Abstract
Background
Guideline‐recommended antiemetic prophylaxis improves nausea and vomiting control in most patients undergoing chemotherapy. Multinational Association of Supportive Care in Cancer/European Society for Medical Oncology (MASCC/ESMO) antiemetic guidelines recommend prophylaxis with a neurokinin‐1 receptor antagonist (NK1RA), a 5‐hydroxytryptamine‐3 receptor antagonist (5‐HT3RA), and dexamethasone for patients receiving highly emetogenic chemotherapy (HEC), including anthracycline‐cyclophosphamide (AC)‐ and carboplatin (considered moderately emetogenic chemotherapy)‐based chemotherapy. Here, we analyze the use of NK1RA–5‐HT3RA–dexamethasone for antiemetic prophylaxis associated with HEC and carboplatin.
Methods
The data source was the Global Oncology Monitor (Ipsos Healthcare). Geographically representative physicians from France, Germany, Italy, Spain, and the U.K. were screened for treatment involvement and number of patients treated per month. Patients’ data from January to December 2018 were collected from medical charts and extrapolated on the basis of the total number of physicians who prescribe chemotherapy. The emetic risk of chemotherapy was classified per MASCC/ESMO guidelines.
Results
Data from 45,324 chemotherapy‐treated patients were collected, representing a total extrapolated prevalence of 1,394,848 chemotherapy treatments included in the analysis. NK1RAs were used in 45%, 42%, and 19% of patients receiving cisplatin‐, AC‐, and carboplatin‐based chemotherapy, respectively; 18%, 24%, and 7% received the guideline‐recommended NK1RA–5‐HT3RA–dexamethasone combination; no antiemetics were prescribed for 12% of the treatments. Often, physicians’ perception of the emetic risk of chemotherapy did not follow MASCC/ESMO guideline classification.
Conclusion
Low adherence to antiemetic guidelines was revealed in clinical practice in five European countries, with 15% of all HEC‐/carboplatin‐based treatments receiving guideline‐recommended NK1RA–5‐HT3RA–dexamethasone prophylaxis and 12% of them receiving no antiemetics. New strategies for improving guideline adherence are urgently needed.
Implications for Practice
Despite recent advances in antiemetic therapy, a substantial proportion of patients experience nausea and vomiting associated with chemotherapy in daily clinical practice. Antiemetic guidelines aim at prevention of chemotherapy‐induced nausea and vomiting (CINV), and guideline‐consistent antiemetic therapy can effectively prevent vomiting and, to a lesser extent, nausea in most patients with cancer. This study reports low adherence to antiemetic guidelines in the highly emetogenic chemotherapy setting in daily clinical practice across five European countries. Opportunity exists to increase adherence to antiemetic guideline recommendations. Implementation of strategies to facilitate guideline adherence can potentially improve CINV control.
Keywords: Physician survey, Chemotherapy‐induced nausea and vomiting, Antiemetic guidelines, Guideline adherence
Short abstract
Adherence to antiemetic guidelines is associated with improved control of chemotherapy‐induced nausea and vomiting. This article analyzes the use of guideline‐recommended NK1RA‐based regimens for antiemetic prophylaxis of highly emetogenic chemotherapy and carboplatin regimens in real‐world clinical practice across Europe.
Introduction
Chemotherapy‐induced nausea and vomiting (CINV) is ranked by patients among the most distressing and persistent side effects of specific anticancer treatments [1, 2] and can negatively affect quality of life of patients with cancer [3]. In some instances, failure to correctly manage CINV can prevent patients from completing chemotherapy [4]. Monitoring of common chemotherapy‐related symptoms, such as nausea and vomiting, among others, through electronic patient‐reported outcomes, and their management has been shown to increase overall survival [5, 6]. Early intervention for symptom control to prevent further unfavorable effects, and the ability of patients to tolerate chemotherapy treatments for longer periods, have been hypothesized to be responsible for the increased survival effects.
According to the time of occurrence after chemotherapy administration, CINV is classified into acute (occurring within the first 24 hours), delayed (between 24 and 120 hours), and overall (between 0 and 120 hours) categories [7].
Chemotherapeutic agents are ranked in international antiemetic guidelines on the basis of the probability for inducing acute emesis in the absence of antiemetic prophylaxis into highly emetogenic chemotherapy (HEC), moderately emetogenic chemotherapy (MEC), low emetogenic chemotherapy (LEC), and minimally emetogenic chemotherapy. HEC, which is associated with a > 90% risk for emesis, includes agents such as cisplatin and the anthracycline‐cyclophosphamide (AC) combination [8, 9], and MEC, which is correlated with a 30%–90% emetic risk, encompasses agents such as carboplatin, oxaliplatin, cytarabine, and ifosfamide, among others [8, 9]. Agents’ classification is revised periodically on the basis of new clinical data. Although antiemetic guidelines of the Multinational Association of Supportive Care in Cancer/European Society for Medical Oncology (MASCC/ESMO) [10, 11] still classify carboplatin‐based regimens as MEC, they consider them as HEC for their antiemetic recommendations.
MASCC/ESMO antiemetic guidelines [10, 11] recommend prophylaxis with a 5‐hydroxytryptamine‐3 receptor antagonist (5‐HT3RA) plus dexamethasone for patients treated with MEC other than carboplatin‐based regimens. For the prevention of CINV associated with HEC (including AC) and carboplatin‐based regimens, the triple combination of a 5‐HT3RA, a neurokinin‐1 receptor antagonist (NK1RA), and dexamethasone is advised, with addition of olanzapine to the triplet when occurrence of nausea associated with HEC and AC regimens is an issue. Similar recommendations have been issued by the National Comprehensive Cancer Network (NCCN) [12] and the American Society of Clinical Oncology (ASCO) [13] for CINV prophylaxis in the HEC and MEC settings. NK1RA‐based regimens are constituted of either aprepitant (oral), fosaprepitant (i.v.), or rolapitant (oral), in combination with 5‐HT3RA–dexamethasone, or the fixed‐combination agent NEPA (oral), comprising the NK1RA netupitant and the 5‐HT3RA palonosetron, combined with dexamethasone alone. Recently, additional formulations of NK1RAs that increase the convenience of administration of antiemetics have been developed and approved in the U.S. in 2018, for example, i.v. NEPA (fixed combination of fosnetupitant and palonosetron) [14], aprepitant emulsion for injection [15], and rolapitant injectable emulsion [16, 17]; i.v. NEPA also recently received approval in Europe [18]. After the occurrence of anaphylaxis, anaphylactic shock, and hypersensitivity reactions in the clinic with rolapitant injectable emulsion, a safety warning was issued [19] that led to the suspension of its distribution [20]. The new i.v. formulations of NEPA and aprepitant have recently been incorporated in the NCCN antiemetic guidelines and are recommended for the HEC and MEC settings [12]. Moreover, i.v. NEPA is advised as an alternative to oral NEPA in the HEC, AC, and carboplatin settings by MASCC/ESMO [10, 11].
