Introduction
Over 120.000 new cases of bladder cancer are diagnosed each year in Europe, with a 5-year prevalence of around 410.000, consequently resulting in an important contribution to health costs1. About 75% of newly diagnosed bladder cancers are non-muscle-invasive (NMIBC), limited to the mucosa (Ta) or the lamina propria (T1).
Even though T1 lesions belong to the group of NMIBC, they are more likely than Ta lesions to progress to muscle invasive tumors, with a subsequent worsening of survival. Among high risk NMIBC, there is a sub-group of very high risk patients characterized by high recurrence and progression rates despite therapy2,3. EORTC and CUETO risk tables are useful to calculate the probability of recurrence and progression4,5, however they have limitations when it comes to predicting the biological behavior of the tumor in order to decide when radical cystectomy is mandatory6. Currently, treatment strategies of NMIBC are essentially based on transurethral resection (TUR) of the bladder and adjuvant intravesical instillations which take into account a patient’s risk of recurrence and progression3.
High rates of residual disease after the first TUR have been reported, ranging from 22% to 74% in Ta disease and from 26.5% to 81.5% in T1 disease7. In order to minimize the risk of residual disease, European Association of Urology (EAU) Guidelines recommend repeat TUR (re-TUR) within 4–6 weeks when lamina propria involvement or high-grade disease is diagnosed at the initial TUR3.
According to the literature, patients with residual disease at re-TUR are considered to have a higher risk of progression and cancer-specific mortality (CSM) compared to those patients without residual disease8. In some series, the risk of progression to muscle invasive or persistent T1 at re-TUR reached 76%9. Since progression is associated with a poor prognosis, radical cystectomy is strongly recommended in patients with residual T1 at re-TUR10. Nevertheless early cystectomy, advocated by some authors as an alternative to BCG, has shown long term survival rates not exceeding 80%11, meaning that it does not guarantee a cure of T1G3 tumors. Furthermore, radical cystectomy (RC) is a complex surgical procedure, affected by significant morbidity rates12. This aspect should be carefully considered in patients with a poor performance status, in which RC is associated with poorer outcomes and a higher incidence of complications13. Patient selection plays a crucial role to correctly balance tumor prognosis and surgical risks. From this perspective, oncological status at re-TUR should be carefully evaluated in order to predict the real risk of progression and consequently to select the best candidates for surgery.
In the current study, we retrospectively analyzed the risk of recurrence, progression and CSM according to the tumor stage at re-TUR in a large cohort of T1G3 patients treated with BCG.
Materials and methods
Design and setting
The study design and patient selection criteria have been already reported by Gontero et al14. Briefly, patients with primary T1G3 (WHO 1973) / T1 high grade (HG) (ISUP 1998/WHO 2004) tumors or secondary T1G3/HG disease in a previously BCG naïve non T1G3/HG NMIBC tumor formed this retrospective study cohort provided they received at least a full induction course of BCG between 1990 and 2011.
The following patient and tumor characteristics were collected: age, gender, smoking history and intensity, exposure to chemical compounds, tumor status (primary or recurrent), previous intravesical chemotherapy, tumor size (< versus ≥ 3 cm), tumor focality (solitary versus multiple), presence of concomitant CIS, prostatic urethra involvement with or without stromal invasion, presence of muscle in the primary tissue specimen, BCG dose and total number of instillations. Information on re-TUR (defined as a second TUR performed within 4–6 weeks after the initial TUR and before BCG administration) was also recorded. Results of pathology at re-TUR were categorized as: no evidence of disease, persistent disease with lower stage (Ta), or persistent T1 disease. Patients with muscle invasive disease at re-TUR did not match the study inclusion criteria and were therefore excluded upfront.
Statistical analysis
Times to events were calculated taking the date of starting BCG as time zero. To take into account patients who died before observing the event of interest (competing risk), times to recurrence, progression and death due to bladder cancer according to residual tumor characteristics at restaging TUR were estimated using cumulative incidence functions and compared using the Cox proportional hazards regression model. Patients without an event or death prior to the event were censored at the last date of follow-up. Percentages were compared using a chi-square test.
