Abstract
Introduction:
The types of surveillance recommended after radical cystectomy and the degree of patient compliance are not well characterized.
Objectives:
The aims were to identify the pattern of post cystectomy surveillance recommended in the oncologic community, and to assess compliance to a predetermined schedule among a small group of urologists.
Material and Methods:
A survey was sent inquiring about the number of patients followed after cystectomy, the physician specialty, the type of practice, whether the follow-up schedule was stage-dependent, the frequency of office visits, and the type of tests. To assess noncompliance to a strict follow-up schedule, we analyzed the records of 647 patients who underwent radical cystectomy.
Results:
The overall response rate to the survey was 37% (123 of 330). Ninety-six percent of the respondents were urologists. Seventy-two percent were from US academic centers, 13% were from non-US academic centers, and 14% were in private practice. Twenty-one percent reported following more than 100 postcystectomy patients yearly, 29% between 51 and 100, and 43% between 1 and 50. Sixty percent of the respondents tailored the follow-up schedule based on pathologic stage. Computerized tomography (CT) scan of the abdomen and pelvis, chest X-ray, and urine cytology are the most frequent tests used. CT scan of the chest, magnetic resonance imaging (MRI), and abdominal ultrasound are used occasionally. There was a significant deviation from a predetermined follow-up schedule. There is no uniformity among urologic oncologists in post cystectomy surveillance and there is lack of compliance to a predetermined follow-up schedule
Keywords: bladder cancer, surveillance, cystectomy, adherence to surveillance
1. Introduction
The purpose of surveillance of patients who undergo a radical cystectomy is to diagnose the development of urethral and upper tract urothelial disease, to detect metastasis and local recurrences, and to identify the presence of long-term complications of urinary diversions. Although an intensive follow-up can result in an early detection, its utility has been challenged because of a lack of evidence that follow-up leads to improved outcomes.
A schedule for surveillance should be dependent on the natural history of the disease and on the impact of early detection on the extent of needed treatment and the ability to effect a cure. The first aim of this study is to identify the pattern of surveillance post-cystectomy in the oncologic community, predominantly among uro-oncologist. The second aim is to assess compliance to a predetermined schedule among a small group of urologists.
2. Material and methods
2.1. Survey
A survey was sent to 330 members of the Society of Urologic Oncology inquiring about the number of patients followed after cystectomy, the specialty (urologist, medical oncologist, radiation oncologist), the type of practice (US academic, non-US academic, private practice), whether the follow-up schedule was stage-dependent, the frequency of office visits, type of tests (CT abdomen and pelvis, CT chest, ultrasound, chest X-ray, MRI, positron emission tomography (PET) scan, urine cytology, and FISH), and whether they would be willing to participate in a prospective randomized study evaluating patient survival and cost based on intensity of follow-up.
2.2. Compliance analysis
To assess noncompliance to a strict follow-up schedule in the absence of a protocol, we analyzed data from 647 patients who underwent radical cystectomy at Memorial Sloan-Kettering Cancer Center from 2000 to 2005. All patients were treated by one of four urologists, and analyses were performed separately for each urologist to allow for differences in follow-up schemes. All visits were categorized in 3-mo intervals up to 24 mo following surgery. Visits occurring 0.1 to 4.5 mo from surgery were categorized as the 3-mo follow-up visit; visits occurring 4.6 to 7.5 mo from surgery were categorized as the 6-mo follow-up visit (ie, 6 mo +/− 1.5 mo), and subsequent visits were assigned similarly for the 9- to 24-mo visits. Patients were considered to be compliant with the follow-up schedule if they showed up for every scheduled visit until the earlier of 24 mo or time of death. Patients were considered to be noncompliant at the time the first scheduled visit was missed. The proportion of patients who were compliant following surgery was estimated using Kaplan-Meier methods. Statistical analyses were conducted using Stata 11.0 (StataCorp LP, College Station, TX).
