Abstract
Despite the importance of pre-pregnancy body mass index (BMI) and a history of gestational diabetes mellitus (GDM) in the progression of GDM to type 2 diabetes, few studies have evaluated the combined effect of high pre-pregnancy BMI and GDM status on the future development of type 2 diabetes in Korean women. This study aimed to examine the relationship of pre-pregnancy BMI and GDM history with the risk of type 2 diabetes among Korean women. In addition, the effects of pre-pregnancy BMI and current BMI on the risk of type 2 diabetes were evaluated. Women who gave birth in the Health Examinees Study of the Korean Genome and Epidemiology Study from 2004 to 2013 (n = 59,258) were included in this study. Multivariable logistic regression was used to examine the association of pre-pregnancy BMI categories (underweight: <18.5 kg/m2; normal: 18.5–22.9 kg/m2; overweight: 23.0–24.9 kg/m2; obese: ≥25.0 kg/m2) and GDM history with the risk of type 2 diabetes after controlling for the following covariates: age, education, income, smoking status before the first pregnancy, alcohol consumption, regular exercise, menarche age, first pregnancy age, and first pregnancy outcome. Compared to women with normal pre-pregnancy BMIs, women with overweight and obese pre-pregnancy BMIs had higher odds of developing type 2 diabetes (adjusted odds ratio [AOR]: 1.13, 95% confidence interval [CI]: 1.02–1.25 and AOR: 1.29, 95% CI: 1.10–1.50, respectively) after controlling for covariates. Women with pre-pregnancy BMIs <23 kg/m2 and current BMIs ≥23 kg/m2 had increased odds of developing type 2 diabetes (AOR: 1.64, 95% CI: 1.51–1.78) compared to those with pre-pregnancy BMIs <23 kg/m2 and current BMIs <23 kg/m2. Among women without a history of GDM, those with overweight and obese pre-pregnancy BMIs had increased odds of developing type 2 diabetes compared to those with normal pre-pregnancy BMIs (AOR: 1.12, 95% CI: 1.01–1.24 and AOR: 1.23, 95% CI: 1.05–1.44, respectively). Among women with GDM, those with obese pre-pregnancy BMIs had increased odds of developing type 2 diabetes (AOR: 3.84, 95% CI: 1.52–9.87). This study showed that there was a higher likelihood of developing type 2 diabetes in women who were overweight or obese before pregnancy with a history of GDM compared to their counterparts without a history of GDM. Furthermore, high pre-pregnancy BMI or high current BMI increased the risk of type 2 diabetes in Korean women, regardless of GDM history. This emphasizes the importance of maintaining a healthy weight status before and after pregnancy to prevent the future risk of type 2 diabetes.
Introduction
Gestational diabetes mellitus (GDM) is defined as glucose intolerance that is first identified during pregnancy [1], and its prevalence has increased globally over the past 10–20 years [2, 3]. GDM, one of the most common pregnancy complications, affected 5.7% to 9.5% in Korea in 2009 and 2011, according to data from the Health and Insurance Review and Assessment database [4]. The Korean national guidelines during that period recommended all pregnant women to perform GDM screening at 24–28 weeks, regardless of underlying GDM risk [5]. GDM influences short- and long-term adverse health outcomes for both mothers and their offspring [6, 7]. A huge economic burden and significant increase in health care costs associated GDM have been documented [8, 9].
Using more than 4 years of follow-up longitudinal study, women with a history of GDM were found to have a faster deterioration of insulin sensitivity over time, and their beta cell compensation continued to deteriorate at a faster rate during follow-up compared to those without a history of GDM [10]. Among women with GDM, the metabolic stress of pregnancy that they experience may unmask a genetic predisposition to type 2 diabetes [11], which may lead to chronic disease states such as impaired glucose levels and type 2 diabetes after pregnancy [12]. Specifically, it has been reported that women with a history of GDM have a 7-fold increased risk of type 2 diabetes. Thus, a diagnosis of GDM can be used to identify women and their offspring who may be more vulnerable to diabetes, obesity, and cardiovascular disease later in their lives [12–16]. The most important modifiable risk factor for GDM is pre-pregnancy body mass index (BMI) [17, 18]. A previous study showed that every unit increase in pre-pregnancy BMI was associated with significantly shorter cord blood telomere length and placental telomere length in a large birth cohort [19]. This indicated that pre-pregnancy weight might influence longevity in the offspring. Furthermore, recent findings suggest that pre-pregnancy BMI is associated with the development of regulation of body weight and thalamic functional brain connectivity in the offspring [20]. Pre-pregnancy BMI may be one of the most important modifiable environmental factors that may dictate the life expectancy of and brain development in newborns, and their later health status and risk of chronic diseases [19, 20].
Despite the importance of the influence of pre-pregnancy BMI, current BMI, and GDM history on the development of type 2 diabetes, limited studies have been conducted to evaluate the combined effect of high pre-pregnancy BMI and GDM status on the development of type 2 diabetes in a large sample of Korean women. Therefore, we examined the relationship of pre-pregnancy BMI and GDM history with the risk of type 2 diabetes. We further explored the combined effects of pre-pregnancy BMI and current BMI on the future development of type 2 diabetes.
