Abstract
1,1,2,2- Cis-diamminedichloroplatinum (II) (cisplatin) is a chemotherapeutic agent widely used in the clinic to treat various cancers. The antitumor activity of cisplatin is generally attributed to its ability to form intrastrand and interstrand DNA-DNA cross-links via sequential platination of two nucleophilic sites within the DNA duplex. However, cisplatin also induces DNA- protein lesions (DPCs) that may contribute to its biological effects due to their ability to block DNA replication and transcription. We previously reported that over 250 nuclear proteins including high mobility group proteins, histone proteins, and elongation factors formed DPCs in human HT1080 cells treated with cisplatin (Ming et al. Chem. Res. Toxicol. 2017, 30, 980–995). Interestingly, cisplatin induced DNA-protein conjugates were reversed upon heating, by an unknown mechanism. In the present work, DNA repair protein O6-alkylguanine DNA alkyltransferase (AGT) was used as a model to investigate the molecular details of cisplatin-mediated DNA-protein cross-linking and to establish the mechanism of their reversal. We found that AGT is readily cross-linked to DNA in the presence of cisplatin. HPLC-ESI+-MS/MS sequencing of tryptic peptides originating from dG-Pt-AGT complexes revealed that the cross-linking occurred at six sites within this protein including Glu110, Lys125, Cys145, His146, Arg147, and Cys150. Cisplatin-induced Lys-Gua cross-links (1,1-cis-diammine-2-(5-amino-5-carboxypentyl)amino-2-(2'-deoxyguanosine-7-yl)-platinum(II) (dG-Pt-Lys) were detected by HPLC-ESI+-MS/MS of total digests of modified protein in comparison with the corresponding authentic standard. Upon heating, dG-Pt-AGT complexes were subject to platination migration from protein to DNA, forming cis-[Pt(NH3)2{d(GpG)}] cross-links which were detected by HPLC-ESI+-MS/MS. Our results provide a new insight into the mechanism of cisplatin-mediated DNA-protein cross-linking and their dynamic equilibrium with the corresponding DNA-DNA lesions.
Keywords: mass spectrometry, DNA-protein cross-links, Cisplatin, AGT
Introduction
Reversible DNA-protein interactions play an important role in normal cell function. Nuclear protein binding to regulatory sequences within DNA controls DNA replication, gene expression, and mediates responses to DNA damage [1–3]. Any interruption of these dynamic interactions can have serious consequences for cell viability and genetic stability [4]. For example, proteins can become covalently trapped on chromosomal DNA as a result of exposure to physical and chemical agents such as formaldehyde [5, 6], ionizing radiation [7], and anticancer drugs [8–14]. The resulting irreversible DNA-protein cross-links (DPCs) are expected to block normal DNA-protein interactions, potentially contributing to toxicity, cancer, and neurodegenerative diseases [4, 15, 16].
The role of DPC lesions in biological effects of bis-alkylating drugs is not well understood due to their inherent complexity and the propensity of these agents to induce other types of DNA damage. For example, the anticancer drug 1,1,2,2-cis-diamminedichloroplatinum (II) (cisplatin) forms interstrand and intrastrand DNA-DNA cross-links [17], monoadducts [18], and DPCs [19] (Scheme 1). While DNA-DNA cross-linking by cisplatin is well characterized and is thought to be responsible for its anticancer mechanism [20], only limited information is available about the corresponding DNA-protein cross-links. DPCs are estimated to constitute < 1% of total DNA damage following exposure to cisplatin [21] and constitute a highly heterogeneous and complex type of DNA damage, making it difficult to evaluate their role in the cytotoxic and mutagenic effects of platinum drugs [22, 23].
Scheme 1.
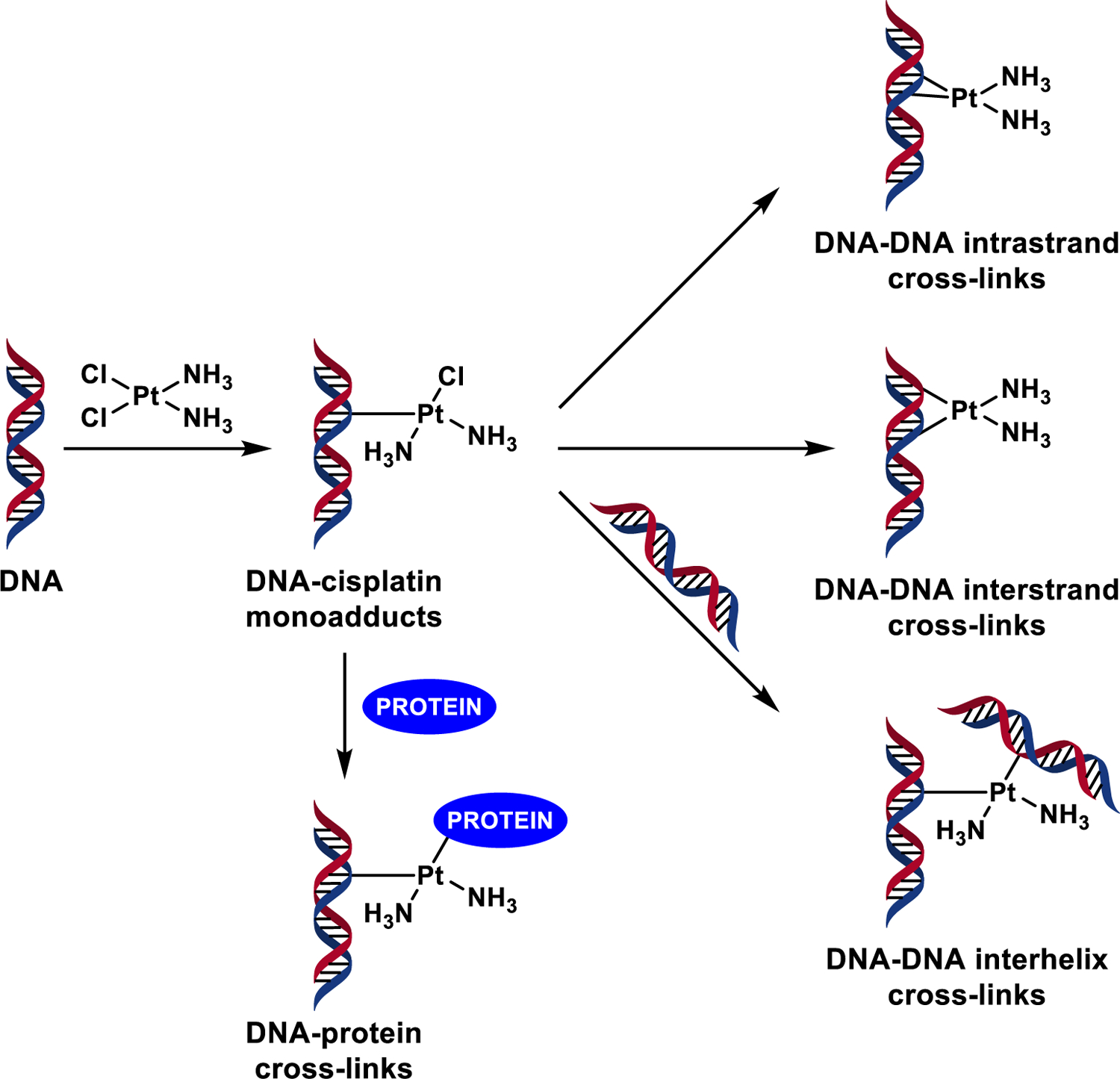
Formation of bifunctional DNA adducts by cisplatin.
We recently conducted a mass spectrometry based proteomics study of cisplatin induced DNA−protein cross-links in human fibrosarcoma (HT1080) cells [24]. Over 250 nuclear proteins cross-linked to chromosomal DNA following treatment with cisplatin were identified, including high mobility group (HMG) proteins, histone proteins, and elongation factors [24]. Interestingly, cisplatin induced DPCs were reversible, with proteins quantitatively released from DNA upon heating. In the present study, we employed O6-Alkylguanine DNA alkyltransferase (AGT) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as model proteins to investigate the structural basis for cisplatin-mediated DPC formation and the mechanism of their reversal upon heating. AGT and GAPDH were previously identified as proteins that form DPC in cisplatin treated cells [24].
Materials and Methods
Chemicals and Reagents.
Cisplatin, dG, and human recombinant glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were purchased from Sigma-Aldrich (St. Louis, MO). Boc-L-Lys-OH was obtained from Fluka (Buchs, Switzerland), and mass spectrometry-grade Trypsin Gold was purchased from Promega (Madison, WI). Adenosine 5'-[γ−32P]-triphosphate was obtained from Perkin-Elmer (Boston, MA). T4 polynucleotide kinase and proteinase K were purchased from New England Biolabs (Beverly, MA). Synthetic DNA oligodeoxynucleotides were prepared at the University of Minnesota Microchemical Facility (Minneapolis, MN). Recombinant C-terminal histidine-tagged hAGT was produced as described previously [25]. Cis-1,1-diammine-2-chloro-2-(2'-deoxyguanosine-7-yl)-platinum (II) (dG-Pt-Cl) monoadducts and cis-1,1-diammine-2,2-bis-(2'-deoxyguanosine-7-yl)platinum (II) (dG-Pt-dG) conjugates were produced according to previous reports [26].
1,1-diammine-2-(5-amino-5-carboxypentyl)amino-2-(2'-deoxyguanosine-7-yl)-platinum(II) (dG-Pt-Lys).
Cisplatin (10 mg, 33.33 μmol) was dissolved in 1 mL of 10 mM Tris-HCl buffer (pH 7.2). AgNO3 (11.3 mg, 66.7 μmol) was added, and the solution was kept in the dark with stirring for 4 h at room temperature. Following centrifugation, to the filtrate was added 2'-deoxyguanosine (dG) (9 mg, 33.3 μmol) and Boc-L-Lysine (8.2 mg, 33.3 μmol), and the resulting mixture was incubated for 48 h at 37 °C. The precipitate was isolated by filtration, and the supernatant was separated by semi-preparative HPLC on a Supelcosil LC-18-DB column (25 cm × 10 mm, 5 μm) eluted with a linear gradient of acetonitrile (B) in 15 mM ammonium acetate, pH 4.9 (A). The solvent composition was changed from 0 to 24% B in 24 min and further to 60% in 6 min. Under these conditions, Boc-protected dG-Pt-Lys eluted at 20.4 min. ESI+-MS/MS (dG-Pt-Lys-Boc): m/z 741.3 [M]+ → m/z 724.2 [M – NH3]+, 624.5 [M – NH3 – Boc]+. Following HPLC purification, the Boc protective group was removed by incubation in 10% TFA (0.5 mL) at room temperature for 30 min. The deprotected dG-Pt-Lys was purified using the same HPLC method. Under these conditions, dG-Pt-Lys eluted at 8.6 min. UV: λmax 260nm, λmin 280 nm (pH 4.9); ESI+-MS/MS (dG-Pt-Lys): m/z 641.2 [M]+ → m/z 624.2 [M – NH3]+, m/z 508.1 [M – NH3 – deoxyribose + H]+, and m/z 357.1 [M – NH3 – deoxyguanosine]+.
Denaturing PAGE of Cisplatin-Induced DNA-Protein Crosslinks.
DNA 18-mer, 5′-GGA GCT GGT GGC GTA GGC-3′ (200 pmol), was 5′-end-labeled with 32P in the presence of [γ−32P]ATP and T4 polynucleotide kinase by standard methods [27], purified by 12% denaturing PAGE, and desalted by size exclusion chromatography. The radiolabeled DNA was mixed with equimolar amounts of the complementary strand (5'-GCCTACGCCACC AGCTCC-3’) in the annealing buffer (10 mM Tris pH=8, 50mM NaCl, 50 μl), heated in a heating block at 95° C for 10 min, and slowly cooled down overnight. The 32P-labeled duplex (0.93 nmol) was incubated with O6-alkylguanine DNA alkyltransferase (AGT) (2.0 μg) or human recombinant glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (2.0 μg) in the presence of 1–100 mol equiv of cisplatin (1, 5, 10, and 20 nmol, respectively) for 3 h at 37 °C. The reaction mixtures were separated by 12% SDS-PAGE, and the radiolabeled products were visualized using a Packard Cyclone Phosphoimager (Packard BioScience, Meridan, CT).
Reaction of Synthetic dG-Pt-Cl Monoadduct with Recombinant AGT Protein.
