Abstract
Highly sensitive nucleic acid amplification tests (NAATs) designed to detect SARS-CoV-2 RNA are the standard of care for the diagnosis of COVID-19. However, the accuracy of these methods for the quantitation of active virus rather than non-infectious RNA fragments that can persist for extended periods of time has been unclear. This issue is particularly relevant for congregate care patients who are unable to return to their home residence until fully negative by NAATs. We tested paired samples from individual patients for the presence of virus at both early and later stages of disease. Culture of nasopharyngeal swab samples for 10 days in Vero E6 cells revealed active virus in only 4 out of 14 (28.6%) patients. The ability to isolate viral plaque-forming units (PFU) correlated with viral RNA loads of >6.79 log genomic copies/ml and only occurred in samples collected from patients early after symptom onset and before development of antibody. Culture in Vero E6 cells lacking the STAT1-dependent interferon signaling pathway increased the numbers of viral PFU detected but did not affect the incidence of positive cultures. We conclude that culturable virus is correlated with SARS-CoV-2 NAATs detection only during early symptom onset and with high viral titers/low antibody titers in non-immunosuppressed patients.
Keywords: SARS-CoV-2, Sub-genomic RNA, RT-qPCR, Plaque assay, Vero STAT1−/−
1. Introduction
The ongoing global COVID-19 pandemic continues to profoundly affect individuals and communities, often overwhelming US health care institutions [1]. One particularly pressing challenge for health care institutions is to effectively isolate infected patients to minimize transmission within the hospital and the community [2,3]. As per Centers for Disease Control and Prevention (CDC) recommendations, nucleic acid amplification tests (NAATs) from nasopharyngeal (NP) and throat swabs are the gold standard for identifying active infection. Most NAATs are highly sensitive methods for detecting SARS-CoV-2 RNA in both symptomatic and asymptomatic patients [3]. However, NAATs detect all SARS-CoV-2 RNA, including genomic RNA, sub-genomic fragments and messenger RNAs produced during viral replication. Cytosolic mRNAs can be protected against nuclease-mediated decay, and thus, are not necessarily indicative of active viral replication [4]. Since NAATs do not distinguish between replicating virions versus persisting viral genomic fragments [2,5], the detection of SARS-CoV-2 using these methods could result in extended and unnecessary isolation of hospitalized patients following the infectious stage [6].
Virus culture methods combined with assays to detect viral plaque-forming units (PFU) are time-consuming and technically challenging, requiring a BSL-3 facility to culture SARS-CoV-2. However, such procedures are most appropriate for clear identification of replicating virions in human samples. Using extended culture periods as well as Vero cells lacking the STAT1-dependent interferon signaling pathway, we tested paired samples from hospitalized congregate care patients at early and later stages of disease and who remained hospitalized for extended periods of time due to NAAT positivity. Our results show that infectious virus can be isolated only in a subset of early-stage patients while all samples from later stage patients failed to show evidence of culturable virus.
2. Materials and methods
2.1. Patients and sample collection
Patients being treated at Albany Medical Center were selected for study if they presented from a congregate care facility (skilled nursing facility or rehabilitation facility, n = 14), had clinical signs and symptoms consistent with COVID-19, and had multiple PCR-positive tests separated by at least 14 days (patient demographics, Table 1 ). Many patients remained hospitalized until their SARS-CoV-2 NAAT results were negative. There were three patients (patients A1, A2, A12) with multiple visits between the hospital and nursing facility, which was counted as one hospital visit. NP swabs were placed in ~3 ml of viral transport medium and transported to the clinical lab for diagnostic testing within 4 hrs of collection.
Table 1.
NP swab samples from COVID-19 RT-qPCR positive congregate care patients.
