Abstract
Objectives:
To evaluate the indications for early and deferred cystectomy and to report the impact of this tailored approach on survival.
Design, setting, and participants:
We retrospectively studied 523 patients seen at our institution who were initially diagnosed with T1 disease between 1990 and 2007.
Measurements:
Variables analyzed included age, gender, multifocality, multifocal T1 disease, carcinoma in situ, grade, recurrence rate, and restaging status. End points were overall and disease-specific survival.
Results and limitations:
A restaging transurethral resection (TUR) was performed in 523 patients. Of the patients who underwent restaging, 106 (20%) were upstaged to muscle-invasive disease and 417 patients were considered true clinical T1 (cT1); 84 of the latter group underwent immediate cystectomy. The median follow-up for survivors was 4.3 yr. The cumulative incidence of disease-specific death at 5 yr was 8% (95% confidence interval [CI], 5–13%), 10% (95% CI, 5–17%), and 44% (95% CI, 35–56%) for those restaged with lower than T1, T1, and T2 disease, respectively. Immediate cystectomy was more likely in patients with cT1 disease at restaging than in those with disease lower than cT1, but there were no other obvious differences in clinical characteristics between those with and without immediate cystectomy. Survival was not statistically different for patients who underwent an immediate cystectomy versus those who were maintained on surveillance with deferred cystectomy if deemed appropriate. Of 333 patients who did not undergo immediate cystectomy, 59 had a deferred cystectomy, and the likelihood of deferred cystectomy was greater in those with T1 disease on restaging TUR (hazard ratio: 2.40; 95% CI, 1.43–4.01; p = 0.001).
Conclusions:
Restaging TUR should be performed in patients diagnosed with cT1 bladder cancer to improve staging accuracy. Patients with T1 disease on restaging are at higher risk of progression and should be considered for early cystectomy.
Keywords: Bladder cancer, Restaging transurethral resection, Stage T1
1. Introduction
Bladder cancer presents as non–muscle-invasive papillary tumors in 75–85% of cases [1]; 20% of such tumors invade the lamina propria (T1) [2]. Although both Ta and T1 tumors commonly recur, T1 cancers are potentially more lethal because 30–50% progress to muscle invasion or metastasis [3]. Multiple tumors, high-grade morphology, large (>3 cm) size, associated carcinoma in situ (CIS), lymphovascular invasion, depth of lamina propria penetration, and presence of tumor at first follow-up cystoscopy are associated with greater risk of stage progression and reduced survival [4,5].
Management of T1 bladder cancer is a dynamic process. Some patients undergo immediate cystectomy while others are placed on long-term surveillance with the possibility of a deferred cystectomy. Although the choice of treatment takes into account several factors including multifocality, associated CIS, the presence of multifocal T1 disease, the presence of residual tumor at restaging transurethral resection (TUR), and prior history of bacillus Calmette-Guérin (BCG) treatment, there are no definite guidelines for selecting patients’ initial treatment. The objective of this study is to define the indications for early cystectomy or surveillance and to report the impact of this approach on survival.
2. Patients and methods
2.1. Subjects and inclusion criteria
An institutional review board–approved, retrospective review of the institutional database was performed to identify patients with clinical T1 (cT1) transitional cell carcinoma (TCC) of the bladder. Diagnosis of cT1 disease was based on cystoscopy, bimanual examination, radiographic tests, and pathologic evaluation. Patients whose pathology slides were not available for review and patients with non-TCC were excluded; however, TCC with aberrant differentiation was included. All tumors were staged according to the TNM system of the International Union Against Cancer and graded according to the World Health Organization/International Society of Urological Pathology 1998 grading system [6]. Multifocality was defined as having a history of Ta tumors or concomitant papillary tumors. Multifocal T1 disease was defined as having concomitant T1 tumors.
We identified 523 patients with an initial T1 diagnosis between 1990 and 2007 who were restaged. Restaging TUR was defined as occurring within 3 mo of initial T1 diagnosis. Immediate cystectomy was defined as a cystectomy performed within 3 mo of the restaging TUR. Deferred cystectomy was defined as a cystectomy performed >3 mo after a restaging TUR in a patient who started conservative management but whose tumor developed characteristics associated with progression or who developed muscle-invasive disease. Patients on surveillance were followed every 3–6 mo with cystoscopy, urine cytology, repeat TURs, and BCG, as necessary. The patients who underwent a radical cystectomy (RC) were followed every 3–6 mo with urinary cytology, computed tomography scan, and chest x-ray.
2.2. Statistical analysis
Separate analyses were conducted for the outcomes of overall survival and disease-specific survival. Survival time was calculated from the time of restaging TUR. Overall survival was estimated using the Kaplan-Meier method and compared with the log-rank test. Disease-specific survival was estimated with the cumulative incidence function, considering death from other and unknown causes as a competing risk, and was compared using a modified χ2 test [7]. Hazard ratios (HRs) with 95% confidence intervals (CIs) were estimated using Cox proportional hazards regression for overall survival and Gray’s regression [8] for disease-specific survival. Statistical analyses were conducted using Stata v.10 (StataCorp, College Station, TX, USA) and R (R Foundation for Statistical Computing, http://www.R-project.org) with the cmprsk package.
