Abstract
Lessons Learned
Fulvestrant is a selective estrogen receptor (ER)‐downregulating antiestrogen that blocks ER transcriptional activity and is approved for ER‐positive breast cancer.
Fulvestrant also induces accumulation of insoluble ER and activates an unfolded protein response; proteasome inhibitors have been shown to enhance these effects in preclinical models.
Background
Fulvestrant is a selective estrogen receptor (ER)‐downregulating antiestrogen that blocks ER transcriptional activity and is approved for ER‐positive (+) breast cancer. Fulvestrant also induces accumulation of insoluble ER and activates an unfolded protein response; proteasome inhibitors have been shown to enhance these effects in preclinical models.
Methods
This is a single‐center phase Ib study with a 3+3 design of fulvestrant and the proteasome inhibitor ixazomib (MLN9708) in patients with advanced ER+ breast cancer that was progressing on fulvestrant. A dose‐escalation design allowed establishment of the ixazomib maximum tolerated dose (MTD). Secondary objectives included progression‐free survival, pharmacokinetics, and tumor molecular analyses.
Results
Among nine evaluable subjects, treatment was well‐tolerated without dose‐limiting toxicities The MTD of ixazomib was 4 mg in combination with fulvestrant. Plasma concentrations of the active form of ixazomib (MLN2238) in the 4‐mg dose cohort had a median (range) maximal concentration (Cmax) of 155 (122–171) ng/mL, time of maximal concentration (Tmax) of 1 (1–1.5) hour, terminal elimination half‐life of 66.6 (57.3–102.6) hour after initial dose, and area under the curve (AUC) of 5,025 (4,160–5,345) ng*h/mL. One partial response was observed, and median progression‐free survival was 51 days (range, 47–137).
Conclusion
This drug combination has a favorable safety profile and antitumor activity in patients with fulvestrant‐resistant advanced ER+ breast cancer that justifies future testing.
Keywords: Breast cancer, Anti‐estrogen, Endocrine therapy, Proteasome inhibitor
Discussion
Approved therapeutics for the treatment of ER‐positive breast cancer include antiestrogens that target ER, such as the selective ER downregulator fulvestrant. Unfortunately, treatment responses to antiestrogen therapy for metastatic disease are unpredictable, and approximately half of these patients experience disease progression within 6 months [1, 2, 3, 4, 5, 6]. The mechanism of fulvestrant action involves antagonizing ER transcriptional activity, inducing degradation of nuclear ER via the proteasome, and inducing the formation of cytoplasmic aggregates containing ER [7, 8, 9]. The accumulation of these aggregates invokes a generalized unfolded protein response that can lead to DNA fragmentation and apoptosis [10]. However, proteasome activity may contribute to resistance to fulvestrant by clearing ER aggregates, thereby protecting cancer cells from death. Therefore, inhibition of proteasomal protein degradation represented a logical therapeutic opportunity to test in combination with fulvestrant [11, 12].
At the time this study was initiated, the oral proteasome inhibitor ixazomib (MLN9708) was in phase III testing in combination with lenalidomide and dexamethasone for multiple myeloma; this combination was subsequently U.S. Food and Drug Administration approved. We conducted a phase Ib trial to test the safety and efficacy of the novel combination of fulvestrant and ixazomib in patients with advanced ER+ breast cancer progressing on fulvestrant. No dose‐limiting toxicities were observed during cycle 1. This study fulfilled its primary objective of establishing a safety profile for ixazomib and identifying a maximum tolerated dose of 4 mg on days 1, 4, 8, and 11 in a 21‐day cycle when combined with fulvestrant. The ixazomib plasma pharmacokinetic profile was generally consistent with prior findings [13]. The addition of ixazomib in patients with disease progression on fulvestrant resulted in a delay of disease progression (progression‐free survival; PFS) in all nine patients by a median of 51 days (maximum 137 days; Fig. 1A). One patient had a partial response by RECIST version 1.1 (Fig. 1B); this patient had previously received four lines of therapy. Comparison of PFS on study treatment with ixazomib plus fulvestrant to that on single‐agent fulvestrant as the prior line of therapy revealed PFS ratios ranging from 0.05 to 0.52 (Fig. 1C), indicating that PFS on study treatment was shorter than PFS on the prior line of (fulvestrant) therapy in all cases.
Figure 1.
