Abstract
Lessons Learned
Apatinib has potential as an effective and safe second‐line or higher treatment for patients with chemotherapy‐refractory esophageal squamous cell carcinoma (ESCC).
Clinical safety is of potential concern when administering apatinib to patients with uncontrolled esophageal lesions or severe invasion of trachea, bronchi, or major blood vessels.
To the best of the authors' knowledge, this is the first prospective phase II study to investigate apatinib for patients with chemotherapy‐refractory ESCC. Apatinib could provide an alternative option for ESCC after first‐line or higher therapy in carefully selected patients.
Background
The aim of this study was to evaluate the efficacy and adverse effects of the oral vascular endothelial growth factor receptor 2 (VEGFR‐2) tyrosine kinase inhibitor apatinib in patients with chemotherapy‐refractory esophageal squamous cell carcinoma (ESCC).
Methods
We enrolled patients with chemotherapy‐refractory ESCC. All patients received continuous apatinib 500 mg once daily.
Results
Between July 2017 and August 2018, 40 patients were recruited, of whom 5 (12.5%) had uncontrolled primary tumors. Additionally, three patients with partial response (PR) and 23 with stable disease (SD) were observed for overall response rate (ORR) of 7.5% and disease control rate (DCR) of 65.0%. Median progression‐free survival (PFS) was 3.8 months (95% confidence interval [CI], 2.2–5.4); median overall survival (OS) was 5.8 months (95% CI, 3.2–8.4). Common adverse effects were fatigue (15%), hypertension (12.5%), and palmar‐plantar erythrodysesthesia syndrome (10%). Two cases of death from massive bronchopulmonary hemorrhage were observed, and esophageal fistula occurred in another two patients. Notably, both patients with esophageal fistula and one patient with massive fatal bronchopulmonary hemorrhage were individuals with uncontrolled primary tumors (3/5, 60%). Fatal bronchopulmonary hemorrhage in a second patient was associated with major blood vessel invasion.
Conclusion
Apatinib has potential as an effective and safe treatment for patients with chemotherapy‐refractory ESCC whose primary tumors are controlled and without severe invasion of trachea, bronchi, or major blood vessels.
Keywords: Apatinib, Esophageal squamous cell carcinoma, Chemotherapy, Phase II
Discussion
The study aimed to assess the efficacy and safety of apatinib, an oral VEGFR‐2 tyrosine kinase inhibitor (TKI), in patients with ESCC refractory to at least one line of prior chemotherapy. Other lines of treatment in patients with chemotherapy‐refractory ESCC usually have a poor response rate, with rapid recurrences and poor long‐term survival.
Apatinib is a small‐molecule TKI that highly selectively binds to and strongly inhibits VEGFR‐2. Previous studies have shown that apatinib treatment significantly improved OS and PFS with an acceptable safety profile in patients with advanced gastric cancer refractory to two or more lines of prior chemotherapy. Studies of apatinib are rare in chemotherapy‐refractory esophageal cancer.
To remedy the lack of prospective studies of apatinib in patients with ESCC, we conducted this phase II clinical trial (ESO‐Shanghai 11). This trial enrolled 40 patients with chemotherapy‐refractory ESCC. All patients received continuous apatinib 500 mg once daily until disease progression, death, intolerable toxicity, or patient request for withdrawal from the study occurred. The primary endpoint was PFS. Secondary endpoints included OS and investigator‐assessed ORR.
Results showed that 3 patients with PR and 23 patients with SD were observed for ORR of 7.5% and DCR of 65.0%. Median PFS was 3.8 months (95% CI, 2.2–5.4); median OS was 5.8 months (95% CI, 3.2–8.4). The common severe adverse effects observed were fatigue (15%), hypertension (12.5%), and palmar‐plantar erythrodysesthesia syndrome (10%). Two cases (5%) of death from massive bronchopulmonary hemorrhage were observed, and esophageal fistula occurred in another two patients (5%). Notably, both patients with esophageal fistula and one patient with massive fatal bronchopulmonary hemorrhage occurred in patients with uncontrolled primary tumors (3/5, 60%). The other fatal bronchopulmonary hemorrhage was associated with major blood vessel invasion. Thus, those patients with chemotherapy‐refractory ESCC, whose primary tumors are not controlled and with severe invasion of trachea, bronchi, or major blood vessels, should be excluded.
The major limitation of our study was the small sample size as a single‐arm phase II trial. Based on our study results, a prospective phase III, randomized comparison of apatinib and placebo should be conducted.
In conclusion, apatinib has potential as an effective and safe second‐line or higher treatment for patients with chemotherapy‐refractory ESCC whose primary tumors are controlled and without severe invasion of trachea, bronchi, or major blood vessels.