Results
A re-TUR was performed in 935 (41.1%) of the 2451 eligible patients. Baseline and disease characteristics of patients who underwent re-TUR are reported in Table 1. Eight hundred and forty-eight patients (90.7%) had a primary T1G3 tumor; concomitant CIS was diagnosed in 241 patients (25.8%). In 624 patients (66.8%) there was muscle in the first TUR specimen. A history of multiple and large (>3cm) tumors was present in 687 (73.5%) and 191 (20.4%) patients, respectively. Persistent disease at re-TUR was documented in 667 patients (71.3%), Ta in 378 (40.4%) and T1 in 289 (30.9%) of the cases (Table 2). 624 patients (66.8%) received only induction BCG. The median follow-up was 5.2 years.
Table 1.
Baseline patient characteristics in 935 patients under going re-TUR
| Age, years | |
| <70, n (%) | 549 (58.7) |
| ≥70, n (%) | 386 (41.3) |
| Median (interquartile range) | 67 (59–74) |
| Sex | |
| Male | 756 (80.9) |
| Female | 179 (19.1) |
| Tumour status | |
| Primary T1G3 | 848 (90.7) |
| Recurrent after non T1G3 | 87 (9.3) |
| Previous intravesical chemotherapy | |
| No | 895 (95.8) |
| Yes | 40 (4.2) |
| Muscle in primary TUR specimen | |
| No | 276 (29.5) |
| Yes | 624 (66.8) |
| Missing/unknown | 35 (3.7) |
| Tumour grade | |
| WHO 1973 G3 | 442 (47.2) |
| WHO 2004 HG | 799 (85.4) |
| G3 and/or HG | 935 (100) |
| Tumour focality | |
| Solitary | 225 (24.1) |
| Multiple | 687 (73.5) |
| Missing/unknown | 23 (2.4) |
| Largest tumour diameter (cm) | |
| <3 | 257 (27.5) |
| ≥3 | 191 (20.4) |
| Missing/unknown | 487 (51.2) |
| Concomitant CIS | |
| No | 694 (74.2) |
| Yes | 241 (25.8) |
| Invasion of prostatic urethra | |
| No | 401 (42.9) |
| Yes, without stromal invasion | 18 (1.9) |
| Yes, with stromal invasion | 3 (0.3) |
| Missing/unknown | 513 (54.9) |
| Pathology at re-staging TUR* | |
| No residual tumour | 267 (28.6) |
| Ta | 378 (40.4) |
| T1 | 289 (30.9) |
| CIS | NA* |
| Missing/unknown | 1 (0.1) |
| Maintenance BCG | |
| No | 624 (66.8) |
| Yes | 311 (33.2) |
Separate information on CIS at re-TUR was not available
Table 2.
Pathology at re-TUR according to the presence or absence of muscle in the specimen of first TUR.
| Variable | No Muscle at first TUR, N (%) | Muscle at first TUR, N (%) | Muscle at first TUR unknown, N (%) | All patients |
|---|---|---|---|---|
| Patients | 276 | 624 | 35 | 935 |
| Pathology at re-TUR* | ||||
| No residual tumour | 39 (14.1) | 217 (34.8) | 11 (31.4) | 267 (28.6) |
| Ta | 126 (45.7) | 240 (38.5) | 12 (34.3) | 378 (40.4) |
| T1 | 111 (40.2) | 166 (26.6) | 12 (34.3) | 289 (30.9) |
| CIS | NA** | NA** | NA** | NA** |
| Missing/unknown | 0 | 1 (.02) | 0 | 1 (0.1) |
Separate information on CIS at re-TUR was not available.
Table 2 presents the pathology at re-TUR according to the presence or absence of muscle at first TUR. In the absence of muscle at first TUR, persistent disease at re-TUR was documented in 237 (85.9%) of 276 cases as compared to 406 (65.1%) of the 624 cases with muscle at the first TUR, p < 0.001. Likewise, the rate of persistent T1 disease was higher when no muscle was reported in the first TUR (40.2%) as compared with that of a primary TUR with muscle in the specimen (26.6%), p < 0.001.