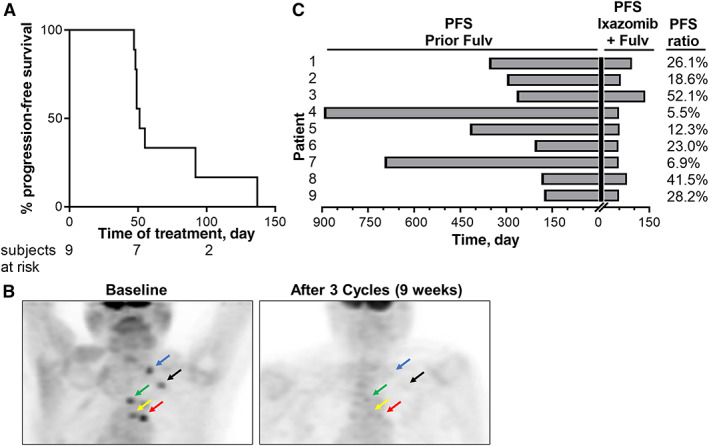
Progression‐free survival and response to ixazomib plus fulvestrant. (A): Distribution of PFS for patients treated with fulvestrant plus ixazomib. (B): The addition of ixazomib induced a partial tumor response in a subject with fulvestrant‐resistant breast cancer. Positron emission tomography imaging results are shown from baseline and after 3 cycles of ixazomib (4 mg) plus fulvestrant. Arrows indicate corresponding tumor locations. (C): Comparison of PFS on prior fulvestrant regimen to fulvestrant/ixazomib regimen. Abbreviations: Fulv, fulvestrant; PFS, progression‐free survival.
Trial Information
| Disease | Breast cancer |
| Stage of Disease/Treatment | Metastatic/advanced |
| Prior Therapy | No designated number of regimens |
| Type of Study | Phase I, 3+3 |
| Primary Endpoint | Maximum tolerated dose |
| Secondary Endpoints | Safety, tolerability, pharmacodynamics, correlative endpoint, efficacy |
| Additional Details of Endpoints or Study Design | |
| Eligibility criteria: Patients were required to be postmenopausal with histologically confirmed ER‐positive, HER2‐negative metastatic or locally advanced breast cancer. Patients were required to have a favorable performance status (ECOG 0–1) without significant hematologic, hepatic, and renal impairment as measured by laboratory measurements, as well as cancer that was progressing by RECIST 1.1 criteria while taking fulvestrant for ≥56 days as the most recent line of treatment. | |
| Adverse event severity was graded using the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 4.03. Dose‐limiting toxicity was defined as any grade 3 or higher nonhematologic toxicity or any grade 4 or higher hematologic toxicity during cycle 1 that was considered related to ixazomib. | |
| Safety (adverse events and hematological/chemistry laboratory parameters) and physical status (including ECOG performance status) were assessed at baseline and at the end of each 21‐day cycle. Adverse event severity was graded using the NCI CTCAE version 4.03. Dose‐limiting toxicity was defined as any grade 3 or higher nonhematologic toxicity or any grade 4 or higher hematologic toxicity during cycle 1 that was considered related to ixazomib. All patients underwent computed tomography (CT) scanning of the chest, abdomen, and pelvis and a bone scan, or a positron emission tomography‐CT scan within 21 days of study entry. Disease reassessments were repeated with the same imaging modality approximately every 6 weeks on study. All lesions measurable by radiologic study or physical exam, up to a maximum of five, were identified as “target lesions” and measured at baseline and after each imaging session. Tumor response was assessed per RECIST criteria v1.1. Clinical benefit was defined as complete response, partial response, or stable disease for ≥24 weeks. PFS was calculated. PFS ratio was then calculated as (PFS on ixazomib + fulvestrant)/(PFS on prior line of fulvestrant). | |
| Investigator's Analysis | Active and should be pursued further |
Drug Information
| Ixazomib | |
| Generic/Working Name | Ixazomib |
| Trade Name | Ninlaro |
| Drug Type | Small molecule |
| Drug Class | Proteasome |
| Dose | 2.3, 3, or 4 mg per flat dose |
| Route | oral (po) |
| Schedule of Administration | Days 1, 4, 8, and 11 of a 21‐day cycle [19, 20] |
| Fulvestrant | |
| Generic/Working Name | Fulvestrant |
| Trade Name | Faslodex |
| Drug Type | Small molecule |
| Drug Class | Estrogen receptor |
