Abstract
Purpose:
To perform a systematic review and pooled meta-analysis of adrenal metastasis stereotactic body radiation therapy (SBRT) outcomes, treatment characteristics, and toxicity to define the efficacy and propose guidelines for intervention.
Methods and Materials:
We performed a comprehensive literature search of the Embase and PubMed databases of studies reporting outcome or toxicity data for photon-based SBRT of adrenal metastases in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. We then conducted a meta-analysis to estimate pooled overall response, local control (LC), and overall survival and analyzed these outcomes in the context of dosimetric parameters and toxicity using metaregression.
Results:
Thirty-nine studies published between 2009 and 2019 reporting outcomes on 1006 patients were included. The median follow-up was 12 months, and the median biological equivalent dose (BED10, alpha/beta = 10) was 67 Gy. The pooled overall response was 54.6% (95% confidence interval [CI], 46.5%-62.5%). The pooled 1- and 2-year rates of LC were 82% (95% CI, 74%-88%) and 63% (95% CI, 50%-74%), respectively, and the pooled 1- and 2-year overall survival rates were 66% (95% CI, 57%-74%) and 42% (95% CI, 31%-53%), respectively. There was a strong positive association between SBRT dose and 1- and 2-year LC(P < .0001, P = .0002) and an association with 2-year OS (P = .03). Based on a metaregression of dose and LC, BED10 of 60Gy, 80 Gy, and 100 Gy predicted 1-year LC of 70.5%, 84.8%, and 92.9% and 2-year LC of 47.8%, 70.1%, and 85.6%, respectively. The overall rate of grade 3 or higher toxicity was 1.8%.
Conclusions:
SBRT of adrenal metastases provides good 1-year LC with an excellent safety profile, and dose escalation may be associated with improved LC. Prospective studies are needed to validate these findings and determine whether there are subsets of patients for whom adrenal metastasis–directed SBRT may confer a survival advantage. Published by Elsevier Inc.
Summary
A systematic review and pooled meta-analysis of studies reporting outcomes of stereotactic body radiation therapy for adrenal metastases was performed. Stereotactic body radiation therapy provided good 1-year local control with an excellent safety profile, and dose escalation was found to correlate with improved local control.
Introduction
In recent years, there has been growing interest in oligometastasis-directed local therapy based on the hypothesis that cytoreductive and ablative treatments may improve the outcomes of patients with a limited burden of systemic disease.1–4 The adrenal glands are a common site of metastasis from lung cancer, renal cell carcinoma, and melanoma, and previous studies have reported good outcomes after surgical adrenalectomy or other invasive approaches such as radiofrequency ablation for the treatment of adrenal metastases.5,6
Stereotactic body radiation therapy (SBRT) has emerged as an important treatment modality that allows conformal delivery of ablative doses of radiation therapy in a limited number of fractions. In the last decade, a growing number of small retrospective series have been published on SBRT treatment of adrenal metastases.7–44 However, because cases of adrenal metastasis that are amenable to SBRT and in an appropriate clinical setting are relatively uncommon, these retrospective reports have been limited in sample size, which has hindered robust estimates of treatment efficacy and identification of optimal dosimetric parameters. In light of these limited data, there are also concerns regarding the safety of SBRT for adrenal metastases, particularly with regard to renal toxicity, adrenal insufficiency,45 and damage to regional gastrointestinal viscera.46 To our knowledge, no comprehensive meta-analysis has been performed on this topic. A prior qualitative systematic review including 10 studies was published in 201547; however, a significant number of additional studies have been published since then, but no quantitative pooled meta-analysis has been performed to date. Thus, the aim of this study was to identify and pool the collective experience in the English-language literature, with a focus on response rate, local control (LC), overall survival (OS), dosimetry, SBRT technique, and toxicity, to define the efficacy and propose guidelines for adrenal metastasis–directed SBRT.
Methods and Materials
Literature search and inclusion and exclusion criteria
A comprehensive search of the English-language literature was conducted in September 2019 using the Embase and PubMed electronic databases with the following query: (sbrt OR stereotactic OR radiosurgery OR sabr OR knife) AND (adrenal/exp OR adrenal) AND (metastasis/exp OR metastasis OR metastases/exp OR metastases OR metastatic). Studies from any period were included. Duplicate and non-English results were removed, and the subsequent list of studies was systematically screened for relevance first by title and then by assessment of the abstract and full text. Studies were excluded from the meta-analysis if (1) there were no outcome or toxicity data specific to stereotactic radiation therapy of adrenal metastases; (2) the study contained technical or dosimetric data only and no patient outcome or toxicity data; (3) the study was a review, editorial, or commentary; (4) the study reported redundant data already reported in another study; (5) the study reported results of proton therapy; or (6) there were fewer than 5 patients in the study. Thus, studies reporting clinical outcome or toxicity data for photon-based stereotactic radiation therapy of adrenal metastases in 5 or more patients were included in this meta-analysis and systematic review.
SBRT was defined as the delivery of higher fractional doses of radiation than conventional fractionation (> 1.8-2.5 Gy) in a relatively small number of fractions, using external beam radiation therapy to a well-defined target and using image guidance or motion management to deliver greater conformality because of sensitive organs at risk. Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines were followed.48
Data extraction
The literature search and study screening were performed by a single investigator (W.C.C.). Studies meeting all inclusion and exclusion criteria were then divided, and data extraction was performed by 4 investigators (W.C.C., J.P., J.D.B., and U.B.). A single investigator (W.C.C.) subsequently re-reviewed extracted data from all included studies and discussed discrepancies with other investors to achieve a consensus. No attempt was made to contact study authors for additional data.
Clinical and dosimetric characteristics were extracted from each study. If median or mean biological equivalent dose using alpha/beta of 10 (BED10) to the target was reported, this was extracted. Otherwise, the study’s representative BED10 was calculated from the reported mean/median prescribed dose and fractionation by applying the standard linear quadratic formula, BED10 = nd × (1 + d/[a/b]), where a/b = 10 and n and d represent the number of fractions and dose per fraction, respectively. An estimated maximum BED10 was calculated by dividing the BED10 reported by the percent isodose prescribed to. For example, a BED10 of 50 prescribed to the 80% isodose line would have an estimated maximum BED10 of 62.5.
If planning target volume (PTV) was not reported, an estimated PTV was calculated based on the gross tumor volume (GTV) and the reported margins used. The GTV was modeled as a sphere, an isotropic margin was applied, and the estimated PTV volume was calculated.
Outcomes extracted included rates of partial and complete response (CR) based on Response Evaluation Criteria in Solid Tumors,49 which were combined for overall response (OR) analyses, 1-year and 2-year actuarial LC and OS, and crude LC or OS. Toxicity data were also collected. Studies were variable in reporting of low-grade toxicity, but the presence or absence of high-grade (Common Terminology Criteria for Adverse Events [CTCAE] version 4.0, grade 3-5) toxicity was uniformly reported.50 Particular attention was paid to report of renal or adrenal toxicity.
Statistical analysis
A pooled analysis was performed to determine the weighted study level rates of 1- and 2-year actuarial LC and OS, as well as rates of CR and OR. Because the reported rates of grade 3+ toxicity were uniformly low and frequently 0, we reported the overall number of adverse events rather than estimating a pooled statistic. Both random effects and fixed effects models were calculated. Because data on the number of patients lost to follow-up were not available, the number of surviving or nonrecurrent patients at 1 and 2 years was estimated simply as the reported actuarial rate multiplied by the sample size. Meta-analysis of binomial proportions was performed using the metaprop function in the meta package (version 4.9-6) in R (version 3.6.1).51 To investigate associations between study characteristics and reported outcomes, univariate metaregression was performed using the metareg function in the meta package.