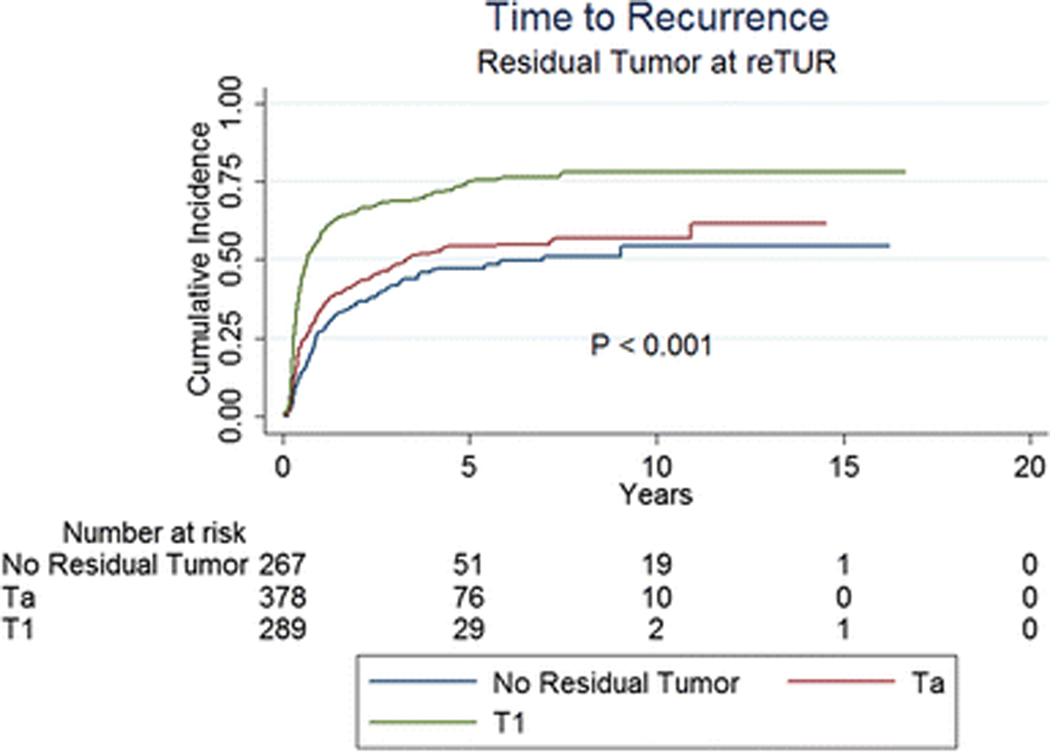
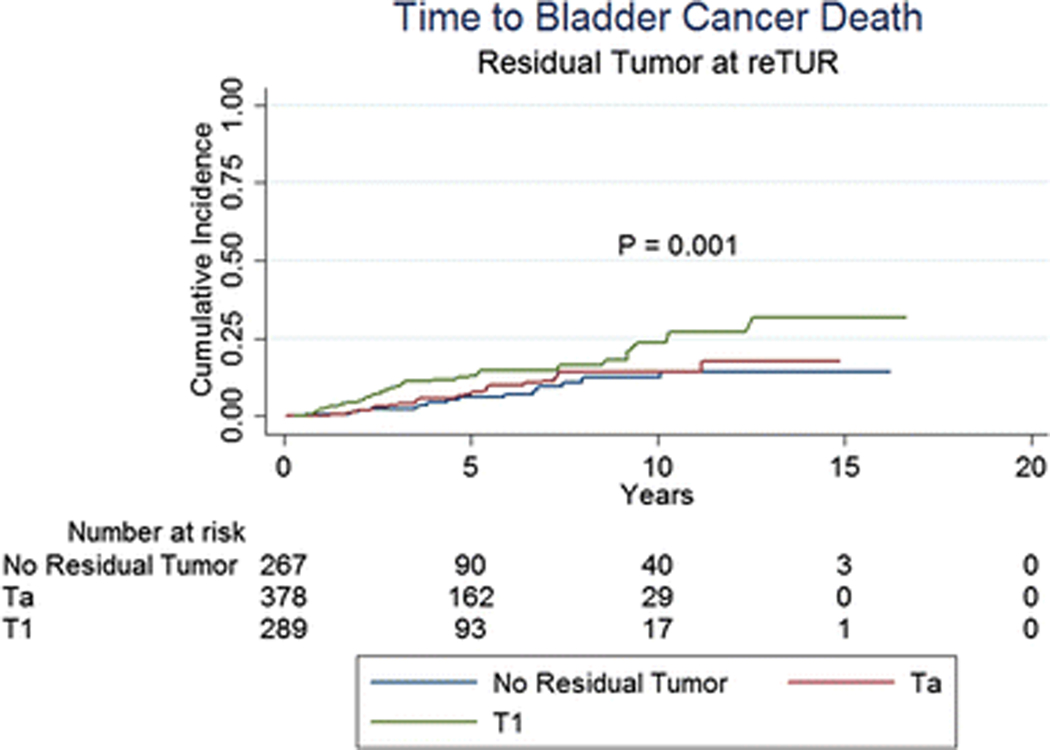
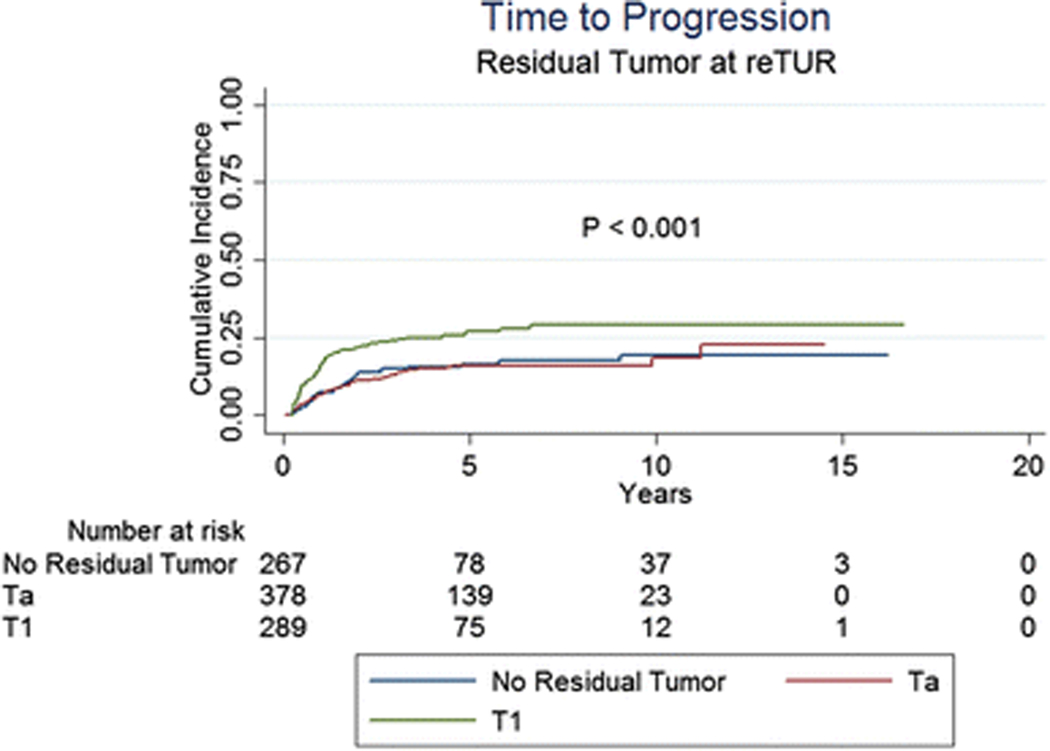
Table 3 and Figures 1 to 3 present the effect of the pathology at re-TUR on event rates and time to event for recurrence, progression and CSM, respectively. Overall, 512 (54.8%) of 934 patients with known pathology recurred and 166 (17.8%) progressed to muscle invasive disease. Patients with T1 tumours were more likely to recur compared to patients with Ta tumours and patients without residual disease (71.6%, 51.1% and 41.9%, respectively) and had a shorter time to recurrence (Figure 1, p < 0.001). Progression rates according to the pathology at re-TUR were higher in T1 patients (25.3% in T1 patients, 14.6% in Ta patients and 14.2% in case of no residual tumour) and the time to progression was shorter (Figure 2, p < 0.001). Similar trends were seen in patients with and in patients without muscle in the original TUR specimen. Overall, 202 patients (21.6%) died, 85 (9.1%) due to bladder cancer. CSM was higher in T1 patients compared to Ta patients and patients without residual disease (13.1%, 8.2% and 6.0%, respectively) and T1 patients had a shorter time to death due to bladder cancer (Figure 3, p = 0.001).