| Dose | 500 mg per flat dose |
| Route | Intramuscular |
| Schedule of Administration | Day 1 of a 28‐day cycle |
Patient Characteristics
| Number of Patients, Male | 0 |
| Number of Patients, Female | 9 |
| Stage | IV |
| Age | Median (range): 60 (37–67) years |
| Number of Prior Systemic Therapies | Median (range): 5 (2–6) |
| Performance Status: ECOG |
0 — 6 1 — 3 2 — 0 3 — 0 Unknown — 0 |
| Other | In addition to fulvestrant, all patients had received other prior endocrine therapy, and four of nine subjects had received prior chemotherapy. Although bone was the most common site of metastatic disease (8/9 subjects), all had accompanying soft‐tissue disease. |
| Baseline characteristic | Subjects, n (%) |
|---|---|
| Median age (range), yr | 60 (37–67) |
| ECOG performance status | |
| 0 | 6 (67) |
| 1 | 3 (33) |
| Receptor status (%) | |
| ER‐positive | 9 (100) |
| PR‐positive | 9 (100) |
| HER2‐positive | 0 (0) |
| Sites of metastatic disease | |
| Bone | 8 (89) |
| Liver | 6 (67) |
| Lung or pleura | 3 (33) |
| Lymph nodes | 2 (22) |
| Omentum | 1 (11) |
| Lines of endocrine therapy in the metastatic setting | |
| 0–1 | 0 (0) |
| 2 | 3 (33) |
| 3 | 3 (33) |
| 4 | 1 (11) |
| 5 | 2 (22) |
| Prior endocrine therapies in the metastatic setting | |
| Aromatase inhibitor(s) | 9 (100) |
| Tamoxifen | 4 (44) |
| Fulvestrant | 9 (100) |
| Everolimus | 1 (11) |
| Palbociclib | 4 (44) |
| Abemaciclib | 2 (22) |
| Lines of chemotherapy in metastatic setting | |
| 0 | 5 (56) |
| 1 | 2 (22) |
| 2 | 1 (11) |
| >2 | 1 (11) |
Abbreviations: ER, estrogen receptor; PR, progesterone receptor.
Primary Assessment Method
| Title | Maximum tolerated dose |
| Number of Patients Screened | 9 |
| Number of Patients Enrolled | 9 |
| Number of Patients Evaluable for Toxicity | 9 |
| Number of Patients Evaluated for Efficacy | 9 |
| Evaluation Method | Adverse events |
| Response Assessment PR | n = 1 (11%) |
| Response Assessment PD | n = 8 (88%) |
| (Median) Duration Assessments PFS | 51 Days, CI: 20 |
| Outcome Notes | |
| We performed a 3+3 dose‐escalation trial to assess the safety and efficacy of fulvestrant with three dose cohorts of oral ixazomib: 2.3 mg (cohort A), 3 mg (cohort B), and 4 mg (cohort C). Subjects were treated with fulvestrant (500 mg) by intramuscular injection once every 28 days starting on day 1. Subjects were treated with ixazomib orally on days 1, 4, 8, and 11 of a 21‐day cycle based on prior clinical studies [19, 20]. The dose of ixazomib started at 2.3 mg, slightly greater than 50% of the phase III dose in another ongoing study at the time (NCT01850524). MLN2238 is the biologically active, boronic acid form of ixazomib citrate; in aqueous systems, the equilibrium shifts from ixazomib citrate to MLN2238. Plasma MLN2238 (ixazomib) concentration versus time profiles were determined [21] during cycle 1 day 1 through cycle 2 day 1. Subjects with visceral or soft‐tissue disease had a tumor biopsy prior to treatment with ixazomib to confirm ER, progesterone receptor (PR), and HER2 status and to provide a baseline specimen for molecular analysis. When safely accessible, a biopsy of the same tumor was obtained on cycle 1 day 11 after dosing with ixazomib. Treatments continued without interruption until disease progression, severe or intolerable toxicity, or participant withdrawal of consent. | |
Adverse Events
| Name | NC/NA | 1 | 2 | 3 | 4 | 5 | All grades |
|---|---|---|---|---|---|---|---|
| Anemia | 67% | 0% | 11% | 22% | 0% | 0% | 33% |
