. LincRNA-p21 promotes mesenchymal stem cell migration capacity and survival through hypoxic preconditioning. Stem Cell Res Ther. 2018;9:280. doi: 10.1186/s13287-018-1031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Hou J, Wang L, Long H, Wu H, Wu Q, Zhong T, Chen X, Zhou C, Guo T, Wang T. Hypoxia preconditioning promotes cardiac stem cell survival and cardiogenic differentiation in vitro involving activation of the HIF-1α/apelin/APJ axis. Stem Cell Res Ther. 2017;8:215. doi: 10.1186/s13287-017-0673-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Abati E, Bresolin N, Comi GP, Corti S. Preconditioning and Cellular Engineering to Increase the Survival of Transplanted Neural Stem Cells for Motor Neuron Disease Therapy. Mol Neurobiol. 2019;56:3356–3367. doi: 10.1007/s12035-018-1305-4. [DOI] [PubMed] [Google Scholar]
- 216.Guo L, Du J, Yuan DF, Zhang Y, Zhang S, Zhang HC, Mi JW, Ning YL, Chen MJ, Wen DL, Sun JH, Liu D, Zeng L, Zhang A, Jiang J, Huang H. Optimal H2O2 preconditioning to improve bone marrow mesenchymal stem cells' engraftment in wound healing. Stem Cell Res Ther. 2020;11:434. doi: 10.1186/s13287-020-01910-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Teng L, Bennett E, Cai C. Preconditioning c-Kit-positive Human Cardiac Stem Cells with a Nitric Oxide Donor Enhances Cell Survival through Activation of Survival Signaling Pathways. J Biol Chem. 2016;291:9733–9747. doi: 10.1074/jbc.M115.687806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Gao F, Hu X, Xie X, Liu X, Wang J. Correction to: Heat Shock Protein 90 Stimulates Rat Mesenchymal Stem Cell Migration via PI3K/Akt and ERK1/2 Pathways. Cell Biochem Biophys. 2019;77:273. doi: 10.1007/s12013-019-00878-1. [DOI] [PubMed] [Google Scholar]
- 219.Noiseux N, Borie M, Desnoyers A, Menaouar A, Stevens LM, Mansour S, Danalache BA, Roy DC, Jankowski M, Gutkowska J. Preconditioning of stem cells by oxytocin to improve their therapeutic potential. Endocrinology. 2012;153:5361–5372. doi: 10.1210/en.2012-1402. [DOI] [PubMed] [Google Scholar]
- 220.Othman FA, Tan SC. Preconditioning Strategies to Enhance Neural Stem Cell-Based Therapy for Ischemic Stroke. Brain Sci. 2020;10 doi: 10.3390/brainsci10110893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sakata H, Niizuma K, Yoshioka H, Kim GS, Jung JE, Katsu M, Narasimhan P, Maier CM, Nishiyama Y, Chan PH. Minocycline-preconditioned neural stem cells enhance neuroprotection after ischemic stroke in rats. J Neurosci. 2012;32:3462–3473. doi: 10.1523/JNEUROSCI.5686-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zhao L, Hu C, Zhang P, Jiang H, Chen J. Melatonin preconditioning is an effective strategy for mesenchymal stem cell-based therapy for kidney disease. J Cell Mol Med. 2020;24:25–33. doi: 10.1111/jcmm.14769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Yin K, Zhu R, Wang S, Zhao RC. Low-Level Laser Effect on Proliferation, Migration, and Antiapoptosis of Mesenchymal Stem Cells. Stem Cells Dev. 2017;26:762–775. doi: 10.1089/scd.2016.0332. [DOI] [PubMed] [Google Scholar]
- 224.Dompe C, Moncrieff L, Matys J, Grzech-Leśniak K, Kocherova I, Bryja A, Bruska M, Dominiak M, Mozdziak P, Skiba THI, Shibli JA, Angelova Volponi A, Kempisty B, Dyszkiewicz-Konwińska M. Photobiomodulation-Underlying Mechanism and Clinical Applications. J Clin Med. 2020;9 doi: 10.3390/jcm9061724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Korshunova I, Rhein S, García-González D, Stölting I, Pfisterer U, Barta A, Dmytriyeva O, Kirkeby A, Schwaninger M, Khodosevich K. Genetic modification increases the survival and the neuroregenerative properties of transplanted neural stem cells. JCI Insight. 2020;5 doi: 10.1172/jci.insight.126268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Liang X, Ding Y, Zhang Y, Chai YH, He J, Chiu SM, Gao F, Tse HF, Lian Q. Activation of NRG1-ERBB4 signaling potentiates mesenchymal stem cell-mediated myocardial repairs following myocardial infarction. Cell Death Dis. 2015;6:e1765. doi: 10.1038/cddis.2015.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ma Y, Lin M, Huang G, Li Y, Wang S, Bai G, Lu TJ, Xu F. 3D Spatiotemporal Mechanical Microenvironment: A Hydrogel-Based Platform for Guiding Stem Cell Fate. Adv Mater. 2018;30:e1705911. doi: 10.1002/adma.201705911. [DOI] [PubMed] [Google Scholar]
