Abstract
Background
In the United States, injection is an increasingly common route of administration for opioids and other substances. Estimates of the number of persons who inject drugs (PWID) are needed for monitoring risk-specific infectious disease rates and health services coverage.
Methods
We reviewed design and instruments for 4 national household surveys, 2012–2016, for their ability to produce unbiased injection drug use (IDU) prevalence estimates. We explored potential analytic adjustments for reducing biases through use of external data on (1) arrest, (2) narcotic overdose mortality, and (3) biomarker-based sensitivity of self-reported illicit drug use.
Results
Estimated national past 12 months IDU prevalence ranged from 0.24% to 0.59% across surveys. All surveys excluded unstably housed and incarcerated persons, and estimates were based on <60 respondents reporting IDU behavior in 3 surveys. No surveys asked participants about nonmedical injection of prescription drugs. Analytic adjustments did not appreciably change IDU prevalence estimates due to suboptimal specificity of data points.
Conclusions
PWID population size estimates in the United States are based on small numbers and are likely biased by undercoverage of key populations and self-report. Novel methods as discussed in this article may improve our understanding of PWID population size and their health needs.
Keywords: PWID, injection behavior, population-based surveys
In the United States, injection is an increasingly common and high-risk route of administration for prescription and illicit opioids [1–5] as well as other drugs such as methamphetamine [6, 7]. Unsafe injection drug use (IDU) behaviors increase risk for bloodborne infectious diseases such as hepatitis C virus (HCV) and human immunodeficiency virus (HIV), making these infectious diseases secondary but deleterious consequences of the opioid epidemic for persons who inject drugs (PWID) [8, 9]. Due to the stigmatized and illicit nature of nonmedical IDU, population-level prevalence is difficult to measure using survey methods typically used to monitor health-related behaviors. However, understanding the national and local scopes of IDU behavior is critical for informing infectious disease prevention efforts among PWID and to help ensure the success of ambitious strategies to eliminate HIV and HCV in the United States [10–13].
Information about the size and characteristics of the PWID population is needed to inform strategies to reduce IDU-associated infectious disease risk at multiple phases of intervention design and monitoring. First, PWID population size estimates are needed to enable data-informed resource allocation and program planning decisions. Second, as prevention and treatment interventions are implemented, estimates of PWID population size can help monitor progress toward coverage goals. Last, PWID population size is needed to monitor infectious disease rates among the at-risk population most in need of health interventions [14, 15].
Evidence of increasing IDU prevalence amplifies the need for current, accurate PWID population size estimates. The national drug overdose death rate increased from 6.1 to 21.7/100 000 during 1999–2017 [16]. Acute HCV infections increased by more than 3.5-fold during 2010–2017, mostly among young adults [17], and IDU-associated increases in HIV infections have recently been observed in rural areas where HIV is rare [1, 2, 18–20]. National increases in infectious conditions typically associated with IDU behavior, such as infectious endocarditis and central nervous system abscesses, have been observed in emergency room and hospital discharge data [21–24]. Indirect but highly correlated indications of IDU behavior suggest further increases in IDU-associated infections are likely.
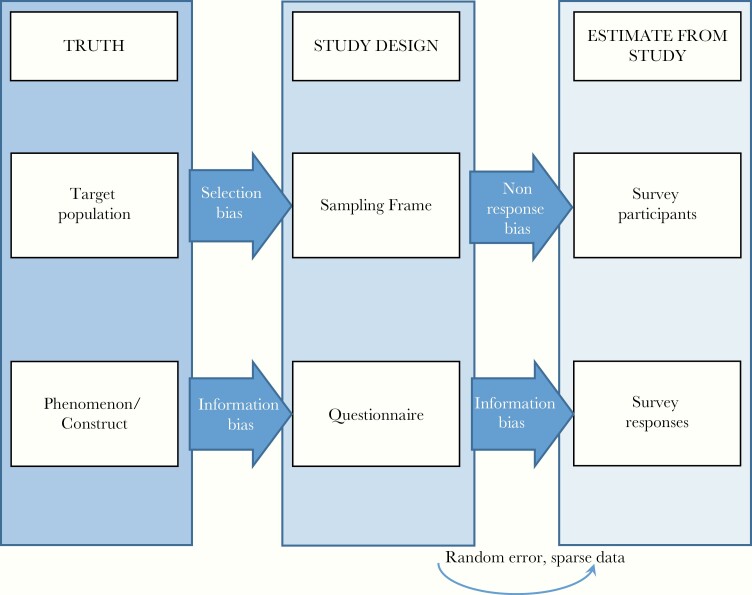
In the United States, like many health-related behaviors, IDU is measured and monitored primarily using household surveys, and prevalence estimates are derived from data from 1 or more surveys [25]. Estimates derived from survey data are generally subject to several types of bias (Figure 1) [26], which may variably affect estimates depending on the condition of interest. Two sources of bias threaten the ability of the study sample to reflect the underlying population of interest: (1) selection bias: when a sampling frame is suboptimally representative of the population, as is the case when, for example, homeless populations who may have relatively high IDU prevalence are not captured in a household survey; and (2) nonresponse bias: when nonresponse of sampled persons is differential by the condition of interest [27, 28]. Two levels of information bias threaten the ability of collected data to represent the construct of interest: (1) questions that are suboptimally designed to measure the construct of interest in the intended sample; and (2) inaccurate participant responses due to misunderstanding of survey questions, limited time or ability to complete the survey, or a desire to provide responses that are socially acceptable or preferred by the interviewer. Due to stigma and legal implications surrounding IDU behavior, response bias is particularly problematic [27, 28]. Random error may further distort estimates, particularly in the context of small sample sizes.
Figure 1.
Framework for evaluating bias in estimates from survey research. Adapted from Hulley et al, Designing Clinical Research, 4th ed [26].
To assess the comparative utility of household survey data sources for estimating PWID population size, we qualitatively evaluated how these biases may influence IDU prevalence estimates from national surveys currently collecting such data. We then conducted a feasibility assessment of potential adjustments to survey-derived estimates using established analytic methods and data external to household surveys. All proposed adjustments have either been previously used in other contexts or are extensions to methods previously used. We conclude with a discussion of possible improvements to survey estimates as well as emerging methods that may be used to estimate PWID population size.