Prevention is the main goal of international antiemetic guidelines. Correct management of nausea and vomiting in the first chemotherapy cycle is critical because CINV occurrence during first administration of emetogenic chemotherapy is associated with increased CINV risk in subsequent cycles [21, 22]. Guideline‐consistent usage of antiemetic regimens can effectively prevent vomiting and, to a lesser extent, nausea in most patients with cancer [23, 24, 25]. Conversely, nonadherence to antiemetic guidelines leads to suboptimal CINV control [25, 26]. However, several studies have reported low guideline adherence for patients receiving HEC and MEC both in Europe [25, 27, 28] and the U.S. [26]. Low adoption in the U.S. of the newly recommended NK1RA–5‐HT3RA–dexamethasone triplet for patients receiving carboplatin‐based regimens was also reported recently [29].
The present study aims to analyze the usage of NK1RA‐based regimens for the prevention of CINV associated with HEC, including AC chemotherapy, and carboplatin‐based (any dose of carboplatin) regimens in five European countries: France, Germany, Italy, Spain, and the U.K.
Materials and Methods
Study Design and Inclusion Criteria
The Global Oncology Monitor from Ipsos Healthcare constituted the source of data for the analysis. The data set contains real‐world prescribing information for all types of tumors, retrieved from patients’ clinical records.
Geographically representative physicians from five European countries (France, Germany, Italy, Spain, and the U.K.) were screened for the following inclusion criteria: being a consultant‐level physician, being the primary decision‐maker for treatment and having direct access to patient clinical records, and treating five or more cancer patients per month with anticancer drug therapy. Additionally, the following country‐specific criteria were used to ensure balanced physician representation: in Germany, 35% and 65% of physicians with office‐ and hospital‐based practices, respectively, across all specialties; in France, 70% and 30% of physicians with public and private practice, respectively, across all specialties; for regional representation in Italy, 45%, 25%, and 30% of physicians from the north, center, and south regions; in Spain, 23%, 29%, 23%, and 25% of physicians from the north, center, middle, and south; and in U.K., 93% of physicians representing old cancer networks regions.
Physicians completed online clinical record forms on all patients seen in consultation during the month of the study, irrespective of the specific tumor type (as per their natural assignment of patients). Physicians were allowed to submit between 5 and 36 records per month. For extrapolation of chemotherapy treatment data, clinical record forms were collected each month.
Treatment‐related data collected by physicians from patient charts between January and December 2018 were compiled. Chemotherapy treatment data were extrapolated on the basis of the total number of physicians in the five countries who treat their patients with chemotherapy, that is, “the doctor universe.” A chemotherapy treatment was defined as at least one dose of a cytotoxic anticancer drug. Rigorous projection methodology was performed, where each patient record was projected individually on the basis of the following factors: (a) physician's specialty/practice type/workload of patients, to reflect correct proportions of treating physicians in the population; (b) number of weeks since the patient was last seen, to ensure that patients on continual therapy are correctly represented despite less frequent visits; (c) cycle length, to adjust for the likelihood of counting an individual patient's record each month; (d) number of cycles administered/number of additional cycles planned, for the best estimate of how often each patient's record can be counted within a year—this is the “duplication factor.” These projections were used to estimate the number of treatments in each emetogenic risk chemotherapy category. The analyses are based on the projected estimates for the prevalence of the total number of chemotherapy treatments classified as HEC‐based (including AC) and carboplatin‐based, that is, therapies requiring prophylaxis with NK1RA‐based regimens, per antiemetic guidelines. Data on prescribed antiemetic regimens for acute CINV prophylaxis are presented. Use of NK1RAs only in the delayed phase is reported in the analysis by country.
Guideline adherence was calculated on the basis of the estimated number of all chemotherapy treatments for which NK1RA‐based antiemetic prophylaxis is recommended per MASCC/ESMO antiemetic guidelines, that is, HEC (including cisplatin‐based, AC‐based, and other HEC) and carboplatin‐based. Separate calculations were also performed for these individual chemotherapy types.
The emetic risk of chemotherapy was classified per MASCC/ESMO antiemetic guidelines, with AC combination being classified as HEC and carboplatin‐based regimens (any dose of carboplatin) as “high” MEC [10, 11]. HEC treatments included cisplatin‐based, AC‐based, and “other HEC” therapies; among MEC treatments, only carboplatin‐based therapies were included. Guideline adherence was defined per the most current MASCC/ESMO antiemetic guideline recommendations, from 2016, at the time the survey was conducted [10].
Statistical Analysis
The data were analyzed using IBM SPSS Data Collection Survey Reporter, version 6.0.1 (SPSS, Hong Kong).
Results
Study Participants and Study Population
Raw data were collected from a total of 610 physicians participating in the study: 154 from France, 111 from Germany, 176 from Italy, 68 from Spain, and 101 from the U.K. (Table 1). Most physicians were medical oncologists (54%), had a practice at an academic hospital (49%), and were located in urban areas (89%). Physicians completed a total of 45,324 clinical patient record forms for patients receiving chemotherapy. The most common cancer types were breast (23%), colorectal (18%), and non‐small cell lung (10%) cancers. Among chemotherapy‐treated patients, 27,417 received HEC or MEC.
Table 1.
Baseline characteristics and demographics of survey respondents
| Characteristic | Total respondents (n = 610), n (%) |
|---|---|
| Country | |
| France | 154 (25) |
| Germany | 111 (18) |
| Italy | 176 (29) |
| Spain | 68 (11) |
| U.K. | 101 (17) |
| Specialty | |
| Medical oncology | 330 (54) |
| Hematology/oncology | 116 (19) |
| Urology | 92 (15) |
| Pulmonology | 24 (4) |
| Hematology | 18 (3) |
| Gastroenterology | 12 (2) |
| Dermatology | 12 (2) |
| Gynecologic oncology | 6 (1) |
| Practice type | |
| Office | 55 (9) |
| Academic hospital | 299 (49) |
| Cancer center | 92 (15) |
| General hospital | 164 (27) |
| Practice location | |
| Urban | 541 (89) |
| Suburban | 46 (8) |
| Rural | 23 (4) |
| Tumor type, percentage of chemotherapy treatments | |
| Breast | 23 |
| Colorectal | 18 |
| Non‐small cell lung | 10 |
| Non‐Hodgkin lymphoma | 7 |
| Urinary and bladder | 5 |
| Ovarian | 4 |
| Pancreas | 4 |
| Other | 29 |
After extrapolation of the data to the total number of registered physicians in these five countries, projected data represent a total of 1,394,848 chemotherapy treatments of any emetic risk (i.e., HEC, MEC, LEC, and minimally emetogenic chemotherapy, excluding hormonal‐only therapy); of these, 882,310 correspond to HEC or MEC regimens. The analyses included 489,049 treatments for which NK1RA‐based antiemetic prophylaxis is recommended per MASCC/ESMO antiemetic guidelines, encompassing 326,607 HEC treatments (67%; cisplatin‐based: 179,698 [37%]; AC‐based: 125,203 [26%]; other HEC: 21,706 [4%]) and 162,442 carboplatin‐based (33%) treatments (Fig. 1).