Material and methods
Study participants
Study participants were recruited from the Health Examinees Study (HEXA) of the Korean Genome and Epidemiology Study (KoGES), a large-scale, community-based prospective cohort study. We used baseline examinations, which were obtained from 2004 to 2013 by the Korea Centers for Disease Control and Prevention. A total of 173,208 study participants aged ≥40 years were recruited from 38 major hospitals and local health examination centers in eight regions of Korea. The HEXA study design has been described in detail elsewhere [21, 22]. An interview-based questionnaire survey was conducted among women to collect information on sociodemographic characteristics, medical history, medication usage, family history, lifestyle factors, diet, physical activity, and reproductive factors. The KoGES study was reviewed and approved by the Institutional Review Board of the Korea Centers for Disease Control and Prevention. All participants enrolled in the study voluntarily and all gave written-informed consent. All study methods and protocols were conducted in accordance with the relevant institutional guidelines and regulations. The study protocol was reviewed and approved by the Institutional Review Board (IRB) of Inha University on January 31, 2020 (IRB No. 200129–1A).
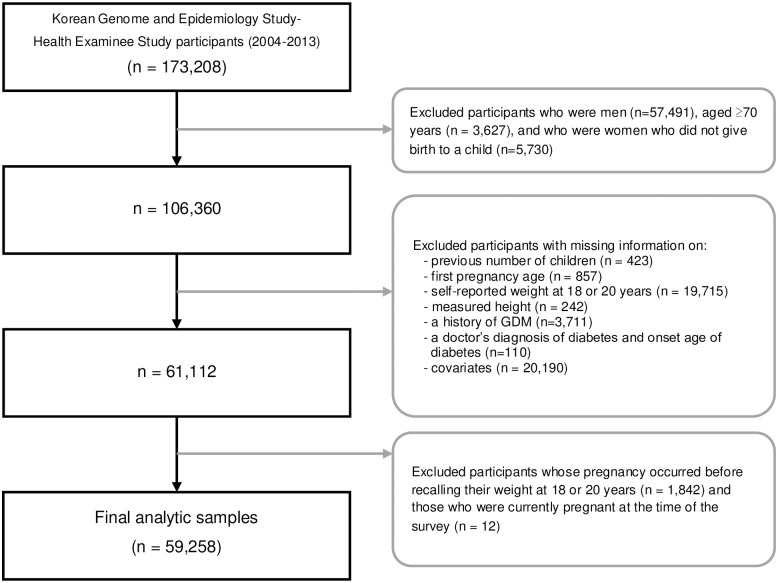
Fig 1 presents flow chart of study participants available in KoGES. At baseline, 173,208 individuals participated in the HEXA study. We excluded participants who were men, aged ≥70 years, and who were women who did not give birth to a child. We also excluded participants with missing information on previous number of children, first pregnancy age, self-reported weight at 18 or 20 years, measured height, a history of GDM, and a doctor’s diagnosis of diabetes and onset age of diabetes, and covariates. Further, we excluded participants whose pregnancy occurred before recalling their weight at 18 or 20 years and those who were currently pregnant at the time of the survey. The final study sample comprised 59,258 women.
Fig 1. Flow chart of study population.
Pre-pregnancy BMI
In the surveys from 2004 to 2006, women had to report their weight at age 20, and in the surveys from 2007 to 2012, women were asked to report their weight at age 18. BMI was calculated by dividing the recalled weight at age 18 or 20, which was before their first childbirth, by the measured height at baseline squared. Pre-pregnancy BMI was categorized as “underweight (<18.5 kg/m2),” “normal weight (18.5–22.9 kg/m2),” “overweight (23.0–24.9 kg/m2)”, and “obese (≥25.0 kg/m2)”, according to the criteria for Asians [23].
Current BMI
Current BMI was calculated by dividing the measured weight by the measured height at baseline squared (kg/m2) at the time of the survey.
Ascertainment of GDM and type 2 diabetes
A woman was considered to have GDM if they responded “yes” to the following question: “have you ever been diagnosed with GDM in the past?” Type 2 diabetes was defined as a woman diagnosed with diabetes by a doctor or a fasting blood glucose level ≥126 mg/dL. After fasting overnight for 12 hours, plasma concentrations of glucose were measured enzymatically using a HITACHI Automatic Analyzer 7600 (Hitachi, Tokyo, Japan).
Confounding factors
Confounding factors included sociodemographic, lifestyle, and reproductive variables. Sociodemographic variables included age, education level, and monthly income. Lifestyle variables included smoking before the first pregnancy, current alcohol consumption, and regular exercise. Reproductive variables included age at menarche, age at first pregnancy, first pregnancy outcome, and number of children. Education level was grouped into three categories: “≤high school,” “vocational school graduate or college dropout,” and “college and above”. Monthly income was classified into four categories: “<1 million Korean won,” “1–2 million Korean won”, “2–3 million Korean won”, and “3 million Korean won and above.” Smoking status before the first pregnancy was classified as “yes” or “no.” Alcohol consumption status was classified into three categories: “non-drinker,” “past drinker,” and “current drinker.” Regular exercise was categorized as “yes (active)” or “no (inactive)”.
Statistical analyses
Descriptive statistics were computed to detect significant differences between the variables according to the status of type 2 diabetes using chi-square tests for categorical variables and t-tests for continuous variables. Multivariable logistic regression was used to compute odds ratios and 95% confidence intervals of the associations of pre-pregnancy BMI with GDM and type 2 diabetes after controlling for confounders. Confounding variables included age, education, income, smoking status before the first pregnancy, alcohol consumption, regular exercise, menarche age, first pregnancy age, and first pregnancy outcome. Furthermore, we stratified the associations between pre-pregnancy BMI and the risk of type 2 diabetes according to the status of GDM history. All the statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA). A two-sided P value of <0.05 was considered statistically significant.
