Abstract
Aim
The aim of this study was to understand whether the dysglycemia associated with SARS-CoV-2 infection persists or reverts when the viral infection resolves.
Methods
We analyzed fasting blood glucose (FBG) after hospital discharge in a cohort of 621 adult cases with suspected COVID-19 pneumonia.
Results
At admission, 18.8% of the patients in our cohort had pre-existing diabetes, 9.3% fasting glucose in the diabetes range without a prior diagnosis (DFG), 26% impaired fasting glucose (IFG), 44.9% normal fasting glucose (NFG), while 2% had no FBG available. FBG categories were similarly distributed in the 71 patients without confirmed COVID-19 pneumonia. During follow-up (median time 6 month) FBG was available for 321 out of the 453 (70.9%) surviving patients and showed a trend to a marginal increase [from 97 (87–116) to 100 (92–114) mg/dL; p = 0.071]. Transitions between FBG categories were analyzed in subjects without pre-existing diabetes (265 out of 321). We identified three groups: (i) patients who maintained or improved FBG during follow-up [Group A, n = 185; from 100 (86–109) to 94 (88–99) mg/dL; p < 0.001]; (ii) patients who moved from the NFG to IFG category [Group B, n = 66: from 89 (85–96) to 106 (102–113) mg/dl; p < 0.001]; (iii) patients who maintained or reached DFG during follow-up [Group C, n = 14: from 114 (94–138) to 134 (126–143) mg/dl; p = 0.035]. Male sex and ICU admission during the hospitalization were more prevalent in Group C compared to Group A or B.
Conclusions
Six months after the SARS-CoV-2 infection DFG was evident in only few patients who experienced severe COVID-19 pneumonia.
Keywords: Fasting blood glucose, COVID-19, Pneumonia, Diabetes
Introduction
Increasing evidences suggest a bidirectional link between COVID-19 pneumonia and diabetes [1–4]. Many studies have confirmed that diabetes and hyperglycemia are risk factors for the progression and poor prognosis of COVID-19 [5–8], including a higher risk for extrapulmonary complications, like cardiac injury [9], end-stage renal disease requiring replacement therapy [10] and thromboembolic events [11]. On the other hand, new-onset diabetes/hyperglycemia [3] and acute metabolic decompensation of pre-existing diabetes [12] are now emerging as complications of SARS-CoV-2 infection and an infection-related diabetes was hypothesized as a result of virus-associated β-cell destruction [13]. In fact, SARS-CoV-2 can infect cells of the human exocrine and endocrine pancreas ex vivo and in vivo [13] since the viral entry receptor angiotensin-converting enzyme 2 (ACE2) is expressed in human pancreatic β-cells and in the human pancreas microvasculature [14, 15]. However, the evidence that the exocrine and endocrine compartments of the pancreas are susceptible to productive SARS-CoV-2 infection does not necessarily imply that in COVID-19 SARS-CoV-2 infection directly affects glucose homoeostasis or triggers diabetes mellitus. For instance, we recently showed that influenza viruses are able to replicate in human pancreatic islets and cause diabetes in animal models [16], but a direct evidence of a correlation between influenza virus infection and diabetes onset in humans was inconsistent [17]. Moreover, the clinical entity of new-onset diabetes/hyperglycemia associated with SARS-CoV-2 infection still has not been adequately characterized and well discriminated from pre-existing diabetes. In fact, it can include previously unrecognized (pre)diabetes (either type 2 or, less likely, type 1 diabetes) [18], since excluding pre-existing diabetes can be difficult in the context of COVID-19 admissions. Most physicians do not request hemoglobin A1c measurements in the absence of a clinical suspicion of diabetes and hemoglobin A1c measurements in the months preceding SARS-CoV-2 infection are almost invariably unavailable for most COVID-19 patients. Many patients may present with stress hyperglycemia or they may be close to the diagnostic criteria for diabetes and exceed the threshold only at the time of SARS-CoV-2 infection. Furthermore, whether SARS-CoV-2 infection affects glucose metabolism more than other infections, as the community-acquired pneumonia, has still to be clarified. To address this gap in our knowledge, we studied a cohort of 621 adult cases hospitalized with suspected COVID-19 pneumonia and assessed the presence of dysglycemia at the time of hospital admission and during post-discharge follow-up to document whether it persists or reverts when the viral infection resolves.
Materials and methods
Study population and data sources
The study population consisted of adult patients (≥ 18 years) with suspected COVID-19 pneumonia admitted between February 25 and May 2, 2020, to the Emergency or Clinical departments of the IRCCS San Raffaele Hospital (Milan, Italy) and for whom a serum sample was stored in our institution biobank. This series of patients is part of an institutional clinical–biological cohort (COVID-BioB; ClinicalTrials.gov Identifier: NCT04318366) of patients with COVID-19 pneumonia [19]. The Institutional Review Board (protocol number 34/int/2020) approved the study. Informed consent was obtained according to IRB guidelines. We defined as a confirmed infection case a patient with SARS-CoV-2 positive reverse-transcriptase polymerase chain reaction from a nasal/throat swab and signs, symptoms and radiological findings suggestive of COVID-19 pneumonia (n = 529). In case of multiple (at least two) SARS-CoV-2 negative reverse-transcriptase polymerase chain reactions in the presence of radiological findings suggestive of COVID-19 pneumonia, subjects were classified with confirmed infection in the presence of positivity for IgM/IgG against SARS-CoV-2 spike protein [20] (n = 21). SARS-CoV-2 infection was excluded in subjects with multiple (at least two) SARS-CoV-2 negative reverse-transcriptase polymerase chain reactions and negativity for IgM/IgG against SARS-CoV-2 spike protein (n = 71). Data were collected through patient interview or medical chart review and entered in a case report form (CRF). Before analysis, CRF data were crosschecked with medical charts and verified by data managers and clinicians for accuracy (last data collection on March 17, 2021).