2.2.1. Immediate and deferred cystectomy
These analyses focused on patients with disease lower than clinical T2 (cT2) on restaging TUR, as all patients with cT2 disease are recommended for immediate cystectomy. Overall and disease-specific survival were compared for those with and without an immediate cystectomy. We then analyzed deferred cystectomy among patients on surveillance. Univariate Cox proportional hazards regression was used to evaluate predictors of deferred cystectomy following restaging TUR. The association of deferred cystectomy with overall survival was assessed by considering deferred cystectomy as a time-dependent covariate in a multivariable Cox proportional hazards model, controlling for stage from the restaging TUR. A landmark time analysis was performed using a landmark time of 3 mo after the date of the restaging TUR, so that immediate cystectomy status could be ascertained; 18 patients who were lost to follow-up within 3 mo of restaging TUR were excluded.
2.2.2. Survival in patients with cT2 disease
Patients who develop cT2 disease can be categorized as either patients upstaged to cT2 disease (ie, those with cT2 disease on restaging TUR) or patients who progressed to cT2 disease while on surveillance. Overall and disease-specific survival following development of cT2 disease were compared between these groups. Those who presented with cT2 disease inherently had longer follow-up than those who progressed while on surveillance (based on median follow-up for survivors at 5 yr and 3 yr); to ensure comparable lengths of follow-up between groups, any patient followed for >3 yr was censored at 3 yr in this analysis.
3. Results
We identified 523 patients with cT1 TCC of the bladder who were restaged (Table 1). The diagnostic TUR was performed at Memorial Sloan-Kettering Cancer Center (MSKCC) for 144 patients (28%). The restaging TUR was performed at MSKCC for 83% of patients (435 of 523) and at an outside institution for the rest. Overall, 106 (20%) patients were upstaged to muscle-invasive disease. A similar proportion of patients were upstaged to T2 disease, regardless of where the restaging TUR was performed (21% if performed at MSKCC [90 of 435] and 18% if performed outside [16 of 88]). In the first TUR, muscle was pathologically confirmed to be present in only 47% (n = 242) of specimens (53% of the diagnosis TURs were performed at MSKCC and 44% were performed outside). In the restaging TUR, muscle was pathologically confirmed to be present in 84% (n = 426) overall (85% of restaging TURs were performed at MSKCC and 76% were performed outside).
Table 1 –
Patient characteristics
| Characteristics at initial T1 diagnosis | N = 523 |
|---|---|
| Age, yr, median (interquartile range) | 66 |
| (57–74) | |
| Female, n (%) | 115 (22) |
| Muscle in TUR specimen | – |
| No, n (%) | 278 (53) |
| Yes, n (%) | 245 (47) |
| Multifocal | – |
| Any multifocal**, n (%) | 130 (25) |
| Multifocal T1, n (%) | 60 (11) |
| Recurrence, n (%) | 38 (7) |
| CIS, n (%) | 122 (23) |
| High grade*, n (%) | 506 (97) |
| Prior BCG, n (%) | 34 (7) |
| Stage from restaging TUR | |
| <T1, n (%) | 260 (50) |
| T1, n (%) | 157 (30) |
| T2, n (%) | 106 (20) |
| Muscle in restaging TUR | |
| No, n (%) | 82 (16) |
| Yes, n (%) | 441 (84) |
Grade was unknown for two patients with a restaging TUR.
Multifocal defined as recurrent tumors and multifocality at the time of T1 diagnosis.
BCG = bacillus Calmette-Guérin; CIS = carcinoma in situ; TUR = transurethral resection.
At last follow-up, 142 patients had died, 81 of them from bladder cancer. Median follow-up for survivors was 4.3 yr. The cumulative incidence of disease-specific death is given in Figure 1 by stage at restage. The patients upstaged to T2 disease had the worst disease-specific survival, while disease-specific survival was similar between patients with disease lower than T1 versus T1 disease at restaging (global p < 0.001). The cumulative incidence of disease-specific death at 5 yr was 8% (95% CI, 5–13%), 10% (95% CI, 5–17%), and 44% (95% CI, 35–56%) for those restaged with lower than T1, T1, and T2 disease, respectively. Similar results were observed for overall survival (Table 2).