Results
Table 1 shows the sociodemographic, lifestyle, and reproductive variables according to the status of type 2 diabetes. Age, education, monthly income, alcohol consumption, regular exercise, menarche age, first pregnancy age, first pregnancy outcome, number of children, and pre-pregnancy BMI significantly differed according to the status of type 2 diabetes. Women with type 2 diabetes were significantly older (57.0 ± 7.1 vs. 51.9 ± 7.5 years), had lower education levels (≤high school: 87.9% vs. 75.1%), had lower monthly income levels (<1 million Korean won: 20.2% vs. 9.75%), were more likely to be non-drinkers of alcohol (77.0% vs. 65.9%), were more likely to engage in regular exercise (54.3% vs. 51.5%), had higher menarche ages (≥16 years: 47.8% vs. 37.7%), had lower first pregnancy ages (≤23 years: 41.7% vs. 30.4%), had more live births (83.9% vs. 81.7%), had more children (2.7 ± 0.9 vs. 2.4 ± 0.7), and higher pre-pregnancy BMIs (21.0 ± 2.4 vs. 20.6 ± 2.2 kg/m2) than those without type 2 diabetes.
Table 1. Distributions of sociodemographic, lifestyle, and reproductive characteristics of the study population in the Health Examinee Study (HEXA) 2004–2013.
| Type 2 Diabetes | Total | P value | |||||
|---|---|---|---|---|---|---|---|
| No | Yes | ||||||
| Mean | SD | Mean | SD | Mean | SD | ||
| Age (years) | 51.9 | 7.5 | 57.0 | 7.1 | 52.2 | 7.6 | < .0001 |
| Number of children | 2.4 | 0.7 | 2.7 | 0.9 | 2.4 | 0.7 | < .0001 |
| Pre-pregnancy BMI (kg/m2) | 20.6 | 2.2 | 21.0 | 2.4 | 20.6 | 2.2 | < .0001 |
| n | % | n | % | n | % | ||
| Education | |||||||
| ≤High school | 41,718 | 75.1 | 3,279 | 87.9 | 44,997 | 75.9 | < .0001 |
| Vocational school graduate or college dropout | 2,593 | 4.7 | 110 | 3.0 | 2,703 | 4.6 | |
| ≥College | 11,215 | 20.2 | 343 | 9.2 | 11,558 | 19.5 | |
| Monthly income | |||||||
| <1 million Korean won | 5,414 | 9.8 | 754 | 20.2 | 6,168 | 10.4 | < .0001 |
| 1–2 million Korean won | 11,048 | 19.9 | 1,018 | 27.3 | 12,066 | 20.4 | |
| 2–3 million Korean won | 12,631 | 22.8 | 805 | 21.6 | 13,436 | 22.7 | |
| ≥3 million Korean won | 26,433 | 47.6 | 1,155 | 31.0 | 27,588 | 46.6 | |
| Smoking before the first pregnancy | |||||||
| No | 55,157 | 99.3 | 3,716 | 99.6 | 58,873 | 99.4 | 0.0826 |
| Yes | 369 | 0.7 | 16 | 0.4 | 385 | 0.7 | |
| Alcohol consumption | |||||||
| Never | 36,589 | 65.9 | 2,875 | 77.0 | 39,464 | 66.6 | < .0001 |
| Past | 913 | 1.6 | 79 | 2.1 | 992 | 1.7 | |
| Current | 18,024 | 32.5 | 778 | 20.9 | 18,802 | 31.7 | |
| Regular exercise | |||||||
| No | 26,933 | 48.5 | 1,706 | 45.7 | 28,639 | 48.3 | 0.001 |
| Yes | 28,593 | 51.5 | 2,026 | 54.3 | 30,619 | 51.7 | |
| Menarche age (years) | |||||||
| ≤14 | 21,789 | 39.2 | 1,165 | 31.2 | 22,954 | 38.7 | < .0001 |
| 15 | 12,794 | 23.0 | 784 | 21.0 | 13,578 | 22.9 | |
| ≥16 | 20,943 | 37.7 | 1,783 | 47.8 | 22,726 | 38.4 | |
| First pregnancy age (years) | |||||||
| ≤23 | 16,853 | 30.4 | 1,555 | 41.7 | 18,408 | 31.1 | < .0001 |
| 24–26 | 23,227 | 41.8 | 1,427 | 38.2 | 24,654 | 41.6 | |
| ≥27 | 15,446 | 27.8 | 750 | 20.1 | 16,196 | 27.3 | |
| First pregnancy outcome | |||||||
| Live birth | 45,348 | 81.7 | 3,132 | 83.9 | 48,480 | 81.8 | 0.0001 |
| Stillbirth | 364 | 0.7 | 29 | 0.8 | 393 | 0.7 | |
| Extrauterine pregnancy | 99 | 0.2 | 7 | 0.2 | 106 | 0.2 | |
| Spontaneous abortion | 3,900 | 7.0 | 264 | 7.1 | 4,164 | 7.0 | |
| Artificial abortion | 5,815 | 10.5 | 300 | 8.0 | 6,115 | 10.3 | |
P values based on t-tests for continuous variables and chi-square tests for categorical variables.
