Abstract
Background
Despite the international endorsement of multidisciplinary tumor boards (MTBs) for breast cancer care, implementation is suboptimal worldwide, and evidence regarding their effectiveness in developing countries is lacking. We assessed the impact on survival and the cost‐effectiveness of implementing an MTB in Mozambique, sub‐Saharan Africa.
Materials and Methods
This prospective cohort study included 205 patients with breast cancer diagnosed between January 2015 and August 2017 (98 before and 107 after MTB implementation), followed to November 2019. Pre‐ and post‐MTB implementation subcohorts were compared for clinical characteristics, treatments, and overall survival. We used hazard ratios and 95% confidence intervals (CI), computed by Cox proportional hazards regression. The impact of MTB implementation on the cost per quality‐adjusted life year (QALY) was estimated from the provider perspective.
Results
We found no significant differences between pre‐ and post‐MTB subcohorts regarding clinical characteristics or treatments received. Among patients with early breast cancer (stage 0–III; n = 163), the 3‐year overall survival was 48.0% (95% CI, 35.9–59.1) in the pre‐MTB and 73.0% (95% CI, 61.3–81.6) in the post‐MTB subcohort; adjusted hazard ratio, 0.47 (95% CI, 0.27–0.81). The absolute 3‐year mean cost increase was $119.83 per patient, and the incremental cost‐effectiveness ratio was $802.96 per QALY, corresponding to 1.6 times the gross domestic product of Mozambique.
Conclusion
The implementation of a MTB in Mozambique led to a 53% mortality decrease among patients with early breast cancer, and it was cost‐effective. These findings highlight the feasibility of implementing this strategy and the need for scaling‐up MTBs in developing countries, as a way to improve patient outcomes.
Implications for Practice
Currently, more than half of the deaths from breast cancer in the world occur in developing countries. Strategies that optimize care and that are adjusted for available resources are needed to improve the outcomes of patients with breast cancer in these regions. The discussion of cases at multidisciplinary tumor boards (MTBs) may improve survival outcomes, but implementation is suboptimal worldwide, and evidence regarding their effectiveness in developing countries is lacking. This study evaluated the impact of implementing an MTB on the care and survival of patients with breast cancer in Mozambique, sub‐Saharan Africa and its cost‐effectiveness in this low‐income setting.
Keywords: Breast neoplasms, Sub‐Saharan Africa, Developing countries, Survival analysis, Cost‐effectiveness analysis
Short abstract
This article evaluates the effect of implementing a multidisciplinary tumor board on the care and survival of patients with breast cancer in Mozambique, Sub‐Saharan Africa, including the cost‐effectiveness of this strategy in a low‐resource setting.
Introduction
Breast cancer is the most frequent cancer among women in the world, and its incidence rates have been increasing over the last decades, especially in developing countries [1]. Specifically in Mozambique, it is currently the second most incident cancer in Maputo City, with an age‐standardized rate of 15.5 per 100,000 women [2].
Breast cancer is still the most frequent cause of cancer death among women in developing regions, and age‐standardized mortality rates are higher in low‐ and medium‐income than in high‐income countries (14.9 vs. 11.6 per 100,000 women, respectively) [1]. In sub‐Saharan Africa, this is partly due to the large proportion of patients with stage III/IV disease at presentation, which was around 75% in a large pooled analysis [3]. Additionally, the percentage of women diagnosed with stage IV disease may also be high, reaching 70% in some series [3]. Moreover, the high prevalence of triple‐negative breast cancer (TNBC) [4] and the limited access to adequate diagnosis and care, namely pathology services, surgical care, radiotherapy, and systemic treatment, also contribute to patients’ dismal prognosis [5].
The discussion of cases at multidisciplinary tumor boards (MTBs) may be one of the strategies to improve outcomes in breast cancer. Most international oncology societies consider multidisciplinarity an essential part of breast cancer care, proposing that all patients should be presented in a MTB before initiating treatment [6]. Nonetheless, there is a wide gap between this universal recommendation and its effective implementation, even in developed countries. A French multicentric study showed that, although all breast cancer cases were presented in an MTB, only 64% were discussed before surgery, with significant differences between academic vs. nonacademic hospitals [7]. In a report using data from the U.S. SEER‐Medicare database, only 70% of patients had access to multidisciplinary care, and this proportion was even lower among those living in a rural area [8]. In an Australian study, only 60% of breast cancer cases diagnosed during 2010–2015 were discussed in MTBs [9]. In China, less than 2% of patients operated for breast cancer during 2006–2016 in a large cancer center were discussed in an MTB [10], and, in Taiwan, only 28% were presented in a multidisciplinary meeting [11]. In a recent survey conducted among members of the African Organisation for Research and Training in Cancer, 80% of the respondents reported they were involved in a multidisciplinary breast cancer team, but this is likely to be an overestimate, as there were only 37 respondents [12]. A total of 72% of the participants in a survey conducted in Arab countries reported having an MTB at their institution, although only 49% of these MTBs met on a weekly basis [13].
Data regarding the impact of breast cancer MTBs on survival is even scarcer. Although a large study from the U.K. and another from Germany demonstrated that multidisciplinary care improved survival among patients with breast cancer [14, 15], a population‐based study from Germany did not present such a survival benefit [16]. Studies from China and Taiwan showed a survival advantage with the implementation of multidisciplinary care for breast cancer [10, 11], but there are no data from other parts of the world. A study from the U.K. revealed that MTBs can be expensive resources [17], and they may be burdensome to some clinical departments, raising the question of their added value [18]. However, we could not find any study assessing the cost‐effectiveness of breast cancer MTBs.
Currently, more than half of the deaths from breast cancer in the world occur in developing countries. Therefore, it is paramount to develop strategies adjusted for the available resources that optimize care, translating into improved breast cancer survival. With this study, we aimed to assess the impact of implementing an MTB on the care and survival of patients with breast cancer in Mozambique. Additionally, its cost‐effectiveness was evaluated in this low‐resource setting.
Subjects, Materials, and Methods
Setting
Mozambique is a low‐income country from Eastern sub‐Saharan Africa with 30 million inhabitants. In 2018, the gross domestic product (GDP) was $499.00 per capita [19]. The average life expectancy is approximately 59 years [20], and 13.2% of adults aged 15–49 years are infected with HIV [21]. The country is now undergoing a surge of noncommunicable diseases, such as diabetes, cardiovascular disease, and cancer [22]. The density of physicians was 0.1/1000 inhabitants in 2017 [23], and the public health system, which is free‐of‐charge, is the largest health care provider. A total of 8.7% of the 2018 Mozambican state budget was allocated to the health sector, which is similar to other low‐income countries. It also relies on external resources (e.g., foreign donations, among others), which constituted 14% of the 2018 health budget [24]. The country has scarce resources for cancer care, namely in terms of facilities and health professionals. In 2015, there were only three pathology departments, two oncology units, and one surgery department with specialized surgical oncologists. Patients had free access to anthracyclines, cyclophosphamide, taxanes, methotrexate, and tamoxifen, although there were occasional interruptions in their supply. Trastuzumab and aromatase inhibitors were not provided by the public health system, and the first radiotherapy unit only opened in August 2019. Since 2013, the Calouste Gulbenkian Foundation has had a cancer care program for Mozambican health practitioners, in which they are trained in Portugal for short periods of time. Additionally, there are Cancer Registries in the Beira and Maputo Cities and in the Maputo Central Hospital [2, 25].
