ABSTRACT
The aim of this research was to determine the total phenolic content (TPC), antioxidation, antiaging, and antibacterial activities of Carissa carandas Linn., and aims at the novel plant sources which is utilized for their cosmeceutical applications. The two conditions (fresh and dried) and three stages (unripe, ripe, and fully ripe) of C. carandas were extracted by ethanolic maceration. Folin–Ciocalteu assay was used for determining the TPC. 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assays were used for estimating antioxidant activity. The inhibitory tyrosinase activities were measured using the modified dopachrome assay. Antiaging was evaluated by inhibition of collagenase and elastase, and antibacterial activities. The result of six extracts from C. carandas showed that the highest phenolic content and elastase inhibition of the fresh fruit in fully ripe stage were 100.31 ± 2.64 mg GAE/g extract and 14.11% ± 0.95%, respectively. The fresh fruit in the unripe stage showed that the strongest percentage of DPPH IC50 and collagenase inhibitory activity were 29.11 ± 0.23 μg/mL and 85.94% ± 2.21%, respectively. The ethanolic extract of unripe dried fruit exhibited the highest antioxidant activity in the of ABTS assay, with an IC50 of 0.17 ± 0.01 μg/mL. The MBC displayed the dried fruit ripe stage anti Cutibacterium acnes, Staphylococcus epidermidis, and Staphylococcus aureus strains were 25.0, 25.0, and 16.25 mg/mL, respectively. The fresh fruit in the ripe stage showed that the strongest inhibition tyrosinase was 93.88% ± 5.64%. The conclusion of this research indicates that the fresh fruit of C. carandas fruit extracts has high potential as a novel cosmeceuticals’ applications to antiaging and skin whitening. The dried fruit in ripe stage extract has the most effective ingredient for antiacne products.
Keywords: Antiaging, antibacterial, Carissa carandas, cosmeceuticals, karanda
INTRODUCTION
Natural beauty products have gained a growing popularity from consumers, correspond to the global cosmetics market forecasts of $429.8 billion by 2022.[1] Skin aging is affected by intrinsic and extrinsic factors such as reduction of regenerative cells, decrease of hormone levels, sunlight, etc.[2] Antioxidants are substances that help eliminate free radicals and reduce the amount of compound not to destroy the cells.[3] The fruit of Karanda (Carissa carandas Linn.) has white color to be pink-red until it is dark-purple too almost black when fully ripe with a sour taste, bitter, and astringent. Moreover, it is useful for wound healing, reduce fever, heart disease, etc.[4] In addition, fruits of C. carandas have found the phytochemical constituents, including Vitamin C,[5] anthocyanin,[6] flavonoids, glycosides, alkaloids, carbohydrates, sterols, terpenoids, tannins, and saponins.[7] Therefore, the objective of this study is designed to research the antioxidant, antityrosinase, antiaging, and antibacterial activities of various C. carandas fruit extracts. The results can provide the benefit to be used as novel cosmeceuticals applications.
MATERIALS AND METHODS
Plant materials
C. carandas fruits were collected by Dr. Juthaporn Kwansang from Prachathipat district, Pathum Thani Province, Thailand., which has been identified and authenticated of plant species by Bangkok herbarium, Plant varieties protection office, Department of Agriculture. C. carandas fruits were picked at each ripening stage; unripe (white color), ripe (pink-red color), and fully ripe (purple-black) fruit stage.
Chemicals
Ascorbic acid, Folin–Ciocalteu reagent (FCR), gallic acid, DPPH, ABTS, mushroom tyrosinase, L-DOPA, collagenase type I (from Clostridium histolyticum), FALGPA, epigallocatechin gallate, elastase from porcine pancreas type I, AAAPVN, Trizma® base, potassium persulfate, clindamycin, ampicillin, dimethyl sulfoxide, NA, NB, TSA, and TSB were purchased from Sigma Chemical Corp and Merck.
Bacterial strains
The bacterial strains in this research were used Cutibacterium acnes Scholz and Kilian (Propionibacterium acnes) (ATCC 11827), Staphylococcus epidermidis (Winslow and Winslow) Evans (ATCC 12228), Staphylococcus aureus subsp.aureus (ATCC 25923). They were obtained from the American Type Culture Collection (ATCC).