| Leukocytes (total WBC) | 78% | 0% | 22% | 0% | 0% | 0% | 22% |
| Lymphopenia | 89% | 0% | 11% | 0% | 0% | 0% | 11% |
| Neutrophil count decreased | 78% | 0% | 22% | 0% | 0% | 0% | 22% |
| Platelets | 56% | 0% | 0% | 22% | 22% | 0% | 44% |
| Anorexia | 89% | 0% | 11% | 0% | 0% | 0% | 11% |
| Arthralgia | 78% | 0% | 22% | 0% | 0% | 0% | 22% |
| Cough | 78% | 0% | 22% | 0% | 0% | 0% | 22% |
| Dizziness | 89% | 0% | 11% | 0% | 0% | 0% | 11% |
| Dysgeusia | 89% | 0% | 11% | 0% | 0% | 0% | 11% |
| Dyspepsia | 89% | 0% | 11% | 0% | 0% | 0% | 11% |
| Dyspnea (shortness of breath) | 67% | 0% | 22% | 11% | 0% | 0% | 33% |
| Alanine aminotransferase increased | 89% | 0% | 11% | 0% | 0% | 0% | 11% |
| Aspartate aminotransferase increased | 89% | 0% | 0% | 11% | 0% | 0% | 11% |
| Blood bilirubin increased | 89% | 0% | 11% | 0% | 0% | 0% | 11% |
| Fatigue (asthenia, lethargy, malaise) | 78% | 0% | 22% | 0% | 0% | 0% | 22% |
| Hot flashes/flushes | 89% | 0% | 11% | 0% | 0% | 0% | 11% |
| Hypertension | 33% | 0% | 67% | 0% | 0% | 0% | 67% |
| Hypotension | 89% | 0% | 11% | 0% | 0% | 0% | 11% |
| Hypoxia | 89% | 0% | 0% | 11% | 0% | 0% | 11% |
| Heart failure | 89% | 0% | 11% | 0% | 0% | 0% | 11% |
| Lip infection | 89% | 0% | 11% | 0% | 0% | 0% | 11% |
| Nausea | 78% | 0% | 11% | 11% | 0% | 0% | 22% |
| Pulmonary hypertension | 89% | 0% | 0% | 11% | 0% | 0% | 11% |
| Rash | 78% | 0% | 11% | 11% | 0% | 0% | 22% |
| Right ventricular dysfunction | 89% | 0% | 11% | 0% | 0% | 0% | 11% |
| Insomnia | 89% | 0% | 11% | 0% | 0% | 0% | 11% |
Cycle 1 toxicities in any dose level.
Abbreviations: NC/NA, no change from baseline/no adverse events; WBC, white blood cell.
Pharmacokinetics/Pharmacodynamics
| Dose level | Dose of drug: Ixazomib | Dose of drug: fulvestrant | Number enrolled | Cmax (ng/mL) mean ± SD | Tmax(h) (min ‐ max) | AUC 0–12 (ng*h/mL) mean ± SD | T ½(h) mean ± SD |
|---|---|---|---|---|---|---|---|
| A | 2.3 mg | 500 mg | 3 | 50.5 ± 25.8 | 0.5–2 | 1978.8 ± 461.6 | 160.2 ± 48.0 |
| B | 3 mg | 500 mg | 3 | 61.5 ± 34.3 | 1–2 | 2,507.1 ± 890.4 | 101.3 ± 26.2 |
| C | 4 mg | 500 mg | 3 | 149.3 ± 25.0 | 1–1.5 | 5,459.4 ± 20.6 | 75.5 ± 23.9 |
Abbreviations: AUC, area under the curve; Cmax, maximal concentration; Tmax, time of maximal concentration.
Assessment, Analysis, and Discussion
| Completion | Did not fully accrue |
| Investigator's Assessment | Active and should be pursued further |
This phase Ib study investigated the safety and efficacy of ixazomib in combination with fulvestrant and revealed no dose‐limiting toxicities (DLTs) during cycle 1. This study fulfilled its primary objective of establishing a safety profile for ixazomib and identifying a maximum tolerated dose of 4 mg p.o. on days 1, 4, 8, and 11 in a 21‐day cycle when combined with fulvestrant in patients with fulvestrant‐resistant metastatic breast cancer. The most common grade 3/4 treatment‐related adverse event was thrombocytopenia, which occurred in four subjects (lasting one cycle each) in a dose‐dependent manner and is consistent with reports of ixazomib use [20, 22, 23]. Other observed grade 2–4 adverse events included anemia (n = 3 subjects), nausea (n = 2), hypertension (n = 6 subjects), dyspnea (n = 3), cough (n = 2), pulmonary hypertension (n = 1), and pneumonia (n = 1). Pulmonary adverse events may also be related to treatment with fulvestrant. For example, the CONFIRM trial found that fulvestrant (500 mg) was associated with cough and dyspnea in 19 of 361 (5.3%) and 16 of 361 (4.4%) of patients, respectively [24].
