Abstract
Context:
The role of surgery in metastatic bladder cancer (BCa) is unclear.
Objective:
In this collaborative review article, we reviewed the contemporary literature on the surgical management of metastatic BCa and factors associated with outcomes to support the development of clinical guidelines as well as informed clinical decision-making.
Evidence acquisition:
A systematic search of English language literature using PubMed-Medline and Scopus from 1999 to 2016 was performed.
Evidence synthesis:
The beneficial role of consolidation surgery in metastatic BCa is still unproven. In patients with clinically evident lymph node metastasis, data suggest a survival advantage for patients undergoing postchemotherapy radical cystectomy with lymphadenectomy, especially in those with measurable response to chemotherapy (CHT). Intraoperatively identified enlarged pelvic lymph nodes should be removed. Anecdotal reports of resection of pulmonary metastasis as part of multimodal approach suggest possible improved survival in well-selected patients. Cytoreductive radical cystectomy as local treatment has also been explored in patients with metastatic disease, although its benefits remain to be assessed.
Conclusions:
Consolidative extirpative surgery may be considered in patients with clinically evident pelvic or retroperitoneal lymph nodal metastases but only if they have had a response to CHT. Surgery for limited pulmonary metastases mayalso be considered in veryselected cases. Best candidates are those with resectable disease who demonstrate measurable response to CHT with good performance status. In the absence of data from prospective randomized studies, each patient should be evaluated on an individual basis and decisions made together with the patient and multidisciplinary teams.
Patient summary:
Surgical resection of metastases is technically feasible and can be safely performed. It may help improve cancer control and eventually survival in very selected patients with limited metastatic burden. In a patient who is motivated to receive chemotherapy and to undergo extirpative surgical intervention, surgery should be discussed with the patient among other consolidation therapies in the setting of multidisciplinary teams.
Keywords: Bladder cancer, Metastatic bladder cancer, Lymph node metastasis, Surgery, Metastasectomy, Lymph node dissection
1. Introduction
In Western countries, muscle-invasive disease accounts for about one-fourth of newly diagnosed urothelial bladder cancer (BCa) cases and approximately 10–30% of nonmuscle invasive BCa that have progressed. Nearly half of patients with muscle-invasive BCa will relapse despite intensive therapies, eventually succumbing to their disease [1–4]. Approximately, three-fourths of these patients relapse with distant failure, with the remaining one-fourth experiencing local recurrence [4,5]. In addition, somewhere between 5% and 15% of patients present with unresectable or metastatic disease at time of diagnosis [6]. When possible, for all patients with primary or secondary metastatic cancer, systemic platinum-based combination chemotherapy (CHT) is the standard treatment [2] resulting in initial response rates of 40–70%, but long-term survival of less than 15% within 5 yr [7,8]. In addition to the unfavorable response to systemic CHT, nearly half of patients are already unfit for this regimen due to renal and other comorbid conditions.
Surgical extirpation of the primary or metastases is part of a multimodal approach in various malignancies yielding potentially better survival and/or quality of life. This concept is increasingly being considered in urology from accepted entities such as testis and kidney cancers to more recently, prostate cancer [5,9]. Nevertheless, the role of surgery in metastatic urothelial carcinoma (UC) is not yet established with most of the experience being accrued from retrospective uncontrolled studies [2]. No pertinent prospective randomized trials have been published on this topic. Therefore, there is a need to better delineate the evidence-based potential oncological benefit of surgical extirpation of the primary in metastatic setting and of metastasectomy.
To address this need, we performed a systematic review of the role of surgery in patients with clinically node-positive BCa, distant metastasectomy, as well as cytoreductive radical cystectomy (RC).
2. Evidence acquisition
This systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses protocols [10]. A systematic literature search of the PubMed-Medline and Scopus databases was performed on November 2016, including literature from 1999 through 2016. We included English language articles only. The search strategy included broad terms in isolation or in combination: “metastatic bladder cancer,” metastatic“ urothelial carcinoma,” locally“ advanced bladder cancer,” “lymph node positive bladder cancer,” clinically“ node positive,”“radical cystectomy,”“cytoreductive radical cystectomy," “metastasectomy,” and “aborted radical cystectomy.”
Relevant articles on surgical management of metastatic BCa were selected. Articles were considered relevant when they included urothelial BCa patients diagnosed with locally advanced disease or clinically evident lymph node metastasis or pulmonary metastasis who underwent RC with or without pelvic or retroperitoneal lymph node dissection or metastasectomy with intention to treat. Review articles, editorials, case reports, comments, and meeting abstracts were excluded. Articles pertinent to the upper tract but not the lower tract were also excluded. If more than one report of the same study population existed, we selected the most recent one for qualitative evidence synthesis. Additional relevant articles were selected from authors’ bibliographies. All studies of interest were obtained as full text articles. European Association of Urology Guidelines 2016 were also reviewed. Study eligibility was determined by two authors (S.F.S and M.A).
We used the MetaProp program (MetaProp NYC, New York, NY, USA) which incorporates the arcsine transformation of proportions in STATA 14.2 (Stata Corp., College Station, TX, USA) to pool the available clinical complete and partial response rates as well as the complete pathological response rates with computation of 95% confidence intervals.
3. Evidence synthesis
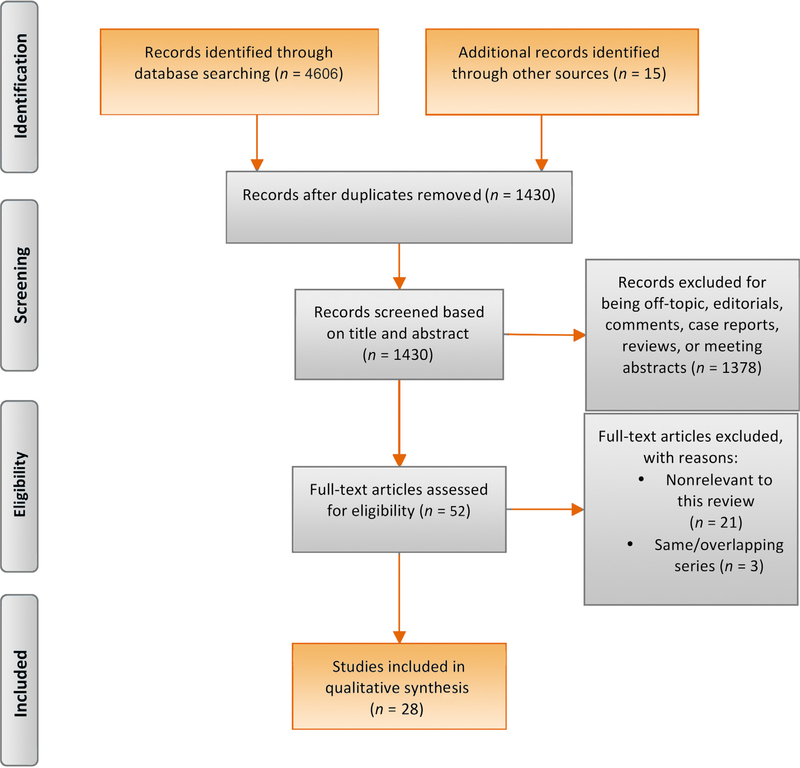
A total of 1430 unique articles were identified, of which 28 were selected and critically analyzed for evidence synthesis based on the Preferred Reporting Items for Systematic Reviews and Meta-analyses protocols (Fig. 1) [11–38].
Fig. 1. Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram detailing the search strategy and identification of studies used in evidence synthesis.