METHODS
We examined 4 national population-based surveys in terms of ability to produce complete, unbiased, nationally representative measures of current nonmedical IDU prevalence. All 4 were US Department of Health and Human Services (HHS) surveys: the National Survey on Drug Use and Health (NSDUH, years 2015–2016) [29], the National Health and Nutrition Examination Survey (NHANES, 2013–2016) [30, 31], the National Survey of Family Growth (NSFG, 2013–2015) [32], and the National Institutes of Alcohol Abuse and Alcoholism (NIAAA)-supported Epidemiologic Survey on Alcohol and Related Conditions-III (NESARC-III, 2012–2013) [33]. Data from NSDUH, NHANES, and NSFG are in the public domain, and NESARC-III data were obtained through a data usage agreement with NIAAA for use of a limited access dataset.
For each survey, we abstracted and compared sampling frames and over-sampling strategies, interview modalities, questionnaire item(s) language, and relevant skip patterns. In order to gauge to what extent random error due to small sample sizes and influential data points may affect estimates and to assess potential for producing stratified prevalence estimates, we compared unweighted numbers of persons reporting past year IDU behavior across surveys. We computed weighted prevalence of past year IDU prevalence with associated 95% confidence intervals, limiting estimates to persons aged 18–64 years with the exception of the NSFG-derived estimate, due to an upper age limit of 44 years in the survey design.
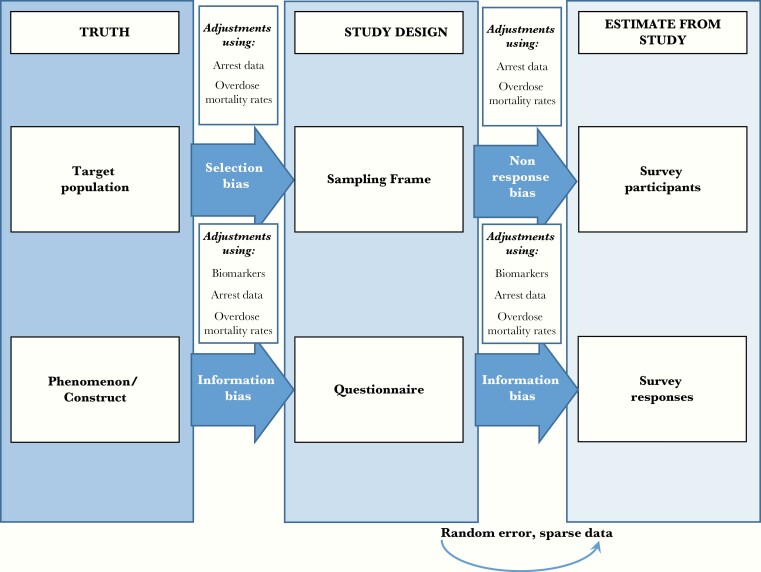
Next, using NSDUH data, we explored the feasibility of making 3 adjustments to population-based estimates of current IDU behavior in order to reduce biases identified (Figure 2). Where possible, we explored use of these adjustments within sex, age, and race/ethnicity strata. Stability of each stratified proportion, p, was assessed using NSDUH guidelines, relative standard error [−ln(p)] < 0.175 [34].
Figure 2.
Framework for evaluating bias in estimates from survey research and potential adjustments for reducing bias. Adapted from Hulley et al, Designing Clinical Research, 4th ed [26].
Biomarker-Based Adjustment Method
To address information biases and threats to internal validity of self-reported IDU (pIDU), we assessed feasibility of computing a standard multiplier for sensitivity of self-reported use of illicit drugs for use in adjusting misclassification of self-reported within demographic strata [14, 35]. We conceptualized such a multiplier as , where sensitivitySR is the number of individuals reporting such drug use within a specific recall period, divided by the total number testing positive for biomarkers of drug use.
We conducted a literature review to identify published estimates of the sensitivity of self-reported illicit (nonmarijuana) drug use. We searched studies published during 1995–2019 and indexed using PubMed. Search terms used were: [self-report(ed), report(ed), sensitivity, accuracy, veracity, validity], [drug (use)], [illegal, illicit, injection], [urinalysis, biomarker, bioassay, screen]. The primary inclusion criterion was reported sensitivity of self-reported nonmarijuana drug use. We excluded studies in drug treatment or rehabilitation settings and those conducted outside the United States. For studies meeting inclusion criteria, we abstracted relevant data and qualitatively evaluated whether a standard multiplier could be computed for improving IDU prevalence estimates.
Arrest Data Adjustment Method
Second, to address selection, nonresponse, and information biases in prevalence estimates, we explored possible use of a multiplier adjustment previously proposed by the National Institute on Drug Abuse. This method uses self-reported arrest and IDU in conjunction with an external estimate of the number of persons arrested in the United States during the past year [36]. Using NSDUH data, we estimated weighted prevalences of IDU in the past year among persons who did and did not report being arrested during the past year, overall and stratified by sex and age. Arrest data were not available by the same race/ethnicity categories used by NSDUH. We then scaled the prevalence of IDU to the US population by applying, within demographic strata, and from NSDUH to the total persons in the United States aged 18–64 years who were (Narrest) and were not (N∼arrest) arrested during the past year using external data from the 2016 Federal Bureau of Investigation Uniform Crime Report [37]. This multiplier is summarized by .
Because information was available only for total number of arrests, rather than number of persons arrested, we followed previous guidance on application of this method and divided the total number of arrests among persons aged 18–64 years in 2016 by the average number of arrests reported per person in NSDUH within sex and age strata [36]. The number of persons not arrested in 2016 was calculated by subtracting the number of persons arrested from the total population in 2016 [38]. Stratum-specific products of were summed for an adjusted PWID population size.
Narcotic Overdose Mortality Adjustment Method
Last, to address selection, nonresponse, and information biases in IDU prevalence estimates, we used temporal growth in narcotic overdose mortality rates as an external data multiplier. Within sex, age, and race/ethnicity strata, we applied the ratio of overdose death rates among US adults aged 18–64 years during 2015–2016 (μ2015−2016) to that during 2010–2011 (μ2010−2011) to the NSDUH IDU prevalence estimate in 2010–2011 . This multiplier is summarized as: .
Narcotic overdose death rates were estimated separately for 2010–2011 and 2015–2016 using National Vital Statistics System Multiple Cause of Death Mortality data and US Census Intercensal data. Multiple Cause of Death Mortality Microdata files (2010–2011 and 2015–2016) were obtained from the National Vital Statistics System. These data include individual death records for persons aged 18–64 years who lived in a US state or the District of Columbia and contained International Classification of Diseases, Tenth Revision (ICD-10) codes for multiple underlying causes of deaths. Narcotic overdose death was defined using the ICD-10 codes for unintentional poisoning by and exposure to narcotics and psychodysleptics (hallucinogens) (X42), unknown intention poisoning by and exposure to narcotics and psychodysleptics (hallucinogens) (Y12), unintentional poisoning by and exposure to other and unspecified drugs, medicaments, and biological substances (X44), or unknown intention poisoning by and exposure to other and unspecified drugs, medicaments, and biological substances (Y14). This definition has previously been shown to be more specific to injection-related overdose deaths than alternatives [39].