Three patients were enrolled into each of the 2.3‐mg, 3‐mg, and 4‐mg ixazomib dose cohorts. No DLTs were observed; thus, the maximum tolerated dose of ixazomib is 4 mg on the schedule used herein in combination with fulvestrant. The adverse event profile among all subjects (grade ≥ 2) is shown in Table 2. Grade 3 or 4 hematologic adverse events included anemia (in 2 subjects) and thrombocytopenia (in 4 subjects). Grade 3 or 4 nonhematologic adverse events included dyspnea, hypoxia, pulmonary hypertension, rash, nausea, and elevated aspartate aminotransferase. Notably, six of nine subjects developed grade 2 hypertension, suggesting that ixazomib plus fulvestrant may elicit cardiovascular complications with longer‐term treatment. Pulmonary hypertension was diagnosed in one subject in the 2.3‐mg ixazomib dose cohort with metastatic breast cancer in lung and axillary lymph nodes. She had a history of bilateral pulmonary emboli diagnosed 10 months prior to study enrollment, and she was prescribed the anticoagulant enoxaparin. At the time of enrollment, she had stable exertional dyspnea and a chronic cough. She was diagnosed with secondary pulmonary hypertension while on study. Imaging showed segmental and subsegmental pulmonary emboli in the lung; the pulmonary emboli and resultant pulmonary hypertension were ascribed to her underlying thromboembolic disease and metastatic breast cancer, and thought to be unrelated to fulvestrant or ixazomib. Pleural effusions and pneumonia were diagnosed in one subject in the 4‐mg cohort with metastatic breast cancer in the brain and lymph nodes. She was hospitalized after 7 weeks of study treatment for hypoxemia secondary to bilateral pleural effusions and pneumonia. Her hypoxemia resolved with diuresis and antibiotics, and she came off study 3 weeks later. The development of pleural effusions and pneumonia in this patient were thought to possibly be due to treatment with fulvestrant and ixazomib. Grade 4 thrombocytopenia was seen in 2 subjects in the 4‐mg cohort. The first subject experienced a drop in platelet counts to 7,000/uL, which required platelet transfusion on cycle 2 day 10. The second subjected experienced a drop in platelet counts to 17,000/uL, which required platelet transfusion on cycle 2 day 14
The ixazomib pharmacokinetic disposition profile (Fig. 2) showed a median Tmax of 1 h, which indicated rapid absorption and is consistent with prior findings [13]. The ixazomib median elimination half‐lives in the 2.3‐mg, 3‐mg, and 4‐mg dose cohorts were 148, 97, and 67 h, respectively. The overall ixazomib exposure, as determined by the area under the curve (AUC)(last), was found to increase with dose, with median values of 1,236, 1,957, and 5,025 ng/mL per hour in the 2.3‐mg, 3‐mg, and 4‐mg cohorts, respectively. The elimination half‐life and the AUC(last) values were similar to those previously reported for ixazomib monotherapy administered twice weekly when pharmacokinetic data were analyzed using noncompartmental analysis [25]. However, the elimination half‐life values in this study were shorter than the cited mean elimination half‐life of 9.5 days (228 hours) [13, 26]. This difference in observed terminal elimination half‐life was most likely due to our inability to fit a multicompartment model to the plasma ixazomib concentration vs. time data in our study, and our limited blood sampling time points. Pharmacodynamic studies showed potent inhibition of proteasome activity in blood following 11 days of combination treatment at all dose levels studied (Fig. 3). Sufficient tumor tissue in biopsy specimens from both baseline and Day 11 time points was available from six study subjects for Ki67 IHC, and from four subjects for ER IHC and TUNEL studies. Significant changes between baseline and Day 11 data were not observed, but TUNEL studies showed a trend of decreasing proportion of apoptotic cells following ixazomib treatment (p=.094; Fig. 4).
Figure 2.
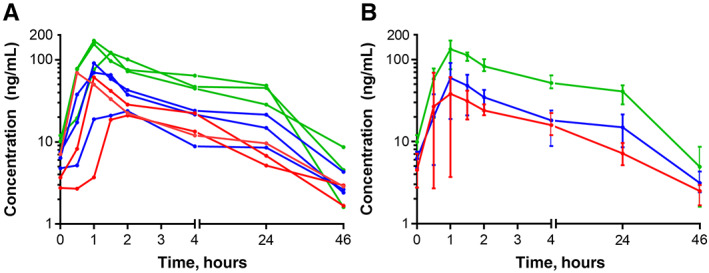
Log plasma ixazomib (MLN2238) concentration versus time profiles. Individual (A) and cohort‐averaged (B) pharmacokinetic data for 9 subjects. In (B), data are shown as mean ± range. Pre‐time point was sampled prior to start of study treatment. Other time points were sampled after the cycle 1 day 11 dose of ixazomib.
Figure 3.