Human recombinant wild-type AGT protein or its variant (C145A/C150S_AGT) (2.3 nmol) was incubated with 10 equiv of synthetic dG-Pt-Cl (23 nmol) in 10 mM Tris-HCl buffer (pH 7.4) for 4 h at 37 °C. Any unreacted dG-Pt-Cl was removed by size exclusion chromatography using Micro Bio-Spin 6 columns (Bio-Rad, Hercules, CA), in which the buffer was exchanged to 0.1% TFA in H2O following manufacturer’s instructions. The cross-linked protein was isolated by HPLC using an Agilent 1100 HPLC system equipped with a DAD UV detector. Agilent Zorbax 300 SB-C3 column (2.1 × 150 mm, 5 µm) was eluted with 0.1% TFA in water (A) and 0.1% TFA in 4:1 acetonitrile/water (B) at a flow rate of 0.4 mL/min at 10 °C. The solvent composition began at 0% B and was linearly changed to 15% B over 15 min and further to 50% from 15 to 25 min. The column was washed at 50% B for 15 min and re-equilibrated to 0% B for 2 min. Under these conditions, the modified AGT proteins eluted as a single peak at ~ 30 min, and the unreacted AGT protein eluted as a single peak at ~ 32 min. HPLC peaks containing modified AGT protein were collected and dried under vacuum, followed by HPLC-ESI+-MS analysis as described below.
Trans-Platination Reactions of dG-Pt-AGT in the Presence with dG.
HPLC-purified AGT-Pt-dG cross-links (~25 μg) were incubated with 10 molar equivalent of dG (~1.1 nmol) in SDS buffer (1% SDS, 10 mM Tris-HCl, 1% glycerol, 50 μM EDTA, pH 8.5) at 70 °C for 10 min or 60 min. Control experiments were conducted in the presence of dG at 37 °C for overnight or in the absence of dG at 70 °C for 10 min. Proteins were subjected to size exclusion chromatography as described above, followed by HPLC-ESI+-MS analysis. To detect cis-1,1-diammine-2,2-bis-(2'-deoxyguanosine-7-yl)-platinum (II) (dG-Pt-dG) cross-links, the solution was passed through Amicon Ultra-0.5 mL Centrifugal Filters (10K MWCO, Millipore, Temecula, CA) to remove proteins before HPLC-ESI+-MS/MS analysis as described below.
Tryptic Digestion of Platinated AGT.
Control or platinated AGT protein (~ 50 μg) was digested with trypsin (2 μg) in 25 mM ammonium bicarbonate buffer (pH 7.9) for 8 h at 37 °C. Samples were dried, desalted by ZipTip C18 purification (ZipTip C18 Pipette Tips, Millipore, Temecula, CA), and finally reconstituted in 0.1% formic acid (25 μL) prior to MS analysis as described below.
Total Digestion of Platinated AGT to Amino Acids.
Tryptic peptides (from ~50 μg of protein) were filtered through Microcon YM-10 membrane filters to remove trypsin. Proteinase K (20 μg) was added to the filtrate, and proteolysis proceeded at 37 °C for 24 h. Samples were dried and subjected to off-line HPLC separation using an Agilent Technologies HPLC system (1100 model) incorporating a diode array detector and a Supelcosil LC-18-DB (4.6 × 250 mm, 5 μm) column (Sigma-Aldrich, St. Louis, MO). The column was eluted at a flow rate of 1 mL/min using 15 mM ammonium acetate, pH 4.9 (A) and acetonitrile (B). The solvent composition was changed linearly from 0 to 24% B over 24 min and further to 60% B in 6 min. HPLC fractions containing dG-Pt-Lys (5–7 min) were collected, dried under vacuum, and reconstituted in 25 µL of 15 mM ammonium acetate buffer for HPLC-ESI+-MS/MS analysis.
Mass Spectrometry.
HPLC-ESI+-MS analysis of modified AGT proteins was performed with an Agilent 1100 capillary HPLC-ion-trap MS system operated in ESI+ mode (m/z 200–2000). For whole protein mass spectrometry, chromatography was achieved using an Agilent Zorbax Extend SB 300-C8 column (150 × 0.3 mm, 3.5 µm) eluted at a flow rate of 12 µL/min with a mobile phase of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B). The solvent composition was held at 30% B for the first 5 min, followed by a linear increase to 80% B over 25 min, and further to 95% B in 5 min. Using these conditions, dG-Pt-Cl-modified AGT proteins (dG-Pt-AGT) eluted ~14.5 min. Deconvolution of the protein charge envelope was performed using the commercial deconvolution software.
AGT tryptic peptides were analyzed by HPLC-ESI+-MS/MS with a Thermo Scientific LTQ Orbitrap Velos mass spectrometer in line with an Eksigent NanoLC-Ultra 2D HPLC system, a nanospray source, and Xcalibur 2.1.0 software for instrument control. Peptide mixtures (8 µL) were loaded on a Symmetry C18 trapping column (180 µm × 20 mm, Waters, Milford, MA) using 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B) at a flow composition of 95% A and 5% B at 5 μL/min for 3 minutes. Following trapping, the flow was reversed and decreased to 0.3 μL/min. The peptides were eluted off the trap column and onto a capillary column (75 µm ID, 10 cm packed bed, 15 µm orifice) created by hand packing a commercially purchased fused-silica emitter (New Objective, Woburn MA) with Zorbax SB-C18 5 µm separation media (Agilent, Santa Clara, CA). The gradient program started at 5% B, followed by a linear increase to 60% B over 60 min, and further to 95% B in 5 min. Liquid chromatography was carried out at an ambient temperature. Centroided MS-MS scans were acquired using an isolation width of 2.5 m/z, an activation time of 30 ms, an activation Q of 0.25, 35% normalized CID collision energy, and 1 microscan with a max ion time of 100 ms for each MS/MS scan. The mass spectrometer was calibrated prior to each analysis, and the spray voltage was adjusted to endure a stable spray. Typically, the tune parameters were as follows: spray voltage of 1.6 kV, a capillary temperature of 275 °C, and an S-lens RF Level of 50%. Peptide MS/MS spectra were collected using data dependent scanning in which one full scan mass spectrum was followed by eight MS/MS spectra. Dynamic exclusion was enabled for 60 s and singly charged species were excluded.
Spectral data were analyzed using Thermo Proteome Discoverer 1.2 (ThermoScientific, San Jose, CA) that linked raw data extraction, database searching, and probability scoring. The raw data were directly uploaded, without any format conversion, to search against human AGT protein FASTA database (http://www.uniprot.org/uniprot/P16455) combined with its reversed counterpart by using the SEQUEST algorithm [28, 29]. Search parameters included trypsin specificity and up to 2 missed cleavage sites. The dG-Pt-AGT conjugates were expected to experience fragmentation at the ESI+ source by a loss of one or two amino groups (due to the fragile nature of coordination between platinum and −NH3 ligands) or a loss of 2-deoxyribose, as well as the possible breakage of the glycosodic bond of dG. Furthermore, dG-Pt-Cl -induced platination at the N-donor residues (N-terminus, histidine, lysine, or arginine), S-donor residues (cysteine or methionine), and O-donor residues (threonine, tyrosine, aspartic acid, or glutamic acid) were specified as the following dynamic modifications to identify spectra of modified peptides: (A) cross-link to dG: +496.115 Da (dG + Pt + 2NH3), +479.087 Da (dG + Pt + NH3, a loss of −NH3), or +462.061 Da (dG + Pt, a loss of 2-NH3); (B) cross-link to guanine: +379.059 Da (Gua + Pt + 2NH3), +361.030 Da (Gua + Pt + NH3, a loss of −NH3), or +345.005 Da (Gua + Pt, a loss of 2-NH3). The following criteria were implemented for all of the identified peptides: mass tolerance ≤ 300 ppm, Xcorr ≥ 2.5, ΔCn ≥ 0.5, and RSp (preliminary score rank) ≤ 3. Peptide sequences with MS/MS spectra not meeting these criteria were removed from the final target list. Platinated peptides were included in the final lists only if they exhibited good quality MS/MS spectra (at least 40% of the observed MS/MS ions should match the theoretical b+ or y+-type peptide fragment ions) and with both platinated and non-platinated fragment ions whose relative abundances were unambiguously higher than the baseline.
HPLC-ESI+-MS/MS of dG-Pt-Lys conjugates was conducted with a Thermo-Finnigan TSQ Vantage mass spectrometer in line with an Eksigent MicroAS autosampler and nanoLC 2D HPLC pump, a heated ESI source, and a Xcalibur 1.4 software for instrument control. Chromatographic separation was accomplished using a Hypercarb column (100 mm × 0.5 mm, 3 μm, ThermoScientific, Waltharm, MA) eluted with a gradient of 15 mM ammonium acetate (A) and 1:1 acetonitrile:water with 1% formic acid (B) at a flow rate of 13 μL/min. The gradient program began at 2% B, followed by a linear increase to 8% B in 10 min, further to 80% B in 18 min, and finally back to 2% B in 2 min. Using this gradient, dG-Pt-Lys eluted at ~14.5 min. ESI was achieved at a spray voltage of 3.2 kV and a capillary temperature of 200°C. CID was performed with Ar as a collision gas (1.0 mTorr) at a collision energy of 25V. The MS parameters were optimized for maximum response during infusion of a standard solution of dG-Pt-Lys and may vary slightly between different experiments. HPLC-ESI+-MS/MS analyses were performed in the selected reaction monitoring (SRM) mode using the transitions corresponding to major fragment ions observed upon CID fragmentation of dG-Pt-Lys using a triple quadrupole mass spectrometer (m/z 641.3 [M]+ → 508.2 [M – NH3 – deoxyribose + H]+, and 340.1 [M – 2NH3 – deoxyguanosine]+).
HPLC-ESI+-MS/MS analysis of dG-Pt-dG cross-links was performed using an Agilent 1100 series capillary LC Ion Trap MS system operated in the ESI+ mode. Auto MS2 was used to isolate and fragment the [M]+ ion of dG-Pt-dG (m/z 762.3). Chromatographic separation was achieved using a Zorbax SB-C18 column (150 mm × 0.5 mm, 5 μm) eluted at a flow rate of 15 μL/min. The mobile phase consisted of 15 mM ammonium acetate, pH 4.9 (A) and acetonitrile (B). The solvent composition was held with a linear gradient of 0 – 15% B over the course of 30 min.
Results
SDS-PAGE Analysis of Cisplatin-Induced DNA-Protein Cross-Links.
To examine the ability of cisplatin to cross-link proteins to DNA, recombinant O6-alkylguanine DNA alkyltransferase (AGT) or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) proteins were incubated with 5'−32P-end labeled DNA duplexes in the presence of increasing amounts of cisplatin, followed by SDS-PAGE analysis. The results presented in Figure 1 reveal a slow migrating species corresponding to protein-DNA conjugates when duplex DNA was exposed to cisplatin in the presence of AGT or GAPDH. No such product was formed in control experiments in which either protein or oligonucleotide was omitted (Lanes 1–3, Figures 1A and 1B). Cross-linking of AGT to DNA by cisplatin displayed a pronounced concentration dependence, with 2–5% of recombinant protein being cross-linked to DNA depending on treatment levels (Lanes 4–8, Figure 1A).
Figure 1.