RESULTS
The estimated overall past year prevalence of IDU among the civilian, noninstitutionalized US population aged 18–64 years ranged from 0.24% in NESARC (2012–2013) to 0.59% in NSFG (2013–2015, among 18–44 year olds) (Table 1). All surveys sampled only persons within households from the civilian, noninstitutionalized population. Unweighted numbers of persons reporting IDU in the past year varied greatly from 38 (NHANES) to 380 persons (NSDUH).
Table 1.
National Estimates of Injection Drug Use in Past 12 Months From Population-Based Surveys and Survey Characteristics
| Survey | Year | Most Recent Weighted Estimate Among 18–64 Year Olds,a % (95% CI) | Unweighted Number With Outcome | Question(s) Used | Population Frame | Populations Over-Sampled | Interview Modality (eg, ACASI) | Skip Pattern Considerations |
|---|---|---|---|---|---|---|---|---|
| National Epidemiologic Survey on Alcohol and Related Conditions (NESARC)-III | 2012–2013 | 0.24 (.16– .31) | 58 | Did you take ANY medicines or drugs that we just talked about (sedatives or tranquilizers, painkillers, cocaine or crack, stimulants, club drugs, hallucinogens, heroin, other) on your own by injection with a needle in the last 12 months? | Civilian, noninstitutionalized persons aged 18 y and older | Census blocks with more than 26% of the population who identify as Hispanic, Black, or Asian | CAPI | Respondents were asked about injection only if they reported any drug use (of sedatives or tranquilizers, painkillers, cocaine or crack, stimulants, club drugs, hallucinogens, heroin, or other) apart from marijuana and/or inhalants/solvents |
| National Survey on Drug Use and Health (NSDUH) | 2015–2016 | 0.41 (.35– .47) | 380 | How long has it been since you last used a needle to inject (cocaine, heroin, methamphetamine, stimulants, any other drug not prescribed to you)? (options: within the past 30 days, more than 30 days ago but within the past 12 months, and more than 12 months ago). | Civilian, noninstitutionalized persons aged 12 y and older | Persons aged 12–25 y | ACASI | Respondents were asked about past year injection only if they reported lifetime use of predetermined drugs (for 2012–2013, drugs were cocaine, heroin, methamphetamine, or stimulants; for 2015–2016, drugs were cocaine, heroin, methamphetamine, or any other drug not prescribed to them) using a needle |
| National Health and Nutrition Examination Survey (NHANES) | 2013–2016 | 0.45 (.27– .63) | 38 | How long ago has it been since you last used a needle to inject a drug not prescribed by a doctor? (Time periods of 12 months or less.) | Civilian, noninstitutionalized persons aged 12–69 y | Persons who identify as Hispanic, non-Hispanic Black, non-Hispanic Asians, non-Hispanic White, or other persons at or below 130% federal poverty level, and persons aged 80 y and over | ACASI | Respondents were only asked if they indicated that they had ever used a needle to inject an illegal drug |
| National Survey of Family Growth (NSFG) | 2013–2015 | 0.59 (.35– .82) | 53 | During the last 12 months, how often have you shot up or injected drugs other than those prescribed for you? By shooting up, we mean anytime you might have used drugs with a needle, by mainlining, skin-popping, or muscling. | Civilianb, noninstitutionalized persons aged 15–44 y | Persons who identify as Black or Hispanic, or who are aged 15–19 y | ACASI | Applicable for all respondents |
Abbreviations: ACASI, audio-computer assisted self-interview; CAPI, computer-assisted personal interviewing; CI, confidence interval.
aAmong 18–44 year olds in NSFG.
bIncludes military living off base.
With regards to survey administration, all were administered using audio-computer assisted self-interview (ACASI) with the exception of NESARC. Questions used to capture past year injection and associated skip patterns varied greatly. NESARC and NSDUH asked participants whether and during what time frame they last injected if they previously reported use of prespecified drugs. NSFG asked everyone if they had injected in the past year, and NHANES asked about time since last injection among everyone who reported ever injecting. Questions from NSDUH, NSFG, and NHANES included language indicating the drugs in question were drugs not prescribed by a doctor or not specifically prescribed to the respondent.
NSDUH was the only survey in which an adequate number of respondents reported past year IDU behavior for computation of stratified prevalence estimates (n = 380), likely due to oversampling of persons aged 18–39 years. Other key advantages of the NSDUH survey are the use of ACASI and its focus on substance use behaviors, which requires specialized staff training for data collection among substance-using populations. For these reasons, NSDUH IDU prevalence estimates were used for feasibility assessment of the following correction factors.
Biomarker-Based Adjustment Method
Self-reported sensitivity of illicit, nonmarijuana drug use varied widely across published studies (Table 2) [40–46]. Biomarkers are used to validate drug use rather than mode of administration, so no studies evaluated sensitivity of self-reported injection behaviors. Seven studies met inclusion criteria; many were excluded because they were conducted in drug treatment or rehabilitation settings. Time frames for data collection ranged from 1996 to 2017.
Table 2.