Trial Information
| Disease | Esophageal cancer |
| Stage of Disease/Treatment | Metastatic/advanced |
| Prior Therapy | One prior regimen |
| Type of Study | Phase II, single arm |
| Primary Endpoint | Progression‐free survival |
| Secondary Endpoints | Overall survival, overall response rate |
| Additional Details of Endpoints or Study Design | |
| This was an open‐label, single‐arm, phase II clinical trial that enrolled patients with ESCC who had evidence of disease progression after one or more lines of chemotherapy. Enrollment criteria were as follows: patient (aged ≥18 years) with histologically confirmed ESCC that was refractory to more than one chemotherapy regimen (the criteria for progression were based on computed tomography [CT] and magnetic resonance imaging [MRI] evaluation); an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2; at least one measurable lesion as defined by RECIST version 1.1 and modified RECIST (mRECIST); and acceptable hematologic, hepatic, and renal function. Patients with uncontrolled blood pressure on medication, with a bleeding tendency, or those receiving thrombolytic therapy or anticoagulants were excluded. | |
| The study protocol was approved by the institutional review board, the Fudan University Shanghai Cancer Center Ethics Committee for Clinical Investigation, and registered on ClinicalTrials.gov as number NCT03274011. The study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines. All patients provided written informed consent before participation. | |
| The primary endpoint was PFS. PFS was defined as the time from beginning apatinib therapy until disease progression or death, whichever occurred first. The time period before progression or death was thus considered the PFS. Secondary endpoints included OS (the time from apatinib taking until death from any cause) and investigator‐assessed ORR (the percentage of patients whose best overall response was either a complete response or partial response). The proportion of patients with DCR was defined as complete response (CR), PR, or SD, and objective response was considered a reduction in tumor size. RECIST version 1.1 was used to assess tumor response every 30 days. If patients had obvious tumor necrosis and cavitation of lung metastasis after treatment, the response was also evaluated by mRECIST. Two independent radiologists who were blind to the treatment had to agree on evidence of efficacy. | |
| Pretreatment evaluation included physical examination, baseline laboratory tests (complete blood count; hepatic and renal function, coagulation function), and MRI or CT scan of measurable lesions at baseline. Assessment of toxicity was performed biweekly. Complete blood counts were performed weekly; hepatic, renal, and coagulation function tests were performed monthly. Physical examinations, MRI, or CT scans of measurable lesions were assessed monthly. MRI or CT scans could be scheduled ahead of time if there was evidence of substantial progression. Patients were observed until death or loss to follow‐up. | |
| The primary endpoint was PFS. Previous data report a median PFS of less than 1 month for patients without treatment. The single‐stage study design was built around a null rate of 25% PFS at 2 months and required 33 patients to detect an absolute difference of 25% for a target rate of 50% PFS at 2 months. The trial was designed to have a 5% chance of a type 1 error and 90% power. The study was considered to reach the primary endpoint if 13 or more patients did not progress at 2 months. We expected a dropout or nonevaluable rate of 20%. An estimated 40 patients needed to be enrolled. Analysis of PFS was performed using Kaplan‐Meier for OS. Significance was set at p = .05. | |
| Investigator's Analysis | Active and should be pursued further |
Drug Information
| Generic/Working Name | Apatinib |
| Company Name | Hengrui Company |
| Drug Type | Small molecule |
| Drug Class | VEGFR |
| Dose | 500 milligrams (mg) per flat dose |
| Route | Oral (p.o.) |
| Schedule of Administration | All patients received continuous apatinib 500 mg once daily until disease progression, death, intolerable toxicity, or patient request for withdrawal from the study occurred. Dose escalation was allowed. The dose decreased to 250 mg in patients who had grade 3 or higher adverse events. |
Patient Characteristics
| Number of Patients, Male | 34 |
| Number of Patients, Female | 6 |
| Stage | Patients with chemotherapy‐refractory ESCC |
| Age | Median (range): 63 (47–76) years |
| Number of Prior Systemic Therapies | Median (range): 2 (1–3) |
| Performance Status: ECOG |
0 — 0 1 — 11 2 — 29 3 — 0 Unknown — 0 |
| Other | |
| From July 2017 to August 2018, we enrolled 40 patients with chemotherapy‐refractory ESCC in the study and observed them until November 2019, when 92.5% (37/40) of OS events were reached. One patient was still alive, whereas the other two patients terminated apatinib because of adverse effects, and we subsequently lost touch with them. Patient baseline characteristics (sex, age, ECOG performance status, smoking history, alcohol use, line of apatinib therapy, surgical history, control of primary tumor, and sites of metastasis) are listed in Table 1. | |
| The median age of these patients was 63 years (range: 47–76). None of the patients in the study had received molecular targeted therapy. An ECOG performance status of 2 was measured in 72.5% (29/40) of patients. Most patients were heavily treated before receiving apatinib, with 57.5% (23/40) of patients receiving second‐line chemotherapy and 7.5% (3/40) receiving third‐line chemotherapy prior to the study. Prior surgery of the primary tumor had been carried out in 21 patients (52.5%), whereas 12.5% (5/40) of patients had an uncontrolled primary tumor. The main recurrent or metastatic sites were the lymph nodes (22%), lungs (40.0%), and liver (32.5%). | |
| Data collection ended on November 6, 2019. During the study, 47.5% (19/40) of patients received 500 mg of once daily apatinib continuously until disease progression or death from any cause. The dose was decreased to 250 mg in 47.5% (19/40) of patients who had intolerable toxicity. Seven of the 40 patients (17.5%) discontinued apatinib treatment in the first 2 weeks, and 52.5% (21/40) of patients had dose reduction or discontinuation, as shown in Table 2. | |
| Cancer Types or Histologic Subtypes | Esophageal squamous cell carcinoma, 40 |
Table 1.