Laboratory variables
Routine blood tests encompassed serum biochemistry [including renal and liver function, lactate dehydrogenase (LDH)], complete blood count with differential and C-reactive protein (CRP) as inflammation marker. Specific antibodies to different SARS-CoV-2 antigens, interferon alpha-4 and glutamic acid decarboxylase (GAD) were measured by a luciferase immunoprecipitation system (LIPS) assay, as previously described [20–23].
Definition of diabetes
Study participants were defined as having: a) pre-existing diabetes if they had a documented diagnosis of diabetes before the hospital admission for COVID-19 pneumonia [fasting plasma glucose (FPG) ≥ 126 mg/dl or HbA1c ≥ 6.5% (48 mmol/mol), or they were prescribed diabetes medications]; b) new-onset hyperglycemia if they had a mean fasting plasma glucose ≥ 126 mg/dl during the hospitalization for COVID-19 pneumonia in the presence of a negative history for diabetes and/or normal glycated hemoglobin level in the last year when available. We computed mean fasting glucose and glucose variability (standard deviation) from all laboratory fasting glucose values measured during hospitalization. Normal Fasting Glucose (NFG), impaired fasting glucose (IFG) and fasting glucose in the diabetes range (DFG) were defined according to ADA criteria.
Statistical analysis
Categorical variables are reported as frequency or percent, continuous variables as median with interquartile range (IQR) in parenthesis. Categorical variables were compared using Chi-square or Fischer’s exact test, as appropriate; continuous variables using the Mann–Whitney test, Wilcoxon signed rank test or Mann–Whitney U test, as appropriate. Survival was estimated according to Kaplan–Meier. The time-to-event was calculated from the date of symptom onset to the date of the event, or of last follow-up visit, whichever occurred first. Two-tailed P values are reported, with P value < 0.05 indicating statistical significance. All confidence intervals are two-sided and not adjusted for multiple testing. Statistical analyses were performed with the SPSS 24 (SPSS Inc. /IBM). Sankey diagram of transitions between glucose tolerance categories was made by Sankey Diagram Generator by Dénes Csala, based on the Sankey plugin for D3 by Mike Bostock; https://sankey.csaladen.es; 2014.
Results
Study participants
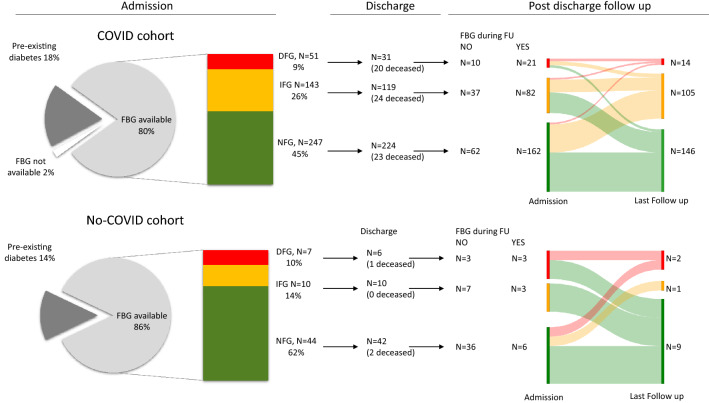
We evaluated a series of 621 adult cases with suspected COVID-19 pneumonia enrolled from February 25 to May 2, 2020, in our institutional clinical–biological cohort (COVID-BioB). A confirmed COVID-19 pneumonia was present in 550 out of 621 (88.6%) of cases (COVID cohort), while the SARS-CoV-2 infection was excluded in the remaining 71 cases (No-COVID cohort). The characteristics of study participants are reported in Table 1. Among 550 patients with confirmed COVID-19, 98 (17.8%) had a pre-existing diabetes [FBG 159 (118–201) mg/dL], 51 (9.3%) had new-onset hyperglycemia [FBG 139 (133–148) mg/dL], 143 (26%) had IFG [FBG 108 (103–116) mg/dL], 247 (44.9%) had NFG [FBG 88 (81–94) mg/dL] while 11 patients had no laboratory fasting glucose measurements. Among 71 patients without confirmed COVID-19 pneumonia, 10 (14.1%) had a pre-existing diabetes [FBG 134 (119–156) mg/dL], seven (9.9%) had new-onset hyperglycemia [FBG 144 (131–174) mg/dL], 10 (14.1%) had IFG [FBG 106 (101–112) mg/dL] and 44 (62%) had NFG [FBG 87 (82–93) mg/dL].
Table 1.