| Patient | Gender and age | Collection day post-hospitalization | Viral gc/ml (log10) |
|---|---|---|---|
| A1 | Male, 60 years | day0 | 8.05 |
| day19 | 3.40 | ||
| A2 | Male, 55 years | day3 | 7.61 |
| day20 | 3.83 | ||
| A3 | Male, 84 years | day0 | 3.79 |
| day16 | 2.76 | ||
| A4 | Male, 81 years | day0 | 6.29 |
| day17 | 1.08 | ||
| A5 | Male, 67 years | day0 | 6.32 |
| day14 | 3.92 | ||
| A6 | Female, 65 years | day0 | 9.72 |
| day16 | 2.59 | ||
| A7 | Male, 89 years | day0 | 6.79 |
| day18 | 4.34 | ||
| A8 | Male, 65 years | day14 | 4.10 |
| day29 | 2.76 | ||
| A9 | Male, 66 years | day1 | 6.84 |
| day20 | 4.04 | ||
| A10 | Female, 75 years | day9 | 7.31 |
| day24 | 2.31 | ||
| A11 | Male, 67 years | day9 | 3.99 |
| day23 | 3.09 | ||
| A12 | Male, 79 years | day10 | 5.56 |
| day26 | 2.49 | ||
| A13 | Male, 71 years | day2 | 2.12 |
| day16 | 2.31 | ||
| A14 | Female, 92 years | day0 | 2.93 |
| day14 | 2.45 |
2.2. Diagnostic NAAT
Testing was performed in the clinical laboratories at Albany Medical Center Hospital (AMCH) using one of four commercial NAATs, three of which are RT-qPCR based: [1] CDC 2019-nCoV Real-Time RT-qPCR Diagnostic Panel on Q-cyclers [Quantabio, Beverly, MA] with easyMAG extraction [bioMerieux, Durham, NC]; [2] Abbott RealTime SARS-CoV-2 assay on the m2000; and [3] BioGX SARS-CoV-2 Reagents [Spaarks, MD] and TNA-3 Extraction Kit [GeneOhm Sciences Canada, Quebec, QC] for BD MAX™ System [Sparks, MD]. The fourth NAAT was transcription-mediated amplification (TMA)-based (Aptima™ SARS-CoV-2 assay on the Panther Fusion [Hologic, San Diego, CA]). After clinical testing at Albany Medical Center Hospital, the samples were frozen and stored at −80 °C.
Viral genomic copies/ml (gc/ml) were determined by normalization of all three RT-qPCR assays to a standard curve derived from viral RNA obtained from Western Gulf Center of Excellence for Vector-Borne Diseases (WRCEVA SARS-CoV-2 USA-WA1/2020). This latter material was measured with the CDC assay using a quantified RNA transcript containing the SCV2 nucleoprotein gene, obtained from the New York State Wadsworth Center Virology Laboratory (Supplemental Table 1). Samples initially tested by TMA were thawed for RT-qPCR testing using BioGX SARS-CoV-2 reagents to obtain a viral load. Likewise, samples selected for viral culture were thawed, kept on ice and immediately transferred to the Albany Medical College (AMC) BSL-3 facility.
2.3. Cell lines and in vitro SARS-CoV-2 infection
Vero E6 wild-type (WT) (CRL-1586™) and STAT1−/− (CCL-81VHG™) cell lines were purchased from the American Type Culture Collection (ATCC). Cell monolayers were prepared in 6-well tissue culture plates and maintained in DMEM that was supplemented with 10% heat inactivated fetal bovine serum (FBS) (Thermo-Scientific, USA), 2 mM l-glutamine, 50 U/mL penicillin, and 50 µg/mL streptomycin. Vero E6 STAT1−/− cell monolayers were cultured in the same medium that was additionally supplemented with nonessential amino acids. The cells were infected under BSL-3 conditions for 1 hr at 37°C with patient samples or as a positive control, supernatants from Vero cells that had been infected with the SARS-CoV-2, Isolate USA-WA1/2020 (provided by the Biodefense and Emerging Infections Repository). An aliquot of the cells was cultured for an additional 10 days in 2 ml of the DMEM culture medium. Culture supernatants were collected both at 1 hr (day 0) and 10 days post-infection and were tested by RT-qPCR (an off-label modification of the CDC assay, see below) and PFU assay. Vero E6 cells cultured without virus served as negative controls and showed no evidence of viral RNA or PFU.
2.4. PFU assay
Vero E6 cell monolayers were washed with DPBS and infected at 37°C with culture supernatants that were diluted 10-fold in DMEM. At 1 hr post-infection, the cell monolayers were overlaid with DMEM supplemented with 2% FBS, penicillin-streptomycin and 1% methylcellulose (4000 centipoise viscosity) (Sigma, USA). Four days later, the cells were fixed with 10% formalin for 1 hr followed by staining with 1% crystal violet. PFU numbers were counted under 10X magnification.
2.5. AMC off-label modification of the CDC RT-qPCR
Extraction of SARS-CoV-2 RNA and RT-qPCR were performed using commercially available kits and CDC recommendations. In brief, extraction of viral RNA directly from NP swabs and from cell-free culture supernatants was performed using the Qiagen QIAamp® viral RNA mini kit as per the manufacturer's instructions. RT-qPCR quantification of viral titers used a single step qPCR kit (Promega catalog #A6101) with primers designed to amplify N1 and N2 nucleic acids, and probes procured from IDT, USA. A known concentration of viral SARS-CoV-2 cDNA was used as a positive control in all experiments. This assay had an upper detection limit of approximately 10 Ct with Ct values of >40 considered negative. No nucleic acid amplification was detected in the absence of RT.