Patient characteristics
| Variables | n (%) |
|---|---|
| Sex | |
| Male | 34 (85.0) |
| Female | 6 (15.0) |
| Age, median (range), years | 63 (47–76) |
| ECOG | |
| 1 | 11 (27.5) |
| 2 | 29 (72.5) |
| Smoking history | |
| Yes | 22 (55.0) |
| No | 18 (45.0) |
| Alcohol use | |
| Yes | 24 (60.0) |
| No | 16 (40.0) |
| No. of previous chemotherapy lines | |
| 1 | 14 (35.0) |
| 2 | 23 (57.5) |
| 3 | 3 (7.5) |
| Prior surgery of primary tumor | |
| Yes | 21 (52.5) |
| No | 19 (47.5) |
| Prior treatment of primary tumor | |
| Surgery | 21 (52.5) |
| Chemoradiotherapy | 17 (42.5) |
| Chemotherapy | 2 (5.0) |
| Sites of metastasis | |
| Lung | 16 (40.0) |
| Liver | 13 (32.5) |
| Adrenal gland | 1 (2.5) |
| Bone | 4 (10.0) |
| Lymph node | 22 (55.0) |
| Intolerant of last‐line treatment at time of enrollment, median (range), months | 3.6 (0.7–20.0) |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Table 2.
Adverse events (n = 40)
| Toxicity | Total (%) | Grade | Severe AEs (%) | Dosage reduction/ Discontinuation (%) | ||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||||
| ALL | 35 (87.5) | 0 | 0 | 0 | 0 | 0 | 23 (57.5) | 21 (52.5) |
| Abdominal Pain | 1 (2.5) | 0 | 0 | 1 | 0 | 0 | 1 (2.5) | 0 |
| ALT increased | 1 (2.5) | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Anal mucosal reaction | 3 (7.5) | 0 | 3 | 0 | 0 | 0 | 0 | 1 (2.5) |
| AST increased | 2 (5.0) | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bleeding Gums | 1 (2.5) | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chest pain | 1 (2.5) | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Constipation | 1 (2.5) | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Diarrhea | 8 (20.0) | 3 | 4 | 1 | 0 | 0 | 1 (2.5) | 1 (2.5) |
| Dizziness | 4 (10.0) | 2 | 1 | 1 | 0 | 0 | 1 (2.5) | 1 (2.5) |
| Dyspepsia | 5 (12.5) | 3 | 2 | 0 | 0 | 0 | 0 | 1 (2.5) |
| Fatigue | 15 (37.5) | 2 | 7 | 6 | 0 | 0 | 6 (15) | 6 (15) |
| Palmar‐plantar erythrodysesthesia syndrome | 11 (27.5) | 5 | 2 | 4 | 0 | 0 | 4 (10) | 3 (7.5) |
| Headache | 1 (2.5) | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Hematuria | 4 (10.0) | 2 | 0 | 2 | 0 | 0 | 2 (5.0) | 0 |
| Bronchopulmonary hemorrhage | 5 (12.5) | 1 | 2 | 0 | 0 | 2 | 2 (5.0) | 3 (7.5) |
| High creatinine | 1 (2.5) | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypertension | 10 (25.0) | 2 | 3 | 5 | 0 | 0 | 5 (12.5) | 0 |
| Nausea | 4 (10.0) | 3 | 1 | 0 | 0 | 0 | 0 | 0 |
| White blood cell decreased | 6 (15.0) | 5 | 1 | 0 | 0 | 0 | 0 | 0 |
| Mediastinoesophageal Fistula | 1 (2.5) | 0 | 0 | 0 | 1 | 0 | 1 (2.5) | 1 (2.5) |
| Oral mucosal reaction | 3 (7.5) | 2 | 1 | 0 | 0 | 0 | 0 | 0 |
| Palpitations | 2 (5.0) | 0 | 1 | 0 | 0 | 0 | 0 | 1 (2.5) |
| Pantalgia | 2 (5.0) | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
| Proteinuria | 5 (12.5) | 0 | 2 | 3 | 0 | 0 | 3 (7.5) | 3 (7.5) |
| Thrombocytopenia | 3 (7.5) | 0 | 2 | 1 | 0 | 0 | 1 (2.5) | 1 (2.5) |
| Tracheoesophageal Fistula | 1 (2.5) | 0 | 0 | 0 | 1 | 0 | 1 (2.5) | 1 (2.5) |
Of the five uncontrolled primary tumors patients, one patient died of massive fatal bronchopulmonary hemorrhage and another two patients had fistula/perforation (they had uncontrolled esophageal lesions adjacent to the main bronchi and mediastinum respectively).
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase.