Table 2 shows the associations of pre-pregnancy BMI with the risk of GDM and type 2 diabetes. Compared to women with normal pre-pregnancy BMIs, those with obese pre-pregnancy BMIs had higher odds of GDM (AOR: 1.63, 95% CI: 1.05–2.55) after controlling for the following covariates: age, education, income, smoking status before the first pregnancy, alcohol consumption, physical activity, menarche age, first pregnancy age, and first pregnancy outcome. Compared to women with normal pre-pregnancy BMIs, those with overweight and obese pre-pregnancy BMIs had higher odds of developing type 2 diabetes (AOR: 1.13, 95%: 1.02–1.25 and AOR: 1.29, 95% CI: 1.10–1.50, respectively) after controlling for the same covariates.
Table 2. Associations of pre-pregnancy BMI with GDM and type 2 diabetes in the Health Examinee Study (HEXA) 2004–2013.
| Pre-pregnancy BMI (kg/m2) | GDM | Type 2 Diabetes |
|---|---|---|
| Underweight (<18.5) | 0.95 (0.77–1.17) | 0.99 (0.89–1.09) |
| Normal (18.5–22.9) | 1.00 (Ref.) | 1.00 (Ref.) |
| Overweight (23–24.9) | 1.19 (0.91–1.56) | 1.13 (1.02–1.25) |
| Obese (≥25) | 1.63 (1.05–2.55) | 1.29 (1.10–1.50) |
Data are presented as adjusted odds ratio (95% confidence intervals) after controlling for age (continuous), education (≤high school, vocational school, graduate or college dropout, ≥college), income (<1 million won, 1–2 million Korean won, 2–3 million Korean won, ≥3 million Korean won), smoking status before the first pregnancy (no, yes), alcohol consumption (never, past, current), regular exercise (no, yes), menarche age (≤14, 15, and ≥16 years), first pregnancy age (≤23, 24–26, and ≥27 years), and first pregnancy outcome (live birth, stillbirth, extrauterine pregnancy, spontaneous abortion, artificial abortion).
GDM: Gestational diabetes mellitus; BMI: Body mass index.
Table 3 presents the distributions of pre-pregnancy BMI categories and risk of type 2 diabetes according to the status of GDM history. The associations between pre-pregnancy BMI and type 2 diabetes according to the GDM status are also presented. Among women with and without GDM, most women had normal pre-pregnancy BMIs (69.2% and 70.8%, respectively). Among women without GDM, those with overweight and obese pre-pregnancy BMIs had higher odds of developing type 2 diabetes than those with normal pre-pregnancy BMIs (AOR: 1.12, 95% CI: 1.01–1.24 and AOR: 1.23, 95% CI: 1.05–1.44, respectively). Among women with GDM, those with obese pre-pregnancy BMIs had higher odds of developing type 2 diabetes than those with normal pre-pregnancy BMIs (AOR: 3.87, 95% CI: 1.52–9.87).
Table 3. Association between pre-pregnancy BMI and type 2 diabetes according to the status of GDM history in the Health Examinee Study (HEXA) 2004–2013.
| Pre-pregnancy BMI (kg/m2) | Without a history of GDM (n = 58,629) | With a history of GDM (n = 629) | ||||
|---|---|---|---|---|---|---|
| n | % | AOR (95% CI) | n | % | AOR (95% CI) | |
| Underweight (<18.5) | 9,402 | 16.0 | 0.99 (0.89–1.09) | 111 | 17.7 | 1.13 (0.65–1.97) |
| Normal (18.5–22.9) | 41,429 | 70.8 | 1.00 (Ref.) | 435 | 69.2 | 1.00 (Ref.) |
| Overweight (23.0–24.9) | 6,002 | 10.2 | 1.12 (1.01–1.24) | 62 | 9.9 | 1.43 (0.75–2.73) |
| Obese (≥25.0) | 1,796 | 3.1 | 1.23 (1.05–1.44) | 21 | 3.3 | 3.87 (1.52–9.87) |
Data are presented as numbers, percentages, and adjusted odds ratio (95% confidence intervals) after controlling for age (continuous), education (≤high school, vocational school, graduate or college dropout, ≥college), income (<1 million Korean won, 1–2 million Korean won, 2–3 million Korean won, ≥3 million Korean won), smoking status before the first pregnancy (no, yes), alcohol consumption (never, past, current), regular exercise (no, yes), menarche age (≤14, 15, and ≥16 years), first pregnancy age (≤23, 24–26, and ≥27 years), and first pregnancy outcome (live birth, stillbirth, extrauterine pregnancy, spontaneous abortion, artificial abortion).
GDM: Gestational diabetes mellitus; BMI: Body mass index; AOR: Adjusted odds ratio; CI: Confidence interval.