Table 3.
Clinical outcomes according to pathology at re-TUR in 934 patients.
| Residual tumour at re-TUR | Recurrence N (%) | Progression N (%) | CSM N (%) |
| No residual tumour (267 patients) | 112 (41.9) | 38 (14.2) | 16 ( 6.0) |
| Ta tumour (378 patients) | 193 (51.1) | 55 (14.6) | 31 ( 8.2) |
| T1 tumour (289 patients) | 207 (71.6) | 73 (25.3) | 38 (13.1) |
| Total (934 patients) | 512 (54.8) | 166 (17.8) | 85 (9.1) |
| P value | P < 0.001 | P < 0.001 | P = 0.01 |
Fig. 1.
Time to recurrence according to the presence or absence of residual disease at re-TUR and the pathology at re-TUR
Fig. 3.
Time to bladder cancer death according to the presence or absence of residual disease at re-TUR and the pathology at re-TUR
Fig. 2.
Time to progression according to the presence or absence of residual disease at re-TUR and the pathology at re-TUR
For all three endpoints of time to recurrence, progression and CSM, pathology at re-TUR was the most important prognostic factor in a multivariate model, which also included tumour multiplicity, the presence or absence of concomitant CIS and whether or not the patient received maintenance BCG.
Discussion
In accordance with the available literature 9,10,15,16, we found that patients with T1 residual disease at re-TUR had higher recurrence and progression rates and shorter times to recurrence and progression compared to patients with no residual disease or with Ta tumours. The presence of residual disease at re-TUR was also associated with a higher CSM and a shorter time to death due to bladder cancer, especially for patients with persistent T1 disease. Subgroup analyses revealed similar outcomes regardless of the presence or absence of muscle in the primary TUR specimen.
The role of pathology at re-TUR as a prognostic factor has been supported by accumulating evidence in high-risk NMIBC, mainly in the worst group of T1G3 lesions. The presence of a T1 residual tumour in the re-TUR specimen has especially been advocated as a strong indication for radical treatment. Herr et al evaluated clinical outcomes according to the pathology at re-TUR in 710 patients9. The progression rate for patients with residual T1 disease was 76%, while in case of no residual tumour or tumour < T1, the risk of disease progression decreased to 14%. One year later, the same group reported a 82% of progression rate in patients with residual T1 disease at re-TUR, with a median survival of 15 months10. Due to this dramatic worsening of prognosis, the authors suggested to completely abandon conservative treatment and do an immediate cystectomy in case of T1 disease at re-TUR. More recently, Bishr et al reached similar conclusions8. In their population of 94 NMIBC patients, the risk of progression was significantly higher in the presence of residual disease at re-TUR. Moreover, the pathological stage at re-TUR was an independent predictor of recurrence-free and progression-free survival in multivariate analysis.
Even if our results have confirmed the increased risk of progression to muscle invasive disease in patients with residual T1 disease, we could not confirm an increased risk of progression in patients with residual Ta disease. Moreover, in patients who were T1 at re-TUR, we found a progression rate of 25.3% as compared to 14% in both patients with no residual tumour and in patients with residual Ta tumours. Similar progression rates were recently obtained by Gordon et al. In their retrospective cohort of 932 patients with high risk NMIBC, those with T1 disease at re-resection had a risk of progression of 22%17. Since these progression rates are not as high as previously reported in the other series, conservative management may still be considered with a very close follow up in these high-risk patients, deferring radical cystectomy except in case of a high recurrence rate or progression to muscle invasive disease.
In the urological community, the timing and patient selection for radical cystectomy in high-risk NMIBC remains an open debate. Dalbagni et al failed to find a significant difference in survival between patients who underwent immediate radical cystectomy and patients treated with delayed surgery, although the stage on restaging TUR was significantly associated with the decision to perform a deferred cystectomy15. These findings suggest that the pathology at re-TUR alone is probably not enough to select the group of patients who could really benefit from an early radical treatment. According to Gontero et al., 79% of T1G3 patients did not progress with up to 10 years of follow up and cystectomy appeared to be an overtreatment in at least 70% of these high-risk patients18. Regardless of the selection criteria, even if cystectomy is given to all high risk NMIBC, the chances of cure are not 100%19,20. The goal of delaying cystectomy is to better balance the risk of dying of disease and the risk of overtreatment with subsequent morbidity. Radical cystectomy still represents an invasive treatment with quite high complication rates, especially in elderly patients since age and pre-operative comorbidities are traditionally considered to be determinant factors for surgical outcome21.
