Abstract
Background
Although specific interventions previously demonstrated benefit in patients with ARDS, use of these interventions is inconsistent, and patient mortality remains high. The impact of variability in center management practices on ARDS mortality rates remains unknown.
Research Question
What is the impact of treatment variability on mortality in patients with moderate to severe ARDS in the United States?
Study Design and Methods
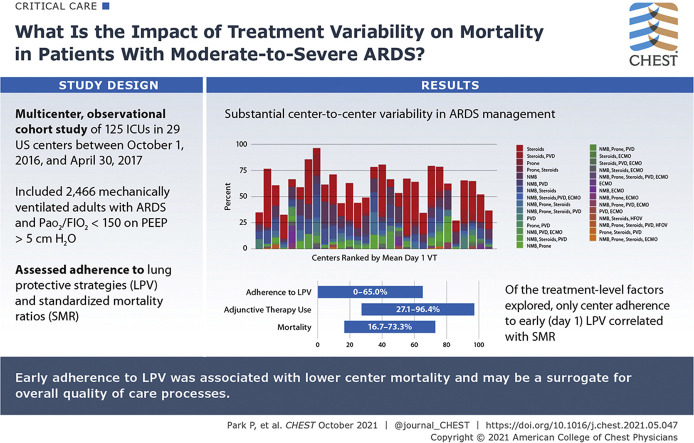
We conducted a multicenter, observational cohort study of mechanically ventilated adults with ARDS and Pao2 to Fio2 ratio of ≤ 150 with positive end-expiratory pressure of ≥ 5 cm H2O, who were admitted to 29 US centers between October 1, 2016, and April 30, 2017. The primary outcome was 28-day in-hospital mortality. Center variation in ventilator management, adjunctive therapy use, and mortality also were assessed.
Results
A total of 2,466 patients were enrolled. Median baseline Pao2 to Fio2 ratio was 105 (interquartile range, 78.0-129.0). In-hospital 28-day mortality was 40.7%. Initial adherence to lung protective ventilation (LPV; tidal volume, ≤ 6.5 mL/kg predicted body weight; plateau pressure, or when unavailable, peak inspiratory pressure, ≤ 30 mm H2O) was 31.4% and varied between centers (0%-65%), as did rates of adjunctive therapy use (27.1%-96.4%), methods used (neuromuscular blockade, prone positioning, systemic steroids, pulmonary vasodilators, and extracorporeal support), and mortality (16.7%-73.3%). Center standardized mortality ratios (SMRs), calculated using baseline patient-level characteristics to derive expected mortality rate, ranged from 0.33 to 1.98. Of the treatment-level factors explored, only center adherence to early LPV was correlated with SMR.
Interpretation
Substantial center-to-center variability exists in ARDS management, suggesting that further opportunities for improving ARDS outcomes exist. Early adherence to LPV was associated with lower center mortality and may be a surrogate for overall quality of care processes. Future collaboration is needed to identify additional treatment-level factors influencing center-level outcomes.
Trial Registry
ClinicalTrials.gov; No.: NCT03021824; URL: www.clinicaltrials.gov
Key Words: ARDS, corticosteroids, extracorporeal membrane oxygenation, mechanical ventilation, neuromuscular blockade, prone positioning
Abbreviations: ABG, arterial blood gas; CHF, congestive heart failure; ESRD, end-stage renal disease; iNO, inhaled nitric oxide; LPV, lung protective ventilation; PBW, predicted body weight; PEEP, positive end-expiratory pressure; PVD, pulmonary vasodilator; SMR, standardized mortality ratio; SOFA, sequential organ failure assessment; UAB, unassisted breathing; VFD, ventilator-free day; VT, tidal volume
Graphical Abstract
FOR EDITORIAL COMMENT, SEE PAGE 1167
ARDS is a potentially fatal condition characterized by acute hypoxemia and bilateral radiographic infiltrates, with a reported mortality of 36% to 47%.1, 2, 3, 4, 5 Specific interventions in ARDS, including lung protective ventilation (LPV)6 and prone positioning,7 previously were demonstrated to improve survival in clinical trials, but remain underused.1 , 8, 9, 10, 11 Simultaneously, treatment methods with unclear benefit and potential, including neuromuscular blockade,12 extracorporeal membrane oxygenation,13 steroids,14 and pulmonary vasodilators,15 , 16 continue to be used in the management of ARDS. Regional variation in adoption of different practices for treating patients with ARDS was described previously.17 , 18 More recently, reports of widely variable treatments for COVID-19-related ARDS have emerged.19 Because of the complexity of ARDS epidemiologic reporting, it is unclear if ARDS mortality has changed over time,5 , 20, 21, 22, 23, 24, 25 and the impact of potential heterogeneity in ARDS management on patient outcomes remains unknown.
To understand the patient- and center-level treatment factors associated with mortality in moderate to severe ARDS in the United States, we conducted a multicenter observational study across US institutions. Center variability in management practices was examined, as well as the association of this variability with patient outcomes. We hypothesized that center management practices would be associated with risk-adjusted 28-day in-hospital mortality.
Methods
Study Design and Setting
The Severe ARDS: Generating Evidence Study was a multicenter observational cohort study (ClinicalTrials.gov Identifier: NCT 03021824) conducted in 125 ICUs at 29 academic and community hospital centers across the United States from October 1, 2016, through April 30, 2017. Institutional review board approval was obtained at each center, and the requirement for informed consent was waived. Study principal investigators were responsible for ensuring data integrity and validity and for reviewing that patients met study criteria. A list of collaborating centers can be viewed in e-Appendix 1.
Participants and Study Design
All consecutive patients 18 years of age or older who were receiving invasive mechanical ventilation at participating ICUs were followed up for 5 days for the development of ARDS by Berlin criteria and a Pao 2 to Fio 2 ratio of ≤ 150.2 Data subsequently were collected for 3 days from the time of inclusion, and patients were followed up for 28 days or until hospital discharge, whichever occurred first. No exclusion criteria were applied. Study day 1 was defined as the first day that patients met enrollment criteria. Inclusion criteria could be met at either the study hospital, if the patient was a direct admission, or at a referring hospital, if the patient was transferred to the study hospital. The initial 6-month study period was extended for 1 additional month to capture ongoing presentation of seasonal respiratory illness at the clinical sites. The primary outcome was 28-day in-hospital mortality. Center variation in ventilator management, adjunctive therapy use, and mortality also were assessed.
Data Collection
Baseline demographic variables, ARDS risk factors, arterial blood gas (ABG) analysis, mechanical ventilator settings, and sequential organ failure assessment (SOFA) scores were collected. Ventilator data and respiratory parameters also were collected for the first 3 days of invasive mechanical ventilation and on days when any adjunctive therapy was initiated. Determination of day 1 adherence to LPV required both documented tidal volume (VT) ventilation of ≤ 6.5 mL/kg predicted body weight (PBW) and plateau pressure of ≤ 30 cm H2O. In cases where plateau pressure was not recorded, the combination of VT < 6.5 mL/kg PBW and peak inspiratory pressure of ≤ 30 cm H2O was considered LPV. For instances in which an adjunctive therapy was initiated at a transferring hospital, the first known date of adjunctive therapy was collected. Outcomes data that were collected included hospital mortality, liberation from mechanical ventilation, discharge from the ICU, discharge from the hospital at 28 days, and SOFA score at day 7. Patients who were discharged from the hospital alive before study day 28 were assumed to be alive at that time point. Additionally, whether evidence was present in the chart of active withdrawal of life-sustaining measures was documented. The full case report form can be viewed in e-Appendixes 2 and 3.