3. Results
3.1. Survey results
The overall response rate to the survey was 37% (123 of 330). Ninety-six percent of the respondents were urologists. Seventy-two percent were from US academic centers, 13% were from non-US academic centers, and 14% were in private practice (Table 1). Twenty-one percent were following more than 100 postcystectomy patients yearly, 29% between 51 and 100, and 43% between 1 and 50. Sixty percent of the respondents tailored the follow-up schedule based on pathologic stage. Eighty percent of the respondents were willing to participate in a prospective study to evaluate patient survival and cost. The follow-up routine for the urologists who indicated that follow-up is or is not dependent on the pathologic stage is shown in supplementary material (http://www.mskcc.org/survey/followup_stage). CT scan of the abdomen and pelvis, chest X-ray, and urine cytology were the most frequent tests used. CT scan of the chest, MRI, and abdominal ultrasound were used occasionally. PET scans and FISH were rarely used.
Table 1—
Characteristics of respondents, overall and by whether follow-up is dependent on stage
| Overall n = 123 |
Not Stage-Dependent n = 47 |
Stage-Dependent n = 74 |
|
|---|---|---|---|
| Specialty | |||
| Missing | 1 (1%) | 1 (2%) | 0 (0%) |
| Medical oncologist | 3 (2%) | 2 (4%) | 1 (1%) |
| Radiation oncologist | 1 (1%) | 1 (2%) | 0 (0%) |
| Urologist | 118 (96%) | 43 (91%) | 73 (99%) |
| Practice | |||
| Missing | 1 (1%) | 0 (0%) | 1 (1%) |
| Non-US academic | 16 (13%) | 5 (11%) | 11 (15%) |
| Private practice | 17 (14%) | 10 (21%) | 7 (9%) |
| US academic | 89 (72%) | 32 (68%) | 55 (74%) |
| Estimated annual volume | |||
| Missing | 2 (2%) | 1 (2%) | 1 (1%) |
| 0 | 6 (5%) | 3 (6%) | 2 (3%) |
| 1 to 50 | 53 (43%) | 28 (60%) | 25 (34%) |
| 51 to 100 | 36 (29%) | 8 (17%) | 28 (38%) |
| >100 | 26 (21%) | 7 (15%) | 18 (24%) |
| Follow-up is dependent on stage | |||
| Missing | 2 (2%) | 0 (0%) | 0 (0%) |
| No | 47 (38%) | 47 (100%) | 0 (0%) |
| Yes | 74 (60%) | 0 (0%) | 74 (100%) |
| Willing to participate in prospective study | |||
| Missing | 5 (4%) | 1 (2%) | 4 (5%) |
| No | 19 (15%) | 13 (28%) | 5 (7%) |
| Yes | 99 (80%) | 33 (70%) | 65 (88%) |
3.2. Compliance results
Table 2 summarizes the responses from each surgeon regarding follow-up strategy separately by pathologic stage and by type of visit (clinic visit, cytology, chest X-ray, and CT of the abdomen/pelvis). In general, the surgeons had similar follow-up strategies, although one surgeon (surgeon 1) tended to be more conservative, with as many or more frequent proposed follow-up visits than the others. Figures 1 and 2 show the proportion of patients who were compliant with every scheduled follow-up visit and CT scan according to surgeon. The curves drop down only at scheduled follow-up visits, because the first time that a patient could become noncompliant was at 3 mo following surgery and all other opportunities to become noncompliant occurred at subsequent follow-up visits.
Table 2.