- 228.Arjmand B, Goodarzi P, Aghayan HR, Payab M, Rahim F, Alavi-Moghadam S, Mohamadi-Jahani F, Larijani B. Co-transplantation of Human Fetal Mesenchymal and Hematopoietic Stem Cells in Type 1 Diabetic Mice Model. Front Endocrinol (Lausanne) 2019;10:761. doi: 10.3389/fendo.2019.00761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Das B, Bayat-Mokhtari R, Tsui M, Lotfi S, Tsuchida R, Felsher DW, Yeger H. HIF-2α suppresses p53 to enhance the stemness and regenerative potential of human embryonic stem cells. Stem Cells. 2012;30:1685–1695. doi: 10.1002/stem.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Qin HH, Filippi C, Sun S, Lehec S, Dhawan A, Hughes RD. Hypoxic preconditioning potentiates the trophic effects of mesenchymal stem cells on co-cultured human primary hepatocytes. Stem Cell Res Ther. 2015;6:237. doi: 10.1186/s13287-015-0218-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Huang Y, Tan F, Zhuo Y, Liu J, He J, Duan D, Lu M, Hu Z. Hypoxia-preconditioned olfactory mucosa mesenchymal stem cells abolish cerebral ischemia/reperfusion-induced pyroptosis and apoptotic death of microglial cells by activating HIF-1α. Aging (Albany NY) 2020;12:10931–10950. doi: 10.18632/aging.103307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Liu W, Li L, Rong Y, Qian D, Chen J, Zhou Z, Luo Y, Jiang D, Cheng L, Zhao S, Kong F, Wang J, Xu T, Gong F, Huang Y, Gu C, Zhao X, Bai J, Wang F, Zhao W, Zhang L, Li X, Yin G, Fan J, Cai W. Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126. Acta Biomater. 2020;103:196–212. doi: 10.1016/j.actbio.2019.12.020. [DOI] [PubMed] [Google Scholar]
- 233.Chu X, Liu D, Li T, Ke H, Xin D, Wang S, Cao Y, Xue H, Wang Z. Hydrogen sulfide-modified extracellular vesicles from mesenchymal stem cells for treatment of hypoxic-ischemic brain injury. J Control Release. 2020;328:13–27. doi: 10.1016/j.jconrel.2020.08.037. [DOI] [PubMed] [Google Scholar]
- 234.Kondo-Nakamura M, Shintani-Ishida K, Uemura K, Yoshida K. Brief exposure to carbon monoxide preconditions cardiomyogenic cells against apoptosis in ischemia-reperfusion. Biochem Biophys Res Commun. 2010;393:449–454. doi: 10.1016/j.bbrc.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 235.Park JH, Lee NK, Lim HJ, Mazumder S, Kumar Rethineswaran V, Kim YJ, Jang WB, Ji ST, Kang S, Kim DY, Van LTH, Giang LTT, Kim DH, Ha JS, Yun J, Kim H, Han J, Mishchenko NP, Fedoreyev SA, Vasileva EA, Kwon SM, Baek SH. Therapeutic Cell Protective Role of Histochrome under Oxidative Stress in Human Cardiac Progenitor Cells. Mar Drugs. 2019;17 doi: 10.3390/md17060368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Carrero R, Cerrada I, Lledó E, Dopazo J, García-García F, Rubio MP, Trigueros C, Dorronsoro A, Ruiz-Sauri A, Montero JA, Sepúlveda P. IL1β induces mesenchymal stem cells migration and leucocyte chemotaxis through NF-κB. Stem Cell Rev Rep. 2012;8:905–916. doi: 10.1007/s12015-012-9364-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Seto SW, Chang D, Jenkins A, Bensoussan A, Kiat H. Angiogenesis in Ischemic Stroke and Angiogenic Effects of Chinese Herbal Medicine. J Clin Med. 2016;5 doi: 10.3390/jcm5060056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Jung JE, Kim GS, Chan PH. Neuroprotection by interleukin-6 is mediated by signal transducer and activator of transcription 3 and antioxidative signaling in ischemic stroke. Stroke. 