2.6. ELISA for NP antibody to SARS-CoV-2 spike protein
Nunc MaxiSorp 96-well plates were coated overnight at 4 °C with 1 μg/ml of purified recombinant SARS-CoV-2 S1/S2 spike protein (Biolegend, catalog #794206) in carbonate/bicarbonate buffer pH = 9.4. The plates were then incubated at room temperature with 1% BSA in PBS/0.05% Tween blocking solution and then with 2-fold dilutions of patient NP samples in the same buffer. A patient serum sample with a known anti-spike antibody titer was used as a positive control and a NP sample from an uninfected patient was used as a negative control. After washing of the wells with 1% BSA in PBS/0.05% Tween, bound antibody was detected using goat anti-human Ig conjugated to horseradish peroxidase (1:5000 dilution; Sigma, catalog #AP120P) followed by 3,3′,5,5′-Tetramethylbenzidine substrate (BD OptEIA™ kit, BD Biosciences, USA). After color development, titers were determined as the last dilution giving an OD value at 450 nm of at least 0.1 above the negative control.
2.7. Ethical statement
Because our study was specifically requested by a public health authority during the COVID-19 pandemic, the Albany Medical Center Institutional Review Board (IRB) deemed this research to be public health research and exempt from IRB approval.
3. Results
3.1. Patient characteristics
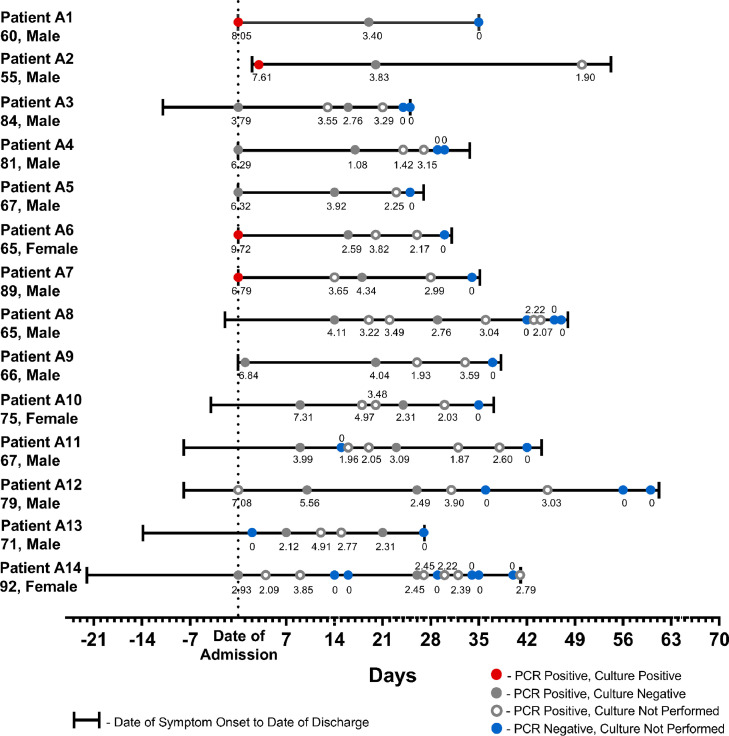
The mean age of the patients was 72.57 years (range 55 to 92), with a mean peak Sequential Organ Failure Assessment score of 5.6 (range 1 to 11). Patients were hospitalized for a mean of 37.0 days (range 25 to 60). Viral RNA was detected by PCR for a mean of 32.9 days (range 19 to 47). The median RNA viral load for the first positive NP swab collected was 5.81 log gc/mL (range 2.12 to 9.72) (Table 1). Viral load decreased after clinical symptom onset (R = −0.69, 95% CI, −0.80 to −0.54). At 26 days of symptoms, no specimens had viral loads greater than 4.00 log gc/mL (Fig. 1 ).
Fig. 1.
Clinical demographics of hospitalized congregate care COVID-19 patients with NAATs tested for SARS-CoV-2 gc/ml in NP swab samples. Symbols represent viral titers (log gc/ml) in NP swab samples quantified by NAATs. Red dots: NP swab samples with live replicating virus in Vero cell culture supernatant at dpi 10. gray solid dots; NP swabs with no live replicating viruses in Vero 10 days culture supernatant. gray open dots: NAATs positive NP swabs samples, not tested for live replicating virus by in vitro Vero cell infection. Blue dots: NAATs negative NP swab samples.