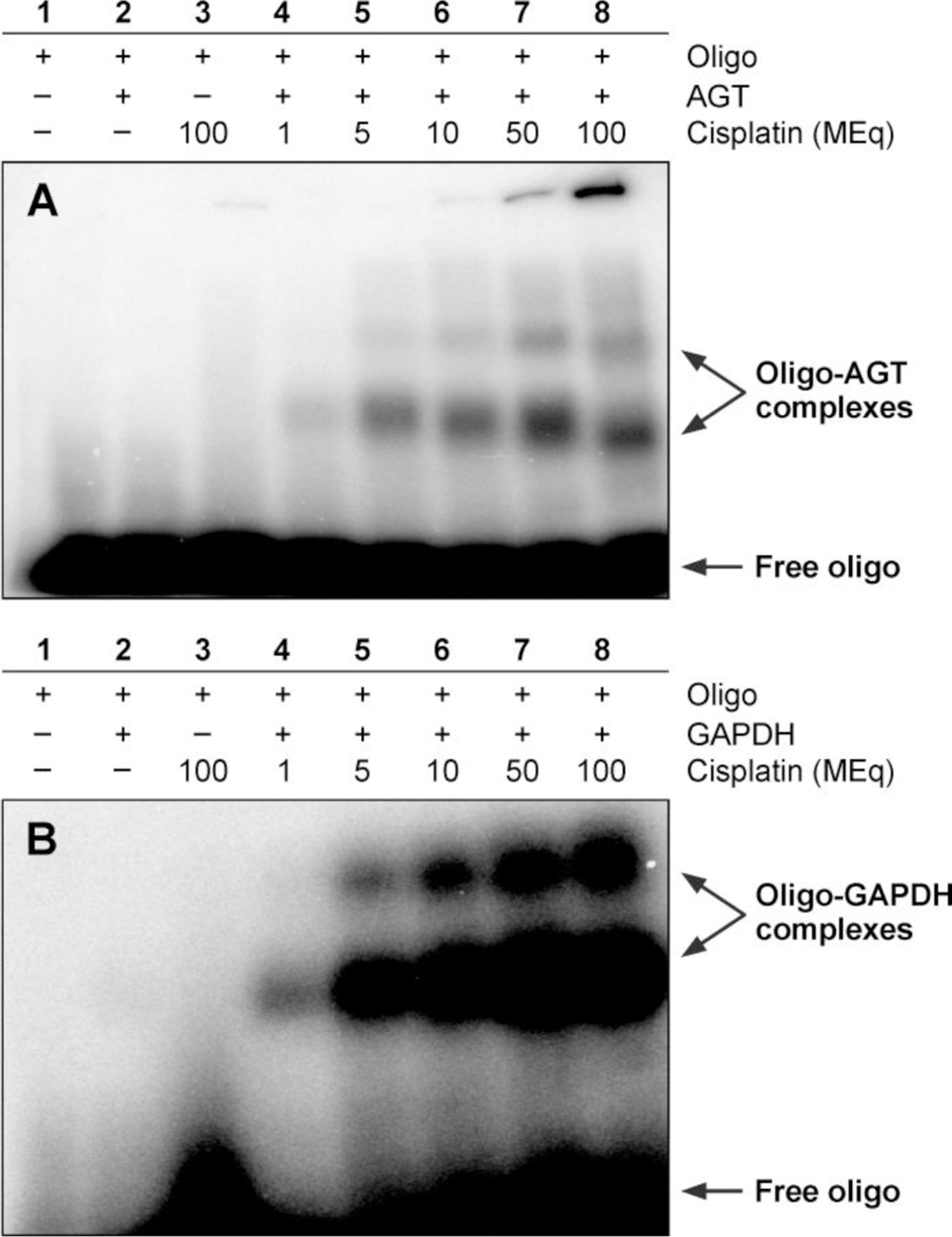
Detection of cisplatin-induced DNA-protein cross-links by gel electrophoresis. 12% SDS-PAGE analysis of 32P-endlabeled DNA duplexes (5'-GGA GCT GGT GGC GTA GGC-3’ +strand) following incubation with (A) recombinant human AGT or (B) recombinant GAPDH protein in the presence of 1 (lane 4), 5 (lane 5), 10 (lane 6), 50 (lane 7) or 100 (lane 8) molar equivalents of cisplatin. Free duplex DNA (labeled “Free oligo”) migrates to the bottom of the gel, whereas DNA-protein cross-links display substantially reduced mobility.
HPLC-ESI+-MS Analysis of dG-Pt-Cl Monoadduct-Induced AGT-dG Cross-Links: Whole Protein Results.
Initial platination of DNA by cisplatin leads to the formation of DNA monoadducts, which retain one reactive Pt functionality (Scheme 1). These monoadducts can subsequently react with nucleophilic sites within proteins, giving rise to DNA-protein cross-links. To gain insight into the nature of cisplatin-induced AGT-DNA linkages, recombinant AGT protein was incubated with synthetic cis-1,1-diammine-2-chloro-2-(2'-deoxyguanosine-7-yl)-platinum (II) (dG-Pt-Cl) as a model of monoplatinated DNA (4 h at 37 °C). Following size exclusion chromatography to remove the bulk of unreacted dG-Pt-Cl, AGT-dG cross-links were separated from unmodified AGT by reverse phase HPLC (Scheme 2 and Figure 2). Three HPLC peaks were observed (Figure 2). HPLC-ESI+-MS/MS analyses revealed that the first peak eluting at 4.5 min corresponded to unreacted dG-Pt-Cl, the second peak (30.1 min) contained AGT-dG cross-links, and the third peak (32.1 min) was due to unreacted AGT protein (Figures 2 and 3). ESI+ spectrum of unreacted AGT contains multiple m/z signals corresponding to the various charge states of the protein (+17 – +37) (Figure 3A). Deconvolution of the mass spectrum results in a molecular weight of 21,878 Da, which matches the theoretical value of 21,877 Da (Figure 3A). A similar spectrum was observed for the peak eluting at 32.1 min (Figure 2), with the exception of a small additional signal at 22,355 Da corresponding to AGT protein containing a single cisplatin cross-link to dG (AGT + Pt + dG + NH3, M = 22355 Da) (Figure 3B). ESI+ analysis of the material eluting at 30.1 min (Figure 2) reveals the presence of AGT containing a single platinum cross-link to dG (AGT + Pt + dG + 2NH3, M = 22372 Da), two platinum cross-links to dG (AGT + 2Pt + 2dG + 2NH3, M = 22832 Da), and a triple platinum cross-link to dG (AGT + 3Pt + 3dG + 4NH3, M = 23328 Da) (Figure 3C). Taken together, those results indicate that AGT protein can be covalently modified by dG-Pt-Cl to form cross-links at a minimum of three different sites of the protein.
Scheme 2.
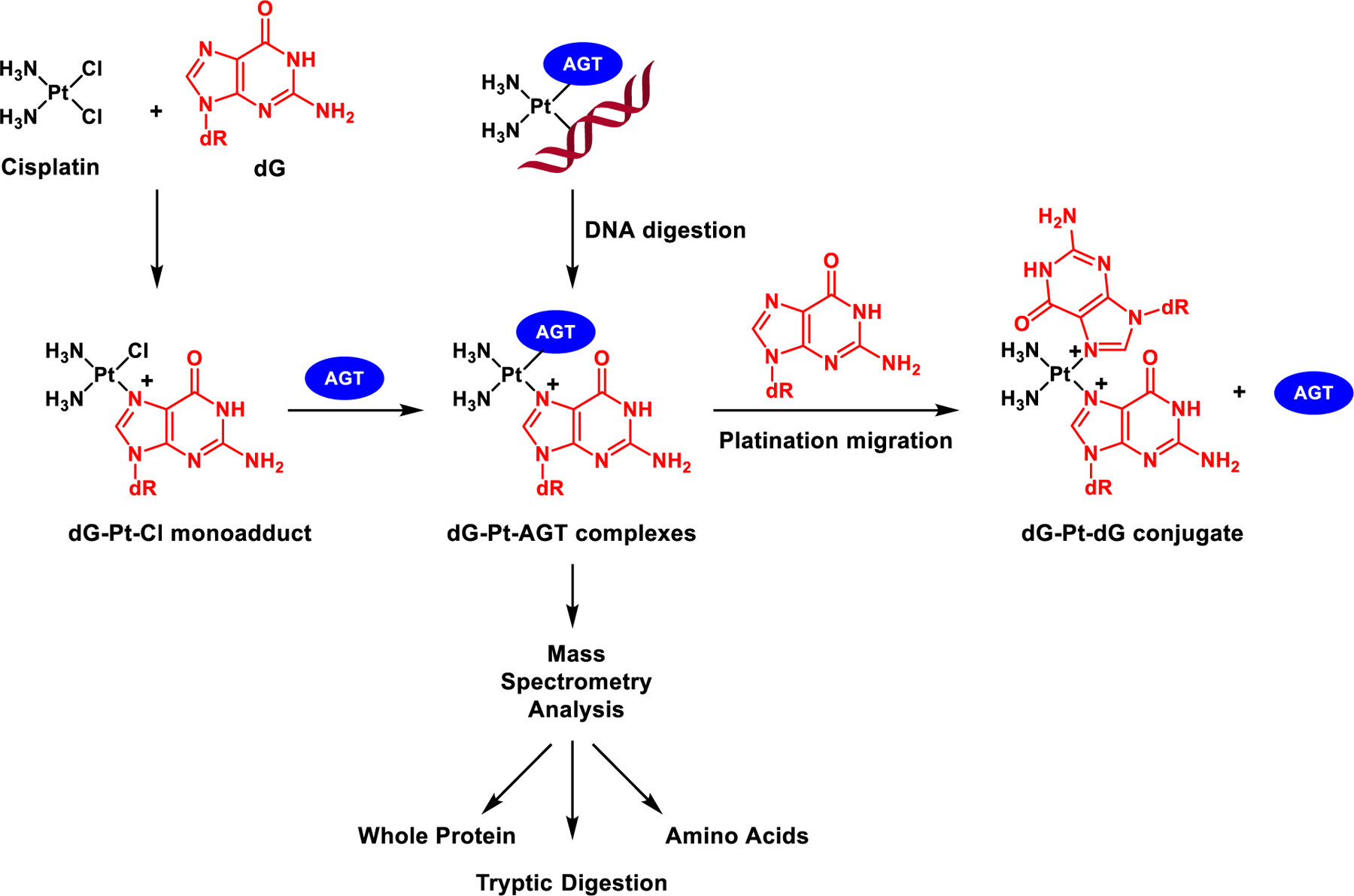
Mass spectrometry-based approach employed to characterize AGT-DNA cross-links of cisplatin and platination migration from AGT protein to dG to release intact AGT and form G-G cisplatin cross-links.
Figure 2.
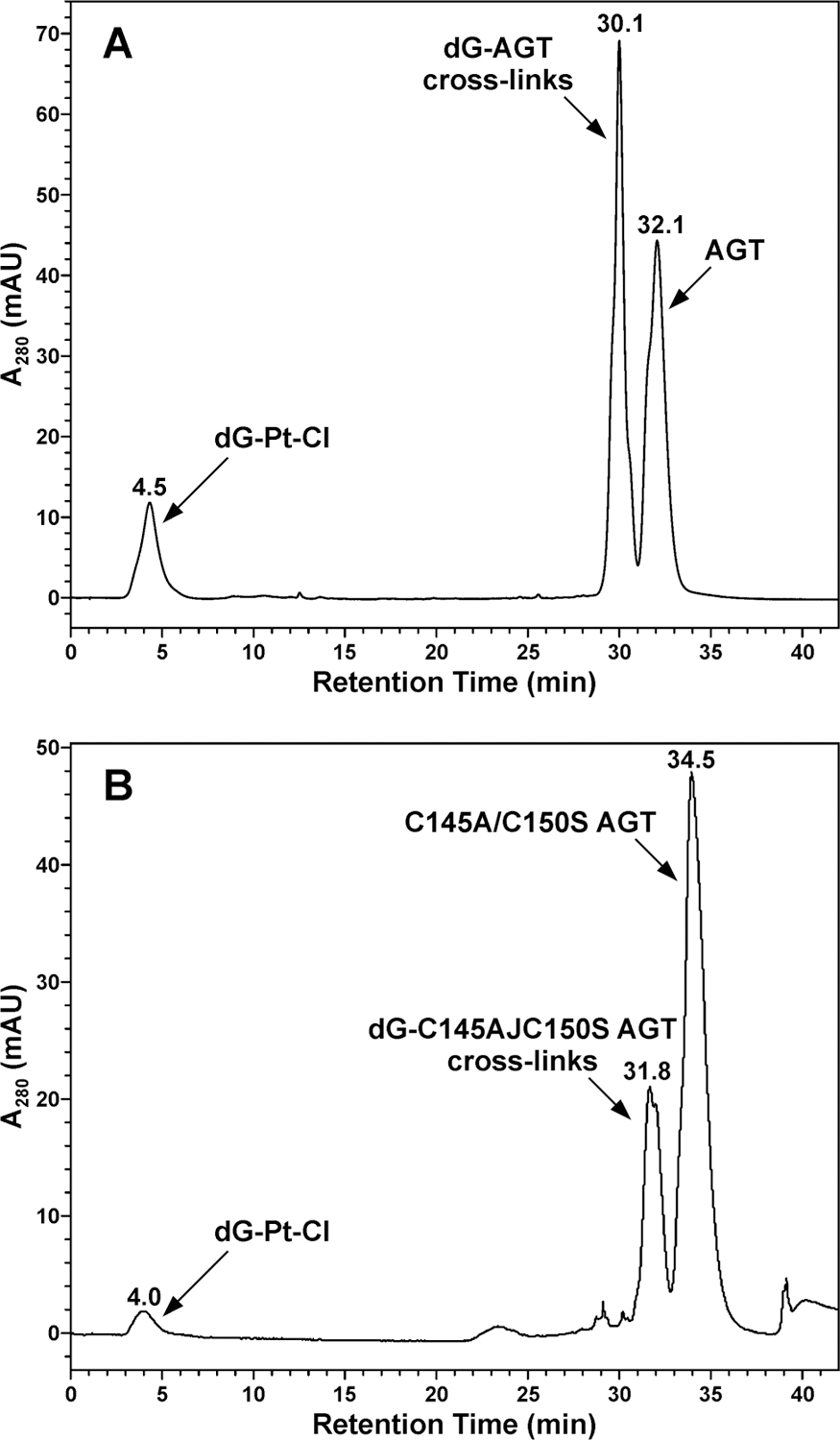
HPLC separation of reaction mixtures, following incubation of recombinant AGT protein with dG-Pt-Cl, as models for monoalkylated DNA to induce cross-linking. AGT-dG conjugates were identified by HPLC-ESI+-MS as shown in Figure 3.