Because these methods and assumptions may have resulted in underestimation of study variances, we validated our pooled analysis of time-to-event data using a previously described parametric bootstrap method.52 In brief, to simulate study-level survival data, parametric exponential models were used to model the survival and censoring processes. For the survival process, a 1-parameter exponential distribution was fit to the available 1-year or 2-year actuarial LC or OS using the least-squares method. The censorship process was also modeled as an exponential distribution with the median equal to the median follow-up time reported. Five hundred simulated survival curves were subsequently randomly generated for each study, and bootstrap estimated standard errors were calculated for 1- and 2-year LC or OS. Standard errors were then used to estimate a pooled weighted summary statistic for the respective outcomes using the metagen function in the meta package in R.
Results
Literature search and study characteristics
A comprehensive search of the English-language literature reporting LC, OS, or toxicity outcomes after SBRT of adrenal metastases was performed in September 2019 (Fig. 1). A total of 569 references were identified, out of which 39 studies published between 2009 and 2019 were identified that met inclusion and exclusion criteria and were ultimately included in this meta-analysis.7–44 Summary study characteristics are reported in Table 1, and study-level characteristics are reported in Table 2. The majority of patients had a primary lung malignancy (Table 1). Concurrent chemo- or immunotherapy was rare, and, although report of these characteristics was uncommon, a sizeable minority of patients had adrenal metastasis as the sole site of metastatic disease, and most patients presented with metachronous rather than synchronous adrenal metastasis (Table 1).
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-analyses flowchart.
Table 1.
Pooled study characteristics
| Characteristics | |
|---|---|
| No. of studies | 39 (13 abstracts, 1 thesis) |
| No. of patients | 1006 (median 24, range 7-58) |
| Median age (29 studies), y | 63.0 (range, 25.8-91.0) |
| Median follow up (32 studies), mo | 12.0 mo (range, 5.5-41.0) |
| Median follow up interval (16 studies), mo | 3.0 mo (range, 2.0-4.0) |
| Clinical characteristics | |
| Primary tumor (38 studies, N = 976) | n patients, % |
| Lung | 641, 65.7 |
| Renal | 62, 6.4 |
| Colorectal | 45, 4.6 |
| Melanoma | 38, 3.9 |
| Liver | 28, 2.9 |
| Breast | 16, 1.6 |
| Other/unknown | 145, 14.8 |
| Concurrent chemotherapy (14 studies, N = 326) | 19, 5.8 (range, 0%-44.4%) |
| Concurrent immunotherapy (8 studies, N = 223) | 2, 0.9 (range, 0%-7.7%) |
| Adrenal metastasis only (9 studies, N = 229) | 100, 43.7 (range, 20.0%-72.2%) |
| Nonadrenal metastasis present (9 studies, N = 229) | 129, 56.3 (range, 27.8%-71.4%) |
| Synchronous adrenal metastasis (6 studies, N = 96) | 26, 27.1 (range, 10.6%-55.5%) |
| Bilateral adrenal metastases (24 studies, N = 661) | 63, 8.3 (range, 0%-28.6%) |
| Dosimetric characteristics | |
| Median BED10 (36 studies), Gy | 67.0 (range, 37.5-112.5) |
| Median dose/fractions (33 studies), Gy | 38.0 (range, 18-60)/5 (range, 1-10) |
| Tumor motion management (28 studies) | n studies, % |
| Fiducial | 6, 21 |
| 4DCT-ITV or similar | 13, 46 |
| Respiratory tracking | 6, 21 |
| Breath hold | 3, 11 |
| Tumor response and toxicity | |
| n patients, % | |
| OR/CR (22/21 studies, N = 474/506): | 287, 55.2/89, 17.1 |
| Pain relief (7 studies, N = 42): | 36, 85.7 |
| Grade 3+ toxicity (39 studies, N = 1006): | 18, 1.8 |
Abbreviations: 4DCT-ITV = 4-dimensional computed tomography internal target volume; BED10 = biologically equivalent dose (alpha/beta = 10); CR = complete response; OR = overall response.
Table 2.
Individual study level characteristics
| Author, year | N | F/u (mo) | Age | Lung, (%) | PTV, cm3 | CR, n (%) | PR, n (%) |
|---|---|---|---|---|---|---|---|
| Chawla, 200940 | 30 | 9.8 | 61.8 | 20 (67) | 1 (3) | 15 (50) | |
| Levin, 201013 | 16 | 13 (81) | 2 (13) | 5 (31) | |||
| Holy, 201141 | 18 | 21.0 | 61.5 | 18 (100) | 176.0 | 8 (44) | |
| Rudra, 201144 | 10 | 14.9 | 58.0 | 8 (80) | 0 (0) | 4 (40) | |
| Scorsetti, 201231 | 34 | 41.0 | 67.0 | 26 (76) | 3 (9) | 13 (38) | |
| Torok, 201143 | 7 | 14.0 | 60.0 | 5 (71) | 1 (14) | 2 (29) | |
| Oshiro, 201116 | 19 | 10.1 | 63.0 | 19 (100) | 9 (47) | 4 (21) | |
| Casamassima, 201242 | 48 | 16.2 | 62.7 | 24 (50) | 5 (10) | 11 (23) | |
| Guiou, 201212 | 9 | 7.3 | 59.2 | 9 (100) | 387.6 | 0 (0) | 6 (67) |
| Engin, 201214 | 24 | 11.0 | 60.0 | 19 (79) | 9 (38) | 14 (58) | |
| Ahmed, 201315 | 13 | 12.3 | 71.0 | 6 (46) | 88.3 | 2 (15) | 9 (69) |
| Li, 201319 | 26 | 17.0 | 64.0 | 7 (27) | 3 (12) | 12 (46) | |
| Rochefort, 201417 | 32 | 15.0 | 15 (47) | ||||
| Lindberg, 201418 | 58 | 12.6 | 64.0 | 31 (53) | |||
| Anchuelo Latorre, 201420 | 14 | 12 (86) | 75.0 | ||||
| Desai, 201521 | 14 | 11.0 | 65.0 | 6 (43) | 1 (7) | 4 (29) | |
| Gamsiz, 201532 | 15 | 16.0 | 56.0 | 15 (100) | 57.4 | 3 (20) | 5 (33) |
| Francis, 201564 | 19 | 9.2 | 72.0 | 9 (47) | |||
| Wang, 201622 | 26 | 66.0 | 16 (62) | ||||
| Celik, 201738 | 15 | 24.0 | 70.0 | 15 (100) | 74.5 | 0 (0) | 8 (53) |
| Chance, 201723 | 43 | 36 (84) | |||||
| Franzese, 201736 | 46 | 7.6 | 46.0 | 30 (65) | 63.0 | 15 (33) | |
| Haidenberger, 201733 | 23 | 23.6 | 63.5 | 11 (48) | 48.6 | ||
| Plichta, 201737 | 10 | 6.0 | 66.0 | 6 (60) | |||
| Lederman, 201726 | 23 | 10.0 | 62.0 | 23 (100) | 44.2 | ||
| Buergy, 201834 | 18 | 10 (56) | |||||
| Katoh, 201825 | 20 | 17.5 | 65.0 | 9 (45) | 148.0 | 3 (15) | 6 (30) |
| Toesca, 201839 | 35 | 37.0 | 66.0 | 17 (49) | 50.5 | ||
| Zhao, 201835 | 30 | 10.7 | 63.0 | 27 (90) | 7 (23) | 10 (33) | |
| Al-Assaf, 201824 | 46 | 12.9 | 66.0 | 26 (57) | |||