Basal characteristics according to COVID-19 diagnosis
| COVID cohort | No-COVID cohort | p | Missing data | |
|---|---|---|---|---|
| N | 550 | 71 | ||
| Age, years | 63 (46–75) | 63 (53–75) | 0.559 | 0 |
| Sex, male [N (%)] | 357 (64.9) | 39 (54.9) | 0.115 | 0 |
| BMI | 27.7 (24.5–31.2) | 24 (21.4–27.3) | 0.005 | 121 |
| Caucasian [N (%)] | 464 (84.4) | 60 (84.5) | 0.840 | 0 |
| Comorbidities [N (%)] | ||||
| Hypertension | 263 (48.4) | 23 (32.4) | 0.011 | 7 |
| Coronary Artery Diseases | 73 (13.4) | 13 (18.3) | 0.276 | |
| Pre-existing diabetes | 98 (18) | 10 (14.1) | 0.508 | |
| COPD | 31 (5.7) | 13 (18.3) | 0.001 | |
| Chronic Kidney Disease | 64 (11.8) | 7 (9.9) | 0.843 | |
| Cancer | 60 (11) | 16 (22.5) | 0.011 | |
| Neurodegenerative disease | 32 (5.9) | 2 (2.8) | 0.411 | |
| Preadmission treatment [N (%)] | ||||
| ASA | 99 (18.8) | 9 (13.2) | 0.317 | 27 |
| Statin | 94 (17.9) | 11 (16.2) | 0.866 | |
| ACE inhibitors | 87 (16.5) | 10 (14.7) | 0.862 | |
| Angiotensin II Receptor Blockers | 78 (14.8) | 8 (11.8) | 0.586 | |
| ACEI and/or ARB | 154 (29.3) | 17 (25) | 0.569 | |
| Calcium channel blockers | 83 (15.8) | 12 (17.6) | 0.725 | |
| Beta blockers | 126 (24) | 14 (20.6) | 0.649 | |
| Clinical outcomes | ||||
| Median time from symptoms to admission, days | 5 (1–14) | 7 (4–10) | 0.061 | 34 |
| Median follow-up, days (95%CI) | 213 (205–220) | 195 (100–289) | 0.063 | 0 |
| Median hospital stay, days | 14 (8–25) | 10 (5–19) | 0.049 | 0 |
| Invasive ventilation/ICU [N (%)] | 80 (14.5) | 0 (0) | < 0.001 | 0 |
| Death in hospital [N (%)] | 95 (17.45) | 5 (7.1) | 0.027 | 0 |
| Death after hospitalization [N (%)] | 1/455 (0.2) | 2/66 (3) | < 0.001 | 0 |
| Swab negativization, days (95%CI) | 39 (37.4–40.6) | - | 6 | |
| Median time from symptoms, days | ||||
| Random fasting glucose (mg/dl) | ||||
| Median | 101 (89–122) | 84 (86–112) | 0.021 | 11 |
| Max | 114 (98–147) | 97 (85–122) | < 0.001 | |
| Min | 89 (75–105) | 89 (79–106) | 0.521 | |
| Glucose variability (SD) | 17 (10–29) | 17 (8–29) | 0.836 | |
| N° of glucose measurements | 2 (1–5) | 1 (1–2) | < 0.001 | |
| New-onset hyperglycemia [N (%)] | 51 (9.5) | 7 (9.9) | 0.9 | 11 |
Post-discharge follow-up
As of March 18, 2021, the median follow-up time after symptoms onset was 213 (95% CI: 205–220) and 195 (100–289) days for the COVID and No-COVID cohorts, respectively.
We recorded fasting blood glucose during the post-discharge follow with outpatient visits at 1, 3, 6, and 9 months. In the COVID cohort, 97 patients died during follow-up (17.6%; 96 during hospitalization, 1 after hospital discharge), 133 (24.2%) had no glucose measurements either during follow-up (n = 122) or at admission (n = 11). Considering the remaining 321 subjects with available data post-hospital discharge, the FBG showed a trend to a marginal increase during follow-up from 97 (87–116) to 100 mg/dl (92–114) (p = 0.071) (median length of follow-up: 6 months). In the No-COVID cohort five (7%) patients died during and after the hospitalization and 50 (70.4%) had no glucose measurements during follow-up. The remaining 16 subjects with available data post-hospital discharge, showed a marginal not significant decrease in FBG during follow-up from 105 (94–134) to 97 mg/dl (88–142) (p = 0.48).
FBG during follow-up stratified by glucose category at the time of admission
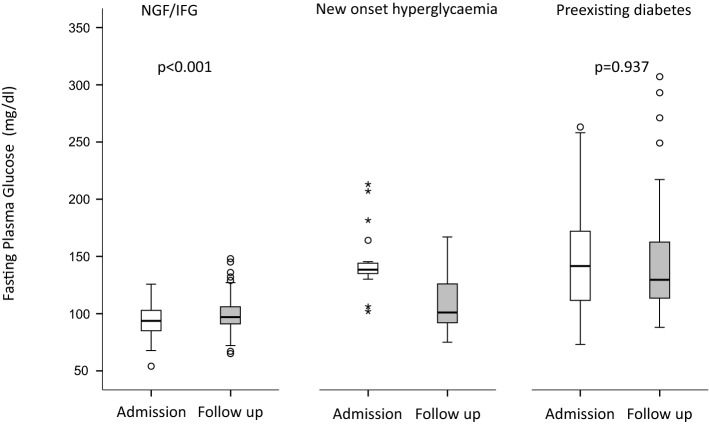
Since the study population was heterogeneous in terms of glycemic glucose levels at baseline, we conducted a sub analysis in the COVID cohort taking into account the dysglycemia state at the time of admission (Fig. 1). Among 98 patients with pre-existing diabetes, 29 died (29.6%) and 13 (13.3%) had no glucose measurements during follow-up. The remaining 56 subjects showed a non-significant decrease in FBG: from 141 (111–172) to 129 mg/dl (113–163) (p = 0.937). Among patients with new-onset hyperglycemia, 20 (39.2%) died and 10 (19.6%) had no glucose measurements during follow-up. Of the remaining 21 subjects, none was prescribed a diabetes treatment during follow-up and they showed a significant decreased of FBG: from 138 (134–145) to 101 mg/dl (91–126) (p = 0.001; Fig. 1). Among patients with IFG/NFG, 47 (12.1%) died and 99 (25.4%) had no glucose measurements during follow-up. The remaining 244 subjects showed a marginal, but significant increase in FBG: from 94 (80–103) to 97 mg/dl (91–106), p < 0.001 (Fig. 1).
