Abstract
Background:
Lowe syndrome is a rare X-linked disease that is characterized by renal dysfunction, developmental delays, congenital cataracts and glaucoma. Mutations in the oculocerebral renal syndrome of Lowe (OCRL) gene are found in Lowe syndrome patients. Although loss of vision is a major concern for families and physicians who take care of Lowe syndrome children, definitive cause of visual loss is still unclear. Children usually present with bilateral dense cataracts at birth and glaucoma, which occurs in more than half of cases, either concurrently or following cataract surgery.
Materials and methods:
A retrospective review was conducted on the prevalence and characteristics of ocular findings among families of patients with Lowe syndrome with 137 uniquely affected individuals.
Results:
Of 137 patients, all had bilateral congenital cataracts. Nystagmus was reported in 69.3% of cases, glaucoma in 54.7%, strabismus in 35.0%, and corneal scar in 18.2% of patients. Glaucoma was reported as the most common cause of blindness (46%) followed by corneal scars (41%). Glaucoma occurred in 54.7% of patients and affected both eyes in the majority of cases. Of these patients, 55% underwent surgery for glaucoma, while the remaining patients used medications to control their eye pressure. Timolol and latanoprost were the most commonly used medications. Although trabeculectomy and goniotomy are commonly used for pressure management, aqueous tube shunts had the best outcomes.
Conclusion:
Ocular manifestations in individuals with Lowe syndrome and carriers with OCRL mutation are reported which may help familiarize clinicians with the ocular manifestations and management of a rare and complex syndrome.
Keywords: Lowe syndrome, congenital cataract, congenital glaucoma, Fanconi syndrome, OCRL gene
The oculocerebrorenal syndrome of Lowe (OMIM #309000) is a rare and pan-ethnic X-linked multisystem disorder first described by Lowe et al.1 in 1952. This severe disorder is characterized by the triad of intellectual disability, congenital cataract, and proximal renal tubule dysfunction (Fanconi-type) that leads to slowly progressive renal failure. The mutation of the causal gene OCRL1 is localized on Xq26.1.2 The estimated prevalence of Lowe syndrome (LS) is approximately 1 in 500,000 in the general population.3,4 The life span of an affected individual with this disorder rarely exceeds 40 years and is mainly limited due to progressive renal failure. Fanconi syndrome may develop in the first months of life and varies in severity between individuals.3 Other cardinal characteristics include isolated kidney dysfunction, severe progressive growth retardation, neonatal hypotonia, areflexia, seizures, arthropathy, and behavioral problems, such as temper tantrums and aggressiveness.5,6
Vision loss is a major manifestation of LS; ocular involvement may happen with prenatal development of cataract with glaucoma with or without buphthalmia, microphthalmia, nystagmus, corneal scarring/keloid formation and amblyopia. Bilateral dense congenital cataracts are often the presenting signs of LS and are typically noted in neonates shortly after birth5 and can be seen in utero as well.7 Although intraocular lens implantation is not recommended,3 early cataract detection and removal is recommended to improve visual stimulation. However, not all ocular conditions may develop prenatally in LS,5 thus precise continued ophthalmic examination should be performed.
In this study, we reviewed the clinical data in a large group of patients with LS regarding the ocular manifestations and clinical management. This comprehensive dataset is subsequently analyzed to provide information on the prevalence of each ophthalmic finding.
Materials and methods
Subjects
The study was conducted in accordance with the principles of the Declaration of Helsinki. All study procedures were defined, and patient consent was obtained as specified by the Institutional Review Board (IRB) protocol 45037 approved by the IRB at Stanford University. This retrospective study was conducted based on an extensive patient-based questionnaire completed by LS families who are members of the Lowe Syndrome Association (LSA) between 2007 and 2013. The survey was completed for 137 unique patients diagnosed with LS. This comprehensive survey includes anthropometric and biochemical parameters, clinical manifestations, treatment options (medical and surgical information), and their families’ subjective/objective evaluation.