Data sources came from the electronic health record at each institution. In some cases (four centers), data variables were extracted electronically when feasible. Data were entered into a Research Electronic Data Capture database housed behind the Duke University School of Medicine firewall.
Site investigators were required to respond to any queries raised by the electronic case report form before finalizing individual entries. Additionally, all entries were screened for outliers, potentially erroneous data, or missing outcomes. Data that could not be verified or corrected by site investigators were not included in the final data set.
Statistical Methods
We summarized the baseline characteristics, day 1 ventilator settings, and 28-day outcomes for the study cohort overall using numbers and percentages for categorical variables and mean ± SD or median (interquartile range) for continuous variables. We then explored the use patterns of various adjunctive therapies by summarizing therapies used in isolation and in combination as well as timing of treatment initiation.
We explored the univariate relationships between early LPV adherence and the primary outcome of 28-day in-hospital mortality via Kaplan-Meier curves and log-rank tests. We used a multivariate generalized mixed-effect model for 28-day in-hospital mortality, with fixed terms for baseline variables and random effects for center to investigate which baseline factors were associated with our primary outcome. Model adjustment terms were fixed a priori and included the following: inclusion Pao 2 to Fio 2 ratio, day 1 SOFA score, age, sex, day 1 VT > 6.5 mL/kg, risk factor for ARDS, and comorbidities.
To further explore the variability in mortality rate across centers while accounting for patient-level factors, we derived and compared standardized mortality ratios (SMRs). The SMR for a given center was calculated as the ratio between the observed mortality rate and the expected mortality rate. Because no appropriate standard population exists for this type of patient population, we used the center-wise average of predicted mortality probabilities from a multivariate logistic regression model, adjusting for the baseline patient-level characteristics listed above, as the best approximation. The 95% CI for SMR was calculated using the Byar approximation.
We described center variability visually and descriptively by summarizing ventilator settings, adjunctive therapy use, and SMR by center. We then explored the relationship between SMR and center median day 1 VT, rate of adherence to LPV, PEEP, and rate of adjunctive therapy use visually and via Pearson correlation. Statistical analysis was performed using SAS version 9.4 software (SAS, Inc.). All P values were two-sided, and P < .05 was considered statistically significant.
Results
Baseline Characteristics
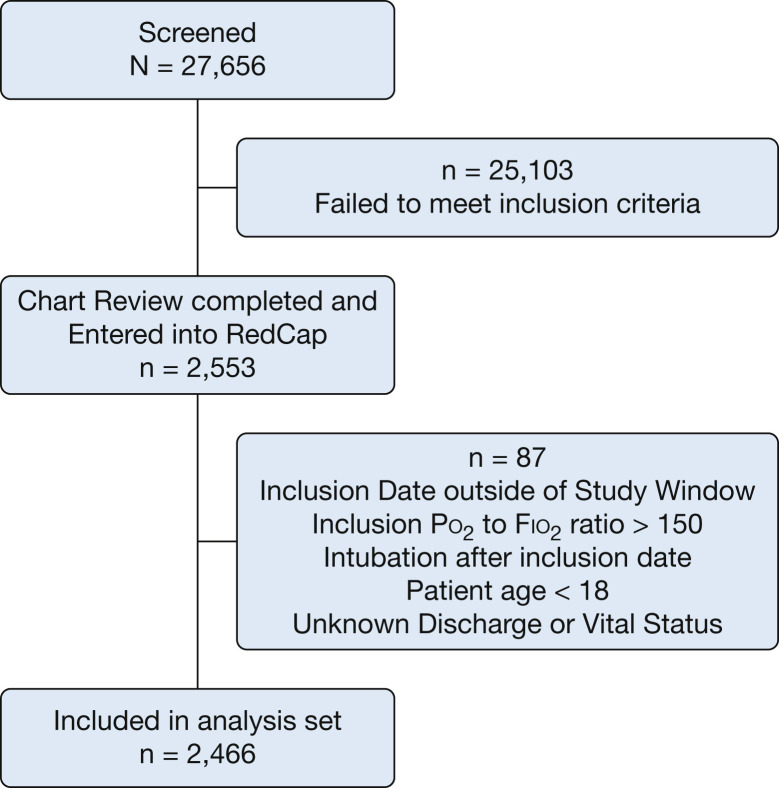
Of 27,656 patients screened, 2,466 (8.9%) were identified as having moderate to severe ARDS (Fig 1 ). Baseline characteristics are summarized in Table 1 . The mean age was 56.9 ± 16.3 years, with 58.6% being men. The median day 1 Pao 2 to Fio 2 ratio was 105 (interquartile range, 78-129), and the most common risk factors for ARDS development were sepsis (62.3%) and pneumonia (62.5%). Shock, as defined by the use of vasopressors, was present in 64.5% of patients. Only 7.1% of patients were enrolled in concurrent ARDS clinical trials. Center characteristics are presented in e-Table 1.
Figure 1.
Flow chart showing patient screening and enrollment.
Table 1.
Baseline Characteristics (N = 2,466 Patients)
| Baseline Characteristic | Data |
|---|---|
| Age, y | 57 ± 16.3 |
| Sex, male | 1,445 (58.6) |
| Height, m | 1.70 ± 0.11 |
| Weight, kg | 88.15 ± 30.1 |
| BMI, kg/m2 | 30.46 ± 9.6 |
| Race or ethnicity | |
| American Indian/Alaskan Native | 32 (1.5) |
| Asian | 96 (4.4) |
| Hispanic or Latino | 241 (9.8) |
| Black | 338 (15.4) |
| Native Hawaiian/Pacific Islander | 12 (0.6) |
| White | 1,712 (77.9) |
| Multiracial | 9 (0.4) |
| Unknown/not reported | 267 (10.8) |
| Transferred from outside hospital | 621 (25.2) |
| Comorbidities | |
| Cirrhosis | 240 (9.7) |
| Hepatic failure | 156 (6.3) |
| ESRD requiring hemodialysis | 140 (5.7) |
| Metastatic carcinoma | 142 (5.8) |
| Lymphoma | 56 (2.3) |
| Leukemia | 84 (3.431) |
| Myeloma | 16 (0.7) |
| AIDS | 44 (1.8) |
| Immunosuppression | 454 (18.4) |
| Chronic lung disease | 572 (23.2) |
| Diabetes mellitus | 724 (29.4) |
| Congestive heart failure | 402 (16.3) |
| Risk factors for ARDS | |
| Sepsis | 1,537 (62.3) |
| Pneumonia | 1,541 (62.5) |
| Shock | 1,429 (58) |
| Aspiration | 658 (26.7) |
| Blood product transfusion | 545 (22.1) |
| Trauma | 240 (9.7) |
| Drug overdose | 127 (5.2) |
| Pancreatitis | 82 (3.3) |
| Othera | 226 (9.2) |
| Unknown | 26 (1.1) |
Data are presented as No. (%) or mean ± SD. ESRD = end-stage renal disease.