Figure 1.
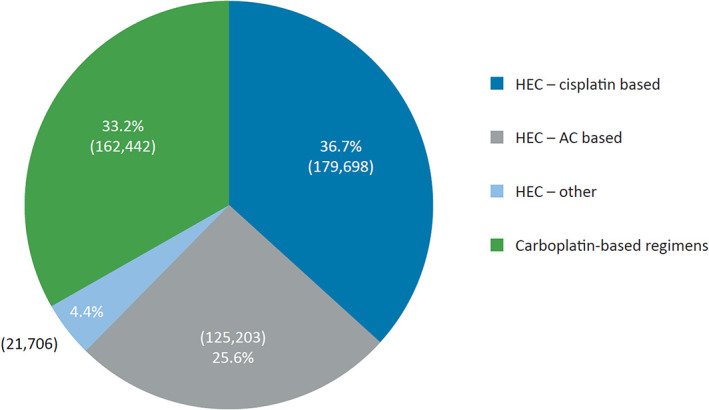
Distribution of chemotherapeutic regimens analyzed for which neurokinin‐1 receptor antagonist–based antiemetic prophylaxis is recommended (n = 489,049 treatments). Abbreviations: AC, anthracycline‐cyclophosphamide; HEC, highly emetogenic chemotherapy.
Use of NK1RA‐Based Antiemetic Regimens per Chemotherapy Setting
NK1RA use was low across all guideline‐recommended chemotherapy settings, with physicians prescribing NK1RA‐based prophylaxis for only 45%, 42%, 17%, and 19% of cisplatin‐based, AC‐based, other HEC, and carboplatin‐based treatments, respectively (Fig. 2).
Figure 2.
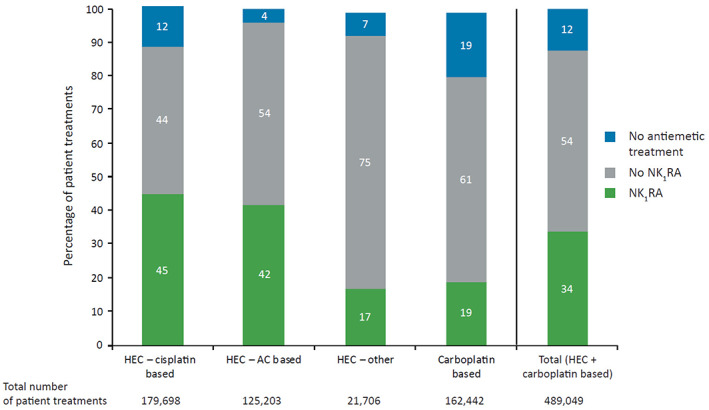
Use of NK1RA‐based antiemetic regimens in the acute phase of chemotherapy‐induced nausea and vomiting (day 1) for prophylaxis of HEC and carboplatin‐based therapy in the overall study population (n = 489,049). Abbreviations: AC, anthracycline‐cyclophosphamide; HEC, highly emetogenic chemotherapy; NK1RA, neurokinin‐1 receptor antagonist.
The guideline‐recommended NK1RA, 5‐HT3RA, and dexamethasone regimen for CINV prevention was given in only 18% of cisplatin‐based, 24% of AC‐based, and 2% of other HEC treatments, and in 7% of carboplatin‐based regimens (Fig. 3A–D). Overall, the guideline‐recommended NK1RA‐based triplet regimen was used in only 15% of HEC‐ and carboplatin‐based treatments (Fig. 3E).
Figure 3.
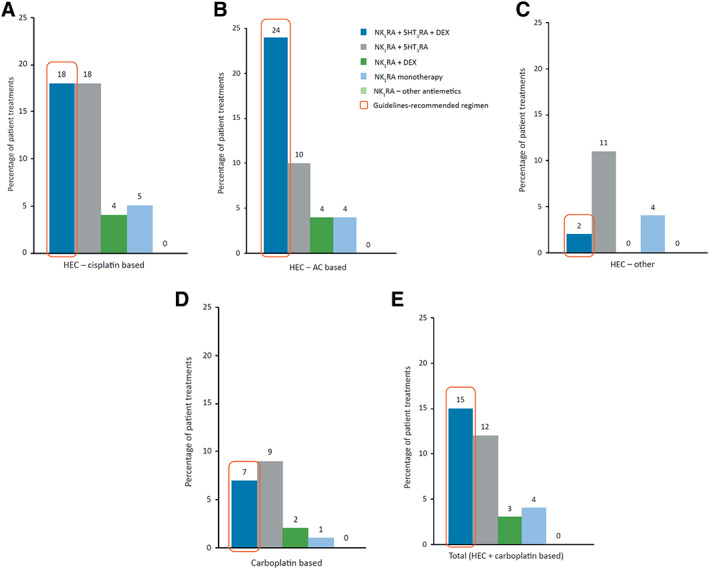
Distribution of NK1RA‐based antiemetic regimens used in the acute phase of chemotherapy‐induced nausea and vomiting (day 1) for prophylaxis of cisplatin‐based HEC (A), AC‐based HEC (B), other HEC (C), carboplatin‐based therapy (D), and any HEC and carboplatin‐based therapy (E). Percentages of patients are reported to the overall study population (n = 489,049).Note: Percentages were rounded to the closest value excluding decimals.Abbreviations: 5‐HT3RA, 5‐hydroxytryptamine‐3 receptor antagonist; AC, anthracycline‐cyclophosphamide; DEX, dexamethasone; HEC, highly emetogenic chemotherapy; NK1RA, neurokinin‐1 receptor antagonist.
Among the 10 most frequent chemotherapeutic regimens for which NK1RA‐based prophylaxis was prescribed, cisplatin‐based regimens (48%) were the most common, followed by AC‐based regimens (39%) and carboplatin‐based regimens (13%). Overall, the most common (>5%) chemotherapeutic treatments receiving NK1RAs for the prevention of acute CINV were cyclophosphamide‐epirubicin, cisplatin‐gemcitabine, and cisplatin monotherapy, which accounted for 12%, 7%, and 6%, respectively, of all NK1RA‐based antiemetic prescriptions (Fig. 4).
Figure 4.