Extraction
Three different ripening stages including unripe, ripe, and fully ripe fruit stage were divided into two conditions as a fresh and dried fruit. The fruits were washed, cut into small pieces, and dried at temperature of 50°C. Extraction powdered samples (100 g each) of fresh and dried karanda fruit (C. carandas) were extracted with 95% ethanol (1:10 w/v) in a conical flask as well as maceration until 1 week. After the mixtures were filtered to obtain the extract, then evaporated by rotary evaporator and water bath to remove the solvent and stored at –20°C, therefore the percentage yield calculates.[8]
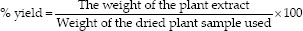
Total phenolic content
Total phenolic content (TPC) was identified using Folin–Ciocalteu following a previous method of Chatatikun and Chiabchalard[9] Briefly, blended 100 μL of C. carandas extracts (1 mg/mL) with 100 μL of 10% FCR and leaving it for 5 min. After that, added 70 μL of 10% Na2CO3 in order to neutralize. And then, kept it in a dark place for 90 min. The absorbance of the blended was measured at 750 nm by a microplate reader.
Antioxidant activities
Determination of the antioxidant capacity by DPPH assay
The DPPH assay of C. carandas extracts was adapted procedure from Lim et al.[10] The DPPH solution was prepared in absolute ethanol. The C. carandas extract concentrations were used in 15.625–1000 μg/mL serial dilution as the test sample. The test sample (100 μL) was mixed with DPPH solution (100 μL) then kept it in a dark place for 30 min. After that, the reaction was measured the absorbance at 520 nm. Trolox was used as a standard. The DPPH scavenging percentage was estimated by the following:

In addition, the antioxidant capacity was expressed as IC50 and calculated by regression analysis.
Determination of the antioxidant capacity by ABTS assay
ABTS activity was achieved as before defined by Kim et al.[11] with some adjustments. The extracts were used in 15.625–1,000 μg/mL serial dilution. The mixture was mixed with 7 mM ABTS + and 2.45 mM K2S2O8 (8:12 v/v ratio), then kept it in a dark place for 16 h. After that, the blend was diluted in absolute ethanol and the absorbance was measured at 750 nm. The ascorbic acid was used as a calibration curve. Finally, added 180 μL of the ABTS + solution to each well. Afterward, kept it in a dark place for 30 min and evaluated absorbance at 750 nm wavelength. The ABTS scavenging percentage was estimated as follows: (1). Moreover, calculations were applied to obtain the IC50.
Tyrosinase inhibition test
The tyrosinase inhibition was done with L-DOPA as substrate following the method by Di Petrillo et al.[12] with some modification. The mixture was contained with 50 μL of 2 mg/mL C. carandas extract concentration, 50 μL of sodium phosphate buffer (0.2 M, pH 6.6), and 50 μL of tyrosinase (50 units/mL). Subsequently, the mixture was incubated at 37°C for 10 min. After, adding a solution of L-DOPA (2.5 mM) volume of 50 μL. Finally, the reaction was immediately monitored at 492 nm. Consequently, the tyrosinase inhibitory percentage was estimated by the following formulation:
% Inhibition = (A − B)/A × 100
where
A = slope of control at 492 nm
B = slope of test sample at 492 nm
Antiaging activities
Collagenase inhibitory activity
The collagenase inhibitory activity was evaluated by method following Liyanaarachchi et al.[13] with modification. The collagenase solution (0.8 units/mL) was dissolved in tricine buffer (50 mM, pH 67.5). The 0.5 mM FALGPA substrate was dissolved in buffer and measured kinetic absorbance at 340 nm for 1 min. The total reaction volume was included plant extracts (20 μL), enzyme solution (20 μL), and substrate (200 μL). Nevertheless, enzymatic activity was measured by reducing absorbance in the time period. The anticollagenase percentage was calculated by the following formula

Elastase inhibitory activity
The elastase inhibitory activity was evaluated by following Chiocchio et al.[14] with modification. The elastase solution (2 units/mL) was dissolved in Tris-HCl buffer (1 M, pH 8).
The 1.6 mM AAAPVN was dissolved in buffer and constantly measured absorbance at 410 nm for 5 min. The total reaction volume was contained plant extracts (40 μL), enzyme solution (20 μL), and substrate (540 μL). The enzymatic activity was measured by reduced kinetic absorbance. The antielastase percentage was calculated by the following formula (2).