| Knippen, 201828 | 33 | 11.0 | 19 (58) | ||||
| Scouarnec, 201930 | 31 | 18.0 | 63.0 | 14 (45) | 10 (32) | 10 (32) | |
| Shah, 201929 | 44 | 5.4 | 61.3 | 29 (66) | 49.7 | 2 (5) | 13 (30) |
| Georgiev, 201927 | 8 | 5.5 | 6 (75) | 28.5 | 2 (25) | 1 (13) | |
| Figura, 20198 | 41 | 10.5 | 67.0 | 25 (61) | 31.7 | ||
| Voglhuber, 201910 | 24 | 11 (46) | |||||
| Chiang, 20197 | 29 | 9.5 | 29 (100) | 79.0 | 3 (10) | 19 (66) | |
| Everett, 201911 | 30 | ||||||
| Cobussen, 20199 | 25 | 11.6 | 62.0 | 21 (84) | |||
| Median dose (range), Gy | Median fractions (range) | BED10 | Isodose prescription | CTV/ITV-PTV, mm | Motion management | Modality | G3+ toxicity, n (%) |
| 40 (16-50) | 10 (4-16) | 56.0 | 80% | 7-10 | Breath hold. ExacTrac | LINAC | 0 (0) |
| 0 (0) | |||||||
| 38 (15-40) | 5 (3-12) | 64.8 | 100% | 5-10 | Radioscopy-ITV | LINAC | 0 (0) |
| 30 (24-50) | 3 (3-10) | 60.0 | 80%-90% | 5-10 | 4DCT-ITV | 0 (0) | |
| 32 (20-45) | 4 (4-18) | 57.6 | 95% | 5 | 4DCT-ITV | LINAC | 0 (0) |
| 18 (10-36) | 1 (1-3) | 41.6 | 80%-94% | 0 | Respiratory tracking | CK | 0 (0) |
| 50 (30-60) | 10 (1-27) | 75.0 | 5-10 | Breath hold | LINAC | 0 (0) | |
| 36 (21.6-54.1) | 3 (1-3) | 79.2 | 70% | 3 | End exp/insp CT ITV | LINAC | 0 (0) |
| 25 (20-37.5) | 5 (5-5) | 37.5 | 4DCT-ITV | LINAC | 0 (0) | ||
| 24 (NR) | 3 (NR) | 43.2 | 78% | CK | 0 (0) | ||
| 45 (33.8-60) | 5 (5-5) | 85.5 | 5 | 4DCT-ITV | LINAC | 0 (0) | |
| 45 (30-50) | 5 (3-5) | 85.5 | 70% | 2-3 | Fiducial. Spine tracking | CK | 0 (0) |
| 45 (NR) | 3 (NR) | 71.6 | 80% | 1 (3.1) | |||
| NR (14-60) | NR (2-15) | 83.7 | 80% | 5-10 | 4DCT-ITV | LINAC | 8 (12.1) |
| 56 (NR) | 7 (NR) | 100.0 | 5 | Fiducial. Exactrac. 4DCT-ITV | LINAC | 0 (0) | |
| 25 (13-30) | 3 (1-5) | 45.8 | Respiratory tracking | CK | 0 (0) | ||
| 30 (30-30) | 3 (3-3) | 60.0 | 90%-100% | 3-5 | Respiratory tracking | LINAC | 0 (0) |
| 36 (8-50) | 3 (1-4) | 79.2 | 4D-CBCT-ITV | LINAC | 0 (0) | ||
| 35 (30-50) | 5 (3-14) | 59.5 | 1 (3.8) | ||||
| 42 (42-42) | 6 (6-6) | 71.4 | 65% | 4 | Respiratory tracking | CK | 0 (0) |
| 60 (NR) | 10 (NR) | 96.0 | 0 (0) | ||||
| 40 (40-40) | 4 (4-4) | 80.0 | 95% | 5 | 4DCT-ITV | LINAC | 0 (0) |
| 22 (20-45) | 1 (1-3) | 70.4 | 60%-70% | 4 | Fiducial. Respiratory tracking | CK | 0 (0) |
| 30 (30-48) | 3 (3-5) | 60.0 | 5 | 4DCT-ITV | LINAC | 0 (10) | |
| 40 (25-45) | 8 (5-8) | 60.0 | 0 (0) | ||||
| 35 (20-60) | 7 (4-25) | 52.5 | 95% | DIBH | LINAC | 0 (0) | |
| 48 (NR) | 8 (NR) | 76.8 | Isocenter | 2 | Fiducial. 4DCT-ITV | Robotic SBRT | 0 (0) |
| 40 (21.4-57.3) | 5 (1-6) | 72.0 | 95% | 2-3 | Fiducial. 4DCT-ITV | LINAC | 0 (0) |
| 44.4 (32-50) | 5 (3-8) | 85.5 | 66%-82% | 3-5 | Respiratory or spine tracking | CK | 1 (3.3) |
| NR (30-48) | (4-6) | 2 (4.3) | |||||
| 67.2 | 90% | Exactrac. 4DCT-ITV | LINAC | 0 (0) | |||
| 45 (30-55) | 3 (3-9) | 112.5 | 62%-90% | 3-5 | Fiducial. 4DCT-ITV | CK | 0 (0) |
| 30 (14-40) | 5 (1-6) | 48.0 | 0-6 | 4DCT-ITV | LINAC | 1 (2.3) | |
| 30 (18-40) | 5 (1-5) | 48.0 | 92% | 3-5 | 4DCT-ITV | LINAC | 0 (0) |
| 50 (NR) | NR | 1 (2.4) | |||||
| 35 (25-40) | 7 (5-8) | 52.5 | 0 (0) | ||||
| 66.7 | 0 (0) | ||||||
| 60.0 | 0 (0) | ||||||
| 50 (24-60) | 5 (3-8) | 100.0 | 3 | Respiratory tracking | MRI-LINAC | 1 (4) |
Abbreviations: 4D = 4-dimensional; BED10 = biologically equivalent dose (alpha/beta = 10); CBCT = cone beam computed tomography; CR = complete response; CT = computed tomography; CTV = clinical target volume; DIBH = deep inspiration breath hold; ITV = internal target volume; LINAC = linear accelerator; MRI = magnetic resonance imaging; NR = no response; PR = partial response; PTV = planning target volume.
Eleven studies (28%) contained information regarding the method of diagnosis; most simply noted that imaging and clinical features were used to diagnose adrenal metastases, with rare cases of tissue confirmation. Only 1 study, that by Li et al,19 reported that a majority of patients received biopsy and tissue confirmation at the same time as fiducial placement. Only 1 study, by Toesca et al,39 specified that all patients received positron emission tomography, which was used for diagnosis of adrenal metastasis.
Dosimetric characteristics and tumor motion management
Dosimetric characteristics and tumor motion strategies from the included studies are reported in Tables 1 and 2. The median prescribed dose was 38 Gy, and the median number of fractions was 5. The median PTV reported was 63.0 cm3 (range, 28.5-387.6 cm3), and a variety of isodose prescriptions and target volume expansions were reported (Table 2).
Twenty-eight studies reported tumor motion management strategies (Table 1). Studies reporting the use of fiducial tracking also reported a higher BED10 (median, 81.2 Gy) compared with studies using 4-dimensional computed tomography (median, 64.8 Gy; P = .017, Student’s t test), respiratory tracking (median, 65.7 Gy) or breath hold (median, 56.0 Gy), as well as a significantly higher 1-year LC (model sum of squares [QM] = 5.74, P = .02, compared with all other studies) and a trend toward a higher 2-year LC (QM = 2.96, P = .09). There was no difference in OS or radiation-related toxicity according to tumor motion management technique. Only 1 study reported the rate of complications related to fiducial placement, which was 10.7% (3 of 26 patients), including 1 hematoma and 2 occurrences of pneumothorax.30
Local control and overall survival after stereotactic body radiation therapy of adrenal metastases
Twenty-four studies comprising 667 patients treated with SBRT to adrenal metastases reported 1-year actuarial LC, and 16 studies comprising 482 patients reported 2-year actuarial LC. The random-effects pooled 1-year and 2-year LC rates were 82% (95% confidence interval [CI], 74%-88%) and 63% (95% CI, 50%-74%), respectively (Fig. 2A, 2B).