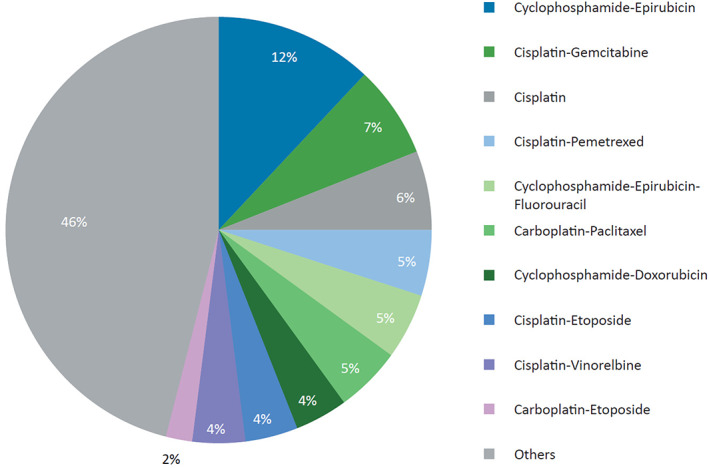
Chemotherapeutic regimens with most frequent use of neurokinin‐1 receptor antagonists (n = 217,528).
No Antiemetic Treatment
Overall, in 12% of all HEC and carboplatin‐based treatments, no antiemetic prophylaxis was administered for the prevention of CINV, including 12% of cisplatin‐, 4% of AC‐, and 19% of carboplatin‐based regimens (Fig. 2).
NK1RA Use by Country
NK1RA use was low across the five European countries, prescribed overall in only 45%, 42%, and 19% of cisplatin‐, AC‐, and carboplatin‐based treatments, respectively (Fig. 5). In general, NK1RA‐based prophylaxis was higher in Germany and France than in Spain, the U.K., and Italy for all chemotherapy settings. For prophylaxis of cisplatin‐based treatments, NK1RA use was 61% in Germany and 54% in France and for AC combination regimens 69% and 58%, respectively. The use of NK1RAs was lowest in the carboplatin setting for all countries, and especially in Italy, Spain, and the U.K., where the prescription rate was ≤10%. Remarkably, NK1RAs were prescribed only in the delayed period in 1% of cisplatin‐based therapies in Germany.
Figure 5.
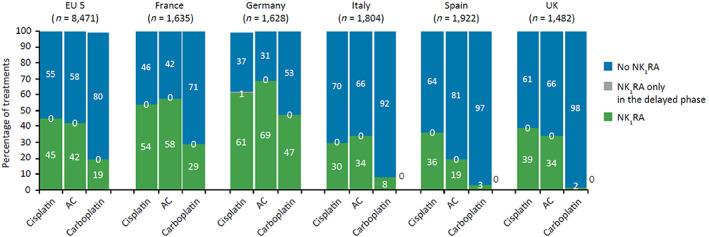
NK1RA usage by country and overall. Abbreviations: AC, anthracycline‐cyclophosphamide; NK1RA, neurokinin‐1 receptor antagonist.
Physicians’ Perception of the Emetogenic Risk of Chemotherapy
Only 55% of cisplatin‐based treatments, 51% of AC‐based treatments, and 24% of carboplatin‐based treatments (classified as “high MEC” by MASCC/ESMO guidelines) were perceived as HEC by physicians (Fig. 6A).
Figure 6.
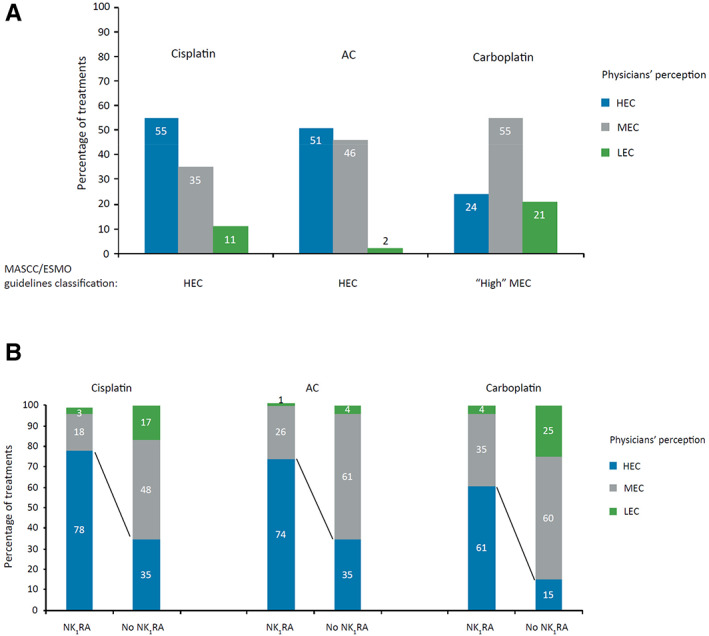
Emetogenic risk of chemotherapy as perceived by all physicians (A) and by physicians who prescribe NK1RAs (B). Abbreviations: AC, anthracycline‐cyclophosphamide; ESMO, European Society for Medical Oncology; HEC, highly emetogenic chemotherapy; LEC, low emetogenic chemotherapy; MASCC, Multinational Association of Supportive Care in Cancer; MEC, moderately emetogenic chemotherapy; NK1RA, neurokinin‐1 receptor antagonist.
From the perspective of physicians who prescribed NK1RAs, 78% of cisplatin‐, 74% of AC‐, and 61% of carboplatin‐based therapies were perceived as HEC (Fig. 6B). Conversely, among physicians who did not administer NK1RA‐based prophylaxis, the perceived emetogenic potential of these chemotherapies was lower, with only 35% of cisplatin‐, 35% of AC‐, and 15% of carboplatin‐based treatments considered HEC.
Discussion
This study showed that during 2018, adherence to 2016 MASCC/ESMO guideline recommendations for the prevention of acute CINV associated with HEC and carboplatin‐based regimens was suboptimal in real‐world clinical practice across five European countries. Noticeable is the reported low use of NK1RAs in the HEC setting. Even though NK1RAs were introduced in MASCC/ESMO guidelines in 2004 for CINV control following HEC and AC (considered MEC at the time) regimens [30], here we show that they were prescribed in only 45% of cisplatin‐ and in 42% of AC‐based treatments during 2018 (Fig. 2). The reported NK1RA use in the carboplatin setting was even lower, with only 19% of carboplatin‐based treatments being associated with NK1RA‐based prophylaxis (Fig. 2). The limited use of NK1RAs in this setting could reflect the shorter period for adoption of this recommendation since its incorporation in MASCC/ESMO guidelines in 2016 [10, 11]. Although NK1RAs were mostly used for cisplatin‐based regimens (both monotherapy and combinations), cyclophosphamide‐epirubicin was the most frequent chemotherapeutic regimen for which NK1RA‐based prophylaxis was prescribed (Fig. 4), which is consistent with breast cancer being the most common tumor type (23%; Table 1).
