Abstract
Background
Both opioid use and COVID-19 affect respiratory and pulmonary health, potentially putting individuals with opioid use disorders (OUD) at risk for complications from COVID-19. We examine the relationship between OUD and subsequent hospitalization, length of stay, risk for invasive ventilator dependence (IVD), and COVID-19 mortality.
Methods
Multivariable logistic and exponential regression models using electronic health records data from the Cerner COVID-19 De-Identified Data Cohort from January through June 2020.
Findings
Out of 52,312 patients with COVID-19, 1.9% (n=1,013) had an OUD. COVID-19 patients with an OUD had higher odds of hospitalization (aOR=3.44, 95% CI=2.81–4.21), maximum length of stay (=1.16, 95% CI=1.09–1.22), and odds of IVD (aOR=1.26, 95% CI=1.06–1.49) than patients without an OUD, but did not differ with respect to COVID-19 mortality. However, OUD patients under age 45 exhibited greater COVID-19 mortality (aOR=3.23, 95% CI=1.59–6.56) compared to patients under age 45 without an OUD. OUD patients using opioid agonist treatment (OAT) exhibited higher odds of hospitalization (aOR=5.14, 95% CI=2.75–10.60) and higher maximum length of stay (=1.22, 95% CI=1.01–1.48) than patients without OUDs; however, risk for IVD and COVID-19 mortality did not differ. OUD patients using naltrexone had higher odds of hospitalization (aOR=32.19, 95% CI=4.29–4,119.83), higher maximum length of stay (=1.59, 95% CI=1.06–2.38), and higher odds of IVD (aOR=3.15, 95% CI=1.04–9.51) than patients without OUDs, but mortality did not differ. OUD patients who did not use treatment medication had higher odds of hospitalization (aOR=4.05, 95% CI=3.32–4.98), higher maximum length of stay (=1.14, 95% CI=1.08–1.21), and higher odds of IVD (aOR=1.25, 95% CI=1.04–1.50) and COVID-19 mortality (aOR=1.31, 95% CI=1.07–1.61) than patients without OUDs.
Interpretation
This study suggests people with OUD and COVID-19 often require higher levels of care, and OUD patients who are younger or not using medication treatment for OUDs are particularly vulnerable to death due to COVID-19.
Research in context:
Evidence before this study
PubMed, JAMA, Taylor & Francis online and ScienceDirect were searched using the following key terms: COVID-19 and opioid overdoses, pulmonary and opioids, comorbidity and opiates, opioid use disorder (OUD) and types of treatment, state regulations and COVID-19 and opioid treatment programs (OTP), federal regulations and OTP and COVID-19. Studies, as well as reports and data from State health agencies, published after January 1, 2000 were included if they explored COVID-19 and at least one of the following variables: opioids, opioid treatment program (OTP), opioid agonist treatment (OAT), and/or OUD. Most studies identified were small and descriptive except one study (n = 12,030) that did not, however, examine patient use of OAT, or outcomes including hospital length of stay or the risk for invasive ventilator dependence.
Added value of this study
To our knowledge, this is the first study characterizing and quantifying the association between OUD and the risk of death and three measures of health services (maximum length of stay, hospitalization, and invasive ventilator dependence) among COVID-19 patients while adjusting for OAT, sociodemographic variables, comorbidities, and medications for COVID-19 treatment. This study suggests people with OUD and COVID-19 often require higher levels of care, and OUD patients who are younger or not using medication treatment for OUDs are particularly vulnerable to death due to COVID-19.
Implications of all the available evidence
This study underscores the precariousness of individuals at the intersection of these health crises; people with OUDs are not only at risk for overdoses, which have soared during the pandemic, this population is also at risk for worse COVID-19 outcomes, especially when not receiving OAT. COVID-19 efforts tailored to populations who use opioids that focus on expanded treatment access may have a significant effect on reducing the inequitable outcomes found in this research.
Alt-text: Unlabelled box
1. Introduction
Studies, from the U.S. and other countries, indicate substance use disorders (SUDs) increase risk for worse COVID-19 outcomes. [1,2] Patients with an SUD diagnosis may not only be at higher risk for poorer outcomes, but also of infection in the first place, more severe illness, and greater rates of death than other patients[3]. The National Institute on Drug Abuse (NIDA) warns that patients with SUDs often have co-occurring conditions that enhance vulnerability to SARS-CoV-2 infections and worse outcomes after infection, including vascular and cardiac, pulmonary, and metabolic diseases [4]. A national U.S. study that included 12,030 COVID-19 patients showed 43.8% of SUD patients with COVID-19 were hospitalized and their mortality rate was 9.6%, compared to 30.1% of patients without an SUD diagnosis who were hospitalized for COVID-19; they also had a lower mortality rate (6.6%) [3]. In this study, patients with opioid use disorders (OUDs) were particularly vulnerable to COVID-19 infection, but risk for hospitalization and mortality were unclear for this subset of SUD patients due to the small sample size. However, patients in this study that had been diagnosed with an SUD also had significantly higher prevalence of asthma, chronic kidney or liver disease, chronic obstructive pulmonary disease (COPD), diabetes, cancer, human immunodeficiency virus (HIV), cardiovascular diseases, and obesity compared to study patients without an SUD [3]. Research on older adults showed tobacco smokers and those with heart disease [5,6], chronic renal disease [7], and COPD [8] faced greater risk for morbidity and mortality when contracting COVID-19, which may explain links between SUD/OUD diagnoses and SARS-CoV-2 infection, hospitalization, and/or mortality.
The physiological effects of opioids and societal factors limiting access to effective treatment and harm reduction resources may make the experience and outcomes of COVID-19 particularly heinous. [9,10] Before the COVID-19 pandemic, 2018 data indicated opioid overdoses led to the death of 47,000 people in the U.S. (CDC 2020) [11]. Preliminary research suggests social isolation due to COVID-19 may fuel a new rise in fatal and non-fatal opioid overdoses [12,13]. In addition to increases in opioid-related morbidity and mortality, researchers also suggest people who use opioids may be at heightened risk for COVID-19 and adverse outcomes related to viral infection due to biological effects of these drugs [14]. Opioids can directly affect the immune system [15] and can have detrimental effects on respiratory [[16], [17], [18]] and pulmonary health [16,18,19]. Opioids slow breathing and with higher doses can cause hypoxemia [20,21], potentially increasing risk for higher illness severity. The respiratory and pulmonary effects of past or present opioid use among people with OUDs may also intersect with weakened lung capacity from COVID-19 to deepen morbidity and mortality risks, and ultimately lead to greater health service use among this vulnerable population. However, whether these outcomes disproportionately occur among OUD patients with COVID-19 is unclear. Analyses have also yet to consider outcome variation among OUD patients by treatment status, such as whether patients who are treated with opioid agonist medications like buprenorphine or methadone differ from those treated with the opioid antagonist naltrexone and those not receiving medication assisted treatment.
This study builds on recent research exploring relationships between SUD and COVID-19 outcomes by considering use of opioid agonist treatment (OAT) and naltrexone, and examines outcomes such as length of stay, risk for requiring respiratory support, and mortality among individuals with OUD admitted to a hospital for COVID-19. This analysis will contribute to a better understanding of health service utilization and mortality due to COVID-19 among this particularly vulnerable population and provide indirect measures of COVID-19 severity among individuals with OUD.
2. Methods
2.1. Settings
De-identified electronic health records (EHR) data for this study were obtained from the Cerner COVID-19 De-Identified Data Cohort, which is a subset of the U.S. Cerner Corporation's Real-World Database™. “Cerner Real-World Data is extracted from the EMR of hospitals in which Cerner has a data use agreement. Encounters may include pharmacy, clinical and microbiology laboratory, admission, and billing information from affiliated patient care locations. All admissions, medication orders and dispensing, laboratory orders and specimens are date and time stamped, providing a temporal relationship between treatment patterns and clinical information. Cerner Corporation has established Health Insurance Portability and Accountability Act-compliant operating policies to establish deidentification for Cerner Real-World Data” [22]. Data were cleaned (in part by a multipoint-match algorithm to capture and remove duplicate records) and standardized across the vast amount of disparate EHR coding systems. Data are updated multiple times monthly and stored in a cloud-based health management platform called the HealtheDataLab™ where aggregate analysis results can be generated [23].