Figure 3.
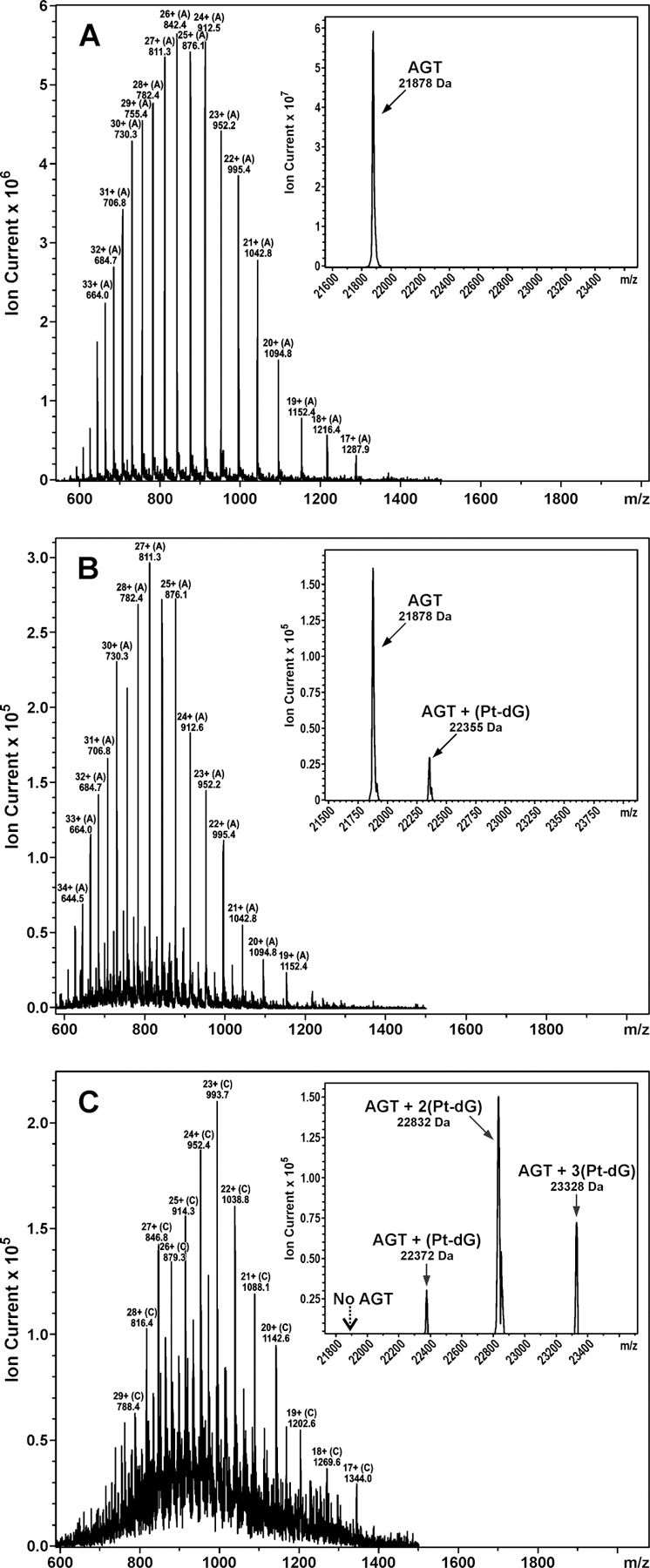
HPLC-ESI+-MS and deconvoluted spectra (inset) of (A) unreacted AGT protein, (B) dG-Pt-Cl monoadduct-treated AGT protein that had been separated by HPLC with retention time 32.1 min in Figure 2, and (C) dG-Pt-Cl monoadduct-treated AGT protein that had been separated by HPLC with retention time 30.1 min in Figure 2. Unmodified AGT (calculated, M = 21876 Da; observed, M = 21878 Da), AGT containing a single platinum cross-link to dG (calculated, M = 22354 Da; observed, M = 22355 Da or calculated, M = 22371 Da; observed, M = 22372 Da), AGT containing two platinum cross-links to dG (calculated, M = 22832 Da; observed, M = 22832 Da), and AGT containing three platinum cross-links to dG (calculated, M = 23327 Da; observed, M = 23328 Da).
To determine whether the cross-linking reaction involved active site cysteine residues of AGT, cross-linking experiments were repeated using C145A/C150S AGT active site mutant. The calculated molecular weight of the C145A/C150S_AGT variant is 22996 Da because of the addition of the N-terminal histidine tag (MRGSHHHHHHGS) (See Supporting Information, S-1). A similar HPLC trace (Figure 2B) was obtained for C145A/C150S_AGT variant that had been treated with synthetic dG-Pt-Cl monoadduct under the same condition as described above. HPLC-ESI+-MS analysis of the HPLC fractions revealed that the peak at 31.6 min contained C145A/C150S_AGT-dG cross-links, including C145A/C150S_AGT containing a single platinum cross-link to dG (AGT + Pt + dG + 2NH3, M = 23493 Da), two platinum cross-links to dG (AGT + 2Pt + 2dG + 3NH3, M = 23968 Da). ESI+ analysis of the material eluting at 34.5 min indicated a single protein species with a deconvoluted mass of 22,996.2 Da, which is consistent with the theoretical value (data not shown). Comparison of Figure 2A with Figure 2B in terms of the relative peak areas of modified proteins against unmodified proteins showed that wild-type AGT produced more adducts than the C145A/C150S_AGT variant, suggesting that Cys145 and Cys150 are involved in cross-linking.
Peptide Mapping by HPLC-ESI+-MS/MS.
Our ESI+-MS results for dG-Pt-Cl-treated AGT protein detected AGT species containing one-, two-, or three- platinum cross-linked to dG (Figure 3C), suggesting the presence of at least three distinct cross-linking sites within the protein. Further insight into the identities of AGT amino acid residues responsible for reaction with dG-Pt-Cl was provided by HPLC-ESI+-MS/MS analysis of tryptic digests of the platinated protein as described below.
Proteolytic digestion of native AGT protein with trypsin provided good protein sequence coverage (82%, Table 1). Only one of the predicted tryptic peptide, representing amino acids 37–96, was not detected due to its large size. In order to locate platinated sites within the protein, dG-Pt-Cl modified AGT (from HPLC peak at 30.1 min at Figure 2, see ESI+-MS spectrum in Figure 3C) was subjected to tryptic digestion, followed by nanoHPLC-nanospray ESI+-MS/MS on an Orbitrap Velos mass spectrometer. Spectral data were analyzed using Thermo Proteome Discoverer 1.2 software to search against human AGT protein FASTA database. Because the cross-linked moiety (dG-Pt-2NH3) is likely to experience fragmentation in the ESI+ source by a loss of one or two amino groups or a loss of 2-deoxyribose, multiple m/z values were specified for each target peptide: (A) cross-link to dG: +496.115 Da (dG + Pt + 2NH3), +479.087 Da (dG + Pt + NH3, a loss of −NH3), or +462.061 Da (dG + Pt, a loss of 2 −NH3); (B) cross-link to guanine: +379.059 Da (Gua + Pt + 2NH3), +361.030 Da (Gua + Pt + NH3, a loss of −NH3), or +345.005 Da (Gua + Pt, a loss of 2 – NH3). Furthermore, dG-Pt-Cl monoadduct-induced platination at the N-donor residues (N-terminus, histidine, lysine, or arginine), S-donor residues (cysteine or methionine), and O-donor residues (threonine, tyrosine, aspartic acid, or glutamic acid) were specified as potential dynamic modifications. The following stringent criteria were further implemented for all of the identified peptides: mass tolerance ≤ 300 ppm, Xcorr ≥ 2.5, ΔCn ≥ 0.5, and RSp (preliminary score rank) ≤ 3. Peptides sequences with MS/MS spectra not obeying with these criteria were removed from the final target list.
Table 1.
HPLC-ESI+-MS/MS analysis of AGT tryptic peptides (unmodified protein).
| Position | Peptide sequence | [M + H]+calculated | [M + 2H]2+calculated | [M + 3H]3+calculated | Observed ions |
|---|---|---|---|---|---|
| 1–8 | MDKDCEMK | 998.38 | 499.6939 | 333.4652 | 500.2 |
| 9–18 | RTTLDSPLGK | 1,086.60 | 543.8039 | 362.8718667 | 363.21, 544.31 |
| 10–18 | TTLDSPLGK | 930.5 | 465.7539 | 310.8385333 | 466.26 |
| 19–32 | LELSGCEQGLHEIK | 1,554.77 | 777.8889 | 518.9285333 | 519.27, 778.39 |
| 33–36 | LLGK | 429.28 | 215.1439 | 143.7652 | 215.43 |
| 37–96 | GTSAADAVEVPAPAAVLGGPEPLMQCTAW LNAYFHQPEAIEEFPVPALHHPVFQQESFTR |
6,468.13 | 3234.5689 | 2156.7152 | ND* |
| 97–101 | QVLWK | 673.40 | 337.2039 | 225.1385333 | 337.21 |
| 102–107 | LLKVVK | 699.51 | 350.2589 | 233.8418667 | 350.26 |
| 108–125 | FGEVISYQQLAALAGNPK | 1,905.00 | 953.0039 | 635.6718667 | 636.01, 935.51 |
| 126–135 | AARAVGGAMR | 959.52 | 480.2639 | 320.5118667 | 320.51, 480.26 |
| 129–135 | AVGGAMR | 661.35 | 331.1789 | 221.1218667 | 331.18 |
| 136–147 | GNPVPILIPCHR | 1,314.72 | 657.8639 | 438.9118667 | 658.37 |
| 148–165 | VVCSSGAVGNYSGGLAVK | 1,666.84 | 833.9239 | 556.2852 | 834.43 |
| 166–175 | EWLLAHEGHR | 1,246.61 | 623.8089 | 416.2085333 | 416.55, 624.32 |
| 176–193 | LGKPGLGGSSGLAGAWLK | 1,667.93 | 834.4689 | 556.6485333 | 566.99 |
| 194–207 | GAGATSGSHHHHHH | 1,428.60 | 714.8039 | 476.8718667 | 715.34 |
Not detected.
HPLC-ESI+-MS/MS analyses detected a prominent, triply charged peptide at m/z 801.12 ([M+3H]3+) corresponding to AGT residues F108GEVISYQQLAALAGNPK125 containing a cisplatin-dG cross-link (calculated M = 2401.28, ΔM = 496.11 Da (dG + Pt + 2NH3), Table 2 and Figure 4A). When this peptide was subjected to collision-induced dissociation (CID), the resulting MS/MS spectrum was consistent with the presence of dG-Pt-2NH3 adduct at Glu110 (Table 2 and Figure 4A). While the m/z values of y2, y3, y5-y10 and y12 fragments were in agreement with theoretical values for unmodified peptide, the mass of the y17 fragment was increased by 495.11 Da (observed M = 2255.05 Da, calculated M = 1759.93 Da for the unmodified y17 peptide fragment). The b32+-b72+ and b92+-b152+ fragments also contained the dG-Pt-2NH3 moiety as indicated by the 496.11 Da mass increase, suggesting the adduct resides at either the 2nd or the 3rd residue of the peptide (G109 or E110). Since Gly is a non-nucleophilic residue, these results are suggestive of Glu110 participation in AGT-dG cross-linking by cisplatin.
Table 2.
Platinated peptide sequences of AGT after treatment with dG-Pt-Cl followed by HPLC-ESI+-MS/MS analysis of the resulting tryptic digests. The assigned platinum binding site is given as bold italic type within peptide sequence.
| Peptide sequence | Pt adduct | Pt adduct mass (Da) |
Charge | SEQUEST parameters | Ions* | MS/MS spectrum | |
|---|---|---|---|---|---|---|---|
| Xcorr | ΔCn | ||||||
| F108GEVISYQQLAALAGNPK125 | +(Pt-dG-2NH3) | 495.11 | +3 | 4.53 | 1.0 | 34/68 | Figure 2.4A |
| F108GEVISYQQLAALAGNPKAAR128 | +(Pt-Gua) | 344.00 | +3 | 3.39 | 1.0 | 28/60 | Figure 2.4B |
| G136NPVPILIPCHR147 | +(Pt-Gua) | 344.00 | +2 | 2.54 | 1.0 | 22/44 | Figure 2.4C |
| G136NPVPILIPCHR147 | +(Pt-Gua-NH3) | 361.03 | +2 | 3.07 | 1.0 | 20/50 | Figure 2.4D |
| G136NPVPILIPCHR147 | +(Pt-dG-2NH3) | 495.11 | +2 | 3.05 | 1.0 | 16/35 | Figure 2.4E |
| V148VCSSGAVGNYSGGLAVK165 | +(Pt-Gua-2NH3) | 378.05 | +3 | 4.95 | 1.0 | 30/68 | Figure 2.4F |
Ratio of the assigned b and y ions to the total number of all possible fragment ions.