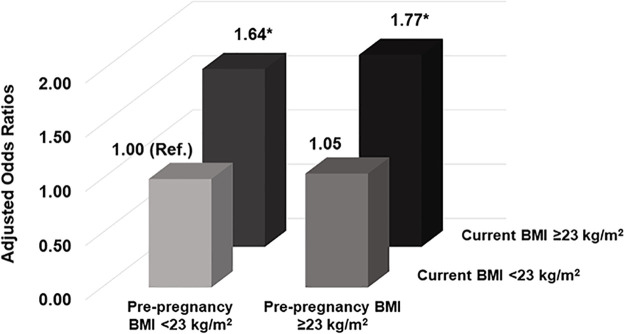
Table 4 shows the combined effects of pre-pregnancy BMI and current BMI on type 2 diabetes. The distributions of the combined effects of pre-pregnancy BMI and current BMI significantly differed according to the status of type 2 diabetes (P <0.0001). A high proportion of women with type 2 diabetes had pre-pregnancy BMI ≥23 kg/m2 and current BMI ≥23 kg/m2 (10.0%). The distribution of type 2 diabetes significantly differed according to the pre-pregnancy BMI and current BMI categories (P <0.0001). The association of the combined effects of pre-pregnancy BMI and current BMI with the risk of type 2 diabetes is presented (Fig 2). Women with pre-pregnancy BMIs <23 kg/m2 and current BMIs ≥23 kg/m2 had higher odds of developing type 2 diabetes (AOR: 1.64, 95% CI: 1.51–1.78) than those with pre-pregnancy BMIs <23 kg/m2 and current BMI <23 kg/m2. In addition, women with pre-pregnancy BMIs ≥23 kg/m2 and current BMIs ≥23 kg/m2 had higher odds of developing type 2 diabetes (AOR 1.77, 95% CI 1.59–1.98) than those with pre-pregnancy BMIs <23 kg/m2 and current BMIs <23 kg/m2.
Table 4. Distributions of pre-pregnancy BMI and current BMI categories according to the status of type 2 diabetes in the Health Examinee Study (HEXA) 2004–2013.
| BMI categories | Type 2 diabetes | P value | |||||
|---|---|---|---|---|---|---|---|
| No | Yes | Total | |||||
| n | % | n | % | n | % | ||
| Pre-pregnancy BMI <23 kg/m2 & Current BMI <23 kg/m2 | 23,921 | 96.1 | 969 | 3.9 | 24,890 | 42.0 | <0.0001 |
| Pre-pregnancy BMI ≥23 kg/m2 & Current BMI <23 kg/m2 | 1,709 | 94.6 | 98 | 5.4 | 1,807 | 3.1 | |
| Pre-pregnancy BMI <23 kg/m2 & Current BMI ≥23 kg/m2 | 24,423 | 92.2 | 2,059 | 7.8 | 26,482 | 44.7 | |
| Pre-pregnancy BMI ≥23 kg/m2 & Current BMI ≥23 kg/m2 | 5,467 | 90.0 | 606 | 10.0 | 6,073 | 10.3 | |
| Total | 55,520 | 93.7 | 3,732 | 6.3 | 59,252 | 100.0 | |
P value based on the chi-square test.
BMI: Body mass index.
Fig 2. Associations of pre-pregnancy BMI and current BMI with the development of type 2 diabetes in the in the Health Examinee Study (HEXA) 2004–2013.
Data are presented as adjusted odds ratios after controlling for age (continuous), education (≤high school, vocational school, graduate or college dropout, ≥college), income (<1 million Korean won, 1–2 million Korean won, 2–3 million won, ≥3 million Korean won), smoking status before the first pregnancy (no, yes), alcohol consumption (never, past, current), regular exercise (no, yes), menarche age (≤14, 15, and ≥16 years), first pregnancy age (≤23, 24–26, and ≥27 years), and first pregnancy outcome (live birth, stillbirth, extrauterine pregnancy, spontaneous abortion, artificial abortion). *P value <0.05. BMI: Body mass index.
Table 5 demonstrates the associations of current BMI and combined effects of pre-pregnancy BMI and current BMI with type 2 diabetes according to the status of GDM history. A higher proportion of women with a history of GDM had obese current BMIs than those without a history of GDM (30.2% vs. 28.0%). Among women without a history of GDM, those with overweight or obese current BMIs had a higher risk of type 2 diabetes than those with normal current BMIs (AOR: 1.29, 95% CI: 1.17–1.41 and AOR: 1.96, 95% CI: 1.81–2.14, respectively). Among women with a history of GDM, those with obese current BMIs were 2.04 more likely to develop type 2 diabetes (AOR: 2.04, 95% CI: 1.24–3.35) than those with normal current BMIs. Among women without a history of GDM, those with pre-pregnancy BMIs <23 kg/m2 and current BMIs ≥23 kg/m2 as well as those with pre-pregnancy BMIs ≥23 kg/m2 and current BMIs ≥23 kg/m2 had a higher risk of type 2 diabetes than those with pre-pregnancy BMIs ≥23 kg/m2 and current BMIs <23 kg/m2 (AOR: 1.63, 95% CI: 1.50–1.77; AOR: 1.73, 95% CI: 1.55–1.93, respectively). Among women with a history of GDM, those with pre-pregnancy BMIs ≥23 kg/m2 and current BMIs ≥23 kg/m2 had a higher risk of type 2 diabetes than those with pre-pregnancy BMIs ≥23 kg/m2 and current BMIs <23 kg/m2 (AOR: 2.54, 95% CI: 1.32–4.89).