Sensitivity of Self-Reported Illicit Drug Use Estimates From Research Studies in Nondrug-Treatment Settings
| Study | Year | Study Population Description | Self-Report Sensitivity, Proportion (n Reported Use/n With Drug Detected) |
Sample Collected for Gold Standard | Biomarker(s) Used for Gold Standard | Duration Used for Self-Report | Notes | ||
|---|---|---|---|---|---|---|---|---|---|
| Methamphetamine | Cocaine | Heroin | |||||||
| White et al [46] | 2010–2012 | 454 black MSM aged 18–40 y from Atlanta area | 1.0 (2/2)a | 0.54 (19/35) | 0.0 (0/0)b | Urine | Cocaine: benzoylecgonine. Heroin/opiates: morphine Methamphetamine: D-methamphetamine |
Past 12 mo | |
| 349 white MSM aged 18–40 y from Atlanta area | 0.82 (9/11) a |
0.84 (16/19) | NA | ||||||
| Ledgerwood et al [43] | 1996–1997 | 839 middle-aged men from a cohort of individuals deployed to Vietnam and a matched nonveteran group, 16.3% black, 78.2% white | 0.44c | 0.46c | 0.78b,c | Hair | Cocaine: cocaine, benzoylecgonine, or cocaethylene Opiates: codeine, morphine glucuronide, or 6-acetylmorphine Methamphetamine: amphetamine and methamphetamine Radioimmunoassay, GC-MS. 5 ng drug/10 g hair cutoff for GC-MS testing |
Past 90 d | 50% of veterans screened positive for drugs at enrollment in 1971c |
| Fendrich et al [40] | 2001–2002 | 627 Chicago residents aged 18–40 y selected by multistage area probability design: 42.8% aged 18–25 y; 25.5% aged 26–30y ; 31.8% aged 31–40 y; 35.6% black; 33.2% white; 22.1% Hispanic; 9.2% other | NA (tested for amphetamine, only 2 tested positive, both for MDMA) | Hair: 0.24c Saliva: 0.40c Urine: 0.46c | Hair: 0.38c Saliva: 0.60c Urine: 0.25c | Hair, saliva, and urine | Heroin: monoacetyl morphine or morphine Substances screened for drugs, then the samples that screened positive were tested by GC-MS |
Past year | |
| Hair: 0.22c Saliva: 0.30c Urine: 0.35c | Hair: 0.38c Saliva: 0.60c Urine: 0.25c | Past 90 d | |||||||
| Hair: 0.14c Saliva: 0.30c Urine: 0.30c | Hair: 0.38c Saliva: 0.60c Urine: 0.25c | Past 30 d | |||||||
| Li et al [44] | 2015–2017 | 1029 individuals aged 16–29 y, assigned male at birth from the Chicago, IL area who identify as gay or bisexual and/or reported sex with a man in the last 12 mo; 34.1% black, 24.9% white, 29.8% Hispanic | 0.33c | 0.37c | 0.27b,c | Urine | Methamphetamine: D-methamphetamine (500 ng/L) Cocaine: benzoylegonine (150 ng/L) Heroin: morphine (300 ng/L) |
Window period lower limit methamphetamine: 3 d, cocaine: 1 d, opiates: 1 d | |
| 0.28c | 0.24c | 0.27b,c | Window period upper limit methamphetamine: 5 d, cocaine: 3 d, opiates: 4 d | ||||||
| 0.44c | 0.53c | 0.727b,c | Past 6 mo | ||||||
| 0.39c | 0.50c | 0.64b,c | Past 30 d | ||||||
| Garg et al [41] | 2012 | 83 pregnant women from New Mexico; 80% Latina; 7% American Indian; 13% other | 0.37a,c | 0.33c | 0.478b,c | Urine | Not specified | Occasional use (once per month or less) | Women were selected on history of substance abusec |
| 0.29c | 0.17b,c | Regular use (more frequently than once per month) | |||||||
| Rendon et al [45] | 2014–2015 | 334 adults aged 18 y and older from Fort Worth, TX with mental health problems and living in supportive housing | 0.44 (8/18) | 0.44 (21/48) | NA | Saliva | Not specified | Past 72 h | |
| 0.67c | 0.60c | Past 90 d | |||||||
| Kim et al [42] | 1999 | 290 black men aged 18–55 y from inner-city Baltimore with documented hypertension or currently taking antihypertensive medications | 0.47 (42/89) | Urine | Not specified | Past 7 d | Drugs tested for were not specifiedc |
Abbreviations: GC-MS, gas chromatography mass spectrometry; MDMA, 3,4-methylenedioxy-methamphetamine; MSM, men who have sex with men; NA, not available.
aAll amphetamines, not just methamphetamine.
bAll opiates, not just heroin.
cn not available.
The studies were heterogeneous across study populations, biomarker(s) used (urine, hair, or saliva), drugs tested for, assays used for testing, and detectable time window of drug use due to recall periods for survey questions, biomarkers, and assays used. Generally, the numbers of people reporting nonmarijuana drug use was small. Self-report sensitivity estimates for methamphetamine ranged from 0.28 to 1.00, estimates for cocaine ranged from 0.14 to 0.60, and estimates for heroin ranged from 0.17 to 0.78. Due to heterogeneity in study designs and associated sensitivity estimates and the inability to stratify sensitivity estimates by demographic characteristics, we concluded it was imprudent to compute a combined correction factor for adjusting household survey IDU estimates.
Arrest Data Adjustment Method
Self-reported IDU prevalence was considerably higher among persons reporting arrest during the past year than among those not reporting arrest (5.55% vs 0.27% overall) (Table 3). Overall, we estimated 9.1 million people, or 4.5% of persons aged 18–64 years, were arrested during 2016 [37]. Applying the percentage of persons self-reporting IDU among arrested and nonarrested persons and summing the products across strata of arrested and nonarrested populations resulted in an adjusted overall IDU prevalence of 0.51%, a 0.10 percentage point increase over the unadjusted estimate. Larger differences were observed between adjusted and unadjusted estimates among males and younger persons.
Table 3.
National Estimates of Injection Drug Use in Past 12 Months Derived From NSDUH and Adjusted Using Ratio Estimator of Injection Drug Use to 2016 Arrest Data
| Characteristic | Col A | Col B | Col C | Col D | Col E | Col F | Col G | Col H | Col I |
|---|---|---|---|---|---|---|---|---|---|
| Survey-Based Estimate for IDU in p12m for Year 2015–2016, % | Survey-Based Estimate for IDU in p12m + Arrested in p12m/Arrested in p12m, % | Survey-Based Estimate for IDU in p12m + Not Arrested in p12m/Not Arrested in p12m, % | Number of People Arrested in Year 2016 | Number of People Not Arrested in Year 2016 | (Col B × Col D) | (Col C × Col E) | Adjusted Estimate for IDU in p12m in Year 2016 (Col F + Col G) | Adjusted % IDU in p12m in Year 2016 | |
| Race | |||||||||
| Non-Hispanic white | 0.52 | 8.33 | 0.35 | NA | NA | NA | NA | NA | |
| Non-Hispanic black | 0.17 | 1.23 | 0.12 | NA | NA | NA | NA | NA | |
| Hispanic | 0.31 | 5.20 | 0.15 | NA | NA | NA | NA | NA | |
| Other | 0.14 | 2.01 | 0.10 | NA | NA | NA | NA | NA | |
| Sex | |||||||||
| Male | 0.59 | 5.82 | 0.39 | 6 597 869 | 93 030 722 | 384 306 | 363 657 | 747 963 | 0.75 |
| Female | 0.23 | 4.91 | 0.15 | 2 471 842 | 98 247 406 | 121 390 | 148 747 | 270 136 | 0.27 |
| Age group, y | |||||||||
| 18–29 | 0.49 | 4.88 | 0.29 | 4 327 018 | 49 324 610 | 211 280 | 140 772 | 352 052 | 0.66 |
| 30–49 | 0.50 | 7.12 | 0.33 | 3 797 443 | 79 687 100 | 270 355 | 266 792 | 537 148 | 0.64 |
| 50–64 | 0.23 | … | 0.16 | 990 365 | 62 221 303 | … | 102 167 | … | … |
| Total | 0.41 | 5.55 | 0.27 | 9 082 056 | 191 265 783 | 503 972 | 510 871 | 1 014 843 | 0.51 |
Arrest data abstracted from the 2016 Federal Bureau of Investigation Uniform Crime Report.