Figure 4.
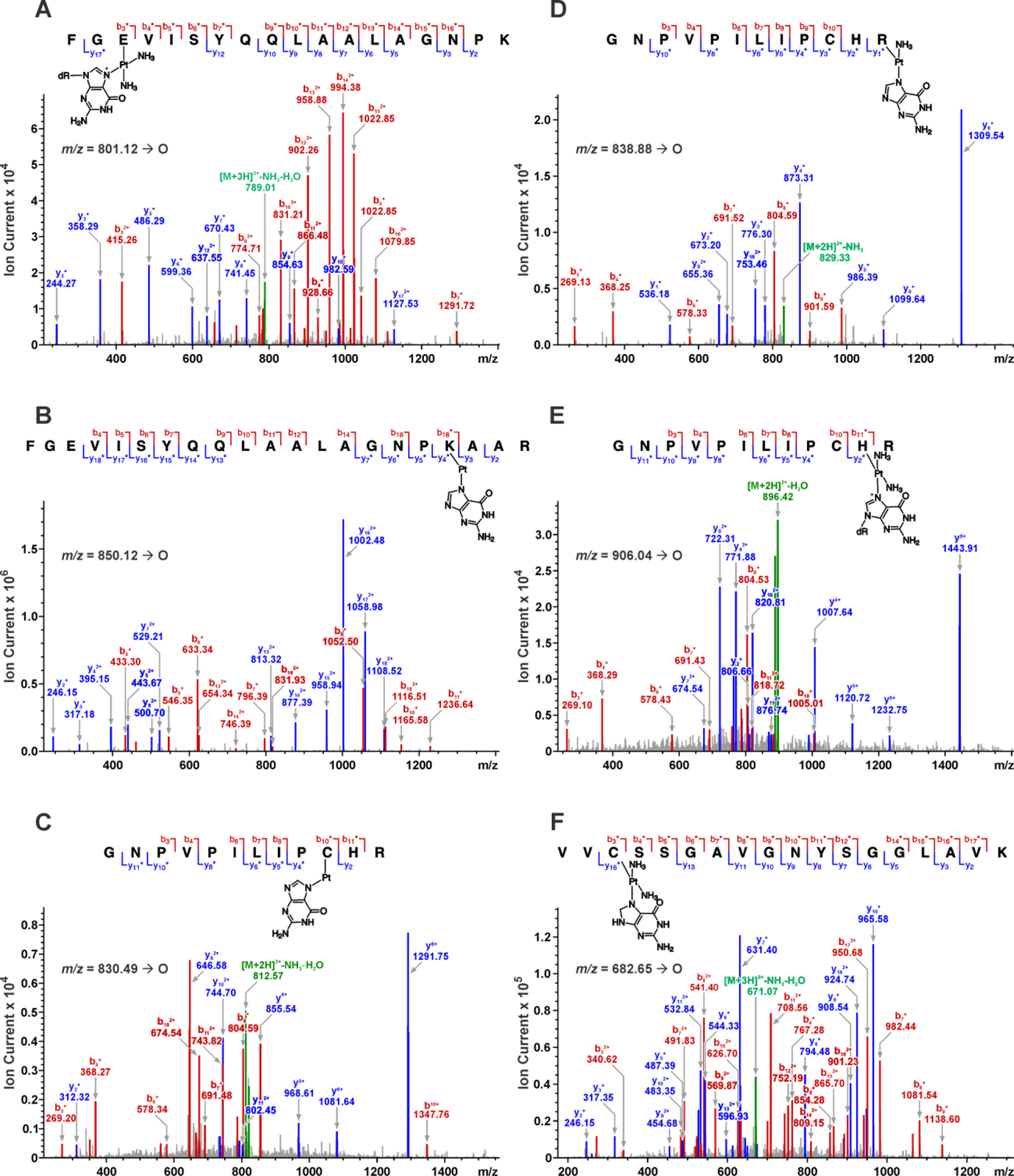
HPLC-ESI+-MS/MS analysis of tryptic peptides derived from cisplatin-induced AGT-dG conjugates at Glu110, Lys125, Cys145, His146, Arg147, and Cys150. Fragment ions containing a cross-linked platinum adduct are indicated by “*”.
The second cross-linking site was located upon detection of the peptide F108GEVISYQQ LAALAGNPKAAR128 containing a single platinum-dG adduct (m/z 850.12 [M+3H]3+; calculated M = 2547.35 Da, ΔM = 344.00 Da (Gua + Pt)) (Table 2 and Figure 4B). The cross-linking site was mapped to Lys125 based on the MS/MS fragmentation patterns, especially the diagnostic y3, y4 and b18 ions at m/z 317.18, 395.15 and 1116.51, respectively (Figure 4B). The y3 ion mass matched the theoretical value for unmodified peptide, while the y4 and b18 both experienced a mass shift corresponding to guanine-platinum adduct, which suggested that the cross-linking took place at Lys125.
Significant cross-linking was observed at three active site residues directly involved in AGT catalysis: Cys145 (Figure 4C), Arg147 (Figure 4D), His146 (Figure 4E), and Cys150 (Figure 4F). While Cys145 serves as alkyl acceptor during AGT repair reaction, His146 acts as a base to deprotonate Cys145. Arg147, together with Pro144 and Leu168, provides a hydrophobic slot for His146. In this hydrophobic environment, His146 accepts a hydrogen bond from water and donates one to the negatively charged Glu172 carboxylate, which pairs with Arg146 within a salt bridge [30]. HPLC-ESI+-MS/MS analyses revealed doubly charged ions corresponding to a guanine-cisplatin cross-link-containing the G136NPVPILIPCHR147 peptide (m/z 838.88 [M+2H]2+, calculated M = 1675.74 Da, ΔM = 361.03 Da (Gua + Pt + NH3), Table 2). CID of the doubly charged ion of the peptide (m/z 838.88 [M+2H]2+, calculated M = 1675.74 Da, ΔM = 361.03 Da (Gua + Pt + NH3)) produced an MS/MS spectrum containing b- (b3, b4, and b6–b10) and y-series ions (y1, y2, y5, y6, y8, and y10), consistent with the presence of a guanine-platinum adduct at Arg147 (Figure 4D).
Figure 4F depicts the CID-MS2 spectrum of the ions at m/z 682.65 ([M+3H]3+), corresponding to the platinated peptide V148VCSSGAVGNYSGGLAVK165 containing a Pt(NH3)2-guanine adduct. The observed b- and y-series ions y2–y3, y5–y11, and y13 ions are in agreement with the fragmentation of unmodified peptide, whereas m/z 340.62 (b32+) and 626.73 (b10+) both experience +379.10 Da (Gua + Pt + 2NH3) mass increase (Figure 4F), making it possible to assign the modification site to Cys150 of the AGT protein.
Taken together, our nanoHPLC-nanospray MS/MS results for tryptic digests of platinated AGT provide evidence for six dG-Pt-Cl binding sites: Glu110, Lys125, Cys145, His146, Arg147, and Cys150 (Table 2 and Figure 4). A crystal structure of human AGT protein bound to DNA is shown in Figure 5 (PDB 1T39). As is apparent from the crystal structures, Cys145, His146, and Arg147 are located directly in the AGT active site pocket (I143PCHRV148). The side chain of Glu110 is in a close proximity to the protein active site. Lys125 is located at the DNA-binding domain of the protein, which interacts with double stranded DNA via helix-turn-helix (HTH) motif. Mutations at Lys125 (i.e. Lys125Ala) have been previously shown to significantly disrupt the interaction of wild type AGT with DNA, indicating Lys125 is essential for DNA binding [30, 31].
Figure 5.
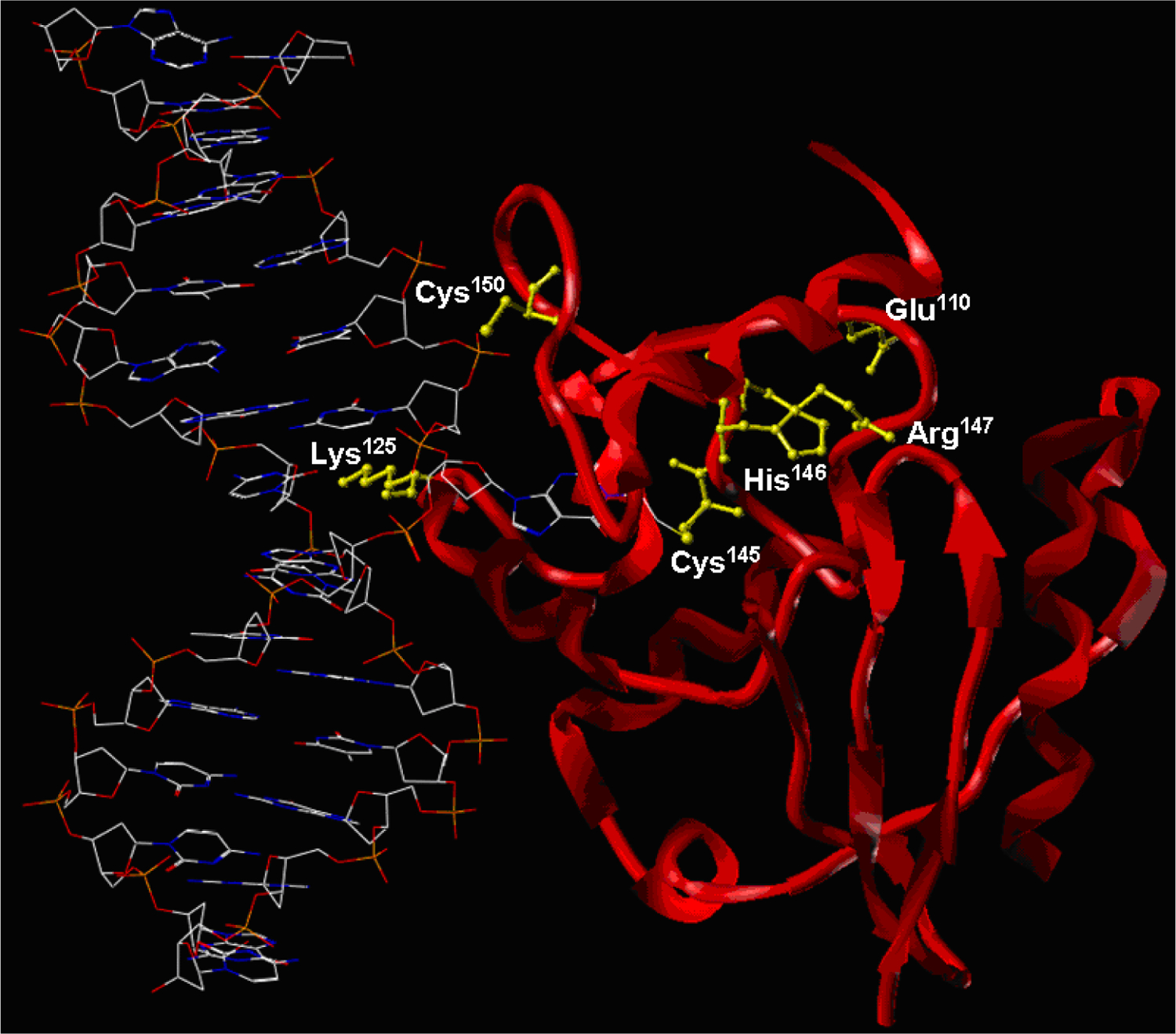
Crystal structure of human AGT protein bound to DNA (PDB 1T39) showing the sites of cisplatin-mediated DNA-protein cross-linking.
Platination Migration from DNA-Pt-Protein to DNA-Pt-DNA.
In our earlier global proteomics study, we observed that unlike other types of DPC adducts, cisplatin-induced DPCs could be reversed by heating [24]. One interesting property of platinum-induced adducts is that the Pt-S and Pt-N coordination bonds are potentially reversible, making it possible to observe “platination migration” from one nucleophilic site within biomolecules to another [32–35]. For example, Reediji and colleagues employed synthetic S-guanosyl-L-homocystine (sgh) as a model compound to examine intramolecular migration of platinum from cysteine thiol to the N7 of guanosine [32]. Similarly, Sadler’s group investigated the intermolecular displacement of Pt-S bound in Pt(dien)2+ model compound by N7-guanosine 5'-monophosphate [33]. In 2000, Reediji et al further demonstrated that the sulfur atom of the platinum-thioether adduct can be substituted by guanine nucleobase within oligonucleotides [34].