3.1. Surgery in patients with lymph node metastasis
3.1.1. Theroleof surgeryinpatientswithclinicallypositivelymph nodes
Patients with clinically positive lymph node (LN) disease are generally considered for induction systemic platinum-based CHT [39–41]. In the absence of visceral metastasis and despite CHT, the reported 5-yr overall (OS) rate was less than 20% [11]. Several groups evaluated RC and pelvic LN dissection (PLND) as a consolidative intervention in patients who experienced complete or significant response to induction systemic CHT [11–19] (Table 1).
Table 1 –
Studies reporting the outcomes of patients with clinically positive lymph node bladder cancer who underwent radical cystectomy and pelvic lymph node dissection
| Reports | Patients (n) | Study population | Chemotherapy |
Surgery | Pathologic response rate | Survival |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Regimen (%) | Median no. of cycles | Clinical response rate | Median follow up (mo) | Rate (%) | Median survival time (mo) | Other findings | |||||
| Herr et al [15] 2001 | 80 | Unresectable or regionally metastatic BCa | MVAC: 75% Others: 25% |
4 | cCR: 30% cPR: 55% |
RC, PLND with or without RPLND | pCR: 62.5%a pCR: 15.9%b |
60 (minimum) | 5-yr OS: 42% | NR | Complete response to CHT and surgery: 41% |
| Nieuwenhuijzen et al.[12] 2005 | 52 | Histologically proven pN+ by aspiration (40%) or by PLND (60%) | MVAC: 59% HD-MVAC: 41% |
4 | cCR: 29% cPR: 57% |
RC with PLND: 19 (36.5%) Only RC 21 (40.4%) | pCR: 73%a
pCR: 14%b |
68 | Overall 5-yr CSS: 23% 5-yr CSS in cCR: 42% 5-yr CSS in cPR: 19% In nonrespondent: 0% |
15.4 | cCR (HR 8) and post CHT cT any, cN0 (HR 2.8) were independently associated with better CSS |
| Kassouf et al [16] 2009 | 37 | pT any, pN+ pts who received preoperative CHT. 18 (48.6%) pts were cN+. | Platinum-based: 30 81% Other treatments: 7 23.3% |
5 | NR | RC with PLND | All pN+ Only one pT0 | 50 | 2-yr OS: 20% 2-yr DSS: 29.2% 2-yr RFS: 13.5% |
OS: 13 DSS: 14.6 RFS: 6 |
Variant histology was associated with shorter OS (p = 0.01) and RFS (p = 0.036) Female sex was associated with OS (HR 0.25, p = 0.006) |
| Ghadjar et al [17] 2011 | 30 | T4cN0cM0: 20% ≥cT4cN+cM0: 70% ≥cT4cN0cM+: 10% | GC: 64% MVAC: 3% Others: 33% |
4 | In cN+ patients cCR: 76% cPR: 5% |
RC with PLND | pCR (pT0: 30%) pCR (pN0:50%) |
28 | 5-yr DFS: 42% 5-yr OS: 46% For patients with pT0: 5-yr DFS: 83% 5-yr OS: 71% |
NR | pT0 was significantly associated with both increased DFS (HR 0.08) and increased OS (HR 0.21) |
| Meijer et al[13] 2014 | 149 | cN+M0: 78% cNx, M1: 22% |
MVAC: 21% HD-MVAC: 40% GC: 13% Others: 26% |
4 | cCR: 34.9% cPR: 47.7% SD: 12.1% PD: 5.4% |
RC with PLND: 79% EBRT: 9% No local treatment: 12% |
pCR: 26.8% pPR: 28.2% pSD: 23.5% |
57 | Overall 5-yr CSS: 29.2% 5-yr CSS in pCR: 63.5% 5-yr CSS in cCR: 43.3% |
CSS for total group: 20 CSS in pCR: 127 CSS in cCR: 36 |
In patients with cCR, residual disease was present in 38.5% pPR (HR 2.31) and pSD (HR 4.02) were independently associated with CSS |
| Urakami et al [11] 2015 | 60 | cN+ BCa: 31 cN+ UTUC: 29 |
GC: 57% Others: 43% |
4 | cCR: 24% cPR: 57% SD: 20% PD 8% |
Consolidative surgery in 85% | pCR: 14% | 22.2 | 5-yr PFS: 39% OS: 42% |
NR | pN0 (HR 6.8), absence of lymphovascular invasion (HR 3.3), negative surgical margins (HR 5.1) and more LNs removed (HR 3.0) were independent postsurgical prognostic factors for OS in the surgical group |
| Ho et al [18] 2016 | 55 | cN+: 53% cM1: 47% |
Cisplatin-based: 92.7% Noncisplatin-based: 7.3% |
5 | cCR: 38.2% cPR: 45.5% SD: 9.1% PD: 1.8% |
RC, PLND, with or without RPLND | pT0:22% pN0: 55% |
58.7 | Overall 5-yr CSS: 40.4% 5-yr CSS rate for pN0: 66% 5-yr CSS rate for pN+: 12% 5-yr RFS: 39% |
CSS: 25.7 (all patients) CSS: 35.5 (cN+) CSS: 22.6 (cM1) |
No differences in response rates after CHT in cN1 and cN2 No difference between cN+ and cM1 in terms of OS Perioperative mortality: 1.8% |
| Zargar-Shoshtari et al [14] 2016 | 304 | cN+ | MVAC: 42% GC: 43% Others: 15% |
4 | NR | RC with PLND | pCR: 14.5% pPR: 27% CNR: 48% |
13 | 50% of patients died during follow up 44% died of BCa | OS: 23 OS for MVAC: 20 OS for GC: 24 OS for other regimens: 19 |
Positive surgical margins (HR 2.96), ≥2 positive LNs (HR 3.26) and number of nodes removed (HR 0.55) were independently associated with OS |
| Galsky et al[19] 2016 | 1104 | cTanyN1–3M0 | Preoperative CHT: 32% Adjuvant CHT: 30% |
NR | NR | RC alone: 37% CHT + RC: 32% RC + CHT: 30% |
cPR (pT0pN 0): 9% CNR (pN0: 37% |
16.6 | 5-yr OS in RC alone: 19% 5-yr OS CHT + RC: 31% 5-yr OS RC + CHT: 26% |
NR | Preoperative CHT (HR 0.8) and postoperative CHT (HR 0.68) were associated with a significant improvement in OS compared to RC alone |
BCa = bladder cancer; cCR = clinical complete response; CHT = chemotherapy; CI = confidence interval; CNR = complete nodal response; CSS = cancer-specific survival; DFS = disease free survival; EBRT = external beam radiotherapy; GC = gemcitabine and cisplatin; HD-MVAC = high-dose methotrexate, vinblastine, doxorubicin, and cisplatin; HR = hazard ratio; MVAC = methotrexate, vinblastine, doxorubicin, and cisplatin; NR = not reported; OS = overall survival; pCR = pathological complete response; PD = progressive disease; PFS = progression free survival; PLND = pelvic lymph node dissection; pPR = pathological partial response; pSD = pathological stable disease; RC = radical cystectomy; RFS = recurrence free survival; RPLND = retroperitoneal lymph node dissection; SD = stable disease; UTUC = upper tract urothelial carcinoma.
In patients clinically defined as complete responders.
In patients clinically defined as partial responders.
The largest series was published by Zargar-Shoshtari et al [14] in which 304 patients received induction CHT. The rate of complete pathological response in LN and bladder specimens, combined, was 14.5%. This rate is lower than the 23–38% reported rates in neoadjuvant CHT series that had not included patients with cN1–3 [42,43]. Complete pathological response in LN (pN0), number of LNs removed (≥15), negative soft tissue surgical margins, and cisplatin-based CHT were independently associated with better OS. Interestingly, there was no statistically significant difference in OS between cN1 and cN2–3. Likewise, there was no statistically significant difference between methotrexate, vinblastine, adriamycin, and cisplatin, and gemcitabine and cisplatin.