In addition to their consistently low use across HEC and MEC settings, NK1RA‐based regimens are largely not prescribed per MASCC/ESMO guidelines, that is, in combination with a 5‐HT3RA and dexamethasone. Specifically, the guideline‐recommended NK1RA–5‐HT3RA–dexamethasone triplet prophylaxis was prescribed in as few as 15% of all eligible treatments, including only 18% of cisplatin‐, 24% of AC‐, and 7% of carboplatin‐based treatments (Fig. 3). Compared with a similar analysis performed in 2017 [31], the use of the triplet during 2018 remained the same in the cisplatin setting (18% vs. 18%), slightly increased in AC (20% vs. 24%), and slightly decreased for carboplatin‐based regimens (11% vs. 7%).
Adherence to 2006 MASCC/ESMO guidelines was analyzed in a prospective observational study enrolling patients at various centers across Europe and showed a 55% adherence rate in the acute phase, with lowest adherence in the HEC (43%) and AC (32%) settings. Similar prospective and retrospective studies in Europe and the U.S. [26, 27, 28] as well as surveys of oncologists [32] and oncology nurses [33, 34] consistently showed low compliance with antiemetic guidelines.
Recently, and in line with our results, low guideline adherence during routine clinical practice was reported in a study analyzing patient data from five prospective noninterventional studies conducted between 2008 and 2015 to develop a tool to predict the risk of CINV [35, 36]. This study included a total of 1,198 patients receiving outpatient chemotherapy, of whom 27.4% and 52.8% received platinum‐ and anthracycline‐based chemotherapy, respectively. Although these HEC regimens represent 80.2% of the total chemotherapy cycles analyzed, the guideline‐recommended NK1RA–5‐HT3RA–dexamethasone triplet was only prescribed in 12.2% of cycles and the olanzapine–NK1RA–5‐HT3RA–dexamethasone quadruplet in 2.1%. Additionally, use of nonprescription antiemetic treatment was reported by 57.7% of all patients. A recent study in the U.S. using an electronic health record database reported low use of the NK1RA–5‐HT3RA–dexamethasone triplet for prophylaxis of carboplatin area under the concentration‐time curve (AUC) ≥4 mg/mL per minute regimens, reclassified as HEC by NCCN and ASCO guidelines since February and August 2017, respectively. The study analyzed antiemetic treatments prescribed for a total of 11,554 carboplatin courses from 2012 to 2018 [29] and showed only a mild increase in the use of the NK1RA–5‐HT3RA–dexamethasone triplet from 16% in mid‐2017 to 26% in the first quarter of 2018, which then dropped to 20% by the third quarter of 2018 [29]. In the two above‐mentioned studies, inadequate prophylaxis was associated with high rates of nausea and vomiting [29, 35].
Remarkable is the high proportion of HEC and MEC treatments for which no associated antiemetic prophylaxis was prescribed (Fig. 2). This alarming result may be explained by physicians’ underestimation of the emetic risk of chemotherapy and by their lack of awareness of antiemetic guidelines (despite being regularly downloaded from the societies’ Web sites). Regarding physicians’ perception of the risk for CINV, here we show that only 55% of cisplatin‐, 51% of AC‐, and 24% of carboplatin‐based treatments were recognized as HEC by physicians (Fig. 6A). As expected, among physicians who prescribe NK1RA‐based regimens, the perceived emetic risk of chemotherapy correlated better with MASCC/ESMO guidelines’ classification, with more than double the number of cisplatin‐ and AC‐based treatments and more than four times the number of carboplatin‐based treatments classed as HEC (Fig. 6B). Several studies have highlighted discrepancies between guideline‐based emetic classification and physician‐ or oncology nurse–based emetic classification of chemotherapy, and a tendency to underrate the occurrence of CINV associated with both MEC [37] and HEC [38, 39]. Underestimation of the emetic potential of chemotherapy was cited as the main reason for uncontrolled CINV in the acute phase by 43% of oncologists participating in a European survey [32]. A potential reason for the underrated CINV risk of chemotherapy by physicians is low awareness of the risk classification described in guidelines. Additionally, patients tend to underreport CINV occurrence [40, 41, 42] because they either consider nausea and vomiting a sign of their chemotherapy being effective [39], fear their chemotherapy dose may be adjusted, or forget it if a long time has elapsed between its occurrence and the next medical appointment [43].
Even when physicians’ perception of the emetic risk of chemotherapy is considered, overall prescription patterns did not follow guideline recommendations. This suggests that a lack of familiarity with antiemetic guidelines is an important driver for nonadherence. For example, although nearly half of cisplatin‐ and AC‐ based therapies and 24% of carboplatin‐based therapies were perceived as HEC, only 18%, 24%, and 7% of them received guideline‐recommended NK1RA‐based triplet prophylaxis, respectively. In addition, although physicians considered the total of cisplatin‐, AC‐, and carboplatin‐based treatments as HEC, MEC, or LEC—thus requiring antiemetics per guidelines—as many as 12% of these chemotherapies were associated with no antiemetic prophylaxis (Fig. 2). In the above‐mentioned oncologists’ survey [32], only approximately a third of European oncologists reported awareness of MASCC/ESMO guidelines. Low guideline awareness was also reported by oncology nurses in Europe [34] and the U.S. [33], with only 40% and 6%, respectively, noting familiarity with MASCC/ESMO guidelines. Similarly, low uptake of the NK1RA–5‐HT3RA–dexamethasone triplet for prophylaxis of carboplatin AUC ≥4‐mg/mL per minute regimens in the U.S. may reflect low awareness of changes in guidelines [29].
NK1RA use was consistently low across all five European countries (France, Germany, Italy, Spain, and the U.K.), with NK1RAs prescribed most in Germany and France (Fig. 5). Distinct traits in prescription patterns are observed depending on the chemotherapy setting. Although NK1RAs were most used overall for cisplatin‐based prophylaxis, they were primarily prescribed in the AC setting in France, Germany, and Italy. Additionally, although their use was lowest in the carboplatin setting in all five countries, usage varied from 47% and 29% in Germany and France, respectively, to ≤10% in Italy, Spain, and the U.K. These discrepancies may be explained by local guideline recommendations or country‐specific reimbursement policies. In the U.K., no country‐level recommendations have been issued by the National Institute for Health and Care Excellence for the management of CINV, and antiemetic usage is determined locally by cancer networks and trusts, usually following MASCC/ESMO guidelines, which may account for the low usage of NK1RAs.