Study Design and Participants
We conducted the prospective Moza‐BC cohort study, which included consecutive patients with newly diagnosed breast cancer with a pathological diagnosis between January 2015 and August 2017 (supplemental online Fig. 1) [26]. Data on socio‐demographic characteristics, risk factors, HIV status, clinical and tumor characteristics, treatment, and survival of patients treated in the oncology unit of the Maputo Central Hospital were prospectively collected until July 2018. Survival data were updated through the Hospital's Cancer Registry, health records, and telephone interviews in November 2019.
Breast tissue samples were collected by surgical biopsy, breast surgery, and fine‐needle aspiration cytology and infrequently by core‐needle biopsies. Handling of breast specimens was standardized according to the American Society of Clinical Oncology/College of American Pathologists recommendations [27]. Expression of the estrogen receptor (ER), the progesterone receptor, and the overexpression and amplification of HER2 was assessed by immunohistochemistry on the primary tumor, as described in the literature [27, 28]. In cases in which the immunohistochemistry score of HER2 was 2+ (HER2‐equivocal), silver in situ hybridization was performed at the Centro Hospitalar São João, Portugal. Tumors were grouped according to the classic subtypes into ER‐positive/HER2‐negative, HER2‐positive (ER‐positive/HER2‐positive and ER‐negative/HER2‐positive), and TNBC (ER‐negative/ progesterone receptor‐negative/HER2‐negative).
Staging was defined by the AJCC TNM 7th edition classification [29]. Clinical staging was performed using clinical examination, mammography or breast ultrasound, chest x‐ray, and abdominal ultrasound. Computed tomography scans were occasionally performed, but bone scans and breast magnetic resonance imaging were not available.
Multidisciplinary Tumor Board
The above‐mentioned training program in cancer care, along with the assembling and follow‐up of this cohort, raised awareness among Maputo Central Hospital physicians regarding the need for a better coordination of breast cancer care. Therefore, in March 2016, conditions were set to start an MTB for breast cancer, which was the first one to ever occur in Mozambique. The MTB meetings were performed on a weekly basis and lasted 1 hour, with the participation of at least one member from the surgery, oncology, pathology, and radiology departments and with trainees from these different specialties. Although radiotherapy was not yet available in Mozambique at the time, its implementation process was already ongoing, and there were Mozambican physicians being trained abroad in radiation oncology. Thus, a radiation oncologist who had recently completed his training was also present at the MTB and would indicate if the patient needed radiotherapy or not, even if only a small fraction of patients could have access to it abroad. A secretary recorded all decisions. With the creation of the MTB, patients’ navigation inside the Maputo Central Hospital changed from erratic to organized (Fig. 1). Every new breast cancer case is now presented in the MTB, and patients with relapse are often discussed as well. The list of cases is sent to the team a few days in advance and the medical oncologist usually does the presentation. Moreover, an effort has been done by the oncology unit team to ensure the adherence of patients to systemic therapy and follow‐up care, which led to an increase in the number of follow‐up visits from three to four visits per year during the first 3 years after diagnosis.
Figure 1.

Implementation of the MTB at the Maputo Central Hospital. Before March 2016 (left panel), upon suspicion of a breast tumor, the patient was referred to the surgical department of the Maputo Central Hospital (MCH), and the surgeon usually asked for a breast fine‐needle aspiration cytology biopsy to be performed at the pathology department. Alternatively, the patient had a biopsy performed outside the MCH or the surgeon performed a surgical biopsy at the MCH, but the tumor sample was analyzed and diagnosed at the pathology department. In those cases, the pathologist referred the patient to the surgical department. The surgeon then decided if the patient was to be operated on first or if she needed neoadjuvant chemotherapy. In the latter situation, the surgeon referred the patient to the oncology unit and the medical oncologist asked for staging exams to be performed at the radiology department. Upon completion of neoadjuvant chemotherapy, the patient was referred back to the surgical department. Following surgery, the patient was sent to the oncology unit again, to decide if she should also undergo adjuvant therapy and to receive long‐term follow‐up. After March 2016 (right panel), the referral scheme of patients from outside the MCH was maintained. However, following the pathological diagnosis of breast cancer, the patient is now directly referred to the oncology unit. She is often seen on the same day by the “on‐duty” oncologist, who asks for staging exams and schedules the MTB discussion within 1–2 weeks. Every new case of breast cancer is presented at the MTB regardless of stage, and patients with relapse are often discussed as well. The MTB team decides if the patient with early breast cancer is to receive neoadjuvant chemotherapy or surgery first. Following completion of neoadjuvant chemotherapy, the oncologist refers the patient to the surgical department, but also verbally informs the surgeon of this referral at the weekly MTB. After surgery, the patient's case is discussed again at the MTB to decide if she should also undergo adjuvant treatment and its type. Dashed arrows: infrequent interaction; full arrows: frequent interaction. Abbreviation: MTB, multidisciplinary tumor board.
Ethics
The National Health Bioethical Committee of Mozambique approved this study (226/CNBS/15). All participants provided written informed consent.
Statistical Analysis
Two subcohorts were defined according to the date of the MTB implementation (March 2016). Baseline patient, tumor, and treatment characteristics were compared between the two subcohorts using χ2 test and Fisher's exact test for categorical variables and through adjusted odds ratios and 95% confidence intervals (CIs), computed using binomial logistic regression.
Overall survival was defined as time from diagnosis (date of pathological confirmation of breast cancer) until death by any cause. Among patients with early stage breast cancer (stage 0–III), disease‐free survival was defined as time from diagnosis until loco‐regional or distant relapse, second primary malignancy or death by any cause.
The median follow‐up duration was 41.0 months (maximum, 47 months) among patients on the pre‐MTB subcohort and 37.8 months (maximum, 43 months) among patients in the post‐MTB subcohort; thus, survival analyses were censored at 36 months and performed using the Kaplan‐Meier estimator. Comparisons of survival between the two subcohorts were accomplished through log‐rank tests and crude and adjusted hazard ratios (HRs) and corresponding 95% CIs, computed using Cox proportional hazards regression. Analyses were carried out in STATA version 15 (StataCorp, College Station, TX). All tests were two‐sided and a p value of <.05 was considered significant.
To estimate the impact of implementing a MTB on the cost per quality‐adjusted life year (QALY), measured over a 3‐year time horizon, a decision tree model was developed that integrated patient‐level data from this cohort study, including survival estimates (supplemental online Methods). All cost calculations assumed 2019 as the reference year. Costs determined in previous years were corrected for inflation using the GDP deflator, as proposed by the World Health Organization [30]; correction factors were extracted from the World Bank [31]. All costs determined in U.S. dollars (USD) or Euros were converted to Mozambique Meticais (MZN), using the exchange rates $1 = 63.8292 MZN and €1 = 70.5682 MZN, respectively; final results were converted back to USD, and are presented in both U.S. dollars and MZN. A detailed description of the cost‐effectiveness analysis methods can be found in the supplemental online Methods. The model was implemented using TreeAgePro 2018 R2.1 (Williamstown, MA).
Results
Patients’ median age was 48.0 years, and most were Black (98.0%), premenopausal (53.7%), and overweight or obese (54.1%) and presented with stage III/IV disease (74.1%). The prevalence of HIV infection was 25.4% (Table 1).
Table 1.