As of June 2020, there were 62 health systems across the U.S. that provided information found in the COVID-19 cohort of Cerner Real-World Data. The data used in this study represent EHR data from January 2020 through June 2020. Patients included in the sample were identified as having an encounter associated with a diagnosis or recent positive lab result (at the encounter or up to two weeks prior) for COVID-19. Additional available medical information was retrieved retrospectively going back to January 1, 2015 in order to gather information on relevant covariates.
The University of Utah Institutional Review Board (IRB #136,696) has determined that this study does not meet the definitions of Human Subjects Research for using secondary data with no intervention or interaction with an individual, and for not having identifiable private information in the data.
2.2. Measurements
The primary outcomes in this study were death due to COVID-19 and three measures of disease severity ascertained by health services among COVID-19 patients: hospitalization, maximum length of hospital stay (maximum LOS), and invasive ventilator dependence (IVD). Each of these outcomes was identified in the EHR data to reflect a unique indication per patient associated with a COVID-19 encounter (i.e., involved a COVID-19 diagnosis or lab indication). Indication of patient death due to COVID-19 was already provided in the Cerner COVID-19 De-Identified Data Cohort, and was constructed as a binary variable indicating whether a patient died from COVID-19 at discharge or any time after leading up to data collection. Maximum LOS was a continuous variable calculated by taking the difference in days between each patients’ hospital encounter start date and end date, and then taking the maximum difference per patient. Maximum LOS was only analyzed for those patients who were hospitalized. Hospitalization was constructed as a binary indication of whether a patient ever had a COVID-19 related length of stay that lasted one day or more. Invasive ventilator dependence was constructed as a binary indication of whether a patient ever had a diagnosis, procedure, or encounter result indication that signified reliance on an invasive ventilator. Indications of less severe ventilator dependence such as CPAP and BIPAP machines were not included as invasive ventilator dependence. The full list of code types and corresponding Current Procedural Terminology (CPT), International Classification of Diseases (ICD), Logical Observation Identifiers Names and Codes (LOINC®), and Systematized Nomenclature of Medicine - Clinical Terms (SNOMED CT) codes is found in Supplemental Table 1. Encounters that had possible ventilator indications, but lacked a positive indication of COVID-19, were not used to indicate IVD.
The main independent variable of interest was an indication of an OUD in the EHR, as measured by past opioid overdose or OUD recorded in ICD-9 or ICD-10 codes. The full list of code types and corresponding codes to identify patients with OUD is found in Supplemental Table 2. Patients were flagged as having an OUD in this binary predictor variable if they had these diagnoses up to one year prior to their first COVID-19 diagnosis, or any time after the COVID-19 diagnosis. Opioid agonist treatment (OAT) and naltrexone use for OUD were identified by using ICD-10 procedural classification system (PCS) codes, healthcare common procedure coding system (HCPCS) codes, national drug codes, Multum drug codes, and review of chart text entries. Specifically, along with codes, OAT was identified by any text entry identifying administration of buprenorphine film or tablets (inclusive of buprenorphine-naloxone) and methadone oral concentrate. Similarly, Naltrexone use was also identified by any text entry of naltrexone administration. The codes and code types used to identify patients treated with OAT and naltrexone are listed in Supplemental Table 3 and Supplemental Table 4.
Demographic predictors included age (in 10-year increments), sex, race and ethnicity (categories: (i) Non-Hispanic Black or African American, (ii) Non-Hispanic White, (iii) Hispanic or Latino with any race, and (iv) Non-Hispanic Other which includes Non-Hispanic American Indian or Alaskan Native, Non-Hispanic Asian or Pacific Islander, Non-Hispanic: other, unknown, or mixed race), type of insurance held (categories: (i) Private, (ii) Medicaid, (iii) Medicare, and (iv) Other which includes Other Government/Misc, Self-Pay, and Missing), and U.S. geographic region (defined by one-digit zip code) including (i) Northeast: 0 (Connecticut, Massachusetts, Maine, New Hampshire, New Jersey, Rhode Island, Vermont), 1 (Delaware, New York, Pennsylvania); (ii) Southeast: 2 (DC, Maryland, North Carolina, South Carolina, Virginia, West Virginia), 3 (Alabama, Florida, Georgia, Mississippi, Tennessee); (iii) Midwest: 4 (Indiana, Kentucky, Michigan, Ohio), 5 (Iowa, Minnesota, Montana, North Dakota, South Dakota, Wisconsin), 6 (Illinois, Kansas, Missouri, Nebraska), 7 (Arkansas, Louisiana, Oklahoma, Texas); (iv) West: 8 (Arizona, Colorado, Idaho, New Mexico, Nevada, Utah, Wyoming), 9 (Alaska, California, Hawaii, Oregon, Washington), and (v) Missing. Additional clinical predictors included history of several chronic diseases identified by ICD-9 and ICD-10 codes: type 2 diabetes, hypertension, cardiovascular disease (CVD), coronary heart disease (CHD), asthma, chronic kidney disease (CKD), acute respiratory distress syndrome (ARDS), and chronic obstructive pulmonary disease (COPD). The final clinical predictors consisted of medications for management of COVID-19: hydroxychlorquine, remdesivir, Decadron or prednisone, aspirin and Plavix, and anticoagulants. These medications were identified by Multum drug codes.
2.3. Statistical analysis
Demographic and clinical characteristics for the COVID-19 positive patient sample were presented based on data through June 2020 in aggregate and stratified by OUD status. Categorical variables were presented with frequencies and percentages, while continuous variables were presented with medians and interquartile ranges (IQRs: Q1-Q3) due to lack of normality in variable distributions. Stratified data were compared using Chi-Square tests for categorical variables with sufficient sample size, Fisher's Exact tests for those with small sample size, and non-parametric continuous variables were compared using Wilcoxon Rank-Sum tests. Mortality and health service outcomes for COVID-19 patients were compared between those with an OUD and those without. Median maximum LOS was compared with a Wilcoxon Rank-Sum test and percentages of hospitalization, death, and invasive ventilator dependence were compared with Chi-Square tests. In addition to being presented for the overall sample, outcome comparisons by OUD status were also further stratified by demographic groups. Because median age was significantly older among COVID-19 patients in the sample with an OUD compared to those without an OUD (Table 1), the relationship between age and OUD was charted for each outcome.
Table 1.
Demographic and clinical characteristics of COVID-19 positive patients by OUD status.