Primary Assessment Method
| Number of Patients Screened | 42 |
| Number of Patients Enrolled | 40 |
| Number of Patients Evaluable for Toxicity | 35 |
| Number of Patients Evaluated for Efficacy | 37 |
| Evaluation Method | RECIST 1.1 |
| Response Assessment CR | n = 0 (0%) |
| Response Assessment PR | n = 3 (7.5%) |
| Response Assessment SD | n = 23 (57.5%) |
| Response Assessment PD | n = 11 (27.5%) |
| Response Assessment OTHER | n = 3 (7.5%) |
| (Median) Duration Assessments PFS | 3.8 months; 95% CI, 2.2–5.4 |
| (Median) Duration Assessments OS | 5.8 months; 95% CI, 3.2–8.4 |
| Outcome Notes | |
| Only one patient is still alive. Two patients terminated apatinib because of adverse effects, and we subsequently lost touch with them. The main causes of treatment failure were disease progression and severe adverse events (AEs). We achieved our primary endpoint of 37 respondents among the 40 patients. Among them, 3 patients with PR and 23 patients with SD were observed for ORR of 7.5% and DCR of 65.0%. Five percent (2/40) of patients had obvious tumor necrosis and cavitation of lung metastasis (Fig. 1). When the responses were evaluated by mRECIST, they were PRs, and the ORR was 12.5% (5/40; Fig. 2B). Median PFS was 3.8 months (95% CI, 2.2–5.4; Fig. 3A), whereas median OS was 5.8 months (95% CI, 3.2–8.4; Fig. 3B). PFS at 2 months was 60.4%, and 3‐month PFS was 57.7%. OS at 6 months was 45.0%, and 12‐month OS was 15.9%. Two cases of massive fatal bronchopulmonary hemorrhage and one case of esophageal fistula occurred in patients with uncontrolled primary tumors (3/5, 60%). We conducted an analysis excluding the patients with uncontrolled primary tumors. This subset analysis suggested that the ORR and DCR were 8.6% (3/35) and 68.6% (24/35), mPFS2 was 3.9 months (95% CI, 3.3–4.5; Fig. 3C), and mOS2 was 6.0 months (95% CI, 3.3–8.7; Fig. 3D). PFS2 was 64.5% at 2 months and 61.4% at 3 months. Six‐month OS2 was 47.2%, and 12‐month OS2 was 14.8%. | |
Figure 1.
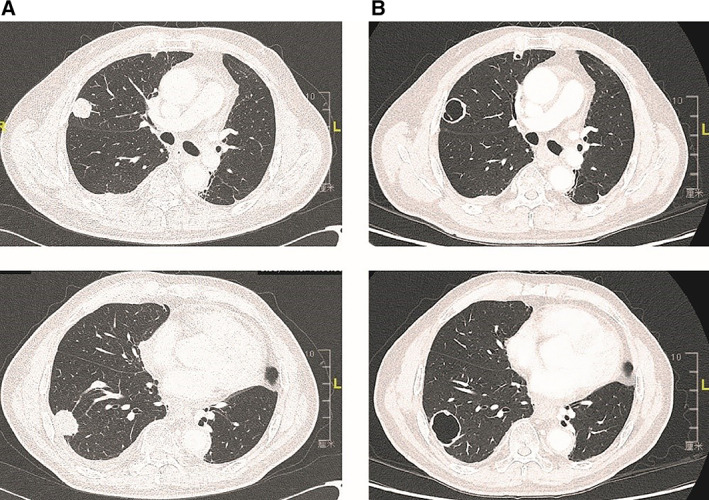
(A): The lung metastases before treatment with apatinib. (B): Thirty days after treatment, the lesions occurred tumor necrosis and cavitation. Only a small anterior portion did uptake contrast medium, which might correspond to the vital tumor tissue.
Figure 2.
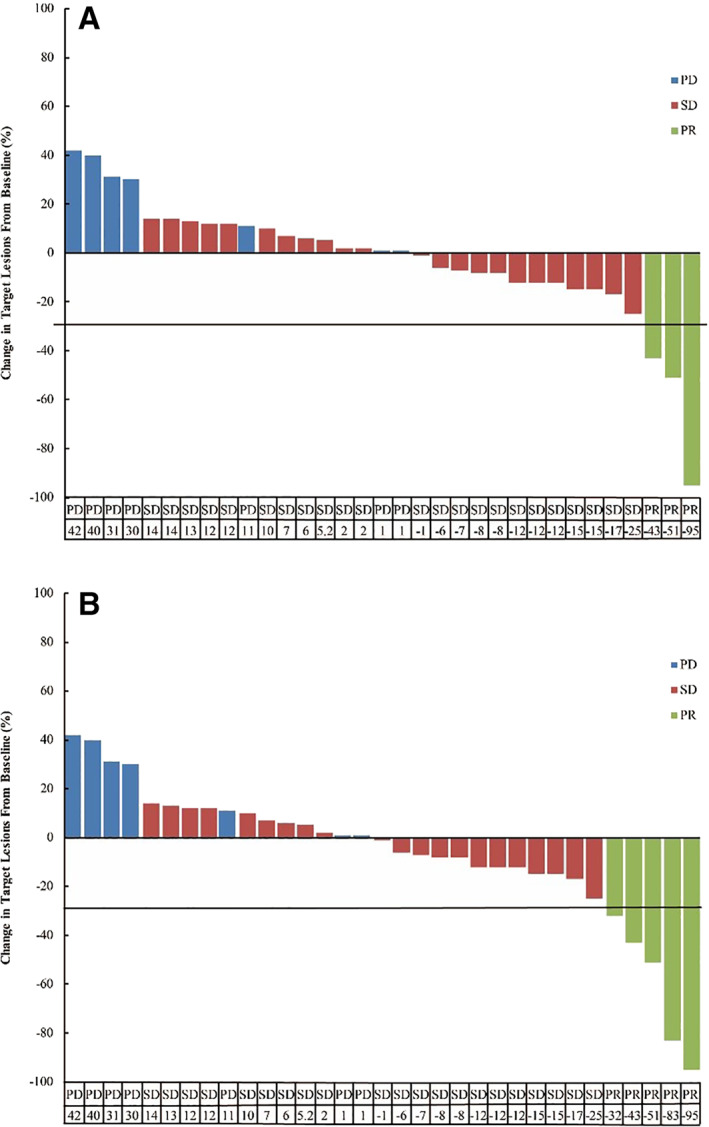
The best percentage from baseline in target lesions for the patients (n = 33) who had undergone at least one tumor assessment after treatment. Among these, three patients (two with PR, one with SD) terminated the treatment because of intolerable toxicity. (A): The response according to RECIST version 1.1: three patients achieved partial response; 23 had stable disease. (B): Five percent (2/40) of patients had obvious tumor necrosis and cavitation of lung metastasis. The response according to modified RECIST was PR.Abbreviations: PD, progression disease; PR, partial response; SD, stable disease.