Abbreviations: Col, column; IDU, injection drug use; NA, not available; NSDUH, National Survey on Drug Use and Health; p12m, past 12 months.
Narcotic Overdose Mortality Adjustment Method
For the narcotic overdose correction factor, we used 2010–2016 growth in the narcotic overdose death rate to scale up self-reported IDU prevalence estimated from 2010–2011 NSDUH (Table 4). IDU prevalence in 2010–2011 was 0.24% overall and, like the more recent estimate, was higher among males, non-Hispanic whites, and young people. Overall, the ratio of narcotic overdose death in 2015–2016 compared to 2010–2011 was 1.44 and was higher among males (1.51), 18–29 year olds (1.50), and non-Hispanic blacks (1.70).
Table 4.
National Estimates of Injection Drug Use in Past 12 Months Derived from NSDUH and Adjusted for Temporal Change in Narcotic Overdose Death During 2010–2016
| Characteristic | Col A | Col B | Col C | Col D | Col E |
|---|---|---|---|---|---|
| Survey-Based Estimate for Year 2010–2011, % | Narcotic Overdose Death Rate per 100 000 During 2010–2011a | Narcotic Overdose Death Rate 100 000 During 2015–2016a | (Col C/ Col B) | Adjusted Estimate for Year 2016 (Col A × Col D), % | |
| Race | |||||
| Non-Hispanic white | 0.31 | 21.21 | 30.78 | 1.45 | 0.44 |
| Non-Hispanic black | 0.11 | 12.99 | 22.02 | 1.70 | 0.19 |
| Hispanic | 0.12 | 7.59 | 11.26 | 1.48 | 0.18 |
| Other | 0.08 | 3.60 | 4.82 | 1.34 | 0.11 |
| Sex | |||||
| Male | 0.32 | 21.28 | 32.23 | 1.51 | 0.49 |
| Female | 0.16 | 12.16 | 15.96 | 1.31 | 0.21 |
| Age group, y | |||||
| 18–29 | 0.36 | 12.81 | 19.24 | 1.50 | 0.55 |
| 30–49 | 0.31 | 19.69 | 28.39 | 1.44 | 0.44 |
| 50–64 | … | 15.88 | 22.46 | 1.41 | … |
| Total | 0.24 | 16.69 | 24.06 | 1.44 | 0.34 |
Abbreviations: Col, column; NSDUH, National Survey on Drug Use and Health.
aOverdose rates are calculated as (2010 death + 2011 death) / (2010 population + 2011 population) × 100 000.
Application of the narcotic overdose death correction factor resulted in an overall adjusted IDU prevalence of 0.34%, ranging from 0.19% among Hispanic persons and non-Hispanic blacks to 0.44% among non-Hispanic whites and 0.21% among females to 0.49% among males. The overall estimate was marginally higher than the NSDUH prevalence 2010–2011 (0.24%) but lower than the unadjusted 2015–2016 estimate (0.41%).
DISCUSSION
The most recent prevalence estimates of past year IDU behavior from US household surveys range from 0.24% to 0.59% but are likely subject to multiple biases. Selection bias is likely introduced to estimates by noninclusion of unstably housed and incarcerated populations in sampling frames [47–50]. Survey nonresponse may also be differential by PWID status due to competing demands for time and fear of stigma and legal repercussions [27, 28]. Information bias may be introduced by suboptimally designed survey items [51] and underreporting of IDU behavior [36]. Apart from the NSDUH estimate, prevalence estimates from population-based surveys were based on just 38–58 respondents, small samples that may be disproportionately affected by random error and are too small for stratification by respondent characteristics.
We observed gaps in national surveys’ questionnaires that suggest their ability to measure IDU prevalence could be improved. First, survey questions should capture information about injection of prescription drugs, which has increased in recent years [3, 4], either using a universal question about any nonmedical injection or specific questions about prescription drug injection. Apart from NESARC-III, the surveys we assessed specified illicit drugs in all questionnaire items regarding injection, and NSDUH, NHANES, and NSFG items stipulated drugs that were “not prescribed.” Second, questionnaire items could be harmonized across surveys to facilitate meta-analyses that combine data from small samples, as was previously carried out by Lansky and colleagues [25]. Third, specialized training should be provided to interviewers to encourage interactions with PWID that avoid stigmatizing language and attitudes and are sensitive to competing demands for time and attention [27]. Due to intensive training needs, surveys focused on substance use, such as NSDUH or NESARC, are likely better suited to measuring IDU behavior than general health surveys including limited IDU questionnaire items.
To the extent biases we identified affect estimates, they may be reduced through the use of adjustments and external data. None of the external adjustments we explored were viable using existing data but may be in the future with additional external data points. Misclassified self-reported IDU behavior may be adjusted by external estimates of self-report sensitivity, although we found very few studies outside drug treatment settings that assessed sensitivity of self-reported illicit drug use. Differences in biomarkers and assays used, including types of drugs that are detectable and over what period of time, made it impractical to compute a standardized multiplier. To our knowledge, biomarker data on drugs that are primarily injected have not been previously used to adjust survey data for PWID population size estimates. More research is needed on sensitivity of self-reported IDU, and harmonization across studies may allow findings to be combined for adjustment of survey-derived prevalence estimates.