Antibacterial activities
Agar disc diffusion method tests
The agar disc diffusion test was played according to the method defined by Gomesa et al.[15] Three bacterial strains, including C. acnes and S. epidermidis, were inoculated into the TSB, and S. aureus was inoculated into the NB, and then incubated for 24 h at 37°C. After that, the incubated bacterial were diluted with TSB and NB and then measured absorbance (0.08–0.10 OD) using a spectrophotometer at 625 nm wavelength. Afterward, the bacterial suspension was spreaded on the surface of the TSA and NA plates. A sterile paper disc (6 mm in diameter) was impregnated with 20 μL of C. carandas extracts (4 mg/mL), the DMSO was used as a negative control. Subsequently, the antibacterial activity was determined by the mean of clear zone diameter (mm including the diameter of the disc).
Determination of minimal inhibitory concentration
The minimal inhibitory concentration (MIC) value was performed by applying a modified microdilution method with Gomesa et al.[15] and Sabooraa et al.[16]. The bacterial inhibitory results from agar disc diffusion were selected to MIC determination. The extracts were used in 3.125–100 mg/mL serial dilution with sterile TSB and NB and added 1.0 mL into a sterile test tube. The 1.0 mL of bacterial suspensions was added into each tube and mix well. After that, incubated at 37°C for 16 h, excluded C. acnes incubated under anaerobic condition. Finally, measured absorbance at 625 nm. The lowest concentration that did not show any growth (turbidity was not observed) of these bacterial was taken as the MIC.
Determination of minimum bactericidal concentration
The minimum bactericidal concentration (MBC) value was explained as the least concentrate of the extract to kill microorganisms. Based on MIC test results, the tubes showing a lack of growth were streaked on TSA and NA plate and incubated at 37°C for 24 h but C. acnes incubated under anaerobic condition. The MBC of antibacterial extracts is prescribed to the lowest concentrations without visible growth.
Statistical analysis
Each experiment was performed three replicates and presented as means ± standard deviation. The significant differences between the extracts were determined using analysis of variance at P < 0.05; furthermore, statistical analysis was done with GraphPad Prism Version 5.01 (San Diego, California, USA).
RESULTS AND DISCUSSION
Extraction
The percent yield of six extracts of C. carandas fruits including fresh fruit in unripe stage, fresh fruit in ripe stage, fresh fruit in fully ripe stage, dried fruit in unripe stage, dried fruit in ripe stage, and dried fruit in the fully ripe stage was presented 5.33, 4.70, 5.28, 32.40, 38.59, and 38.30, respectively. The dried fruit extracts showed that the highest percentage was significant. Maybe due to the dried plant, it was removing excess water and enzyme action in the cell. Therefore, it gives free bonding and low viscosity which useful in the extraction process.
Total phenolic content
Total phenolic content of C. carandas fruits, including fresh fruit in unripe stage, fresh fruit in ripe stage, fresh fruit in fully ripe stage, dried fruit in unripe stage, dried fruit in ripe stage, and dried fruit in the fully ripe stage was shown that TPC varied from 55.32 ± 1.81 to 100.31 ± 2.64 mg GAE/g of the extract [Figure 1] by the extract of fresh fruit in fully ripe stage showed the highest phenolic content. It may be concluded that the color of fruit is another factor affecting the total phenolic content of fruits. This is consistent with the research of Athipornchai and Jullapo.[17] In contrast, when comparing the fresh and dried condition of extract, it found that the extract of dried condition in unripe stage indicated an increase in the total of phenolic content. This may be because the heating process can be changed some polyphenol in C. carandas extracts. However, these results are in accordance with Tagouelbe, et al.[18] who recommended that a heating temperature enhances the total phenolic content. Consequently, the temperature is one of the factors that affect the amount of phenolic in both positive and negative. If the excessive temperature is used, the cell walls and the bound of phenolic compounds are destroyed that causing the loss of phenolic. On the other hand, drying at the right temperature, it can be increasing of the total phenolic content.
Figure 1.