Figure 3.
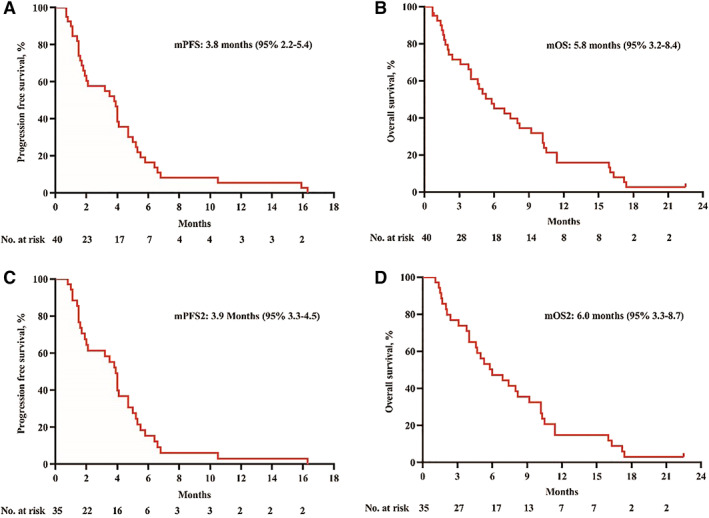
The x‐axis depicts months from first dose of study drug administration and number of patients at risk. The y‐axis depicts the probability of being progression‐free/alive. (A): Investigator‐assessed Kaplan‐Meier estimated mPFS was 3.8 months (95% confidence interval [CI], 2.2–5.4). (B): Investigator‐assessed Kaplan‐Meier estimated mOS was 5.8 months (95% CI, 3.2–8.4). (C): Excluding the patients with uncontrolled primary tumors, investigator‐assessed Kaplan‐Meier estimated mPFS2 was 3.9 months (95% CI, 3.3–4.5). (D): Excluding the patients with uncontrolled primary tumors, investigator‐assessed Kaplan‐Meier estimated mOS2 was 6.0 months (95% CI, 3.3–8.7).Abbreviations: mOS, median overall survival; mPFS, median progression‐free survival.
Adverse Events
| All Cycles Name | NC/NA, % | Grade 1, % | Grade 2, % | Grade 3, % | Grade 4, % | Grade 5, % | All grades, % |
|---|---|---|---|---|---|---|---|
| Bronchopulmonary hemorrhage | 87 | 3 | 5 | 0 | 0 | 5 | 13 |
| Diarrhea | 79 | 8 | 10 | 3 | 0 | 0 | 21 |
| Dizziness | 89 | 5 | 3 | 3 | 0 | 0 | 11 |
| Dyspepsia | 87 | 8 | 5 | 0 | 0 | 0 | 13 |
| Fatigue | 62 | 5 | 18 | 15 | 0 | 0 | 38 |
| Hematuria | 90 | 5 | 0 | 5 | 0 | 0 | 10 |
| Hypertension | 74 | 5 | 8 | 13 | 0 | 0 | 26 |
| Nausea | 89 | 8 | 3 | 0 | 0 | 0 | 11 |
| Palmar‐plantar erythrodysesthesia syndrome | 72 | 13 | 5 | 10 | 0 | 0 | 28 |
| Proteinuria | 87 | 0 | 5 | 8 | 0 | 0 | 13 |
|
White blood cell decreased |
84 | 13 | 3 | 0 | 0 | 0 | 16 |
Abbreviation: NC/NA, no change from baseline/no adverse event.
Serious Adverse Events
| Name | Grade | Attribution |
|---|---|---|
| Bronchopulmonary hemorrhage | 5 | Definite |
| Esophageal fistula | 4 | Definite |
| Abdominal Pain | 3 | Probable |
| Diarrhea | 3 | Definite |
| Fatigue | 3 | Definite |
| Fatigue | 3 | Probable |
| Palmar‐plantar erythrodysesthesia syndrome | 3 | Definite |
| Hematuria | 3 | Definite |
| Hypertension | 3 | Definite |
| Pantalgia | 3 | Definite |
| Pantalgia | 3 | Probable |
| Proteinuria | 3 | Definite |
| Thrombocytopenia | 3 | Definite |
| Adverse events were assessed throughout the treatment in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. Most instances of toxicity were generally well tolerated. Detailed AEs are presented in Tables 2 and 3. Severe AEs occurred in 23 patients (57.5%). The most common severe AEs were fatigue (15%), hypertension (12.5%), and palmar‐plantar erythrodysesthesia syndrome (10%). Dose was decreased to 250 mg in 47.5% (19/40) of patients who experienced intolerable toxicity. The 500 mg once daily apatinib treatment was discontinued by 17.5% (7/40) of patients less than 2 weeks after beginning therapy, and 52.5% (21/40) of patients had dose reduction or discontinuation. The most common reasons for dose reduction or discontinuation were fatigue (15%), palmar‐plantar erythrodysesthesia syndrome (7.5%), bronchopulmonary hemorrhage (7.5%), and proteinuria (7.5%). Fatigue was the most common adverse effect among patients enrolled in this study. Three patients achieved partial response (7.5%). Among them, one patient terminated apatinib treatment because of severe fatigue. Hematologic toxicities were mostly moderate, and grade 3 to 4 hematologic toxicities were rarely noted. Three patients had an anal mucosal reaction (grade 2) that had not been reported before; symptomatic treatment was effective for them. | ||
| In our trial, five patients had a baseline progressive esophageal lesion. Among these patients, one died of massive fatal bronchopulmonary hemorrhage, and esophageal fistula occurred in two patients (Fig. 4; 3/5, 60.0%). Of the 40 patients enrolled in this study, two patients (5%) died of massive fatal bronchopulmonary hemorrhage, and two patients (5%) developed esophageal fistulas. Notably, both esophageal fistula cases and one massive fatal bronchopulmonary hemorrhage case occurred in patients with uncontrolled primary tumors (3/5, 60%). The other fatal bronchopulmonary hemorrhage was associated with major blood vessel invasion, highlighting potential safety concerns when administering apatinib to patients with uncontrolled esophageal lesions or with severe invasion of trachea, bronchi, or major blood vessels. | ||
Table 3.