Survey responses for 4 MSKCC urologists
| pT1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Clinic Visit | Cytology | Xray | CT Abdomen | |||||
| Year 1 | Year 2 | Year 1 | Year 2 | Year 1 | Year 2 | Year 1 | Year 2 | |
| Surgeon1 | 3 months | 6 months | 3 months | 6 months | 3 months | 3 months | 6 months | 6 months |
| Surgeon2 | 3 months | 6 months | 3 months | 6 months | never | never | 12 mo | 12 mo |
| Surgeon3 | 3 months | 6 months | 3 months | 6 months | 12 mo | 12 mo | 12 mo | 12 mo |
| Surgeon4 | 3 months | 6 months | 3 months | 6 months | 12 mo | 12 mo | 12 mo | 12 mo |
| pT2 | ||||||||
| Clinic Visit | Cytology | Xray | CT Abdomen | |||||
| Year 1 | Year 2 | Year 1 | Year 2 | Year 1 | Year 2 | Year 1 | Year 2 | |
| Surgeon1 | 3 | 3 | 3 months | 3 months | 3 months | 3 months | 6 months | 6 months |
| Surgeon2 | 3 | 6 | 3 | 6 | never | never | 6 | 6 months |
| Surgeon3 | 3 | 6 | 3 months | 6 months | 3 months | 6 months | 3 months | 6 months |
| Surgeon4 | 3 months | 6 months | 3 months | 6 months | 3 months | 6 months | 3 months | 6 months |
| pT3+ | ||||||||
| Clinic Visit | Cytology | Xray | CT Abdomen | |||||
| Year 1 | Year 2 | Year 1 | Year 2 | Year 1 | Year 2 | Year 1 | Year 2 | |
| Surgeon1 | 3 months | 3 months | 3 months | 3 months | 3 months | 3 months | 3 months | 3 months |
| Surgeon2 | 3 | 3 | 3 | 3 | never | never | 3 months | 3 months |
| Surgeon3 | 3 | 3 | 3 months | 3 months | sometimes | sometimes | 3 months | 6 months |
| Surgeon4 | 3 | 3 months | 3 months | 3 months | sometimes | sometimes | 3 months | 6 months |
| pN1 | ||||||||