Total phenolic content of Carissa carandas extracts determined by the Folin–Ciocalteu assay
Antioxidant activities by DPPH and ABTS assay
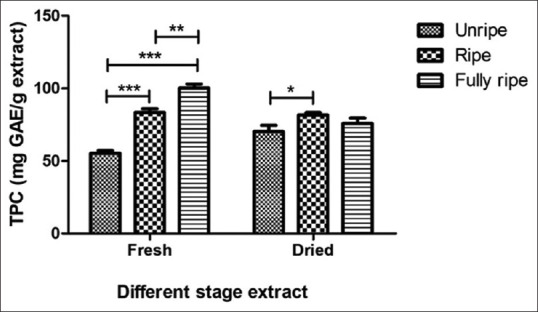
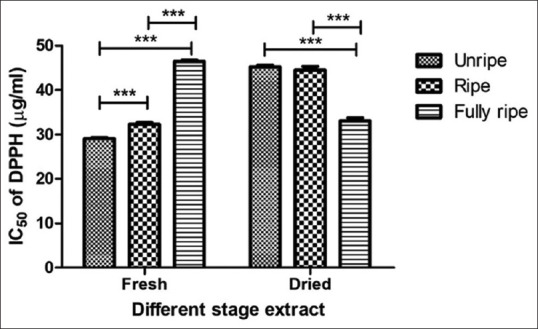
The DPPH scavenging activity of C. carandas extracts is depicted in Figure 2. The results exhibited that IC50 DPPH values were found to vary degree ranging from 29.11 ± 0.23 to 46.49 ± 0.34 μg/mL and standard Trolox in this assay was 11.33 ± 2.23 μg/mL and the ABTS scavenging activity of C. carandas extracts is shown in Figure 3. The dried unripe fruit extract presented the greatest ABTS free radical scavenger with IC50 values of 0.17 ± 0.01 μg/mL and standard ascorbic acid in this evaluate was 0.03 ± 0.00 μg/mL. The findings of the DPPH scavenging assay showed the fresh condition of C. carandas extracts, giving better effect than dried condition by the fresh unripe extract was shown the highest scavenging (IC50 29.11 ± 0.23 μg/mL), while the fresh fully ripe extract was displayed weakly active (IC50 46.49 ± 0.34 μg/mL). Thus, the results expressed that the unripe extract was more efficient in resisting free radicals than ripe and fully ripe fruit extract may be due to unripe extract from C. carandas fruit has a good source of vitamin C.[19] Vitamin C is a natural phytochemical composite with antioxidant qualities with the ability to provide hydrogen atoms and create reasonably stable ascorbyl free radicals.[20]
Figure 2.
Antioxidant activity of Carissa carandas extracts determined by DPPH method
Figure 3.
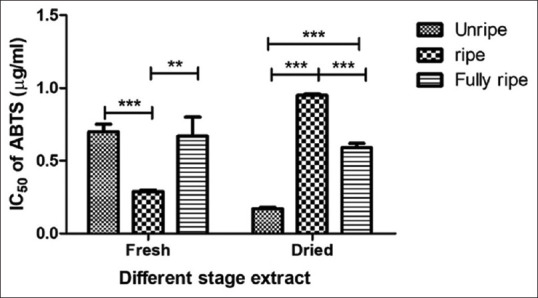
Antioxidant activity of Carissa carandas extracts determined by ABTS method
Tyrosinase inhibition test
The inhibition tyrosinase enzyme of C. carandas extracts was presented strong inhibition of mushroom tyrosinase with a percentage from 83.00% ± 1.89% to 93.88% ± 5.64%. The most active extract level was the fresh ripe extract and 100.00% ± 0.00% of ascorbic acid are summarized in Figure 4. Tyrosinase inhibition effect of C. carandas fruit extracts indicated the potential for use as a skin whitening ingredient in cosmetics. Since C. carandas fruit extracts show more than 80% of the enzyme inhibitors, the result of the fresh ripe extract is best expressed in the inhibitory effect similar to the results of Khunchalee[21] that the 70% ethanol of C. carandas fruit extracts showed the greatest tyrosinase inhibition at 77.65% may be due to the C. carandas fruit extracts are rich of flavonoid,[22] vitamin C, and anthocyanin[23] which flavonoid and anthocyanins have the ability to inhibited tyrosinase and melanogenesis.[24,25,26] Moreover, Vitamin C and flavonoid can be antioxidant, antielastase,[27] and anticollagenase effect.[28]
Figure 4.