Similarly, Meijer et al [13] reported that clinical and pathological responses to CHT are predictive of better cancer-specific survival (CSS). Complete pathological response was achieved in about one-fourth of patients with a 5-yr CSS of 63.5%. Complete radiological response was documented in about one-third of patients with a lower 5-yr CSS. However, patients with partial radiological or pathological response had similar 5-yr CSS (25% and 26.6%, respectively).
Nieuwenhuijzen et al [12] evaluated the outcomes of surgery in patients with histologically proven LN metastasis. Complete and partial response after CHT were independently associated with CSS. This study demonstrated that LN status after CHT was a more powerful predictor than local tumor stage. Clinically negative LNs after induction CHT without local bladder response were independently associated with better survival. Moreover, no patient with pathologically positive LN (pN+) survived more than 2 yr after surgery, underlining the importance of response in LN as a surrogate indicator for OS. Indeed, the independent effect of pN0 on OS was confirmed by other groups [11,14,18]. Meijer et al [13] also reported better CSS in isolated nodal response without reaching statistical significance. In fact, the combined local and nodal response had a better outcome compared with either response alone (p < 0.0001). A recent study reported a five-fold higher CSS in patients who attained pN0 compared to patients with pN+ [18].
A series from Memorial Sloan Kettering Cancer Center showed that 92% of patients who did not undergo surgery after major response to CHT died of metastatic disease, whereas a third of patients who achieved complete response to CHT and surgery had long-term survival [15].
Kassouf et al [16] retrospectively evaluated the outcomes of persistent pN+ despite preoperative CHT. Of the 150 patients with pN+ disease, 37 received preoperative CHT. In 18 patients, radiologically positive LNs were present and were independently associated with shorter disease-specific survival (13.5 mo vs 19.9 mo, hazard ratio: 2.84) and recurrence-free survival (RFS; 4.9 mo vs 10.8 mo, hazard ratio: 2.58). The presence of pN+ after preoperative CHT and RC abrogated the effect of pathological T stage on OS, disease-specific survival, and RFS. However, the positive surgical margin retained its independent prognostic significance on all survival outcomes [11,14,16]. Recently, Galsky et al [19] used the National Cancer Data Base to evaluate a large number of patients with clinically positive LN who underwent CHT and/or RC [19]. A multimodal approach integrating perioperative CHT was associated with betteroutcomes than RC alone. The crude 5-year OS for preoperative CHT and RC, RC and adjuvant CHT, and RC alone being 31%, 26%, and 19%, respectively.
The pooled percentage of complete and partial clinical response in the regional LNs were 33% (95% confidence interval [CI]: 27–40%) and 44% (95% CI: 22–66%), respectively. The pooled pathological response rate was 18% (95% CI: 13–23%). These results should be interpreted with caution since data from these cohorts were heterogeneous in term of treatment protocols. Nevertheless, the cumulative evidence suggests a benefit for surgery after complete or major response to CHT. Multiple factors are likely to be associated with improved outcomes such as cisplatin-based CHT, number of cycles, number of LNs removed, and pathological response in LN.
3.1.2. The role of surgery in case of intraoperative finding of LN metastasis
It is estimated that about 40% of patients with clinically localized BCa will be upstaged to locally advanced at time of RC [44]. Herr et al [20] underscored the benefit of surgery in patients with intraoperatively identified grossly enlarged LN who were treated with RC and PLND alone. Approximately, one-fourth of patients had no evidence of disease at a median follow-up of 10 yr. The benefit was more pronounced in patients with pathologically organ-confined disease. The authors encouraged extensive surgical approach—when possible—if the surgeon encountered enlarged LN at time of surgery. Similarly, a previous report from the same center concluded that patients with organ-confined disease and minimal nodal involvement benefited most from complete surgical removal of all cancer sites [45]. Obviously, these data came from a high volume center with large expertise in RC and PLND, so these results probably cannot be translated to everyday practice for surgeons who perform RC less frequently.
Results from series evaluating the outcomes in patients who had surgery aborted due to gross LN involvement and/ or extensive extravesical extension revealed poor outcomes [21,22] (Table 2). However, these series demonstrated benefit in few patients undergoing salvage RC after responding to CHT [21] and a trend toward improved survival in patients undergoing retroperitoneal LND (RPLND) [22]. However, bulky LN disease is unlikely to be missed with modern imaging.
Table 2 –
Studies reporting the outcomes of patients after aborted radical cystectomy for intraoperative findings of grossly enlarged lymph nodes and locally advanced tumor
| Reference | No. of cases | Findings | PLND at time of aborted RC | Chemotherapy after aborted RC | Median follow-up (mo) | Survival | Comment |
|---|---|---|---|---|---|---|---|
| Guzzo et al [21] | 35 | Grossly enlarged LNs: 91% pT4:23% |
31 | 30 | 18.5 | 60% died from the disease 31% alive with evidence of disease persistence or re-progression. 9% alive with no evidence of disease |
Subsequent salvage RC: 23%. 43% had no evidence of disease progression at a mean time of 10 mo after salvage RC |
| Yafi et al [22] | 31 | Clinical T≥2: 45% Clinical N1: 10% Pathological T4b: 55 Pathological N2–3: 45% |
20 | 23 | 10 | 2-yr OS: 41% 5-yr OS: 0% Patients who underwent RPLND trended toward improved OS (24 vs 10 mo, p = 0.09) |
35% received CHT with intention to surgical consolidation, only 9% rendered resectable |
CHT = chemotherapy; LN = lymph node; OS = overall survival; PLND = pelvic lymph node dissection; RC = radical cystectomy.
3.2. Metastasectomy
Despite the limited long-term survival of patients with metastatic UC treated with combination CHT, about 15% seemed to survive beyond 5 yr reflecting the heterogeneity of tumor biology and its sensitivity to CHT [7,8,46]. To predict survival, several factors were identified to be independently associated with worse outcomes such as poor performance status, visceral metastasis (lung, liver, or bone), and number of sites of visceral metastases [7,47,48]. The presence of favorable prognostic factors has encouraged surgeons to integrate surgery in treatment strategies to improve oncological outcomes.
3.2.1. Pulmonary metastasectomy
Several groups evaluated the role of surgical resection of pulmonary metastatic deposits as a part of multidisciplinary approach [23–33] (Table 3).