| Clinic Visit | Cytology | Xray | CT Abdomen | |||||
| Year 1 | Year 2 | Year 1 | Year 2 | Year 1 | Year 2 | Year 1 | Year 2 | |
| Surgeon1 | 3 months | 3 months | 3 months | 3 months | 3 months | 3 months | 3 months | 3 months |
| Surgeon2 | 3 months | 3 months | 3 months | 3 months | never | never | 3 months | 3 months |
| Surgeon3 | 3 | 3 months | 3 months | sometimes | sometimes | sometimes | 3 months | 3 months |
| Surgeon4 | 3 | 3 | 3 months | sometimes | sometimes | sometimes | 3 months | 3 months |
Figure 1—
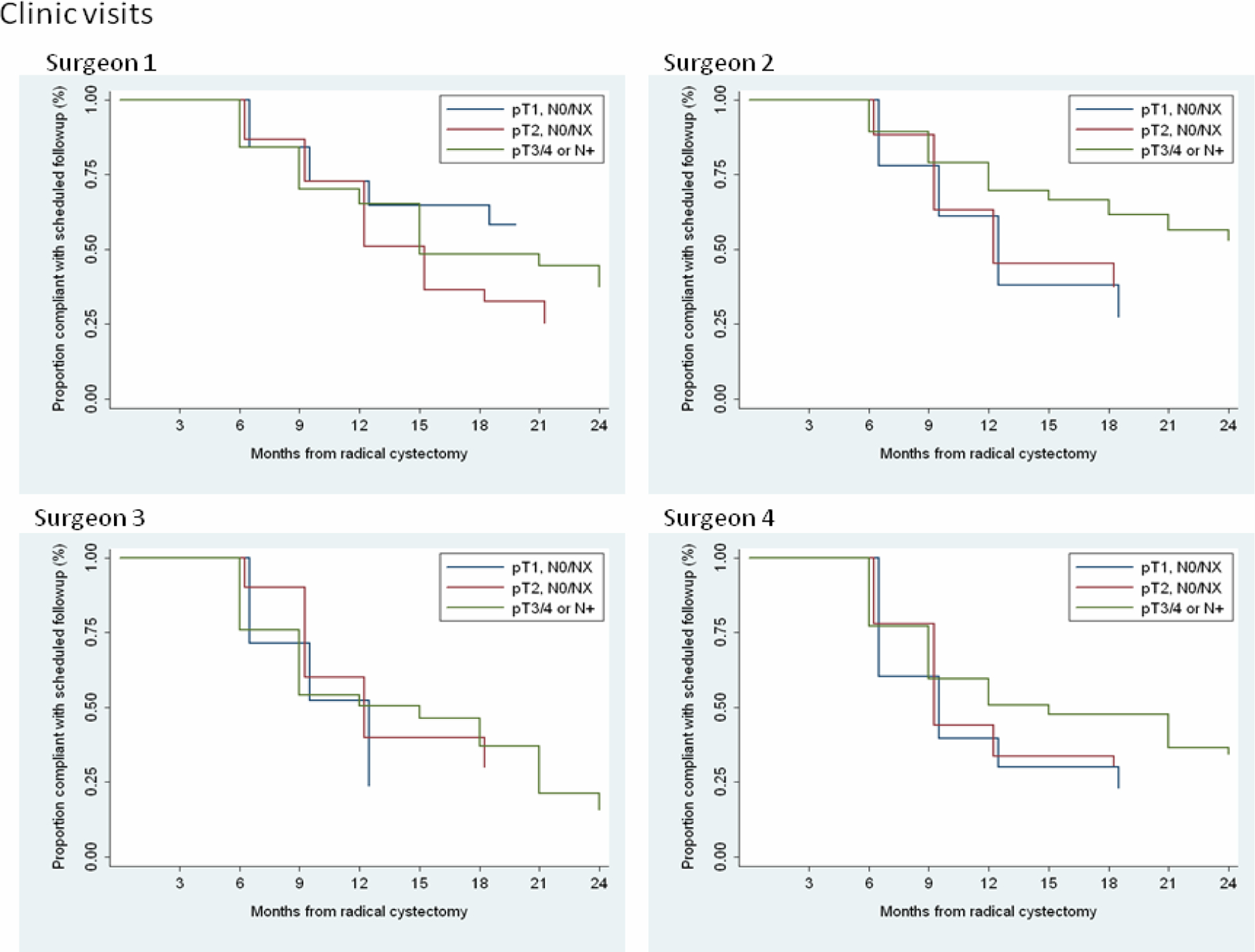
Proportion of patients compliant with every scheduled follow-up visit by surgeon
Figure 2—
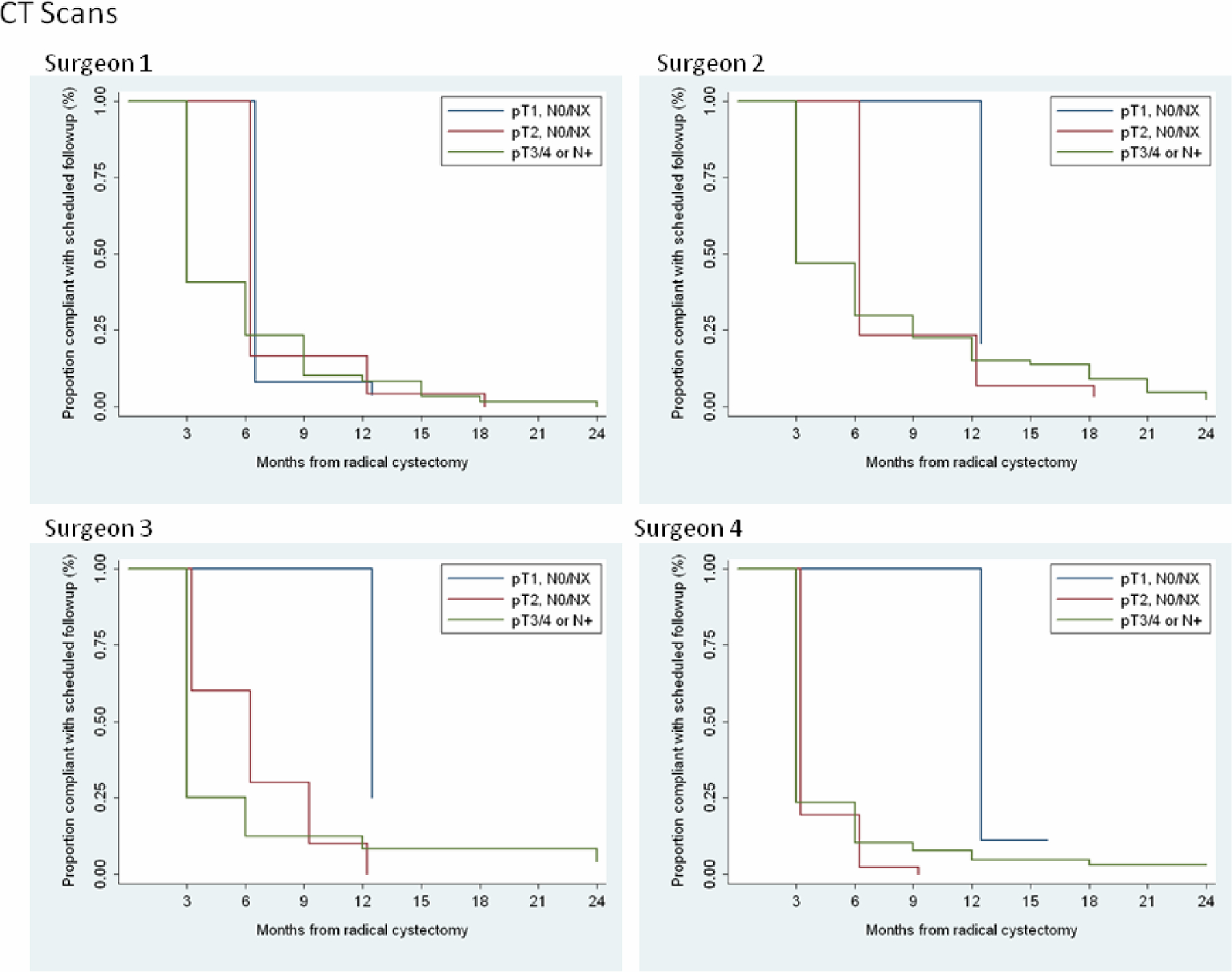
Proportion of patients compliant with every CT scan by surgeon.
4. Discussion
We have shown that there is no uniformity among urologic oncologists in the post-cystectomy follow-up and have also shown that there is lack of compliance to a predetermined follow-up schedule. But do we really need to follow those patients?
Urethral recurrence rates suggest that we do not. Urethral recurrences following a radical cystectomy range from 0.7–19%[1] The incidence depends on certain risk factors. Tongaonkar categorized patients into three groups: a high-risk group with tumors involving the prostate, an intermediate group with multiple tumors, associated carcinoma in situ (CIS), or tumors involving the bladder neck or trigone, and a low-risk group with solitary tumors away from the trigone or bladder neck[2]. The urethral recurrence rate was 70% in the high-risk group, 9.6% in the intermediate group, and 1.2% in the low-risk