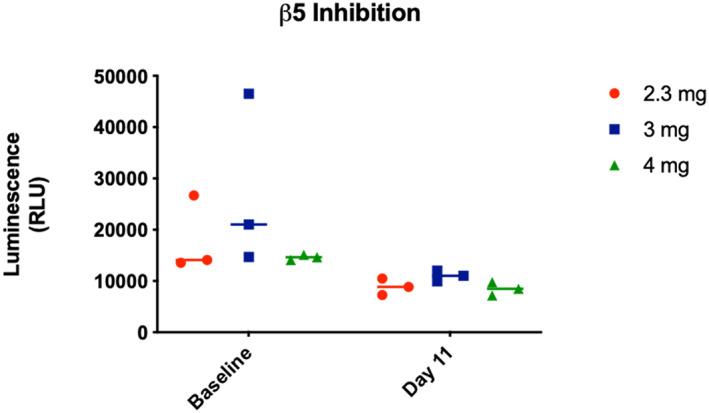
Ixazomib suppresses blood 20S proteasome activity. Whole‐blood samples acquired at baseline and on cycle 1 day 11 at 2 hours postdosing were analyzed by β5 chymotrypsin‐like proteasome activity assay. Although proteasome activity was widely variable at baseline, postixazomib samples showed a significant decrease of proteasome activity compared with baseline (Fig. 2; two‐way analysis of variance [ANOVA] time effect p = .026). The postixazomib samples showed residual proteasome activity, which was not significantly different between dose cohorts, suggesting that maximal proteasome inhibition in blood was achieved with the lowest dose (2.3 mg) of ixazomib in this study. Luminescence per sample is shown according to dose cohort. Data were analyzed by two‐way ANOVA followed by Tukey‐adjusted comparisons between dose cohorts, which revealed only a statistically significant effect of time on proteasome activity. Abbreviation: RLU, relative luminescence unit.
Figure 4.
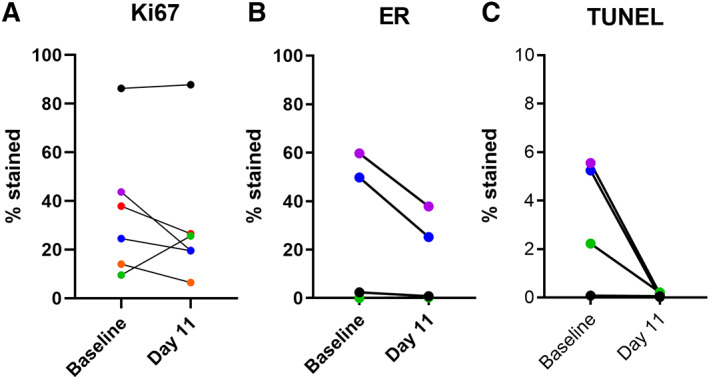
Immunohistochemical (IHC) analysis of paired baseline and cycle 1 day 11 tumor tissue specimens. Sufficient tumor tissue in biopsy specimens from both baseline and day 11 time points was available from six study subjects for Ki67 IHC and from four subjects for ER IHC and TUNEL studies. IHC scoring of Ki67 (A) and ER (B), and TUNEL scoring (C) from tumor specimens obtained at baseline or on cycle 1 day 11. Tissue specimens are color‐coded by subject across panels. Significant changes between baseline and day 11 data were not observed, but TUNEL studies showed a trend of decreasing proportion of apoptotic cells following ixazomib treatment (paired t test p = .094). Tumor specimens from one subject were ER‐negative despite this subject having a history of ER+ metastatic disease. Abbreviations: ER, estrogen receptor; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling.
Despite encouraging preclinical data, clinical studies of proteasome inhibitors in solid tumors including breast cancer have thus far failed to demonstrate efficacy [27]. Engel et al. reported the results of a phase II trial of single‐agent bortezomib in subjects with metastatic breast cancer, and they saw no objective responses among 12 evaluable subjects [28]. Trinh et al. reported on a phase l study in which subjects with hormone receptor‐positive metastatic breast cancer received the combination of bortezomib and an aromatase inhibitor (8 subjects), or bortezomib and tamoxifen (1 subject). There were no objective responses, but two of the nine subjects experienced stable disease for ≥15 weeks [29]. Based on these results, the activity of single‐agent ixazomib or the combination of ixazomib with an aromatase inhibitor or tamoxifen in metastatic breast cancer was hypothesized to be very low. Alternatively, the addition of proteasome inhibitors to fulvestrant has been shown to increase apoptosis in breast cancer cell lines and mouse xenografts [11, 12], leading to the initiation of clinical testing of this combination in breast cancer. Adelson et al. reported the results of a randomized phase II trial comparing bortezomib plus fulvestrant to fulvestrant alone, and showed an improved 12‐month progression‐free survival (PFS; 28.1% vs. 13.6%; p = .03) [18].