2011;42:3574–3579. doi: 10.1161/STROKEAHA.111.626648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Don CW, Murry CE. Improving survival and efficacy of pluripotent stem cell-derived cardiac grafts. J Cell Mol Med. 2013;17:1355–1362. doi: 10.1111/jcmm.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Urnukhsaikhan E, Cho H, Mishig-Ochir T, Seo YK, Park JK. Pulsed electromagnetic fields promote survival and neuronal differentiation of human BM-MSCs. Life Sci. 2016;151:130–138. doi: 10.1016/j.lfs.2016.02.066. [DOI] [PubMed] [Google Scholar]
- 241.Zhu Z, Gan X, Fan H, Yu H. Mechanical stretch endows mesenchymal stem cells stronger angiogenic and anti-apoptotic capacities via NFκB activation. Biochem Biophys Res Commun. 2015;468:601–605. doi: 10.1016/j.bbrc.2015.10.157. [DOI] [PubMed] [Google Scholar]
- 242.Hafizi M, Hajarizadeh A, Atashi A, Kalanaky S, Fakharzadeh S, Masoumi Z, Nazaran MH, Soleimani M. Nanochelating based nanocomplex, GFc7, improves quality and quantity of human mesenchymal stem cells during in vitro expansion. Stem Cell Res Ther. 2015;6:226. doi: 10.1186/s13287-015-0216-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Hafizi M, Kalanaky S, Fakharzadeh S, Janzamin E, Arjmandi T, Atashi A, Nazaran MH. GFc7 as a Smart Growth Nanofactor for ex vivo Expansion and Cryoprotection of Humans' Hematopoietic Stem Cells. Int J Nanomedicine. 2020;15:6263–6277. doi: 10.2147/IJN.S256104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kaufmann KB, Büning H, Galy A, Schambach A, Grez M. Gene therapy on the move. EMBO Mol Med. 2013;5:1642–1661. doi: 10.1002/emmm.201202287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Choi KA, Choi Y, Hong S. Stem cell transplantation for Huntington's diseases. Methods. 2018;133:104–112. doi: 10.1016/j.ymeth.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 246.Navarro Quiroz E, Navarro Quiroz R, Ahmad M, Gomez Escorcia L, Villarreal JL, Fernandez Ponce C, Aroca Martinez G. Cell Signaling in Neuronal Stem Cells. Cells. 2018;7 doi: 10.3390/cells7070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Hsu MN, Chang YH, Truong VA, Lai PL, Nguyen TKN, Hu YC. CRISPR technologies for stem cell engineering and regenerative medicine. Biotechnol Adv. 2019;37:107447. doi: 10.1016/j.biotechadv.2019.107447. [DOI] [PubMed] [Google Scholar]
- 248.Kim H, Bae C, Kook YM, Koh WG, Lee K, Park MH. Mesenchymal stem cell 3D encapsulation technologies for biomimetic microenvironment in tissue regeneration. Stem Cell Res Ther. 2019;10:51. doi: 10.1186/s13287-018-1130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Zhang L, Li B, Liu B, Dong Z. Co-transplantation of Epidermal Neural Crest Stem Cells and Olfactory Ensheathing Cells Repairs Sciatic Nerve Defects in Rats. Front Cell Neurosci. 2019;13:253. doi: 10.3389/fncel.2019.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kallekleiv M, Larun L, Bruserud Ø, Hatfield KJ. Co-transplantation of multipotent mesenchymal stromal cells in allogeneic hematopoietic stem cell transplantation: A systematic review and meta-analysis. Cytotherapy. 2016;18:172–185. doi: 10.1016/j.jcyt.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 251.Naudot M, Barre A, Caula A, Sevestre H, Dakpé S, Mueller AA, Devauchelle B, Testelin S, Marolleau JP, Le Ricousse S. Co-transplantation of Wharton's jelly mesenchymal stem cell-derived osteoblasts with differentiated endothelial cells does not stimulate blood vessel and osteoid formation in nude mice models. J Tissue Eng Regen Med. 2020;14:257–271. doi: 10.1002/term.2989. [DOI] [PubMed] [Google Scholar]