group. The degree of prostate involvement correlated with subsequent tumor recurrence. Hardeman reported no recurrence with urethral involvement, compared with 25% with ductal involvement and 64% with stromal invasion[3]. Levinson reported a urethral recurrence of 4.8% 6 to 40 mo (median 23.5 mo) after radical cystectomy. Urethral recurrence was found in 1.5% of patients who presented with a solitary tumor, 4.5% of patients with CIS or multifocality, 17% of patients with disease in the prostate, and 30% with stromal invasion[4]. Most recurrences occur during the first 5 yr after a radical cystectomy; however, recurrences as late as 20 yr after cystectomy have been reported[5].
Does an early detection of urethral recurrence result in better outcome? Lin et al reported no significant survival difference between patients diagnosed by urethral washing vs those who were diagnosed by the presence of symptoms. In that series, cystectomy pathology was the only significant parameter of disease-free survival[6]. These results were confirmed by Knapik et al who found no difference in the rate of disease progression between the patients monitored with urethral wash and those who were not[7]. These results question the routine use of urethral washes after a radical cystectomy. One must be cautious however when making firm conclusions from these studies. The number of recurrences however overall in each series is small and when the patients with urethral recurrences are divided into noninvasive and invasive groups, there is no ability to detect a statistically significant difference in outcome.