Fig. 1 –
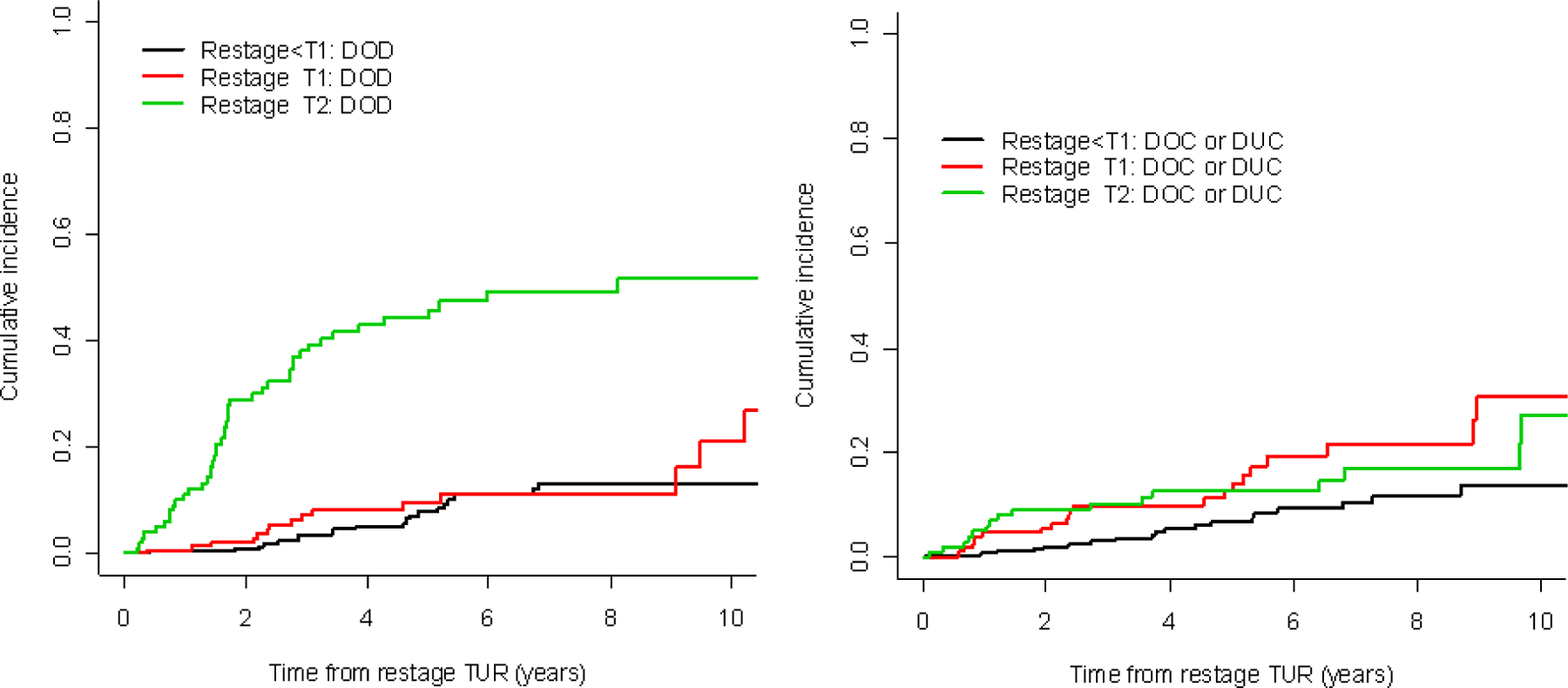
Bladder-cancer-specific death according to stage from restaging transurethral resection (TUR).
DOD = death from bladder cancer; DOC = death from other causes; DUC = death from unknown causes.
Table 2 –
Cumulative incidence disease-specific mortality and overall mortality, separately by stage from restaging transurethral resection (TUR)
| Years from initial T1 diagnosis | Stage from restaging TUR | ||
|---|---|---|---|
| <T1 | T1 | T2 | |
| Disease-specific mortality, % (95% confidence interval) | |||
| 1 | 0(0–3) | 1 (0–5) | 11 (6–20) |
| 2 | 1 (0–3) | 2 (1–7) | 29 (21–40) |
| 3 | 3 (2–7) | 7 (4–14) | 38 (29–49) |
| 4 | 5 (3–9) | 8 (5–15) | 43 (34–55) |
| 5 | 8 (5–13) | 10 (5–17) | 44 (35–56) |
| Overall mortality, % (95% confidence interval) | |||
| 1 | 1 (0–4) | 6 (3–11) | 16 (10–25) |
| 2 | 3 (1–6) | 8 (4–14) | 38 (29–49) |
| 3 | 6 (4–11) | 17 | 48 (39–59) |
| (12–25) | |||
| 4 | 11 | 18 | 56 (46–66) |
| (7–16) | (12–26) | ||
| 5 | 15 | 22 | 57 (47–68) |
| (10–21) | (16–32) | ||
3.1. Immediate cystectomy versus surveillance
Of the 417 patients considered to have true cT1 disease or lower, 84 underwent an immediate cystectomy. Immediate cystectomy was more likely among patients with cT1 disease at restage (58 of 157, 37%) than among those with disease lower than cT1 at restage (26 of 260, 10%) (p < 0.001). Other than the stage at restaging TUR, no obvious differences in clinical characteristics were observed between those with and without immediate cystectomy (Table 3). Additionally, disease-specific survival did not differ significantly between these two groups (Fig. 2; p = 0.7). Similar results were observed for overall survival: Overall survival at 5 yr was 79% (95% CI, 66–88%) and 84% (95% CI, 78–88%) for patients with and without immediate cystectomy, respectively. To verify that our results were not affected by restaging TURs performed outside, we repeated all analyses for the 435 patients with restaging TUR performed at MSKCC; none of the results or conclusions changed. Pathologic stage is given in Table 4 for the 84 patients who underwent immediate cystectomy. Overall, 23% (n = 18) were pT2 or higher; of those, 20% (n = 6) were lower than T1 on restaging TUR and 24% (n = 14) were T1 on restaging TUR.