Twelve patients came from skilled nursing facilities, one patient (patient A2) came from an adult care home, and one patient (patient A1) was homeless and later transferred to a rehabilitation facility. All patients had at least one comorbidity, with hypertension as the most common comorbidity and chronic heart disease as the second most common comorbidity. Ten patients were chronically bedbound (Patients A5-A14). All patients had respiratory symptoms, characterized by cough, congestion, and/or dyspnea. Thirteen patients exhibited findings on chest radiography consistent with a COVID-19 diagnosis. Four patients had at least a one-night stay in the ICU (Patients 2, 5, 10, 12). Fortunately, no patients expired during their hospital stay.
3.2. Detection of SARS COV-2 replication in cultures of NP swabs
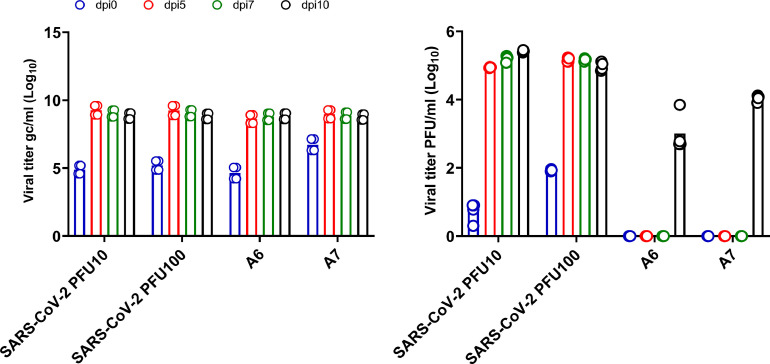
Exposure of Vero E6 cells to NP swab extracts for 1 hr and culture for 4 days failed to yield infectious virus as determined by PFU assay. We considered the possibility that the 4-day culture period was insensitive for detection of low amounts of active virus in the tested samples. Thus, Vero cells exposed to patient NP swab extracts were cultured for 10 days and the PFU assays were repeated. We found that of the samples tested, four out of 14 (28.6%) showed evidence of replicating virus 10 days after culture (patients A1, A2, A6 and A7, Table 2 ). All four positive samples were obtained within 12 days of COVID-19 symptom onset (Fig. 1). Positivity by PFU assay correlated with greater levels of virus genomic copies (gc). A time course study of two patient samples showed that viral RNA copy numbers increased substantially within 5 days of culture but PFU were only detected after 10 days (Fig. 2 ). All samples from the four PFU-positive patients but collected 13 days after symptom onset failed to show evidence of either increased viral nucleic acid or the presence of PFU after a 10 day culture. The samples from all remaining 10 patients did not show evidence of increased viral nucleic acid in culture supernatants nor active virus at either Day 4 or Day 10 of culture. The data from all 14 patients are summarized in Fig. 1.
Table 2.
SARS-CoV-2 RT-qPCR and PFU from patient NP swab extracts after growth in Vero E6 cell monolayers.
| Patient | Day of sample collection | 0 dpi viral gc/ml (log10) | 10 dpi viral gc/ml (log10) | 10 dpi PFU (log10) |
|---|---|---|---|---|
| A1 | day0 | 6.25 | 10.33 | 3.44 |
| day19 | 2.99 | 2.69 | 0 | |
| A2 | day3 | 6.77 | 9.61 | 3.69 |
| day20 | 3.24 | 5.81 | 0 | |
| A3 | day0 | 2.82 | 4.45 | 0 |
| day16 | 3.0 | 3.91 | 0 | |
| A4 | day0 | 3.43 | 4.40 | 0 |
| day17 | 2.96 | 3.48 | 0 | |
| A5 | day0 | 3.75 | 5.19 | 0 |
| day14 | 3.17 | 3.19 | 0 | |
| A6 | day0 | 7.05 | 9.63 | 2.73 |
| day16 | 2.89 | 3.74 | 0 | |
| A7 | day0 | 4.84 | 9.53 | 3.9 |
| day18 | 3.28 | 3.37 | 0 | |
| A8 | day14 | 3.38 | 3.42 | 0 |
| day29 | 2.26 | 4.20 | 0 | |
| A9 | day1 | 5.97 | 5.82 | 0 |
| day20 | 2.66 | 3.64 | 0 | |
| A10 | day9 | 5.31 | 5.33 | 0 |
| day24 | 2.90 | 3.45 | 0 | |
| A11 | day9 | 3.63 | 3.45 | 0 |
| day23 | 2.92 | 3.03 | 0 | |
| A12 | day10 | 4.43 | 4.04 | 0 |
| day26 | 3.15 | 2.24 | 0 | |
| A13 | day2 | 2.24 | 3.50 | 0 |
| day16 | 2.31 | 3.69 | 0 | |
| A14 | day0 | 3.89 | 3.43 | 0 |
| day14 | undetermined | 3.65 | 0 | |
| +ve control | NA | 8.48 | 10.25 | 5.77 |
Fig. 2.