To investigate the possibility that AGT-DNA cross-links may undergo platination migration to form guanine-guanine cross-links to release the intact AGT protein, HPLC-purified dG-AGT conjugates (dG-Pt-AGT) were incubated with excess dG at 70 °C for varying times, followed by capillary HPLC-ESI+-MS analysis of the products (Scheme 2). HPLC-ESI+-MS analysis of the HPLC-purified dG-Pt-AGT confirmed that AGT was completely modified by dG-Pt-Cl, with no unreacted AGT protein present (Figure 3C). Deconvoluted ESI+ MS spectra revealed AGT protein bearing one (AGT + Pt + dG + NH3, M = 22355 Da), two (AGT + 2Pt + 2dG + 2NH3, M = 22832 Da) or three-platinum adducts cross-linked to dG (AGT + 3Pt + 3dG + 4NH3, M = 23328 Da) (Figure 3C). In contrast, HPLC-ESI+-MS analysis of dG-Pt-AGT cross-links incubated in the presence of free dG revealed the presence of free AGT protein (M = 21879 Da) (Figure 6A). When the incubation time was extended to 60 min, the unreacted AGT species (M = 21877 Da) became the dominant species (Figure 6B). In contrast, no unmodified AGT species was observed when dG-Pt-AGT complexes were heated in the absence of dG (Figure 6C).
Figure 6.
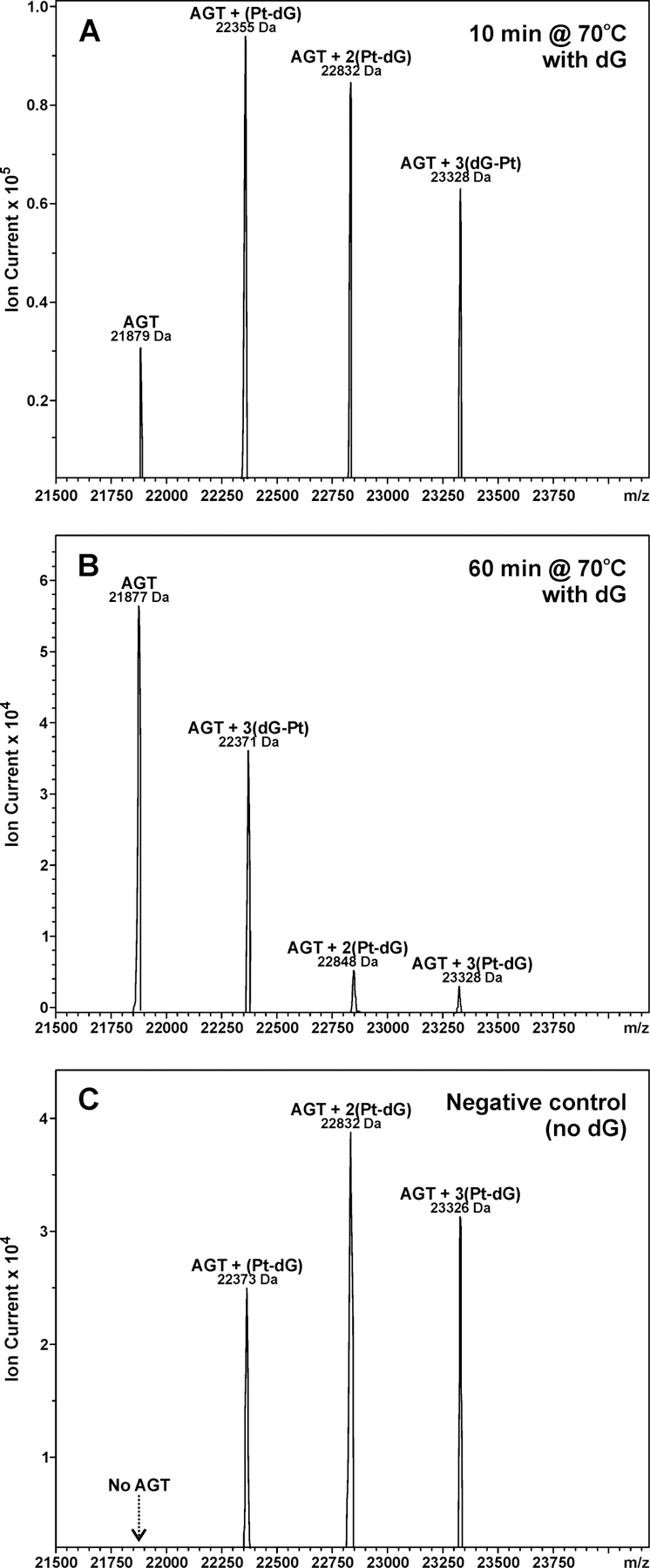
Platination migration from AGT to dG. (A) Deconvoluted ESI+-MS spectra of dG-Pt-AGT that was incubated with dG for 10 min at 70 °C. (B) Spectra of dG-Pt-AGT that was incubated with dG for 60 min at 70 °C. (C) Spectra of dG-Pt-AGT complexes was incubated in the absence of dG for 10 min at 70 °C.
Further experimental evidence for platination migration from protein to DNA was obtained by direct detection of dG-Pt-dG conjugates. HPLC-ESI+-MS/MS of AGT-DNA cross-links after heating detected a prominent peak co-eluting with the authentic standard of cis-1,1-diammine-bis-(2'-deoxyguanosine-7-yl)-platinum (II) (dG-Pt-dG, m/z 762.3) in samples treated with dG for 60 min at 70 °C (Figure 7) but not in control samples incubated either in the presence of dG at 37 °C overnight or in the absence of dG (results not shown). MS/MS fragmentation of m/z 762.3 yielded the product ions corresponding to the loss of ammonia (m/z 745.2 [M-NH3]+), the removal of 2'-deoxyribose and ammonia (m/z 629.1 [M-NH3-dR+H]+), and the loss of one dG (m/z 495.1 [M-dG]+). This compound had the same MS/MS fragmentation pattern and HPLC retention time as a synthetically prepared standard of dG-Pt-dG (Figure 7). In contrast, no dG-Pt-dG cross-link peak was detected from the control sample in which no dG was added (results not shown). Taken together, our results indicate that cisplatin-induced DNA-protein cross-links can be transformed into DNA-DNA cross-links upon heating, releasing intact proteins.
Figure 7.
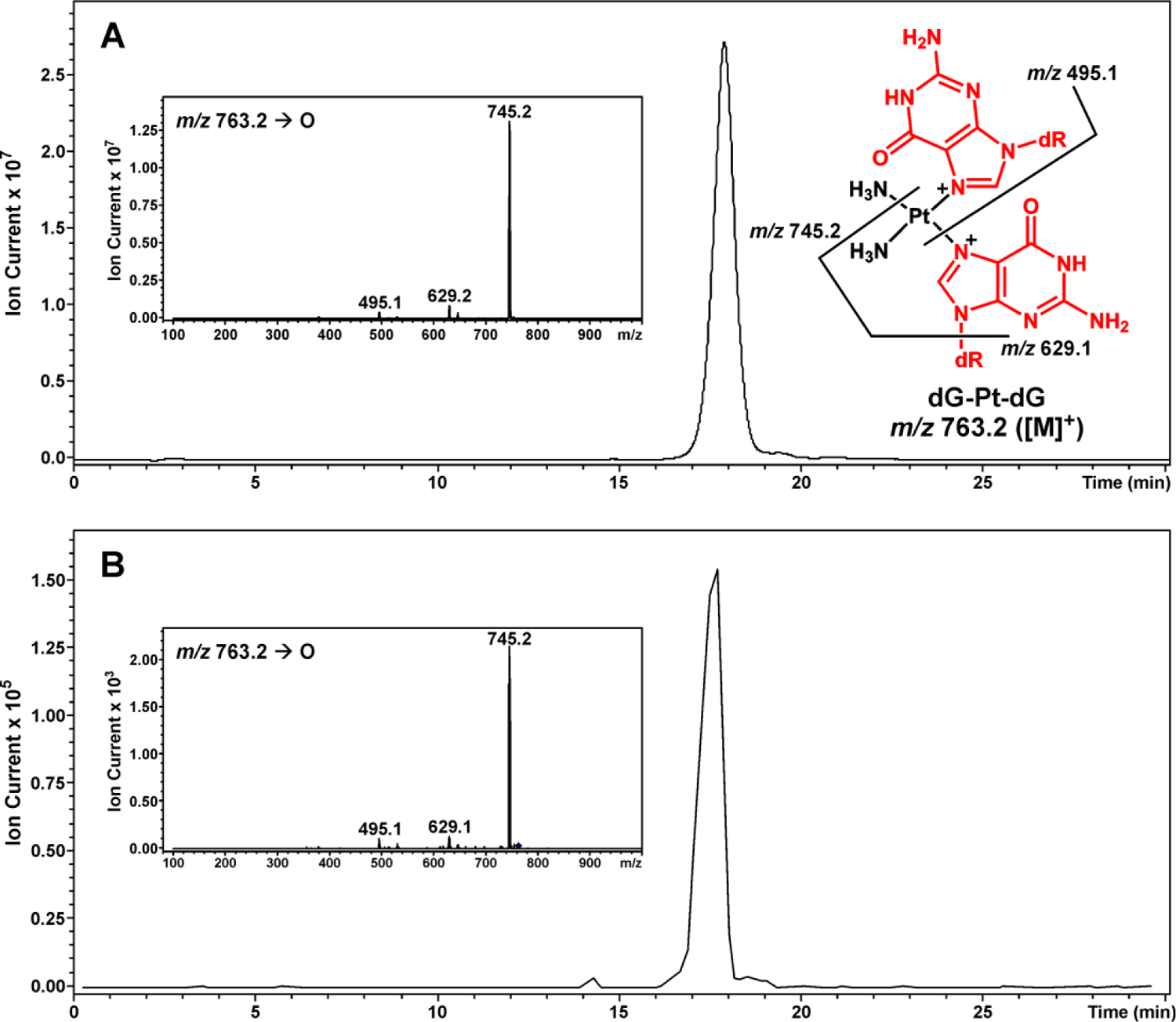
HPLC-ESI+-MS/MS analysis of dG-Pt-dG conjugates produced as a result of platination migration from dG-Pt-AGT cross-links to dG. Extracted ion chromatogram of dG-Pt-dG (m/z 763.2 [M]+). Inset: MS/MS fragmentation. (A) Synthetic dG-Pt-dG; (B) Sample treated with dG for 60 min at 70 °C
A similar platination migration experiments were conducted with HPLC-purified C145A/C150S AGT-dG conjugates. However, HPLC-ESI+-MS analysis of dG-Pt-C145A/C150S_AGT cross-links incubated in the presence of free dG revealed that only a small amount of free C145A/C150S_AGT protein (M = 22996 Da) was released from the cross-links, even after heating at 70 °C for 60 min (Figure S-2). This is consistent with platination migration from sulfhydryl chains of Cys145 and Cys150 to the N7 position of guanine.
Capillary HPLC-ESI+-MS/MS Analysis of dG-Pt-Lys Conjugates in Total Protein Digests.
To confirm that cisplatin-induced AGT-DNA cross-linking involves covalent modification of proteins and DNA, HPLC-ESI+-MS/MS analysis of the total enzymatic digests of platinated protein was performed. Synthetic 1,1-cis-diammine-2-(5-amino-5-carboxypentyl)amino-2-(2'-deoxyguanosine-7-yl)-platinum (II) (dG-Pt-Lys) was used as an authentic standard. Following cross-linking reaction, AGT protein was subjected to complete digestion in the presence of trypsin and proteinease K, followed by HPLC-ESI+-MS/MS analysis of the resulting amino acid mixtures in parallel with authentic dG-Pt-Lys.
Representative extracted ion chromatograms for capillary HPLC-ESI+-MS/MS analysis of dG-Pt-Lys conjugates in digests of cisplatin AGT-dG cross-links are shown in Figure 8. HPLC-ESI+-MS/MS of the digest mixtures detected a prominent peak (Figure 8B) co-eluting with the authentic standard of dG-Pt-Lys (Figure 8A). These data confirm that cisplatin-induced DNA-protein cross-linking can take place between the N7 position of guanine in DNA and the ε-amino group of lysines in AGT protein.
Figure 8.