Table 3 –
Characteristics of patients considered true cT1 disease or lower based on restaging transurethral resection (TUR), stratified by immediate cystectomy*
| Immediate cystectomy | p value | ||
|---|---|---|---|
| No, n = 333 | Yes, n = 84 | ||
| Characteristics at initial T1 diagnosis | – | – | – |
| Age, median (interquartile range) | 65 | 67 | 0.8** |
| (57–74) | (59–73) | ||
| Female, n (%) | 72 (22) | 16 (19) | 0.7 |
| Multifocal | – | – | – |
| Any multifocal, n (%) | 90 (27) | 20 (24) | 0.6 |
| Multifocal T1, n (%) | 35 (11) | 9 (11) | 1.0 |
| Recurrence, n (%) | 28 (8) | 6 (7) | 0.8 |
| CIS, n (%) | 84 (25) | 15 (18) | 0.2 |
| High grade***, n (%) | 317 (96) | 84 (100) | 0.08 |
| Prior BCG, n (%) | 25 (8) | 3 (4) | 0.2 |
| Stage from restaging TUR | – | – | <0.001 |
| <T1, n (%) | 234 (70) | 26 (31) | – |
| T1, n (%) | 99 (30) | 58 (69) | – |
| Muscle in restaging TUR | – | – | 0.16 |
| No, n (%) | 25 (30) | 72 (22) | – |
| Yes, n (%) | 59 (70) | 261 (78) | – |
BCG = bacillus Calmette-Guérin; CIS = carcinoma in situ.
p values were calculated by Fisher exact test for all variables except as indicated.
p value was determined by the Mann-Whitney test.
Grade was unknown for two patients without immediate cystectomy.
Fig. 2 –
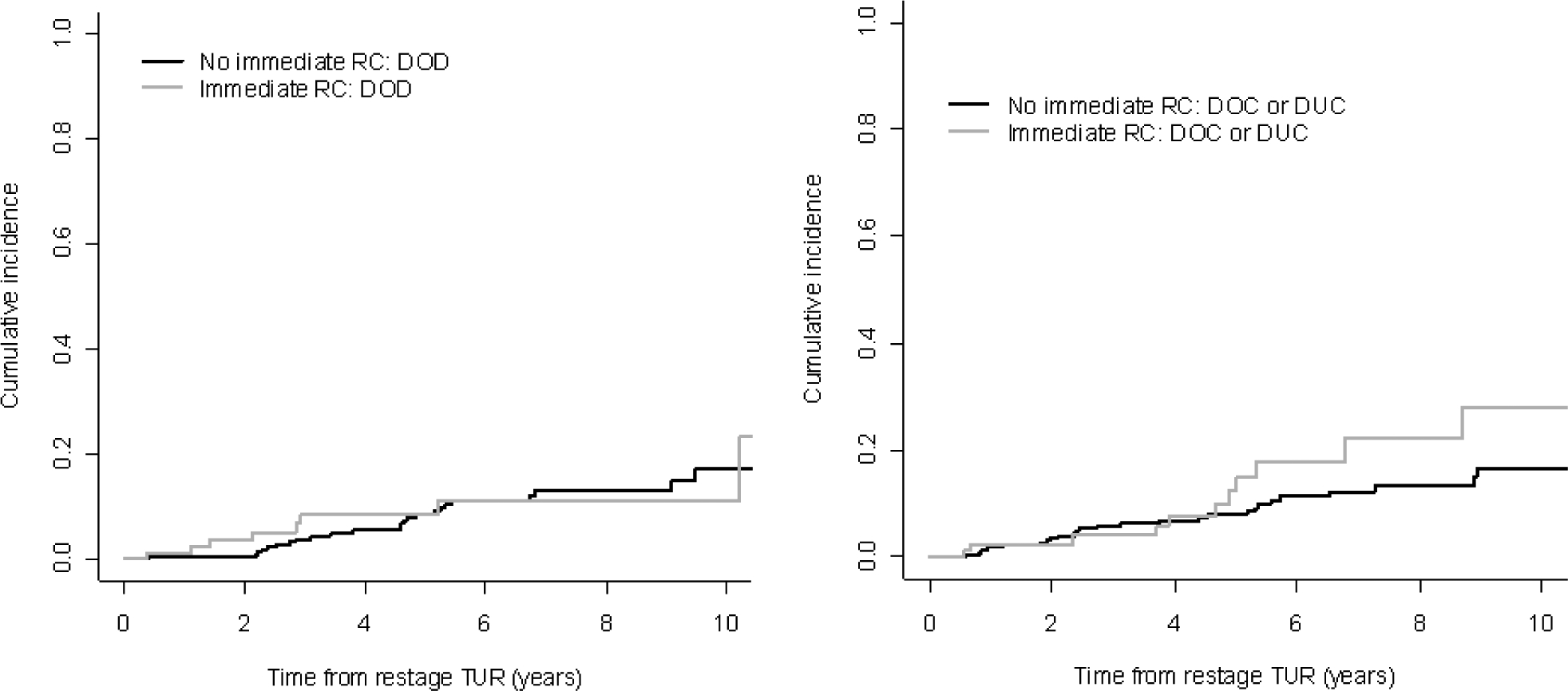
Bladder-cancer-specific death among patients with clinical T1 disease or lower on restaging transurethral resection (TUR), stratified by immediate cystectomy.