In terms of the other 2 adjustments we explored, the arrest data adjustment method assumes persons are equally likely to underreport IDU behavior and being arrested, which is unlikely. Additionally, arrest data were not available at the person level so the number of persons arrested had to be estimated from episode-level data. Adjusting IDU prevalence using narcotic overdose mortality data may have more potential for use in the future. In our demonstration, using 2010–2016 growth in narcotic overdose mortality as an adjustment to the 2010–2011 NSDUH IDU prevalence estimate resulted in an estimate that was implausibly lower than the 2015–2016 survey-derived prevalence estimate. This adjustment in its current form is problematic because case fatality varies by drugs used or injected. Synthetic opioids, which account for a large proportion of narcotic overdose deaths in the United States, likely have higher case fatality at the same rate of injection [52, 53]. Coverage of harm reduction and overdose prevention programs also varies across time and place, which modifies the association between injection behavior and overdose mortality [54, 55], and local resource and policies (eg, medical examiner vs coroner) for death certifications may also lead to underreporting of deaths due to narcotic overdose [56]. Additionally, selection and information bias in the 2010–2011 estimate carry over to the 2015–2016 estimate, although incorporating growth in an external indicator is still likely to produce a less underestimated IDU prevalence than using the survey data alone. Data on change in types of drugs used and overdose prevention programs may increase usefulness of narcotic overdose mortality for adjusting survey-derived IDU prevalence, but due to wide geographical variation in these data points, this adjustment may be more appropriate for local versus national estimates.
Ultimately, methods apart from national surveys are needed to better estimate PWID population size. Several types of emerging methods have previously been used to estimate hard-to-reach populations. Capture-recapture methods use joint probabilities of an individual appearing in multiple “captures” of a target population to estimate population size and have been carried out using both physical evidence of “captures” or appearances in multiple, linked datasets [57–65]. Service usage multipliers combine the percentage of target population members reporting usage of a particular service with the number of people receiving that service to inflate survey data to population size [51, 59, 66, 67]. Respondent-driven sampling methods have been used with successive sampling and weighting schemes to estimate the underlying population size from the size and characteristics of sampled networks [68–70]. Quantitative methods facilitating use of these techniques have advanced in recent years [71–74], but most still require intensive fieldwork and primary data collection.
Ideally, PWID population size estimates should be geographically specific enough to inform local programs but standardized across geographies to allow for comparative monitoring and optimal resource allocation. To our knowledge, standardized local estimates of PWID population size have only been estimated twice in the United States. Brady and colleagues estimated PWID population size in 96 metropolitan statistical areas through the use of an index created from external data sources anchored to previous estimates of PWID population size from 1992 and 1998 [75]. Estimates were later extended to 2007 using similar methods [76]. Current use of these estimates is limited, however, by their urban and suburban geographic focus and outdated parameters.
For future work in this area, robust analysis of large administrative data sources may offer promise for improved PWID population size estimation with fewer resource requirements compared to survey-based methods. For example, electronic medical records have been used to detect change in the frequency of conditions, such infectious endocarditis, that cause morbidity among PWID [21, 23, 24]. Additionally, injection behavior associated with narcotic overdose may be increasingly detectable from text analysis of emergency medical services, emergency room, and police response data [77]. Combining data sources may be most informative, for example using capture-recapture approaches with linked administrative data for estimation and temporal monitoring of PWID population size [60, 62, 63].
PWID are a hard-to-reach and likely growing population with complex health needs. Meaningful, population-level declines in bloodborne infections such as HIV and HCV will not be possible without increasing attention to disease surveillance and prevention in this risk group. To better serve PWID with infectious disease prevention and other health services, a much better understanding is needed of the number and characteristics of PWID in the United States, including in terms of variation across time and place. IDU prevalences derived from population surveys are subject to multiple sources of bias, and the extent of underestimation imposed by these biases is unclear. Improvements to surveys and the use of external data adjustments have potential to reduce bias in estimates, but innovative and robust nonsurvey-based strategies for estimating PWID population size are urgently needed.
Notes
Acknowledgment. This article was prepared using a limited-access dataset obtained from the National Institute on Alcohol Abuse and Alcoholism (NIAAA).
Disclaimer. This article does not reflect the opinions or views of the NIAAA or the US Government.
Supplement sponsorship. This supplement is sponsored by the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Conrad C, Bradley HM, Broz D, et al. ; Centers for Disease Control and Prevention (CDC) . community outbreak of HIV infection linked to injection drug use of oxymorphone–Indiana, 2015. MMWR Morb Mortal Wkly Rep 2015; 64:443–4. [PMC free article] [PubMed] [Google Scholar]
- 2. Cranston K, Alpren C, John B, et al. Notes from the field: HIV diagnoses among persons who inject drugs - Northeastern Massachusetts, 2015–2018. MMWR Morb Mortal Wkly Rep 2019; 68:253–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jones CM. Trends and key correlates of prescription opioid injection misuse in the United States. Addict Behav 2018; 78:145–52. [DOI] [PubMed] [Google Scholar]
- 4. Jones CM, Christensen A, Gladden RM. Increases in prescription opioid injection abuse among treatment admissions in the United States, 2004–2013. Drug Alcohol Depend 2017; 176:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Young AM, Havens JR. Transition from first illicit drug use to first injection drug use among rural Appalachian drug users: a cross-sectional comparison and retrospective survival analysis. Addiction 2012; 107:587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Al-Tayyib A, Koester S, Langegger S, Raville L. Heroin and methamphetamine injection: an emerging drug use pattern. Subst Use Misuse 2017; 52:1051–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Glick SN, Burt R, Kummer K, Tinsley J, Banta-Green CJ, Golden MR. Increasing methamphetamine injection among non-MSM who inject drugs in King County, Washington. Drug Alcohol Depend 2018; 182:86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. National Academies of Sciences, Engineering, and Medicine. Integrating responses at the intersection of opioid use disorder and infectious disease epidemics: Proceedings of a workshop. Washington, DC: National Academies Press, 2018. [PubMed] [Google Scholar]
- 9. Reardon S. The US opioid epidemic is driving a spike in infectious diseases. Nature 2019; 571:15–6. [DOI] [PubMed] [Google Scholar]
- 10. Division of Viral Hepatitis, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. Progress toward viral hepatitis elimination in the United States, 2017. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, Office of Infectious Diseases, NCHHSTP, 2017. [Google Scholar]
- 11. Fauci AS, Redfield RR, Sigounas G, Weahkee MD, Giroir BP. Ending the HIV epidemic: a plan for the United States. JAMA 2019; 321:844–5. [DOI] [PubMed] [Google Scholar]
- 12. New York State. New York State hepatitis C elimination campaign. https://www.endhepcny.org/. Accessed 6 May 2019.
- 13. Lerner AM, Fauci AS. Opioid injection in rural areas of the United States: a potential obstacle to ending the HIV epidemic [published online ahead of print 1 August 2019]. JAMA doi: 10.1001/jama.2019.10657. [DOI] [PubMed] [Google Scholar]
- 14. Rothman KJ, Greenland S, Lash TL. Modern epidemiology. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2008. [Google Scholar]
- 15. Choi J, Ki M, Kwon HJ, et al. Health indicators related to disease, death, and reproduction. J Prev Med Public Health 2019; 52:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Centers for Disease Control and Prevention, National Center for Health Statistics. Drug overdose mortality by state, 2019. https://www.cdc.gov/nchs/pressroom/sosmap/drug_poisoning_mortality/drug_poisoning.htm. Accessed 25 July 2019.