| Characteristic | Total n (%1) | Opioid use disorder indication n (%1) | No opioid use disorder indication n (%1) | p-value10 |
|---|---|---|---|---|
| Total | 52,3122 | 1013 (1.93) | 51,299 (98.13) | |
| Demographic Characteristics | ||||
| Age (Years)4 | 53 (35–68) | 60 (48–70) | 53 (35–68) | <0.00111 |
| Sex | 0.90 | |||
| Female | 26,512 (50.7) | 511 (50.4) | 26,001 (50.7) | |
| Male | 25,800 (49.3) | 502 (49.6) | 25,298 (49.3) | |
| Race and Ethnicity | <0.001 | |||
| Non-Hispanic Black or African American | 10,617 (20.3) | 226 (22.3) | 10,391 (20.3) | |
| Non-Hispanic White | 15,027 (28.7) | 572 (56.5) | 14,455 (28.2) | |
| Non-Hispanic Other5 | 8257 (15.8) | 71 (7.0) | 8186 (16.0) | |
| Hispanic or Latino | 18,411 (35.2) | 144 (14.2) | 18,267 (35.6) | |
| Insurance Type | <0.001 | |||
| Private | 17,969 (34.3) | 150 (14.8) | 17,819 (34.7) | |
| Medicaid | 8587 (16.4) | 225 (22.2) | 8362 (16.3) | |
| Medicare | 11,768 (22.5) | 401 (39.6) | 11,367 (22.2) | |
| Other6 | 13,988 (26.7) | 237 (23.4) | 13,751 (26.8) | |
| Region7 | <0.001 | |||
| Northeast | 11,794 (22.5) | 216 (21.3) | 11,578 (22.6) | |
| Southeast | 17,935 (34.3) | 306 (30.2) | 17,629 (34.4) | |
| Midwest | 7699 (14.7) | 149 (14.7) | 7550 (14.7) | |
| West | 12,323 (23.6) | 237 (23.4) | 12,086 (23.6) | |
| Missing | 2561 (4.9) | 105 (10.4) | 2456 (4.8) | |
| Clinical Characteristics | ||||
| OUD Indication8 | 1013 (1.9) | – | – | |
| History of chronic disease9 | ||||
| Type 2 Diabetes (DM) | 14,119 (27.0) | 474 (46.8) | 13,645 (26.6) | <0.001 |
| Hypertension | 23,218 (44.4) | 739 (73.0) | 22,479 (43.8) | <0.001 |
| Cardiovascular Disease (CVD) | 27,682 (52.9) | 861 (85.0) | 26,821 (52.3) | <0.001 |
| Coronary Heart Disease (CHD) | 4360 (8.3) | 207 (20.4) | 4153 (8.1) | <0.001 |
| Asthma | 9165 (17.5) | 438 (43.2) | 8727 (17.0) | <0.001 |
| Chronic Kidney Disease (CKD) | 7014 (13.4) | 315 (31.1) | 6699 (13.1) | <0.001 |
| Acute Respiratory Distress Syndrome (ARDS) | 1880 (3.6) | 105 (10.4) | 1775 (3.5) | <0.001 |
| Chronic Obstructive Pulmonary Disease (COPD) | 6529 (12.5) | 392 (38.7) | 6137 (12.0) | <0.001 |
| Medications | ||||
| Hydroxychloroquine | 7468 (14.3) | 152 (15.0) | 7316 (14.3) | 0.53 |
| Remdesivir | 399 (0.8) | 5 (0.5) | 394 (0.8) | 0.4612 |
| Decadron or Prednisone | 4166 (8.0) | 154 (15.2) | 4012 (7.8) | <0.001 |
| Aspirin and Plavix | 894 (1.7) | 37 (3.7) | 857 (1.7) | <0.001 |
| Anticoagulant | 17,756 (33.9) | 533 (52.6) | 17,223 (33.6) | <0.001 |
n (column%) except when otherwise noted;.
Has a diagnosis for COVID-19 or a positive lab indication of COVID-19;.
Out of total: 52,312;.
median (Q1-Q3);.
Non-Hispanic American Indian or Alaskan Native, Non-Hispanic Asian or Pacific Islander, Non-Hispanic: other, unknown, or mixed race;.
Other Government/Misc, Self-Pay, Missing,.
Northeast: 0 (Connecticut, Massachusetts, Maine, New Hampshire, New Jersey, Rhode Island, Vermont), 1 (Delaware, New York, Pennsylvania); Southeast: 2 (DC, Maryland, North Carolina, South Carolina, Virginia, West Virginia), 3 (Alabama, Florida, Georgia, Mississippi, Tennessee); Midwest: 4 (Indiana, Kentucky, Michigan, Ohio), 5 (Iowa, Minnesota, Montana, North Dakota, South Dakota, Wisconsin), 6 (Illinois, Kansas, Missouri, Nebraska), 7 (Arkansas, Louisiana, Oklahoma, Texas), West: 8 (Arizona, Colorado, Idaho, New Mexico, Nevada, Utah, Wyoming), 9 (Alaska, California, Hawaii, Oregon, Washington);.
indication of opioid overdose or opioid use disorder diagnosis up to one year prior to first COVID-19 diagnosis and any time after;.
positive indications of disease history identified by ICD 9/10 codes;.
Chi-Square test (unless otherwise noted);.
Wilcoxon Rank-Sum test;.
Fisher's Exact test.
Adjusted models assessed the association between OUD and clinical and health service outcomes among those with COVID-19 while controlling for confounding factors. All hypothesis tests were two-sided with a significance level of 5% and R version 3.6.1 (R Foundation for Statistical Computing) was used to perform all analyses. Death due to COVID-19, hospitalization, and invasive ventilator dependence were assessed using multivariable logistic regression models. Adjusted odds ratios (aORs) and corresponding 95% confidence intervals (CIs) were estimated for each outcome. Models were adjusted for demographic and clinical characteristics that were significantly different between OUD and non-OUD groups, or were considered to be of clinical relevance. Variables were removed if they showed evidence of high multicollinearity. The area under the receiver operating characteristic (ROC) curve (AUC) was calculated to indicate the performance of each logistic model's ability to correctly classify outcome groups.
The fourth outcome, maximum LOS, was assessed using multivariable exponential regression models because this outcome followed a continuous exponential distribution. Adjusted exponentiated slope coefficients (relating to the percentage change in expected maximum LOS) with 95% CIs were estimated for this outcome. The coefficient of determination (R2) value was calculated to estimate the percent of variation in maximum LOS as explained by the model predictors.
To further explore associations between OUD and outcomes among COVID-19 patients by different demographic groups, the same four outcome models were again fit by stratifying models by each of the following demographics: categorical age (<45, 45–64, >=65), sex, race/ethnicity, insurance type, and region. For sufficient sample sizes, each model was adjusted for all other predictors previously used in the main adjusted models. For insufficient sample sizes, stratified models were adjusted only for necessary demographic variables to avoid overfitting. Adjusted odds ratios for those with a history of OUD (compared to those without) and 95% CIs were again presented for each demographic-stratified model.
A sensitivity analysis was also conducted to assess the role of treatment among OUD patients and whether it affected the outcomes assessed in this study. We defined treatment by indications of OAT or naltrexone use. This analysis divided COVID-19 patients into four levels: those with an OUD using OAT, those with an OUD using naltrexone (and not OAT), those with an OUD not using either treatment, and those with no OUD. The unadjusted and adjusted associations with the four outcomes of interest were calculated. Unadjusted maximum LOS was compared between the four groups with a Kruskall–Wallis test, and fit with an exponential regression model for the adjusted results. The other unadjusted outcomes (hospitalization, invasive ventilator dependence, and death) were compared between the four groups with a Chi-Square test, and fit in logistic regression models for adjusted results.
2.4. Funding
This study had no funding source. FQ and BT have full access to the data of this study and take complete responsibility for the integrity of the data and the accuracy of the data analysis.
3. Results
3.1. Sample characteristics
There was a total of 52,312 unique patients in the COVID-19 positive cohort. Table 1 provides descriptive statistics for the entire sample, as well as stratified by whether the patient had an OUD. The median patient age was 53 years and 50.7% of patients were females. More than a third (35.2%) of COVID-19 patients in the sample were Hispanic or Latino, followed by non-Hispanic White patients (28.7%) and non-Hispanic Black or African American patients (20.3%). More than a third of patients (34.3%) had private health insurance, while 22.5% had Medicare, 16.4% had Medicaid, and 26.7% had other insurance characteristics (other government/miscellaneous, self-pay, or missing). The region with the greatest representation of patients in the sample was the Southeast United States (34.3%).
Among individuals in the COVID-19 sample, 1.9% (n = 1013) met the criteria for OUD within one year of their first COVID-19 diagnosis. Additional medical history identified in the sample included 27.0% with a history of type 2 diabetes, 17.5% with asthma, 12.5% with COPD, 8.3% with CHD, and 3.6% with ARDS. Some form of an anticoagulant was used as treatment for COVID-19 among 33.9% of sample patients, and 14.3% were treated with hydroxychloroquine, 8.0% were treated with Decadron or prednisone, 1.7% were jointly treated with aspirin and Plavix, and 0.8% were treated with Remdesivir (Table 1).
Stratifying the sample characteristics by whether the patient had an OUD showed that COVID-19 patients with an OUD differed from those without an OUD in terms of demographics, history of all selected chronic diseases, and three medications (Table 1). These unadjusted comparisons showed that COVID-19 patients with an OUD were significantly older, had significantly lower percentages of private insurance, and were less numerous in the Southeastern U.S. region. Patients with an OUD also had significantly higher representation among individuals receiving Decadron/Prednisone, aspirin/Plavix, and anticoagulant treatments. There were no significant differences in hydroxychloroquine and Remdesivir treatment between the two groups (Table 1). OUD patients with COVID-19 also exhibited significant differences across all four outcomes (Table 2). More patients with an OUD were admitted for hospitalization, were hospitalized for longer periods (7.1 days vs. 6.0 days), had greater likelihood of invasive ventilator dependence (18.6% vs. 11.6%), and had greater mortality (12.8% vs. 8.9%, p < 0.001) compared to those without an OUD.