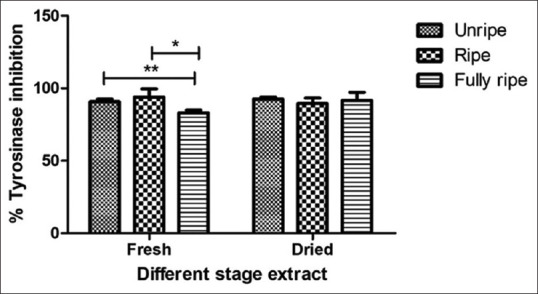
Tyrosinase inhibition assay of Carissa carandas extracts
Antiaging activities by collagenase and elastase inhibitory assay
The percentage of collagenase inhibition of C. carandas extracts at 1 mg/mL 71.88 ± 4.42 to 85.94 ± 2.21. The fresh unripe extract had a high percentage of collagenase inhibition and standard epigallocatechin 3 gallate (EGCG) in this assay was 71.88 ± 0.00 [Figure 5]. The elastase inhibitory activity assay performed by taking EGCG as a standard showed that C. carandas extracts with the weakly elastase inhibition that percentage 4.23 ± 0.72 to 10.01 ± 0.92 and standard EGCG in this assay was 95.89 ± 0.22 [Figure 6]. Collagen and elastin are elements of the extracellular matrix. Collagen has the properties of the skin to be strong, tensile strength, and well-weighted of the skin, while elastin gives the elasticity to tissues. Hence, inhibiting the enzymes collagenase and elastase which breakdown these components may have antiaging benefits.[29] Our results indicate the various of C. Carandas ethanolic extracts have potential to reduce the degradation of collagen but slightly inhibited elastase. The fresh unripe extract is great capable of inhibiting collagenase. Therefore, the results indicate that the unripe extract of C. carandas fruit especially unripe fruit can be used as an active ingredient for antiaging products because the effects correlated with previous tests for antioxidant activity and also completely protected DNA damage.[30]
Figure 5.
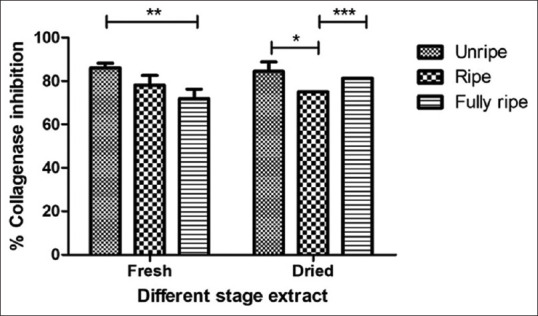
Collagenase inhibitory activity of Carissa carandas extracts
Figure 6.
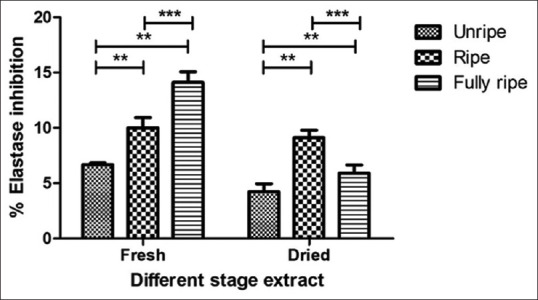
Elastase inhibitory activity of Carissa carandas extracts
Antibacterial activities
Acne is one of the causes associated with skin damage. Acne can occur for many reasons, such as hormone imbalance, sebum production, follicular hyperkeratinization, inflammation, and the presence of the main bacteria C. acnes, S. epidermidis, and S. aureus that causes of acne skin.[31,32,33] The results of antibacterial activities of six ethanolic extracts to anti-C. acnes, S. epidermidis, and S. aureus are displayed in Table 1. The dried unripe extract had the maximum inhibition zone at 8.00 ± 1.00, 7.67 ± 0.58, and 12.33 ± 1.15 mm, respectively, the MIC of the dried ripe extract showed the best effect between 6.25 and 12.50 mg/mL, and the MBC values of the extracts showed inhibitory activity at 6.25 and 25.0 mg/mL. Summary, the dried ripe and fully ripe extracts have the best potential to inhibit C. acnes growth, the dried ripe extract showed good MBC against S. epidermidis, and all extracts in dried condition showed greatly inhibit S. aureus. Hence, the study of this research suggests that the dried ripe extract can be applied as an active ingredient for antiacne products because of active phytochemicals present in C. carandas fruit ethanolic extracts including alkaloids, flavonoids, glycosides, terpenoids, tannins, and saponin.[34] Tannins, flavonoids, alkaloids, and polyphenols were remarkably anti propionibacteriu property.[35,36]
Table 1.