Table 3 –
Studies reporting the outcomes of patients with metastatic bladder cancer who underwent pulmonary metastasectomy
| Reports | Patients (n) | Study population | Chemotherapy |
Surgery | Pathologic response rate | Survival |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Regimen (%) | Median no. of cycles | Median follow-up (mo) | Rate (%) | Median survival time (mo) | Other findings | |||||
| Otto et al. 2001 [34] | 70 | Metastatic BCa refractory to CHT | M-VAC | 3 | RC:100% Pulmonary resection: 30% Peritoneal deposit resection: 14% Liver resection: 11% |
0% | NR | 1-yr survival: 30% 2-yr survival: 19% |
7 | Symptomatic patients: 73% Asymptomatic patients: 27% No survival advantage for surgery Perioperative mortality: 4% |
| Bekku at al 2013 [23] | Original cohort: 47 Study population: 12 |
Patients with cPR to CHT who underwent surgery cM+ BCa (8) and UTUC (4) | GCP or MVAC 75% of patients received adjuvant CHT. | 3 | Primary surgery: 50% RPLND: 83% Pulmonary resection: 17%. |
pCR in pulmonary metastasis in one (50%) patient. pCR after RPLND in 8 (80%) patients |
33.6 | 3-yr PFS in patients with salvage surgery: 39.8% 3-yr PFS without salvage surgery was 0% 3-yr OS in surgery group: 71.6% 3-yr OS without surgery group: 12.1% |
Time to progression in patients undergoing salvage surgery 23 CSS in patients undergoing salvage surgery: 47.2 |
Resection of metastasis was significantly associated with CSS and time to progression |
| Matsuguma et al. 2011 [24] | 32 | UC with Pulmonary metastasis 81% had solitary metastasis | 50% patients received preoperative CHT | NR | Lobectomy: 47% wedge resection: 44% Segmentectomy: 9% |
NR | NR | 5-yr PFS: 26% 5-yr modified PFS: 40% 5-yr OS: 50% |
NR | 17 (53%) patients had recurrence after metastasectomy 9 (28%) patients survived without recurrence >5 yr |
| Han et al. 2002 [25] | 16 | UC with Pulmonary metastasis. | Adjuvant CHT GC | 3 | Wedge resection: 50% segmentectomy: 13% lobectomy: 37% |
NR | NR | 5-yr OS: 65.3% 5-yr DSS: 37.5% |
NR | 5 (31%) of patients with pulmonary recurrence underwent repeated metastasectomy and had a median survival of 31 mo No perioperative mortality |
| Siefker-Radtke et al. 2004 [26] | 31 | cM+ BCa (24) and UTUC (7) Lung metastasis: 77% Distant LNs: 13% Brain: 7% Subcutaneous tissue: 3% |
Preoperative CHT: 71% Adjuvant CHT: 13% Surgery alone: 29% |
NR | Primary surgery: 90% Complete pulmonary resection: 22/24 (90%). |
6.5% | 16 | 3-yr survival: 33% | OS from diagnosis: 31 OS from resection: 23 Time to recurrence: 7 |
No perioperative mortality |
| Abe et al. 2007 [27] | 48 | cM+ BCa (23), UTUC (16), synchronous BCa and UTUC (8), urethral carcinoma (1). | MVAC: 21% MEC: 63% |
4 | Resection of the primary site: 75% Metastasectomy: 21% (83% of them received preoperative CHT) |
Lung: 0% LN: 33% |
NR | NR | Whole cohort: 17 Patients who underwent metastasectomy: 42 Patients who did not undergo metastasectomy: 10 | Absence of liver, bone and local recurrence, >5 CHT cycles, and resection of metastasis were independent predictors of prolonged OS. |
| Lehmann et al. 2009 [28] | 44 patients | cM+ BCa (35) and UTUC (9) | Preoperative CHT: 50% Adjuvant CHT: 41% |
NR | Surgery alone: 20.5% Surgery+CHT: 29.5% CHT+surgery: 36.4% CHT+surgery+CHT: 13.6% |
After RPLND: 18% | 63 | 5-yr OS survival from diagnosis: 28% 5-yr OS survival from metastasis resection: 27.7% 5-yr PFS survival from diagnosis: 23.6% 5-yr PFS survival from metastasis resection: 24% |
OS from diagnosis, metastasis, and surgical resection: 34.7, 34.3 and 27.2 respectively | No perioperative death 7 patients survived >2 yr and remained free from tumor progression Metastasis site: RPLN (57%), distant LN (11%), lung (18%) and other (14%) |
| Kanzaki et al [29] 2010 | 18 | Pulmonary metastasis in patients with BCa (9), UTUC (6) and synchronous BCa and UTUC (3). | Perioperative platinum-based CHT 8 (44.4%) | NR | Sublobar resection: 77.8% Lobectomy: 22% Primary surgery TURB: 6% RC: 44% RNU:50% |
NR | 52 | 3-yr OS: 59.8% 5-yr OS: 46.5% 5-yr OS for solitary metastasis: 85.7% The 5-yr OS for multiple metastases: 20% |
NR | No perioperative mortality |
| Nakagawa et al [30] 2013 | 114 | Patients with local or distant recurrence after RC and PLND | Post recurrence platinum-based CHT: 53% | NR | Metastasectomy: 11.4% Lung: 7% LN: 3% Ileal conduit: 0.8% Brain: 0.8% |
NR | 11a | 1-yr OS: 48% 3-yr OS: 12% |
11 | Time to recurrence (≥1 yr, HR: 0.58), symptoms at recurrence (HR: 2.44), metastatic organs at recurrence (≥2, HR: 2.1), postrecurrence CHT (HR: 0.48) and metastasectomy (HR: 0.37) were independent predictors of postrecurrence OS |
| Abe et al. 2014 [31] | 42 | cM+ BCa (21), UTUC (18), synchronous BCa and UTUC (3) | Preoperative CHT: 81% | NR | RPLND: 36% Distant LN: 12% Pulmonary resection: 29% |
35.3% | 28 | 5-yr OS after metastasectomy: 31% | OS from metastasectomy: 26 | Patient who had metastasectomy in solitary LN or solitary lung lesion had significantly longer survival (81 versus 19) |
| Kim et al. 2015 [32] | 30 | cM+ BCa (14), UTUC (16) | Preoperative CHT: 6.7% Adjuvant CHT: 37% |
NR | Lung: 80% Liver: 7% Bone: 3% LN: 10% |
NR | 54 | 3-yr survival: 41%. | OS: 30 time to disease progression: 15.2 | Patients with nonpulmonary visceral metastases did not appear to benefit from surgery Initial stage IV disease (HR 4.28), pure urothelial pathology (HR 5.24), and nonpulmonary metastasectomy (HR 5.12) were independent predictors of time to disease progression |
| Luzzi et al. 2016 [33] | 69 | Pulmonary metastasis in BCa (55) and UTUC (14) | GC Carboplatin in case RNU | NR | Wedge or segmental resection: 64% Lobectomy: 36% |
NR | 50 | Overall 5-yr OS: 52% Overall 5-yr OS in BCa patients: 54% Overall 5-yr OS in UTUC pts: 48% |
OS: 62 disease-free interval: 37 |
No perioperative mortality Pulmonary metastasis <3 cm (HR 0.65) was independently associated with 5-yr OS Disease-free interval > 24 mo significantly related to a better 5-yr OS |
CHT = chemotherapy; CR = complete response; GC = gemcitabine and cisplatin; GCP = gemcitabine/cisplatin/paclitaxel-based; MVAC = methotrexate, vinblastine, doxorubicin, and cisplatin; MEC = mitoxantrone, etoposide, and intermediate-dose Ara-C; NR = not reported; OS = overall survival; PFS = progression-free survival; PLND = pelvic lymph node dissection; PR = partial response; RC = radical cystectomy; RNU = radical nephroureterectomy; RPLND = retroperitoneal lymph node dissection; TURB = transuretheral resection of the bladder; UC = urothelial carcinoma; UTUC = upper tract urothelial carcinoma.
Median follow-up (after recurrence) was 11 mo for all patients and 47 mo in 15 survivors at final follow-up.
Otto et al [34] reported on the role of surgery in patients with metastatic disease who experienced disease progression during or after CHT. Lung was the most common site of metastasis after LNs. There was no survival advantage for metastasectomy and no difference in survival between different metastatic sites. Notably, patients with symptomatic metastasis benefited from surgery in terms of quality of life and performance improvement while asymptomatic hpatients, however, complained from a reduced sense of well-being.