Table 2.
Mortality and health service outcomes between those with/without an OUD among COVID-19 positive patients (stratified by demographics1).
| Hospitalization |
Maximum LOS (Days)2 |
Ventilator dependence |
Death |
|||||
|---|---|---|---|---|---|---|---|---|
| OUD n (%3) | No OUD n (%) | OUD Median (Q1-Q3) | No OUD Median (Q1-Q3) | OUD n (%) | No OUDn (%) | OUD n (%) | No OUD n (%) | |
| Overall | 880 (86.9) | 26,833 (52.3) | 7.1 (3.4–13.7) | 6.0 (3.1–11.5) | 188 (18.6) | 5948 (11.6) | 130 (12.8) | 4555 (8.9) |
| Age | ||||||||
| <45 | 172 (78.5) | 5956 (31.0) | 5.7 (2.8–11.9) | 4.0 (2.3–7.5) | 33 (15.1) | 870 (4.5) | 9 (4.1) | 186 (1.0) |
| 45–64 | 365 (86.3) | 8901 (52.6) | 7.1 (3.6–14.3) | 6.1 (3.2–11.8) | 87 (20.6) | 2158 (12.8) | 31 (7.3) | 961 (5.7) |
| >=65 | 343 (92.5) | 11,976 (79.0) | 7.8 (4.1–14.0) | 7.1 (3.9–13.2) | 68 (18.3) | 2920 (19.3) | 90 (24.3) | 3408 (22.5) |
| Sex | ||||||||
| Female | 435 (85.1) | 12,872 (49.5) | 7.0 (3.6–13.4) | 5.8 (3.0–10.7) | 98 (19.2) | 2374 (9.1) | 61 (11.9) | 1901 (7.3) |
| Male | 445 (88.6) | 13,961 (55.2) | 7.3 (3.3–14.1) | 6.3 (3.3–12.2) | 90 (17.9) | 3574 (14.1) | 69 (13.7) | 2654 (10.5) |
| Race and Ethnicity | ||||||||
| Non-Hispanic Black or African American | 194 (85.8) | 5904 (56.8) | 7.2 (3.1–14.2) | 6.4 (3.4–12.0) | 45 (19.9) | 1330 (12.8) | 31 (13.7) | 1036 (10.0) |
| Non-Hispanic White | 508 (88.8) | 9292 (64.3) | 7.1 (3.5–13.6) | 6.0 (3.1–10.9) | 97 (17.0) | 1921 (13.3) | 73 (12.8) | 1922 (13.3) |
| Non-Hispanic Other | 59 (83.1) | 4326 (52.8) | 6.9 (3.8–10.2) | 6.8 (3.4–12.9) | 14 (19.7) | 1263 (15.4) | 9 (12.7) | 785 (9.6) |
| Hispanic or Latino | 119 (82.6) | 7311 (40.0) | 6.6 (3.8–13.1) | 5.6 (2.9–10.9) | 32 (22.2) | 1434 (7.9) | 17 (11.8) | 812 (4.4) |
| Insurance Type | ||||||||
| Private | 132 (88.0) | 6910 (38.8) | 7.8 (2.9–18.3) | 5.2 (2.9–10.2) | 45 (30.0) | 1489 (8.4) | 14 (9.3) | 662 (3.7) |
| Medicaid | 187 (83.1) | 4015 (48.0) | 5.6 (2.9–12.2) | 5.1 (2.8–10.0) | 35 (15.6) | 813 (9.7) | 12 (5.3) | 354 (4.2) |
| Medicare | 353 (88.0) | 9070 (79.8) | 7.4 (4.1–13.2) | 7.1 (4.0–13.0) | 70 (17.5) | 2136 (18.8) | 70 (17.5) | 2528 (22.2) |
| Other | 208 (87.8) | 6838 (49.7) | 7.5 (3.4–13.4) | 5.9 (3.0–11.4) | 38 (16.0) | 1510 (11.0) | 34 (14.3) | 1011 (7.4) |
| Region | ||||||||
| Northeast | 189 (87.5) | 6901 (59.6) | 6.7 (3.1–13.4) | 6.6 (3.5–12.0) | 44 (20.4) | 1678 (14.5) | 31 (14.4) | 1555 (13.4) |
| Southeast | 258 (84.3) | 7365 (41.8) | 7.6 (3.9–13.7) | 5.8 (3.0–11.2) | 52 (17.0) | 1520 (8.6) | 36 (11.8) | 1100 (6.2) |
| Midwest | 126 (84.6) | 4257 (56.4) | 7.0 (3.7–12.4) | 5.8 (3.1–10.9) | 33 (22.1) | 1031 (13.7) | 22 (14.8) | 720 (9.5) |
| West | 206 (86.9) | 6189 (51.2) | 6.9 (3.6–15.7) | 6.1 (3.2–11.4) | 38 (16.0) | 1406 (11.6) | 29 (12.2) | 982 (8.1) |
| Missing | 101 (96.2) | 2121 (86.4) | 8.0 (3.1–15.8) | 5.4 (11.5) | 21 (20.0) | 313 (12.7) | 12 (11.4) | 198 (8.1) |
Maximum LOS compared with Wilcoxon Rank-Sum test; all outcome percentages compared with Chi-Square test; bolded results were significantly different.
“Overall” is using OUD/no OUD results out of total n (52,312) but each ensuing demographic result is using OUD/no OUD results restricted to only that group n;.
Restricted to only those patients that were hospitalized (n = 27,713).
n (column%);.
Significant differences in outcomes between those with and without OUDs were also observed when further stratifying by age, sex, race/ethnicity, insurance, and region (Table 2). For example, among COVID-19 patients who were less than 45 years of age, 78.5% (n = 172) of those with an OUD were hospitalized while only 31.0% (n = 5956) of those without an OUD were hospitalized (P<0.001). Males with an OUD exhibited higher rates of hospitalization and death due to COVID-19 and higher maximum LOS than females. Non-Hispanic (NH) Black and NH White patients with an OUD exhibited greater rates of hospitalization and death due to COVID-19 and higher maximum LOS, while Hispanic/Latino patients with an OUD exhibited the highest rates of invasive ventilator dependence. Differences between OUD status were seen in other outcomes as well, but were not statistically significant.
3.2. Model results
Results from the main analysis of the adjusted models (Table 3) suggest COVID-19 patients with an OUD significantly differ from individuals without an OUD for three of the primary outcomes assessed in this study. The models show COVID-19 patients with an OUD had significantly greater maximum LOS (=1.16, 95% CI=1.09, 1.22), greater odds of hospitalization (aOR=3.44, 95% CI=2.81, 4.21), and greater odds of invasive ventilator dependence (aOR=1.26, 95% CI=1.06, 1.49) than individuals without OUD after adjusting for confounding factors. The R-squared for the maximum LOS regression analysis indicated that the model explained 12% of the variance in maximum LOS (R2=0.12). The AUCs for the logistic regression analyses were 0.85 for hospitalization and 0.78 for invasive ventilator dependence, indicating adequate model fit.
Table 3.
Adjusted associations of OUD and OUD treatment with hospitalization, maximum LOS, invasive ventilator dependence, and death among COVID-19 positive patients.
| Variables | Hospitalization | Maximum LOS | Invasive Ventilator Dependence | Death |
|---|---|---|---|---|
| aOR1 (95% CI) | 2 (95% CI) | aOR1 (95% CI) | aOR1 (95% CI) | |
| Main Analysis3: OUD status | ||||
| No | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Yes | 3.44 (2.81, 4.21) | 1.16 (1.09, 1.22) | 1.26 (1.06, 1.49) | 1.15 (0.94, 1.41) |
| AUC | 0.85 | – | 0.78 | 0.85 |
| R2 | – | 0.12 | – | – |
| Sensitivity Analysis3: OUD Treatment status | ||||
| No OUD | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| OUD without treatment | 4.05 (3.32, 4.98) | 1.14 (1.08, 1.21) | 1.25 (1.04, 1.50) | 1.31 (1.07, 1.61) |
| OUD with naltrexone | 32.19 (4.29, 4119.83) | 1.59 (1.06, 2.38) | 3.15 (1.04, 9.51) | 1.87 (0.39, 8.91) |
| OUD with OAT | 5.14 (2.75, 10.60) | 1.22 (1.01, 1.48) | 1.04 (0.54, 2.00) | 0.52 (0.19, 1.46) |
| OUD without treatment | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| OUD with naltrexone | 7.95 (1.05, 1019.45) | 1.39 (0.93, 2.09) | 2.52 (0.82, 7.71) | 1.43 (0.30, 6.90) |
| OUD with OAT | 1.27 (0.66, 2.68) | 1.07 (0.88, 1.31) | 0.83 (0.42, 1.64) | 0.40 (0.14, 1.13) |
| OUD with naltrexone | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| OUD with OAT | 0.16 (0.01, 1.39) | 0.77 (0.49, 1.20) | 0.33 (0.09, 1.19) | 0.28 (0.04, 1.81) |
| AUC | 0.85 | – | 0.78 | 0.83 |
| R2 | – | 0.12 | – | – |
Adjusted odds ratio from logistic regression model;.