Kinetic analysis of SARS-CoV-2 viral genome copies and PFU in Vero E6 cell supernatants. (a) Viral RNA levels (gc/ml) at various days post-infection (dpi) of Vero E6 cells (b) Viral PFU at various days post-infection of Vero E6 cells. Symbols on each bar represent individual technical repeats used for each sample.
3.3. Culture with Vero STAT1−/− cells results in greater numbers of PFU but not lower CT values
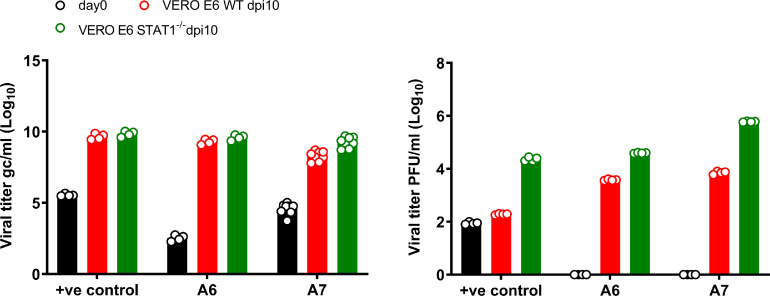
Interferon (IFN)-stimulated genes induced through STAT1 signaling are highly expressed during COVID-19 infection and are well-known to have anti-viral activity [7]. We therefore considered the possibility that Vero cell-derived IFN might limit viral replication during in vitro culture and thus, be responsible for the lack of PFU observed in many of our cultures. To test this, we compared growth of virus from NP swab extracts in Vero WT versus STAT1−/− cells. All NP swab samples that showed no viral growth in WT Vero cells remained negative when tested with Vero STAT1−/- cells (Table 3 ). However, those samples that showed viral PFU in WT Vero cells produced 10–100-fold greater numbers of PFU in Vero STAT1−/− cells (Table 3 and Fig. 3 ). Interestingly, RT-qPCR analysis of all samples showed comparable production of viral gc in both WT and STAT1−/- cells. Taken together, these results indicate that IFN signaling in host cells inhibits virus production, likely at a post-transcriptional stage of replication. Nevertheless, IFN signaling does not completely inhibit viral cytopathic effects and thus, was not the reason for failure to detect active virus in 10 out of 14 of the congregate care patient samples.
Table 3.
Differential role of STAT1 in levels of SARS-CoV-2 viral RNA and PFU.
| Patient/Day of sample collection | 0 dpi Vero WT | 10 dpi Vero WT | 10 dpi Vero STAT1−/− | |||
|---|---|---|---|---|---|---|
| viral gc/ml (log10) | PFU (log10) | viral gc/ml (log10) | PFU (log10) | viral gc/ml (log10) | PFU (log10) | |
| A1/ D19 | 3.05 | 0 | 3.10 | 0 | 2.73 | 0 |
| A3/ D16 | 2.92 | 0 | 2.32 | 0 | 3.03 | 0 |
| A4/ D17 | 2.85 | 0 | 2.34 | 0 | 3.21 | 0 |
| A6/ D0 | 2.63 | 0 | 9.28 | 3.56 | 9.58 | 4.59 |
| A7/ D0 | 4.84 | 0 | 8.55 | 3.84 | 9.55 | 5.78 |
| A11/D9 | 4.60 | 0 | 4.18 | 0 | 3.4 | 0 |
| A14/D0 | 4.74 | 0 | 3.97 | 0 | 3.08 | 0 |
| +ve control | 5.51 | 2 | 9.64 | 2.27 | 9.83 | 4.30 |
Fig. 3.
Comparative analysis of SARS-CoV-2 virus growth in Vero E6 WT and STAT1−/−cells. (a) RT-qPCR quantification of gc/ml using SARS-COVID-19 primers targeting N1 and N2. (b) PFU quantitation at Days 0 or 10 post-infection of Vero E6 WT and STAT1−/− cells. Samples A6 and A7 were NP swabs from congregate care patients and a stock SARS-CoV-2 virus preparation was used as a positive (+ve) control. Data were pooled from two independent experiments, each with duplicate or triplicate technical replicates. The results are presented as means. Symbols represent individual technical repeats for each sample.