Patients’ characteristics and treatment according to date of diagnosis (pre‐ vs. post‐MTB implementation)
| Characteristics | Pre‐MTB, n = 98 | Post‐MTB, n = 107 | p a | aOR b (95% CI) |
|---|---|---|---|---|
| Age in years, n (%) | .945 | |||
| <40 | 26 (26.5) | 28 (26.2) | 1 | |
| 40–49 | 29 (29.6) | 28 (26.2) | 0.81 (0.36–1.80) | |
| 50–59 | 21 (21.4) | 25 (23.4) | 1.10 (0.48–2.56) | |
| ≥60 | 22 (22.4) | 26 (24.3) | 1.07 (0.46–2.47) | |
| Race, n (%) | .623 | |||
| Black | 97 (99.0 | 104 (97.2) | 1 | |
| Other c | 1 (1.0) | 3 (2.8) | 2.34 (0.22–25.41) | |
| Education in years, n (%) | .618 | |||
| 0 | 18 (22.0) | 23 (25.3) | 1 | |
| 1–4 | 14 (17.1) | 11 (12.1) | 0.63 (0.22–1.83) | |
| >4 | 50 (61.0) | 57 (62.6) | 1.09 (0.45–2.67) | |
| Missing | 16 | 16 | ||
| Place of residence, n (%) | .089 | |||
| South (including Maputo) | 80 (86.0) | 96 (94.1) | 1 | |
| Center/North | 13 (14.0) | 6 (5.9) | 0.30 (0.10–0.88) | |
| Missing | 5 | 5 | ||
| Menopausal status, n (%) | .889 | |||
| Premenopausal | 52 (53.1) | 58 (54.2) | 1 | |
| Postmenopausal | 46 (46.9) | 49 (45.8) | 0.47 (0.13–1.70) | |
| Body mass index, n (%) | .460 | |||
| Under/normal weight, <25 kg/m2 | 33 (37.9) | 44 (43.6) | 1 | |
| Overweight/obese, ≥25 kg/m2 | 54 (62.1) | 57 (56.4) | 0.81 (0.42–1.59) | |
| Missing | 11 | 6 | ||
| HIV status, n (%) d | .873 | |||
| Negative/unknown | 74 (75.5) | 79 (73.8) | 1 | |
| Positive | 24 (24.5) | 28 (26.2) | 1.28 (0.65–2.53) | |
| Classic subtype, n (%) | .028 | |||
| ER+/HER2− | 46 (46.9) | 45 (42.1) | 1 | |
| HER2+ | 22 (22.4) | 22 (20.6) | 0.98 (0.46–2.05) | |
| TNBC | 23 (23.5) | 17 (15.9) | 0.72 (0.33–1.56) | |
| Unknown e | 7 (7.1) | 23 (21.5) | 3.66 (1.39–9.64) | |
| Stage at diagnosis, n (%) | .538 | |||
| 0‐II f | 23 (23.5) | 30 (28.0) | 1 | |
| III | 52 (53.1) | 58 (54.2) | 0.92 (0.45–1.86) | |
| IV | 23 (23.5) | 19 (17.8) | 0.53 (0.22–1.31) | |
| Time from symptoms to diagnosis, n (%) | .598 | |||
| <180 days | 33 (54.1) | 32 (50.0) | 1 | |
| ≥180 days | 28 (45.9) | 33 (50.8) | 1.23 (0.57–2.64) | |
| Missing | 37 | 42 | ||
| Time from diagnosis to treatment, n (%) | .324 | |||
| <45 days | 42 (44.7) | 55 (51.9) | 1 | |
| ≥45 days | 52 (55.3) | 51 (48.1) | 0.69 (0.38–1.25) | |
| Missing | 4 | 1 | ||
| No treatment received: yes, n (%) | 3 (3.1) | 1 (0.9) | .351 | 0.32 (0.03–3.52) |
| Type of first treatment received, n (%) | .999 | |||
| Surgery | 24 (25.5) | 26 (24.5) | 1 | |
| Chemotherapy/endocrine therapy | 70 (74.5) | 80 (75.5) | 0.94 (0.48–1.86) | |
| Missing | 1 | 0 | ||
| Surgery (ever): yes, n (%) g | 79 (80.6) | 88 (82.2) | .858 | 1.44 (0.59–3.50) |
| Type of breast surgery, n (%) h | .257 | |||
| Total mastectomy | 75 (94.9) | 79 (89.8) | 1 | |
| Tumorectomy | 4 (5.1) | 9 (10.2) | 2.44 (0.65–9.23) | |
| Status of surgical margins, n (%) | .575 | |||
| Clean | 61 (88.4) | 72 (92.3) | 1 | |
| Positive | 8 (11.6) | 6 (7.7) | 0.66 (0.20–2.19) | |
| Missing i | 10 | 10 | ||
| Histological grade at surgery, n (%) | .422 | |||
| 1 | 16 (22.2) | 20 (27.0) | 1 | |
| 2 | 35 (48.6) | 28 (37.8) | 0.62 (0.25–1.51) | |
| 3 | 21 (29.2) | 26 (35.1) | 1.22 (0.47–3.19) | |
| Missing i | 7 | 14 | ||
| Axillary surgery: type, n (%) h | 1.0 | |||
| Sentinel lymph node biopsy | 0 | 1 (1.3) | ||
| Axillary dissection | 70 (100) | 77 (98.7) | ||
| Axillary surgery: completeness, n (%) j | .423 | |||
| Not done | 9 (11.7) | 9 (10.7) | 0.93 (0.30–2.89) | |
| Incomplete | 23 (29.9) | 18 (21.4) | 0.58 (0.27–1.24) | |
| Complete | 45 (58.4) | 57 (67.9) | 1 | |
| Missing | 2 | 4 | ||
| Lymph node status, n (%) | .828 | |||
| (y)pN0 | 18 (27.7) | 22 (32.4) | 1 | |
| (y)pN1 | 23 (35.4) | 25 (36.8) | 0.97 (0.39–2.43) | |
| (y)pN2 | 13 (20.0) | 13 (19.1) | 0.94 (0.30–2.93) | |
| (y)pN3 | 11 (16.9) | 8 (11.8) | 0.58 (0.17–2.04) | |
| Missing i | 14 | 20 | ||
| Chemotherapy (ever): yes, n (%) | 90 (91.8) | 103 (96.3) | .237 | 2.36 (0.65–8.58) |
| Intent of first‐line CT, n (%) k | .174 | |||
| Neoadjuvant only | 8 (8.9) | 20 (19.4) | 1 | |
| Neoadjuvant + adjuvant | 45 (50.0) | 45 (43.7) | 0.50 (0.19–1.31) | |
| Adjuvant only | 16 (17.8) | 20 (19.4) | 0.64 (0.21–1.96) | |
| Palliative | 21 (23.3) | 18 (17.5) | ||
| Neoadjuvant CT: outcome, n (%) | .682 | |||
| Same stage | 19 (42.2) | 22 (37.9) | 1 | |
| Upstaging | 8 (17.8) | 8 (13.8) | 0.72 (0.20–2.60) | |
| Downstaging | 18 (40.0) | 28 (48.3) | 1.35 (0.51–3.59) | |
| Missing l | 8 | 7 | ||
| pCR rate after neoadjuvant CT, n (%) | .494 | |||
| No pCR | 48 (90.6) | 56 (86.2) | 1 | |
| pCR only in the breast (ypT0/is) | 3 (5.7) | 3 (4.6) | 0.71 (0.11–4.75) | |
| pCR in the breast and lymph nodes (ypT0/is, ypN0) | 2 (3.8) | 6 (9.2) | 2.00 (0.34–11.96) | |
| Type of first‐line CT regimen, n (%) k | .393 | |||
| Anthracycline‐based only | 44 (48.9) | 45 (43.7) | 1 | |
| Anthracyclines + taxanes based | 40 (44.4) | 55 (53.4) | 1.22 (0.61–2.43) | |
| Other m | 6 (6.7) | 3 (2.9) | 0.37 (0.07–1.80) | |
| First‐line CT dose‐intensity, n (%) k | .881 | |||
| <85% | 48 (57.8) | 60 (59.4) | 1 | |
| ≥85% | 35 (42.2) | 41 (40.6) | 0.95 (0.50–1.79) | |
| Endocrine therapy (ever): yes, n (%) | 50 (51.0) | 54 (50.5) | .888 | 0.87 (0.45–1.66) |
| Radiotherapy (ever): yes, n (%) | 2 (2.0) | 7 (6.5) | .175 | 3.88 (0.74–20.27) |
p value for the univariate comparison between the two subcohorts. Values in bold correspond to statistically significant differences.