All 137 patients were diagnosed with clinical phenotype of LS by detailed assessment of extraocular signs at multiple international centers.
Statistical analysis
Statistical analyses were performed using Stata 12.1 (StataCorp, College Station, TX, USA) software; Student’s two-tail t test was used to test for the difference between the groups.
Results
A majority of patients (118/137) had a skin biopsy which was assessed for inositol polyphosphate 5-phosphatase activity of OCRL-1 in cultured skin fibroblasts. In total, 54 cases were positively identified with reduced enzymatic function (<10% of normal). In addition, 79 patients had confirmed molecular genetic sequence analysis of the OCRL-1 gene. Furthermore, 16 mothers of LS patients were identified with another affected son, and 22 patients have positive family history of LS. About 88% (n=123) of them were White; three were Asian, two were African American; three were Hispanic; two were American Indian, and four cases had another race/ethnicity.
A novel Lowe patient-specific treatment score, called the clinical effective score, was used. It was based on the feedback by Lowe patients’ families surveyed. Scores are determined on a scale of 1–5 by the patient’s family from 1 considered as least effective to 5 which was considered as most effective.
Ocular manifestations and treatment
Table 1 depicts the ocular findings of our patients. While all patients had bilateral cataracts, strabismus, nystagmus, glaucoma, and corneal scar were reported among 48 (35.0%), 95 (69.3%), 75 (54.7%), and 25 (18.2%) of patients, respectively.
Table 1.
Characteristics and comparison of ocular symptoms.
| Cataracts | Glaucoma | Strabismus | Nystagmus | Corneal scars | |
|---|---|---|---|---|---|
| Median of age at diagnosis (years) | 0.01 | 0.3 | – | – | 7.0 |
| Patients no. (%) | 137 (100) | 75 (54) | 48 (35) | 95 (69) | 25 (18) |
| Unilateral no. (%) | – | 13 (9) | 14 (10) | 4 (2) | 13 (9) |
| Bilateral no. (%) | 137 (100) | 62 (45) | 34 (24) | 91 (66) | 11 (8) |
| Caused vision loss no. (%) | – | 25 (18) | 11 (8) | 24 (17) | 14 (10) |