3.4. Increased levels of NP anti-spike protein antibody are associated with undetectable viral replication
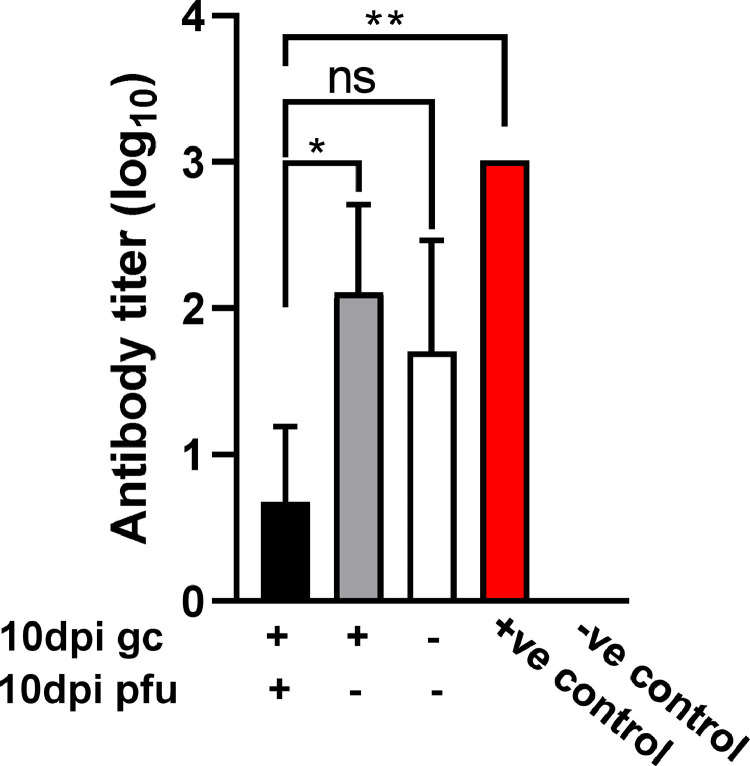
Recent studies have identified neutralizing antibodies reactive with SARS-CoV-2 spike protein in host mucosal tissues [8,9]. Such antibodies are detectable within 6–15 days post-infection [9]. We thus evaluated the potential presence of antibodies in our patient NP samples by ELISA. Specifically, we compared samples that produced differential gc and PFU results after 10 days of culture in Vero cells. Consistent with the observations of Cervia et al. [9], we did detect anti-spike antibodies in all NP swab samples tested (Fig. 4 ). Samples that were positive in both Day 10 gc and PFU assays showed low levels of antibody while all NP samples that were negative for PFU contained relatively high titers of anti-spike antibody, regardless of whether they were positive or negative by RT-PCR. The association between high antibody titers in NP swabs and lack of culturable virus suggests that any virions present in NP can be neutralized by anti-spike antibody and therefore, do not replicate and mediate cytotoxic effects. These results could partially explain our negative PFU results but nevertheless, indicate that the patients are noninfectious despite the presence of viral RNA.
Fig. 4.
SARS-CoV-2 spike protein-specific antibody titers in COVID-19 patient NP swab samples. Samples that were positive for both gc and PFU after 10 days culture in Vero E6 WT cells were compared to samples positive for gc but negative for PFU or negative for both gc and PFU. Each group consisted of 3–4 patient NP samples. A patient plasma sample with a known anti-spike antibody titer was used as a positive control and a NP sample from an uninfected patient was used as a negative control. Mean titers are shown and the error bars represent SD. Statistical significance was assessed by ANOVA. *p<0.05, **p<0.01, ns=not significant.
4. Discussion
We tested sequential NP samples from hospitalized, congregate care COVID-19 patients for the differential identification of viral nucleic acid versus actively replicating virus. Consistent with previous studies [10,11], Vero E6 cells were found to be susceptible to SARS-CoV-2 virus replication and damage but extended culture for 10 days was necessary to detect PFU in cultures infected with clinical samples. A comparative evaluation of viral replication in WT versus STAT1−/− Vero E6 cells showed that absence of IFN signaling enhanced viral replication and development of plaques. Samples initially positive for viral replication in WT Vero cells were also positive for replication in STAT1−/- cells, but samples negative for viral growth in WT cells remained negative when tested in STAT1−/− cells. Importantly, all samples obtained from patients who remained hospitalized for extended periods of time due to NAAT positivity, were uniformly negative for active viral replication as indicated by no PFU growth.