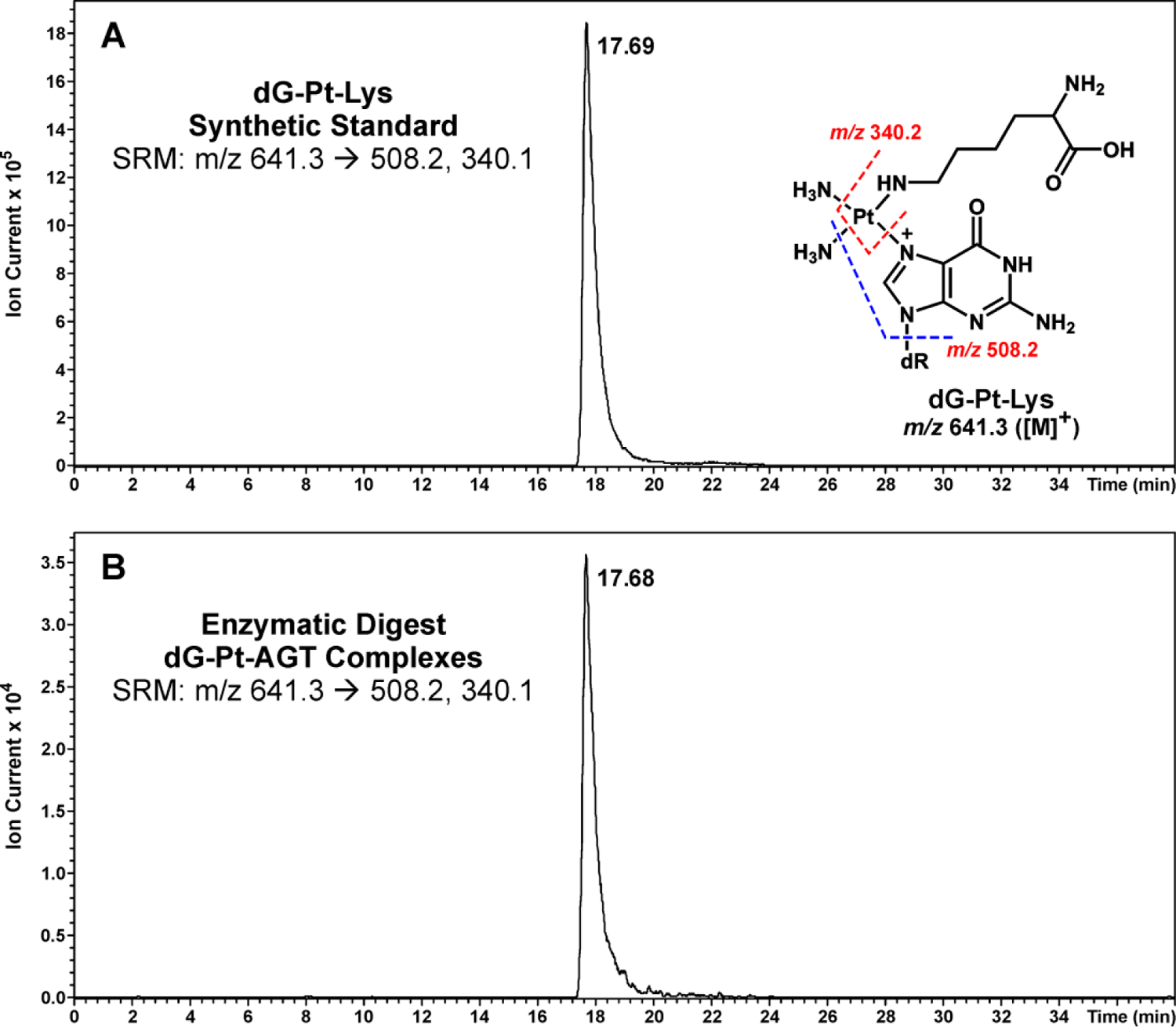
HPLC-ESI+-MS/MS analysis of dG-Pt-Lys conjugates in total proteolytic digests of AGT treated with dG-Pt-Cl to generate cross-links. Following HPLC purification of dG-Pt-AGT complexes, they were subjected to enzymatic digestion to release amino acid-nucleobase conjugates. (A) Synthetic dG-Pt-Lys; (B) Enzymatic digests of dG-Pt-AGT complexes.
Discussion
AGT is an important DNA repair protein that removes promutagenic DNA O6-alkylguanine lesions formed as a result of exposure to chemotherapeutic drugs and environmental toxins [36]. AGT transfers the O6-alkyl group from O6-alkylguanines in DNA to a cysteine residue within the protein active site (Cys145), thus restoring normal guanine [31]. Crystal structures of AGT-DNA complexes reveal that during the alkyl transfer reaction, the alkylated nucleotide is flipped out of the DNA base stack to enter the AGT active site [31].
AGT is readily cross-linked to DNA in the presence of various bis-electrophiles, probably due to its high affinity for DNA and the remarkable nucleophilicity of AGT Cys145, which is activated to a thiolate anion via a hydrogen bonding network in the protein active site [37]. As a result, the cytotoxicity and mutagenicity of bis-electrophiles such as 1,2-dibromoethane, dibromomethane, and 1,2,3,4-diepoxybutane (DEB), are enhanced in bacteria over-expressing human AGT protein due to the formation of toxic AGT-DNA cross-links [38, 39].
Based on the known ability of AGT to form toxic DPC lesions [39, 40], this protein was selected for our in vitro studies of DNA-protein cross-linking by cisplatin. Gel shift experiments with wild type and mutant AGT proteins revealed multiple sites of AGT can participate in cross-linking (Scheme 2, Figures 1–3). Subsequent HPLC-ESI+-MS/MS sequencing of tryptic peptides originating from AGT treated with dG-Pt-Cl monoadduct demonstrated that the cross-linking can occur at three sites within this protein, including Glu110, Lys125, and Arg147 (Figure 4A – C). Finally, the exact chemical structure of the amino acid-nucleoside conjugates was established as 1,1-cis-diammine-2-(5-amino-5-carboxypentyl) amino- 2-(2'-deoxyguanosine-7-yl)- platinum (II) (dG-Pt-Lys) based on the HPLC-ESI+-MS/MS analysis of amino acid-nucleoside conjugates in total protein digests in comparison with the corresponding authentic standard (Figure 8).
Our experiments were conducted with dG-Pt-Cl as a model of monoalkylated DNA because platination of N7 position of guanine accounts for over 98% among all the DNA-damage induced by cisplatin [18]. However, similar specificity is expected for AGT-DNA cross-linking in cells, resulting in N7-guanine-AGT cross-links. Our laboratory previously employed mass spectrometry-based methods to characterize AGT-DNA cross-linking by antitumor nitrogen mustards [11] and diepoxybutane (DEB) [41]. For both bis-electrophiles, cross-linking took place specifically at the two active site cysteine residues within the protein: Cys145 and Cys150 [11, 41]. Additional sites within AGT were identified to be involved in cisplatin-induced cross-linking, including the non-thiol side chains of Glu110, Lys125, and Arg147. This is indicative of a higher reactivity of cisplatin towards proteins and a different cross-linking chemistry characteristic for platinum compounds. Indeed, our recent mass spectrometry-based proteomics experiments of cisplatin-induced DPCs identified over 250 proteins covalently trapped to chromosomal DNA [24].
Previous studies investigated the reactions of model proteins such as cytochrome C [42], myoglobin [43], insulin [44, 45], ubiquitin [46], and human serum proteins [47] with platinum-based drugs. These researchers identified multiple Pt binding sites at N-donor residues (His and Lys), S-donor residues (Cys and Met), and O-donor residues (Thr, Tyr, Asp, and Glu). This is consistent with our results for AGT-dG cross-linking (Table 2 and Figure 4).
Crystal structures of human AGT protein reveal two distinct domains: the N-terminal domain (residues 1–85) and the C-terminal domain (residues 86–207) [31]. The N-terminal domain is composed of three β-sheets separated by two α-helixes, whereas the C-terminal domain contains two β-sheets and five α-helixes [31] (Figure 5). The C-terminal domain is involved in DNA-binding via the helix-turn-helix (HTH) motif and is responsible for DNA repair function. AGT active site contains the conserved active site cysteine motif (I143PCHRV148), and the O6-alkylguanine-binding channel, that are critical for nucleotide flipping and alkyl transfer reaction [30, 48].
The observed specificity of cisplatin-mediated cross-linking at Glu110, Lys125, Arg147, Cys145, and Cys150 of the AGT protein may be a result of their proximity to the AGT active site. These residues are found in the C-terminal domain of AGT (Figure 5) [30]. Arg147 is a nucleophilic active site residue that is located in the immediate proximity to DNA in the AGT-DNA complex (Figure 5) [30]. While the side chain of Glu110 is close to the reactive site pocket (I143PCHRV148) in space, Lys125 is directly located in the DNA-binding domain (Figure 5). In contrast, the other three cysteines within AGT, Cys5, Cys24 and Cys62 are not identified as the primary cross-linking sites, confirming our hypothesis that cross-linking could potentially take place at residues in close proximity DNA or directly involved in DNA-binding.
Our results reveal a potentially transient nature of dG-AGT cross-links, which can be converted to dG-dG cross-links upon heating (Figures 6 and S-1). Historically, there has been a long-term debate over the competition of purine bases, protein side chains, and glutathione for coordination sites of platinum [49]. The Hard Soft Acid Base (HSAB) theory predicts that the preferential binding ligands for platinum are S-containing biomolecules such as methionine and cysteine residues of proteins or glutathione [50]. This should leave little opportunity for platinum to coordinate with N-donor ligands in DNA, since numerous S-donor biomolecules present in cytosol (e.g. glutathione) should capture the drug before it enters the nucleus. Indeed, in vitro studies have demonstrated kinetically favored affinity of cisplatin toward S over N [33]. However, it is generally accepted that the ultimate biological target of cisplatin is DNA due to the ability of this drug to form DNA intra- and interstrand cross-links between N7 of the purine bases [20].
To determine how cisplatin can induce DNA-DNA cross-links despite its potential reactions with glutathione, peptides, and proteins in the cytosol before reaching the nucleus, Reedijk [32] and Sadler [33] conducted intra- and intermolecular competition experiments with S-ligands and nucleobases. These studies detected platination migration from S-ligands to the N7 of guanine. Deubel et al [49] employed density functional theory to calculate Pt-L bond energy in [Pt(NH3)3L]2+ complexes, where L represents peptide side chains, sulfur-containing protein agents, and guanine bases of DNA. These results revealed that platinum prefers N-ligands over S-ligands in terms of orbital interactions, electrostatics, and intramolecular hydrogen binding [49].
In summary, our results are consistent with previous studies indicating that there is a balance between Pt-S and Pt-N binding, where Pt-S binding is kinetically favored but Pt-N binding is more thermodynamically stable [35]. As a result, platinum initially bound to proteins may further react with DNA to yield Pt-DNA complexes and to release intact proteins. Indeed, our results presented above (Figures 6 and 7) provide evidence that AGT-DNA cross-links can rearrange to DNA-DNA cross-links, with N7-G winning the competition for the coordination sites of cisplatin upon heating of dG-AGT complexes. Although platination migration was not observed upon incubation at physiological conditions for 1 h, further studies are needed to establish whether platination migration from AGT to DNA takes place in cells [34].
In conclusion, our study demonstrates that cisplatin is capable of sequentially platinating nucleophilic sites within dG and AGT protein to form covalent cross-links between the N7 position of guanine and several residues within AGT including Glu110, Lys125, Cys145, His146, Arg147, and Cys150. To our knowledge, this is the first report of specific, structurally defined DNA-protein cross-linking involving cisplatin and a first observation of platination migration from DPC to DNA. These results are important because cisplatin-mediated DPC formation is likely to contribute to both on-target and off-target toxicity of this drug.
Supplementary Material
Acknowledgements
We thank Professor Anthony E. Pegg (Penn State University) for generously providing AGT protein, Brock Matter (Masonic Cancer Center, University of Minnesota) for assistance with MS experiments, Jason A. Kuchar (ThermoScientific) and Pratik Jagtap (Minnesota Supercomputing Insitute, University of Minnesota) for help with proteomic data analyses, Prof. Christine Chow and Bett Kimutai (Wayne State Univ.) for help with MS/MS data interpretation, and Robert Carlson for preparing figures for this manuscript. Funding for this research was provided by NIH grants R01-ES023350 and R01-CA-1006700 and a faculty development grant from the University of Minnesota Academic Health Center. X.M. and E.M.R. and were partially supported by the NIH Chemistry-Biology Interface Training Grant (T32-GM08700), University of Minnesota Masonic Cancer Center, and University of Minnesota Graduate School.