A weakness of the study is that the total number of chemotherapy treatments analyzed are extrapolated from patient charts on the basis of the total number of physicians in the five European countries analyzed. Nevertheless, use of a large database of more than 45,000 patients provides robustness to the results presented here. Another limitation is that antiemetic prophylaxis was only analyzed for the acute phase following chemotherapy. Consistent with previous reports, lower rates of guideline adherence would be expected for the delayed phase [25, 28, 33, 34]. Analysis of patient records for delayed CINV prophylaxis would provide a more detailed picture of prescription patterns in the real world for the overall CINV risk period. Finally, patients receiving other MEC regimens besides carboplatin, such as oxaliplatin, who may be eligible for NK1RA‐based prophylaxis but for whom no clear consensus has been reached in guidelines, were excluded from the analysis.
This study underscores the need to implement novel strategies to improve adherence to antiemetic guidelines for patients receiving HEC and carboplatin‐based regimens, with a special focus on increasing the use of NK1RAs. “Physician preference” was identified as the key barrier for implementation of guideline‐recommended prophylaxis by oncology nurses in the U.S. and Europe [33, 34], and changing that preference is often difficult. Increasing health care providers’ awareness of antiemetic guidelines through educational programs combined with lectures by “experts,” feedback on institutional prescription patterns [30], and information on patients’ CINV outcomes [44] have been shown to modify physicians’ behavior and improve adherence. Collaboration between the multidisciplinary team, including clinicians, nurses, and pharmacists, in the implementation of standardized antiemetics orders on the basis of chemotherapy type has been shown to improve adherence at the institutional level [45]. In addition, application of standardized physician order‐entry systems developed at medical centers in routine practice may increase compliance.
Complex administration schedules have been reported to interfere with use of guideline‐recommended antiemetics [32, 34]. Some contributing factors include nurse‐patient miscommunication during the explanation of complex schedules, and the desire of some patients to reduce the pill burden, leading them to only take their medication once symptoms appear. NEPA is the only fixed combination of an NK1RA and a 5‐HT3RA and has the simplest administration schedule, offering high convenience of administration for most patients. Simple administration schedules not only could facilitate adherence by physicians but also could prevent patients from making medication mistakes, a recurring problem during home administration in the delayed phase [32]. In line with this, a recent noninterventional study evaluating patient‐reported outcomes during the use of NEPA in routine clinical practice showed similar antiemetic efficacy as in the controlled pivotal trials, suggesting high treatment compliance by patients under real‐world conditions [46]. On the other hand, a recent phase IV study demonstrated lower antiemetic effectiveness of the aprepitant/fosaprepitant‐palonosetron‐dexamethasone regimen in the real world as compared with the reported efficacy in randomized controlled trials [47, 48]. Reduced patient compliance with the more complex aprepitant‐based regimen in the real‐world setting may account for this difference, at least in part.
Approaches to prevent patients from underreporting nausea and vomiting would provide physicians more realistic data about the incidence and severity of CINV and correct their perception of the emetic risk of chemotherapy. Use of electronic questionnaires, and phone‐ or Web‐based applications for reporting of symptoms associated with chemotherapy have been shown to promote communication between patients and physicians during clinical visits [49, 50, 51]. Application of such tools in routine clinical practice may prove beneficial for appropriate reporting of nausea and vomiting by patients.
Besides improving adherence to antiemetic guidelines, correct patient assessment and management are needed at all stages of the cancer treatment pathway for optimal supportive care [52]. Uptake of supportive care guidelines is usually low, and most studies aim at increasing overall survival instead of focusing on improving symptom management. Recently, a study that evaluated remote patient monitoring through the electronic collection of chemotherapy‐related symptoms in real time combined with timely feedback and medical intervention for symptom management demonstrated improved clinical outcomes, including overall survival [6]. There is a clear need for more studies that prospectively evaluate supportive care procedures and for the inclusion of patient‐reported outcomes as endpoints in trials evaluating anticancer treatments, in order to ensure optimal patient care across the continuum of disease.
Conclusion
Our study results indicate low adherence to MASCC/ESMO antiemetic guidelines in real‐world clinical practice in Europe. Only 15% of all HEC and carboplatin‐based treatments for which NK1RA‐based prophylaxis is recommended received the guideline‐compliant NK1RA–5‐HT3RA–dexamethasone triplet. Of note, no antiemetic prophylaxis was prescribed for as many as 12% of the treatments. New strategies to improve guideline adherence, such as increasing awareness of guidelines, using simple and convenient antiemetic regimens, and preventing patients from underreporting CINV episodes, are urgently needed.
Author Contributions
Conception/design: Matti Aapro, Florian Scotté, Yolanda Escobar, Luigi Celio, Richard Berman, Karin Jordan
Provision of study material or patients: Matti Aapro, Florian Scotté, Yolanda Escobar, Luigi Celio, Richard Berman, Alessandra Franceschetti, Danielle Bell, Karin Jordan
Collection and/or assembly of data: Alessandra Franceschetti, Danielle Bell