Fig. 1.
Fasting blood glucose during follow-up according to fasting glucose category at the time of hospital admission. Glucose measurements at admission and at last follow in patients with pre-existing diabetes (n = 56), new-onset hyperglycemia (n = 21) or IFG/NGF (n = 244) at admission. Depicted are box and whisker plots. The horizontal line within the box is the median; the lower and upper border of the box are the 25th and 75th percentile of the data points, respectively, Whiskers extend to the lower and upper fence. Circles indicate outliers (calculated as 3rd quartile + 1.5 × interquartile range or 1st quartile—1.5 × interquartile range). Asterisks indicate extreme outliers (calculated as 3rd quartile + 3 × interquartile range or 1st quartile—3 × interquartile range). Statistical analysis: Wilcoxon signed-rank test
To identify whether SARS-CoV-2 infection was able to induce dysglycemia in susceptible individuals in the COVID cohort, a Sankey diagram of transitions between FBG categories was drawn for patients without pre-existing diabetes (Fig. 2). Of the 21 patients with new-onset hyperglycemia at admission 6 showed normalization of their fasting glucose [28.5%; 86 mg/dl (83–90)], 9 had IFG [43%; 101 mg/dl (100–110)] while 6 were confirmed as having DFG [28.5%; 141 (126–148) mg/dl]. Of the 82 patients with IFG 48 showed normalization of their fasting glucose [58.5%; 93 mg/dl (87–96)], 30 were confirmed as having IFG [36.6%; 109 (103–117) mg/dl] while four worsened their fasting plasma glucose by entering the DFG range [4.9%; 137 (127–147) mg/dl]. Of the 162 patients with NFG at admission 92 were confirmed as having NFG [56.8%; 92 (85–96) mg/dl], while 70 worsened moving to the IFG [n = 66, 40.7%; 106 (102–113) mg/dl] or DFG [n = 4, 2.5%; 129 mg/dl (126–135)] range. The same analysis was performed also in the No-COVID cohort with similar results (see Fig. 2).
Fig. 2.
Fasting blood glucose during admission and post-discharge follow-up. Valid glucose measurements during follow-up (median 6 month) were available for 265 out of 374 and 12 out of 58 surviving patients without pre-existing diabetes in the COVID and No-COVID cohort, respectively. Sankey diagram of transitions between glucose tolerance categories were drawn using the Sankey Diagram Generator by Dénes Csala, based on the Sankey plugin for D3 by Mike Bostock; https://sankey.csaladen.es; 2014. Left side of each panel is the proportion of individuals with FBG categories upon admission. Right side of each panel is the proportion of individuals with FBG categories at last follow-up. NFG: Normal Fasting Glucose, < 100 mg/dL; IFG: Impaired Fasting Glucose 100–125 mg/dL; DFG: Diabetes Fasting Glucose ≥ 126 mg/dL
Clinical characteristics according to glucose category during follow-up
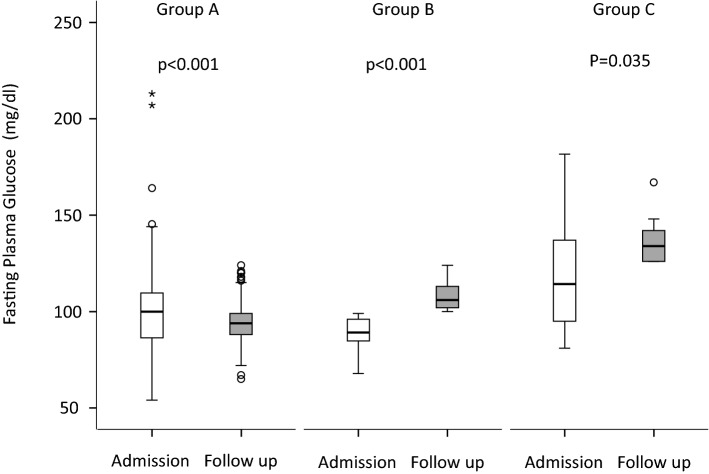
According to the transitions between FBG categories during follow-up, patients in the COVID cohort were divided into three groups (Fig. 3). Group A (n = 185) included patients who maintained or improved their fasting blood glucose category during follow-up (NFG to NFG, n = 92; IFG to IFG/NFG, n = 78; DFG to IFG/NFG, n = 15) with FBG going from 100 (86–109) at admission to 94 (88–99) mg/dl at the last follow-up (p < 0.001). Group B (n = 66) included patients who shifted from the NFG to IFG category in which FBG went from 89 (85–96) at admission to 106 (102–113) mg/dl and at the last follow-up (p < 0.001). Group C (n = 14) included subjects who maintained or reached DFG during follow-up (NFG to DFG, n = 4; IFG to DFG, n = 4; DFG to DFG, n = 6), with FBG going from 114 (94–138) at admission to 134 (126–143) mg/dl at the last follow-up (p = 0.035). The characteristics of the study participants according to these three subgroups are reported in Tables 2 and 3. Male sex and the need of ICU admission during hospitalization showed a significantly higher prevalence in Group C compared to Group A or B.
Fig. 3.