Fig. 2.
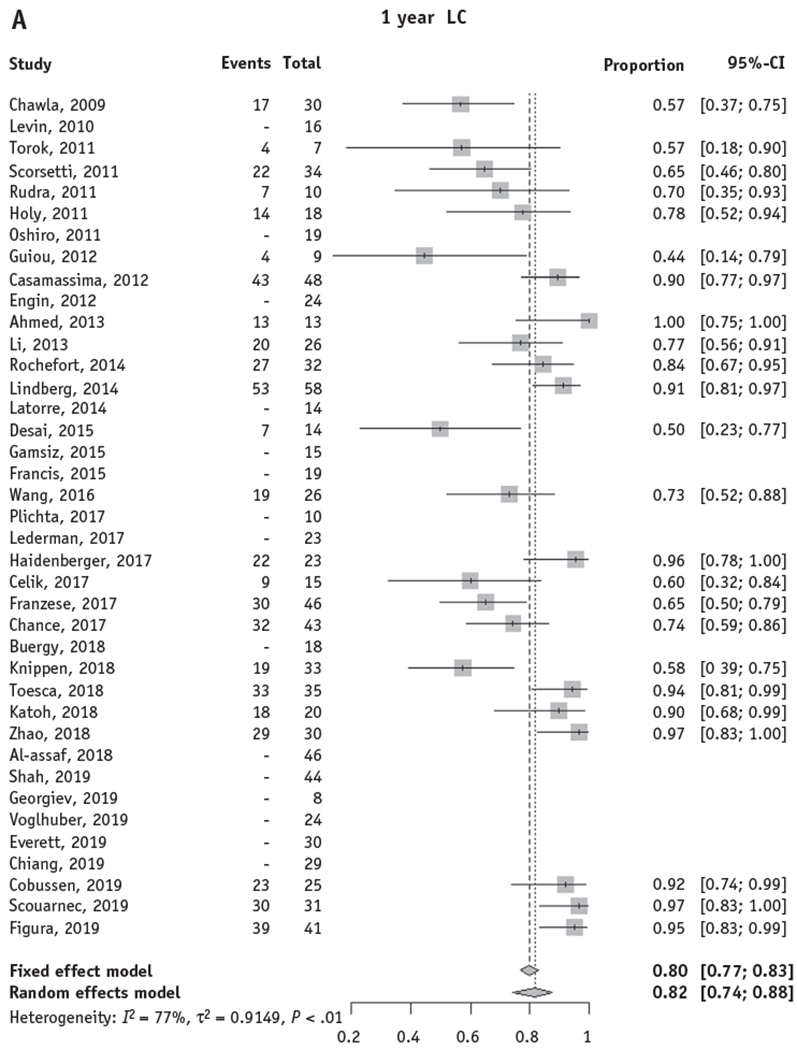

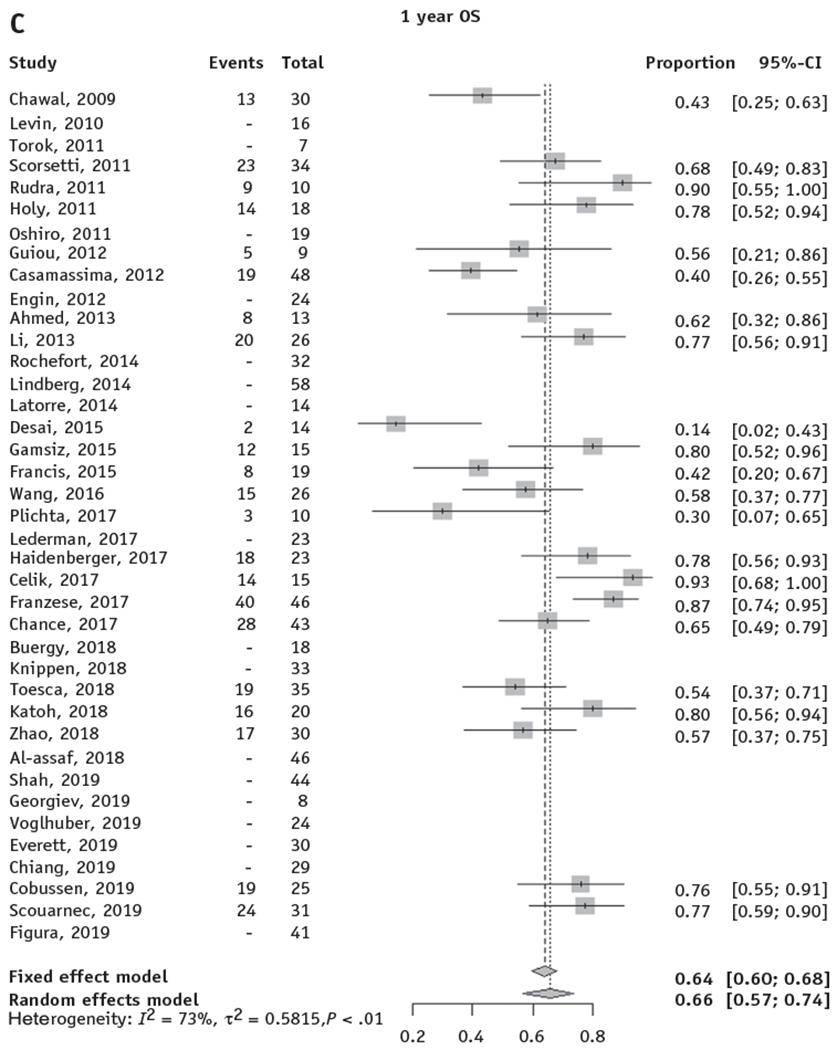
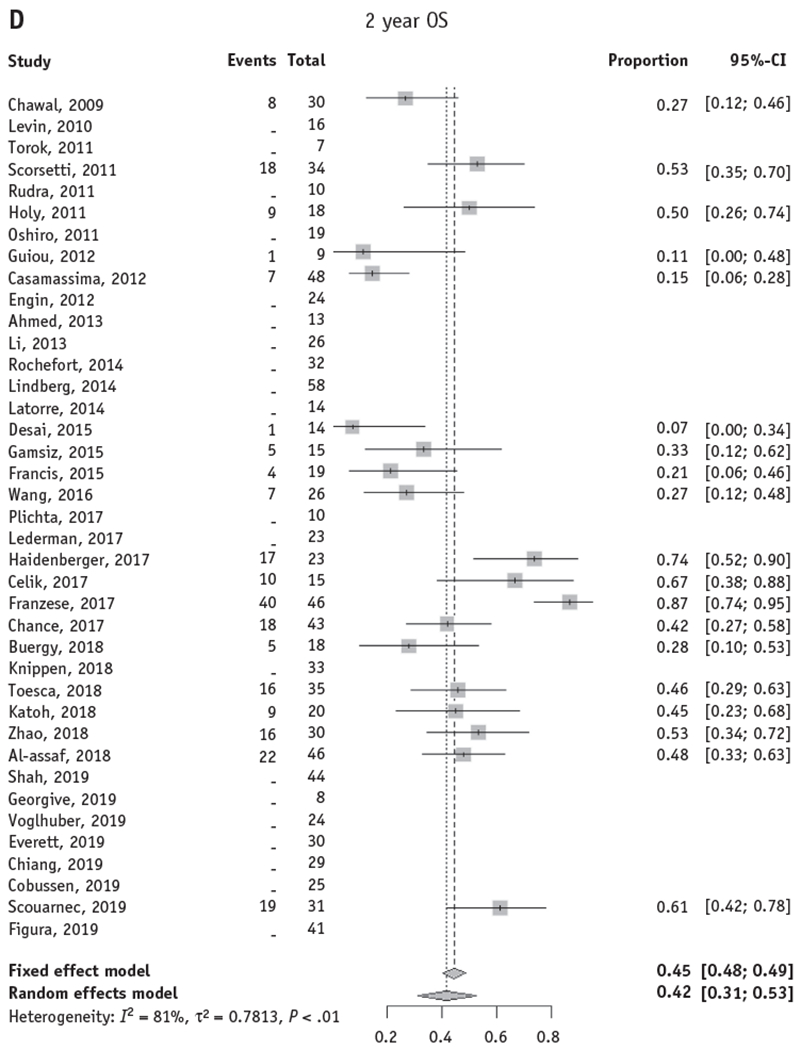
Forest plots for local control and overall survival of stereotactic body radiation therapy for adrenal metastases. Forest plots are shown for actuarial LC and OS at 1 and 2 years. Only studies with reported actuarial 1- and/or 2-year LC or OS were included in the pooled model. (A, B) The random effects pooled estimate of 1- and 2-year LC was 82% (95% CI, 74%-88%) and 63% (95% CI, 50%-74%), respectively. (C, D) The random effects pooled estimate of 1- and 2-year OS was 66% (95% CI, 57%-74%) and 42% (95% CI, 31%-53%), respectively. Abbreviations: CI = confidence interval; LC = local control; OS = overall survival.