Adjusted exponentiated coefficient (exponential regression model) relating to change in the ratio of expected maximum LOS (i.e., “OUD with OAT” coefficient is the ratio of the expected maximum LOS for those with an OUD on OAT over expected maximum LOS for those without an OUD, so maximum LOS is 22% greater for patients with an OUD on OAT compared to patients without an OUD);.
Models of sufficient sample sizes adjusted for age, gender, race/ethnicity, insurance, region, Diabetes mellitus (DM), asthma, hypertension, hydroxychloroquine, Remdesivir, Decadron or Prednisone, aspirin and Plavix; models of insufficient sample sizes had medications and disease histories removed to avoid overfitting.
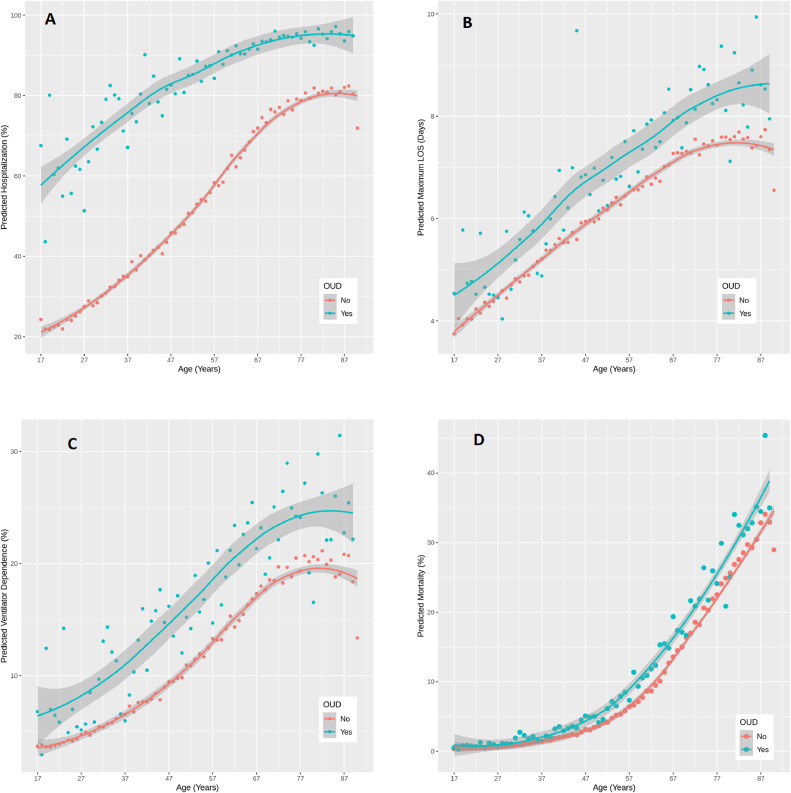
Overall, there was no significant association between having an OUD and odds of death due to COVID-19 (aOR=1.15, 95% CI=0.94, 1.41). However, stratifying by age indicated patients younger than 45 with a history of OUD exhibited significantly higher odds of death (aOR=3.23, 95% CI=1.59, 6.56) than patients without an OUD (Table 4). Figs. 1.a-1.d depict the relationship between age and OUD against model predicted hospitalization (Fig. 1.a), maximum LOS (Fig. 1.b), invasive ventilator dependence (Fig. 1.c), and death (Fig. 1.d). As age increases, the predicted outcomes increase in each part of the figure, and patients with an OUD have higher death and health service use predictions on average compared to those without an OUD across the age distribution.
Table 4.
Adjusted odds of hospitalization, maximum LOS, invasive ventilator dependence, and death for those with OUD histories compared to those without among COVID-19 positive patients (stratified by demographics).
| Variables1 | Hospitalization | Maximum LOS | Invasive Ventilator Dependence | Death |
|---|---|---|---|---|
| aOR (95% CI) | (95% CI) | aOR (95% CI) | aOR (95% CI) | |
| Age | ||||
| <45 | 5.10 (3.58, 7.27)1 | 1.31 (1.16, 1.49) | 2.70 (1.83, 3.99) | 3.23 (1.59, 6.56) |
| 45–64 | 3.43 (2.53, 4.65) | 1.13 (1.04, 1.24) | 1.50 (1.17, 1.92) | 0.90 (0.61, 1.32) |
| >=65 | 2.44 (1.63, 3.66) | 1.06 (0.97, 1.16) | 0.95 (0.72, 1.24) | 1.24 (0.97, 1.59) |
| Sex | ||||
| Female | 2.86 (2.17, 3.77) | 1.15 (1.06, 1.24) | 1.49 (1.18, 1.88) | 1.33 (0.99, 1.78) |
| Male | 4.23 (3.12, 5.74) | 1.16 (1.07, 1.26) | 1.03 (0.81, 1.31) | 1.19 (0.90, 1.56) |
| Race and Ethnicity | ||||
| Non-Hispanic White | 3.27 (2.46, 4.36) | 1.18 (1.09, 1.27) | 1.02 (0.81, 1.28) | 1.10 (0.85, 1.44) |
| Non-Hispanic Black or African American | 3.56 (2.34, 5.42) | 1.09 (0.97, 1.23) | 1.54 (1.09, 2.16) | 1.35 (0.90, 2.03) |
| Non-Hispanic Other | 3.69 (1.90, 7.14) | 1.03 (0.82, 1.30) | 1.19 (0.65, 2.19) | 1.07 (0.50, 2.27) |
| Hispanic or Latino | 4.58 (2.80, 7.48) | 1.20 (1.03, 1.40) | 2.41 (1.58, 3.67) | 1.83 (1.05, 3.18) |
| Insurance | ||||
| Private | 8.01 (4.63, 13.86) | 1.36 (1.18, 1.58) | 3.81 (2.60, 5.57) | 2.04 (1.13, 3.70) |
| Medicaid | 3.79 (2.60, 5.54) | 1.10 (0.97, 1.25) | 1.31 (0.89, 1.91) | 0.93 (0.50, 1.71) |
| Medicare | 2.05 (1.48, 2.84) | 1.05 (0.96, 1.15) | 0.96 (0.74, 1.26) | 0.98 (0.75, 1.28) |
| Other | 3.75 (2.41, 5.84) | 1.16 (1.03, 1.32) | 1.27 (0.88, 1.82) | 1.68 (1.13, 2.50) |
| Region | ||||
| Southeast | 3.26 (2.30, 4.62) | 1.16 (1.04, 1.30) | 1.39 (1.02 (1.90) | 1.27 (0.88, 1.86) |
| Northeast | 3.99 (2.54, 6.26) | 1.08 (0.96, 1.22) | 1.54 (1.09, 2.17) | 1.13 (0.75, 1.70) |
| Midwest | 2.35 (1.45, 3.80) | 1.14 (0.99, 1.32) | 1.68 (1.12, 2.51) | 1.21 (0.74, 1.99) |
| West | 4.16 (2.75, 6.31) | 1.16 (1.03, 1.30) | 1.40 (0.98, 2.01) | 1.26 (0.83, 1.91) |
| Missing | 2.69 (0.96, 7.55) | 1.21 (1.01, 1.46) | 1.67 (1.01, 2.80) | 1.75 (0.90, 3.39) |
Odds of column outcome for those with OUD history compared to those without OUD history among row group, adjusted for all other predictors in Table 3 for sufficient sample sizes, COVID-19 medications and disease history (upon need) removed for insufficient sample sizes; Bolded results were significantly different.