- 17. Centers for Disease Control and Prevention. Viral hepatitis surveillance United States, 2017. Atlanta, GA: Centers for Disease Control and Prevention, 2019. [Google Scholar]
- 18. Bradley H, Hogan V, Agnew-Brune C, et al. Increased HIV diagnoses in West Virginia counties highly vulnerable to rapid HIV dissemination through injection drug use: a cautionary tale. Ann Epidemiol 2019; 34:12–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nash B. Cabell HIV cluster growing at consistent rate. Herald-Dispatch 22 August 2019. [Google Scholar]
- 20. Paquette CE, Pollini RA. Injection drug use, HIV/HCV, and related services in nonurban areas of the United States: a systematic review. Drug Alcohol Depend 2018; 188:239–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Collier MG, Doshani M, Asher A. Using population based hospitalization data to monitor increases in conditions causing morbidity among persons who inject drugs. J Community Health 2018; 43:598–603. [DOI] [PubMed] [Google Scholar]
- 22. New York State. Ending the AIDS epidemic in New York State. https://www.health.ny.gov/diseases/aids/ending_the_epidemic/. Accessed 6 May 2019.
- 23. Schranz AJ, Fleischauer A, Chu VH, Wu LT, Rosen DL. Trends in drug use-associated infective endocarditis and heart valve surgery, 2007 to 2017: a study of statewide discharge data. Ann Intern Med 2019; 170:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wurcel AG, Anderson JE, Chui KK, et al. Increasing infectious endocarditis admissions among young people who inject drugs. Open Forum Infect Dis 2016; 3:ofw157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lansky A, Finlayson T, Johnson C, et al. Estimating the number of persons who inject drugs in the United States by meta-analysis to calculate national rates of HIV and hepatitis C virus infections. PLoS One 2014; 9:e97596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hulley SB, Cumming SR, Browner WS, Grady DG, Newman TB. Designing clinical research. 4th ed. Philadelphia, PA: Lippincott, Williams, & Wilkins, 2013. [Google Scholar]
- 27. Johnson TP. Sources of error in substance use prevalence surveys. Int Sch Res Notices 2014; 2014:923290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McCabe SE, West BT. Selective nonresponse bias in population-based survey estimates of drug use behaviors in the United States. Soc Psychiatry Psychiatr Epidemiol 2016; 51:141–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. US Department of Health and Human Services. Substance abuse and mental health data archive. National survey on drug use and health (NSDHU). https://datafiles.samhsa.gov/. Accessed 10 June 2020.
- 30. Centers for Disease Control and Prevention. National health and nutrition examination survey 2015–2016 data documentation, codebook, and frequencies 2017. https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/HEPC_I.htm. Accessed 1 January 2018.
- 31. Centers for Disease Control and Prevention. National health and nutrition examination survey 2013–2014 data documentation, codebook, and frequencies 2017. https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/HEPC_H.htm. Accessed 1 January 2018.
- 32. Centers for Disease Control and Prevention. National survey of family growth 2013–2015 public use data files, codebooks, and documentation, 2016. https://www.cdc.gov/nchs/nsfg/nsfg_2013_2015_puf.htm. Accessed 1 January 2018.
- 33. Grant B, Chu A, Sigman R, et al. Source and accuracy statement: National epidemiologic survey on alcohol and related conditions-III (NESARC-III). Rockville, MD: National Institute on Alcohol Abuse and Alcoholism, 2014. [Google Scholar]
- 34. US Department of Health and Human Services, Substance Abuse and Mental Health Services Administration. NSDUH 2016 methodological resource book (MRB), 2016. https://www.samhsa.gov/data/report/nsduh-2016-methodological-resource-book-mrb. Accessed 10 June 2020.
- 35. Greenland S. Basic methods for sensitivity analysis of biases. Int J Epidemiol 1996; 25:1107–16. [PubMed] [Google Scholar]
- 36. Harrison L, Hughes A. NIDA research monograph: the validity of self-reported drug use: improving the accuracy of estimates. Rockville, MD: National Institute on Drug Abuse, 1997. [PubMed] [Google Scholar]
- 37. US Department of Justice, Federal Bureau of Investigation. 2016 Crime in the United States. https://ucr.fbi.gov/crime-in-the-u.s/2016/crime-in-the-u.s.-2016/. Accessed 10 June 2020.
- 38. Summary File Data: 2012-2016 ACS 5-year Estimates. https://www.census.gov/programs-surveys/acs/data/summary-file.2016.html. Accessed 1 February 2018.