DOD = death from bladder cancer; DOC = death from other causes; DUC = death from unknown causes; RC = radical cystectomy.
Table 4 –
Clinical and pathologic stage for patients with lower than T2 disease at restaging and who underwent immediate cystectomy
| <T1 | T1 | |
|---|---|---|
| No. with RC | 26 | 58 |
| RC stage | – | – |
| pT0/Ta/Tis, n (%) | 12 (46) | 23 (40) |
| pT1, n (%) | 9 (35) | 21 (36) |
| pT2, n (%) | 3 (12) | 6 (10) |
| ≥pT3, n (%) | 2 (8) | 8 (14) |
RC = radical cystectomy.
3.2. Bacillus Calmette-Guérin in patients on surveillance
Patients were considered to be treated with early BCG if they started BCG treatment within 90 d of restaging TUR. Of 308 patients with true cT1 disease or lower who did not undergo an immediate cystectomy and who did not receive prior BCG, 138 (46%) were treated with early BCG. There were 51 overall deaths in this group of patients, 29 from bladder cancer. On univariate analysis, early BCG was not significantly associated with disease-specific survival (HR: 1.03; 95% CI, 0.44–2.43; p = 0.9) or overall survival (HR: 0.71; 95% CI, 0.40–1.25; p = 0.2).
3.3. Deferred cystectomy
Deferred cystectomy was defined as a cystectomy deemed necessary in patients who were placed on surveillance. Of 333 patients with true cT1 disease or lower who did not undergo an immediate cystectomy, 59 had a deferred cystectomy. The deferred RC was performed for progression to muscle-invasive disease in 12 patients (20%), for recurrent T1 tumors in 36 patients (61%), and for recurrent tumors lower than T1 in 11 patients (19%). The stage on restaging TUR was significantly associated with deferred cystectomy (HR: 2.40 for T1 vs lower than T1; 95% CI, 1.43–4.01; p = 0.001). The 2-yr probability of deferred cystectomy was 11% (95% CI, 8–16%) for patients lower than T1 on restaging TUR, compared to 28% (95% CI: 20–39%) for patients with T1 disease on restaging TUR. No other variables known at the time of restaging TUR were significant predictors of deferred cystectomy. Those who underwent a deferred cystectomy had worse overall survival than those still on surveillance, even when controlling for stage on restaging TUR (HR: 1.89 for deferred cystectomy vs none; 95% CI, 1.08–3.29; p = 0.025); this is likely because patients selected for deferred cystectomy had unfavorable disease that became apparent sometime after restaging TUR. The likelihood of deferred cystectomy according to present or absent muscle in the restaging TUR did not differ significantly (HR: 1.02; 95% CI, 0.52–1.95; p = 0.9). We performed an analysis comparing survival following cystectomy for the patients who underwent an immediate cystectomy versus those who underwent a deferred cystectomy and found no significant difference (p = 0.3).
3.4. Survival in patients with cT2 disease
There were 106 patients upstaged to T2 (ie, on restaging TUR) and 29 patients who progressed to T2 on surveillance. In the subgroup of patients who underwent RC, those who progressed to T2 disease on surveillance had a higher percentage of non-organ-confined disease (58%) compared to those who were upstaged to T2 on restaging TUR (45%) (Table 5). Although this difference was not statistically significant (p = 0.5), an important proportion of patients on surveillance had non-organ-confined disease, which suggests that close surveillance does not always guarantee detection while disease is still organ confined. These data, however, must be interpreted with caution because of the limited number of cases. Five patients (four at presentation and one on surveillance) were excluded from the survival analysis because their last follow-up date was the date they were diagnosed with T2 disease. Disease-specific survival did not differ significantly between patients who progressed to T2 on surveillance compared to those with T2 on restaging TUR, although past 1.5 yr, the curves started to separate in favor of patients on surveillance (p = 0.08; Fig. 3). Similar results were observed for overall survival. Two-year overall survival, for example, was 62% (95% CI, 51–71%) and 76% (95% CI, 54–88%) for patients with pT2 disease at presentation and on surveillance, respectively.
Table 5 –
Pathologic stage for patients with T2 disease at restaging versus pathologic stage for patients who progressed to T2 disease while on surveillance
| T2 on Restaging TUR | Progressed to T2 on surveillance | p value* | |
|---|---|---|---|
| No. of patients | 106 | 29 | – |
| No. with RC | 77 | 12 | – |
| RC stage | – | – | 0.5 |
| ≤pT2, n (%) | 42 (55) | 5 (42) | – |
| ≥pT3, n (%) | 35 (45) | 7 (58) | – |
RC = radical cystectomy; TUR = transurethral resection.
p value was determined by Fisher exact test.