Adverse events occurring in ≥10% of patients
| Toxicity | Total, n (%) | Grade, n | Severe AEs, n (%) | Dosage reduction/discontinuation, n (%) | ||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||||
| Diarrhea | 8 (20.0) | 3 | 4 | 1 | 0 | 0 | 1 (2.5) | 1 (2.5) |
| Proteinuria | 5 (12.5) | 0 | 2 | 3 | 0 | 0 | 3 (7.5) | 3 (7.5) |
| Hypertension | 10 (25.0) | 2 | 3 | 5 | 0 | 0 | 5 (12.5) | 0 |
| Bronchopulmonary hemorrhage | 5 (12.5) | 1 | 2 | 0 | 0 | 2 | 2 (5.0) | 3 (7.5) |
| Dizziness | 4 (10.0) | 2 | 1 | 1 | 0 | 0 | 1 (2.5) | 1 (2.5) |
| Dyspepsia | 5 (12.5) | 3 | 2 | 0 | 0 | 0 | 0 | 1 (2.5) |
| Hematuria | 4 (10.0) | 2 | 0 | 2 | 0 | 0 | 2 (5.0) | 0 |
| Nausea | 4 (10.0) | 3 | 1 | 0 | 0 | 0 | 0 | 0 |
| Palmar‐plantar erythrodysesthesia syndrome | 11 (27.5) | 5 | 2 | 4 | 0 | 0 | 4 (10) | 3 (7.5) |
| White blood cell decreased | 6 (15.0) | 5 | 1 | 0 | 0 | 0 | 0 | 0 |
| Fatigue | 15 (37.5) | 2 | 7 | 6 | 0 | 0 | 6 (15) | 6 (15) |
Figure 4.
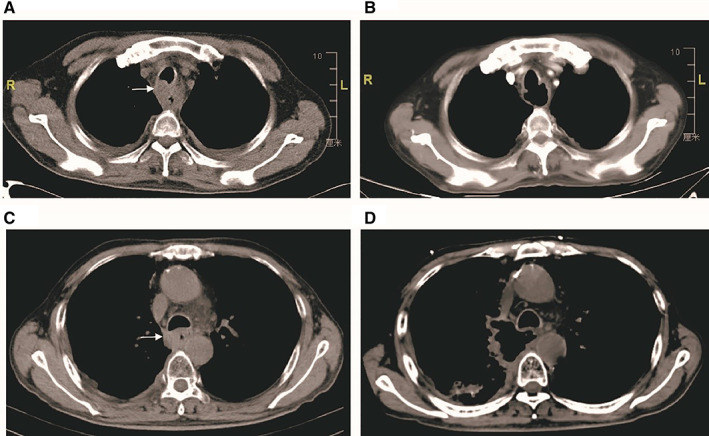
(A): The computed tomography (CT) scan of the patient before apatinib showed the uncontrolled primary tumor (white arrow). (B): The patient with tracheal esophageal fistula, 25 days after treatment. (C): The CT scan of the patient before apatinib showed the uncontrolled primary tumor (white arrow). (D): The patient with mediastinoesophageal fistula, 30 days after treatment.
Assessment, Analysis, and Discussion
| Completion | Study completed |
| Investigator's Assessment | Active and should be pursued further |
To the best of our knowledge, this is the first prospective phase II study to investigate apatinib for patients with chemotherapy‐refractory esophageal squamous cell carcinoma (ESCC). As the results demonstrated, we believe apatinib could provide an alternative option for carefully selected patients with ESCC after first‐line or higher therapy.
Esophageal cancer is one of the leading causes of cancer‐related death in China [1]. China has about half of all ESCC cases on earth; more than 95% of esophageal cancer in China is squamous cell carcinoma [2]. ESCC remains a significant cause of cancer death worldwide, with a poor survival rate mainly because of a high rate of both local recurrences and distant metastasis, even in patients with a previously resectable disease.
Cisplatin‐based combination regimens were established as the first‐line chemotherapy for these patients [3, 4, 5]. However, no standard chemotherapy or target regimen has been established for other lines of treatment in patients with chemotherapy‐refractory ESCC.