Fig. 1.
A-1.D. Predicted health service outcomes and mortality vs. age (by OUD status) among COVID-19 positive patients.
Across demographic groups, patients with both COVID-19 and an OUD generally exhibited greater health service use compared to those without an OUD, but greater COVID-19 mortality rates were only observed for some demographic sub-groups. Table 4 reports aORs for each outcome, stratified by demographics. The reference group for each aOR is the corresponding demographic category without an OUD. Younger COVID-19 patients with OUDs displayed the greatest differences in health service use and mortality when compared to their counterparts without an OUD. In particular, OUD patients under age 45 exhibited 223% greater odds of COVID-19 mortality (aOR=3.23, 95% CI=1.59–6.56) when compared to those without an OUD. Older patients only exhibited significant differences in some outcomes. OUD patients age 45–64 had greater odds of hospitalization and invasive ventilator dependence and higher maximum LOS, but patients 65 years of age and older with an OUD only exhibited greater rates of hospitalization (aOR=2.44, 95% CI=1.63, 3.66).
Stratification by insurance type, sex, race/ethnicity, and geographic region also demonstrated several significant associations with the health service outcomes measured in this study, but only some categories demonstrated differences in COVID-19 mortality (Table 4). COVID-19 patients with an OUD who had private insurance were 8.01 times more likely to be hospitalized compared to their counterparts without an OUD (aOR=8.01, 95% CI=4.63, 13.86). Male and female COVID-19 patients with an OUD displayed higher odds of hospitalization and higher maximum LOS, while female patients with an OUD also displayed higher odds of hospitalization invasive ventilator dependence compared to their counterparts without an OUD. Hispanic/Latino patients with COVID-19 and an OUD exhibited 83% higher odds of death due to COVID-19 compared to Hispanic/Latino COVID-19 patients without an OUD (aOR=1.83, 95% CI=1.05, 3.18), as well as greater odds of hospitalization and invasive ventilator dependence and higher maximum LOS. NH White COVID-19 patients with an OUD had greater odds of hospitalization and stayed longer in the hospital, and NH Black patients with an OUD also had higher odds of hospitalization, as well as invasive ventilator dependence compared to their counterparts without an OUD. Private insurance holders with an OUD also consistently displayed significantly worse outcomes across all measures, such as 104% higher odds of dying due to COVID-19 compared to their counterparts without an OUD (aOR=2.04, 95% CI=1.13, 3.70). Finally, region was significantly associated with the health service outcomes for COVID-19 patients with an OUD, but not strongly associated with COVID-19 mortality differences between patients with and without OUDs.
3.3. Treatment sensitivity analysis
A sensitivity analysis that further stratified OUD patients by whether their EHR indicated use of either OAT or naltrexone demonstrated that these treatments affect the extent to which patients with OUD differ from patients without an OUD. Unadjusted analyses (Table 5) show that OUD patients using OAT and naltrexone had the highest percentages of hospitalization among the patient groups, yet OUD patients treated with naltrexone and OUD patients not treated with medication stayed longer in the hospital than those treated with OAT. OUD patients treated with naltrexone and OUD patients not treated with medication also had higher percentages of invasive ventilator dependence and death due to COVID-19 than both OUD patients treated with OAT and patients without an OUD (see Table 5).
Table 5.
Unadjusted estimates of health service outcomes and mortality between those with OUD using OAT, those with OUD using naltrexone, those with OUD not using treatment, and those without OUD among COVID-19 positive patients.
| OUD using OAT n (%)1 | OUD using naltrexone n (%) | OUD not using treatment n (%) | No OUD n (%) | p-value3 | |
|---|---|---|---|---|---|
| Total | 84 | 17 | 912 | 51,299 | |
| Hospitalized | 74 (88.1) | 17 (100.0) | 789 (86.5) | 26,833 (52.3) | <0.001 |
| Maximum LOS (Days)2 | 5.9 (2.4–11.8) | 13.4 [3.1–27.2] | 6.0 (2.3–12.1) | 1.4 (0.1–6.3) | <0.0014 |
| Invasive Ventilator Dependence | 11 (13.1) | 6 (35.3) | 171 (18.8) | 5948 (11.6) | <0.001 |
| Death | 4 (4.8) | 2 (11.8) | 124 (13.6) | 4555 (8.9) | <0.001 |
n (column%) unless otherwise noted;.
median (Q1-Q3), maximum LOS is restricted to only hospitalized patients (n = 27,713).
Chi-Square test unless otherwise noted;.
Kruskall–Wallis test.
After adjusting for confounding factors, significant differences remained (Table 3). OUD patients using OAT exhibited higher odds of hospitalization (aOR=5.14, 95%=CI 2.75–10.60) and maximum LOS (=1.22, 95% CI=1.01–1.48) compared to patients without OUDs; however, risk for IVD and death did not differ. OUD patients using naltrexone also showed much higher odds of hospitalization (aOR=32.19, 95% CI=4.29–4119.83), higher maximum LOS (=1.59, 95% CI=1.06–2.38), and higher odds of IVD (aOR=3.15, 95% CI=1.04–9.51) compared to patients without OUDs, but no significant difference in death was found. OUD patients who did not use these treatment medications had higher odds of hospitalization (aOR=4.05, 95% CI=3.32–4.98), higher maximum LOS (=1.14, 95% CI=1.08–1.21), and higher odds of both IVD (aOR=1.25, 95% CI=1.04–1.50) and COVID-19 mortality (aOR=1.31, 95% CI=1.07–1.61) compared to patients without an OUD. The risk for the four outcomes did not differ between OUD patients with OAT vs. OUD patients with naltrexone or between OUD patients without treatment vs. those using OAT (p > 0.05).
4. Discussion
This study showed that COVID-19 patients with OUDs are more likely to require more intensive health services than other COVID-19 patients, but did not find that they face higher COVID-19 mortality risk. Medication treatment for OUDs may have some relationship to the magnitude of these differences. A sensitivity analysis indicated that patients using OAT or naltrexone demonstrated greater differences compared to patients without OUDs in terms of risk for hospitalization and maximum length of stay, while OUD patients who did not use medication exhibited greater risk for death and invasive ventilator dependence compared to patients without an OUD.
The trends in the main analysis demonstrating greater health service use held within many demographic groups as well; however, significant increases in mortality were isolated to particular sub-groups of OUD patients with COVID-19. Increased COVID-19 mortality risk was identified among COVID-19 patients who are younger, Hispanic/Latino, or privately insured. While previous research indicates that patients with OUD are at heightened risk for SARS-CoV-2 infection and that COVID-19 patients with a variety of SUDs may experience greater risk of COVID-19 hospitalization and death [3], this study builds on these findings by utilizing a database with a large sample of OUD patients to highlight how OUD patients in particular may require more health services when contracting the virus and estimates their vulnerability to death due to COVID-19. The greater representation of males, higher median age, and higher prevalence of chronic conditions among OUD patients that are associated with worse COVID-19 outcomes, such as CVD, CKD, and respiratory disorders, may contribute to the poorer outcomes and complications demonstrated in the unadjusted analyses. These traits are shown in other reports to increase COVID-19 risks [24]. But this study also presents adjusted analyses for each outcome that control for age, sex, and histories of relevant chronic conditions, and in these models OUD patients with COVID-19 still exhibit greater mortality due to COVID-19 and health service utilization.
The findings of this study related to OAT are somewhat surprising. The pharmacological effects of opiate-induced respiratory depression with hypoxia are proposed as factors contributing to worse outcomes among SUD patients. Opioid-related respiratory depression may increase the risks of hypoxemia from coronavirus pneumonia and opioids may depress the immune system, making individuals with OUD more susceptible to opportunistic infections [25,14]. These effects imply patients using OAT could be at greater risk for poor outcomes, yet this study finds they are only at greater risk for hospitalization and higher maximum length of stay. Compared to patients without an OUD, COVID-19 patients with OUD who were treated with naltrexone demonstrated even higher risk for hospitalization, length of stay, and invasive ventilator dependence, but not death. Naltrexone has been proposed as a possible therapeutic candidate for COVID-19 due to its ability to act as a host-targeted broad-spectrum antiviral therapy [26]; however, the small sample of patients treated with naltrexone in this study do not show clear mortality advantages but do show more intensive health service use. COVID-19 patients with OUDs who do not have charts indicating medication treatment exhibit greater risk for all health service outcomes and greater mortality compared to their counterparts without an OUD. The sample of patients with OUD who do not use OAT likely mix two groups of people: those who have an untreated OUD and those who have an OUD and pursue forms of non-medication treatment. The data used in this study cannot parse out whether one of these sub-groups drives these results. However, it is possible patients with an untreated OUD are actively engaging in riskier forms of opioid use that increase their likelihood for poor outcomes.