Fig. 3 –
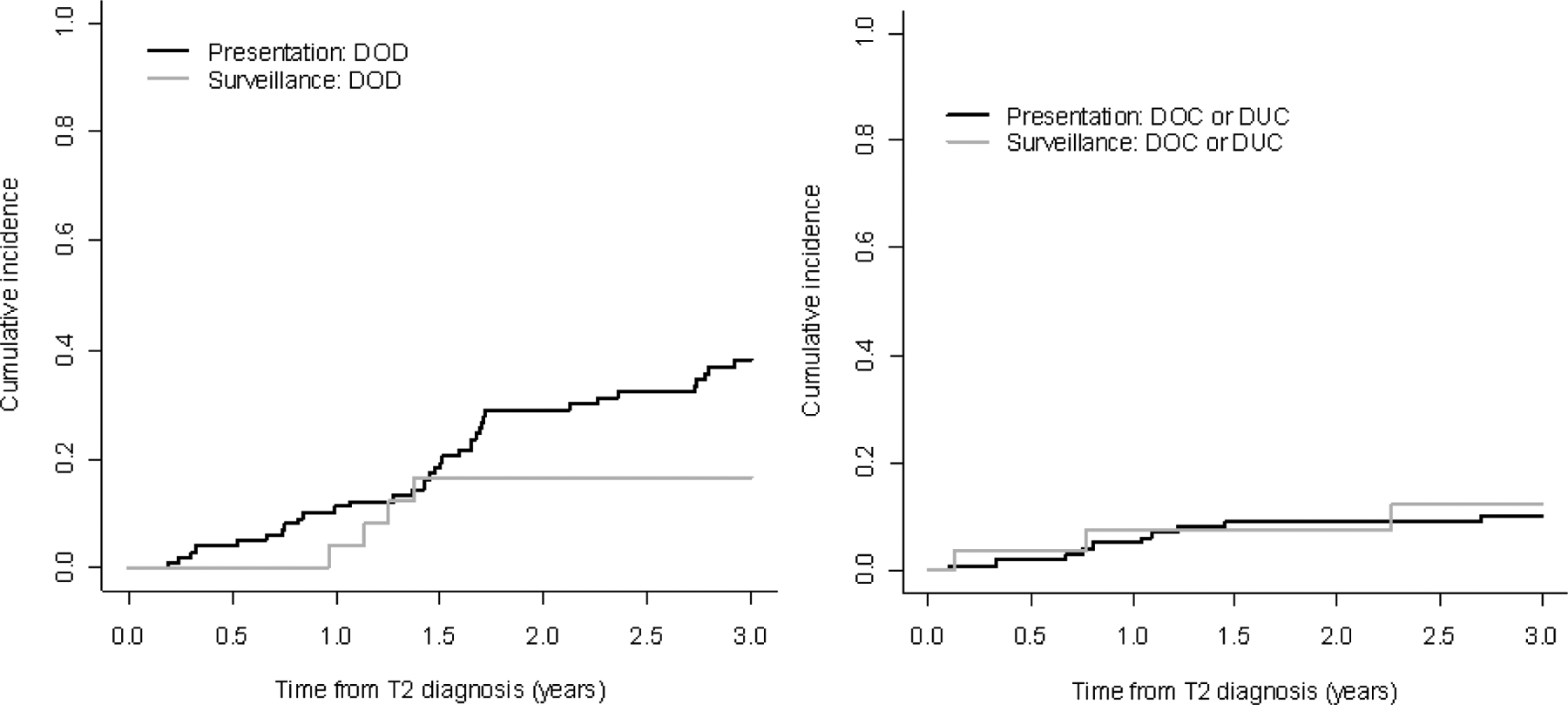
Bladder-cancer-specific death among patients who presented with T2 disease versus those who developed T2 disease on surveillance.
DOD = death from bladder cancer; DOC = death from other causes; DUC = death from unknown causes.
4. Discussion
Conservative management of T1 bladder cancer seeks to preserve the bladder while preventing death from progressive bladder cancer. It is a dynamic process that, to be successful, requires (1) careful selection of appropriate patients based on accurate initial pathologic staging and restaging with TUR; (2) identification of prognostic indicators, which include pathologic characteristics of the tumors, biologic markers, and the clinical response to BCG; and (3) adequate treatment, including intravesical BCG for those patients not undergoing immediate cystectomy and close surveillance with deferred cystectomy if high risk tumors recur or progress after BCG treatment. This retrospective review was a critical analysis of our clinical approach to patients with cT1 disease to better define clinical and pathologic factors that influence clinical decisions and their effects on patient outcome.
For better staging, we strongly advocate a restaging TUR in all patients with an initial diagnosis of bladder cancer. Collective data from this and several earlier studies provide compelling evidence that a restaging TUR decreases errors in staging and potentially affects further management of patients with non-muscle-invasive bladder cancer [9–16]. In our cohort, the concordance index of the new stage for overall survival was 0.71, indicating that the new stage provides diagnostic information to risk-stratify patients.