Adjusted for age (<40 vs. 40–49 vs. 50–59 vs. ≥60 years), HIV status (negative/unknown vs. positive), stage at diagnosis (0–II vs. III vs. IV), and classic subtypes (ER+/HER2− vs. HER2+ vs. TNBC vs. unknown). The reference is the “pre‐MTB” implementation period. Values in bold correspond to statistically significant differences.
Includes mixed and Asian race.
Eight patients had unknown HIV status; among HIV‐positive patients, 38 (74%) had been previously diagnosed with HIV infection; the median time since HIV diagnosis was 3.93 years (range, 0.1–12.2); 48 (98.1%) patients were under antiretroviral treatment (ART) when starting chemotherapy, mostly with the TDF + 3TC + EFV regimen (33 patients); the median time under ART was 2.00 years (range, 0.1–12.2); the median CD4+ cells count was 439 cells/μL (range, 43–1,104), and 46 (90%) patients had a CD4+ cell count >200/μL.
Biomarker determination unknown due to carcinoma in situ or pathological complete response (i.e., no residual invasive tumor): n = 8 (n = 3 in the pre‐MTB and n = 5 in the post‐MTB subcohort); inadequate tissue fixation: n = 2 (0/2); unavailable samples: n = 20 (4/16).
Among patients within the category 0–II stage, one patient had stage 0 and four patients had stage I.
Includes 12 patients submitted to surgery outside the Maputo Central Hospital for whom complete pathological information regarding the surgical specimen is not available.
Patients submitted to a tumorectomy followed by a mastectomy were included in the "Mastectomy" group and those receiving a sentinel lymph node biopsy followed by an axillary dissection were included in the “Axillary dissection” group.
Cases with missing data for surgical margins, histological grade, or lymph node status include patients submitted to surgery outside the Maputo Central Hospital for whom complete pathological information is not available regarding the surgical specimen; patients with pathological complete response; patients for whom axillary surgery was not performed; and patients for whom these assessments at the Maputo Central Hospital are missing.
Among patients receiving any type of breast surgery (n = 166). Not done: not done or no isolated lymph nodes; incomplete: 1–5 isolated lymph nodes (in case of axillary lymph node dissection); complete: ≥6 isolated lymph nodes (in case of axillary lymph node dissection) or ≥ 1 isolated lymph nodes with ≤2 positive lymph nodes (in case of sentinel lymph node biopsy).
First line of chemotherapy that the patient received includes neoadjuvant, adjuvant, or palliative treatment. If the patient received part of chemotherapy as neoadjuvant (e.g., doxorubicin plus cyclophosphamide [AC] regimen) and another part as adjuvant chemotherapy (e.g., paclitaxel), the type of regimen and dose‐intensity refer to the entire scheme (neoadjuvant plus adjuvant).
Cases with missing data regarding clinical staging or patient abandoned treatment.
Includes taxane‐based chemotherapy (three patients), nonanthracycline/nontaxane‐based chemotherapy (five patients), and unknown regimen (one patient). The preferred anthracycline‐containing regimen was AC (cyclophosphamide 600 mg/m2 and doxorubicin 60 mg/m2 every 3 weeks), and the preferred taxane used was paclitaxel (175 mg/m2 every three weeks); dose‐dense regimens were not used because of the unpredictable availability of granulocyte‐colony stimulating factors.
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; CT, chemotherapy; ER, estrogen receptor; MTB, multidisciplinary tumor board; pCR, pathological complete response; TNBC, triple‐negative breast cancer.
A total of 98 patients were included in the pre‐MTB subcohort and 107 patients were included in the post‐MTB subcohort. Patients in the post‐MTB subcohort had a higher chance of having an unknown breast cancer subtype and of living in the South of Mozambique, but there were no significant differences regarding other clinico‐pathological characteristics. Nearly half of the patients were diagnosed more than 6 months after the first symptoms, and half of them started treatment more than 45 days after diagnosis. The MTB implementation led to a slight increase in the use of chemotherapy (from 91.8% to 96.3%), the use of neoadjuvant chemotherapy only (from 8.9% to 19.4%), tumor downstaging with chemotherapy (from 40.0% to 48.3%), and the use of taxanes with anthracyclines (from 44.4% to 53.4%); however, these differences were not significant. There were also nonsignificant increases in the proportions of clean surgical margins (from 88.4% to 92.3%) and in complete axillary surgery (from 58.4% to 67.9%).
Overall Survival
A total of 23 women (11.2%) were lost to follow‐up, as their last contact with the hospital was more than 12 months before the survival cut‐off date, but they were included in the analysis. The 3‐year overall survival was 44.8% (95% CI, 34.2–54.8) in the pre‐MTB and 62.6% (95% CI, 51.8–71.6) in the post‐MTB subcohort (Fig. 2A). When restricting the analysis to patients with early breast cancer (stage 0–III), 3‐year overall survival was 48.0% (95% CI, 35.9–59.1) in the pre‐MTB and 73.0% (95% CI, 61.3–81.6) in the post‐MTB subcohort (Fig. 2B). Among patients with metastatic breast cancer, there was no survival benefit with the introduction of the MTB: median overall survival was 19.4 months (95% CI, 15.6–23.2) before and 13.6 months (95% CI, 9.2–17.9) after MTB implementation (Fig. 2C).
Figure 2.

Kaplan‐Meier curves for overall survival among all patients, patients with early breast cancer and patients with metastatic breast cancer; and for disease‐free survival among patients with early breast cancer. (A): Overall survival in the total population: 3‐year overall survival was 44.8% (95% confidence interval [CI], 34.2–54.8) in the pre‐MTB subcohort versus 62.6% (95% CI, 51.8–71.6) in the post‐MTB subcohort. (B): Overall survival among patients with early breast cancer (stage 0–III): 3‐year overall survival was 48.0% (95% CI, 35.9–59.1) in the pre‐MTB subcohort versus 73.0% (95% CI, 61.3–81.6) in the post‐MTB subcohort. (C): Overall survival among patients with metastatic breast cancer (stage IV): median overall survival was 19.4 months (95% CI, 15.6–23.2) in the pre‐MTB subcohort versus 13.6 months (95% CI, 9.2–17.9) in the post‐MTB subcohort. (D): Disease‐free survival among patients with early breast cancer (stage 0–III): 3‐year disease‐free survival was 41.7% (95% CI, 30.2–52.8) in the pre‐MTB subcohort versus 56.8% (95% CI, 45.3–66.8) in the post‐MTB subcohort. Abbreviation: MTB, multidisciplinary tumor board.
In the multivariable analysis, the adjusted HR for death in the post‐MTB vs. the pre‐MTB subcohort was 0.77 (95% CI, 0.49–1.19) in the overall population and 0.47 (95% CI, 0.27–0.81) among patients with early breast cancer (Fig. 3; supplemental online Tables 1–2). Factors significantly associated with overall survival in both the overall population and patients with early breast cancer were stage, tumor subtype, and type of first‐line chemotherapy regimen.
Figure 3.