Siefker-Rradtke et al [26] reported on 31 patients with primary bladder and upper tract UC (24 and 7, respectively); pulmonary metastases were the most common site for surgical resection (n = 24). Thirty patients had their tumor completely resected with negative surgical margins. There was no significance survival difference according to the site of metastasis with a 5-yr OS of 33%. Abe et al [27] reported on another heterogeneous cohort of 48 patients. Resection of pulmonary metastasis was also the most commonly performed procedure after CHT (n = 12). All patients were found to have viable tumor after pulmonary metastasis resection. The median OS in patients who underwent metastasectomy was significantly higher than patients who did not (42 mo vs 10 mo). Similarly, in patients who experienced local or distance recurrence after RC, postrecurrence CHT and metastasectomy was found to be independent predictor of better postrecurrence OS [30]. Factors that independently predict prolonged survival were number of chemotherapy cycles (5 or more), metastasectomy and absence of liver, bone, and local recurrence. Two series underlined the impact of metastatic deposit size [24,33]. They found that resection of metastatic deposit greater than 3 cm was independently associated with poor survival (Table 3).
These studies suggest that the absence of visceral metastasis, small volume disease, and the use of perioperative CHT are associated with favorable response [28,29,31– 33,36] (Table 3). Moreover, patients with metastatic disease in multiple organs or metastasis in bone, brain, and liver may not benefit from consolidative surgery in terms of survival. Consequently, the indication of surgical intervention in these patients remains mostly palliative and the current therapeutic armamentarium to deal with these lesions comprises nonsurgical approaches [2,24]. Itis worthwhile to mention that although BCa and upper tract UC may have different biological behaviors [49], similar results were reported in metastatic upper tract UC [23,26,29,31–33,50].
3.2.2. RPLND
According to the seventh edition of the tumor, node, metastasis classification of malignant tumors, LN involvement above the common iliac LN is considered M1 disease [2]. The presence of LN metastasis outside the true pelvis has been always thought of not curable. However, new data show that the number of involved LNs is a worse prognosticator than the level of involvement [51–53]. In Meijer et al’s report [13], patients with cM1 had a similar median CSS as those who had cN2–3 M0 (15 mo). Likewise, patients with cM1 were found to have a similar outcome compared with patients with T3–4N+M0 [54]. Several investigators evaluated the outcomes of patients with metastatic BCa who underwent RPLND [23,31,35–37] (Table 4).
Table 4 –
Studies reporting the outcomes of patients with metastatic bladder cancer who underwent retroperitoneal lymph node dissection
| Reports | Patients (n) | Study Population | Chemotherapy |
Surgery | Pathologic response rate | Survival |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Regimen (%) | Median no. of cycles | Clinical response rate | Median follow up (mo) | Rate (%) | Median survival time (mo) | Other findings | |||||
| Bekku et al. 2013 [23] | Original cohort: 47 Study population: 12 | Patients with cPR to CHT who underwent surgery. cM+ BCa (8) and UTUC (4) | GCP MVAC 9 (75%) patients received adjuvant CHT | 3 | cCR: 9% cPR: 57%a | Primary surgery: 50% RPLND: 83% Pulmonary resection: 17% |
pCR after RPLND: 8 (80%) patients pCR in pulmonary metastasis: one (50%) patient | 33.6 | 3-yr PFS in patients with salvage surgery: 39.8% 3-yr PFS without salvage surgery: 0% 3-yr OS in surgery group: 71.6% 3-yr OS without surgery group: 12.1% |
Time to progression in patients undergoing salvage surgery: 23 CSS in patients undergoing salvage surgery: 47.2 |
Patients who underwent RPLND or pulmonary resection had a significantly longer OS compared to those who did not underwent without salvage surgery |
| Sweeney et al. 2003 [35] | 11 | cM1 BCa patients | Not standardized | 8 | cCR: 8.5% cPR: 57.4% |
RC, PLND and bilateral RPLND concurrent RC and RPLND: 64% | 18% | 14 | 4-yr CSS: 36% 4-yr RFS: 27% |
Time to recurrence: 7 RFS: 7 CSS: 14 |
No perioperative mortality Viable tumor in ≤2 LNs correlated with better CSS and RFS |
| De Vries et al. 2009 [36] | 14 | cM1 BCa patients | MVAC: 57% HD-MVAC: 36% GC: 7% |
4 | cCR: 36% cPR: 64% |
RC, PLND, and bilateral RPLND. | pCR: 80%b
In cPR: pCR in bladder: 33% pCR in LN: 56% |
30 | 3-yr survival: 36% 5-yr survival: 24% |
10.1 | Post CHT surgery in selected patients with supra-regional LN metastases can result in durable long-term survival |
| Necchi et al. 2013 [37] | Original cohort: 59 Study population: 28 |
Locally advanced or metastatic BCa (17) and UTUC (11) with at least SD post CHT | Modified MVAC | 4–6 | cCR: 25% cPR: 61% SD:14% |
PLND: 14 RPLND: 11 Both: 3 |
28.6% | 88 | 5-yr PFS: 35.2% 5-yr OS: 48.7% |
PFS: 18 OS: 37 |
cCR was independent predictorofPFS (HR: 2.42) Post CHT surgery was independently associated with better PFS (HR 0.43) and OS (HR 0.37). pCR did not affect PFS or OS (p = 0.1 and 0.3, respectively) |
| Abe et al. 2014 [31] | 42 | cM+ BCa: 21, UTUC: 18 synchronous BCa and UTUC: 3 | MVAC Preoperative CHT: 91% Adjuvant CHT: 16% |
NR | NR | RPLND 36% (below and above bifurcation of Aorta (40% and 60%), respectively) Distant LN: 12% Pulmonary resection: 29% Other: 33% | 29% | For all patients: 28 For metastasectomy group: 22 |
5-yr OS after metastasectomy: 31% | OS for all patients: 29 OS from metastasectomy: 26 |
No perioperative mortality Patient who had metastasectomy in solitary LN or solitary lung lesion had significantly longer survival (81 vs 19 mo) |
cCR = clinical complete response; CHT = chemotherapy; cPR = clinical partial response; EBRT = external beam radiotherapy; GC = gemcitabine and cisplatin; GCP = gemcitabine/cisplatin/paclitaxel-based; HD-MVAC = high-dose methotrexate, vinblastine, doxorubicin, and cisplatin; LN = lymph node; MVAC = methotrexate, vinblastine, doxorubicin, and cisplatin; NA = not available; NR = not reported; OS = overall survival; PLND = pelvic lymph node dissection; pCR = pathological complete response; RC = radical cystectomy; RPLND = retroperitoneal lymph node dissection; SD = stable disease.
In the original cohort.
In patients with complete clinical response.
Sweeney et al [35] reported on 11 patients with biopsy proven subdiaphragmatic LN involvement without evidence of visceral metastasis. Patients underwent bilateral RPLND after response to CHT. The presence of more than two viable LNs at time of RPLND was associated with worse oncological outcome. The 4-yr CSS and RFS were 36% and 27%, respectively.
Necchi et al [37] demonstrated similar results in 28 patients who underwent RPLND (n = 14) or PLND (n = 14) following complete, partial response, or at least stable disease after CHT. Other 31 patients underwent consolidation CHT, radiotherapy, or observation. Surgery was significantly associated with higher median progression-free survival (PFS; 18 mo vs 11 mo) and was an independent prognosticator of both PFS and OS. Response to CHT was also associated with better PFS and showed a trend towards significance for OS.
The pooled percentage of complete and partial clinical response in the retroperitoneal LNs were 17% (95% CI: 6– 28%) and 68% (95% CI: 59–77%), respectively. The pooled pathological response rate was 46% (95% CI: 26–66%).
It should be stressed that especially after multiple CHT courses (6 to sometimes 10), surgical removal of the LNs and extensive RPLND can be surgically challenging, with fibrotic and desmoplastic reactions similar to post-CHT surgery in seminomatous testis cancer. Surgery clearly should be limited to large volume centers accustomed to this type of surgery, especially as in contrast to young patients with testicular cancer, patients with metastatic BCa tend to be older with multiple comorbidities.