The rationale for monitoring the upper tract is to detect the presence of recurrent malignancies in the upper tract and to identify the presence of benign ureteral strictures that would result in loss of renal function. The incidence of upper tract recurrences following cystectomy ranges from 1% to 9%[8]. . Tran et al have shown that the 3-year risk of recurrence remains at 4% to 6% after cystectomy and does not change over time[9]. There is strong evidence that outcome is related to pathologic stage of upper tract TCC.
In addition, a significant number of patients with ileal conduit diversion develop stenosis with deterioration of renal function. The incidence of benign anastomotic strictures has been reported to range from 1% to 9%[10]. However, the stenosis can also result from a local recurrence. Tsuji et al reported hydronephrosis in 26% of the patients. In 8% the obstruction causing the hydronephrosis was due to a recurrent malignancy and in 18% it was due to a benign stricture. The postoperative period before the recurrent malignancy ranged between 34 and 118 mo (mean 69 mo) and before the benign stricture between 1 and 20 mo (mean 5.1 mo)[10]. The routine use of upper tract studies has not been effective in the early detection of those tumors and did not result in better clinical outcome. Hastie et al followed 180 patients with routine IVP[11]. Ten patients developed upper tract tumors and all were diagnosed with onset of symptoms, undercutting the rationale for the routine use of IVP after cystectomy. Meissner et al concluded that the efficiency of IVP was 0.75%. In their series of 322 patients followed by routine IVP, 15 patients developed upper tract tumors and only 8 were diagnosed by IVP[12]. Holmang et al detected the presence of ureteral carcinoma in 16 patients out of 680 after at least 5 yr of follow-up and 6 of these patients underwent surgery for ureteral stricture. The majority of tumors were detected by symptoms despite routine IVP. The researchers concluded that routine imaging is not indicated[13] To date, no definitive study using modern CT urography, the current postoperative imaging modality of choice, has documented its ability to detect recurrent upper tract lesions at an early time point that would lead to improved survival. CT urography has been found to be more sensitive for detecting upper tract TCC compared to IVP.
Local recurrence occurs in 6% of patients after a radical cystectomy. It was 2% for superficial tumors, 5% for muscle invasive organ confined disease, 6% for extravesical disease. The mean interval from cystectomy to recurrence was 8.3 months (median 9, range 1 to 17 months). In this study, CT scan was not done routinely on all the patients and the mean followup was 23.9 months[14]. Similarly, Schoenberg reported a 5% local recurrence following a nerve sparing radical cystoprostatectomy. The median follow-up was 67 mo[15]. However, Greven reported an 18% actuarial local recurrence rate at 5 yr. Fifty-one percent of patients with pT3b disease had local recurrences vs 15% for pT1, 6% for pT2, and 18% for pT3a. The time until local recurrence ranged from 3 to 62 mo (median 9 mo) and the follow-up period ranged from 0 to 167 mo (median 56 mo)[16]. However, even with early detection, local recurrence has a dismal prognosis and nearly all patients will die of their disease. Bostrom et al reported their 20 yr experience of radical cystectomy. The mortality rate for both local recurrence and metastatic disease was 93%[17]. Patients who develop a local recurrence after a radical cystectomy have a median survival of 4 to 8 mo despite systemic chemotherapy and local intervention[18].
There has been a significant improvement in the median survival of patients with metastatic disease with cisplatin-based chemotherapy agents. Bajorin et al indentified two prognostic factors that affect long-term survival in patients with metastatic disease. Karnofsky performance status and visceral metastasis (lung, liver, and bone) were independent prognostic factors, with a median survival of 33, 23.4, and 9.3 mo for those patients with 0, 1, or 2 factors[19]. However, there is no evidence to suggest that earlier detection would have affected risk-group allocation or improved survival.