Table 5. Association between current BMI and type 2 diabetes according to the status of GDM history in the Health Examinee Study (HEXA) 2004–2013.
| Without a history of GDM (n = 58,623) | With a history of GDM (n = 629) | |||||
| Current BMI (kg/m2) | n | % | AOR (95% CI) | n | % | AOR (95% CI) |
| Underweight (<18.5) | 1,072 | 1.8 | 0.86 (0.60–1.23) | 15 | 2.4 | 0.53 (0.06–4.34) |
| Normal (18.5–22.9) | 25,365 | 43.3 | 1.00 (Ref.) | 245 | 39.0 | 1.00 (Ref.) |
| Overweight (23.0–24.9) | 15,768 | 26.9 | 1.29 (1.17–1.41) | 179 | 28.5 | 1.06 (0.63–1.80) |
| Obese (≥25.0) | 16,418 | 28.0 | 1.96 (1.81–2.14) | 190 | 30.2 | 2.04 (1.24–3.35) |
| BMI categories | n | % | AOR (95% CI) | n | % | AOR (95% CI) |
| Pre-pregnancy BMI <23 kg/m2 & Current BMI <23 kg/m2 | 24,646 | 42.0 | 1.00 (Ref.) | 244 | 38.8 | 1.00 (Ref.) |
| Pre-pregnancy BMI ≥23 kg/m2 & Current BMI <23 kg/m2 | 1,791 | 3.1 | 1.03 (0.82–1.28) | 16 | 2.5 | 1.46 (0.42–5.04) |
| Pre-pregnancy BMI <23 kg/m2 & Current BMI ≥23 kg/m2 | 26,180 | 44.7 | 1.63 (1.50–1.77) | 302 | 48.0 | 1.41 (0.89–2.25) |
| Pre-pregnancy BMI ≥23 kg/m2 & Current BMI ≥23 kg/m2 | 6,066 | 10.3 | 1.73 (1.55–1.93) | 67 | 10.7 | 2.54 (1.32–4.89) |
Data are presented as numbers, percentages, and adjusted odds ratios (95% confidence intervals) after controlling for age (continuous), education (≤high school, vocational school, graduate or college dropout, ≥college), income (<1 million won, 1–2 million Korean won, 2–3 million Korean won, ≥3 million Korean won), smoking status before the first pregnancy (no, yes), alcohol consumption (never, past, current), regular exercise (no, yes), menarche age (≤14, 15, and ≥16 years), first pregnancy age (≤23, 24–26, and ≥27 years), and first pregnancy outcome (live birth, stillbirth, extrauterine pregnancy, spontaneous abortion, artificial abortion).
GDM: Gestational diabetes mellitus; BMI: Body mass index; AOR: Adjusted odds ratio; CI: Confidence interval.
Discussion
This study aimed to examine the associations of pre-pregnancy BMI, current BMI, and GDM history with type 2 diabetes in Korean women. We examined the relationships of pre-pregnancy BMI and GDM with type 2 diabetes. Among 59,258 women, pre-pregnancy BMI was associated with an increased risk of GDM and type 2 diabetes. In accordance with our findings, using data from the U.S. Pregnancy Risk Assessment Monitoring System, women with obese pre-pregnancy BMIs (≥30 kg/m2) had increased odds of GDM (AOR: 2.78, 95% CI: 2.60–2.96) [18]. In a retrospective longitudinal cohort study from the U.S., the prevalence of GDM was higher in women who were obese (17.1%) or overweight (12.0%) before pregnancy than in those who had normal weights (7.4%) [24]. A meta-analysis [25] that included 70 studies involving 671,945 women revealed that those who were overweight, moderately obese, and morbidly obese before pregnancy had higher risks of GDM (OR: 1.97, 95% CI: 1.77–2.19; OR: 3.01, 95% CI: 2.34–3.87; and OR: 5.55, 95% CI: 4.27–7.21, respectively).
When stratified by GDM history, women with pre-pregnancy obesity and a history of GDM were 3.87 times more likely to develop type 2 diabetes than those with normal pre-pregnancy BMIs. However, women with pre-pregnancy obesity and without a history of GDM were 1.23 times more likely to develop type 2 diabetes, which was less than one-third of the odds of those with a history of GDM. This highlighted that the combination of pre-pregnancy obesity and GDM history may be associated with a greater likelihood of developing type 2 diabetes in the future.
Furthermore, we evaluated the combined effect of pre-pregnancy BMI and current BMI on the risk of type 2 diabetes. Interestingly, women with pre-pregnancy BMI <23 kg/m2 and current BMI ≥23 kg/m2 had 1.64-fold increased risk of type 2 diabetes compared to those women with both pre-pregnancy BMI and current BMI <23 kg/m2. When women were not overweight or obese before pregnancy, but became overweight or obese in their later lives, this increases the risk of type 2 diabetes emphasizing the role of current weight status. We found that the combination of pre-pregnancy BMI ≥23 kg/m2 and current BMI ≥23 kg/m2 was associated with a 1.77-fold increased risk of type 2 diabetes compared to pre-pregnancy BMI <23 kg/m2 and current BMI <23 kg/m2 after controlling for covariates. Both high BMI before pregnancy and high current BMI were associated with the risk of type 2 diabetes, and type 2 diabetes was closely linked with the presence of obesity before and after pregnancy. The association between obesity and type 2 diabetes has been well-established, and the major basis for this association is insulin resistance [26]. Insulin resistance in obesity and type 2 diabetes is caused by decreased insulin-stimulated glucose transport and metabolism in adipocytes [27]. Specifically, the impaired ability of insulin to lower blood glucose levels is partially due to disruptions in the translocation of the Glut4 glucose transporter to the surface membrane of muscle cells [28, 29] which in turn reduces glucose uptake from the bloodstream [30].