Data analysis and interpretation: Matti Aapro, Florian Scotté, Yolanda Escobar, Luigi Celio, Richard Berman, Alessandra Franceschetti, Danielle Bell, Karin Jordan
Manuscript writing: Matti Aapro, Florian Scotté, Yolanda Escobar, Luigi Celio, Richard Berman, Alessandra Franceschetti, Danielle Bell, Karin Jordan
Final approval of manuscript: Matti Aapro, Florian Scotté, Yolanda Escobar, Luigi Celio, Richard Berman, Alessandra Franceschetti, Danielle Bell, Karin Jordan
Disclosures
Matti Aapro: Eisai, Helsinn, Merck, Mundipharma, Roche, Tesaro (C/A, H), Helsinn, Merck, Roche, Tesaro (RF); Florian Scotté: Roche, Amgen, Tesaro, Vifor, Merck Sharp & Dohme, Pierre Fabre Oncology, LEO Pharma, Sanofi, Helsinn, Pfizer (C/A); Yolanda Escobar: Merck Serono, Merck Sharp & Dohme, Tesaro, Vifor Pharma, Kyowa Kirin, Grünenthal, Fresenius Kabi (C/A); Luigi Celio: Italfarmaco SpA, Kyowa Kirin (C/A); Richard Berman: Chugai, Tesaro (H); Alessandra Franceschetti: Ipsos Healthcare (E); Danielle Bell: Ipsos Healthcare (E); Karin Jordan: Helsinn Healthcare, Tesaro, Merck/Merck Sharp & Dohme, Amgen, MEUpdate, Glenmark, Art Tempi (C/A, H).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
Editorial and medical writing assistance was provided by Iratxe Abarrategui, Ph.D., C.M.P.P., and Oana Draghiciu, Ph.D., C.M.P.P., from Aptitude Health, The Hague, The Netherlands, funded by Helsinn Healthcare SA. The authors are fully responsible for all content and editorial decisions for this manuscript. Helsinn Healthcare SA subscribed to access Ipsos Healthcare. The nature of these syndicated, real‐world data further validates the unbiased analysis of the current prescribing of supportive care in oncology. Partial results of this survey were presented at the European Society for Medical Oncology Annual Congress, October 19–23, 2018, Munich, Germany.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Kuchuk I, Bouganim N, Beusterien K et al. Preference weights for chemotherapy side effects from the perspective of women with breast cancer. Breast Cancer Res Treat 2013;142:101–107. [DOI] [PubMed] [Google Scholar]
- 2. Sun CC, Bodurka DC, Weaver CB et al. Rankings and symptom assessments of side effects from chemotherapy: Insights from experienced patients with ovarian cancer. Support Care Cancer 2005;13:219–227. [DOI] [PubMed] [Google Scholar]
- 3. Fernández‐Ortega P, Caloto MT, Chirveches E et al. Chemotherapy‐induced nausea and vomiting in clinical practice: Impact on patients’ quality of life. Support Care Cancer 2012;20:3141–3148. [DOI] [PubMed] [Google Scholar]
- 4. Van Laar ES, Desai JM, Jatoi A. Professional educational needs for chemotherapy‐induced nausea and vomiting (CINV): Multinational survey results from 2388 health care providers. Support Care Cancer 2015;23:151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Basch E, Deal AM, Kris MG et al. Symptom monitoring with patient‐reported outcomes during routine cancer treatment: A randomized controlled trial. J Clin Oncol 2016;34:557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Basch E, Deal AM, Dueck AC et al. Overall survival results of a trial assessing patient‐reported outcomes for symptom monitoring during routine cancer treatment. JAMA 2017;318:197–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jordan K, Gralla R, Jahn F et al. International antiemetic guidelines on chemotherapy induced nausea and vomiting (CINV): Content and implementation in daily routine practice; Eur J Pharmacol 2014;722:197–202. [DOI] [PubMed] [Google Scholar]
- 8. Hesketh PJ, Kris MG, Grunberg SM et al. Proposal for classifying the acute emetogenicity of cancer chemotherapy. J Clin Oncol 1997;5:103–109. [DOI] [PubMed] [Google Scholar]
- 9. Grunberg SM, Warr D, Gralla RJ et al. Evaluation of new antiemetic agents and definition of antineoplastic agent emetogenicity–state of the art. Support Care Cancer 2011;19(suppl 1):S43–S47. [DOI] [PubMed] [Google Scholar]
- 10. Roila F, Molassiotis A, Herrstedt J et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy‐ and radiotherapy‐induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol 2016;27:v119–v133. [DOI] [PubMed] [Google Scholar]
- 11.Multinational Association of Supportive Care in Cancer. MASCC/ESMO Antiemetic Guidelines. 2019. Available at https://www.mascc.org/antiemetic-guidelines. Accessed May 12, 2020.
- 12. National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Antiemesis. Version 2. 2020. 2020. Available at https://www.nccn.org/professionals/physician_gls/PDF/antiemesis.pdf. Accessed May 12, 2020.
- 13. Hesketh PJ, Kris MG, Basch E et al. Antiemetics: American Society of Clinical Oncology Clinical Practice Guideline update. J Clin Oncol 2017;35:3240–3261. [DOI] [PubMed] [Google Scholar]
- 14. Akynzeo (netupitant and palonosetron) capsules; Akynzeo (fosnetupitant and palonosetron) for injection [prescribing information]. Dublin, Ireland: Helsinn Birex Pharmaceuticals Ltd; 2020. [Google Scholar]
- 15. Cinvanti (aprepitant) injectable emulsion [prescribing information]. San Diego, CA: Heron Therapeutics, Inc; 2019. [Google Scholar]
- 16. Varubi (rolapitant) tablets; Varubi (rolapitant) injectable emulsion [prescribing information]. Lake Forest, IL: TerSera Therapeutics LLC; 2018. [Google Scholar]
- 17. Jordan K. New formulation, new drug? The importance of assessing the safety of new supportive care formulations in oncology. Ann Oncol 2018;29:1494–1496. [DOI] [PubMed] [Google Scholar]
- 18.European Medicines Agency. Summary of Opinion (Post Authorisation). Akynzeo (Fosnetupitant/Palonosetron). EMA/CHMP/670824/2019. Available at https://www.ema.europa.eu/en/documents/smop‐initial/chmp‐summary‐positive‐opinion‐akynzeo_en.pdf. Accessed May 12, 2020.
- 19. Tesaro, Inc . Anaphylaxis, anaphylactic shock and other serious hypersensitivity reactions associated with use of VARUBI (rolapitant) injectable emulsion. 2018. Available at https://www.fda.gov/media/110258/download. Accessed May 12, 2020.
- 20. ASD Healthcare . Varubi IV distribution suspension. 2018. Available at https://www.asdhealthcare.com/AsdHealthcare/media/AsdLibrary/pdfs/News/Varubi‐Final‐Web‐Notice.pdf. Accessed May 12, 2020.