Noncompliance with surveillance protocols has been previously described. Yu et al reported poor compliance which degraded with time among patients with testis cancer who were placed on surveillance or were on postadjuvant follow-up[20]. Thirty percent of patients on surveillance did not receive abdominal imaging. Shrag et al have shown that the actual practice of follow-up among patients with bladder cancer differs substantially from the recommended standards[21]. It is not surprising that we have a high rate of noncompliance in postcystectomy follow-up, particularly when there is no evidence for its utility.
Currently, there is no level I evidence to suggest that intensive surveillance results in better outcomes among patients who have undergone a radical cystectomy. Volkmer et al failed to demonstrate a survival benefit for an early detection before developing symptoms. The overall survival at 2 yr was 10% in asymptomatic patients vs 8% in symptomatic patients, but the difference was insignificant[22]. Conversely, Giannarini et al suggested that patients diagnosed with asymptomatic recurrences have a slightly higher survival than patients with symptoms[23]. The intensity of the current follow-up schedule could also be explained as a safeguard against malpractice liability. It is quite obvious that until a concerted effort among urologic oncologists is undertaken, we will continue to wonder whether routine surveillance is effective in producing better outcomes.
5. Conclusions
There is no uniformity among urologic oncologists in postcystectomy surveillance and there is lack of compliance to a predetermined follow-up schedule. One might hypothesize that this finding may be due to the lack of evidentiary data showing that early detection of asymptomatic urethral, upper tract or local recurrences of disease will impact survival outcomes. One area where early detection may be beneficial is the detection of asymptomatic ureteral obstruction which if left undetected may result in the unnecessary loss of a renal unit. Prospective studies examining functional and disease specific outcomes between variant surveillance schedules are needed.
Acknowledgment:
This project was initiated at the Bladder Cancer Advocacy Network Think Tank Meeting 2009. We would like to acknowledge the input of the other members of the Standardization of Treatment Working Group of the Think Tank for their support: Gary Steinberg, Dean Bajorin, Mark Schoenberg, Walter Stadler, Wassim Kassouf, Alon Weizer, Theresa Koppie, Mike Porter, Alexandre Zlotta, Steven Wong, Rick Vitti, Bruce Malkowicz, and Deborah Bradley
Supported by:
The Sidney Kimmel Center for Prostate and Urologic Cancers
Glossary
- CT
Computerized tomography
- MRI
magnetic resonance imaging
- PET
positron emission tomography
- FISH
fluorescent insitu hybridization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental material (http://www.mskcc.org/survey/followup_stage
References
- 1.Donat SM. Staged based directed surveillance of invasive bladder cancer following radical cystectomy: Valuable and effective? World J Urol 2006;24:557–64. [DOI] [PubMed] [Google Scholar]
- 2.Tongaonkar HB, Dalal AV, Kulkarni JN, et al. Urethral recurrences following radical cystectomy for invasive transitional cell carcinoma of the bladder. Br J Urol 1993;72:910–4. [DOI] [PubMed] [Google Scholar]
- 3.Hardeman SW, Soloway MS. Urethral recurrence following radical cystectomy. J Urol 1990;144:666–9. [DOI] [PubMed] [Google Scholar]
- 4.Levinson AK, Johnson DE, Wishnow KI. Indications for urethrectomy in an era of continent urinary diversion. J Urol 1990;144:73–5. [DOI] [PubMed] [Google Scholar]