Antibacterial activities of different Carissa carandas fruit extracts
| Carissa carandas fruit extracts | Cutibacterium acnes | Staphylococcus epidermidis | Staphylococcus aureus | ||||||
|---|---|---|---|---|---|---|---|---|---|
| IZ (mm) | MIC (mg/mL) | MBC (mg/mL) | IZ (mm) | MIC (mg/mL) | MBC (mg/mL) | IZ (mm) | MIC (mg/mL) | MBC (mg/mL) | |
| Fresh unripe | 9.00±1.00 | 6.25 | 12.5 | 7.33±0.58 | 12.5 | 50.0 | ND | - | - |
| Fresh ripe | 7.67±0.58 | 6.25 | 12.5 | 7.00±0.00 | 12.5 | 50.0 | ND | - | - |
| Fresh fully ripe | 7.00±0.00 | 6.25 | 12.5 | 7.00±0.00 | 12.5 | 50.0 | ND | - | - |
| Dried unripe | 12.33±1.15 | 6.25 | 12.5 | 7.67±0.58 | 12.5 | 50.0 | 8.00±1.00 | 12.5 | 25.0 |
| Dried ripe | 11.33±1.53 | 6.25 | 6.25 | 7.33±0.58 | 12.5 | 25.0 | 7.33±0.58 | 12.5 | 25.0 |
| Dried fully ripe | 6.67±0.58 | 6.25 | 6.25 | 7.00±0.00 | 12.5 | 100.0 | 6.67±0.58 | 12.5 | 25.0 |
| Ampicillin 10 ug/ml | - | - | - | 9.00±1.00 | 0.0625 | >0.5 | 21.00±1.00 | 0.03125 | >0.25 |
| Clindamycin 2 ug/ml | 26.33±0.58 | 0.0125 | 0.05 | - | - | - | - | - | - |
IZ: Inhibition zone, MIC: Minimum inhibitory concentration, MBC: Minimal bactericidal concentration, ND: Not detected; -: Not tested
CONCLUSION
C. carandas fresh fruit extracts are highly potent for antiaging and skin whitening properties. In addition, the dried ripe extracts have the most active ingredient in antiacne products. Therefore, C. carandas fruits can be used as a new cosmeceutical agent.
Financial support and sponsorship
This work was supported by the Rajamangala University of Technology Thanyaburi, Thailand (Project code: IRF01126006).
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Rajput N. Cosmetics Market by Category (Skin & Sun Care Products, Hair Care Products, Deodorants, Makeup & Color Cosmetics, Fragrances) and by Distribution Channel (General departmental store, Supermarkets, Drug stores, Brand outlets)-Global Opportunity Analysis and Industry Forecast, 2014 – 2022, Market Research Report. 2016. [Last accessed on 2020 Mar 03]. Available from: https://www.alliedmarketresearch.com/cosmetics-market .