The main clinical implication of our findings is that a T1 tumour at re-TUR should not be considered alone as an absolute contraindication to a bladder sparing approach, as indicated by Herr et al., who has suggested to completely abandon conservative treatment and to do an immediate cystectomy in case of T1 disease at re-TUR.
Some limitations of this study are to be acknowledged. First and foremost, this is a retrospective analysis. Consequently, the accuracy in reporting prognostic factors and tumour characteristics suffered from missing data for some variables, for example tumour size, and a lack of standardized assessment. Moreover, there was no central pathology review. Secondly, our series has been derived from a large retrospective T1G3 population14 in which re-TUR was not determined by randomisation. Our re TUR population mainly presented multiple and large tumours at first resection; moreover an incomplete first TUR may have affected the decision to perform a re TUR. All these aspects make the re TUR population a selected one17 and could explain the high rate of residual disease we found in our patients. By the way, rates of residual disease up to 60% were recently showed in a retrospective series of high risk NMIBC17. Moreover, a recent review on re TUR confirmed rates of residual disease ranging from 20% to 71% for T1 tumours22. Lastly, patients diagnosed with muscle-invasive disease at re-TUR were excluded by our study design and consequently no data about their prognosis are available.
Further studies are needed to individualize the clinical, pathological and molecular markers that are able to accurately select the best candidates for an immediate radical cystectomy.
Conclusion
In patients with T1G3 tumours who are treated with BCG, those with no residual disease or Ta tumour at re-TUR have better recurrence, progression and CSM rates than those with T1 tumour. Furthermore, a progression rate of 25.3% in case of T1 tumour at re-TUR is far lower than previously reported and does not a priori exclude the conservative management of these patients.
Acknowledgements
The authors declare that the development of the manuscript was not supported by an honorarium, a grant, or any other sources of support, including sponsorship or any material sources of support.
Informed consent
For this type of study, informed consent is not required.
Research involving human participants and/or animals
The study has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Footnotes
Compliance with ethical standards
Conflict of interest
None.
References
- 1.Svatek RS, Hollenbeck BK, Holmäng S, et al. The economics of bladder cancer: costs and considerations of caring for this disease. Eur Urol. 2014;66(2):253–262. doi: 10.1016/j.eururo.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Burger M, Catto JWF, Dalbagni G, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63(2):234–241. doi: 10.1016/j.eururo.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 3.Babjuk M, Böhle A, Burger M, et al. EAU Guidelines on Non–Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur Urol. 2017;71(3):447–461. doi: 10.1016/j.eururo.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 4.Sylvester RJ, Van Der Meijden APM, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: A combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49(3):466–475. doi: 10.1016/j.eururo.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez-Gomez J, Madero R, Solsona E, et al. Predicting Nonmuscle Invasive Bladder Cancer Recurrence and Progression in Patients Treated With Bacillus Calmette-Guerin: The CUETO Scoring Model. J Urol 2009;182(5):2195–203 doi: 10.1016/j.juro.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 6.Xylinas E, Kent M, Kluth L, et al. Accuracy of the EORTC risk tables and of the CUETO scoring model to predict outcomes in non-muscle-invasive urothelial carcinoma of the bladder. Br J Cancer 2013;109(6):1460–6.doi: 10.1038/bjc.2013.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramirez-Backhaus M, Dominguez-Escrig J, Collado A, Rubio-Briones J, Solsona E. Restaging transurethral resection of bladder tumor for high-risk stage Ta and T1 bladder cancer. Curr Urol Rep. 2012;13(2):109–114. doi: 10.1007/s11934-012-0234-4. [DOI] [PubMed] [Google Scholar]