This study also demonstrated worse COVID-19 health service outcomes and mortality among OUD patients in some age groups and racial/ethnic populations. Observing that younger COVID-19 patients with an OUD had significantly higher odds of dying from COVID-19 compared to their counterparts without an OUD aligns with research showing risk for opioid overdoses is significantly higher among younger adults. Individuals age 18–44 comprise 57.9% of non-fatal opioid overdoses [27] and 65.9% of fatal opioid overdoses occur among individuals age 15–44 [28], suggesting younger patients may be involved in riskier forms of opioid use that also result in worse COVID-19 outcomes.
Significant differences between COVID-19 patients with OUD and without OUD were observed consistently within Hispanic/Latino patient populations. Differences within other racial/ethnic groups varied depending on the outcome measured. While this study presents comparisons between patients with OUD and those without OUD within racial groups, this stratified analysis indicates significant differences between COVID-19 patients with OUD and without OUD observed in the main analysis are maintained in some groups, while not maintained in others. Limited access to OUD treatment for patients of color may fuel the poorer health service outcomes observed in these data. Access to treatment and recovery services remains inequitable for many communities of color [29]. Although U.S. Black and Hispanic populations have relatively lower rates of opioid misuse compared to non-Hispanic White populations, rates of misuse have grown since 1999 among Black populations and remained relatively flat among Hispanic populations [30]. However, 2014–2017 overdose deaths involving synthetic opioids increased by 617% among Hispanic populations [31]. Despite these trends, patients of color receive buprenorphine prescriptions at lower rates than non-Hispanic White patients [32] and pregnant Hispanic and non-Hispanic Black patients are shown in multiple studies to be less likely to receive methadone or buprenorphine compared to non-Hispanic White patients [33,34]. Even among patients admitted to a hospital for an opioid overdose, non-Hispanic Black and Hispanic patients are less likely to receive timely follow-up care in the form of treatment medication initiation or use of inpatient or outpatient treatment [35], Across all race and ethnicity groups, Hispanic COVID-19 patients in this study with co-occurring OUD had the highest odds of hospitalization, invasive ventilator dependence, and death due to COVID-19 compared to their counterparts without an OUD. Similarly, Black or African American COVID-19 patients with an OUD in this study also had significantly higher odds of hospitalization and invasive ventilator dependence than African American patients without an OUD. Given elevated rates of health service use and death among OUD patients in the study sample who were not treated with OAT or naltrexone, limited access to treatment medication may play a role in the racial and ethnic inequities identified in these analyses.
Adjusted analyses also demonstrated that sex, insurance type, and region also affect health service outcomes among COVID-19 patients with OUD. OUD patients with private insurance and other insurance characteristics (i.e., self-pay, missing data) also exhibited greater COVID-19 mortality than similar patients without an OUD. Insurance status is a determinant of utilization and access to care in the U.S., with uninsured people forgoing care at higher rates than people with public or private insurance [36]. Even with public insurance through Medicaid or Medicare, low-income patients may still struggle with access and exhibit lower utilization of some health services, although use of emergency departments are higher among Medicaid patients than among the privately insured [37]. Higher use of health services among privately insured COVID-19 patients with OUD in this study compared to their counterparts without OUD may indicate better access to care among this insurance group. Future research may further examine why such factors influence health service outcomes and mortality among patients with OUDs.
Data from patient EHR have key limitations due to ICD coding errors and because these data are primarily utilized as a tool for billing. EHR data frequently do not include all relevant patient diagnoses, and may only document primary patient complaints, leading to incomplete data and under-counting of key components of patient health history. The EHR database used for this analysis also combines race and ethnicity into a single category and it is unknown how patient race or ethnicity data are collected, making the quality of this variable unclear. Patients from rural areas are also less likely to be represented in EHR databases because adoption of EHR and health information technology in rural and remote areas lags behind urban health facilities. Despite these weaknesses, EHR data also provide large sample sizes that allow for analyses of rare patient populations that may be particularly difficult to identify and recruit through other means, such as patients with OUD and COVID-19.
The findings of this study provide mortality and health service estimates for OUD patients diagnosed with COVID-19. These analyses highlight how people with OUD are particularly vulnerable to death due to COVID-19 and often need higher levels of care than other patients when contracting COVID-19. This study also shows OUD patients treated with methadone or buprenorphine appear to have worse outcomes in terms of hospitalization and length of hospital stay, but better outcomes related to COVID-19 mortality and risk for requiring invasive ventilator dependence compared to OUD patients not receiving OAT. The concurrent overdose crisis and global pandemic require special attention to people who use drugs. This study underscores the precariousness of individuals at the intersection of these health crises; people with OUDs are not only at risk for overdoses, which have soared during the pandemic, this population is also at risk for worse COVID-19 outcomes, especially when not receiving OAT. COVID-19 efforts tailored to populations who use opioids that focus on expanded treatment access may have a significant effect on reducing the inequitable outcomes found in this research.
Funding
This study had no funding source.
Contributors
Conceptualization, F.Q.; project administration, F.Q., methodology, F.Q.; accessed and verified the underlying data, F.Q., B.T., formal analysis, B.T.; software, B.T., validation, F.Q., B.T., A.I.S.; visualization, B.T., writing—original draft preparation, F.Q., B.T., E.F.M., R.B.; writing—review and editing, F.Q., B.T., E.F.M., R.B., C.A.P., K.E, A.I.S.; All authors read and approved the manuscript.
Data sharing
The datasets generated during and/or analyzed during the current study are not publicly available due to restrictions by Cerner, the owner of the data. Data could be accessed by signing a data sharing agreement with Cerner and covering any costs that may be involved.
Declaration of Competing Interest
Dr. Porucznik reports personal fees from McKesson Corporation, outside the submitted work. All other authors declare no competing interests.
Acknowledgments
The authors acknowledge Cerner and Amazon Web Services for awarding Dr. Qeadan free data access and computation capabilities. We also thank Tracy Rees, TR, who provided editorial assistance. We acknowledge NIH for partly supporting Drs. Qeadan, Madden, and English (Grant number 5R61DA04938202). The content is solely the responsibility of the authors and does not necessarily represent the official views of Cerner, the NIH, or University of Utah.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.100938.
Appendix. Supplementary materials
References
- 1.Baillargeon J., Polychronopoulou E., Kuo Y.F., Raji M.A. The impact of substance use disorder on COVID-19 outcomes. Psychiatr Serv. 2020;72(5):578–581. doi: 10.1176/appi.ps.202000534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei Y., Shah R. Substance use disorder in the COVID-19 pandemic: a systematic review of vulnerabilities and complications. Pharmaceuticals. 2020;13(7):155. doi: 10.3390/ph13070155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Q.Q., Kaelber D.C., Xu R., Volkow N.D. COVID-19 risk and outcomes in patients with substance use disorders: analyses from electronic health records in the United States. Mol Psychiatry. 2021;26(1):30–39. doi: 10.1038/s41380-020-00880-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Institute on Drug Abuse. Health consequences of drug misuse (2020). https://www.drugabuse.gov/related-topics/healthconsequences-drug-misuse. Retrieved September 20, 2020.