- 2.Zhang S, Duan E. Fighting against skin aging: The Way from bench to bedside. Cell Transplant. 2018;27:729–38. doi: 10.1177/0963689717725755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Painupong A. Free radicals and anti-oxidants in human health. PKRU Sci Tech J. 2017;1:20–7. [Google Scholar]
- 4.Arif M, Kamal M, Jawaid T, Khalid M, Saini SK, Kumar A, et al. Carissa carandas Linn.(Karonda): An exotic minor plant fruit with immense value in nutraceutical and pharmaceutical industries. Asian J Biomed Pharm. 2016;6:14–9. [Google Scholar]
- 5.Debashis D, Amit N, Dushyant M, Nisha V, Pawan K. Phytochemical studies on antioxidant activities of two types of Karonda (Carissa carandas) during storage. Indian J Hortic. 2016;73:623–6. [Google Scholar]
- 6.Le TX, Huynh TM, Pham NT, Than TV, Toan QT, Bach GL, et al. Optimization of total anthocyanin content, stability and antioxidant evaluation of the anthocyanin extract from Vietnamese Carissa carandas L.fruits. Processes. 2019;7:468. [Google Scholar]
- 7.Kasturi P, Satyanarayana B, Devi SP. Phytochemical studies and TLC finger print profiling of different bioactive compounds from Carissa carandas L. A medicinally important plant. Int Res J Pharm. 2018;9:115–20. [Google Scholar]
- 8.Anosike CA, Igboegwu ON, Nwodo OF. Antioxidant properties and membrane stabilization effects of methanol extract of Mucuna pruriens leaves on normal and sickle erythrocytes. J Tradit Complement Med. 2019;9:278–84. doi: 10.1016/j.jtcme.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatatikun M, Chiabchalard A. Thai plants with high antioxidant levels, free radical scavenging activity, anti-tyrosinase and anti-collagenase activity. BMC Complement Altern Med. 2017;17:487. doi: 10.1186/s12906-017-1994-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim S, Choi AH, Kwon M, Joung EJ, Shin T, Lee SG, et al. Evaluation of antioxidant activities of various solvent extract from Sargassum serratifolium and its major antioxidant components. Food Chem. 2019;278:178–84. doi: 10.1016/j.foodchem.2018.11.058. [DOI] [PubMed] [Google Scholar]
- 11.Kim GN, Shin MR, Shin SH, Lee AR, Lee JY, Seo BI, et al. Study of antiobesity effect through inhibition of pancreatic lipase activity of Diospyros kaki fruit and Citrus unshiu Peel. Biomed Res Int 2016. 2016:1–7. doi: 10.1155/2016/1723042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Petrillo AD, González-Paramás AM, Era B, Medda R, Pintus F, Santos-Buelga C, et al. Tyrosinase inhibition and antioxidant properties of Asphodelus microcarpus extracts. BMC Complement Altern Med. 2016;16:453. doi: 10.1186/s12906-016-1442-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liyanaarachchi DG, Samarasekera RR, Mahanama RR, Hemalal PD. Tyrosinase, elastase, hyaluronidase, inhibitory and antioxidant activity of Sri Lankan medicinal plants for novel cosmeceuticals. Ind Crops Prod. 2018;111:597–605. [Google Scholar]
- 14.Chiocchio I, Mandrone M, Sanna C, Maxia A, Tacchini M, Poli F. Screening of a hundred plant extracts as tyrosinase and elastase inhibitors, two enzymatic targets of cosmetic interest. Ind Crops Prod. 2018;122:498–505. [Google Scholar]
- 15.Gomesa F, Martins N, Barros L, Rodriguesa ME, Oliveirac PP, Henriques M, et al. Plant phenolic extracts as an effective strategy to control Staphylococcus aureus, the dairy industry pathogen. Ind Crops Prod. 2018;112:515–20. [Google Scholar]
- 16.Sabooraa A, Sajjadia ST, Mohammadib P, Fallahib Z. Antibacterial activity of different composition of aglycone and glycosidic saponins from tuber of Cyclamen coum Miller. Ind Crops Prod. 2019;140:111662. Available from: https://doi.org/10.1016/j.indcrop.2019.111662 . [Google Scholar]
- 17.Athipornchai A, Jullapo N. Tyrosinase inhibitory and antioxidant activities of Orchid (Dendrobium spp.) S Afr J Bot. 2018;119:188–92. [Google Scholar]
- 18.Tagouelbe T, Jean Y, Yao B, Augustin A. Drying temperature effect on total phenols and flavonoids content, and antioxidant activity of Borassus aethiopum mart ripe fruits’ pulp. J Food Res. 2017;6:50–64. [Google Scholar]
- 19.Singh S, Bajpai M, Mishra P. Carissa carandas L.– Phyto-pharmacological review. J Pharm Pharmacol. 2020;72:1694–714. doi: 10.1111/jphp.13328. [DOI] [PubMed] [Google Scholar]
- 20.Pehlivan FE. Vitamin C: An antioxidant agent. IntechOpen. 2017. Available from: https://dx.doi.org/10.5772/intechopen.69660 .