Abbreviations
- Cisplatin
1,1,2,2-cis-diamminedichloroplatinum (II)
- AGT
O6-alkylguanine DNA alkyltransferase
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- dG-Pt-Lys
1,1-cis-diammine-2-(5-amino-5-carboxypentyl)amino-2-(2'-deoxyguanosine-7-yl)-platinum(II)
- DEB
1,2,3,4-diepoxybutane
- DPC
DNA-protein cross-link
- d(GpG)
cis-[Pt(NH3)2{d(GpG)}] adducts
- HPLC-ESI+-MS/MS
high performance liquid chromatography electrospray ionization tandem mass spectrometry
Bibliography
- [1].Bain G, Maandag EC, Izon DJ, Amsen D, Kruisbeek AM, Weintraub BC, Krop I, Schlissel MS, Feeney AJ, van Roon M, et al. , E2A proteins are required for proper B cell development and initiation of immunoglobulin gene rearrangements, Cell, 79 (1994) 885–892. [DOI] [PubMed] [Google Scholar]
- [2].Dynlacht BD, Regulation of transcription by proteins that control the cell cycle, Nature, 389 (1997) 149–152. [DOI] [PubMed] [Google Scholar]
- [3].Accili D, Arden KC, FoxOs at the crossroads of cellular metabolism, differentiation, and transformation, Cell, 117 (2004) 421–426. [DOI] [PubMed] [Google Scholar]
- [4].Barker S, Weinfeld M, Murray D, DNA-protein crosslinks: their induction, repair, and biological consequences, Mutation Research, 589 (2005) 111–135. [DOI] [PubMed] [Google Scholar]
- [5].Shaham J, Bomstein Y, Meltzer A, Kaufman Z, Palma E, Ribak J, DNA-protein crosslinks, a biomarker of exposure to formaldehyde—in vitro and in vivo studies, Carcinogenesis, 17 (1996) 121–126. [DOI] [PubMed] [Google Scholar]
- [6].Qiu H, Wang Y, Exploring DNA-binding proteins with in vivo chemical cross-linking and mass spectrometry, Journal of Proteome Research, 8 (2009) 1983–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Barker S, Weinfeld M, Zheng J, Li L, Murray D, Identification of mammalian proteins cross-linked to DNA by ionizing radiation, Journal of Biological Chemistry, 280 (2005) 33826–33838. [DOI] [PubMed] [Google Scholar]
- [8].Ewig RA, Kohn KW, DNA-protein cross-linking and DNA interstrand cross-linking by haloethylnitrosoureas in L1210 cells, Cancer Research, 38 (1978) 3197–3203. [PubMed] [Google Scholar]
- [9].Kloster M, Kostrhunova H, Zaludova R, Malina J, Kasparkova J, Brabec V, Farrell N, Trifunctional dinuclear platinum complexes as DNA−protein cross-linking agents, Biochemistry, 43 (2004) 7776–7786. [DOI] [PubMed] [Google Scholar]
- [10].Baker JM, Parish JH, Curtis JPE, DNA-DNA and DNA-protein crosslinking and repair in Neurospora crassa following exposure to nitrogen mustard, Mutation Research, 132 (1984) 171–179. [DOI] [PubMed] [Google Scholar]
- [11].Loeber R, Michaelson E, Fang Q, Campbell C, Pegg AE, Tretyakova N, Cross-linking of the DNA repair protein O6-alkylguanine DNA alkyltransferase to DNA in the presence of antitumor nitrogen mustards, Chemical Research in Toxicology, 21 (2008) 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Loeber RL, Michaelson-Richie ED, Codreanu SG, Liebler DC, Campbell CR, Tretyakova NY, Proteomic analysis of DNA−protein cross-linking by antitumor nitrogen mustards, Chemical Research in Toxicology, 22 (2009) 1151–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Michaelson-Richie ED, Ming X, Codreanu SG, Loeber RL, Liebler DC, Campbell C, Tretyakova NY, Mechlorethamine-induced DNA–protein cross-linking in human fibrosarcoma (HT1080) cells, Journal of Proteome Research, 10 (2011) 2785–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Groehler A, Villalta PW, Campbell C, Tretyakova N, Covalent DNA–protein cross-linking by phosphoramide mustard and nornitrogen mustard in human cells, Chemical Research in Toxicology, 29 (2016) 190–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Oleinick NL, Chiu SM, Ramakrishnan N, Xue LY, The formation, identification, and significance of DNA-protein cross-links in mammalian cells, Br J Cancer Suppl, 8 (1987) 135–140. [PMC free article] [PubMed] [Google Scholar]
- [16].Tretyakova NY, Groehler A, Ji S, DNA–protein cross-links: Formation, structural identities, and biological outcomes, Accounts of Chemical Research, 48 (2015) 1631–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Goggin M, Loeber R, Park S, Walker V, Wickliffe J, Tretyakova N, HPLC−ESI+-MS/MS analysis of N7-guanine−N7-guanine DNA cross-links in tissues of mice exposed to 1,3-butadiene, Chemical Research in Toxicology, 20 (2007) 839–847. [DOI] [PubMed] [Google Scholar]
- [18].Jamieson ER, Lippard SJ, Structure, recognition, and processing of cisplatin−DNA adducts, Chemical Reviews, 99 (1999) 2467–2498. [DOI] [PubMed] [Google Scholar]
- [19].Chválová K, Brabec V, Kašpárková J, Mechanism of the formation of DNA–protein cross-links by antitumor cisplatin, Nucleic Acids Research, 35 (2007) 1812–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jung Y, Lippard SJ, Direct cellular responses to platinum-induced DNA damage, Chemical Reviews, 107 (2007) 1387–1407. [DOI] [PubMed] [Google Scholar]
- [21].Sherman SE, Lippard SJ, Structural aspects of platinum anticancer drug interactions with DNA, Chemical Reviews, 87 (1987) 1153–1181. [Google Scholar]
- [22].Zwelling LA, Anderson T, Kohn KW, DNA-protein and DNA interstrand cross-linking by cis- and trans-platinum(II) diamminedichloride in L1210 mouse leukemia cells and relation to cytotoxicity, Cancer Research, 39 (1979) 365–369. [PubMed] [Google Scholar]
- [23].Zwelling LA, Bradley MO, Sharkey NA, Anderson T Kurt W. Kohn, Mutagenicity, cytotoxicity and DNA crosslinking in V79 Chinese hamster cells treated with cis- and trans-Pt(II) diamminedichloride, Mutation Research, 67 (1979) 271–280. [DOI] [PubMed] [Google Scholar]
- [24].Ming X, Groehler A, Michaelson-Richie ED, Villalta PW, Campbell C, Tretyakova NY, Mass spectrometry based proteomics study of cisplatin-induced DNA–protein cross-linking in human fibrosarcoma (HT1080) cells, Chemical Research in Toxicology, 30 (2017) 980–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bender K, Federwisch M, Loggen U, Nehls P, Rajewsky MF, Binding and repair of O6-ethylguanine in double-stranded oligodeoxynucleotides by recombinant human O6-alkylguanine-DNA alkyltransferase do not exhibit significant dependence on sequence context, Nucleic Acids Res, 24 (1996) 2087–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Eastman A, Separation and characterization of products resulting from the reaction of cis-diamminedichloroplatinum(II) with deoxyribonucleosides, Biochemistry, 21 (1982) 6732–6736. [DOI] [PubMed] [Google Scholar]
- [27].Sambrook J, Fritsch EF, Maniatis T, Molecular Cloning. A Laboratory Manual, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y., 1989. [Google Scholar]
- [28].Yates JR, Eng JK, McCormack AL, Schieltz D, Method to correlate tandem mass spectra of modified peptides to amino acid sequences in the protein database, Analytical Chemistry, 67 (1995) 1426–1436. [DOI] [PubMed] [Google Scholar]
- [29].Yates JR, Eng JK, McCormack AL, Mining genomes: Correlating tandem mass spectra of modified and unmodified peptides to sequences in nucleotide databases, Analytical Chemistry, 67 (1995) 3202–3210. [DOI] [PubMed] [Google Scholar]
- [30].Daniels DS, Mol CD, Arvai AS, Kanugula S, Pegg AE, Tainer JA, Active and alkylated human AGT structures: a novel zinc site, inhibitor and extrahelical base binding, EMBO J, 19 (2000) 1719–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Daniels DS, Woo TT, Luu KX, Noll DM, Clarke ND, Pegg AE, Tainer JA, DNA binding and nucleotide flipping by the human DNA repair protein AGT, Nature Structural & Molecular Biology, 11 (2004) 714–720. [DOI] [PubMed] [Google Scholar]
- [32].van Boom SSGE, Reedijk J, Unprecedented migration of [Pt(dien)]2+(dien = 1,5-diamino-3-azapentane) from sulfur to guanosine-N7 in S-guanosyl-L-homocysteine (sgh), Journal of the Chemical Society, Chemical Communications, (1993) 1397–1398.
- [33].Barnham KJ, Djuran MI, del Socorro Murdoch P, Sadler PJ, Intermolecular displacement of S-bound L-methionine on platinum(II) by guanosine 5′-monophosphate: implications for the mechanism of action of anticancer drugs, Journal of the Chemical Society, Chemical Communications, (1994) 721–722.
- [34].Teuben J-M, Reedijk J, Reaction of DNA oligonucleotides with [Pt(dien)GSMe]2+ (GSMe =S-methylated glutathione) and cis-[Pt(NH3)2(GSMe)2]2+: evidence of oligonucleotide platination via sulfur-coordinated platinum intermediates, Journal of Biological Inorganic Chemistry, 5 (2000) 463–468. [DOI] [PubMed] [Google Scholar]
- [35].Reedijk J, Why does cisplatin reach guanine-N7 with competing S-donor ligands available in the cell?, Chemical Reviews, 99 (1999) 2499–2510. [DOI] [PubMed] [Google Scholar]
- [36].Pegg AE, Repair of O6-alkylguanine by alkyltransferases, Mutation Research, 462 (2000) 83–100. [DOI] [PubMed] [Google Scholar]
- [37].Guengerich FP, Principles of covalent binding of reactive metabolites and examples of activation of bis-electrophiles by conjugation, Archives of Biochemistry and Biophysics, 433 (2005) 369–378. [DOI] [PubMed] [Google Scholar]
- [38].Liu L, Hachey DL, Valadez G, Williams KM, Guengerich FP, Loktionova NA, Kanugula S, Pegg AE, Characterization of a mutagenic DNA adduct formed from 1,2-dibromoethane by O6-alkylguanine-DNA alkyltransferase, Journal of Biological Chemistry, 279 (2003) 4250–4259. [DOI] [PubMed] [Google Scholar]
- [39].Valadez JG, Liu L, Loktionova NA, Pegg AE, Guengerich FP, Activation of bis-electrophiles to mutagenic conjugates by human O6-alkylguanine-DNA alkyltransferase, Chemical Research in Toxicology, 17 (2004) 972–982. [DOI] [PubMed] [Google Scholar]
- [40].Kalapila AG, Loktionova NA, Pegg AE, Alkyltransferase-mediated toxicity of 1,3-butadiene diepoxide, Chemical Research in Toxicology, 21 (2008) 1851–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Loeber R, Rajesh M, Fang Q, Pegg AE, Tretyakova N, Cross-linking of the human DNA repair protein O6-alkylguanine DNA alkyltransferase to DNA in the presence of 1,2,3,4-diepoxybutane, Chemical Research in Toxicology, 19 (2006) 645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhao T, King FL, Direct determination of the primary binding site of cisplatin on cytochrome c by mass spectrometry, Journal of the American Society for Mass Spectrometry, 20 (2009) 1141–1147. [DOI] [PubMed] [Google Scholar]
- [43].Zhao T, King FL, A mass spectrometric comparison of the interactions of cisplatin and transplatin with myoglobin, Journal of Inorganic Biochemistry, 104 (2010) 186–192. [DOI] [PubMed] [Google Scholar]
- [44].Moreno-Gordaliza E.a., Cañas B, Palacios M.a.A., Gómez-Gómez MM, Top-down mass spectrometric approach for the full characterization of insulin−cisplatin adducts, Analytical Chemistry, 81 (2009) 3507–3516. [DOI] [PubMed] [Google Scholar]
- [45].Moreno-Gordaliza E, Canas B, Palacios MA, Gomez-Gomez MM, Novel insights into the bottom-up mass spectrometry proteomics approach for the characterization of Pt-binding proteins: The insulin-cisplatin case study, Analyst, 135 (2010) 1288–1298. [DOI] [PubMed] [Google Scholar]
- [46].Zhao T, King FL, Mass-spectrometric characterization of cisplatin binding sites on native and denatured ubiquitin, Journal of Biological Inorganic Chemistry, 16 (2011) 633–639. [DOI] [PubMed] [Google Scholar]
- [47].Will J, Wolters DA, Sheldrick WS, Characterisation of cisplatin binding sites in human serum proteins using hyphenated multidimensional liquid chromatography and ESI tandem mass spectrometry, ChemMedChem, 3 (2008) 1696–1707. [DOI] [PubMed] [Google Scholar]
- [48].Tubbs JL, Pegg AE, Tainer JA, DNA binding, nucleotide flipping, and the helix-turn-helix motif in base repair by O6-alkylguanine-DNA alkyltransferase and its implications for cancer chemotherapy, DNA Repair, 6 (2007) 1100–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Deubel DV, On the competition of the purine bases, functionalities of peptide side chains, and protecting agents for the coordination sites of dicationic cisplatin derivatives, Journal of the American Chemical Society, 124 (2002) 5834–5842. [DOI] [PubMed] [Google Scholar]
- [50].Pearson RG, Recent advances in the concept of hard and soft acids and bases, J Chem Educ, 64 (1987) 561–567. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