Fasting blood glucose according to the transitions between FBG categories during follow-up. Glucose measurements upon admission and at last follow in patients who maintained or improved their fasting blood glucose category during follow-up (Group A, n = 185; NFG to NFG, n = 92; IFG to IFG/NFG, n = 78; DFG to IFG/NFG, n = 15), who moved from NFG to IFG category (Group B, n = 66), who maintained or shifted to DFG during follow-up (Group C, n = 14; NFG to DFG, n = 4; IFG to DFG, n = 4; DFG to DFG, n = 6). Depicted are box and whisker plots. The line in the box is the median; the lower and upper border of the box are the 25th and 75th percentile of the data points, respectively. Whiskers extend to the lower and upper fence. Circles indicate outliers (calculated as 3rd quartile + 1.5 × interquartile range or 1st quartile—1.5 × interquartile range). Asterisks indicate extreme outliers (calculated as 3rd quartile + 3 × interquartile range or 1st quartile—3 × interquartile range). Statistical analysis: Wilcoxon signed-rank test
Table 2.
COVID cohort baseline characteristics according to the change of fasting glucose category during follow-up of
| Group A | Group B | Group C | p | Missing | |
|---|---|---|---|---|---|
| NFG to NFG | NFG to IFG | NFG to DFG | |||
| IFG to IFG/NFG | IFG to DFG | ||||
| DFG to IFG/NFG | DFG to DFG | ||||
| N | 185 | 66 | 14 | ||
| Age, years | 61 (52–70) | 63 (51–75) | 55 (50.7–57.5) | 0.11 | 0 |
| Sex, male [N (%)] | 123 (66.5) | 42 (63.6) | 14 (100) | 0.026 | 0 |
| BMI | 27.7 (25–31.1) | 28.2 (25.1–31.5) | 29.1 (27.2–31.4) | 0.51 | 2 |
| Ethnicity [N (%)] | |||||
| Caucasian | 155 (83.8) | 53 (80.3) | 92.9) | 0.27 | 0 |
| Hispanic | 19 (10.3) | 12 (18.2) | 0 | ||
| Asian | 4 (2.2) | 0 | 0 | ||
| African | 7 (3.8) | 1 (1.5) | 1 (7.1) | ||
| Comorbidities [N (%)] | |||||
| Hypertension | 76 (41.1) | 29 (43.9) | 4 (28.6) | 0.57 | 0 |
| Coronary Artery Diseases | 13 (7) | 7 (10.6) | 2 (14.3) | 0.47 | |
| COPD | 5 (2.7) | 4 (6.1) | 0 | 0.33 | |
| Chronic Kidney Disease | 11 (5.9) | 8 (12.1) | 1 (7.1) | 0.26 | |
| Cancer | 11 (5.9) | 4 (6.1) | 3 (21.4) | 0.082 | |
| Neurodegenerative disease | 3 (1.6) | 1 (1.5) | 1 (7.1) | 0.33 | |
| Preadmission treatment [N (%)] | |||||
| ASA | 22 (11.9) | 10 (15.4) | 4 (28.6) | 0.19 | 1 |
| Statin | 21 (11.4) | 12 (18.2) | 3 (21.4) | 0.26 | |
| ACE inhibitors | 26 (14.1) | 10 (15.4) | 3 (21.4) | 0.74 | |
| Angiotensin II Receptor Blockers | 26 (14.1) | 6 (9.2) | 1 (7.1) | 0.49 | |
| ACEI and/or ARB | 46 (24.9) | 13 (20) | 4 (28.6) | 0.67 | |
| Calcium channel blockers | 24 (13) | 9 (13.8) | 1 (7.1) | 0.79 | |
| Beta blockers | 34 (18.4) | 14 (21.5) | 3 (21.4) | 0.84 | |
| Admission to the hospital | |||||
| Median time from symptoms to admission, days | 7 (5–10) | 8 (5–11) | 10 (4.5–10) | 0.94 | 0 |
| Symptoms at onset [N (%)] | |||||
| Fever | 170 (94.4) | 61 (92.4) | 13 (92.9) | 0.83 | 5 |
| Dyspnea | 128 (71.1) | 55 (83.3) | 11 (78.6) | 0.14 | |
| Cough | 125 (69.4) | 49 (74.2) | 9 (64.3) | 0.88 | |
| Fatigue/malaise | 117 (65) | 46 (69.7) | 8 (57.1) | 0.62 | |
| Hypo/dysgeusia | 94 (52.8) | 38 (58.2) | 8 (57.1) | 0.72 | |
| Hypo/anosmia | 80 (44.4) | 37 (56.1) | 7 (50) | 0.27 | |
| Myalgia/arthralgia | 66 (36.7) | 25 (37.9) | 3 (21.4) | 0.49 | |
| Headache | 52 (28.9) | 17 (25.8) | 4 (28.6) | 0.89 | |
| Chest pain | 44 (24.4) | 16 (24.2) | 3 (21.4) | 0.97 | |
| Diarrhea | 67(37.2) | 23 (34.8) | 6 (35.7) | 0.94 | |
| Sore throat | 34 (18.9) | 8 (12.1) | 2 (14.3) | 0.44 | |
| Vomiting/nausea | 39 (21.7) | 13 (19.7) | 3 (21.4) | 0.94 | |
| Conjunctivitis | 33 (18.3) | 11 (16.7) | 4 (28.6) | 0.58 | |
| Abdominal pain | 21 (11.7) | 7 (10.6) | 4 (28.6) | 0.16 | |
| Skin rash | 8 (4.7) | 6 (9.7) | 0 | 0.24 | |
Table 3.