Weight status, especially obesity, plays a key role in the pathogenesis of type 2 diabetes [31, 32]. In line with our findings, previous studies have reported that past and present weight status are significantly associated with the risk of type 2 diabetes. In 2,927 Japanese adults with a mean age of 59.3 years, previous and current obesity were risk factors for diabetic nephropathy (OR: 1.66, 95% CI: 1.32–2.07 and OR: 2.48, 95% CI: 1.96–3.14, respectively) [33]. In a national cohort of 8,545 adults in the U.S. from the National Health and Nutrition Examination Survey Epidemiology Follow-up Study [34], the associations between weight change over approximately 10 years and the incidence of diabetes were investigated. Participants who gained 11–20 kg or more than 20 kg had a higher risk of developing diabetes (hazard ratio [HR]: 2.57, 95% CI: 1.84–3.85 and HR: 3.85, 95% CI: 2.04–7.22, respectively) than those whose weights remained stable over the 10-year period. Similarly, in Korean adults with a mean age of 56 years [35], a history of obesity and increased upper body adiposity were associated with non-insulin-dependent diabetes mellitus.
We further examined the associations of pre-pregnancy BMI and current BMI with type 2 diabetes stratified by GDM history. We found that women with a history of GDM, who were overweight or obese before pregnancy, and were currently obese were 2.54 times more likely to develop type 2 diabetes than those who were overweight or obese before pregnancy and currently had normal weights or were underweight. However, women with a history of GDM, who were overweight and obese before pregnancy, and currently underweight or had normal weights did not have an increased risk of type 2 diabetes. This illustrated that women who are previously diagnosed with GDM may need to be cautious about their current weight if they were overweight or obese before their pregnancies. This also emphasized that current weight status may prevail over pre-pregnancy weight status when considering the risk of type 2 diabetes.
There were several limitations in our study. First, the mean age of the study population was 52.2 years, and women were asked to report their weight when they were 18 or 20 years, which might have resulted in a recall bias. However, in a study that validated self-reported pre-pregnancy weight in a representative sample of pregnant women in the U.S. [36], the mean (standard error of the mean) difference between self-reported and imputed pre-pregnancy weight was -1.7 (0.1) kg, with an r = 0.98 (P <0.001) and κ = 0.78, which indicated substantial agreement. Additionally, to define type 2 diabetes, no information was available on glycated hemoglobin (HbA1c) levels and medications such as diabetic pills or insulin injection status. Lastly, a history of GDM information was based on self-reports, not proved by any blood tests.
Despite these limitations, to the best of our knowledge, the present study was the first to identify the combined effects of high pre-pregnancy BMI, high current BMI, and GDM history on the increased risk of type 2 diabetes using a large sample of Korean women (n = 59,258) in the HEXA cohort.
Conclusions
In conclusion, elevated pre-pregnancy BMI was significantly associated with an increased risk of GDM and type 2 diabetes in Korean women. We found that women with a history of GDM who were overweight or obese before pregnancy and currently had normal weights were not associated with a higher risk of type 2 diabetes. However, women who were overweight or obese and also currently overweight or obese had a 2.54-fold increased risk of type 2 diabetes, highlighting the importance of weight management throughout the life course. Furthermore, when a woman was overweight or obese before pregnancy and remained overweight or obese about 30 years after pregnancy, there was a greater risk of developing type 2 diabetes. These findings are of great public health significance given the increasing prevalence of GDM and type 2 diabetes among Korean women. Furthermore, there is a need for the increased effort to screen for undiagnosed GDM, especially when pregnant women are overweight or obese. Interventions to achieve a healthy weight status before pregnancy and throughout the rest of one’s life may prevent the future risk of type 2 diabetes, especially in women with a history of GDM.
Acknowledgments
This study was conducted with biosources from the National Biobank of Korea, the Centers for Disease Control and Prevention, Republic of Korea (KBN-2020-016).
Abbreviations
- AOR
Adjusted odds ratio
- BMI
Body mass index
- CI
Confidence interval
- GDM
Gestational diabetes mellitus
- HEXA
Health Examinees Study
- KoGES
Korean Genome and Epidemiology Study
Data Availability
Data underlying the results of our study are not publicly available due to KoGES data policy. Data are available from the Division of Genetic Epidemiology and Health Index, NIH, Korea Centers for Disease Control and Prevention (contact via Mi-Jin Cho at whalwls0227@korea.kr) for researchers who meet the criteria for access to confidential data.