Includes smoke inhalation, near drowning, burn, or other known risk factors not listed above.
Ventilator Management
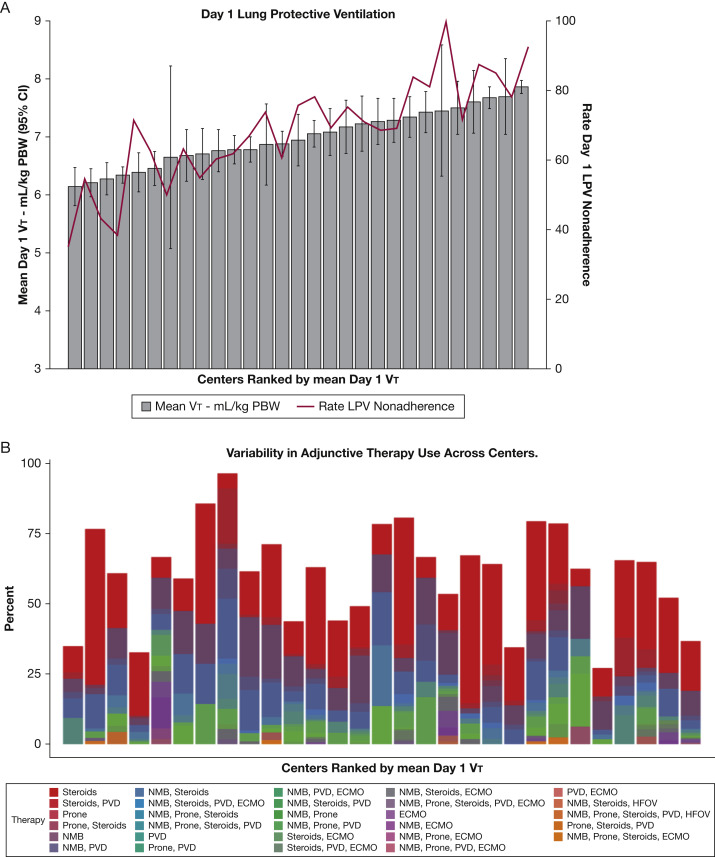
For the overall cohort, mean day 1 VT was 7 mL/kg PBW (SD, 1.5 mL/kg PBW), with 56.3% of patients receiving a VT of > 6.5 mL/kg PBW and 21.7% receiving > 8 mL/kg PBW (Table 2 ). Substantial variation among centers was noted in mean Vt (6.2-7.9 mL/kg PBW) as well as initial rate of adherence to LPV (0-65%) (Fig 2 A). Mean day 1 PEEP was 9.2 cm H2O (SD, 3.9 cm H2O). The overall rate of measurement of the plateau pressure on day 1 was 49.6%, with 85% of recorded values being ≤ 30 mm H2O. When plateau pressure was not recorded, peak inspiratory pressure of ≤ 30 cm H2O was used to permit classifying LPV adherence in > 90% of the remaining cohort. Overall adherence to LPV in this group was 31.4%, with more than half of the nonadherence to LPV the result of use of Vt values of > 6.5 mL/kg PBW. Characteristics of patients who received LPV on day 1 and those who did not were similar, with the exception of day 1 VT (7.6 mL/kg PBW vs 5.9 mL/kg PBW; Cohen’s d, –1.49), day 1 peak pressure (32 cm H2O vs 26 cm H2O; Cohen’s d, –0.76), day 1 plateau pressure (24 cm H2O vs 23 cm H2O; Cohen’s d, –0.35), and height (1.68 cm vs 1.73 cm; Cohen’s d, 0.42) (e-Table 2). Additional physiologic characteristics are presented in e-Table 3.
Table 2.
Clinical Characteristics, Ventilator Management, and Outcomes (N = 2,466 Patients)
| Variable | Data |
|---|---|
| Day 1 clinical characteristics | |
| Pao2 to Fio2 ratio | 105 (78-129) |
| Fio2 | 80 (60-100) |
| SOFA score | 12 ± 4 |
| Vasopressors (infusion lasting > 1 h) | 1,572 (64.5) |
| Missing | 28 |
| PEEP, mm H2O | 9 ± 4 |
| Missing | 92 |
| Plateau pressure, mm H2O | 24 (20-28) |
| Missing | 1,223 |
| Peak pressure, mm H2O | 29 (24-35) |
| Missing | 243 |
| Vt, mL/kg PBW | 7 ± 1.5 |
| Missing | 154 |
| Vt > 6.5 mL/kg PBW | 1301 (56.3) |
| Vt > 8 mL/kg PBW | 502 (21.7) |
| LPV adherencea | 712 (31.4) |
| Outcomes | |
| Ventilator-free days to day 28b | 1 (0-22) |
| Mechanical ventilation duration (d)c | 6 (3-13) |
| Missing | 28 |
| Duration of initial ICU stay, dc | 9 (4-16) |
| Missing | 38 |
| Alive day 7 | 1,890 (76.7) |
| SOFA score day 7d | 9 ± 4 |
| Alive and with UAB day 28 | 1,166 (47.9) |
| Missing | 34 |
| Hospital-free days to day 28b | 0 (0-12) |
| Hospital length of stay, dc | 13 (6-25) |
| Missing | 16 |
| 28-day hospital mortality | 1,003 (40.7) |
Data are presented as No. (%), mean ± SD or median (interquartile range). If data are missing present, this is listed below the specific variable. LPV = lung protective ventilation; PBW = predicted body weight; PEEP = positive end-expiratory pressure; SOFA = Sequential Organ Failure Assessment; UAB = unassisted breathing; Vt = tidal volume.
Defined as Vt ≤ 6.5 mL/kg PBW and plateau pressure ≤ 30 mm H2O. If plateau pressure was missing, peak pressure was used. Either Vt or plateau pressure and peak pressure were missing for 197 patients.
If the patient died during the study period, ventilator- and hospital-free days were set to 0, or else ventilator- or hospital-free days were the difference between 28 and the duration of invasive mechanical ventilation or hospitalization.
Study follow-up truncated at 28 days after inclusion (durations range, 0-28 d). Length of stay for patients who died was defined as days from study inclusion to day of death.
Only among those alive and data available on day 7 (n = 1,890).
Figure 2.
A, B, Graphs showing ARDS management. A, Mean day 1 tidal volume (Vt) and percentage nonadherence to early lung protective ventilation across centers. B, Variability in frequency of adjunctive therapy use by combinations by center. Twenty-nine different combinations of therapy were used, with different frequencies at each center. ECMO = extracorporeal membrane oxygenation; HFOV = high frequency oscillatory ventilation; LPV = lung protective ventilation; NMB = neuromuscular blockade; PBW = predicted body weight; PVD = pulmonary vasodilator.