COVID cohort clinical laboratory profile and clinical outcome according to dysglycemia during follow-up
| Group A | Group B | Group C | p | Missing | |
|---|---|---|---|---|---|
| NFG to NFG | NFG to IFG | NFG to DFG | |||
| IFG to IFG/NFG | IFG to DFG | ||||
| DFG to IFG/NFG | DFG to DFG | ||||
| N | 185 | 66 | 14 | ||
| Clinical outcomes | |||||
| Median follow-up, days (95%CI) | 226 (215–236) | 218 (184–251) | 233 (97–368) | 0.85 | 0 |
| After admission [N (%)] | |||||
| Discharged | 20 (10.8) | 6 (9.1) | 2 (14.3) | 0.20 | |
| Hospitalized ≤ 7 days | 34 (18.4) | 20 (30.3%) | (7.1) | ||
| Hospitalized > 7 days | 131 (70.8) | 37 (60.6) | 7 (78.6) | ||
| Invasive ventilation/ICU | 22 (11.9) | 10 (15.1) | 4 (28.6) | 0.025 | |
| Median hospital stay, days | 12 (6–29) | 12 (3.75–23.5) | 14 (8–36) | 0.60 | |
| Swab negativization, days (95%CI) | 41 (38–43) | 39 (36–42) | 39 (33–44) | 0.71 | 0 |
| Median time from symptoms, | |||||
| Random fasting glucose (mg/dL) | |||||
| Median | 100 (86–109) | 89 (85–96) | 114 (94–138) | < 0.001 | 0 |
| Max | 109 (95–125) | 101.5 (96–114) | 138 (110–145) | 0.002 | |
| Min | 88 (74–102) | 77 (72–87) | 94 (79–135) | < 0.001 | |
| Glucose variability (SD) | 13.4 (7–21.4) | 13 (9.6–21.6) | 19.7 (11–38.8) | 0.27 | |
| N° of glucose determinations | 2 (1–4) | 3 (2–5.2) | 3.5 (1–7.5) | 0.31 | |
| FPG follow-up (mg/dL) | 94 (88–99) | 106 (102–113) | 134 (126–143) | < 0.001 | |
| Laboratory at admission: | |||||
| White blood cells (× 109/L) | 6.65 (4.92–9.02) | 6.55 (5.2–8.5) | 6.7 (4.6–8.25) | 0.62 | 11 |
| Neutrophil (× 109/L) | 4.85 (3.3–7.22) | 4.9 (3.45–6.59 | 4.4 (2.8–5.8) | 0.49 | 20 |
| Lymphocytes (× 109/L) | 1 (0.7–1.3) | 1.1 (0.7–1.5) | 0.9 (0.72–1.55) | 0.64 | 20 |
| Monocytes (× 109/L) | 0.5 (0.3–0.6) | 0.5 (0.35–0.6) | 0.5 (0.32–0.6) | 0.99 | 20 |
| Hemoglobin (g/L) | 13.6 (12.3–14.7) | 13.4 (12.1–14.1) | 14.1 (12.6–14.8) | 0.33 | 11 |
| Platelets (× 109/L) | 251 (186–342) | 215 (180–283) | 231 (207–333) | 0.084 | 11 |
| Creatinine (mol/L) | 71.7 (58.7–90.7) | 70.1 (55.7–91.5) | 76.2 (67.9–92.3) | 0.48 | 16 |
| Aspartate transaminase (U/L) | 47 (32–72.25) | 39 (28–62) | 48 (41–75) | 0.18 | 33 |
| Alanine transaminase (U/L) | 49 (28–77) | 37 (24–56.7) | 49 (30–82) | 0.17 | 33 |
| Lactate dehydrogenase (U/L) | 356 (278–465) | 321 (240–401) | 318 (294–457) | 0.054 | 44 |
| Total bilirubin (mg/dL) | 0.64 (0.39–0.86) | 0.5 (0.36–0.81) | 0.67 (0.43–1.25) | 0.21 | 44 |
| Phosphatase alkaline (U/L) | 67 (51–94.) | 66.5 (55–80) | 79 (60–274) | 0.41 | 134 |
| C reactive protein (mg/dL) | 55.3 (19.9–121.6) | 46.8 (14–119.3) | 48.5 (6.4–120) | 0.57 | 11 |
| Humoral immune response [N (%)] | |||||
| Sampling time from symptoms, days | 11.5 (8–17) | 13 (7.5–16) | 12.5 (9.5–18) | 0.85 | |
| Anti-GAD antibody | 5 (2.7) | 2 (3) | 1 (7.1) | 0.55 | 1 |
| Interferon alpha-4 Antibody | 10 (5.4) | 2 (3) | 0 | 0.51 | 0 |
| SARS-Cov2 RBD IgG | 124 (67) | 40 (60.6) | 9 (64.3) | 0.64 | 0 |
| SARS-Cov2 RBD IgM | 146 (78.9) | 48 (72.7) | 10 (71.4) | 0.52 | 0 |
| SARS-Cov2 RBD IgA | 130 (70.3) | 39 (59.1) | 11 (78.6) | 0.17 | 0 |
| SARS-Cov2 S1 + S2 IgG | 140 (75.7) | 45 (68.2) | 10 (71.4) | 0.49 | 0 |
| SARS-Cov2 S1 + S2 IgM | 159 (85.9) | 52 (78.8) | 12 (85.7) | 0.39 | 0 |
| SARS-Cov2 S1 + S2 IgA | 166 (89.7) | 55 (83.3) | 13 (92.9) | 0.33 | 0 |
| SARS-Cov2 NP IgG | 137 (74.1) | 49 (74.2) | 11 (78.6) | 0.93 | 0 |
Discussion
Whether diabetes/hyperglycemia associated with SARS-CoV-2 infection should be considered a specific clinical entity is a matter of discussion. To address this issue, we studied a cohort of 621 adult cases with suspected COVID-19 pneumonia, assessing the presence of dysglycemia at the time of admission and whether this persisted or reverted when the viral infection resolved. Our study generated several interesting findings. First, the prevalence of different FBG categories was similar between patients in the COVID and No-COVID cohorts. The No-COVID cohort patients were admitted to the hospital because of clinical signs of infection and respiratory insufficiency, but the diagnosis of COVID-19 was excluded by both molecular and serological testing. Thus, the No-COVID cohort represents a suitable sex and age matched control population to verify whether precipitating factors other than SARS-CoV-2 infection can induce dysglycemia. The results confirmed that dysglycemia per se is not unique to COVID-19, an expected finding as acute intercurrent illness of any kind are associated with metabolic abnormalities including impaired glucose use as well as decreased insulin secretion or increased counter-regulation. Second, new-onset hyperglycemia associated with COVID-19 pneumonia reversed in most patients after the viral infection resolved. A similar behavior