Although efficacy was not a primary endpoint of our study, we found that the addition of ixazomib in patients with disease progressing on fulvestrant resulted in a delay of disease progression in all patients by a median of 51 days, up to as many as 137 days. Furthermore, one patient had a partial response by RECIST version 1.1. Of note, this patient had previously received 3 lines of endocrine therapy including letrozole plus palbociclib, single‐agent anastrozole, and single‐agent fulvestrant, as well as chemotherapy with docetaxel and cyclophosphamide. Although the overall rate of clinical benefit (11%) of ixazomib in combination with fulvestrant observed in this study is modest, it is worth noting that this patient population has disease resistant to multiple agents with recent progression on fulvestrant. This is unlike the majority of clinical trials for fulvestrant‐containing regimens in advanced breast cancer, which typically involve patients who have progressed on a single endocrine agent such as an aromatase inhibitor and have not had previous exposure to fulvestrant. Additionally, four of nine (44%) subjects in our study population had disease that progressed on combination therapy with palbociclib and an endocrine agent, which is now considered a standard regimen for advanced endocrine‐resistant hormone receptor‐positive disease. Based on our observation of a response in one subject in our study population, and a PFS in one subject of 137 days in the lowest dose cohort studied, ixazomib with fulvestrant in fulvestrant‐naive patients with advanced endocrine‐resistant disease may be considered for testing by other investigators.
Disclosures
Gary N. Schwartz: Takeda Pharmaceuticals (RF); Lionel D. Lewis: G1 Therapeutics, 7 Hills Pharma, LLC (C/A, H), Bristol‐Myers Squibb, AstraZeneca, Polynoma (RF); Todd W. Miller: Takeda Pharmaceuticals (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Figures
Acknowledgments
We thank Takeda Pharmaceuticals for providing research grant funding and ixazomib citrate for this study, and the Norris Cotton Cancer Center for additional grant funding.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Footnotes
- ClinicalTrials.gov Identifier: NCT02384746
- Sponsor: Dartmouth‐Hitchcock Medical Center
- Principal Investigators: Todd Miller, Gary Schwartz
- IRB Approved: Yes
Contributor Information
Gary Schwartz, Email: todd.w.miller@dartmouth.edu, Email: gary.n.schwartz@hitchcock.org.
Todd Miller, Email: todd.w.miller@dartmouth.edu.
References
- 1. Robertson JF, Lindemann JP, Llombart‐Cussac A et al. Fulvestrant 500 mg versus anastrozole 1 mg for the first‐line treatment of advanced breast cancer: Follow‐up analysis from the randomized ‘FIRST’ study. Breast Cancer Res Treat 2012;136:503–511. [DOI] [PubMed] [Google Scholar]
- 2. Robertson JF, Llombart‐Cussac A, Rolski J et al. Activity of fulvestrant 500 mg versus anastrozole 1 mg as first‐line treatment for advanced breast cancer: Results from the FIRST study. J Clin Oncol 2009;27:4530–4535. [DOI] [PubMed] [Google Scholar]
- 3. Thürlimann B, Hess D, Köberle D et al. Anastrozole (‘arimidex’) versus tamoxifen as first‐line therapy in postmenopausal women with advanced breast cancer: Results of the double‐blind cross‐over SAKK trial 21/95 ‐ A sub‐study of the TARGET (Tamoxifen or ‘Arimidex’ Randomized Group Efficacy and Tolerability) trial. Breast Cancer Res Treat 2004;85:247–254. [DOI] [PubMed] [Google Scholar]
- 4. Nabholtz JM, Buzdar A, Pollak M et al. Anastrozole is superior to tamoxifen as first‐line therapy for advanced breast cancer in postmenopausal women: Results of a North American multicenter randomized trial. J Clin Oncol 2000;18:3758–3767. [DOI] [PubMed] [Google Scholar]
- 5. Mouridsen H, Gershanovich M, Sun Y et al. Superior efficacy of letrozole versus tamoxifen as first‐line therapy for postmenopausal women with advanced breast cancer: Results of a phase III study of the international letrozole breast cancer group. J Clin Oncol 2001;19:2596–2606. [DOI] [PubMed] [Google Scholar]
- 6. Paridaens R, Dirix L, Lohrisch C et al. Mature results of a randomized phase II multicenter study of exemestane versus tamoxifen as first‐line hormone therapy for postmenopausal women with metastatic breast cancer. Ann Oncol 2003;14:1391–1398. [DOI] [PubMed] [Google Scholar]
- 7. Dauvois S, White R, Parker MG. The antiestrogen ICI 182780 disrupts estrogen receptor nucleocytoplasmic shuttling. J Cell Sci 1993;106:1377–1388. [DOI] [PubMed] [Google Scholar]