NP swab samples from 14 SARS-CoV-2 NAAT-positive congregate care patients at both early and later stages of disease were utilized for quantification of live replicating virions by the PFU assay. The viral RNA levels in early-stage samples were found to be higher than in later stage samples. However, none of the samples induced Vero E6 cytopathic effects within 4 days of culture. This finding was surprising since in our hands and those of others [12], testing of NP swab samples from various other COVID-19 patients showed evidence of PFU on Day 0. This led us to determine whether the congregate care samples used in the current study retained virus at levels below detection, which could be increased by extended culture. Indeed, 4 of the 14 patient samples demonstrated increased gc values and detectable PFUs after culture for 10 days in Vero cells, despite initially showing no replicating virus. All of the PFU-positive cultures were derived from patients with viral loads >6.8 log10 gc/ml, which agrees with earlier reports that found a dissociation between viral RNA production and positive viral cultures [12]. In fact, our findings are nearly identical to those of Bullard et al. [13], who reported that 28.9% of samples from COVID-19 patients showed viral growth in Vero cells but samples from patients with Ct values of >24 or symptom onset >8 days lacked replicating virus. Of note, our experimental approach was novel in that it directly compared sequential samples from the same patient at various stages of disease. In our study, all NAAT positive patients tested negative by viral culture at later symptom onset, although admittedly, we only examined NP swabs, and other sample sources such as sputum could conceivably contain more active virions. An explanation for the consistent disconnect between levels of viral genome copies and active virus described by us and other groups is offered by a recent finding indicating long-term production of genomic and sub-genomic fragments of RNA following SARS-CoV-2 infection of host cells [4]. The authors of this latter study suggested that sub-genomic RNAs are nuclease-resistant possibly due to protection by intracellular cell membranes. However, they did not use culture methods to directly measure infectious virus.
We further tested whether IFN-mediated host immunity in Vero cells could be responsible for an inability to detect active virus after an extended hospital stay. Thus, we determined viral growth in STAT1−/− Vero E6 cells, which lack the intrinsic IFN signaling pathway known to inhibit viral replication, including replication of SARS-CoV-2 ([14, 15]). Notably, all samples that were PFU-negative remained negative after extended culture in these cells. Conversely, the positive samples remained positive but yielded ~10–100-fold more PFU than the same samples grown in WT cells. In addition, the actual plaques were larger and better defined compared to those developed in WT Vero cells. It is unclear whether type I [16] or type III [17] IFN was responsible for these inhibitory effects, especially since Vero E6 cells are known to be deficient in type I IFN responsiveness [17,18]. In either case, we conclude that IFN produced by Vero cells can limit viral replication but is not ultimately responsible for culture negativity. The mechanism that mediates IFN-dependent inhibition of virus growth is unknown, particularly since IFN is known to upregulate expression of the ACE2 virus receptor, which would be predicted to increase infectivity [19]. It is likely that IFN-mediated virus inhibition occurs post-transcriptionally since virus PFU-negative cultures still contained viral RNA.
We also tested for the possible presence of anti-spike antibody in the patient NP samples to determine whether such antibodies might influence our ability to successfully obtain culture-positive virus. In fact, varying levels of anti-spike antibodies were readily detected in the NP samples. Patient samples that were PFU-positive after 10 days in culture showed the lowest levels of antibody, while samples that were PFU-negative, regardless of whether they were gc-positive or -negative, demonstrated higher levels of antibody. Considering the mucosal site of antibody expression, it is likely that they were of the non-inflammatory IgA isotype, consistent with the results of Cervia et al. [9]. These results provide an explanation for the data obtained – antibody present in NP can prevent growth of virus from fresh NP samples and only those samples containing low levels of antibody allow a positive PFU result after 10 days of culture. The obvious implication is that virus may be present during early stages of infection in patients that have a low level of anti-spike antibody, but after production of larger amounts of antibody later during disease the virus is effectively neutralized and the patient becomes noninfectious.
In conclusion, we have used sequential NP swab samples to demonstrate that hospitalized congregate care patients showed active virus only during early symptom onset even though the patients remained RNA positive for weeks after initial infection. This was not due to lack of assay sensitivity nor inhibitory IFN production by the host cells but is likely related to the presence of anti-spike protein antibody.
Author contributions
Experiments performed by AKS and TKB. Patient information and samples provided by KAS, TA and MJW. Data analysis and interpretation by AKS, DWM, TA, MJW, MDR, and KAS. Manuscript preparation by AKS and DWM.
Declaration of Competing Interest
M. Robek reports financial relationships with CaroGen Corp. and research funding from Gilead Sciences, outside of this work. The other authors declare no competing financial interests.
Acknowledgements
We thank the Department of Immunology and Microbial Disease BSL-3 facility for assistance in the viral culture assays.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jcv.2021.104879.