| Surgery no. (%) | CE 132 (96) Artificial lens implants 18 (13) |
Glaucoma surgery 41 (29) | Muscle surgery 21 (15) | Muscle surgery 5 (3) | Keloid removal 2 (1) Corneal transplant 1 (0.7) |
Percentages have been “rounded”. “−” =absent; CE: cataract extraction.
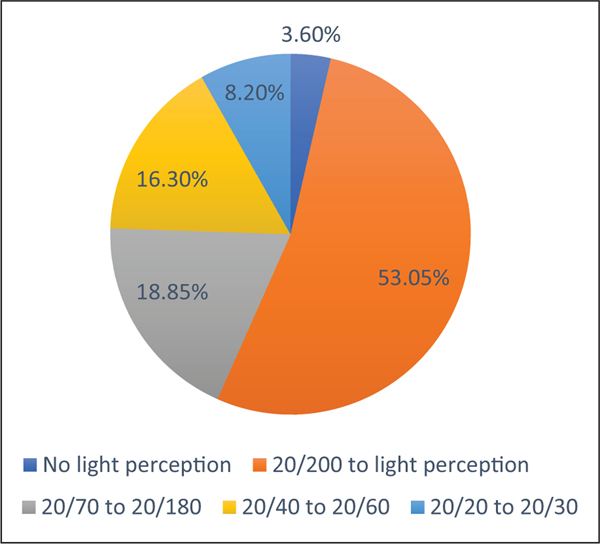
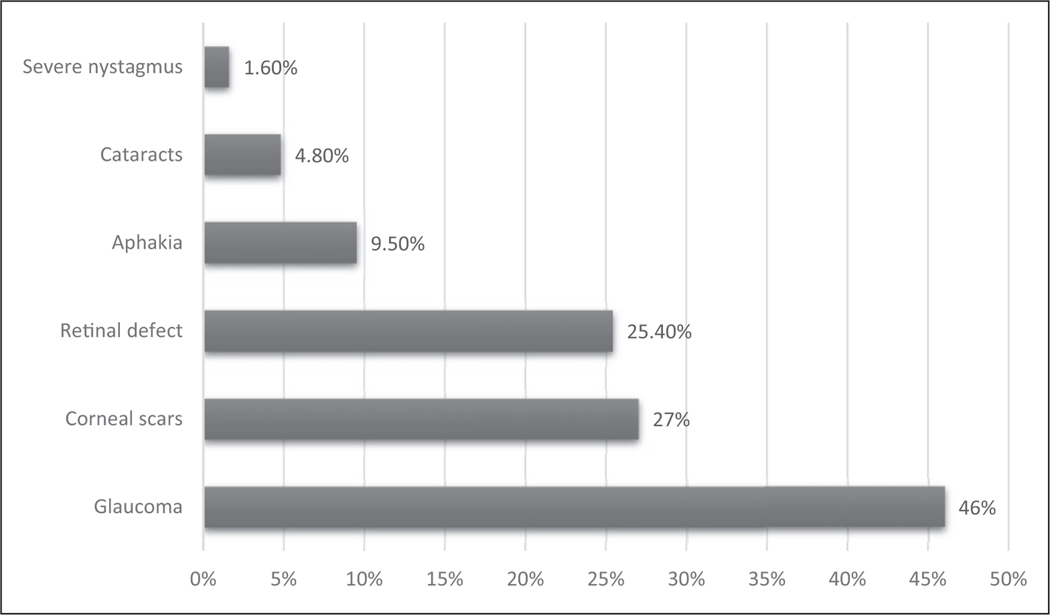
Visual acuity was collected in 196; the information on the other 78 eyes was not available (Figure 1). Of 196 eyes, 53.05% had 20/200 to light perception, 18.85% had best-corrected visual acuity (BCVA) of 20/70–20/180, 16.30% had 20/40–20/60, 8.20% had 20/20–20/30, and 3.60% had no light perception. A large number of patients (63/137) were diagnosed with legal blindness, which was defined as the BCVA of 20/200 or less in the better eye or peripheral vision less than central 20. Glaucoma was reported as the most common cause of blindness (46%), which was the parent-perceived cause of blindness and not confirmed with ophthalmology records, followed by corneal scars (27%), and retinal pathology (25.40%; Figure 2).
Figure 1.
Corrected visual acuity (i.e. glasses, contacts) of 196 eyes with Lowe syndrome.
Figure 2.
The main causes for 63 patients diagnosed with blindness. “Blindness” means central visual acuity of 20/200 or less in the better eye with the use of a correcting lens. An eye that is accompanied by a limitation in the fields of vision so that the widest diameter of the visual field subtends an angle no greater than 20° which is considered as having a central visual acuity of 20/200 or less. Blindness in some patients is caused by multiple factors, so the percentage sum is not 100%.
Congenital cataract
The median age at which cataracts were diagnosed was 0.01 years indicating quartile 2 (Q2=50th percentile; Table 1). Approximately 75% of patients were diagnosed with cataract within 1 month of birth (Figure 3). Cataract extraction was performed on 132 patients with a mean age of 4 months. The majority of patients who underwent cataract surgery were aphakic and only 18 patients had intraocular lens implantation.