was also evident in the No-COVID cohort and it is reasonable to speculate that reversible transient factors, such as inflammation-induced insulin resistance, may be causing hyperglycemia in those patients [24]. Third, a small group of patients without pre-existing diabetes in COVID cohort maintained or achieved DFG during follow-up. These subjects corresponded to 4.3% of patients and showed a high prevalence of male sex and admission to intensive care, suggesting an association of DFG with a worse respiratory function during the acute phase of the disease. Fourth, a larger group of patients (about 20% of the COVID cohort) showed a modest increase in fasting blood glucose during follow-up, resulting in their shift to the IFG category. Of note, group B and group C (subjects whose glycemia increases) show a tendency toward a higher BMI. Even if this difference is not statistically significant, the presence of a higher insulin resistance linked to the weight could be one of the predisposing factors for the development or maintenance of dysglycemia during follow-up. On the other hand, BMI was associated with greater severity of COVID [25], and could be indirectly linked as a proxy for severe infection [26]. This second hypothesis is less likely. In fact, these patients did not differ from the remaining COVID cohort in terms of IgG, IgM and IgA responses to the SARS-CoV-2 spike protein (RBD or S1 + S2), IgG response to NP, autoimmune antibodies anti-GAD, anti-interferon alpha-4, virus clearance, laboratory variables associated with COVID-19 pneumonia severity (C reactive protein, white blood cells, lymphocytes, lactate dehydrogenase), markers of liver and kidney function, comorbidities, preadmission treatments or symptoms at onset (see Tables 2, 3). The interpretation of these results is not simple but they could support the hypothesis that SARS-CoV-2 cannot directly cause dysglycemia and an attribution beyond a random variability of FBG remains unlikely.
Our study has some limitations. First, FBG during follow-up was unavailable for 24% of patients in the COVID cohort. Even if FBG categories were similarly distributed at admission in the patients with or without glucose measurements, we cannot exclude a selection bias. Second, our COVID cohort did not include asymptomatic or pauci-symptomatic patients, as it only includes patients with COVID-19 pneumonia requiring hospital admission. Third, we only assessed fasting blood glucose. Studies on insulin secretion and resistance would have provided relevant information, but conducting such studies during the first wave of the COVID-19 pandemic was essentially impossible. Fourth, the lack of HbA1c measurements in our cohort is an additional limitation. The evidence of normal HbA1c at the time of admission would indicate no history of recent hyperglycemia and confirm the diagnosis of new-onset hyperglycemia. Fifth, the number of subjects included in No-COVID cohort is unbalanced respect to COVID cohort (71 vs 550) and this may prevent you from detecting small differences.
In conclusion, new-onset diabetes/hyperglycemia was documented as a complication of COVID-19 pneumonia. It is clear from our study that only a small proportion of patients without pre-existing diabetes maintained or shifted to DFG during follow-up and this finding may not be related to the SARS-CoV-2 infection. We cannot dismiss the possibility that secondary diabetes can be a distinct clinical entity within ‘long COVID’ or PARS (post-acute sequelae SARS-CoV-2 infection); however, our data suggest that the frequency of this event would be low. Large epidemiological studies in the next years will be required to clarify whether COVID-19 induce permanent diabetes and the alarmistic claims regarding the diabetes risk associated with COVID-19 should be interpreted with caution.
Acknowledgement
Professor Lorenzo Piemonti is the guarantor of this work and, as such, had full access to all the data presented in the study and takes responsibility for the integrity of data and the accuracy of data analysis.
Authors contribution
LP, VL and MS contributed to the conception of the study, wrote the manuscript, researched data and contributed to the discussion. AL, AC and CM, contributed to the acquisition, analysis and interpretation of clinical data, contributed to the design of the study and critically reviewed/edited the manuscript. PRQ and GL contributed to the collection of biological samples, managed the biobanking activities, critically revised the manuscript, recruited patients and managed sample biobanking.
Funding
This work was funded by the IRCCS Ospedale San Raffaele (Program Project COVID-19) and the Italian Ministry of Health (Ministero della Salute COVID-2020–12371617).