- 8. Htun H, Holth LT, Walker D et al. Direct visualization of the human estrogen receptor alpha reveals a role for ligand in the nuclear distribution of the receptor. Mol Biol Cell 1999;10:471–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pick H, Jankevics H, Vogel H. Distribution plasticity of the human estrogen receptor alpha in live cells: Distinct imaging of consecutively expressed receptors. J Mol Biol 2007;374:1213–1223. [DOI] [PubMed] [Google Scholar]
- 10. Schröder M. Endoplasmic reticulum stress responses. Cell Mol Life Sci 2008;65:862–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ishii Y, Papa L, Bahadur U et al. Bortezomib enhances the efficacy of fulvestrant by amplifying the aggregation of the estrogen receptor, which leads to a proapoptotic unfolded protein response. Clin Cancer Res 2011;17:2292–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Germain D, Bahadur U, Halpern M et al. Bortezomib enhances the efficacy of fulvestrant by promoting the aggregation of the estrogen receptor in the cytoplasm. Cancer research 2009;69(suppl):5142.19491278 [Google Scholar]
- 13. Gupta N, Hanley MJ, Xia C et al. Clinical pharmacology of ixazomib: The first oral proteasome inhibitor. Clin Pharmacokinet 2019;58:431–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Howell A, Osborne CK, Morris C et al. ICI 182,780 (Faslodex) ‐ Development of a novel, “pure” antiestrogen. Cancer 2000;89:817–825. [DOI] [PubMed] [Google Scholar]
- 15. Robertson JFR. ICI 182,780 (fulvestrant) ‐ The first oestrogen receptor down‐regulator ‐ Current clinical data. Brit J Cancer 2001;85(suppl 2):11–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Buzdar AU, Robertson JFR. Fulvestrant: Pharmacologic profile versus existing endocrine agents for the treatment of breast cancer. Ann Pharmacother 2006;40:1572–1583. [DOI] [PubMed] [Google Scholar]
- 17. Stenoien DL, Patel K, Mancini MG et al. Frap reveals that mobility of oestrogen receptor‐alpha is ligand‐ and proteasome‐dependent. Nat Cell Biol 2001;3:15–23. [DOI] [PubMed] [Google Scholar]
- 18. Adelson K, Ramaswamy B, Sparano JA et al. Randomized phase II trial of fulvestrant alone or in combination with bortezomib in hormone receptor‐positive metastatic breast cancer resistant to aromatase inhibitors: A New York Cancer Consortium trial. NPJ Breast Cancer 2016;2:16037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Richardson PG, Hofmeister CC, Rosenbaum CA et al. Twice‐weekly ixazomib in combination with lenalidomide‐dexamethasone in patients with newly diagnosed multiple myeloma. Br J Haematol 2018;182:231–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Richardson PG, Baz R, Wang M et al. Phase 1 study of twice‐weekly ixazomib, an oral proteasome inhibitor, in relapsed/refractory multiple myeloma patients. Blood 2014;124:1038–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gupta N, Hanley MJ, Venkatakrishnan K et al. The effect of a high‐fat meal on the pharmacokinetics of ixazomib, an oral proteasome inhibitor, in patients with advanced solid tumors or lymphoma. J Clin Pharmacol 2016;56:1288–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kumar SK, LaPlant B, Roy V et al. Phase 2 trial of ixazomib in patients with relapsed multiple myeloma not refractory to bortezomib. Blood Cancer J 2015;5:e338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dhakal B, D'Souza A, Hamadani M et al. Phase I/II trial of bendamustine, ixazomib, and dexamethasone in relapsed/refractory multiple myeloma. Blood Cancer J 2019;9:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Di Leo A, Jerusalem G, Petruzelka L et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor‐positive advanced breast cancer. J Clin Oncol 2010;28:4594–4600. [DOI] [PubMed] [Google Scholar]
- 25. Smith DC, Kalebic T, Infante JR et al. Phase 1 study of ixazomib, an investigational proteasome inhibitor, in advanced non‐hematologic malignancies. Invest New Drugs 2015;33:652–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ixazomib. Prescribing information. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/208462lbl.pdf. Accessed December 10, 2020.
- 27. Huang Z, Wu Y, Zhou X et al. Efficacy of therapy with bortezomib in solid tumors: A review based on 32 clinical trials. Future Oncol 2014;10:1795–1807. [DOI] [PubMed] [Google Scholar]
- 28. Engel RH, Brown JA, Von Roenn JH et al. A phase II study of single agent bortezomib in patients with metastatic breast cancer: A single institution experience. Cancer Invest 2007;25:733–737. [DOI] [PubMed] [Google Scholar]
- 29. Trinh XB, Sas L, Van Laere SJ et al. A phase II study of the combination of endocrine treatment and bortezomib in patients with endocrine‐resistant metastatic breast cancer. Oncol Rep 2012;27:657–663. [DOI] [PubMed] [Google Scholar]