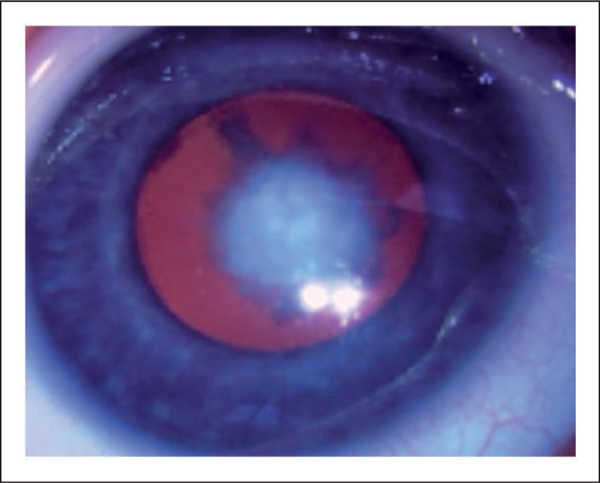
Figure 3.
Cataract in a 3-month-old boy with Lowe syndrome presented with bilateral decrease in red reflex.
Corneal keloid
Corneal keloids were diagnosed at a median age of 7.0 (3.3–11.3) years (Q2 (quartile 1 - quartile 3)). Of 25 patients, two corneal keloids were removed and one required corneal transplant. Further characterization showed that two patients regularly used eye drops and topical antibiotic ointment. Vision was compromised in 56% of cases with corneal scars (Table 1) and 41% experienced blindness due to corneal scars, and likely secondary amblyopia (Figure 2).
Nystagmus and strabismus
Nystagmus and strabismus occurred in 69.3% of patients. Five cases underwent muscle surgery for the treatment of nystagmus. In the strabismus group, 21 of 48 (44%) cases underwent extraocular muscle surgery. Extraocular muscle surgery and patching were the most commonly used treatments for strabismus. The mean age of patients receiving extraocular muscle surgery was 2.8 years (Table 2).
Table 2.
Comparison of various treatment methods for strabismus.
| Treatment | No. of patients | Mean age of treatment | Clinical effective score |
|---|---|---|---|
| Surgery | 21 | 2.8 | 3.8 |
| Patching | 21 | 1.7 | 2.6 |
| Corrective glasses | 13 | 2.6 | 4.3 |
Clinical effective score: 1 = not effective, 5 = very effective. These data are based on the subjective or objective evaluation of patients and clinicians.
Congenital glaucoma
Congenital glaucoma occurred in our patients with the median age of 0.3 (0.1–2.0) years at diagnosis. In total, 41% of patients had a history of glaucoma at the time of the first or second cataract surgery. All glaucoma cases were confirmed by ophthalmologists. Glaucoma was reported as the main cause of blindness. In addition, among patients with glaucoma, 33% had vision loss shown in Table 1. Glaucoma treatment has been summarized in Table 3. Timolol and latanoprost were the most commonly used medications for the treatment of glaucoma in 25 (18.2%) and 24 (17.5%) patients, respectively. The three drugs including betaxolol, levobunolol, and timolol have been used for the longest period of time which was 15, 12.5, and 8.9 years, respectively. The clinical effective score for levobunolol was 5, for travoprost with benzalkonium 4.25, and for latanoprost 4.2 for latanoprost. Our results showed that 41/75 (55%) of patients with glaucoma had undergone at least one surgery for glaucoma (Table 1). Although the threshold of proceeding with glaucoma surgeries was optic nerve cupping, axial length elongation, or elevated intraocular pressure (IOP); the decision was made based on individual patients.
Table 3.
Medications and clinical effective score of 75 glaucoma patients with Lowe syndrome.
| Medication | No. of patients | Mean of medication using time (year) | Clinical effective score |
|---|---|---|---|
| Timolol | 25 | 8.9 | 3.8 |
| Brinzolamide | 4 | 3.7 | 4 |
| Latanoprost | 24 | 4.1 | 4.2 |
| Levobunolol | 2 | 12.5 | 5 |
| Brimonidine | 6 | 7 | 3.5 |
| Betaxolol | 4 | 15 | 3.5 |
| Bimatoprost | 8 | 2.8 | 3.5 |
| Dorzolamide | 10 | 4.4 | 3.75 |
| Travoprost | 4 | 2.4 | 4.25 |
| Dorzolamide/timolol | 15 | 4.9 | 4.1 |
| Pilocarpine | 4 | 1 | 2.25 |
Clinical effective score: 1 = not effective, 5 = very effective. These data are based on the subjective or objective evaluation of patients and clinicians.