Twenty-two studies comprising 540 patients treated with SBRT to adrenal metastases reported 1-year actuarial OS, and 19 studies comprising 520 patients reported 2-year actuarial OS. The random-effects pooled 1-year and 2-year OS rates were 66% (95% CI, 57%-74%) and 42% (95% CI, 31%-53%), respectively (Fig. 2C, 2D).
The proportion-based meta-analysis described does not take into account patients who are lost to follow-up or censored and may underestimate study-level variances. Thus, the meta-analysis was validated using stricter assumptions and a bootstrap method to simulate study-level LC and OS curves and estimate study-level variances based on reported median follow-up durations (see Methods, Fig. E1–E4, available online at https://doi.org/10.1016/j.ijrobp.2020.01.017). According to this approach, the pooled 1- and 2-year LC and OS rates were similar (1-year LC: 84% [95% CI, 78%-89%]; 2-year LC: 70% [95% CI, 59%-81%]; 1-year OS: 72% [95% CI, 63%-81%]; 2-year OS: 47% [95% CI, 35%-59%]).
Tumor response and pain relief after stereotactic body radiation therapy of adrenal metastases
The random-effects pooled CR and OR rates were 14.7% (95% CI, 9.8%-21.5%) and 54.6% (95% CI, 46.5%-62.5%), respectively (Table 1; Fig. E5 and E6, available online at https://doi.org/10.1016/j.ijrobp.2020.01.017). Qualitative pain relief was reported as an outcome in 7 studies, and 85.7% of patients for whom pain was evaluated experienced improvement of their symptoms after SBRT (Table 1). Quantitative data on baseline pain and responses to treatment were rarely reported, which precluded statistical analysis.
Association of biological equivalent dose with local control and survival
A higher median biological equivalent dose (BED10, alpha/beta = 10) was significantly associated with improved 1-year LC (N = 23 studies, QM = 16.78, P < .0001, Fig. 3) and 2-year LC (N = 15 studies, QM = 13.54, P = .0002). Based on a metaregression model of BED10 and LC, a BED10 of 60 Gy, 80 Gy, and 100 Gy predicted 1-year LC of 70.5%, 84.8%, and 92.9% and 2-year LC of 47.8%, 70.1%, and 85.6%, respectively (Fig. 3).
Fig. 3.

One-year local control is positively associated with the median stereotactic body radiation therapy dose for adrenal metastases. The median reported prescription biological equivalent dose associated with 1-year local control (QM = 16.78, P < .0001). BED10 is plotted against the logit-transformed 1-year local control. The size of circles is proportional to the sample size of each study. The dashed line is the regression line (intercept 1.67, slope 0.042 per unit increase in BED10).
Interestingly, BED10 was not significantly associated with 1-year OS (N = 22 studies, QM = 2.69, P = .10) but was positively associated with 2-year OS (N = 18 studies, QM = 4.48, P = .03; Fig. E7 and E8, available online at https://doi.org/10.1016/j.ijrobp.2020.01.017). Based on a metaregression model of BED10 and OS, a BED10 of 60 Gy, 80 Gy, and 100 Gy predicted a 2-year OS of 34.0%, 47.2%, and 60.1%, respectively.
Although most studies did not report the percent isodose line prescribed to, we sought to investigate the effect of this factor on LC by calculating an estimated maximum BED10. Incorporation of the percent isodose prescribed to did not alter the relationship between dose and LC, and the estimated maximum BED10 was similarly associated with improved 1-year LC (N = 16 studies, QM = 10.0, P = .0015) and 2-year LC (N = 10 studies, QM = 25.1, P < .0001).
Next, we sought to account for the effect of tumor histology. There was a trend between the proportion of lung malignancy and 1-year LC (N = 24 studies, QM = 2.7, P = .09) and 2-year LC (N = 16, QM = 3.2, P = .07), with poorer LC among studies with a greater proportion of lung primary. BED10 remained robustly associated with 1-year LC (N = 23 studies, P < .0001), 2-year LC (N = 15 studies, P = .0004), and 2-year OS (N = 18 studies, P = .02) after accounting for proportion of lung malignancy.
Similarly, we sought to account for the effect of tumor size on LC. Because many studies did not consistently report tumor size or PTV, we combined reported PTV with calculation of an estimated PTV based on reported GTV and margins. In 5 studies reporting both GTV and PTV, the mean percent error of this estimate was 4.4%, and the mean absolute percent error was 13.0% ± 1.1% (standard error of the mean). Using the combined reported/estimated PTV, there was no significant association between PTV and reported median BED10 (N = 22, adjusted R2 = 0.06, P = .31). There was also no significant association on univariate metaregression between PTV and 1-year LC (N = 15, QM = 2.0, P = .16). BED10 remained a significant predictor of 1-year LC (N = 14 studies, P = .002) and 2-year LC (N = 10 studies, P = .004) after accounting for PTV.
Toxicity
The overall rate of CTCAE grade 3 or higher toxicity was 1.8% (N = 18 patients). Fifteen patients experienced CTCAE grade 3 toxicity. These included nausea (N = 3), diarrhea (N = 1), gastrointestinal bleeding (N = 1), esophageal ulcer (N = 1), gastric/duodenal ulcers responding to medical management (N = 2), hypertensive emergency (N = 1), and unknown (N = 6). Neither dose, laterality, proportion of bilateral tumors treated, reported/estimated PTV, nor tumor motion management strategy were associated with increased toxicity.
Only 2 patients (0.2%) experienced CTCAE grade 4 toxicity (gastrointestinal bleeding from a duodenal ulcer, and a perforated pyloric ulcer 14 months after radiation). There was a single possible CTCAE grade 5 toxicity in a patient receiving nivolumab with SBRT who developed an immune reaction to nivolumab after SBRT that was accompanied by severe abdominal pain with diarrhea.22 Unfortunately, neither a cause of death nor dosimetric data on the radiation treatment of this patient, or specifics regarding the timing of immunotherapy, SBRT, and death, were reported.