The first prospective, randomized, controlled study comparing doublet with single‐agent chemotherapy in patients with advanced ESCC progressing after systemic cytotoxic therapies showed that the median PFS was significantly longer in the irinotecan plus S‐1 group than in the S‐1 monotherapy group: 3.8 versus 1.7 months. The objective response rates (ORRs) were 24.6% in the irinotecan plus S‐1 group and 9.7% in the S‐1 monotherapy group (p = .002). The median OS in the irinotecan plus S‐1 group and the S‐1 monotherapy group were 7.1 versus 6.2 months [6].
Although paclitaxel, docetaxel, and irinotecan‐based chemotherapy, as well as salvage radiation and supportive treatment, could be used as second‐line or higher treatment, these therapies still have a poor response rate, with rapid recurrences and poor long‐term survival [7, 8, 9, 10, 11, 12, 13].
The KEYNOTE‐181 study showed that pembrolizumab was approved for recurrent locally advanced or metastatic ESCC in patients whose tumors express PD‐L1 (combined positive score ≥ 10) after one or more prior lines of systemic therapy [14]. The ATTRACTION‐3 study also showed that a survival benefit occurred with nivolumab regardless of the patients' levels of tumor PD‐L1 expression [15]. Both of the studies showed a little better or even worse progression‐free survival (PFS) in the immunotherapy group. The median PFS in the ESCC group was 2.2 months (95% confidence interval [CI], 2.1–3.2) and 1.7 months (95% CI, 1.5–2.7), respectively. Furthermore, the overall survival (OS) of intent to treat in the pembrolizumab group was similar to that of the chemotherapy group. The ORR remained low, at 13.1%, compared with 19% in the ATTRACTION‐3 nivolumab study group. The patients in these studies primarily had an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1 and had experienced first‐line treatment. Studies of three or more lines of treatment and patients with ECOG performance status 2 are still lacking.
The efficacy of targeted treatment in advanced ESCC is poorly defined, and there are few data in the Chinese population. No targeted drugs have been approved for ESCC by the U.S. Food and Drug Administration so far. Apatinib is a small‐molecule tyrosine kinase inhibitor (TKI) that highly selectively binds to and strongly inhibits vascular endothelial growth factor receptor 2 (VEGFR‐2). This results in a decrease in VEGF‐mediated endothelial cell migration, proliferation, and microvascular tumor density. The previous phase II and III studies showed that apatinib could significantly improve OS and PFS with an acceptable safety profile in patients with advanced gastric cancer refractory to two or more lines of prior chemotherapy. The ORR was 2.84% with apatinib versus 0% with placebo [16, 17]. Studies of apatinib are rare in chemotherapy‐refractory esophageal cancer.
To remedy the lack of prospective studies of apatinib in patients with ESCC, we conducted this phase II clinical trial (ESO‐Shanghai 11). The study aimed to assess the efficacy and safety of apatinib, an oral VEGFR‐2 TKI, in patients with ESCC refractory to at least one line of prior chemotherapy.
Our result showed that median PFS was 3.8 months (95% CI, 2.2–5.4) and that median OS was 5.8 months (95% CI, 3.2–8.4). PFS was 60.4% at 2 months and 57.7% at 3 months, which reached our anticipated improvement. Of the patients receiving apatinib, 65% (26/40) achieved disease control, which is comparable to the treatment outcomes of antiangiogenic agents in advanced gastric cancer and other solid tumors. Three patients with partial response (PR) were observed for ORR of 7.5%. Five percent (2/40) of patients had obvious tumor necrosis and cavitation of lung metastasis (Fig. 1). When the responses were evaluated by mRECIST, they were PRs, and the ORR was 12.5% (5/40; Fig. 2B).
Excluding the patients with uncontrolled primary tumors, this subset analysis suggested that the ORR and disease control rate were 8.6% (3/35) and 68.6% (24/35), mPFS2 was 3.9 months (95% CI, 3.3–4.5), and mOS2 was 6.0 months (95% CI, 3.3–8.7). PFS2 was 64.5% at 2 months and 61.4% at 3 months. Six‐month OS2 was 47.2%, and 12‐month OS2 was 14.8%.
The study demonstrated that apatinib was effective and safe as second‐line or higher treatment for patients with chemotherapy‐refractory ESCC. It was especially effective for those patients whose primary tumors were controlled and without severe invasion of trachea, bronchi, or major blood vessels.
Previous studies showed that antiangiogenic drugs such as bevacizumab are contraindicated for treatment of squamous cell lung cancer because squamous cell tumors are more frequently centrally located and close to major blood vessels and have a high incidence of severe hemorrhage. Furthermore, squamous cell tumors have a greater tendency to cavitate compared with adenocarcinomas.
Whether ESCC can be treated with antiangiogenic drugs is controversial because of the risk of hemorrhage and fistula. This concern is due serious consideration because, in this study, two patients died of massive fatal bronchopulmonary hemorrhage, and esophageal fistula occurred in another two patients (4/40, 10%). These four patients had regular follow‐ups and showed no significant abnormality in baseline laboratory tests. Both patients with esophageal fistula and one patient with a massive fatal bronchopulmonary hemorrhage case had uncontrolled primary tumors (3/5, 60%). In conclusion, those patients with progressive primary tumors or metastatic lymph nodes adjacent to the bronchi or large vessels may need to be excluded from apatinib treatment. This was consistent with the report of two cases from the affiliated Qianfoshan Hospital of Shandong University in 2018 [18]. The two patients' computed tomography images before their deaths also indicated that the tumor continued to progress and invaded the bronchial artery. Furthermore, in our trial, a patient with lymph node metastases and invasion of the main bronchi also died of massive fatal bronchopulmonary hemorrhage.