Different surgical approaches were used to treat glaucoma as depicted in Table 4. Trabeculectomy and goniotomy were the most commonly used glaucoma surgeries; both were done in 20 of 41 (49%) patients. The next most common was aqueous tube shunt implantation (Ahmed valve and Baerveldt implant) which was performed in 17 of 41 (41%) patients. Cryotherapy was applied in five (12%) patients. Goniotomy which is the earliest operation considered by ophthalmologists, was performed at an average age of 0.7 years. Iridectomy was performed only in one patient.
Table 4.
Surgical and clinical effective score of 75 glaucoma patients with Lowe syndrome.
| Surgical | No. of patients | Mean age of surgery | Clinical effective score |
|---|---|---|---|
| Goniotomy | 20 | 0.7 | 3.1 |
| Trabeculotomy | 20 | 1.4 | 3 |
| Cryotherapy | 5 | 2.8 | 3.3 |
| Aqueous tube shunt implantation | 17 | 3.7 | 4.1 |
| Iridectomy | 1 | 1.8 | 5 |
Clinical effective score: 1 = not effective, 5 = very effective. These data are based on the subjective or objective evaluation of patients and clinicians.
Refractive errors
Glasses were used by the majority (85%) of patients with average age of 2.4 years and clinical effective score of 3.9. A total of 43% of patients had a history of wearing contact lens at an average age of 0.6 years and the highest evaluation score which was 4. Monocles and magnifiers were also used in clinics with a usage rate of 2% and 9%, respectively. The mean age for the two latter methods was approximately 10 years of age with the evaluation score of 3.0 and 3.3, respectively (Table 5).
Table 5.
Treatment methods for refractive error and effective evaluation of 137 patients.
| Treatment | Usage rate (%) | Mean age at first prescription | Clinical effective score |
|---|---|---|---|
| Glasses | 85 | 2.4 | 3.9 |
| Contacts | 43 | 0.6 | 4.0 |
| Monocle | 2 | 10.0 | 3.0 |
| Magnifier | 9 | 10.4 | 3.3 |
Carriers of LS present with lenticular opacities
Subcapsular plaques were reported in 25.0% (30/120) of the patients’ mothers (Table 6). Among all tested mothers with premature cataract, 26 cases were identified as carriers, three remained an unclear carrier status, and only one mother was determined as not an obligate carrier. Most of the mothers who were diagnosed with cataract were in the childbearing age with an average age of 26 years. Age for one carrier was unknown. About 85% (22/26) of them had bilateral cataracts and 11 obligate carriers underwent cataract surgery at a mean age of 38 years.
Table 6.
Clinical features of 30 patients’ mothers with premature cataracts.
| No. | Detected age (years) | Which eye | Age when cataract removed/eye | Carrier |
|---|---|---|---|---|
| 1 | 7 | OU | 34/OD | + |
| 2 | 34 | OU | 39/OD | – |
| 3 | 29 | OU | – | + |
| 4 | 30 | OU | – | + |
| 5 | 26 | OU | 39/OD | + |
| 6 | 8 | OU | – | + |
| 7 | 45 | OU | – | + |
| 8 | 29 | OU | 32/OD | + |
| 9 | 35 | OU | – | + |
| 10 | 27 | OU | – | + |
| 11 | 17 | OD | 39/OD | + |
| 12 | 54 | OU | – | + |
| 13 | 23 | OS | – | + |
| 14 | 30 | OS | – | + |
| 15 | 15 | OU | 24/OS | + |
| 16 | 12 | OU | 20/OU | + |
| 17 | 32 | OU | – | + |
| 18 | 36 | OU | 46/OD, 47/OS | + |
| 19 | 27 | OS | – | + |
| 20 | 10 | OU | 42/OU | + |
| 21 | 30 | OU | 49/OS | + |
| 22 | 30 | OU | – | + |
| 23 | 26 | OU | – | + |
| 24 | 38 | OU | 61/OD, 60/OS | + |
| 25 | 28 | OU | – | + |
| 26 | 35 | OU | 37/OU | + |
| 27 | N/A | OU | N/A | + |
“+” = present; “−” = not a carrier or without surgery; N/A: not available; OU: oculus uterque (both eyes); OD: oculus dexter (the right eye); OS: oculus sinister (the left eye).