Few studies directly addressed renal or adrenal toxicity. One study reported a mean decline in estimated glomerular filtration rate of 2.6 ± 8 mL/min/1.73 m2 after SBRT.39 Five total patients (0.5%) who developed grade 2 adrenal insufficiency were identified in 2 studies, and 1 patient was identified who developed grade 1 adrenal insufficiency.
Discussion
Key findings
To our knowledge, this is the first pooled meta-analysis of the outcomes, treatment characteristics, and toxicity of SBRT for adrenal metastases. We performed a comprehensive search of the literature and identified over 1000 patients treated with SBRT for adrenal metastases in 39 studies published between 2009 and September 2019. We found that SBRT for adrenal metastases was associated with excellent 1-year LC, was effective in palliation of pain and reduction of tumor volume, and was safe, with a clinically significant toxicity rate of only 1.8%. Importantly, there were no reports of severe renal or adrenal toxicity. There did not appear to be an association between higher dose, proportion of bilateral tumors, volume treated, or SBRT technique and a greater risk of toxicity. One study did report a single death in the setting of nivolumab and SBRT, but the specifics of timing, cause of death, dosimetry, and other important clinical information were not reported. Although the safety and efficacy of combined immunotherapy and SBRT has been the subject of several phase 1 and 2 studies,52–55 we suggest that attention should be paid to examining the risk of immune-related adverse events in patients receiving immunotherapy and SBRT to adrenal metastases in future studies.
Dose response of stereotactic body radiation therapy for adrenal metastases
Interestingly, we identified a strong dose response between the reported study-level median or mean BED10 and 1- and 2-year LC and a weaker but significant association between BED10 and 2-year OS. These findings should be interpreted with caution. First, patient-level data were not available, and the study-level median dose did not capture heterogeneity in technique, prescription dose, treatment method, or fractionation. Thus, our reported BED10 values are not easily generalizable given the inconsistency in radiation technique and dosimetry and the inherent biological inaccuracies of BED calculation via the linear quadratic formula. Confounding clinical variables were also not consistently reported, which precluded performance of a multivariate metaregression accounting for factors such as metastasis synchronicity, treatment timing, performance status, systemic disease burden, brain metastases, and prior or concurrent/adjuvant chemo- or immunotherapy.
Nevertheless, the strong association between the study-level dose and LC that we identified is consistent with previously reported dose-response relationships in the early-stage non-small cell lung cancer (NSCLC) SBRT literature. For example, the widely cited HypoFXSRT multi-institutional study reported a local recurrence rate of 8.4% for stage I NSCLC treated with SBRT and a BED10 of 100 Gy or more, compared with 42.9% for BED10 <100 Gy.56 A wide variety of prescribed dose and fractionations were also used in the HypoFXSRT study, ranging from 18 to 75 Gy in 1 to 22 fractions. The similarity between the dose relationship identified in our study and the NSCLC literature is not surprising given that the majority of patients in our study had lung primaries, and the majority of these were NSCLC (data not shown). Thus, given the abundance of lung cancer metastases in our study, our finding of a dose response for adrenal metastasis SBRT may be reflective of intrinsic lung cancer biology. Interestingly, there was a trend in our study toward worse LC in studies with a greater proportion of lung cancer. Given the well-described dose relationship for SBRT in early-stage NSCLC, as well as the dose relationship identified in our analysis, adrenal metastases from a lung primary may stand to benefit more from safe dose escalation.
Our findings are of particular relevance and timeliness in light of the growing momentum behind oligometastasis-directed SBRT.57–60 Multiple phase 1 and 2 trials have demonstrated the relative safety of this approach,45–48 and randomized trials are underway to investigate ablative SBRT to oligometastatic disease in lung (NCT03137771) and breast61 and other malignancies, with the ultimate goal of improving patient survival, freedom from progression, and quality of life. Although literature exists on SBRT dose response and toxicity in the lung, liver, and brain, limited SBRT dose-response data are available for adrenal metastases, owing to the relatively small sample sizes of existing studies. Interestingly, the SARON trial,62 a UK-based randomized trial of consolidative SBRT versus chemotherapy in oligometastatic NSCLC, allows for a prescription dose for adrenal metastases between 30 to 45 Gy in 3 fractions with at least 95% PTV coverage, citing a study by Holy et al,41 which was included in the present meta-analysis. This spectrum of doses corresponds to a wide range of BED10 (60-100 Gy). NRG-LU002, a similar US-based randomized trial, is an expansion of the phase 1 NRG-BR001 study that demonstrated the safety of common SBRT dose schedules61; the dose used for abdominopelvic tumors in NRG-LU002, which presumably include adrenal metastases, was 45 Gy in 3 fractions (BED10 112.5 Gy). SABR-COMET,63 a recently reported randomized trial allowing oligometastatic-directed SBRT in a variety of primary malignancies, used a dose of 60 Gy in 8 fractions (BED10 108 Gy) for adrenal metastases. Thus, our data would seem to support the use of these higher doses in the treatment of adrenal metastases when higher LC is of concern and when these doses can be delivered safely. It remains to be seen, based on the future results of these randomized trials, whether improved LC of oligometastatic disease with ablative SBRT will translate to improved survival or freedom from progression. Until that time, our discovery of an association between BED10 and 2-year OS should be interpreted with caution.
Limitations
As discussed, there are multiple limitations to the current study. First, as a pooled meta-analysis combining retrospective and observational studies, this study is limited by the inherent shortcomings of combining investigations with heterogenous patient populations, treatment details, and reported clinical characteristics. We attempted to mitigate this by using multiple methods of estimating pooled statistics, including the traditional fixed and random-effects models and a more stringent parametric bootstrap approach.
Our analysis of dose and outcomes is limited by the aforementioned caveats regarding confounding clinical variables and heterogeneity of dosimetry and SBRT technique across institutions. In addition, we cannot exclude selection bias as an explanation for the correlation between dose and LC or survival. Certainly, younger and healthier patients with smaller tumors or a lower systemic disease burden may have been biased to receive more aggressive treatment, including higher SBRT doses. We attempted to account for this through sensitivity analyses, and to this end we did not find any significant association between BED10 and the study year, median reported age, proportion of lung primary, median/mean PTV, proportion of synchronous adrenal metastasis, or proportion of isolated adrenal metastasis. Nevertheless, absent patient-level data, and without more consistent reporting of these study level variables, our findings should be interpreted with caution.
Our study included studies reporting a wide range of fractionation and doses. Given our search strategy, these studies self-identified as delivering SBRT; this strategy may have missed studies that did not include certain keywords in their text or may include studies that may not meet stricter definitions of SBRT. That said, in sensitive areas of anatomy, delivery of fractional doses of radiation above conventional doses necessitates a higher level of image guidance or motion management, smaller margins, and a lower error tolerance, which we believe distinguishes SBRT from “hypofractionation.” This certainly applies to radiation to the adrenal glands. Thus, for the purposes of this study, we used a relatively broad definition of SBRT similar to the HypoFXSRT study, rather than a specific cutoff based on number of fractions or dose per fraction. Similarly, a definition of an “oligometastatic” clinical state was beyond the scope of this work; however, to this end, 10 studies did include a definition of “oligometastatic” disease, which was uniformly defined as 5 or fewer metastases. One study additionally specified 5 or fewer metastases in a maximum of 2 organs.
Finally, our results regarding dose escalation should be interpreted and applied with caution; the safe delivery of SBRT should be ensured on a patient-to-patient basis, depending on individualized assessment of patient anatomy and detailed delineation of sensitive organs at risk such as the stomach, duodenum, porta hepatis, liver, and others.