- 8.Bishr M, Lattouf JB, Latour M, Saad F. Tumour stage on re-staging transurethral resection predicts recurrence and progression-free survival of patients with high-risk non-muscle invasive bladder cancer. J Can Urol Assoc. 2014;8(5–6):6–10. doi: 10.5489/cuaj.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herr HW, Donat SM. A re-staging transurethral resection predicts early progression of superficial bladder cancer. BJU Int. 2006;97(6):1194–1198. doi: 10.1111/j.1464-410X.2006.06145.x. [DOI] [PubMed] [Google Scholar]
- 10.Herr HW, Donat SM, Dalbagni G. Can Restaging Transurethral Resection of T1 Bladder Cancer Select Patients for Immediate Cystectomy? J Urol 2007;177(1):75–9; doi: 10.1016/j.juro.2007.08.070. [DOI] [PubMed] [Google Scholar]
- 11.Hautmann RE, Volkmer BG, Gust K. QuantiWcation of the survival beneWt of early versus deferred cystectomy in high-risk non-muscle invasive bladder cancer (T1 G3). World J Urol. 2009;27:347–351. doi: 10.1007/s00345-009-0402-4. [DOI] [PubMed] [Google Scholar]
- 12.Roghmann F, Trinh Q-D, Braun K, et al. Standardized assessment of complications in a contemporary series of European patients undergoing radical cystectomy. Int J Urol. 2014;21(2):143–149. doi: 10.1111/iju.12232. [DOI] [PubMed] [Google Scholar]
- 13.Nayak JG, Gore JL, Holt SK, Wright JL, Mossanen M, Dash A. Patient-centered risk stratification of disposition outcomes following radical cystectomy. Urol Oncol Semin Orig Investig. 2016;34(5):235.e17–235.e23. doi: 10.1016/j.urolonc.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Gontero P, Sylvester R, Pisano F, et al. The impact of re-transurethral resection on clinical outcomes in a large multicentre cohort of patients with T1 high-grade/Grade 3 bladder cancer treated with bacille Calmette-Guérin. BJU Int 2016;118(1):44–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalbagni G, Vora K, Kaag M, et al. Platinum Priority – Bladder Cancer Clinical Outcome in a Contemporary Series of Restaged Patients with Clinical T1 Bladder Cancer. Eur Urol 2009;56(6):903–10. doi: 10.1016/j.eururo.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herr HW. Role of re-resection in non-muscle-invasive bladder cancer. ScientificWorldJournal. 2011;11:283–288. doi: 10.1100/tsw.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordon P, Thomas PC, Noon AP et al. Long-term outcomes from re-resection for high risk non muscle invasive bladder cancer: a potential to rationalize use. Eur Urol Focus 2017, pii: S2405–4569(17)30241–9. [DOI] [PubMed] [Google Scholar]
- 18.Gontero P, Sylvester R, Pisano F, et al. Platinum Priority – Urothelial Cancer Prognostic Factors and Risk Groups in T1G3 Non–Muscle-invasive Bladder Cancer Patients Initially Treated with Bacillus Calmette-Gué rin: Results of a Retrospective Multicenter Study of 2451 Patients. Eur Urol. 2015;7(7):4–8. doi: 10.1016/j.eururo.2014.06.040. [DOI] [PubMed] [Google Scholar]
- 19.Denzinger S, Fritsche H-M, Otto W, Blana A, Wieland W- F, Burger M. Early Versus Deferred Cystectomy for Initial High-Risk pT1G3 Urothelial Carcinoma of the Bladder: Do Risk Factors Define Feasibility of Bladder-Sparing Approach? Eur Urol. 2008;53(1):146–152. doi: 10.1016/j.eururo.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 20.Hautmann RE, Volkmer BG, Gust K. Quantification of the survival benefit of early versus deferred cystectomy in high-risk non-muscle invasive bladder cancer (T1 G3). World J Urol. 2009;27(3):347–351. doi: 10.1007/s00345-009-0402-4. [DOI] [PubMed] [Google Scholar]
- 21.Novotny V, Zastrow S, Koch R, Wirth MP. Radical cystectomy in patients over 70 years of age: impact of comorbidity on perioperative morbidity and mortality. WJUrol 2012;30(6):769–76.doi: 10.1007/s00345-011-0782-0. [DOI] [PubMed] [Google Scholar]
- 22.Cumberbatch MGK, Foerster Catto JWF. Repeat Transurethral Resection in Non–muscle-invasive Bladder Cancer: A Systematic Review. Eur Urol 2018, article in press. [DOI] [PubMed] [Google Scholar]