Use of Adjunctive Therapies
Adjunctive therapies were used in 57.5% of patients. Among those receiving adjunctive therapy, systemic steroids were the most commonly used (41.5%), followed by neuromuscular blockade (27.4%), pulmonary vasodilators (11.7%), prone positioning (5.8%), and extracorporeal membrane oxygenation (4.5%) (Table 3 ). Adjunctive therapies were used most often in combination, and most were initiated before or within 1 day of ARDS development. Although the indication for steroid use was not recorded, 71.8% of patients who received steroids also were noted to have shock requiring vasopressors, and 30.9% had immunosuppression listed as a comorbidity. Substantial center-to-center variation was found in the specific type and rate of adjunctive therapy use (27.1%-96.4%) (Fig 2B). Twenty-nine different combinations of therapy were administered during the study period, with different frequencies at each center.
Table 3.
Adjunctive Therapy Use
| Variable | NMB (n = 675 [27.4%]) | Prone Positioning (n = 143 [5.8%]) | Steroid (n = 1,015 [41.2%]) | PVD (n = 289 [11.7%]) | ECMO (n = 110 [4.5%]) |
|---|---|---|---|---|---|
| Individual therapy is the only one given | 215 (31.9) | 10 (7) | 591 (58.2) | 36 (12.5) | 16 (14.6) |
| Individual therapy is first used | 503 (75.5) | 28 (20.4) | 751 (74.8) | 81 (28.3) | 43 (39.1) |
| No. receiving therapy with known start date | 666 | 137 | 1004 | 286 | 110 |
| Study day of therapy start | 1 (1-2) | 2 (1-3) | 1 (0-2) | 1 (1-2) | 1 (1-2) |
| Therapy started before study day 1 | 90 (13.5) | 12 (8.8) | 283 (28.2) | 54 (18.9) | 25 (22.7) |
| Therapy started by study day 1 | 429 (64.4) | 61 (44.5) | 652 (64.9) | 175 (61.2) | 72 (65.5) |
| Therapy started by study day 3 | 572 (85.9) | 112 (81.8) | 844 (84.1) | 238 (83.2) | 92 (83.6) |
| Therapy started by study day 7 | 629 (94.4) | 127 (92.7) | 943 (93.9) | 257 (89.9) | 105 (95.5) |
Data are presented as No. (%) or median (interquartile range). ECMO = extracorporeal membrane oxygenation; NMB = neuromuscular blockage; PVD = pulmonary vasodilator.
Patient Outcomes
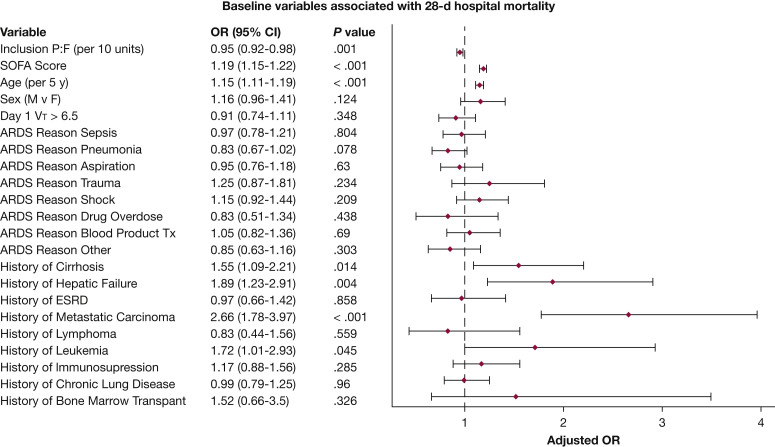
Overall 28-day in-hospital mortality was 40.7%, with higher mortality in patients with severe ARDS compared with those with moderate ARDS (44.4% vs 36.0%; P < .001, log-rank test). One thousand one hundred eleven patients (45.1%) were discharged alive from the hospital, and 352 patients (14.3%) remained hospitalized at day 28 (Table 2). Of those discharged alive from the hospital, 10.4% continued receiving mechanical ventilation, whereas 89.6% were breathing unassisted at discharge. Among the patients still hospitalized, 43.3% continued receiving mechanical ventilation. Life-sustaining measures were known to be withdrawn or limited for 24.7% of patients. In the multivariate generalized mixed-effect model, baseline factors found to be associated with 28-day in-hospital mortality were baseline Pao 2 to Fio 2 ratio, day 1 SOFA score, age, and the following comorbidities: cirrhosis, hepatic failure, metastatic carcinoma, and leukemia (Fig 3 ).
Figure 3.
Forest plot showing baseline variables associated with 28-day hospital mortality. Results of primary outcome multivariate generalized mixed-effect model for 28-day hospital mortality, with fixed terms for baseline variables and random effect for center to investigate which baseline factors are associated with primary outcome. ESRD = end-stage renal disease; F = female; M = male; P:F = Pao2 to Fio2 ratio; SOFA, Sequential Organ Failure Assessment; Tx = transfusion; VT = tidal volume.