3.3. Theroleof cytoreductiveRCinpatientswithdistant metastasis
Cytoreductive surgery is the standard in a few malignancies such as renal cell carcinoma and ovarian cancer [55,56], whereas local treatment in form of RC or irradiation is currently not part of standard management in metastatic BCa [57]. Nevertheless, cytoreductive RC is being explored in specialized centers when there is low volume metastatic burden [2,57]. Using the National Cancer Data Base, Seisen et al [38] addressed the outcome of local treatment in 3753 patients who received multiagent systemic CHT for metastatic BCa at presentation. Two hundred and ninety-seven (7.9%) patients received systemic CHT with high-intensity local treatment (radical cystectomy or ≥50-Gy radiation therapy) and the remaining had conservative treatment (no treatment, radiation < 50 Gy or transurethral resection) in addition to CHT. Using propensity weighting, the authors reported a significant survival benefit for patients with high-intensity local treatment (14.92 mo vs 9.95 mo, p < 0.001). Moreover, OS was better when local treatment was performed after systemic CHT (consolidative strategy). Of note, this study included patients with supraregional LN as well as visceral or bone metastasis. To date, no study has specifically examined the effect of cytoreductive RC in patients with visceral metastasis. It should also be noted that consolidation can be performed by other means than surgery and radiation can be explored especially when combined by CHT. This can be performed locally in selected patients and some ongoing trials currently address the role of radiation therapy on metastatic sites [58]. Important aspects of treatment are the performance status of the patient and the motivation to undergo toxic CHT followed by local treatment.
3.4. Therationaleof integratingsurgeryinmultimodal treatment
The rationale behind post-CHT surgery is multifactorial. First, although UC is a chemosensitive tumor, systemic CHT is rarely curative [8]. Secondary, surgery is the best method to assess response to CHT because radiological evaluation is not always accurate [13,18,54]. Third, surgery can eliminate residual tumor and achieves a complete response in partial responders or patients who have been inaccurately staged as complete responders [12,13,18,54]. Then, the pattern of relapse in patients with locoregional metastasis who responded to CHT supports this concept since approximately three-fourth of patients experience relapse at the site of initial response to CHT [26].
In high-risk patients with resectable disease (cT3b and cT4a), a phase 3 trial confirmed the benefit of combining surgery and CHT. It also revealed that the timing of CHT with respect to surgery did not show any differences on the basis of therapy sequence [59]. An extension for the rational of multimodality treatment has been reported by several groups demonstrating survival benefit and even long-term survival in patients with more advanced disease, initially unresectable BCa, after methotrexate, vinblastine, adriamycin, and cisplatin, and definitive surgery when there is response to CHT [15,60]. In addition, despite a pathological complete response in the bladder (pT0) after definitive preoperative CHT for locally advanced or regionally metastatic BCa, patients were more likely to have persistent disease within the regional LNs in comparison to patients who received neoadjuvant CHT and achieved pT0 [41].
In contrast to metastatic UC, the role of metastasectomy with curative intent is well-established in some chemosensitive tumors such as testicular and colorectal carcinoma as well as some chemoresistant tumors such as renal cell carcinoma [2,24,25,29,61]. Although the aforementioned justifications give a strong argument in favor of the role of surgery, they do not provide final proof or evidence.
3.5. Theroleof imaging
The previously mentioned data showed that between 25% and 40% of patients with complete radiological response to CHT were found to harbor viable tumor at the time of surgical resection [12,13,15,18]. Moreover, no tumor could be detected at final pathology specimen in 10–75% of partial responders [12,23]. This suggests that response as assessed by currently available imaging modalities still has limited sensitivity and misclassifies patients.
Contrast-enhanced computed tomography (CT) or magnetic resonance imaging are recommended to stage locally advanced disease and distant metastasis with CT being preferred for diagnosing pulmonary metastasis. In LN staging, both techniques showed similar results with sensitivity ranging from 48% to 87% [2,62]. These techniques are based on the size and shape of enlarged LN. Currently, pelvic LNs >8 mm and abdominal nodes >10 mm in maximum short-axis diameter are regarded as pathological. Their accuracy is hampered by the inability to detect metastasis in normal or minimally enlarged LNs. The introduction of more advanced functional imaging such as positron emission tomography/CT and diffusion-weighted magnetic resonance imaging seems to allow for better identification of the true extent of the disease and possibly identification of early response to CHT [2,63,64]. Despite promising results, data are insufficient to recommend such imaging modalities [2].
Since currently available imaging techniques are limited in detecting LN metastasis, LN dissection remains the most accurate form of nodal staging and response evaluation after CHT. In this review, the protocol in many studies was to evaluate the clinical response, radiologically, after every two cycles of CHT [12,13,18,35,54]. In some series, diagnosis of LN metastasis was established, in addition to imaging, by fine needle aspiration or LN dissection [12,13,18,35,36].
3.6. Limitations, challenges, and an insight into the future
The selection criteria were similar in most studies. The optimum candidates for surgery were patients with limited resectable metastatic burden, major or complete response to CHT, no evidence of progressive disease elsewhere, and good performance status. In fact, this represents a selection bias that overestimates the effect of surgery. The retrospective design further overestimates success rates due to selection bias. Small sample size, heterogeneous case-mix, nonstandardization of CHT regimens, and the extent of PLND and RPLND are other methodological biases that make drawing a definitive conclusion from these studies difficult.
One of the major challenges is to predict response to CHT accurately; patients who are not responding to CHT may suffer from unnecessary toxicityand may miss the chance to be offered earlier palliative intervention. Many investigators evaluated the role of biomarkers in predicting BCa outcome [65]. In fact, models integrating biomarkers gives more individualized insight into tumor biology and may help to predict clinical behaviors such as sensitivity to CHT and tumor aggressiveness. Other challenges are to determine the optimal CHT regimen, number of cycles, and optimal interval between last cycle and surgery.
Currently, the optimal strategy to ascertain the role of surgery and consequently improving patients’ outcome is to address these limitations and to launch a multicenter collaboration with centralized data and/or a prospective randomized trial. Standardization of reporting response to CHT is also important such as scoring according to Response Evaluation Criteria in Solid Tumors criteria [11].
Finally, immunotherapy provides new hope that can change the outlook of patients with this disease state possibly changing the role of surgery. In fact, checkpoint inhibitors such as atezolizumab, durvalumab, nivolumab, and pembrolizumab have been approved in patients with locally advanced or metastatic UC whose disease progressed during or following combination CHT or within 12 mo of neoadjuvant or adjuvant CHT [66–69]. Furthermore, the indication of atezolizumab was recently extended to include untreated cisplatin ineligible patients who have locally advanced or metastatic UC [70].
4. Conclusions
Metastatic BCa is still a lethal disease with little improvement in outcomes since the introduction of cisplatin-based combination CHT. Cumulative but still limited evidence suggests a role for surgery and/or other consolidation therapies in managing a subgroup of patients with metastatic BCa as an integral part of sequenced multidisciplinary approach. Results are consistently pointing toward improved survival in patients with low volume disease after measurable response to CHT in the lung, pelvic, and retroperitoneal LN metastases. Evidence shows that surgical resection is technically feasible with acceptable morbidity and can achieve long-term cancer control in well-selected patients. Further evidence is needed to identify the role of surgery in patients with metastatic BCa, specifically in the era of immunotherapeutic that is upon us.