For the patients who had confirmed cT1 disease or lower on restaging TUR, we could not detect a difference in survival among patients who underwent immediate cystectomy versus those who were placed on surveillance with or without deferred cystectomy. This finding confirms earlier reports by Dinney et al, who showed no difference in survival and advocated conservative management, with deferred cystectomy for patients who progressed or became refractory to conservative management [17]. Similar results were observed in a study of 89 patients with first-time T1 tumors who received treatment at MSKCC. Fourteen patients underwent immediate cystectomy, and 75 were initially managed with TUR with or without intravesical BCG. The tumor characteristics of these two groups were not statistically different, although a higher proportion of the patients undergoing immediate RC had CIS. During follow-up (median: 31 mo for survivors), 34 of the conservatively managed patients underwent RC, all performed within 3 mo of the initial endoscopic resection. Nine patients died of bladder cancer. Disease-specific survival did not differ significantly between the patients undergoing immediate cystectomy and those managed conservatively [18]. In our cohort, The similar outcomes in patients on immediate cystectomy versus those on surveillance with deferred cystectomy suggest that the more aggressive tumors are successfully eliminated by performing an early intervention. In a retrospective nonrandomized study, Thalmann et al compared the outcome of patients with T1 disease treated with TUR and BCG (92 patients) versus immediate cystectomy (29 patients) [19]. The median follow-up in all patients was 6.9 yr. The overall and disease-specific survival at 5 yr was 69% and 80%, respectively, for patients on conservative management and 54% and 69%, respectively, for patients treated with immediate cystectomy. Twenty-nine percent of the patients on conservative management underwent a deferred cystectomy. These results suggest that patients undergoing an early cystectomy are more likely to be selected because of their intrinsic aggressive biology.
In our study, those with and without early cystectomy had no statistically significant differences in grade, presence of CIS, multifocal T1 disease, multifocality, recurrence rate, or prior BCG. The early cystectomy group, however, had a higher proportion of patients with cT1 disease on restaging TUR, which should be considered a surrogate for tumor load. These earlier cystectomies might have prevented a worse outcome. This point is illustrated in a report by Herr et al on their experience with a cohort of patients presenting with T1 bladder cancer on initial TUR [20]. Of the 352 patients with T1 tumors, 92 had residual T1 cancer on restaging. Eighty-two percent progressed to muscle invasion within 5 yr, compared to 19% (49 of 260) of those who had non-T1 tumors detected on restaging TUR. In our study, there was an imbalance between the two groups: 30% of the patients with cT1 tumors at restaging underwent immediate cystectomy versus 10% for the rest, which could explain the similar outcomes in patients who underwent immediate cystectomy versus those on surveillance with deferred cystectomy. A higher proportion of the patients with cT1 tumors at restaging, which are the more worrisome tumors, underwent an immediate cystectomy.
For surveillance to be successful, patients should undergo deferred cystectomy before progressing to muscle-invasive disease. We have shown that patients who were upstaged to cT2 did as poorly as those who developed muscle-invasive disease while on surveillance. These data confirmed previous reports showing no difference in survival between patients with progressive versus de novo muscle-invasive tumors [21,22]. Schrier et al, however, reported a 67% cancer-specific survival in the primary group versus 37% in the progressive group [23]. As expected, patients with pathologic T1 or lower had better survival than those with pathologic T2 or higher (data not shown). This makes a strong argument for performing an RC before the tumors progress to muscle-invasive disease. This is no easy task, and we have shown that despite diligent surveillance, extravesical disease is present in a clinically significant proportion of the surveillance group; however, we have shown that the presence of residual T1 disease at restage is associated with a higher incidence of deferred cystectomy.
We carefully performed several analyses to characterize the outcomes of patients with immediate or deferred cystectomy. First, we did not find a difference in survival for patients who underwent an immediate cystectomy versus those on surveillance (Fig. 2). Second, we did find a difference in survival among the patients still on surveillance versus those who underwent a deferred cystectomy. Third, we performed an analysis comparing survival following cystectomy for the patients who underwent an immediate cystectomy versus those who underwent a deferred cystectomy and found no significant difference (p = 0.3). Denzinger et al reported a 10-yr cancer-specific survival rate of 78% in high-risk T1G3 patients undergoing an immediate cystectomy versus 51% for a deferred cystectomy; it appears that all of the patients in this analysis eventually underwent a cystectomy. Hautmann et al reported a 10-yr cancer-specific survival of 78.7% for patients undergoing an early cystectomy versus 64.5% for those undergoing deferred cystectomy [24]. The upstaging for the patients with deferred cystectomy was 63.6%, which was significantly higher than our series (20% upstaging) and could explain the different results.
A limitation of this study is its retrospective nature and the heterogeneity of the patient population.
5. Conclusions
Conservative management of cT1 bladder cancer depends on careful selection and monitoring of patients. All patients being considered for conservative management should undergo restaging TUR to allow for better risk assessment. Patients with T1 disease on restaging are at high risk of progression and should be considered for early cystectomy.
Take-home message.
Conservative management of clinical T1 bladder cancer depends on careful selection and monitoring of patients. All patients being considered for conservative management should undergo restaging transurethral resection to allow for better risk assessment. Patients with T1 disease on restaging are at high risk of progression and should be considered for early cystectomy.