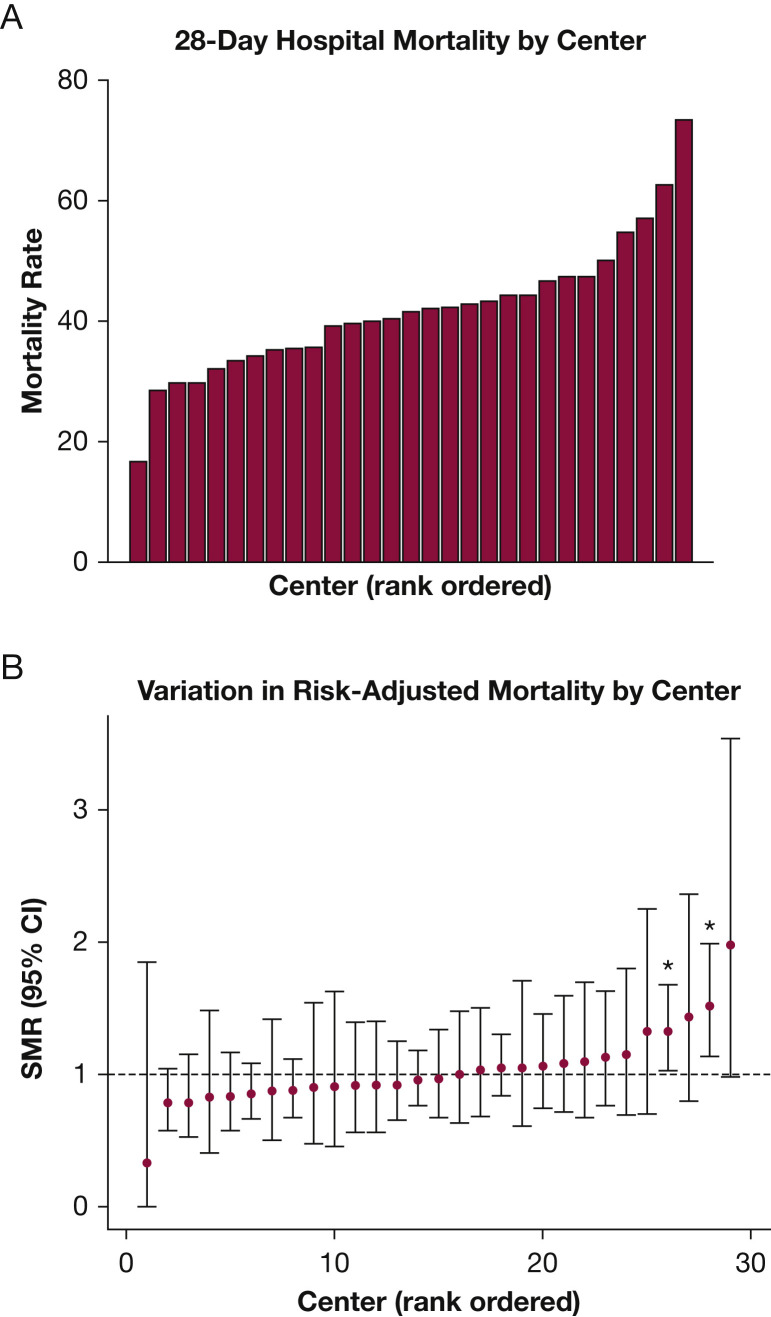
Center Mortality
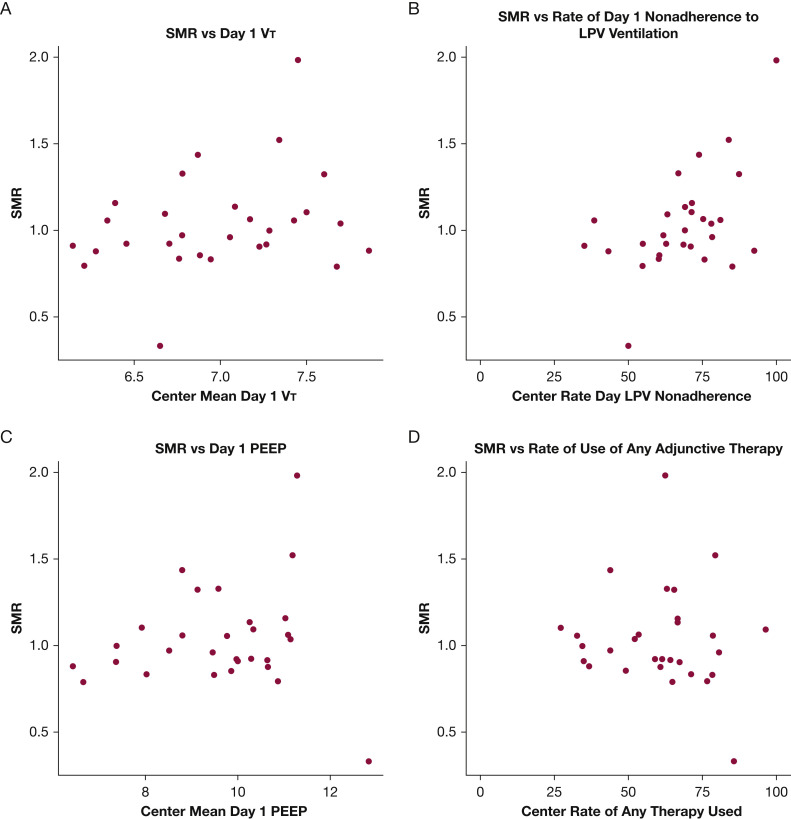
Mortality varied widely across centers, ranging from 16.7% to 73.3% (Fig 4 A). Evaluation of SMRs across centers showed that significant variation persisted (0.33-1.98) after adjustment for baseline factors (Fig 4B). At the center level, higher SMR correlated with nonadherence with day 1 LPV (Pearson r = 0.52; 95% CI, 0.19-0.74; P = .016, adjusted for multiple comparisons), but not with any other individual treatment-level factor, including mean day 1 VT (r = 0.27), mean day 1 PEEP (r = 0.08), or rate of adjunctive therapy use (r = –0.09) (Fig 5 ). The use of any specific adjunctive therapy also did not correlate strongly with SMR (neuromuscular blockade, r = 0.09; prone positioning, r = 0.28; steroids, r = –0.28; PVD, r = 0.21; extracorporeal membrane oxygenation, r = 0.16) (e-Fig 1).
Figure 4.
A, B, Graphs showing variability in center mortality. A, Twenty-eight-day hospital mortality by center. Unadjusted 28-day hospital mortality ranged from 16.7% to 73.3%. B, Variation in risk-adjusted mortality by center. Standardized mortality ratios (SMRs) varied by center, between 0.33 and 1.98.
Figure 5.
A-D, Scatterplots showing SMR vs center metrics. A, SMR vs day 1 VT. B, SMR vs rate of day 1 nonadherence to LPV (VT < 6.5 mL/kg predicted body weight, plateau pressure plateau pressure or peak inspiratory pressure ≤ 30 cm H2O). C, SMR vs day 1 PEEP. D, SMR vs rate of use of any adjunctive therapy. LPV = lung protective ventilation; PEEP = positive end-expiratory pressure; SMR = standardized mortality ratio; VT = tidal volume.
The primary comparison of center-level adherence with day 1 LPV and SMR assumes that LPV adherence was similar for patients with known or unknown adherence status at the same site. As a sensitivity analysis, we examined two extreme scenarios, one in which all patients missing LPV adherence status were assumed to be adherent and another in which all were assumed to be nonadherent. We found the association between day 1 LPV adherence and SMR remained significant whether all patients with unknown status were assumed to be adherent (r = 0.38; 95% CI, 0.02-0.65; P = .042) or nonadherent (r = 0.49; 95% CI, 0.16-0.73; P = .006). Hence, under all scenarios examined, an association seems to exist between center-level day 1 LPV adherence and SMR.
Discussion
To our knowledge, the Severe ARDS: Generating Evidence Study is the largest US observational cohort study of patients with moderate to severe ARDS reported to date. The primary findings of this study include the following: (1) extensive center-to-center variability exists in the early use of LPV and adjunctive therapies in moderate to severe ARDS, with improved, but imperfect, adherence to early lung protective strategies; (2) center mortality rates for patients with moderate to severe ARDS are highly variable; (3) center-level adherence to early LPV was associated with lower standardized mortality rates; and (4) the influence of variations in other specific treatment-level factors on variations in mortality remains unclear. The recent COVID-19 pandemic has underscored the variation in care among institutions, with widely disparate reports of outcomes and responses to therapy.19 However, these variations in ARDS management are neither unique to COVID-19 nor new, as our study demonstrated. Ultimately, variation in center-specific risk-adjusted performance suggests that real opportunity still exists for improving ARDS outcomes.