Conclusions
This systematic review and pooled meta-analysis of SBRT for adrenal metastases demonstrated a high pooled 1-year LC rate, tumor response and pain relief rate, and excellent safety profile in over 1000 patients. A strong association was identified between the BED10 and 1- and 2-year LC, and a weaker but significant association was also observed between BED10 and 2-year OS, although these results should be interpreted cautiously given the heterogeneity of the combined studies and inability of our analysis to account for confounders and other sources of bias. Ultimately, prospective randomized trials in carefully selected populations who may benefit from ablative SBRT of oligometastatic disease are needed.
Supplementary Material
Footnotes
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Disclosures: none.
Supplementary material for this article can be found at https://doi.org/10.1016/j.ijrobp.2020.01.017.
References
- 1.Conti A, D’Elia C, Cheng M, et al. Oligometastases in genitourinary tumors: Recent insights and future molecular diagnostic approach. Eur Urol Suppl 2017;16:309–315. [Google Scholar]
- 2.Corbin KS, Hellman S, Weichselbaum RR. Extracranial oligometastases: A subset of metastases curable with stereotactic radiotherapy. J Clin Oncol 2013;31:1384–1390. [DOI] [PubMed] [Google Scholar]
- 3.Guerrero E, Ahmed M. The role of stereotactic ablative radiotherapy (SBRT) in the management of oligometastatic non small cell lung cancer. Lung Cancer 2016;92:22–28. [DOI] [PubMed] [Google Scholar]
- 4.Pitroda SP, Khodarev NN, Huang L, et al. Integrated molecular subtyping defines a curable oligometastatic state in colorectal liver metastasis. Nat Commun 2018;9:1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wood BJ, Abraham J, Hvizda JL, Alexander HR, Fojo T. Radiofrequency ablation of adrenal tumors and adrenocortical carcinoma metastases. Cancer 2003;97:554–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanvetyanon T, Robinson LA, Schell MJ, et al. Outcomes of adrenalectomy for isolated synchronous versus metachronous adrenal metastases in non-small-cell lung cancer: A systematic review and pooled analysis. J Clin Oncol 2008;26:1142–1147. [DOI] [PubMed] [Google Scholar]
- 7.Chiang Y, Cheng J,Hsu F. Stereotactic ablative radiotherapy outcomes for metastatic adrenal gland tumors in lung cancer based on histology subtype and molecular profile. Int J Radiat Oncol Biol Phys 2019;105:E501. [Google Scholar]
- 8.Figura NB, Oliver DE, Johnstone PA, Hoffe SE, Frakes J. Local control following stereotactic body radiotherapy to adrenal oligometastases. J Clin Oncol 2019;37:447. [DOI] [PubMed] [Google Scholar]
- 9.Cobussen P, Palacios MA, Spoelstra FOB, et al. OC-0072 Clinical outcomes of stereotactic MR-guided adaptive radiation therapy for adrenal oligometastases. Radiother Oncol 2019;133:S33–S34. [Google Scholar]
- 10.Voglhuber T, Combs SE, Kessel KA, et al. EP-1625 Patterns-of-care and outcome-analysis of the stereotactic body RT (SBRT) of adrenal-glandmetastases. Radiother Oncol 2019;133:S876. [Google Scholar]
- 11.Everett A, Popple R, Willey C, et al. Fractionated stereotactic body radiation therapy (SBRT) provides excellent local control for periadrenal and adrenal metastases. Int J Radiat Oncol Biol Phys 2019; 105:E565–E566. [Google Scholar]
- 12.Guiou M, Mayr N, Kim E, et al. Stereotactic body radiotherapy for adrenal metastases from lung cancer. J Radiat Oncol 2012;1:155–163. [Google Scholar]
- 13.Levin KJ, Ajlouni M, Walls N, Movsas B, Ryu S. SBRT for adrenal gland metastasis using single dose vs. fractionated dose regimen. Int J Radiat Oncol 2010;78:S377–S378. [Google Scholar]
- 14.Engin K, Kucuk N, Guden M, Ayata H, Kilic C, Ceylan C. EP-1299 image guided stereotactic body radiotherapy for adrenal metastases. Radiother Oncol 2012;103:S493–S494. [Google Scholar]
- 15.Ahmed K, Barney B, Macdonald O, et al. Stereotactic body radiotherapy in the treatment of adrenal metastases. Am J Clin Oncol 2013; 36:509–513. [DOI] [PubMed] [Google Scholar]
- 16.Oshiro Y, Takeda Y, Hirano S, Ito H, Aruga T. Role of radiotherapy for local control of asymptomatic adrenal metastasis from lung cancer. Am J Clin Oncol 2011;34:249–253. [DOI] [PubMed] [Google Scholar]
- 17.Rochefort P, Tanguy R, Ayadi M, Carrie C, Sunyach M. Local control and role of fractionation in stereotactic body radiation therapy for adrenal gland metastasis. Int J Radiat Oncol 2014;90:S907. [Google Scholar]
- 18.Lindberg K, Grozman V, Haasbeek C, et al. Outcome after SBRT of adrenal gland metastases: A multicenter retrospective analysis of 60 adrenal metastases. Int J Radiat Oncol 2014;90:S326. [Google Scholar]
- 19.Li J, Shi ZR, Wang Z, et al. Treating adrenal tumors in 26 patients with CyberKnife: A mono-institutional experience. PLoS One 2013;8: e80654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anchuelo Latorre J, Hernando O, Sánchez E, et al. PO-0767: Clinical experience in the treatment of adrenal metastases with stereotactic body radiation therapy (SBRT). Radiother Oncol 2014;111: S46–S47. [Google Scholar]
- 21.Desai A, Rai H, Haas J, et al. A retrospective review of CyberKnife stereotactic body radiotherapy for adrenal tumors (primary and metastatic): Winthrop University Hospital experience. Front Oncol 2015; 5:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang WGA, Sura K, Robertson J, Krauss DJ, Chen PY, Grills IS. Outcomes and toxicity for treatment of adrenal metastases with radiation therapy. Int J Radiat Oncol 2016;96:E273–E274. [Google Scholar]
- 23.Chance WW, Nguyen QN, Mehran R, et al. Stereotactic ablative radiotherapy for adrenal gland metastases: factors influencing outcomes, patterns of failure, and dosimetric thresholds for toxicity. Pract Radiat Oncol 2017;7:e195–e203. [DOI] [PubMed] [Google Scholar]
- 24.Al-Assaf H, Cheung P, Chung HT, et al. Stereotactic body radiation therapy (SBRT) for adrenal metastasis provides more than local control. Int J Radiat Oncol 2018;102:e90. [Google Scholar]
- 25.Katoh N, Onishi H, Uchinami Y, et al. Real-time tumor-tracking radiotherapy and general stereotactic body radiotherapy for adrenal metastasis in patients with oligometastasis. Technol Cancer Res Treat 2018;17:1533033818809983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lederman AJ, Arbit DJ, Lee A, et al. Response of oligometastatic non-small cell lung cancer to the adrenal gland treated with stereotactic body radiosurgery. Int J Radiat Oncol 2017;99:E472. [Google Scholar]
- 27.Georgiev D, Gesheva-Atanasova N, Lalova S, et al. EP-1619 SBRT and the treatment of adrenal gland metastasis. Radiother Oncol 2019; 133:S872–S873. [Google Scholar]
- 28.Knippen S, Burjakow K, Putz F, et al. PO-0771: Fractionated SBRT for adrenal metastasis: contributing to local tumor control with modest toxicity. Radiother Oncol 2018;127:S398–S399. [Google Scholar]
- 29.Shah MM, Isrow D, Fareed MM, et al. Single institution experience treating adrenal metastases with stereotactic body radiation therapy. J Cancer Res Ther 2019;15:S27–S32. [DOI] [PubMed] [Google Scholar]