- 5.Sarosdy MF. Management of the male urethra after cystectomy for bladder cancer. Urol Clin North Am 1992;19:391–6. [PubMed] [Google Scholar]
- 6.Lin DW, Herr HW, Dalbagni G. Value of urethral wash cytology in the retained male urethra after radical cystoprostatectomy. J Urol 2003;169:961–3. [DOI] [PubMed] [Google Scholar]
- 7.Knapik JA, Murphy WM. Urethral wash cytopathology for monitoring patients after cystoprostatectomy with urinary diversion. Cancer 2003;99:352–6. [DOI] [PubMed] [Google Scholar]
- 8.Braslis KG, Soloway MS. Management of ureteral and renal pelvic recurrence after cystectomy. Urol Clin North Am 1994;21:653–9. [PubMed] [Google Scholar]
- 9.Tran W, Serio AM, Raj GV, et al. Longitudinal risk of upper tract recurrence following radical cystectomy for urothelial cancer and the potential implications for long-term surveillance. J Urol 2008;179:96–100. Epub 2007 Nov 13. [DOI] [PubMed] [Google Scholar]
- 10.Tsuji Y, Nakamura H, Ariyoshi A. Upper urinary tract involvement after cystectomy and ileal conduit diversion for primary bladder carcinoma. Eur Urol 1996;29:216–20. [PubMed] [Google Scholar]
- 11.Hastie KJ, Hamdy FC, Collins MC, et al. Upper tract tumours following cystectomy for bladder cancer. Is routine intravenous urography worthwhile? Br J Urol 1991;67:29–31. [DOI] [PubMed] [Google Scholar]
- 12.Meissner C, Giannarini G, Schumacher MC, et al. The efficiency of excretory urography to detect upper urinary tract tumors after cystectomy for urothelial cancer. J Urol 2007;178:2287–90. Epub 007 Oct 22. [DOI] [PubMed] [Google Scholar]
- 13.Holmang S, Hedelin H, Anderstrom C, et al. Long-term followup of a bladder carcinoma cohort: Routine followup urography is not necessary. J Urol 1998;160:45–8. [PubMed] [Google Scholar]
- 14.Wishnow KI, Dmochowski R. Pelvic recurrence after radical cystectomy without preoperative radiation. J Urol 1988;140:42–3. [DOI] [PubMed] [Google Scholar]
- 15.Schoenberg MP, Walsh PC, Breazeale DR, et al. Local recurrence and survival following nerve sparing radical cystoprostatectomy for bladder cancer: 10-year followup. J Urol 1996;155:490–4. [PubMed] [Google Scholar]
- 16.Greven KM, Spera JA, Solin LJ, et al. Local recurrence after cystectomy alone for bladder carcinoma. Cancer 1992;69:2767–70. [DOI] [PubMed] [Google Scholar]
- 17.Bostrom PJ, Mirtti T, Kossi J, et al. Twenty-year experience of radical cystectomy for bladder cancer in a medium-volume centre. Scand J Urol Nephrol 2009;43:357–64. [DOI] [PubMed] [Google Scholar]
- 18.Bochner BH, Montie JE, Lee CT. Follow-up strategies and management of recurrence in urologic oncology bladder cancer: Invasive bladder cancer. Urol Clin North Am 2003;30:777–89. [DOI] [PubMed] [Google Scholar]
- 19.Bajorin DF, Dodd PM, Mazumdar M, et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol 1999;17:3173–81. [DOI] [PubMed] [Google Scholar]
- 20.Yu HY, Madison RA, Setodji CM, et al. Quality of surveillance for stage i testis cancer in the community. J Clin Oncol 2009;27:4327–32. Epub 2009 Aug 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schrag D, Hsieh LJ, Rabbani F, et al. Adherence to surveillance among patients with superficial bladder cancer. J Natl Cancer Inst 2003;95:588–97. [DOI] [PubMed] [Google Scholar]
- 22.Volkmer BG, Kuefer R, Bartsch GC Jr., et al. Oncological followup after radical cystectomy for bladder cancer-is there any benefit? J Urol 2009;181:1587–93; discussion 93. Epub 2009 Feb 23. [DOI] [PubMed] [Google Scholar]
- 23.Giannarini G, Kessler TM, Thoeny HC, et al. Do patients benefit from routine follow-up to detect recurrences after radical cystectomy and ileal orthotopic bladder substitution? Eur Urol 2010;4:4. [DOI] [PubMed] [Google Scholar]