Of ARDS management practices, LPV may be the best established as a supportive measure that improves survival,6 , 26 and has more recently been proposed as a center-level performance measure in the care of patients with ARDS.27 Although mean day 1 VT (7 mL/kg PBW) in the present cohort was lower than that seen in recent multicenter observational studies (7.2-7.6 mL/kg PBW),1 , 28 the rate of initial LPV nonadherence remained high. Despite the diagnosis of moderate to severe ARDS, more than half of patients received VT of > 6.5 mL/kg PBW on day 1, and nearly one-quarter received VT of > 8 mL/kg PBW. As in prior studies, plateau pressures were measured inconsistently.1 , 29
The finding that higher adherence to early use of LPV on a center level was associated with lower SMR suggests that adherence to LPV may be a surrogate for overall quality of care processes. A number of clinician-level factors influencing LPV use have been identified previously, including underrecognition of ARDS as well as physicians’ perceptions of LPV,30, 31, 32 suggesting that nonadherence indeed may be correctable, and thus a target for quality improvement. In the setting of ARDS, a syndrome with considerable variability in its management, the use of day 1 LPV adherence as a metric also could be a pragmatic starting point for quality assessment and improvement. However, it may be insufficient, because day 1 LPV may not reflect the subsequent course of ventilator settings during the patient’s hospitalization. A study examining detailed electronic health record data of mechanically ventilated patients found that, despite a low mean Vt in the cohort (6.8 mL/kg PBW), 40% of patients were exposed to VT of > 8 mL/kg PBW, and prolonged exposure to high Vt was associated with increased mortality.33
Regarding adjunctive therapy, the overall pattern of use was similar in the United States to that of a recent international cohort, with frequent use of systemic steroids (41.5% vs 19.4%) and neuromuscular blockade (27.4% vs 25.6%), but low rates of prone positioning (5.8% vs 7.9%),1 the adjunctive therapy that has most clearly demonstrated benefit in moderate to severe ARDS.7 Although underuse of evidence-based therapies in ARDS has been noted previously,18 the number of identified combinations and variation in rates of use within centers implies that clinicians are individualizing treatment, rather than strictly following institutional ARDS management protocols. Clinician-level factors such as expertise with particular methods34 have been found to influence the use of different adjunctive therapies, suggesting that rates of their use may be highly modifiable. However, the impact of systems-level barriers, such as staffing ratios and availability of various methods, remain less well known and warrant further investigation.35
Although variation in center-specific risk-adjusted performance suggests that the potential for improvement in ARDS care exists, many questions remain about how to continue to optimize management in this vulnerable patient population. Although center-level adherence to early LPV was found to be correlated strongly with SMR, no such correlation was found for any individual adjunctive therapy. Although the extreme heterogeneity in recorded site practice patterns in this cohort may have made the impact of any individual adjunctive therapy difficult to assess, another possibility is that additional processes may need to be examined to guide institutional performance improvement. The impact of other aspects of ventilator management, such as the use of PEEP, also may warrant further evaluation. Indeed, the mean PEEP in our cohort was only 9 cm H2O, despite higher PEEP being associated with decreased mortality in moderate to severe ARDS.36 Adherence to supportive measures previously found to be beneficial may be similarly important, such as conservative fluid management,37 sedation, and mobilization practices,38 , 39 as well as details that may be challenging to explore, such as clinician time at bedside and expertise with ventilator management.
Further research clearly is needed before specific, meaningful recommendations for standardization of care can be made, but it is important to note that only 7% of patients were enrolled in ARDS-related clinical trials. This finding warrants further examination, because nearly 80% of the participating centers were university hospitals, suggesting that such trials indeed were accessible. Because ARDS clinical trials involve adherence to protocolized care, low enrollment may have been a missed opportunity to reduce nontherapeutic variability in care. However, if a large number of patients were not enrolled because of a lack of eligibility, the generalizability of clinical trial results may need to be re-evaluated.
This study has several strengths. With 2,466 patients, it comprises the largest US cohort to date of patients with moderate to severe ARDS. Prior large observational studies included cohorts of patients with ARDS in diverse, international health care systems, limiting generalizability to the US population.1 , 7 , 13 , 40 Because generalizability was an important consideration in study design, we included a national group of clinical centers and allowed for the enrollment of an expansive patient population. Both academic and community centers were represented, as were a variety of ICU types, including medical, surgical, cardiothoracic, and mixed units. By eschewing exclusion criteria, we were able to evaluate better the characteristics, treatment, and mortality of the overall US population, as opposed to clinical trial cohorts. The success of the collaborative network also illustrates the potential to measure and benchmark ARDS care between centers.
This study has a number of limitations. This study represents a single 7-month sampling frame , before the COVID-19 pandemic, without the benefit of repeated measures at each center. Despite including a large number of diverse ICUs from around the United States, site recruitment through the Discovery Network may have resulted in selection biases with most centers including academic hospitals that may not truly reflect the national distribution. Because no exclusion criteria were applied, a number of patients in whom life-sustaining care was limited or withdrawn (24.7%) were included in our cohort. Withdrawal of life-sustaining support can be influenced by a patient’s prior wishes, family desires, or the physician caring for the patient, with the resulting limitations likely playing a role in ventilator management and selection of adjunctive therapies. The a priori selection of variables for the mortality risk adjustment was based on well-established risk factors associated with mortality in ARDS, but may still have omitted important unmeasured confounders, potentially obscuring findings in the patient-level analysis. Outcomes also were truncated at 28 days, and thus were unknown for the patients with longer hospitalizations (14.3%). Similarly, the assumption that patients discharged from the hospital before day 28 were alive is a further limitation. Additionally, the impact of systems-based factors such as hospital type, ICU type, staffing models, and ARDS case volume were not assessed. Other limitations include the lack of assessment of additional supportive measures that may benefit patients with ARDS, such as sedation practices and fluid management.
Interpretation
The management and mortality of patients with moderate to severe ARDS remain highly variable at US hospitals. Early adherence to LPV at a center level was associated with improved center performance. Differences in center-specific, risk-adjusted performance suggest that decreasing nontherapeutic variation in care and identifying appropriate targets for treatment optimization represent opportunities to reduce ARDS mortality. Further collaboration is needed to understand the impact of specific treatment-level factors on ARDS mortality, to understand the changes through the COVID-19 pandemic, and to inform rational decision-making in clinical care.
Take-home Points.
Study Question: What is the impact of treatment variability on mortality in patients with moderate-to-severe ARDS in the United States?
Results: In this multicenter observational study of 2,466 adults, considerable center-to-center variation was seen in the use of early lung protective ventilation (LPV, 0%-65%) as well as adjunctive therapies (27.1%-96.4%). Center mortality (16.7%-73.3%) and standardized mortality ratios (SMR, 0.33-1.98) also varied widely; center adherence to early LPV was associated with decreased SMR.
Interpretation: Substantial center-to-center variability exists in ARDS management practices and mortality. Differences in center adherence to early LPV are associated with risk-adjusted mortality, suggesting that real opportunity remains for improving ARDS outcomes.
Acknowledgments
Author contributions: The study was designed by N. Q., P. K. P., R. R. B., and M. N. G. Data was acquired from individual sites by the following investigators: N. Q., R. R. B., C. L. H., M. J. L., V. M. B.-G., J.-T. C., S. G., D. G., M. W. S., N. H., J. K., A. D., A. K. K., R. K., A. K., S. Y. C., J. E. T., H. L. A., J. M. L., J. M. M., P. E. M., A. G., I. K. L., M. T., R. S. S., A. M. E., D. J. D., A. M., K. E. S., W. B., A. T., P. K. G. and S. H. R. R. B. and M. L. C. had full access to all of the data in the study and performed the data analyses. The initial manuscript was drafted by N. Q. and P. K. P. All authors provided critical input into manuscript revisions. P. K. P. is the guarantor of the paper.