Financial disclosures:
I certify that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor:
The Sidney Kimmel Center for Prostate and Urologic Cancers supported this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Babjuk M, Oosterlinck W, Sylvester R, Kaasinen E, Böhle A, Palou-Redorta J; European Association of Urology (EAU). EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder. Eur Urol 2008;54:303–14. [DOI] [PubMed] [Google Scholar]
- [2].Kirkali Z, Chan T, Manoharan M, et al. Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology 2005;66:4–34. [DOI] [PubMed] [Google Scholar]
- [3].Millan-Rodriguez F, Chechile-Toniolo G, Salvador-Bayarri J, Palou J, Vicente-Rodríguez J. Multivariate analysis of the prognostic factors of primary superficial bladder cancer. J Urol 2000;163:73–8. [DOI] [PubMed] [Google Scholar]
- [4].Epstein JI, Amin MB, Reuter V. Bladder biopsy interpretation. Philadelphia, PA: Lippincott Williams & Wilkins; 2004. [Google Scholar]
- [5].Reading J, Hall RR, Parmar MK. The application of a prognostic factor analysis for Ta.T1 bladder cancer in routine urological practice. Br J Urol 1995;75:604–7. [DOI] [PubMed] [Google Scholar]
- [6].Epstein JI, Amin MB, Reuter VR, Mostofi FK. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am J Surg Pathol 1998;22:1435–48. [DOI] [PubMed] [Google Scholar]
- [7].Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988;16:1141–54. [Google Scholar]
- [8].Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- [9].Herr HW. The value of a second transurethral resection in evaluating patients with bladder tumors. J Urol 1999;162:74–6. [DOI] [PubMed] [Google Scholar]
- [10].Klan R, Loy V, Huland H. Residual tumor discovered in routine second transurethral resection in patients with stage T1 transitional cell carcinoma of the bladder. J Urol 1991;146:316–8. [DOI] [PubMed] [Google Scholar]
- [11].Schwaibold HE, Sivalingam S, May F, Hartung R. The value of a second transurethral resection for T1 bladder cancer. BJU Int 2006;97:1199–201. [DOI] [PubMed] [Google Scholar]
- [12].Brauers A, Buettner R, Jakse G. Second resection and prognosis of primary high risk superficial bladder cancer: is cystectomy often too early? J Urol 2001;165:808–10. [PubMed] [Google Scholar]
- [13].Ozen H, Ekici S, Uygur MC, Akbal C, Sahin A. Repeated transurethral resection and intravesical BCG for extensive superficial bladder tumors. J Endourol 2001;15:863–7. [DOI] [PubMed] [Google Scholar]
- [14].Schips L, Augustin H, Zigeuner RE, et al. Is repeated transurethral resection justified in patients with newly diagnosed superficial bladder cancer? Urology 2002;59:220–3. [DOI] [PubMed] [Google Scholar]
- [15].Rigaud J, Karam G, Braud G, Glemain P, Buzelin JM, Bouchot O. T1 bladder tumors: value of a second endoscopic resection [in French]. Prog Urol 2002;12:27–30. [PubMed] [Google Scholar]
- [16].Grimm MO, Steinhoff C, Simon X, Spiegelhalder P, Ackermann R, Vögeli TA. Effect of routine repeat transurethral resection for superficial bladder cancer: a long-term observational study. J Urol 2003;170:433–7. [DOI] [PubMed] [Google Scholar]
- [17].Dinney CP, Babkowski RC, Antelo M, et al. Relationship among cystectomy, microvessel density and prognosis in stage T1 transitional cell carcinoma of the bladder. J Urol 1998;160:1285–90. [PubMed] [Google Scholar]
- [18].Dalbagni G, Parekh DJ, Ben-Porat L, Potenzoni M, Herr HW, Reuter VE. Prospective evaluation of p53 as a prognostic marker in T1 transitional cell carcinoma of the bladder. BJU Int 2007;99:281–5. [DOI] [PubMed] [Google Scholar]
- [19].Thalmann GN, Markwalder R, Shahin O, Burkhard FC, Hochreiter WW, Studer UE. Primary T1G3 bladder cancer: organ preserving approach or immediate cystectomy? J Urol 2004;172:70–5. [DOI] [PubMed] [Google Scholar]
- [20].Herr HW, Donat SM, Dalbagni G. Can restaging transurethral resection of T1 bladder cancer select patients for immediate cystectomy? J Urol 2007;177:75–9; discussion 79. [DOI] [PubMed] [Google Scholar]
- [21].Ferreira U, Matheus WE, Nardi Pedro R, et al. Primary invasive versus progressive invasive transitional cell bladder cancer: multicentric study of overall survival rate. Urol Int 2007;79:200–3. [DOI] [PubMed] [Google Scholar]
- [22].Türkölmez K, Tokgöz H, Reşorlu B, Köse K, Bedük Y. Muscle-invasive bladder cancer: predictive factors and prognostic difference between primary and progressive tumors. Urology 2007;70:477–81. [DOI] [PubMed] [Google Scholar]
- [23].Schrier BP, Hollander MP, van Rhijn BW, Kiemeney LA, Witjes JA. Prognosis of muscle-invasive bladder cancer: difference between primary and progressive tumours and implications for therapy. Eur Urol 2004;45:292–6. [DOI] [PubMed] [Google Scholar]
- [24].Hautmann RE, Volkmer BG, Gust K. Quantification of the survival benefit of early versus deferred cystectomy in high-risk non-muscle invasive bladder cancer (T1 G3). World J Urol 2009;27:347–51. [DOI] [PubMed] [Google Scholar]