Multivariable analyses of prognostic factors for overall survival (left panel) and disease‐free survival (right panel) among patients with early breast cancer (stage 0–III). All models included the following variables: MTB implementation period (pre vs. post), age (<40 vs. 40–49 vs. 50–59 vs. ≥60 years), HIV status (negative/unknown vs. positive), stage at diagnosis (0–II vs. III), and tumor subtype (ER+/HER2− vs. HER2+ vs. TNBC vs. unknown). ¥Among patients receiving any type of breast surgery. Not done: not done or no isolated lymph nodes; incomplete: 1–5 isolated lymph nodes (in case of axillary lymph node dissection); complete: ≥6 isolated lymph nodes (in case of axillary lymph node dissection) or ≥1 isolated lymph nodes with ≤2 positive lymph nodes (in case of sentinel lymph node biopsy). §First‐line of chemotherapy that the patient received includes neoadjuvant and/or adjuvant treatment. If the patient received part of chemotherapy as neoadjuvant (e.g., doxorubicin plus cyclophosphamide [AC] regimen) and another part as adjuvant chemotherapy (e.g., paclitaxel), the type of regimen refers to the entire scheme (neoadjuvant plus adjuvant). Abbreviations: CI, confidence interval; CT, chemotherapy; ER, estrogen receptor; HR, hazard ratio; MTB, multidisciplinary tumor board; TNBC, triple‐negative breast cancer.
There was a benefit in MTB implementation among most subgroups of patients with early breast cancer (Fig. 4). There were apparently no benefits among those with lower education, HER2‐positive disease, grade 1 tumors, or receiving surgery as first treatment, but the low precision of the estimates precludes bold statements regarding stratum‐specific results or potential interactions.
Figure 4.
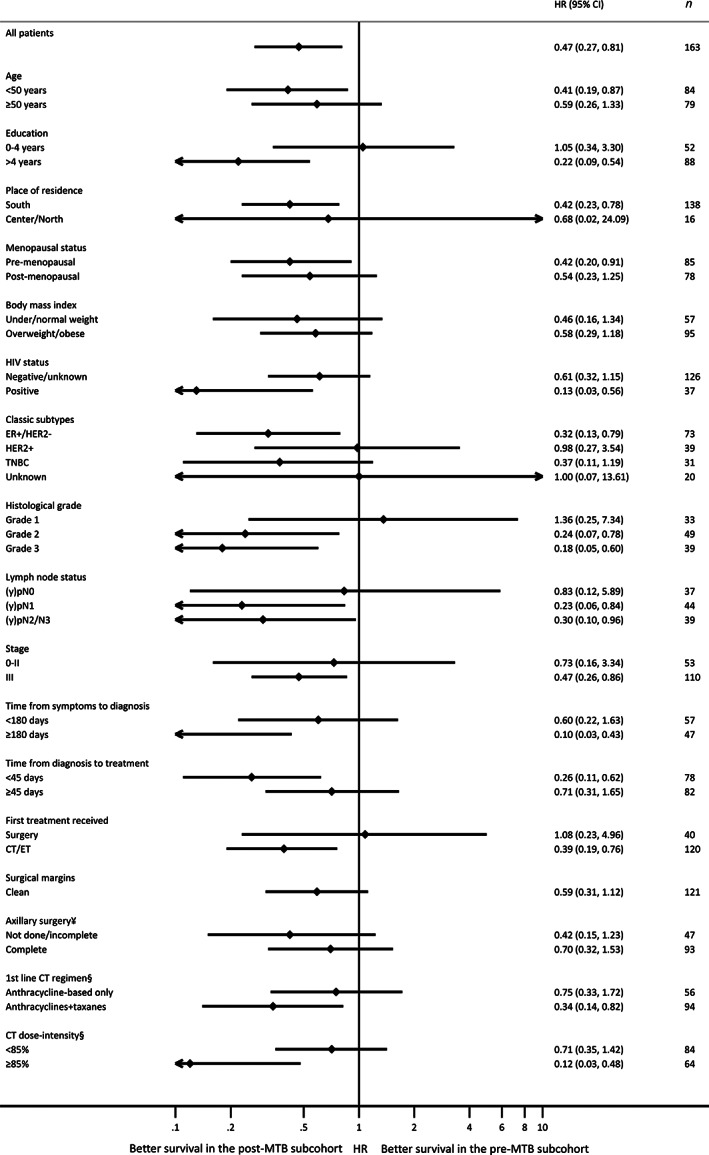
Multivariable analysis of overall survival in the post‐MTB versus pre‐MTB implementation periods within each subgroup of patients with early breast cancer (stage 0–III). All models included the following variables: MTB implementation period (pre vs. post), age (<40 vs. 40–49 vs. 50–59 vs. ≥60 years), HIV status (negative/unknown vs. positive), stage at diagnosis (0–II vs. III), and tumor subtype (ER+/HER2− vs. HER2+ vs. TNBC vs. unknown). ¥Among patients receiving any type of breast surgery. Not done/incomplete: 0–5 isolated lymph nodes (in case of axillary lymph node dissection); complete: ≥6 isolated lymph nodes (in case of axillary lymph node dissection) or ≥ 1 isolated lymph nodes with ≤2 positive lymph nodes (in case of sentinel lymph node biopsy). §First‐line of chemotherapy that the patient received includes neoadjuvant and/or adjuvant treatment. If the patient received part of chemotherapy as neoadjuvant (e.g., doxorubicin plus cyclophosphamide [AC] regimen) and another part as adjuvant chemotherapy (e.g., paclitaxel), the type of regimen and dose‐intensity refer to the entire scheme (neoadjuvant plus adjuvant). Abbreviations: CI, confidence interval; CT, chemotherapy; ER, estrogen receptor; ET, endocrine therapy; HR, hazard ratio; MTB, multidisciplinary tumor board; TNBC, triple‐negative breast cancer.
Disease‐Free Survival
Among patients with early breast cancer, 48% had a disease‐free survival event: 18% had loco‐regional relapse, 8% had distant relapse, 6% had both loco‐regional and distant relapse, 1% had a second primary cancer, and 16% died. The 3‐year disease‐free survival was 41.7% (95% CI, 30.2–52.8) in the pre‐MTB and 56.8% (95% CI, 45.3–66.8) in the post‐MTB subcohort (Fig. 2D). The proportion of patients with loco‐regional relapses (with or without concomitant distant relapse) or death as the first disease‐free survival event was numerically higher in the pre‐MTB vs. the post‐MTB subcohort (29% vs. 18%; and 23% vs. 10%, respectively), but these differences were not statistically significant (p = .07). In the multivariable analysis, the adjusted HR for relapse or death was 0.72 (95% CI, 0.46–1.13) in the post‐MTB compared with the pre‐MTB subcohort (Fig. 3; supplemental online Table 3). Tumor characteristics (subtype, lymph node status, and stage), quality of surgery (surgical margins status and axillary surgery completeness), and type of first‐line chemotherapy regimen were significantly associated with disease‐free survival.
Cost‐Effectiveness Analysis
The absolute 3‐year mean cost increase of implementing a MTB was $119.83 per patient (7648.42 MZN per patient). The incremental cost‐effectiveness ratio was $802.96 per QALY (51252.57 MZN per QALY), corresponding to 1.6 times the GDP of Mozambique (Table 2). Assuming a willingness‐to‐pay threshold of three times the national GDP (1497.00 USD), MTB implementation is a cost‐effective measure in this setting. Sensitivity analyses showed similar findings (supplemental online Table 4).
Table 2.