Financial/nonfinancial disclosures: None declared.
∗Severe ARDS: Generating Evidence (SAGE) Study Investigators (alphabetical, by center): Baystate Medical Center: Jay S Steingrub, Mark Tidswell; Beth Israel Deaconess Medical Center: Valerie M Banner-Goodspeed, Kristin Brierley, Julia L Larson, Ariel Mueller, Tereza Pinkhasova, Daniel Talmor; Brigham and Women’s Hospital: Imoigele Aisiku, Rebecca Baron, Lauren Fredenburgh, Alissa Genthon, Peter Hou, Anthony Massaro, Raghu Seethala; Cleveland Clinic: Abhijit Duggal, Duncan Hite, Ashish K Khanna; Columbia University: Daniel Brodie, Irene K Louh, Briana Short; Duke University Medical Center: Raquel Bartz, Mary L Cooter, Jordan C Komisarow; East Carolina University: Anupama Tiwari; Emory University: William Bender, James Blum; Grady Memorial Hospital: Annette Esper, Greg S. Martin; Harborview Medical Center: Eileen Bulger, Catherine L Hough, Anna Ungar; Intermountain Medical Center: Samuel M Brown, Colin K Grissom, Eliotte L Hirshberg, Michael J Lanspa, Ithan D Peltan; Johns Hopkins University: Roy G Brower, Sarina K Sahetya, R Scott Stephens; Mayo Clinic Florida: Pramod K Guru; Mayo Clinic Rochester: John K Bohman, Hongchuan Coville, Ognjen Gajic, Rahul Kashyap, John C O’Horo; Montefiore Medical Center: Jorge-Bleik Ataucuri-Vargas, Jen-Ting Chen, Michelle N Gong, Fiore Mastroianni; Northwell Health System: Negin Hajizadeh, Jamie Hirsch, Michael Qui, Molly Stewart; Oregon Health Sciences University: Akram Khan, Ebaad Haq, Makrina Kamel, Olivia Krol, Kimberly Lerner; Regions Hospital: David J Dries, John Marini; St. Agnes Hospital: Valentina Chiara Bistolfi Amaral, Anthony Martinez; St. Joseph’s Medical Center: Harry L Anderson, III, Jill Brown, Michael Brozik, Heidi Kemmer, Janet Obear; Temple University: Nina Gentile, Kraftin E Shreyer; University of Arizona - Banner Health: Charles Cairns, Cameron Hypes, Josh Malo, Jarrod Mosier, Bhupinder Natt; University of California Los Angeles: Steven Y Chang, Scott Hu, Ishan Mehta, Nida Qadir; University of Cincinnati: Richard Branson, Dina Gomaa, Betty Tsuei; University of Kentucky: Sanjay Dhar, Ashley Montgomery-Yates, Peter Morris; University of Michigan: Tina Chen, Sinan Hanna, Pauline K Park, Michael W Sjoding; University of Southern California: Alfredo Lee Chang, Perren Cobb, Janice M. Liebler; University of Utah: Estelle Harris, Nate Hatton, Gia Lewis, Stephen McKellar, Sanjeev Raman, Joseph Tonna; University of Washington: Ellen Caldwell, Sarah Dean, Shewit Giovanni.
Additional information: The e-Appendixes, e-Figure, and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
Drs Qadir and Bartz contributed equally to this manuscript as first authors. Drs Gong and Park contributed equally to this manuscript as senior authors.
FUNDING/SUPPORT: The authors have reported to CHEST that no funding was received for this study.
Contributor Information
the Severe ARDS: Generating Evidence (SAGE) Study Investigators:
Jay S. Steingrub, Mark Tidswell, Valerie M. Banner-Goodspeed, Kristin Brierley, Julia L. Larson, Ariel Mueller, Tereza Pinkhasova, Daniel Talmor, Imoigele Aisiku, Rebecca Baron, Lauren Fredenburgh, Alissa Genthon, Peter Hou, Anthony Massaro, Raghu Seethala, Abhijit Duggal, Duncan Hite, Ashish K. Khanna, Daniel Brodie, Irene K. Louh, Briana Short, Raquel Bartz, Mary L. Cooter, Jordan C. Komisarow, Anupama Tiwari, William Bender, James Blum, Annette Esper, Greg S. Martin, Eileen Bulger, Catherine L. Hough, Anna Ungar, Samuel M. Brown, Colin K. Grissom, Eliotte L. Hirshberg, Michael J. Lanspa, Ithan D. Peltan, Roy G. Brower, Sarina K. Sahetya, R Scott Stephens, Pramod K. Guru, John K. Bohman, Hongchuan Coville, Ognjen Gajic, Rahul Kashyap, John C. O’Horo, Jorge-Bleik Ataucuri-Vargas, Jen-Ting Chen, Michelle N. Gong, Fiore Mastroianni, Negin Hajizadeh, Jamie Hirsch, Michael Qui, Molly Stewart, Akram Khan, Ebaad Haq, Makrina Kamel, Olivia Krol, Kimberly Lerner, David J. Dries, John Marini, Valentina Chiara Bistolfi Amaral, Anthony Martinez, Harry L. Anderson, III, Jill Brown, Michael Brozik, Heidi Kemmer, Janet Obear, Nina Gentile, Kraftin E. Shreyer, Charles Cairns, Cameron Hypes, Josh Malo, Jarrod Mosier, Bhupinder Natt, Steven Y. Chang, Scott Hu, Ishan Mehta, Nida Qadir, Richard Branson, Dina Gomaa, Betty Tsuei, Sanjay Dhar, Ashley Montgomery-Yates, Peter Morris, Tina Chen, Sinan Hanna, Pauline K. Park, Michael W. Sjoding, Alfredo Lee Chang, Perren Cobb, Janice M. Liebler, Estelle Harris, Nate Hatton, Gia Lewis, Stephen McKellar, Sanjeev Raman, Joseph Tonna, Ellen Caldwell, Sarah Dean, and Shewit Giovanni
Supplementary Data
References
- 1.Bellani G., Laffey J.G., Pham T. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 2.Ferguson N.D., Fan E., Camporota L. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38(10):1573–1582. doi: 10.1007/s00134-012-2682-1. [DOI] [PubMed] [Google Scholar]
- 3.Rubenfeld G.D., Caldwell E., Peabody E. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 4.Villar J., Blanco J., Añón J.M. The ALIEN study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med. 2011;37(12):1932–1941. doi: 10.1007/s00134-011-2380-4. [DOI] [PubMed] [Google Scholar]
- 5.Ike J.D., Kempker J.A., Kramer M.R., Martin G.S. The association between acute respiratory distress syndrome hospital case volume and mortality in a U.S. cohort, 2002-2011. Crit Care Med. 2018;46(5):764–773. doi: 10.1097/CCM.0000000000003015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Acute Respiratory Distress Syndrome Network. Brower R.G., Matthay M.A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 7.Guerin C., Reignier J., Richard J.C. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 8.Duan E.H., Adhikari N.K.J., D’Aragon F. Management of acute respiratory distress syndrome and refractory hypoxemia. A multicenter observational study. Ann Am Thorac Soc. 2017;14(12):1818–1826. doi: 10.1513/AnnalsATS.201612-1042OC. [DOI] [PubMed] [Google Scholar]
- 9.Li X., Scales D.C., Kavanagh B.P. Unproven and expensive before proven and cheap: extracorporeal membrane oxygenation versus prone position in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2018;197(8):991–993. doi: 10.1164/rccm.201711-2216CP. [DOI] [PubMed] [Google Scholar]
- 10.Guerin C., Beuret P., Constantin J.M. A prospective international observational prevalence study on prone positioning of ARDS patients: the APRONET (ARDS Prone Position Network) study. Intensive Care Med. 2018;44(1):22–37. doi: 10.1007/s00134-017-4996-5. [DOI] [PubMed] [Google Scholar]