- 5.Shi S., Qin M., Shen B. Association of cardiac injury with mortality in hospitalized patients With COVID-19 in Wuhan, China. JAMA Cardiology. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Preliminary estimates of the prevalence of selected underlying health conditions among patients with Coronavirus disease 2019 — United States, February 12–March 28, 2020. MMWR Morbidity Mortal Weekly Rep 2020; 69:382–6. doi: 10.15585/mmwr.mm6913e2external icon [DOI] [PMC free article] [PubMed]
- 7.Alqahtani J.S., Oyelade T., Aldhahir A.M., Alghamdi S.M., Almehmadi M., Alqahtani A.S., Quaderi S., Mandal S., Hurst J.R. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PLoS One. 2020;15(5) doi: 10.1371/journal.pone.0233147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang, J., Wang, X., Jia, X., Li, J., Hu, K., Chen, G., Wei, J., Gong, Z., Zhou, C., Yu, H., Yu, M., Lei, H., Cheng, F., Zhang, B., Xu, Y., Wang, G., & Dong, W. (2020). Risk factors for disease severity, unimprovement, and mortality in COVID-19 patients in Wuhan, China. Clinical microbiology and infection; the official publication of the European Society of Clinical Microbiology and Infectious Diseases, 26(6), 767–72. 10.1016/j.cmi.2020.04.012 [DOI] [PMC free article] [PubMed]
- 9.Ornell F., Moura H.F., Scherer J.N., Pechansky F., Kessler F.H.P., von Diemen L. The COVID-19 pandemic and its impact on substance use: implications for prevention and treatment. Psychiatry Res. 2020;289 doi: 10.1016/j.psychres.2020.113096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volkow N.D. Collision of the COVID-19 and addiction epidemics. Ann Intern Med. 2020;173(1):61–62. doi: 10.7326/M20-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. (2020, May 5) Opioid overdoses. Retrieved from: https://www.cdc.gov/drugoverdose/index.html
- 12.Ochalek T.A., Cumpston K.L., Wills B.K., Gal T.S., Moeller F.G. Nonfatal opioid overdoses at an urban emergency department during the COVID-19 pandemic. JAMA. 2020 doi: 10.1001/jama.2020.17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slavova, S., Rock, P., Bush, H.M., Quesinberry, D., and Walsh, S.L. (2020). Signal of increased opioid overdose during COVID-19 from emergency medical services data. Drug Alcohol Depend, 214, 108176. 10.1016/j.drugalcdep.2020.108176 [DOI] [PMC free article] [PubMed]
- 14.Schimmel J., Manini A.F. Opioid use disorder and COVID-19: biological plausibility for worsened outcomes. Subst Use Misuse. 2020;55(11):1900–1901. doi: 10.1080/10826084.2020.1791184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman H., Newton C., Klein T.W. Microbial infections, immunomodulation, and drugs of abuse. Clin Microbiol. Rev. 2003;16:209–219. doi: 10.1128/CMR.16.2.209-219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hauer P. Systemic effects of methamphetamine use. S D Med. 2010;63(8):285–287. [PubMed] [Google Scholar]
- 17.Hulin J., Brodie A., Stevens J., Mitchell C. Prevalence of respiratory conditions among people who use illicit opioids: a systematic review. Addiction. 2020;115(5):832–849. doi: 10.1111/add.14870. [DOI] [PubMed] [Google Scholar]
- 18.Radke J.B., Owen K.P., Sutter M.E., Ford J.B., Albertson T.E. The effects of opioids on the lung. Clin Rev Allergy Immunology. 2014;46(1):54–64. doi: 10.1007/s12016-013-8373-z. [DOI] [PubMed] [Google Scholar]
- 19.Zamanian R.T., Hedlin H., Greuenwald P., Wilson D.M., Segal J.I., Jorden M., Kudelko K., Liu J., Hsi A., Rupp A. Features and outcomes of methamphetamine associated pulmonary arterial hypertension. Am J Respir Crit Care Med. 2018;197:788–800. doi: 10.1164/rccm.201705-0943OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walker J.M., Farney R.J., Rhondeau S.M. Chronic opioid use is a risk factor for the development of central sleep apnea and ataxic breathing [published correction appears in J Clin. Sleep Med. 2007 Oct 15;3(6):table of contents] J Clin Sleep Med. 2007;3(5):455–461. [PMC free article] [PubMed] [Google Scholar]
- 21.Kiyatkin E.A. Respiratory depression and brain hypoxia induced by opioid drugs: morphine, oxycodone, heroin, and fentanyl. Neuropharmacology. 2019;151:219–226. doi: 10.1016/j.neuropharm.2019.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cerner. COVID-19 De-Identified Data Cohort Access Offer for Academic Researchers. 2020. Available online: https://www.cerner.com/-/media/covid-19/response/2263471793_covid-19-de-identified-data-cohort-access-offer-faq_v1.aspx (accessed on 1 November 2020)
- 23.Ehwerhemuepha L., Gasperino G., Bischoff N., Taraman S., Chang A., Feaster W. HealtheDataLab - a cloud computing solution for data science and advanced analytics in healthcare with application to predicting multi-center pediatric readmissions. BMC Med Inform Decis Mak. 2020;20(1):115. doi: 10.1186/s12911-020-01153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng Z., Peng F., Xu B. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;81(2):e16–e25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.NIDA. 2020, April 6. COVID-19: potential Implications for Individuals with Substance Use Disorders. Retrieved from https://www.drugabuse.gov/about-nida/noras-blog/2020/04/covid-19-potential-implications-individuals-substance-use-disorders on 2020, December 24
- 26.Choubey A., Dehury B., Kumar S., Medhi B., Mondal P. Naltrexone a potential therapeutic candidate for COVID-19. J Biomol Struct Dyn. 2020:1–8. doi: 10.1080/07391102.2020.1820379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olfson M., Wall M., Wang S., Crystal S., Blanco C. Risks of fatal opioid overdose during the first year following nonfatal overdose. Drug Alcohol Depend. 2018;190:112–119. doi: 10.1016/j.drugalcdep.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scholl L., Seth P., Kariisa M., Wilson N., Baldwin G. Drug and opioid-involved overdose deaths—United States, 2013–2017. Morbid Mortal Week Rep. 2019;67(51–52):1419. doi: 10.15585/mmwr.mm675152e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Substance Abuse and Mental Health Services Administration: The Opioid Crisis and the Hispanic/Latino Population: an Urgent Issue. Publication No. PEP20-05-02-002. Office of Behavioral Health Equity. Substance Abuse and Mental Health Services Administration, 2020.
- 30.Schuler M.S., Schell T.L., Wong E.C. Racial/ethnic differences in prescription opioid misuse and heroin use among a national sample, 1999-2018. Drug Alcohol Depend. 2021;221 doi: 10.1016/j.drugalcdep.2021.108588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Substance Abuse and Mental Health Services Administration (SAMHSA). SAMHDA [Internet]. Rockville, MD: SAMHSA Center for Behavioral Health Statistics and Quality; 2019 [cited 2020 May 28]. Available from: https://datafiles.samhsa.gov/
- 32.Lagisetty P.A., Ross R., Bohnert A., Clay M., Maust D.T. Buprenorphine treatment divide by race/ethnicity and payment. JAMA Psychiatry. 2019;76(9):979–981. doi: 10.1001/jamapsychiatry.2019.0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schiff D.M., Nielsen T., Hoeppner B.B., Terplan M., Hansen H., Bernson D., Taveras E.M. Assessment of racial and ethnic disparities in the use of medication to treat opioid use disorder among pregnant women in Massachusetts. JAMA Netw Open. 2020;3(5):e205734. doi: 10.1001/jamanetworkopen.2020.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peeler M., Gupta M., Melvin P., Bryant A.S., Diop H., Iverson R., Schiff D.M. Racial and ethnic disparities in maternal and infant outcomes among opioid-exposed mother–infant dyads in Massachusetts (2017–2019) Am J Public Health. 2020;110(12):1828–1836. doi: 10.2105/AJPH.2020.305888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kilaru A.S., Xiong A., Lowenstein M., Meisel Z.F., Perrone J., Khatri U., Delgado M.K. Incidence of treatment for opioid use disorder following nonfatal overdose in commercially insured patients. JAMA Netw Open. 2020;3(5):e205852. doi: 10.1001/jamanetworkopen.2020.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaiser Family Foundation . The Henry J. Kaiser Family Foundation; Menlo Park, CA: 2016. Key Facts About the Uninsured Population.https://www.kff.org/uninsured/fact-sheet/key-facts-about-the-uninsured-population [April 15, 2021] [Google Scholar]
- 37.Allen H., Gordon S.H., Lee D., Bhanja A., Sommers B.D. Comparison of utilization, costs, and quality of medicaid vs subsidized private health insurance for low-income adults. JAMA Netw Open. 2021;4(1) doi: 10.1001/jamanetworkopen.2020.32669. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.