Appendix. Supplementary materials
References
- 1.Li C., Zhao C., Bao J., Tang B., Wang Y., Gu B. Laboratory diagnosis of coronavirus disease-2019 (COVID-19) Clin. Chim. Acta. 2020;510:35–46. doi: 10.1016/j.cca.2020.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jung Y., Park G.S., Moon J.H., Ku K., Beak S.H., Lee C.S., et al. Comparative analysis of primer-probe sets for RT-qPCR of COVID-19 causative virus (SARS-CoV-2) ACS Infect. Dis. 2020;6(9):2513–2523. doi: 10.1021/acsinfecdis.0c00464. [DOI] [PubMed] [Google Scholar]
- 3.Tahamtan A., Ardebili A. Real-time RT-PCR in COVID-19 detection: issues affecting the results. Expert Rev. Mol. Diagn. 2020;20(5):453–454. doi: 10.1080/14737159.2020.1757437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexandersen S., Chamings A., Bhatta T.R. SARS-CoV-2 genomic and subgenomic RNAs in diagnostic samples are not an indicator of active replication. Nat. Commun. 2020;11(1):6059. doi: 10.1038/s41467-020-19883-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3) doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Muller M.A., et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 7.Yang D., Chu H., Hou Y., Chai Y., Shuai H., Lee A.C., et al. Attenuated interferon and proinflammatory response in SARS-CoV-2-infected human dendritic cells is associated with viral antagonism of STAT1 phosphorylation. J. Infect. Dis. 2020;222(5):734–745. doi: 10.1093/infdis/jiaa356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sterlin D., Mathian A., Miyara M., Mohr A., Anna F., Claer L., et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci. Transl. Med. 2021;13(577) doi: 10.1126/scitranslmed.abd2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cervia C., Nilsson J., Zurbuchen Y., Valaperti A., Schreiner J., Wolfensberger A., et al. Systemic and mucosal antibody responses specific to SARS-CoV-2 during mild versus severe COVID-19. J. Allergy Clin. Immunol. 2021;147(2):545–557. doi: 10.1016/j.jaci.2020.10.040. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaye M. SARS-associated coronavirus replication in cell lines. Emerg. Infect. Dis. 2006;12(1):128–133. doi: 10.3201/eid1201.050496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harcourt J., Tamin A., Lu X., Kamili S., Sakthivel S.K., Murray J., et al. Severe Acute Respiratory Syndrome Coronavirus 2 from patient with coronavirus disease, United States. Emerg. Infect. Dis. 2020 doi: 10.3201/eid2606.200516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fontana L.M., Villamagna A.H., Sikka M.K., McGregor J.C. Understanding viral shedding of severe acute respiratory coronavirus virus 2 (SARS-CoV-2): review of current literature. Infect. Control Hosp. Epidemiol. 2020:1–10. doi: 10.1017/ice.2020.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bullard J., Dust K., Funk D., Strong J.E., Alexander D., Garnett L., et al. Predicting infectious SARS-CoV-2 from diagnostic samples. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zornetzer G.A., Frieman M.B., Rosenzweig E., Korth M.J., Page C., Baric R.S., et al. Transcriptomic analysis reveals a mechanism for a prefibrotic phenotype in STAT1 knockout mice during severe acute respiratory syndrome coronavirus infection. J. Virol. 2010;84(21):11297–11309. doi: 10.1128/JVI.01130-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lokugamage K.G., Hage A., de Vries M., Valero-Jimenez A.M., Schindewolf C., Dittmann M., et al. Type I interferon susceptibility distinguishes SARS-CoV-2 from SARS-CoV. J. Virol. 2020;94(23) doi: 10.1128/JVI.01410-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanifer M.L., Kee C., Cortese M., Zumaran C.M., Triana S., Mukenhirn M., et al. Critical role of type III interferon in controlling SARS-CoV-2 infection in human intestinal epithelial cells. Cell Rep. 2020;32(1) doi: 10.1016/j.celrep.2020.107863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prescott J., Hall P., Acuna-Retamar M., Ye C., Wathelet M.G., Ebihara H., et al. New World hantaviruses activate IFNlambda production in type I IFN-deficient vero E6 cells. PLoS One. 2010;5(6):e11159. doi: 10.1371/journal.pone.0011159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osada N., Kohara A., Yamaji T., Hirayama N., Kasai F., Sekizuka T., et al. The genome landscape of the african green monkey kidney-derived vero cell line. DNA Res. 2014;21(6):673–683. doi: 10.1093/dnares/dsu029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ziegler C.G.K., Allon S.J., Nyquist S.K., Mbano I.M., Miao V.N., Tzouanas C.N., et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181(5):1016–1035. doi: 10.1016/j.cell.2020.04.035. e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.