- 11.Weiss C.H., Baker D.W., Weiner S. Low tidal volume ventilation use in acute respiratory distress syndrome. Crit Care Med. 2016;44(8):1515–1522. doi: 10.1097/CCM.0000000000001710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Heart, Lung, and Blood Institute PETALS Clinical Trials Network. Moss M., Huang D.T. Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019;380(21):1997–2008. doi: 10.1056/NEJMoa1901686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Combes A., Hajage D., Capellier G. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378(21):1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 14.Villar J., Ferrando C., Martinez D. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8(3):267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 15.Gebistorf F., Karam O., Wetterslev J., Afshari A. Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) in children and adults. Cochrane Database Syst Rev. 2016;(6):CD002787. doi: 10.1002/14651858.CD002787.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Afshari A., Bastholm Bille A., Allingstrup M. Aerosolized prostacyclins for acute respiratory distress syndrome (ARDS) Cochrane Database Syst Rev. 2017;7:CD007733. doi: 10.1002/14651858.CD007733.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laffey J.G., Madotto F., Bellani G. Geo-economic variations in epidemiology, patterns of care, and outcomes in patients with acute respiratory distress syndrome: insights from the LUNG SAFE prospective cohort study. Lancet Respir Med. 2017;5(8):627–638. doi: 10.1016/S2213-2600(17)30213-8. [DOI] [PubMed] [Google Scholar]
- 18.Duggal A., Rezoagli E., Pham T. Patterns of use of adjunctive therapies in patients with early moderate to severe ARDS: insights from the LUNG SAFE Study. Chest. 2020;157(6):1497–1505. doi: 10.1016/j.chest.2020.01.041. [DOI] [PubMed] [Google Scholar]
- 19.Gupta S., Hayek S.S., Wang W. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;180(11):1436–1447. doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhatraju P.K., Ghassemieh B.J., Nichols M. Covid-19 in critically ill patients in the Seattle region—case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grasselli G., Zangrillo A., Zanella A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cochi S.E., Kempker J.A., Annangi S., Kramer M.R., Martin G.S. 10th. 13(10) Ann Am Thorac Soc.; 2016. Mortality trends of acute respiratory distress syndrome in the United States from 1999-2013; pp. 1742–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maca J., Jor O., Holub M. Past and present ARDS mortality rates: a systematic review. Respir Care. 2017;62(1):113–122. doi: 10.4187/respcare.04716. [DOI] [PubMed] [Google Scholar]
- 25.Pham T., Rubenfeld G.D. Fifty years of research in ARDS. The epidemiology of acute respiratory distress syndrome. A 50th birthday review. Am J Respir Crit Care Med. 2017;195(7):860–870. doi: 10.1164/rccm.201609-1773CP. [DOI] [PubMed] [Google Scholar]
- 26.Needham D.M., Colantuoni E., Mendez-Tellez P.A. Lung protective mechanical ventilation and two year survival in patients with acute lung injury: prospective cohort study. BMJ. 2012;344:e2124. doi: 10.1136/bmj.e2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Artis K.A., Dweik R.A., Patel B. Performance measure development, use, and measurement of effectiveness using the guideline on mechanical ventilation in acute respiratory distress syndrome. An official American Thoracic Society Workshop report. Ann Am Thorac Soc. 2019;16(12):1463–1472. doi: 10.1513/AnnalsATS.201909-665ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lanspa M.J., Gong M.N., Schoenfeld D.A. Prospective assessment of the feasibility of a trial of low-tidal volume ventilation for patients with acute respiratory failure. Ann Am Thorac Soc. 2019;16(3):356–362. doi: 10.1513/AnnalsATS.201807-459OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Urner M., Juni P., Hansen B., Wettstein M.S., Ferguson N.D., Fan E. Time-varying intensity of mechanical ventilation and mortality in patients with acute respiratory failure: a registry-based, prospective cohort study. Lancet Respir Med. 2020;8(9):905–913. doi: 10.1016/S2213-2600(20)30325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rubenfeld G.D., Cooper C., Carter G., Thompson B.T., Hudson L.D. Barriers to providing lung-protective ventilation to patients with acute lung injury. Crit Care Med. 2004;32(6):1289–1293. doi: 10.1097/01.ccm.0000127266.39560.96. [DOI] [PubMed] [Google Scholar]
- 31.Mikkelsen M.E., Dedhiya P.M., Kalhan R., Gallop R.J., Lanken P.N., Fuchs B.D. Potential reasons why physicians underuse lung-protective ventilation: a retrospective cohort study using physician documentation. Respir Care. 2008;53(4):455–461. [PubMed] [Google Scholar]
- 32.Dennison C.R., Mendez-Tellez P.A., Wang W., Pronovost P.J., Needham D.M. Barriers to low tidal volume ventilation in acute respiratory distress syndrome: survey development, validation, and results. Crit Care Med. 2007;35(12):2747–2754. doi: 10.1097/01.CCM.0000287591.09487.70. [DOI] [PubMed] [Google Scholar]
- 33.Sjoding M.W., Gong M.N., Haas C.F., Iwashyna T.J. Evaluating delivery of low tidal volume ventilation in six ICUs using electronic health record data. Crit Care Med. 2019;47(1):56–61. doi: 10.1097/CCM.0000000000003469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alhurani R.E., Oeckler R.A., Franco P.M., Jenkins S.M., Gajic O., Pannu S.R. Refractory hypoxemia and use of rescue strategies. A U.S. national survey of adult intensivists. Ann Am Thorac Soc. 2016;13(7):1105–1114. doi: 10.1513/AnnalsATS.201508-560OC. [DOI] [PubMed] [Google Scholar]
- 35.Qadir N., Chen J.T. Adjunctive therapies in ARDS: the disconnect between clinical trials and clinical practice. Chest. 2020;157(6):1405–1406. doi: 10.1016/j.chest.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Briel M., Meade M., Mercat A. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA. 2010;303(9):865–873. doi: 10.1001/jama.2010.218. [DOI] [PubMed] [Google Scholar]
- 37.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Wiedemann H.P., Wheeler A.P. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 38.Girard T.D., Kress J.P., Fuchs B.D. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371(9607):126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 39.Devlin J.W., Skrobik Y., Gelinas C. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825–e873. doi: 10.1097/CCM.0000000000003299. [DOI] [PubMed] [Google Scholar]
- 40.Papazian L., Forel J.M., Gacouin A. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363(12):1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.