Discussion
Currently, there exists few clinical reviews of LS, with no large study available for ocular manifestations and management. Based on a LSA patient family survey, we reviewed the clinical manifestations of 137 cases affected with LS.
Due to lyonization, X-linked diseases may present in female carriers of mutations on the X-chromosome. In LS, female carriers have been found to have lenticular opacities that range from punctate to focal subscapular cataracts.8,9 The lenticular abnormalities consist of cortical dots of various shapes and size, ranging from microns to several millimeters, which increase in number with progressive age of the carriers. Our study showed that lens opacities are present in most obligate carriers of LS. They represent a partial clinical expression of the X-linked inherited gene as postulated by Lyon.10 Only approximately two-thirds of mothers of LS male patients are carriers of the condition while 32% of LS causing mutations in OCRL are spontaneous.11–17 About 5% of carrier females have germ line mosaicism, thus even if present, mutations cannot be detected in all mothers of affected patients. In this context, it is advised that all mothers of affected boys by LS, should consider prenatal testing for possible germline mosaicism, which can guide genetic counseling.
The results of our study showed that 88% of cataracts in carriers were bilateral and almost half of the mothers had cataract surgery. Cataracts were usually diagnosed in carriers in the second to third decade of life. The typical white punctate opacities of Lowe carriers are most frequent in the deep layers of the cortex, just outside the nucleus. This indicates that the development of these opacities begins in early infancy.8 In addition, our data demonstrated that three carriers were diagnosed with cataracts during childhood, aged 7, 8, and 10 years (Table 1).
Cataracts in children with LS may develop in utero and are thought to be caused by altered migration of the crystalline embryonic epithelium.18 Surgery at young ages has been reported as a risk factor for the development of glaucoma.19–21 In addition, patients with LS are more susceptible to the development of glaucoma, primarily due to filtration angle anomalies. In 41% of patients, glaucoma was present at the time of the first or second cataract surgery. In spite of this, cataracts should be removed early in patients with oculocerebrorenal syndrome to improve visual stimulation and avoid amblyopia. Furthermore, eyes should be monitored closely for changes in IOP, optic nerve cupping, and refractive error, so that glaucoma can be detected and treated promptly.
Congenital glaucoma is the leading cause of blindness and is often severe and refractory in patients with LS. Patients who were diagnosed as having glaucoma within the first few weeks of life often required surgery. In this study, glaucoma occurred in 54.7% of patients and affected both eyes in the majority of cases (45%). Of these patients, 29% underwent surgery for glaucoma. In addition, patients with later glaucoma developing after cataract surgery were treated medically to control their eye pressure. Timolol and latanoprost were the most commonly used medications. Levobunolol, travoprost with benzalkonium, and latanoprost on the contrary showed the best response, per the patient-reported clinical score (Table 3). Although trabeculectomy and goniotomy are commonly used for pressure management, aqueous tube shunts had the best evaluation with score 4.1 (Table 4).