- 30.Scouarnec C, Pasquier D, Luu J, et al. Usefulness of stereotactic body radiation therapy for treatment of adrenal gland metastases [e-pub ahead of print]. Front Oncol. 10.3389/fonc.2019.00732, Accessed November 1, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scorsetti M, Alongi F, Filippi AR, et al. Long-term local control achieved after hypofractionated stereotactic body radiotherapy for adrenal gland metastases: A retrospective analysis of 34 patients. Acta Oncol 2012;51:618–623. [DOI] [PubMed] [Google Scholar]
- 32.Gamsiz H, Beyzadeoglu M, Sager O, et al. Evaluation of stereotactic body radiation therapy in the management of adrenal metastases from non-small cell lung cancer. Tumori 2015;101:98–103. [DOI] [PubMed] [Google Scholar]
- 33.Haidenberger A, Heidorn S-C, Kremer N, Muacevic A, Fürweger C. Robotic radiosurgery for adrenal gland metastases. Cureus 2017;9:e1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buergy D, Rabe L, Siebenlist K, et al. Treatment of adrenal metastases with conventional or hypofractionated image guided radiation therapy e patterns and outcomes. Anticancer Res 2018;38:4789–4796. [DOI] [PubMed] [Google Scholar]
- 35.Zhao X, Zhu X, Fei J, et al. Short-term outcomes and clinical efficacy of stereotactic body radiation therapy (SBRT) in treatment of adrenal gland metastases from lung cancer. Radiat Oncol 2018;13:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Franzese C, Franceschini D, Cozzi L, et al. Minimally invasive stereotactical radio-ablation of adrenal metastases as an alternative to surgery. Cancer Res Treat 2017;49:20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plichta K, Camden N, Furqan M, et al. SBRT to adrenal metastases provides high local control with minimal toxicity. Adv Radiat Oncol 2017;2:581–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Celik E, Semrau R, Baues C, Trommer-Nestler M, Baus W, Marnitz S. Robot-assisted extracranial stereotactic radiotherapy of adrenal metastases in oligometastatic nonsmall cell lung cancer. Anticancer Res 2017;37:5285–5291. [DOI] [PubMed] [Google Scholar]
- 39.Toesca DAS, Koong AJ, von Eyben R, Koong AC, Chang DT. Stereotactic body radiation therapy for adrenal gland metastases: Outcomes and toxicity. Adv Radiat Oncol 2018;3:621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chawla S, Chen Y, Katz AW, et al. Stereotactic body radiotherapy for treatment of adrenal metastases. Int J Radiat Oncol Biol Phys 2009; 75:71–75. [DOI] [PubMed] [Google Scholar]
- 41.Holy R, Piroth M, Pinkawa M, Eble MJ. Stereotactic body radiation therapy (SBRT) for treatment of adrenal gland metastases from non-small cell lung cancer. Strahlenther Onkol 2011;187:245–251. [DOI] [PubMed] [Google Scholar]
- 42.Casamassima F, Livi L, Masciullo S, et al. Stereotactic radiotherapy for adrenal gland metastases: University of Florence experience. Int J Radiat Oncol Biol Phys 2012;82:919–123. [DOI] [PubMed] [Google Scholar]
- 43.Torok J, Wegner RE, Burton SA, Heron DE. Stereotactic body radiation therapy for adrenal metastases: A retrospective review of a noninvasive therapeutic strategy. Future Oncol 2011;7:145–151. [DOI] [PubMed] [Google Scholar]
- 44.Rudra S, Malik R, Ranck MC, et al. Stereotactic body radiation therapy for curative treatment of adrenal metastases. Technol Cancer Res Treat 2013;12:217–224. [DOI] [PubMed] [Google Scholar]
- 45.Cihan YB. Adrenal insufficiency in bilateral adrenal metastasis implemented SBRT. J Radiol Oncol 2018;2:9–11. [Google Scholar]
- 46.Onishi H, Ozaki M, Kuriyama K, et al. Serious gastric ulcer event after stereotactic body radiotherapy (SBRT) delivered with concomitant vinorelbine in a patient with left adrenal metastasis of lung cancer. Acta Oncol 2012;51:624–628. [DOI] [PubMed] [Google Scholar]
- 47.Ippolito E, D’Angelillo RM, Fiore M, Molfese E, Trodella L, Ramella S. SBRT: A viable option for treating adrenal gland metastases. Rep Pract Oncol Radiother 2015;20:484–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J Clin Epidemiol 2009;62:e1–e34. [DOI] [PubMed] [Google Scholar]
- 49.Schwartz LH, Litière S, De Vries E, et al. RECIST 1.1-Update and clarification: From the RECIST committee. Eur J Cancer 2016;62: 132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.National Institutes of Health. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. Bethesda, MD: National Institutes of Health; 2009. [Google Scholar]
- 51.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2016. [Google Scholar]
- 52.Bi N, Shedden K, Zheng X, Kong FS. Comparison of the effectiveness of radiofrequency ablation with stereotactic body radiation therapy in inoperable stage I non-small cell lung cancer: A systemic review and pooled analysis. Int J Radiat Oncol Biol Phys 2016;95:1378–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Theelen WSME, Peulen HMU, Lalezari F, et al. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: Results of the PEMBRO-RT phase 2 randomized clinical trial [e-pub ahead of print]. JAMA Oncol. 10.1001/jamaoncol.2019.1478. Accessed November 1, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maity A, Mick R, Huang AC, et al. A phase I trial of pembrolizumab with hypofractionated radiotherapy in patients with metastatic solid tumours. Br J Cancer 2018;119:1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luke JJ, Lemons JM, Karrison TG, et al. Safety and clinical activity of pembrolizumab and multisite stereotactic body radiotherapy in patients with advanced solid tumors. J Clin Oncol 2018;36:1611–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: Updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol 2007;2:S94–S100. [DOI] [PubMed] [Google Scholar]
- 57.Scorsetti M, Comito T, Tozzi A, et al. Final results of a phase II trial for stereotactic body radiation therapy for patients with inoperable liver metastases from colorectal cancer. J Cancer Res Clin Oncol 2014;141:543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schefter TE, Rusthoven KE, Kavanagh BD, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol 2009;27:1572–1578. [DOI] [PubMed] [Google Scholar]
- 59.Iyengar P, Kavanagh BD, Wardak Z, et al. Phase II trial of stereotactic body radiation therapy combined with erlotinib for patients with limited but progressive metastatic non-small-cell lung cancer. J Clin Oncol 2014;32:3824–3830. [DOI] [PubMed] [Google Scholar]
- 60.Collen C, Christian N, Schallier D, et al. Phase II study of stereotactic body radiotherapy to primary tumor and metastatic locations in oligometastatic nonsmall-cell lung cancer patients. Ann Oncol 2014;25: 1954–1959. [DOI] [PubMed] [Google Scholar]
- 61.Chmura SJ, Winter KA, Al-Hallaq HA, et al. NRG-BR002: A phase IIR/III trial of standard of care therapy with or without stereotactic body radiotherapy (SBRT) and/or surgical ablation for newly oligometastatic breast cancer (NCT02364557). J Clin Oncol 2019;37. [Google Scholar]
- 62.Conibear J, Chia B, Ngai Y, et al. Study protocol for the SARON trial: A multicentre, randomised controlled phase III trial comparing the addition of stereotactic ablative radiotherapy and radical radiotherapy with standard chemotherapy alone for oligometastatic non-small cell lung cancer. BMJ Open 2018;8:e020690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet 2019;393:2051–2058. [DOI] [PubMed] [Google Scholar]
- 64.Francis S, Contreras J, Bowers J, et al. Stereotactic body radiation therapy (SBRT) for adrenal metastases. J Radiat Oncol 2015;4:3. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