- 39. Rosenberg ES, Rosenthal EM, Hall EW, et al. Prevalence of hepatitis C virus infection in US States and the District of Columbia, 2013 to 2016. JAMA Network Open 2018; 1:e186371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fendrich M, Johnson TP, Wislar JS, Hubbell A, Spiehler V. The utility of drug testing in epidemiological research: results from a general population survey. Addiction 2004; 99:197–208. [DOI] [PubMed] [Google Scholar]
- 41. Garg M, Garrison L, Leeman L, et al. Validity of self-reported drug use information among pregnant women. Matern Child Health J 2016; 20:41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kim MT, Hill MN. Validity of self-report of illicit drug use in young hypertensive urban African American males. Addict Behav 2003; 28:795–802. [DOI] [PubMed] [Google Scholar]
- 43. Ledgerwood DM, Goldberger BA, Risk NK, Lewis CE, Price RK. Comparison between self-report and hair analysis of illicit drug use in a community sample of middle-aged men. Addict Behav 2008; 33:1131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li DH, Janulis P, Mustanski B. Predictors of correspondence between self-reported substance use and urinalysis screening among a racially diverse cohort of young men who have sex with men and transgender women. Addict Behav 2019; 88:6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rendon A, Livingston M, Suzuki S, Hill W, Walters S. What’s the agreement between self-reported and biochemical verification of drug use? A look at permanent supportive housing residents. Addict Behav 2017; 70:90–6. [DOI] [PubMed] [Google Scholar]
- 46. White D, Rosenberg ES, Cooper HL, et al. Racial differences in the validity of self-reported drug use among men who have sex with men in Atlanta, GA. Drug Alcohol Depend 2014; 138:146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bronson J, Stroop J, Zimmer S, Berzofsky M. Drug use, dependence, and abuse among state prisoners and jail inmates, 2007–2009. Washington, DC: US Department of Justice, Bureau of Justice Statistics, 2017. [Google Scholar]
- 48. Linton SL, Celentano DD, Kirk GD, Mehta SH. The longitudinal association between homelessness, injection drug use, and injection-related risk behavior among persons with a history of injection drug use in Baltimore, MD. Drug Alcohol Depend 2013; 132:457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Metraux S, Metzger DS, Culhane DP. Homelessness and HIV risk behaviors among injection drug users. J Urban Health 2004; 81:618–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stone J, Fraser H, Lim AG, et al. Incarceration history and risk of HIV and hepatitis C virus acquisition among people who inject drugs: a systematic review and meta-analysis. Lancet Infect Dis 2018; 18:1397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Althubaiti A. Information bias in health research: definition, pitfalls, and adjustment methods. J Multidiscip Healthc 2016; 9:211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. O’Donnell JK, Gladden RM, Seth P. Trends in deaths involving heroin and synthetic opioids excluding methadone, and law enforcement drug product reports, by census region - United States, 2006–2015. MMWR Morb Mortal Wkly Rep 2017; 66:897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths - United States, 2013–2017. MMWR Morb Mortal Wkly Rep 2018; 67:1419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Platt L, Minozzi S, Reed J, et al. Needle and syringe programmes and opioid substitution therapy for preventing HCV transmission among people who inject drugs: findings from a Cochrane review and meta-analysis. Addiction 2018; 113:545–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schranz AJ, Barrett J, Hurt CB, Malvestutto C, Miller WC. Challenges facing a rural opioid epidemic: treatment and prevention of HIV and hepatitis C. Curr HIV/AIDS Rep 2018; 15:245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tote KM, Bradley H, Martin EG, Yucel R, Rosenberg ES. Factors associated with incomplete toxicology reporting in drug overdose deaths, 2010–2016. Ann Epidemiol 2019; 38:65–9. [DOI] [PubMed] [Google Scholar]
- 57. Allen ST, O’Rourke A, White RH, Schneider KE, Kilkenny M, Sherman SG. Estimating the number of people who inject drugs in a rural county in Appalachia. Am J Public Health 2019; 109:445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Des Jarlais D, Khue PM, Feelemyer J, et al. Using dual capture/recapture studies to estimate the population size of persons who inject drugs (PWID) in the city of Hai Phong, Vietnam. Drug Alcohol Depend 2018; 185:106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kimber J, Hickman M, Degenhardt L, Coulson T, van Beek I. Estimating the size and dynamics of an injecting drug user population and implications for health service coverage: comparison of indirect prevalence estimation methods. Addiction 2008; 103:1604–13. [DOI] [PubMed] [Google Scholar]
- 60. King R, Bird SM, Hay G, Hutchinson SJ. Estimating current injectors in Scotland and their drug-related death rate by sex, region and age-group via Bayesian capture–recapture methods. Stat Methods Med Res 2009; 18:341–59. [DOI] [PubMed] [Google Scholar]
- 61. King R, Bird SM, Overstall A, Hay G, Hutchinson SJ. Injecting drug users in Scotland, 2006: Listing, number, demography, and opiate-related death-rates. Addict Res Theory 2013; 21:235–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Leclerc P, Vandal AC, Fall A, et al. Estimating the size of the population of persons who inject drugs in the island of Montréal, Canada, using a six-source capture-recapture model. Drug Alcohol Depend 2014; 142:174–80. [DOI] [PubMed] [Google Scholar]
- 63. Raag M, Vorobjov S, Uuskula A. Prevalence of injecting drug use in Estonia 2010–2015: a capture-recapture study. Harm Reduct J 2019; 16:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ruiz MS, O’Rourke A, Allen ST. Using capture-recapture methods to estimate the population of people who inject drugs in Washington, DC. AIDS Behav 2016; 20:363–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Xu Y, Fyfe M, Walker L, Cowen LL. Estimating the number of injection drug users in greater Victoria, Canada using capture-recapture methods. Harm Reduct J 2014; 11:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Johnston LG, Prybylski D, Raymond HF, Mirzazadeh A, Manopaiboon C, McFarland W. Incorporating the service multiplier method in respondent-driven sampling surveys to estimate the size of hidden and hard-to-reach populations: case studies from around the world. Sex Transm Dis 2013; 40:304–10. [DOI] [PubMed] [Google Scholar]
- 67. Larney S, Hickman M, Guy R, et al. Estimating the number of people who inject drugs in Australia. BMC Public Health 2017; 17:757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Crawford FW, Wu J, Heimer R. Hidden population size estimation from respondent-driven sampling: a network approach. J Am Stat Assoc 2018; 113:755–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Johnston LG, McLaughlin KR, El Rhilani H, et al. Estimating the size of hidden populations using respondent-driven sampling data: case examples from Morocco. Epidemiology 2015; 26:846–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Li L, Assanangkornchai S, Duo L, McNeil E, Li J. Risk behaviors, prevalence of HIV and hepatitis C virus infection and population size of current injection drug users in a China-Myanmar border city: results from a respondent-driven sampling survey in 2012. PLoS One 2014; 9:e106899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bird SM, King R. Multiple systems estimation (or capture-recapture estimation) to inform public policy. Annu Rev Stat Appl 2018; 5:95–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. King R, Bird SM, Brooks SP, Hutchinson SJ, Hay G. Prior information in behavioral capture-recapture methods: demographic influences on drug injectors’ propensity to be listed in data sources and their drug-related mortality. Am J Epidemiol 2005; 162:694–703. [DOI] [PubMed] [Google Scholar]
- 73. King R, Brooks SP. On the Bayesian estimation of a closed population size in the presence of heterogeneity and model uncertainty. Biometrics 2008; 64:816–24. [DOI] [PubMed] [Google Scholar]
- 74. Overstall AM, King R. A default prior distribution for contingency tables with dependent factor levels. Stat Methodol 2014; 16:90–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Brady JE, Friedman SR, Cooper HL, Flom PL, Tempalski B, Gostnell K. Estimating the prevalence of injection drug users in the U.S. and in large U.S. metropolitan areas from 1992 to 2002. J Urban Health 2008; 85:323–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tempalski B, Pouget ER, Cleland CM, et al. Trends in the population prevalence of people who inject drugs in US metropolitan areas 1992–2007. PLoS One 2013; 8:e64789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Centers for Disease Control and Prevention. Enhanced state opioid overdose surveillance, 2019. https://www.cdc.gov/drugoverdose/foa/state-opioid-mm.html. Accessed 28 June 2019.