- 21. Schwartzberg L, Szabo S, Gilmore J et al. Likelihood of a subsequent chemotherapy‐induced nausea and vomiting (CINV) event in patients receiving low, moderately or highly emetogenic chemotherapy (LEC/MEC/HEC). Curr Med Res Opin 2011;27:837–845. [DOI] [PubMed] [Google Scholar]
- 22. Molassiotis A, Aapro M, Dicato M et al. Evaluation of risk factors predicting chemotherapy‐related nausea and vomiting: Results from a European prospective observational study. J Pain Symptom Manage 2014;47:839–848. [DOI] [PubMed] [Google Scholar]
- 23. Navari RM, Aapro M. Antiemetic prophylaxis for chemotherapy‐induced nausea and vomiting. N Engl J Med 2016;374:1356–1367. [DOI] [PubMed] [Google Scholar]
- 24. Bošnjak SM, Gralla RJ, Schwartzberg L. Prevention of chemotherapy‐induced nausea: The role of neurokinin‐1 (NK1) receptor antagonists. Support Care Cancer 2017;25:1661–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aapro M, Molassiotis A, Dicato M et al. The effect of guideline‐consistent antiemetic therapy on chemotherapy‐induced nausea and vomiting (CINV): The Pan European Emesis Registry (PEER). Ann Oncol 2012;23:1986–1992. [DOI] [PubMed] [Google Scholar]
- 26. Gilmore JW, Peacock NW, Gu A et al. Antiemetic guideline consistency and incidence of chemotherapy‐induced nausea and vomiting in US community oncology practice: INSPIRE study. J Oncol Pract 2014;10:68–74. [DOI] [PubMed] [Google Scholar]
- 27. Molassiotis A, Saunders MP, Valle J et al. A prospective observational study of chemotherapy‐related nausea and vomiting in routine practice in a UK cancer centre. Support Care Cancer 2008;16:201–208. [DOI] [PubMed] [Google Scholar]
- 28. Burmeister H, Aebi S, Studer C et al. Adherence to ESMO clinical recommendations for prophylaxis of chemotherapy‐induced nausea and vomiting. Support Care Cancer 2012;20:141–147. [DOI] [PubMed] [Google Scholar]
- 29. Navari RM, Ruddy KJ, LeBlanc TW et al. Physician concordance with update to ASCO guidelines for antiemetic use with carboplatin AUC ≥4. J Clin Oncol 2019;37(suppl 15):11595a. [Google Scholar]
- 30. Roila F, Hesketh PJ, Herrstedt J et al. Prevention of chemotherapy‐ and radiotherapy‐induced emesis: Results of the 2004 Perugia International Antiemetic Consensus Conference. Ann Oncol 2006;17:20–28. [DOI] [PubMed] [Google Scholar]
- 31. Aapro MS, Scotté F, Escobar Y et al. Evaluation of practice patterns for prevention of chemotherapy (CT)‐induced nausea and vomiting (CINV) and antiemetic guidelines (GLS) adherence based on real‐world prescribing data. Ann Oncol 2018;29(suppl 8):3439a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aapro M, Ruffo P, Panteri R et al. Oncologist perspectives on chemotherapy‐induced nausea and vomiting (CINV) management and outcomes: A quantitative market research‐based survey. Cancer Rep 2018;1:e1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Clark‐Snow R, Affronti ML, Rittenberg CN. Chemotherapy‐induced nausea and vomiting (CINV) and adherence to antiemetic guidelines: Results of a survey of oncology nurses. Support Care Cancer 2018;26:557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dielenseger P, Börjeson S, Vidall C et al. Evaluation of antiemetic practices for prevention of chemotherapy‐induced nausea and vomiting (CINV): Results of a European oncology nurse survey. Support Care Cancer 2019;27:4099–4106. [DOI] [PubMed] [Google Scholar]
- 35. Dranitsaris G, Molassiotis A, Clemons M et al. The development of a prediction tool to identify cancer patients at high risk for chemotherapy‐induced nausea and vomiting. Ann Oncol 2017;28:1260–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Scotté F. Identifying predictive factors of chemotherapy‐induced nausea and vomiting (CINV): A novel approach. Ann Oncol 2017;28:1165–1167. [DOI] [PubMed] [Google Scholar]
- 37. Escobar Y, Cajaraville G, Virizuela JA et al. Incidence of chemotherapy‐induced nausea and vomiting with moderately emetogenic chemotherapy: ADVICE (Actual Data of Vomiting Incidence by Chemotherapy Evaluation) study. Support Care Cancer 2015;23:2833–2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Majem M, Moreno ME, Calvo N et al. Perception of healthcare providers versus patient reported incidence of chemotherapy‐induced nausea and vomiting after the addition of NK‐1 receptor antagonists. Support Care Cancer 2011;19:1983–1990. [DOI] [PubMed] [Google Scholar]
- 39. Salsman JM, Grunberg SM, Beaumont JL et al. Communicating about chemotherapy‐induced nausea and vomiting: A comparison of patient and provider perspectives. J Natl Compr Canc Netw 2012;10:149–157. [DOI] [PubMed] [Google Scholar]
- 40. Vidall C, Fernández‐Ortega P, Cortinovis D et al. Impact and management of chemotherapy/radiotherapy‐induced nausea and vomiting and the perceptual gap between oncologists/oncology nurses and patients: A cross‐sectional multinational survey. Support Care Cancer 2015;23:3297–3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Di Maio M, Gallo C, Leighl NB et al. Symptomatic toxicities experienced during anticancer treatment: Agreement between patient and physician reporting in three randomized trials. J Clin Oncol 2015;33:910–915. [DOI] [PubMed] [Google Scholar]
- 42. Childs DS, Looker S, Le‐Rademacher J et al. What occurs in the other 20% of cancer patients with chemotherapy‐induced nausea and vomiting (CINV)? A single‐institution qualitative study. Support Care Cancer 2019;27:249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vidall C, Dielenseger P, Farrell C et al. Evidence‐based management of chemotherapy‐induced nausea and vomiting: A position statement from a European cancer nursing forum. Ecancermedicalscience 2011;5:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mertens WC, Higby DJ, Brown D et al. Improving the care of patients with regard to chemotherapy‐induced nausea and emesis: The effect of feedback to clinicians on adherence to antiemetic prescribing guidelines. J Clin Oncol 2003;21:1373–1378. [DOI] [PubMed] [Google Scholar]
- 45. Nolte MJ, Berkery R, Pizzo B et al. Assuring the optimal use of serotonin antagonist antiemetics: The process for development and implementation of institutional antiemetic guidelines at Memorial Sloan‐Kettering Cancer Center. J Clin Oncol 1998;16:771–778. [DOI] [PubMed] [Google Scholar]
- 46. Karthaus M, Oskay‐Özcelik G, Wülfing P et al. Real‐world evidence of NEPA, netupitant‐palonosetron, in chemotherapy‐induced nausea and vomiting prevention: Effects on quality of life. Future Oncol 2020;16:939–953. [DOI] [PubMed] [Google Scholar]
- 47. Schwartzberg LS, Marks SM, Gabrail NY et al. Real‐world effectiveness of palonosetron‐based antiemetic regimens: Preventing chemotherapy‐induced nausea and vomiting. J Comp Eff Res 2019;8:657–670. [DOI] [PubMed] [Google Scholar]
- 48. Navari RM, Gray SE, Kerr AC. Olanzapine versus aprepitant for the prevention of chemotherapy‐induced nausea and vomiting: A randomized phase III trial. J Support Oncol 2011;9:188–195. [DOI] [PubMed] [Google Scholar]
- 49. Velikova G, Booth L, Smith AB et al. Measuring quality of life in routine oncology practice improves communication and patient well‐being: A randomized controlled trial. J Clin Oncol 2004;22:714–724. [DOI] [PubMed] [Google Scholar]
- 50. Breen S, Kofoed S, Ritchie D et al. Remote real‐time monitoring for chemotherapy side‐effects in patients with blood cancers. Collegian 2017;24: 541–549. [Google Scholar]
- 51. Peltola MK, Lehikoinen JS, Sippola LT et al. A novel digital patient‐reported outcome platform for head and neck oncology patients–a pilot study. Clin Med Insights Ear Nose Throat 2016;9:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jordan K, Aapro M, Kaasa S et al. European Society for Medical Oncology (ESMO) position paper on supportive and palliative care. Ann Oncol 2018;29:36–43. [DOI] [PubMed] [Google Scholar]