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to concerns about data confidentiality, but are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors have no conflict of interest to disclose in relation to the topic of this manuscript. The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Ethics Committee of the IRCCS Ospedale San Raffaele (protocol number 34/int/2020).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shang L, Shao M, Guo Q, et al. Diabetes mellitus is associated with severe infection and mortality in patients with COVID-19: a systematic review and meta-analysis. Arch Med Res. 2020;51(7):700–709. doi: 10.1016/j.arcmed.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- 3.Rubino F, Amiel SA, Zimmet P, et al. New-onset diabetes in Covid-19. N Engl J Med. 2020;383(8):789–790. doi: 10.1056/NEJMc2018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muniangi-Muhitu H, Akalestou E, Salem V, Misra S, Oliver NS, Rutter GA. Covid-19 and diabetes: a complex bidirectional relationship. Front Endocrinol. 2020;11:758. doi: 10.3389/fendo.2020.582936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mantovani A, Byrne CD, Zheng M-H, Targher G. Diabetes as a risk factor for greater COVID-19 severity and in-hospital death: a meta-analysis of observational studies. Nutr Metab Cardiovasc Dis. 2020;30(8):1236–1248. doi: 10.1016/j.numecd.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hussain S, Baxi H, Jamali MC, Nisar N, Hussain MS. Burden of diabetes mellitus and its impact on COVID-19 patients: a meta-analysis of real-world evidence. Diabetes Metab Syndr. 2020;14(6):1595–1602. doi: 10.1016/j.dsx.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Figliozzi S, Masci PG, Ahmadi N, et al. Predictors of adverse prognosis in COVID-19: a systematic review and meta-analysis. Eur J Clin Investig. 2020;50(10):e13362. doi: 10.1111/eci.13362. [DOI] [PubMed] [Google Scholar]
- 8.Aggarwal G, Lippi G, Lavie CJ, Henry BM, Sanchis-Gomar F. Diabetes mellitus association with coronavirus disease 2019 (COVID-19) severity and mortality: a pooled analysis. J Diabetes. 2020;12(11):851–855. doi: 10.1111/1753-0407.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee MH, Wong C, Ng CH, Yuen DC, Lim AY, Khoo CM. Effects of hyperglycaemia on complications of COVID-19: a meta-analysis of observational studies. Diabetes Obes Metab. 2021;23(1):287–289. doi: 10.1111/dom.14184. [DOI] [PubMed] [Google Scholar]
- 10.Fu EL, Janse RJ, de Jong Y, et al. Acute kidney injury and kidney replacement therapy in COVID-19: a systematic review and meta-analysis. Clin Kidney J. 2020;13(4):550–563. doi: 10.1093/ckj/sfaa160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piazza G, Campia U, Hurwitz S, et al. Registry of arterial and venous thromboembolic complications in patients with COVID-19. J Am Coll Cardiol. 2020;76(18):2060–2072. doi: 10.1016/j.jacc.2020.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, Wang X, Chen J, Zuo X, Zhang H, Deng A. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes Metab. 2020;22(10):1935–1941. doi: 10.1111/dom.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muller JA, Gross R, Conzelmann C, et al. SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat Metab. 2021;3(2):149–165. doi: 10.1038/s42255-021-00347-110.1038/s42255-021-00347-1[pii]. [DOI] [PubMed] [Google Scholar]
- 14.Liu F, Long X, Zhang B, Zhang W, Chen X, Zhang Z. ACE2 expression in pancreas may cause pancreatic damage after SARS-CoV-2 infection. Clin Gastroenterol Hepatol. 2020;18(9):2128–2130.e2122. doi: 10.1016/j.cgh.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fignani D, Licata G, Brusco N, et al. SARS-CoV-2 receptor angiotensin I-converting enzyme type 2 (ACE2) is expressed in human pancreatic β-cells and in the human pancreas microvasculature. Front Endocrinol. 2020;11:876. doi: 10.3389/fendo.2020.596898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Capua I, Mercalli A, Pizzuto MS, et al. Influenza A viruses grow in human pancreatic cells and cause pancreatitis and diabetes in an animal model. J Virol. 2013;87(1):597–610. doi: 10.1128/JVI.00714-12JVI.00714-12[pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capua I, Mercalli A, Romero-Tejeda A, et al. Study of 2009 H1N1 pandemic influenza virus as a possible causative agent of diabetes. J Clin Endocrinol Metab. 2018;103(12):4343–4356. doi: 10.1210/jc.2018-008625091458[pii]. [DOI] [PubMed] [Google Scholar]
- 18.Accili D. Can COVID-19 cause diabetes? Nat Metab. 2021;3(2):123–125. doi: 10.1038/s42255-020-00339-710.1038/s42255-020-00339-7[pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ciceri F, Castagna A, Rovere-Querini P, et al. Early predictors of clinical outcomes of COVID-19 outbreak in Milan. Italy. Clin Immunol. 2020;217:108509. doi: 10.1016/j.clim.2020.108509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Secchi M, Bazzigaluppi E, Brigatti C, et al. COVID-19 survival associates with the immunoglobulin response to the SARS-CoV-2 spike receptor binding domain. J Clin Invest. 2020;130(12):6366–6378. doi: 10.1172/JCI142804142804[pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bastard P, Rosen LB, Zhang Q, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020 doi: 10.1126/science.abd4585science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lampasona V, Secchi M, Scavini M, et al. Antibody response to multiple antigens of SARS-CoV-2 in patients with diabetes: an observational cohort study. Diabetologia. 2020;63(12):2548–2558. doi: 10.1007/s00125-020-05284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dispinseri S, Lampasona V, Secchi M, et al. Robust neutralizing antibodies to SARS-CoV-2 develop and persist in subjects with diabetes and COVID-19 pneumonia. J Clin Endocrinol Metab. 2021;106(5):1472–1481. doi: 10.1210/clinem/dgab055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sathish T, Tapp RJ, Cooper ME, Zimmet P. Potential metabolic and inflammatory pathways between COVID-19 and new-onset diabetes. Diabetes Metab. 2020 doi: 10.1016/j.diabet.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kass DA, Duggal P, Cingolani O. Obesity could shift severe COVID-19 disease to younger ages. London England: Lancet; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rizza S, Coppeta L, Grelli S, et al. High body mass index and night shift work are associated with COVID-19 in health care workers. J Endocrinol Invest. 2021;44(5):1097–1101. doi: 10.1007/s40618-020-01397-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to concerns about data confidentiality, but are available from the corresponding author on reasonable request.