- 21.Khunchalee J. The study of free radical scavenging, total phenolic contents and tyrosinase inhibition activity of crude extract from Carissa carandas Linn. SNRU J Sci Tech. 2018;11:26–34. [Google Scholar]
- 22.Dhar G, Akther S, Sultana A, May U, Islam MM, Dhali M, et al. Effect of extraction solvents on phenolic contents and antioxidant capacities of Artocarpus chaplasha and Carissa carandas fruits from Bangladesh. J Appl Biol Biotechnol. 2017;5:039–44. [Google Scholar]
- 23.Sajjabut S, Pewlong W, Eamsiri J, Chookaew S. Antioxidant potential of Carissa carandas seed comparing to the dietary supplement products. FAB J. 2018;6:95–104. [Google Scholar]
- 24.Şöhretoğlu D, Sari S, Barut B, Özel A. Tyrosinase inhibition by some flavonoids: Inhibitory activity, mechanism by in vitro and in silico studies. Bioorg Chem. 2018;81:168–74. doi: 10.1016/j.bioorg.2018.08.020. [DOI] [PubMed] [Google Scholar]
- 25.Zolghadri S, Bahrami A, Hassan Khan MT, Munoz-Munoz J, Garcia-Molina F, Garcia-Canovas F, et al. A comprehensive review on tyrosinase inhibitors. J Enzyme Inhib Med Chem. 2019;34:279–309. doi: 10.1080/14756366.2018.1545767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karunarathne WA, Molagoda IM, Park SR, Kim JW, Lee OK, Kwon HY, et al. Anthocyanins from Hibiscus syriacus L. Inhibit melanogenesis by activating the ERK signaling pathway. Biomolecules. 2019;9:645. doi: 10.3390/biom9110645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pientaweeratch S, Panapisal V, Tansirikongkol A. Antioxidant, anti-collagenase and anti-elastase activities of Phyllanthus emblica, Manilkara zapota and silymarin: An in vitro comparative study for anti-aging applications. Pharm Biol. 2016;54:1865–72. doi: 10.3109/13880209.2015.1133658. [DOI] [PubMed] [Google Scholar]
- 28.Widowati W, Fauziah N, Herdiman H, Afni M, Afifah E, Kusuma HS, et al. Antioxidant and anti aging assays of Oryza sativa extracts, vanillin and coumaric acid. J Nat Remedies. 2016;16:88–99. [Google Scholar]
- 29.Bahadır AÖ, Ilhan M, Kurtul E, Šmejkal K, Küpeli AE. Inhibitory activity of Podospermum canum and its active components on collagenase, elastase and hyaluronidase enzymes. Bioorg Chem. 2019;93:103330. doi: 10.1016/j.bioorg.2019.103330. Available from: https://doi.org/10.1016/j.bioorg.2019.103330 . [DOI] [PubMed] [Google Scholar]
- 30.Afizah IN, Ahmad W, Jamshed F, Al-Jasabi S. Quantitative biochemical analysis of antioxidant properties of Carissa carandas fruit ethanolic and N-hexane extracts. Middle East J Sci Res. 2016;24:2418–23. [Google Scholar]
- 31.Vora J, Srivastava A, Modi H. Antibacterial and antioxidant strategies for acne treatment through plant extracts. Inform Med Unlocked. 2018;13:128–35. [Google Scholar]
- 32.Kumar B, Pathak R, Mary PB, Jha D, Sardana K, Gautam HK. New insights into acne pathogenesis: Exploring the role of acne-associated microbial populations. Dermatologica Sin. 2016;34:67–73. [Google Scholar]
- 33.Patel SD, Shah S, Shah N. A review on herbal drugs acting against acne vulgaris. J Pharm Sci Biosci Res. 2016;5:165–71. [Google Scholar]
- 34.Kumar V, Tarpada P, Sadariya K, Goswami S. Comparative phytochemical and antioxidant activities of methanol and petroleum ether extract of Carissa carandas leaves, fruit and seed. Vivechan Int J Res. 2017;8:70–5. [Google Scholar]
- 35.Kim S, Oh S, Noh HB, Ji S, Lee SH, Koo JM, et al. In vitro antioxidant and anti-Propionibacterium acnes activities of cold water, hot water, and methanol extracts, and their respective ethyl acetate fractions, from Sanguisorba officinalis L.roots. Molecules. 2018;23:3001. doi: 10.3390/molecules23113001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pummarin T, Noysang C. Evaluation of phytochemicals and pharmacological activities of Ben-Cha-Lo-Ka-Wi-Chian remedy. Appl Mech Mater. 2019;891:52–9. [Google Scholar]