Costs, benefits, costs per unit of benefit and incremental cost‐effectiveness ratios, by strategy (no multidisciplinary tumor board [MTB] vs. MTB), in a provider perspective, over a 3‐year time horizon
| Strategy | Probability of being alive without relapse at 3 years | Probability of being alive with relapse at 3 years | Mean 3‐year cost per patient treated a | Mean 3‐year incremental cost per patient treated a | Mean QALYs per patient | ICER (cost/QALY) b |
|---|---|---|---|---|---|---|
| No MTB (usual care) | 0.4166 | 0.0635 | $993.1803; 63,393.9057 MZN | 1.46225 | ||
| MTB | 0.5681 | 0.1615 |
$1,113.0067; 71,042.3278 MZN |
$119.8264; 7,648.4221 MZN |
1.61148 | $802.9646; 51,252.5772 MZN |
Future health benefits and costs were discounted at 3% annually, assuming a time‐horizon of 3‐years, based on the World Health Organization recommendations [30].
The conversion between U.S. dollars (USD) and MZN was performed using the exchange rate 1 USD = 63.8292 MZN.
Abbreviations: ICER, incremental cost‐effectiveness ratio; MTB, multidisciplinary tumor board; MZN, Mozambique Meticais; QALY, quality‐adjusted life year.
Discussion
To the best of our knowledge, this is the first study reporting the survival benefit and cost‐effectiveness analysis of a MTB for breast cancer in a low‐income country and in Africa. Our prospective cohort study showed that the implementation of a MTB, after the training of a few key health professionals, led to a significant reduction in mortality among patients with early breast cancer. This benefit remained significant after adjusting for other prognostic factors. Given the baseline poor prognosis of these patients, the absolute improvement in 3‐year overall survival among patients with early breast cancer was 25.0% (from 48.0% to 73.0%). Moreover, we have demonstrated that this is a cost‐effective intervention.
Interestingly, time from diagnosis until treatment or the proportion of each treatment received were similar in both the pre‐MTB and post‐MTB subcohorts. Although not statistically significant, there were improvements in the quality of care (i.e., clean surgical margins, use of neoadjuvant chemotherapy, among others). Thus, the survival benefit brought by the MTB might be from the added effect of each of these small improvements and by the truly integrated multidisciplinary care of patients, generating high‐value clinical practices and better patient navigation within the system. This is suggested by the fact that patients receiving neoadjuvant chemotherapy benefited more from MTB implementation than those receiving surgery first, probably because those patients would have had a more difficult navigation in the pre‐MTB era, potentially leading to delays between treatments and a worse outcome.
In contrast, the absence of survival benefit among patients with stage IV breast cancer may be related to their baseline poor prognosis and the low availability of effective systemic therapies. This highlights the need to diagnose breast tumors at an earlier stage and to provide more effective treatment strategies to women with de novo metastatic disease in low‐resource settings.
Studies assessing the composition, functioning, and impact of MTBs in different cancer types in high‐income countries have heterogeneous results in terms of survival impact and cost‐effectiveness [14, 15, 16, 32, 33]. These discrepancies may be due to different study designs, patient population, or MTB models. For instance, differences between two large studies in the U.K. [14] and Germany [16] in terms of inclusion periods (1990–2000 vs. 2004–2010, respectively), median follow‐up time (7.0 vs. 3.3 years), and diverse multidisciplinary care models may explain why the former showed a survival advantage of this intervention and the latter did not. In developing countries, data regarding MTBs are much scarcer, especially in sub‐Saharan Africa. A study from Botswana showed a reduction in the time from biopsy to start of radiation therapy among patients with cervical cancer [34]. After the implementation of gastro‐intestinal MTBs in Saudi Arabia and Ethiopia, adherence to evidence‐based guidelines improved [35, 36]. Unfortunately, these studies did not provide survival data.
One of the major strengths of the present analysis is being based in the prospective follow‐up of a cohort, unlike most breast cancer studies in sub‐Saharan African, which are usually retrospective [5, 37, 38, 39]. We collected information regarding the entire spectrum of cancer care, from the presentation of symptoms to diagnosis, comorbidities, treatment, and survival outcomes. There were also fewer losses to follow‐up (11.2%) than in previous larger retrospective studies (losses of 22%–53%) [38, 39, 40]. This reflects the efforts by the oncology unit staff to improve follow‐up completeness, by recording multiple telephone numbers per patient and actively contacting those who missed appointments.
During the study, there was no improvement in the access to certain drugs (e.g., trastuzumab remained unavailable) or to other treatments (e.g., radiotherapy), thus not confounding the effect estimated for the introduction of the MTB. There were also no significant differences between patients regarding prognostic factors, such as age, stage, HIV status, or proportion of tumor subtypes.
However, our study has limitations that should be acknowledged. We compared patients diagnosed during the 15 months preceding the MTB implementation to patients diagnosed in the 18 months that followed it. Given the time proximity between the two subcohorts, there are patients in the pre‐MTB subcohort who may have benefited from changes in cancer care brought by the training of professionals and MTB implementation and who may have been discussed in the MTB upon relapse. However, this might have contributed to underestimate the effectiveness of MTB implementation, and thus, this study provides conservative estimates of its effect.
There were no differences in disease‐free survival between the two subcohorts, but this might be related to the timing of relapse diagnosis. Patients in the pre‐MTB were not followed as closely as those in the post‐MTB subcohort, and, therefore, documentation of relapse may have occurred later compared with patients in the post‐MTB subcohort. This is reinforced by the fact that most disease‐free survival events consisted of loco‐regional disease, a type of relapse that is easily recognized by both patients and doctors, when compared with distant metastases. Thus, a more frequent clinical examination can detect loco‐regional relapse earlier, without the need for imaging exams. In addition, the proportion of patients with death as the first disease‐free survival event was numerically higher in the pre‐MTB vs. the post‐MTB subcohort, suggesting that these patients probably had an earlier relapse that was not detected by the physician, possibly because of the less close follow‐up in the pre‐MTB subcohort. Even if there were no significant differences in disease‐free survival, there was a significant benefit in overall survival, which is considered to be a more objective survival measure.
Surprisingly, there was a higher proportion of patients with tumors with unknown subtype in the post‐MTB compared with the pre‐MTB subcohort (21.5% vs. 7.1%, respectively). The most common reason was the unavailability of samples (i.e., there were no cell blocks from fine‐needle aspiration biopsies, core‐needle biopsies, or surgical specimens available to perform subtype determination). This could be because some patients were diagnosed and/or received surgery outside the Maputo Central Hospital and that there were fewer patients with stage IV disease operated to their breast tumor and a slightly higher number of patients with pathological complete response (i.e., without residual invasive tumor) in the post‐MTB subcohort.
In this study, we only analyzed patients treated and followed at a single center. Nonetheless, our population had many of the characteristics demonstrated in other sub‐Saharan African studies, such as a high proportion of stage III/IV at presentation [3], a delay of several months between first symptoms and diagnosis [41], a high proportion of TNBC [4], a high prevalence of HIV infection [40], and low survival [42]. This supports the possibility of generalizing our findings to other hospitals and settings in this region.
We showed that improving the continuum of breast cancer care leads to significant gains in survival among patients with early breast cancer, who are potentially curable. These results reflect (a) the training of key professionals in specialized breast cancer care; (b) a better coordination of care, through the implementation of a MTB; and (c) an active follow‐up of patients, leading to an improved adherence to treatment. Some of these solutions do not need significant extra funding to be implemented, but just a better organizational model.
We demonstrated that beyond traditional clinico‐pathological factors, not only does the performance of surgery or chemotherapy matter, but its quality is also paramount to improve survival. This is another argument to adequately train surgeons and oncologists in the best cancer care practices, as it can improve survival without a large increase in cost. Additionally, strategies to increase access to taxanes and chemotherapy dose‐intensity, such as an adequate supply of drugs and supportive treatment, should also be considered. These could lead to an increase in the proportion of patients with pathological complete response after neoadjuvant chemotherapy, which we have previously shown to be low across all tumor subtypes [26].