Footnotes
Financial disclosures: Shahrokh F. Shariat certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
References
- [1].Rink M, Lee DJ, Kent M, et al. Predictors of cancer-specific mortality after disease recurrence following radical cystectomy. BJU Int 2013;111:E30–6. [DOI] [PubMed] [Google Scholar]
- [2].Alfred Witjes J, Lebret T, Compérat EM, et al. Updated 2016 EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer. Eur Urol 2017;71:462–75. [DOI] [PubMed] [Google Scholar]
- [3].Shariat SF, Karakiewicz PI, Palapattu GS, et al. Outcomes of radical cystectomy for transitional cell carcinoma of the bladder: a contemporary series from the Bladder Cancer Research Consortium. J Urol 2006;176:2414–22. [DOI] [PubMed] [Google Scholar]
- [4].Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1054 patients. J Clin Oncol 2001;19:666–75. [DOI] [PubMed] [Google Scholar]
- [5].Abufaraj M, Gust K, Moschini M, et al. Management of muscle invasive, locally advanced and metastatic urothelial carcinoma of the bladder: a literature review with emphasis on the role of surgery. Transl Androl Urol 2016;5:735–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kamat AM, Hegarty PK, Gee JR, et al. ICUD-EAU International Consultation on Bladder Cancer 2012: screening, diagnosis, and molecular markers. Eur Urol 2013;63:4–15. [DOI] [PubMed] [Google Scholar]
- [7].Bajorin DF, Dodd PM, Mazumdar M, et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol 1999;17:3173–81. [DOI] [PubMed] [Google Scholar]
- [8].von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 2005;23:4602–8. [DOI] [PubMed] [Google Scholar]
- [9].Heidenreich A, Wilop S, Pinkawa M, Porres D, Pfister D. Surgical resection of urological tumor metastases following medical treatment. Dtsch Arztebl Int 2012;109:631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Urakami S, Yuasa T, Yamamoto S, et al. Clinical response to induction chemotherapy predicts improved survival outcome in urothelial carcinoma with clinical lymph nodal metastasis treated by consolidative surgery. Int J Clin Oncol 2015;20:1171–8. [DOI] [PubMed] [Google Scholar]
- [12].Nieuwenhuijzen JA, Bex A, Meinhardt W, et al. Neoadjuvant methotrexate, vinblastine, doxorubicin and cisplatin for histologically proven lymph node positive bladder cancer. J Urol 2005;174:80–4. [DOI] [PubMed] [Google Scholar]
- [13].Meijer RP, Mertens LS, van Rhijn BW, et al. Induction chemotherapy followed by surgery in node positive bladder cancer. Urology 2014;83:134–9. [DOI] [PubMed] [Google Scholar]
- [14].Zargar-Shoshtari K, Zargar H, Lotan Y, et al. A multi-institutional analysis of outcomes of patients with clinically node positive urothelial bladder cancer treated with induction chemotherapy and radical cystectomy. J Urol 2016;195:53–9. [DOI] [PubMed] [Google Scholar]
- [15].Herr HW, Donat SM, Bajorin DF. Post-chemotherapy surgery in patients with unresectable or regionally metastatic bladder cancer. J Urol 2001;165:811–4. [PubMed] [Google Scholar]
- [16].Kassouf W, Agarwal PK, Grossman HB, et al. Outcome of patients with bladder cancer with pN+ disease after preoperative chemotherapy and radical cystectomy. Urology 2009;73:147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ghadjar P, Burkhard FC, Gautschi O, Thalmann GN, Studer UE. Induction chemotherapy for unresectable urothelial carcinoma of the bladder. BJU Int 2011;107:894–7. [DOI] [PubMed] [Google Scholar]
- [18].Ho PL, Willis DL, Patil J, et al. Outcome of patients with clinically node-positive bladder cancer undergoing consolidative surgery after preoperative chemotherapy: the M.D. Anderson Cancer Center experience. Urol Oncol 2016;34, 59.e1–8. [DOI] [PubMed] [Google Scholar]
- [19].Galsky MD, Stensland K, Sfakianos JP, et al. Comparative effectiveness of treatment strategies for bladder cancer with clinical evidence of regional lymph node involvement. J Clin Oncol 2016; 34:2627–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Herr HW, Donat SM. Outcome of patients with grossly node positive bladder cancer after pelvic lymph node dissection and radical cystectomy. J Urol 2001;165:62–4. [DOI] [PubMed] [Google Scholar]
- [21].Guzzo TJ, Rogers CG, Deng CY, et al. Outcomes of patients after aborted radical cystectomy for intraoperative findings of metastatic disease. BJU Int 2008;102:1539–43. [DOI] [PubMed] [Google Scholar]
- [22].Yafi FA, Duclos M, Correa JA, et al. Contemporary outcome and management of patients who had an aborted cystectomy due to unresectable bladder cancer. Urol Oncol 2001;29:309–13. [DOI] [PubMed] [Google Scholar]
- [23].Bekku K, Saika T, Kobayashi Y, et al. Could salvage surgery after chemotherapy have clinical impact on cancer survival of patients with metastatic urothelial carcinoma? Int J Clin Oncol 2013;18:110–5. [DOI] [PubMed] [Google Scholar]
- [24].Matsuguma H, Yoshino I, Ito H, et al. Is there a role for pulmonary metastasectomy with a curative intent in patients with metastatic urinary transitional cell carcinoma? AnnThorac Surg 2011;92:449–53. [DOI] [PubMed] [Google Scholar]
- [25].Han WS, Kim K, Park JS. Result of surgical resection for pulmonary metastasis from urothelial carcinoma. Korean J Thorac Cardiovasc Surg 2012;45:242–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Siefker-radtke AO, Walsh GL, Pisters LL, et al. Is There a role for surgery in the management of metastatic urothelial cancer? The M. D. Anderson experience. J Urol 2004;171:145–8. [DOI] [PubMed] [Google Scholar]
- [27].Abe T, Shinohara N, Harabayashi T, et al. Impact of multimodal treatment on survival in patients with metastatic urothelial cancer. Eur Urol 2007;52:1106–14. [DOI] [PubMed] [Google Scholar]
- [28].Lehmann J, Suttmann H, Albers P, et al. Surgery for metastatic urothelial carcinoma with curative intent: The German Experience (AUO AB 30/05). Eur Urol 2009;55:1293–9. [DOI] [PubMed] [Google Scholar]
- [29].Kanzaki R, Higashiyama M, Fujiwara A, et al. Outcome of surgical resection of pulmonary metastasis from urinary tract transitional cell carcinoma. Interact Cardiovasc Thorac Surg 2010;11:60–4. [DOI] [PubMed] [Google Scholar]
- [30].Nakagawa T, Hara T, Kawahara T, et al. Prognostic risk stratification of patients with urothelial carcinoma of the bladder with recurrence after radical cystectomy. J Urol 2013;189:1275–81. [DOI] [PubMed] [Google Scholar]
- [31].Abe T, Kitamura H, Obara W, et al. Outcome of metastasectomy for urothelial carcinoma: a multi-institutional retrospective study in Japan. J Urol 2014;191:932–6. [DOI] [PubMed] [Google Scholar]
- [32].Kim T, Ahn J-H, You D, et al. Pulmonary metastasectomy could prolong overall survival in select cases of metastatic urinary tract cancer. Clin Genitourin Cancer 2015;13:e297–304. [DOI] [PubMed] [Google Scholar]
- [33].Luzzi L, Marulli G, Solli P, et al. Long-term results and prognostic factors of pulmonary metastasectomy in patients with metastatic transitional cell carcinoma. Thorac Cardiovasc Surg In press. 10.1055/s-0036-1583271. [DOI] [PubMed] [Google Scholar]
- [34].Otto T, Krege S, Suhr J, et al. Impact of surgical resection of bladder cancer metastases refractory to systemic therapy on performance score: a phase II trial. Urology 2001;57:55–9. [DOI] [PubMed] [Google Scholar]