The nystagmus in these patients is most likely sensory secondary to their reduced vision arising from deprivation amblyopia and retinal dysfunction. Corneal scarring and keloids developed without prior trauma in about 18.2% of patients around the age of 7.0 years. The etiology of keloids in LS remains obscure, and therefore difficult to treat. Three of the patients who received surgery did not respond well to the procedure, neither corneal transplantation nor corneal keloid resection.
Surgery and patching were the most common treatments for strabismus. In total, 44% of patients received extraocular muscle surgery. The surgical mean age was 2.8 years while the patching treatment age was 1.7 years. Although, outcomes of strabismus surgery did not affect long-term visual acuity,22 it is clear that surgery has a good outcome in the treatment of strabismus (Table 2).
Early prescription of eyeglasses is important for aphakia. In total, 85% of patients used glasses and average age of first-time use was 2.4 years and with the evaluation score of 3.9. The average age of contact lens use was younger (0.6 years). Some authors prefer spectacles3,23 over contact lenses because contact lenses are associated with a higher risk of corneal keloid formation. In the entire cohort, eight had a history of contact lenses and also suffered from corneal keloid; seven developed corneal keloid after wearing contact lenses. Among these seven patients, the median age of first-time contact lens wear was 0.08 years old; the keloids developed some 5–7 years later.
Conclusion
The results of this study can aid pediatric ophthalmologists in the early detection of ocular abnormalities and the management of patients with LS and OCRL carriers. It also may be helpful in understanding the complexity of this syndrome.
Acknowledgements
The authors acknowledge the contributions of Dr Michael Gaynon for thoughtful comments during the preparation of this manuscript.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH/NEI K08-EY022058 (Y.S.), R01-EY025295 (Y.S.), VA merit CX001298 (Y.S.), Ziegler Foundation for the Blind (Y.S.), Showalter Foundation (Y.S.), Children’s Health Research Institute Award (Y.S.). Research for Prevention of Blindness Unrestricted grant (Stanford Ophthalmology), American Glaucoma Society (Y.S.), Lowe syndrome association (Y.S.), and Knights Templar Eye Foundation (Y.S.). P30 Vision Center grant to Stanford Ophthalmology Department. Y.S. is a Laurie Kraus Lacob Faculty Scholar in Pediatric Translational Medicine. R01-EY-023295 and R01-EY024932 (Y.H.).
Footnotes
Availability of data and materials
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Consent for publication
Patient consent for publication was obtained as specified by the IRB protocol 45037 approved by the Institutional Review Board at Stanford University.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval and consent to participate
All participants gave informed consent, and the study was approved by our local ethics committee. All study procedures were defined, and patient consent was obtained as specified by the IRB protocol 45037 approved by the Institutional Review Board at Stanford University.
References
- 1.Lowe CU, Terrey M and MacLachlan EA. Organicaciduria, decreased renal ammonia production, hydrophthalmos, and mental retardation; a clinical entity. AMA Am J Dis Child 1952; 83(2): 164–184. [DOI] [PubMed] [Google Scholar]
- 2.Attree O, Olivos IM, Okabe I, et al. The Lowe’s oculocerebrorenal syndrome gene encodes a protein highly homologous to inositol polyphosphate-5-phosphatase. Nature 1992; 358(6383): 239–242. [DOI] [PubMed] [Google Scholar]
- 3.Loi M. Lowe syndrome. Orphanet J Rare Dis 2006; 1: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bokenkamp A and Ludwig M. The oculocerebrorenal syndrome of Lowe: an update. Pediatr Nephrol 2016; 31(12): 2201–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Couser NL, Masood MM, Aylsworth AS, et al. Ocular manifestations in the X-linked intellectual disability syndromes. Ophthalmic Genet 2017; 38(5): 401–412. [DOI] [PubMed] [Google Scholar]