The success of this first MTB in Mozambique has recently led to the formal endorsement of multidisciplinarity as an essential part of cancer care, both in the Mozambican Breast Cancer Guidelines and in the Mozambican Cancer Control Plan 2019–2029 [43, 44]. Moreover, there are now separate MTBs for thoracic conditions, gynecological cancer, head and neck cancer, and esophageal cancer at the Maputo Central Hospital.
We will continue to update the survival follow‐up of this cohort using data from the Hospital's Cancer Registry. Yet, given the early and wide separation of the overall survival curves among patients with early breast cancer, it is expected that the survival benefit brought by the MTB will be maintained in the future.
We acknowledge the wide variations in available resources for cancer care among sub‐Saharan African countries and regions. Nonetheless, our proposed MTB model is simple, easy to implement, and cost‐effective, so we believe that it could be scaled up to other countries and settings, in which MTB implementation remains suboptimal.
Future research should also focus on ways to improve the effectiveness of MTBs in low‐resource settings, by identifying the processes most associated with survival gain. Moreover, the selection of patients that benefit the most from multidisciplinarity is also warranted. This future knowledge could also be used to improve the effectiveness of MTBs in high‐income countries.
Conclusion
Within this prospective cohort study in Mozambique, we showed that the implementation of an MTB led to a 53% mortality decrease among patients with early breast cancer. This intervention led to an absolute gain in 3‐year overall survival of 25.0% and was cost‐effective. These findings highlight the feasibility of implementing this strategy and the need for expanding the use of MTBs in developing countries as a way to improve patient outcomes. Given the high proportion of women with stage IV disease in our cohort, we also point to the need of diagnosing breast cancer at an earlier stage, to optimize patient outcomes.
Author Contributions
Conception/design: Mariana Brandão, Assucena Guisseve, Carla Carrilho, Nuno Lunet
Provision of study material or patients: Mariana Brandão, Assucena Guisseve, Genoveva Bata, Josefo Ferro, Carlos Garcia, Clésio Zaqueu, Astrilde Jamisse, Jotamo Come, Otília Soares, Alberto Gudo‐Morais, Satish Tulsidás, Carla Carrilho
Collection and/or assembly of data: Mariana Brandão, Assucena Guisseve, Genoveva Bata, Josefo Ferro, Carlos Garcia, Clésio Zaqueu, Cesaltina Lorenzoni, Dina Leitão, Otília Soares, Alberto Gudo‐Morais, Fernando Schmitt, Satish Tulsidás, Carla Carrilho
Data analysis and interpretation: Mariana Brandão, Assucena Guisseve, João Firmino‐Machado, Carla Carrilho, Nuno Lunet
Manuscript writing: Mariana Brandão, Assucena Guisseve
Final approval of manuscript: Mariana Brandão, Assucena Guisseve, Genoveva Bata, João Firmino‐Machado, Matos Alberto Josefo Ferro, Carlos Garcia, Clésio Zaqueu, Astrilde Jamisse, Cesaltina Lorenzoni, Martine Piccart‐Gebhart Dina Leitão, Jotamo Come, Otília Soares, Alberto Gudo‐Morais, Fernando Schmitt, Satish Tulsidás, Carla Carrilho, Nuno Lunet
Disclosures
Mariana Brandão: Radius, AstraZeneca, Eli Lilly & Co, Merck Sharpe & Dohme, GlaxoSmithKline/Novartis, Roche/GNE, Synthon, Servier, Pfizer (RF [Institution]). Roche/GNE (Other‐travel grant, H); Martine Piccart‐Gebhart: AstraZeneca, Camel‐IDS, Crescendo Biologics, Debiopharm, G1 Therapeutics, Genentech, Huya, Immunomedics, Eli Lilly & Co, Menarini, Merck Sharpe & Dohme, Novartis, Odonate, Periphagen, Pfizer, Roche, Seattle Genetics (C/A, H), Oncolytics (SAB), IJB from Radius, AstraZeneca, Lilly, Merck Sharp & Dohme, GlaxoSmithKline/Novartis, Roche/GNE, Synthon, Servier, Pfizer (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figure 1
Appendix S1 Supporting Information.
Supplementary Table S1 Multivariable analysis of prognostic factors for overall survival among the total population
Supplementary Table S2 Multivariable analysis of prognostic factors for overall survival among patients with early stage breast cancer (stage 0‐III)
Supplementary Table S3 Multivariable analysis of prognostic factors for disease‐free survival among patients with early stage breast cancer (stage 0‐III)
Supplementary Table S4 Cost‐effectiveness sensitivity analysesa
Acknowledgments
The Calouste Gulbenkian Foundation supported the training of professionals (J.C., A.J., A.G.) in breast cancer care at the Centro Hospitalar Universitário de São João, under the Project “Atenção Integrada ao Doente Oncológico.”
We posthumously thank Dr. João Fumane, former head of the Oncology Unit (2000–2011) and Director of the Maputo Central Hospital (from 2012 until his death, in 2017), for his continuous support to this cohort study.
We thank Dr. Samantha Morais, from the Instituto de Saúde Pública, Universidade do Porto, for proof‐reading the manuscript.
This study was approved by the National Health Bioethical Committee of Mozambique (reference number 226/CNBS/15). All participants provided written informed consent. This study was performed in accordance with the Declaration of Helsinki.
The Moza‐BC cohort study was supported by the Beginning Investigator Grant for Catalytic Research (BIG Cat) program, an African Organisation for Research and Training in Cancer program with support from the U.S. National Cancer Institute (grant number 59–210–6‐004). The funding source had no role in the study design, in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Bray F, Ferlay J, Soerjomataram I et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. Lorenzoni CF, Ferro J, Carrilho C et al. Cancer in Mozambique: Results from two population‐based cancer registries. Int J Cancer 2020;147:1629–1637. [DOI] [PubMed] [Google Scholar]
- 3. Jedy‐Agba E, McCormack V, Adebamowo C et al. Stage at diagnosis of breast cancer in sub‐Saharan Africa: a systematic review and meta‐analysis. Lancet Glob Health 2016;4:e923–e935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eng A, McCormack V, dos‐Santos‐Silva I. Receptor‐Defined subtypes of breast cancer in Indigenous populations in Africa: A systematic review and meta‐analysis. PLOS Med 2014;11:e1001720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vanderpuye V, Grover S, Hammad N et al. An update on the management of breast cancer in Africa. Infect Agent Cancer 2017;12:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cardoso F, Kyriakides S, Ohno S et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2019;30:1194–1220. [DOI] [PubMed] [Google Scholar]
- 7. Pons‐Tostivint E, Daubisse‐Marliac L, Grosclaude P et al. Multidisciplinary team meeting and EUSOMA quality indicators in breast cancer care: A French regional multicenter study. Breast 2019;46:170–177. [DOI] [PubMed] [Google Scholar]
- 8. Quyyumi FF, Wright JD, Accordino MK et al. Factors associated with multidisciplinary consultations in patients with early stage breast cancer. Cancer Invest 2019;37:233–241. [DOI] [PubMed] [Google Scholar]
- 9. Atwell D, Vignarajah DD, Chan BA et al. Referral rates to multidisciplinary team meetings: Is there disparity between tumour streams? J Med Imaging Radiat Oncol 2019;63:378–382. [DOI] [PubMed] [Google Scholar]
- 10. Lu J, Jiang Y, Qian M et al. The improved effects of a multidisciplinary team on the survival of breast cancer patients: Experiences from China. Int J Environ Res Public Health 2020;17:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kung PT, Tsai WC. P0213 Effects of multidisciplinary care on survival of breast cancer: Results from a national cohort study. Eur J Cancer 2014;50(suppl 4):e69. [Google Scholar]
- 12. Dadzie MA. P214: Assessment of breast cancer management in sub Saharan AFRICA. AORTIC International Conference on Cancer in Africa. November 5–8, 2019, Maputo, Mozambique.