- [35].Sweeney P, Millikan R, Donat M, et al. Is There a therapeutic role for post-chemotherapy retroperitoneal lymph node dissection in metastatic transitional cell carcinoma of the bladder? J Urol 2003;169:2113–7. [DOI] [PubMed] [Google Scholar]
- [36].de Vries RR, Nieuwenhuijzen JA, Meinhardt W, Bais EM, Horenblas S. Long-term survival after combined modality treatment in metastatic bladder cancer patients presenting with supra-regional tumor positive lymph nodes only. Eur J Surg Oncol 2009;35:352–5. [DOI] [PubMed] [Google Scholar]
- [37].Necchi A, Giannatempo P, Lo Vullo S, et al. Postchemotherapy lymphadenectomy in patients with metastatic urothelial carcinoma: long-term efficacy and implications for trial design. Clin Genitourin Cancer 2015;13, 80–6.e1. [DOI] [PubMed] [Google Scholar]
- [38].Seisen T, Sun M, Leow JJ, et al. Efficacy of high-intensity local treatment for metastatic urothelial carcinoma of the bladder: a propensity score-weighted analysis from the National Cancer Data Base. J Clin Oncol 2016;34:3529–36. [DOI] [PubMed] [Google Scholar]
- [39].Sternberg CN. Are nomograms better than currently available stage groupings for bladder cancer? J Clin Oncol 2006;24:3819–20. [DOI] [PubMed] [Google Scholar]
- [40].von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 2000;18:3068–77. [DOI] [PubMed] [Google Scholar]
- [41].Kaag MG, Milowsky MI, Dalbagni G, et al. Regional lymph node status in patients with bladder cancer found to be pathological stage T0 at radical cystectomy following systemic chemotherapy. BJU Int 2011;108:E272–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003;349:859–66. [DOI] [PubMed] [Google Scholar]
- [43].Zargar H, Espiritu PN, Fairey AS, et al. Multicenter assessment of neoadjuvant chemotherapy for muscle-invasive bladder cancer. Eur Urol 2015;67:241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Shariat SF, Palapattu GS, Karakiewicz PI, et al. Discrepancy between clinical and pathologic stage: impact on prognosis after radical cystectomy. Eur Urol 2007;51:137–51. [DOI] [PubMed] [Google Scholar]
- [45].Vieweg J, Gschwend JE, Herr HW, Fair WR. Pelvic lymph node dissection can be curative in patients with node positive bladder cancer. J Urol 1999;161:449–54. [PubMed] [Google Scholar]
- [46].Sternberg CN, de Mulder P, Schornagel JH, et al. Sevenyear update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur J Cancer 2006;42:50–4. [DOI] [PubMed] [Google Scholar]
- [47].Apolo AB, Ostrovnaya I, Halabi S, et al. Prognostic model for predicting survival of patients with metastatic urothelial cancer treated with cisplatin-based chemotherapy. J Natl Cancer Inst 2013;105:499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Taguchi S, Nakagawa T, Uemura Y, et al. Validation of major prognostic models for metastatic urothelial carcinoma using a multi-institutional cohort of the real world. World J Urol 2016;34:163–71. [DOI] [PubMed] [Google Scholar]
- [49].Green DA, Rink M, Xylinas E, et al. Urothelial carcinoma of the bladder and the upper tract: disparate twins. J Urol 2013;189:1214–21. [DOI] [PubMed] [Google Scholar]
- [50].Inokuchi J, Naito S, Fujimoto H, et al. Impact of multimodal treatment on prognosis for patients with metastatic upper urinary tract urothelial cancer: Subanalysis of the multi-institutional nationwide case series study of the Japanese Urological Association. Int J Urol 2016;23:224–30. [DOI] [PubMed] [Google Scholar]
- [51].Tarin TV, Power NE, Ehdaie B, et al. Lymph node-positive bladder cancer treated with radical cystectomy and lymphadenectomy: effect of the level of node positivity. Eur Urol 2012;61:1025–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Herr HW, Faulkner JR, Grossman HB, et al. Surgical factors influence bladder cancer outcomes: a cooperative group report. J Clin Oncol 2004;22:2781–9. [DOI] [PubMed] [Google Scholar]
- [53].Steven K, Poulsen AL. Radical cystectomy and extended pelvic lymphadenectomy: survival of patients with lymph node metastasis above the bifurcation of the common iliac vessels treated with surgery only. J Urol 2007;178:1218–24. [DOI] [PubMed] [Google Scholar]
- [54].Meijer RP, Nieuwenhuijzen JA, Meinhardt W, et al. Response to induction chemotherapy and surgery in non-organ confined bladder cancer: a single institution experience. Eur J Surg Oncol 2013;39:365–71. [DOI] [PubMed] [Google Scholar]
- [55].Bex A, Ljungberg B, van Poppel H, Powles T. The role of cytoreductive nephrectomy: European Association of Urology Recommendations in 2016. Eur Urol 2016;70:901–5. [DOI] [PubMed] [Google Scholar]
- [56].du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials. Cancer 2009;115:1234–44. [DOI] [PubMed] [Google Scholar]
- [57].Galsky MD, Domingo-Domenech J, Sfakianos JP, Ferket BS. Definitive management of primary bladder tumors in the context of metastatic disease: who, how, when, and why? J Clin Oncol 2016;34:3495–8. [DOI] [PubMed] [Google Scholar]
- [58].Kulkarni GS, Hermanns T, Wei Y, et al. Propensity score analysis of radical cystectomy versus bladder-sparing trimodal therapy in the setting of a multidisciplinary bladder cancer clinic. J Clin Oncol 2017;35:2299–305. [DOI] [PubMed] [Google Scholar]
- [59].Millikan R, Dinney C, Swanson D, et al. Integrated therapy for locally advanced bladder cancer: final report of a randomized trial of cystectomy plus adjuvant M-VAC versus cystectomy with both preoperative and postoperative M-VAC. J Clin Oncol 2001;19:4005–13. [DOI] [PubMed] [Google Scholar]
- [60].Dodd PM, McCaffrey JA, Herr H, et al. Outcome of postchemotherapy surgery after treatment with methotrexate, vinblastine, doxorubicin, and cisplatin in patients with unresectable or metastatic transitional cell carcinoma. J Clin Oncol 1999;17:2546–52. [DOI] [PubMed] [Google Scholar]
- [61].Tonyali S, Yazici S. Does solitary- and organ-confined metastasectomy really improve survival in advanced urologic malignancies? Int Urol Nephrol 2016;48:671–80. [DOI] [PubMed] [Google Scholar]
- [62].Vargas HA, Akin O, Schöder H, et al. Prospective evaluation of MRI, 11C-acetate PET/CTand contrast-enhanced CT for staging of bladder cancer. Eur J Radiol 2012;81:4131–7. [DOI] [PubMed] [Google Scholar]
- [63].Lu Y-Y, Chen J-H, Liang J-A, et al. Clinical value of FDG PET or PET/CT in urinary bladder cancer: A systemic reviewand meta-analysis. Eur J Radiol 2012;81:2411–6. [DOI] [PubMed] [Google Scholar]
- [64].Yoshida S, Koga F, Kobayashi S, et al. Role of diffusion-weighted magnetic resonance imaging in predicting sensitivity to chemoradiotherapy in muscle-invasive bladder cancer. Int J Radiat Oncol 2012;83:e21–7. [DOI] [PubMed] [Google Scholar]
- [65].Shariat SF, Chade DC, Karakiewicz PI, et al. Combination of multiple molecular markers can improve prognostication in patients with locally advanced and lymph node positive bladder cancer. J Urol 2010;183:68–75. [DOI] [PubMed] [Google Scholar]
- [66].Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016;387:1909–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol 2017;18:312–22. [DOI] [PubMed] [Google Scholar]
- [68].Massard C, Gordon MS, Sharma S, et al. Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol 2016;34:3119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 2017;376:1015–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017;389:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]