The limitation of our study was its small sample size as a single‐arm phase II trial. To validate these results, based on our study results, a prospective phase III, randomized comparison of apatinib and placebo is necessary for patients with chemotherapy‐refractory ESCC whose primary tumors are controlled and without severe invasion of trachea, bronchi, or major blood vessels.
Disclosures
The authors indicated no financial relationships.
Figures and Tables
Acknowledgments
We thank all the patients, their families, and the institutions for supporting this study. The research was supported by National Key R&D Plan in China (MOST‐2016YFC1303200, 2017YFC0107600) and the National Natural Science Foundation of China (21172043, 81872454)
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Footnotes
- ClinicalTrials.gov Identifier: NCT03274011
- Sponsor: Shanghai Cancer Center, Fudan University
- Principal Investigators: Kuaile Zhao, Li Chu
- IRB Approved: Yes
References
- 1. Torre LA, Bray F, Siegel RL et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 2. Abnet CC, Arnold M, Wei WQ. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology 2018;154:360–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang X, Wang XW, Huang J et al. Irinotecan plus fluorouracil‐based regimen as second or third‐line chemotherapy for recurrent or metastatic esophageal squamous cell carcinoma. Thoracic Cancer 2016;7:246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lorenzen S, Schuster T, Porschen R et al. Cetuximab plus cisplatin‐5‐fluorouracil versus cisplatin‐5‐fluorouracil alone in first‐line metastatic squamous cell carcinoma of the esophagus: A randomized phase II study of the Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol 2009;20:1667–1673. [DOI] [PubMed] [Google Scholar]
- 5. Ajani JA, Ilson DH, Daugherty K et al. Activity of taxol in patients with squamous cell carcinoma and adenocarcinoma of the esophagus. J Natl Cancer Inst 1994;86:1086–1091. [DOI] [PubMed] [Google Scholar]
- 6. Huang J, Xu B, Liu Y et al. Irinotecan plus S‐1 versus S‐1 in patients with previously treated recurrent or metastatic esophageal cancer (ESWN 01): A prospective randomized, multicenter, open‐labeled phase 3 trial. Cancer Commun (Lond) 2019;39:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nomura M, Iwasa S, Tsushima T et al. Active salvage chemotherapy versus best supportive care for patients with recurrent or metastatic squamous cell carcinoma of the esophagus refractory or intolerable to fluorouracil, platinum, and taxane. Cancer Chemother Pharmacol 2016;78:1209–1216. [DOI] [PubMed] [Google Scholar]
- 8. Shirakawa T, Kato K, Kengo N et al. A retrospective study of docetaxel or paclitaxel in patients with advanced or recurrent esophageal squamous cell carcinoma who previously received fluoropyrimidine‐ and platinum‐based chemotherapy. Cancer Chemother Pharmacol 2014;74:1207–1215. [DOI] [PubMed] [Google Scholar]
- 9. Burkart C, Bokemeyer C, Klump B et al. A phase II trial of weekly irinotecan in cisplatin‐refractory esophageal cancer. Anticancer Res 2007;27:2845–2848. [PubMed] [Google Scholar]
- 10. Lordick F, Schilling CV, Bernhard H et al. Phase II trial of irinotecan plus docetaxel in cisplatin‐pretreated relapsed or refractory oesophageal cancer. Br J Cancer 2003;89:630–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou ZG, Zhen CJ, Bai WW et al. Salvage radiotherapy in patients with local recurrent esophageal cancer after radical radiochemotherapy. Radiat Oncol 2015;10:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim YS, Lee CG, Kim KH et al. Re‐irradiation of recurrent esophageal cancer after primary definitive radiotherapy. Radiat Oncol J 2012;30:182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ku GY. Systemic therapy for esophageal cancer: Chemotherapy. Chin Clin Oncol 2017;6:49. [DOI] [PubMed] [Google Scholar]
- 14. Kojima T, Shah MA, Muro K et al.; KEYNOTE‐181 Investigators. Randomized phase III KEYNOTE‐181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol 2020;38:4138–4148. [DOI] [PubMed] [Google Scholar]
- 15. Kato K, Cho BC, Takahashi M et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION‐3): A multicentre, randomised, open‐label, phase 3 trial. Lancet Oncol 2019;20:1506–1517. [DOI] [PubMed] [Google Scholar]
- 16. Li J, Qin S, Xu J et al. Apatinib for chemotherapy‐refractory advanced metastatic gastric cancer: Results from a randomized, placebo‐controlled, parallel‐arm, phase II trial. J Clin Oncol 2013;31:3219–3225. [DOI] [PubMed] [Google Scholar]
- 17. Li J, Qin SK, Yu H et al. Randomized, double‐blind, placebo‐controlled phase III trial of apatinib in patients with chemotherapy‐ refractory advanced or metastatic adenocarcinoma of the stomach or gastoesophageal junction. J Clin Oncol 2016;34:1448–1454. [DOI] [PubMed] [Google Scholar]
- 18. Wang W, Zhang L, Xie Y et al. Fatal hemoptysis in patients with advanced esophageal cancer treated with apatinib. Onco Targets Ther 2018;11:2565–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]