- 13. Saghir NSE, El‐Asmar N, Hajj C et al. Survey of utilization of multidisciplinary management tumor boards in Arab countries. Breast 2011;20(suppl 2):S70–S74. [DOI] [PubMed] [Google Scholar]
- 14. Kesson EM, Allardice GM, George WD et al. Effects of multidisciplinary team working on breast cancer survival: retrospective, comparative, interventional cohort study of 13 722 women. BMJ 2012;344:e2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kreienberg R, Wöckel A, Wischnewsky M. Highly significant improvement in guideline adherence, relapse‐free and overall survival in breast cancer patients when treated at certified breast cancer centres: An evaluation of 8323 patients. Breast 2018;40:54–59. [DOI] [PubMed] [Google Scholar]
- 16. Schrodi S, Tillack A, Niedostatek A et al. No survival benefit for patients with treatment in certified breast centers—A population‐based evaluation of German cancer registry data. Breast J 2015;21:490–500. [DOI] [PubMed] [Google Scholar]
- 17. De Ieso PB, Coward JI, Letsa I et al. A study of the decision outcomes and financial costs of multidisciplinary team meetings (MDMs) in oncology. Br J Cancer 2013;109:2295–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Munro AJ. Multidisciplinary team meetings in cancer care: An idea whose time has gone? Clin Oncol 2015;27:728–731. [DOI] [PubMed] [Google Scholar]
- 19. GDP per capita (current US$) – Mozambique. The World Bank. Available at https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=MZ&most_recent_value_desc=false. Accessed March 10, 2020.
- 20. Life expectancy at birth, total (years) – Mozambique. The World Bank. Available at https://data.worldbank.org/indicator/SP.DYN.LE00.IN?locations=MZ. Accessed March 26, 2020.
- 21. Ministério da Saúde, Instituto Nacional de Estatística, ICF. Inquérito de Indicadores de Imunização, Malária e HIV/SIDA em Moçambique (IMASIDA) 2015. Maputo, Mozambique: Ministério da Saúde, 2018. Available at https://dhsprogram.com/publications/publication‐ais12‐ais‐final‐reports.cfm. Accessed March 26, 2020.
- 22. Ministério da Saúde . Doenças Crónicas e Não Transmissíveis em Moçambique ‐ Relatório Nacional 2018. Maputo, Mozambique: Ministério da Saúde, 2018. Available at https://static1.squarespace.com/static/55d4de6de4b011a1673a40a6/t/5b36457388251bc29f1b1b8b/1530283379635/Relatorio+Final_Portugues.pdf. Accessed March 10, 2020.
- 23. Physicians (per 1,000 people) ‐ South Africa, Mozambique. The World Bank. Available at https://data.worldbank.org/indicator/SH.MED.PHYS.ZS?locations=ZA‐MZ. Accessed March 26, 2020.
- 24. UNICEF . Health Budget Brief of Mozambique 2018. New York: UNICEF. Available at https://www.unicef.org/mozambique/sites/unicef.org.mozambique/files/2019‐04/2018‐Budget‐Brief‐Health.pdf. Accessed September 24, 2020. [Google Scholar]
- 25. Carrilho C, Fontes F, Tulsidás S et al. Cancer incidence in Mozambique in 2015‐2016: data from the Maputo Central Hospital Cancer Registry. Eur J Cancer Prev 2019;28:373–376. [DOI] [PubMed] [Google Scholar]
- 26. Brandão M, Guisseve A, Bata G et al. Breast cancer subtypes: Implications for the treatment and survival of patients in Africa—A prospective cohort study from Mozambique. ESMO Open 2020;5:e000829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hammond MEH, Hayes DF, Dowsett M et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 2010;28:2784–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wolff AC, Hammond MEH, Allison KH et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J Clin Oncol 2018;36:2105–2122. [DOI] [PubMed] [Google Scholar]
- 29. Edge S, Byrd D, Compton C et al. AJCC cancer staging manual, 7th edition. New York: Springer, 2010. [Google Scholar]
- 30. World Health Organization . Making Choices in Health: WHO Guide to Cost‐Effectiveness Analysis. Geneva, Switzerland: World Health Organization, 2003. Available at https://www.who.int/choice/book/en/. Accessed February 15, 2020. [Google Scholar]
- 31. Inflation, GDP deflator (annual %) – Mozambique. The World Bank. Available at https://data.worldbank.org/indicator/NY.GDP.DEFL.KD.ZG?locations=MZ. Accessed December 7, 2019.
- 32. Pillay B, Wootten AC, Crowe H et al. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: A systematic review of the literature. Cancer Treat Rev 2016;42:56–72. [DOI] [PubMed] [Google Scholar]
- 33. Ke KM, Blazeby JM, Strong S et al. Are multidisciplinary teams in secondary care cost‐effective? A systematic review of the literature. Cost Eff Resour Alloc 2013;11:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grover S, Chiyapo SP, Puri P et al. Multidisciplinary Gynecologic Oncology Clinic in Botswana: A model for multidisciplinary oncology care in low‐ and middle‐income settings. J Glob Oncol 2017;3:666–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. AlFarhan HA, Algwaiz GF, Alzahrani HA et al. Impact of GI tumor board on patient management and adherence to guidelines. J Glob Oncol 2018;4:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Deressa BT, Cihoric N, Tefesse E et al. Multidisciplinary cancer management of colorectal cancer in Tikur Anbessa Specialized Hospital, Ethiopia. J Glob Oncol 2019;5:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thomas AS, Kidwell KM, Oppong JK et al. Breast cancer in Ghana: Demonstrating the need for population‐based cancer registries in low‐ and middle‐income countries. J Glob Oncol 2017;3:765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kantelhardt EJ, Zerche P, Mathewos A et al. Breast cancer survival in Ethiopia: a cohort study of 1,070 women. Int J Cancer 2014;135:702–709. [DOI] [PubMed] [Google Scholar]
- 39. Lopes LV, Miguel F, Freitas H et al. Stage at presentation of breast cancer in Luanda, Angola ‐ A retrospective study. BMC Health Serv Res 2015;15:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cubasch H, Dickens C, Joffe M et al. Breast cancer survival in Soweto, Johannesburg, South Africa: A receptor‐defined cohort of women diagnosed from 2009 to 11. Cancer Epidemiol 2018;52:120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Frie KG, Kamaté B, Traoré CB et al. Factors associated with time to first healthcare visit, diagnosis and treatment, and their impact on survival among breast cancer patients in Mali. PLoS One 2018;13:e0207928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ssentongo P, Lewcun JA, Candela X et al. Regional, racial, gender, and tumor biology disparities in breast cancer survival rates in Africa: A systematic review and meta‐analysis. PLoS One 2019;14:e0225039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ministério da Saúde . Normas nacionais para o controlo e tratamento do cancro da mama. Maputo, Mozambique: Health Ministry of Mozambique, 2019. [Google Scholar]
- 44. Ministério da Saúde . Plano nacional de controlo do cancro 2019‐2029. Maputo, Mozambique: Health Ministry of Mozambique, 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figure 1
Appendix S1 Supporting Information.
Supplementary Table S1 Multivariable analysis of prognostic factors for overall survival among the total population
Supplementary Table S2 Multivariable analysis of prognostic factors for overall survival among patients with early stage breast cancer (stage 0‐III)
Supplementary Table S3 Multivariable analysis of prognostic factors for disease‐free survival among patients with early stage breast cancer (stage 0‐III)
Supplementary Table S4 Cost‐effectiveness sensitivity analysesa