- 6.David S, De Waele K, De Wilde B, et al. Hypotonia and delayed motor development as an early presentation of Lowe syndrome: case report and literature review. Acta Clin Belg 2018; 3: 1–5. [DOI] [PubMed] [Google Scholar]
- 7.Schurman SJ and Scheinman SJ. Inherited cerebrorenal syndromes. Nat Rev Nephrol 2009; 5(9): 529–538. [DOI] [PubMed] [Google Scholar]
- 8.Cibis GW, Waeltermann JM, Whitcraft CT, et al. Lenticular opacities in carriers of Lowe’s syndrome. Ophthalmology 1986; 93(8): 1041–1045. [DOI] [PubMed] [Google Scholar]
- 9.Tomčíková D, Gerinec A, Bzdúch V, et al. Ophthalmological finding in a patient with Lowe syndrome. Cesk Slov Oftalmol. Winter 2018; 74(3): 104–106. [DOI] [PubMed] [Google Scholar]
- 10.LYON MF. Sex chromatin and gene action in the mammalian X-chromosome. Am J Hum Genet 1962; 14: 135–148. [PMC free article] [PubMed] [Google Scholar]
- 11.Tripathi RC, Cibis GW and Tripathi BJ. Pathogenesis of cataracts in patients with Lowe’s syndrome. Ophthalmology 1986; 93(8): 1046–1051. [DOI] [PubMed] [Google Scholar]
- 12.Perez Y, Gradstein L, Flusser H, et al. Isolated foveal hypoplasia with secondary nystagmus and low vision is associated with a homozygous SLC38A8 mutation. Eur J Hum Genet 2014; 22(5): 703–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou FQ, Wang QW, Liu ZZ, et al. Novel mutation in OCRL leading to a severe form of Lowe syndrome. Int J Ophthalmol 2019; 12(7): 1057–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shalaby AK, Emery-Billcliff P, Baralle D, et al. Identification and functional analysis of a novel oculocerebrorenal syndrome of Lowe (OCRL) gene variant in two pedigrees with varying phenotypes including isolated congenital cataract. Mol Vis 2018; 24: 847–852. [PMC free article] [PubMed] [Google Scholar]
- 15.Prosseda PP, Luo N, Wang B, et al. Loss of OCRL increases ciliary PI(4,5)P2 in Lowe oculocerebrorenal syndrome. J Cell Sci 2017; 130(20): 3447–3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song E, Luo N, Alvarado JA, et al. Ocular pathology of oculocerebrorenal syndrome of Lowe: novel mutations and genotype-phenotype analysis. Sci Rep 2017; 7(1): 1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo N, Conwell MD, Chen X, et al. Primary cilia signaling mediates intraocular pressure sensation. Proc Natl Acad Sci U S A 2014; 111(35): 12871–12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vashist P, Senjam SS, Gupta V, et al. Definition of blindness under national programme for control of blindness: do we need to revise it. Indian J Ophthalmol 2017; 65(2): 92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balekudaru S, Agarkar S, Guha S, et al. Prospective analysis of the predictors of glaucoma following surgery for congenital and infantile cataract. Eye (Lond) 2018; 33(5): 796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuhli-Hattenbach C, Luchtenberg M, Kohnen T, et al. Risk factors for complications after congenital cataract surgery without intraocular lens implantation in the first 18 months of life. Am J Ophthalmol 2008; 146(1): 1–7. [DOI] [PubMed] [Google Scholar]
- 21.Khan AO and Al-Dahmash S. Age at the time of cataract surgery and relative risk for aphakic glaucoma in nontraumatic infantile cataract. J AAPOS 2009; 13(2): 166–169. [DOI] [PubMed] [Google Scholar]
- 22.Bothun ED, Lynn MJ, Christiansen SP, et al. Strabismus surgery outcomes in the Infant Aphakia Treatment Study (IATS) at age 5 years. J AAPOS 2016; 20(6): 501–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis RA, Nussbaum RL and Brewer ED. Lowe syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al. (eds) GeneReviews((R)). Seattle, WA: University of Washington, Seattle, 1993. [PubMed] [Google Scholar]