Funding Statement
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2020R1G1A1004940). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Metzger BE, Coustan DR, Committee O. Summary and recommendations of the fourth international workshop-conference on gestational diabetes mellitus. Diabetes Care. 1998;21:B161. [PubMed] [Google Scholar]
- 2.Ferrara A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care. 2007;30 Suppl 2:S141–6. Epub 2007/07/13. doi: 10.2337/dc07-s206 . [DOI] [PubMed] [Google Scholar]
- 3.Anna V, van der Ploeg HP, Cheung NW, Huxley RR, Bauman AE. Sociodemographic correlates of the increasing trend in prevalence of gestational diabetes mellitus in a large population of women between 1995 and 2005. Diabetes Care. 2008;31(12):2288–93. Epub 2008/09/24. doi: 10.2337/dc08-1038 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koo BK, Lee JH, Kim J, Jang EJ, Lee C-H. Prevalence of gestational diabetes mellitus in Korea: a national health insurance database study. PLoS One. 2016;11(4):e0153107. doi: 10.1371/journal.pone.0153107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korean Diabetes Association. Treatment guideline for diabetes. 6 ed: Korean Diabetes Association; 2019. [Google Scholar]
- 6.Catalano PM, McIntyre HD, Cruickshank JK, McCance DR, Dyer AR, Metzger BE, et al. The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes care. 2012;35(4):780–6. doi: 10.2337/dc11-1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Metzger BE. Long-term outcomes in mothers diagnosed with gestational diabetes mellitus and their offspring. Clinical Obstetrics and Gynecology. 2007;50(4):972–9. doi: 10.1097/GRF.0b013e31815a61d6 [DOI] [PubMed] [Google Scholar]
- 8.Dall TM, Yang W, Gillespie K, Mocarski M, Byrne E, Cintina I, et al. The Economic Burden of Elevated Blood Glucose Levels in 2017: Diagnosed and Undiagnosed Diabetes, Gestational Diabetes Mellitus, and Prediabetes. Diabetes Care. 2019;42(9):1661–8. doi: 10.2337/dc18-1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolu P, Raitanen J, Rissanen P, Luoto R. Health care costs associated with gestational diabetes mellitus among high-risk women—results from a randomised trial. BMC Pregnancy Childbirth. 2012;12:71-. doi: 10.1186/1471-2393-12-71 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiang AH, Takayanagi M, Black MH, Trigo E, Lawrence JM, Watanabe RM, et al. Longitudinal changes in insulin sensitivity and beta cell function between women with and without a history of gestational diabetes mellitus. Diabetologia. 2013;56(12):2753–60. Epub 2013/09/14. doi: 10.1007/s00125-013-3048-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Catalano PM, Kirwan JP, Haugel-de Mouzon S, King J. Gestational diabetes and insulin resistance: role in short-and long-term implications for mother and fetus. The Journal of nutrition. 2003;133(5):1674S–83S. doi: 10.1093/jn/133.5.1674S [DOI] [PubMed] [Google Scholar]
- 12.Casagrande SS, Linder B, Cowie CC. Prevalence of gestational diabetes and subsequent type 2 diabetes among US women. Diabetes Research and Clinical Practice. 2018;141:200–8. doi: 10.1016/j.diabres.2018.05.010 [DOI] [PubMed] [Google Scholar]
- 13.Daly B, Toulis KA, Thomas N, Gokhale K, Martin J, Webber J, et al. Increased risk of ischemic heart disease, hypertension, and type 2 diabetes in women with previous gestational diabetes mellitus, a target group in general practice for preventive interventions: A population-based cohort study. PLoS Med. 2018;15(1):e1002488. Epub 2018/01/18. doi: 10.1371/journal.pmed.1002488 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Page KA, Luo S, Wang X, Chow T, Alves J, Buchanan TA, et al. Children exposed to maternal obesity or gestational diabetes mellitus during early fetal development have hypothalamic alterations that predict future weight gain. Diabetes Care. 2019;42(8):1473–80. doi: 10.2337/dc18-2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herath H, Herath R, Wickremasinghe R. Gestational diabetes mellitus and risk of type 2 diabetes 10 years after the index pregnancy in Sri Lankan women—A community based retrospective cohort study. PloS One. 2017;12(6):e0179647. doi: 10.1371/journal.pone.0179647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rayanagoudar G, Hashi AA, Zamora J, Khan KS, Hitman GA, Thangaratinam S. Quantification of the type 2 diabetes risk in women with gestational diabetes: a systematic review and meta-analysis of 95,750 women. Springer; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jovanovic L, Pettitt DJ. Gestational diabetes mellitus. Jama. 2001;286(20):2516–8. Epub 2001/11/28. doi: 10.1001/jama.286.20.2516 . [DOI] [PubMed] [Google Scholar]
- 18.Shin D, Song WO. Prepregnancy body mass index is an independent risk factor for gestational hypertension, gestational diabetes, preterm labor, and small- and large-for-gestational-age infants. J Matern Fetal Neonatal Med. 2015;28(14):1679–86. Epub 2014/09/12. doi: 10.3109/14767058.2014.964675 . [DOI] [PubMed] [Google Scholar]
- 19.Martens DS, Plusquin M, Gyselaers W, De Vivo I, Nawrot TS. Maternal pre-pregnancy body mass index and newborn telomere length. BMC Med. 2016;14(1):148. Epub 2016/10/19. doi: 10.1186/s12916-016-0689-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spann MN, Scheinost D, Feng T, Barbato K, Lee S, Monk C, et al. Association of Maternal Prepregnancy Body Mass Index With Fetal Growth and Neonatal Thalamic Brain Connectivity Among Adolescent and Young Women. JAMA Netw Open. 2020;3(11):e2024661. Epub 2020/11/04. doi: 10.1001/jamanetworkopen.2020.24661 Institutes of Health outside the submitted work. No other disclosures were reported. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim Y, Han BG. Cohort Profile: The Korean Genome and Epidemiology Study (KoGES) Consortium. Int J Epidemiol. 2017;46(2):e20. Epub 2016/04/17. doi: 10.1093/ije/dyv316 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Health Examinees Study Group. The Health Examinees (HEXA) study: rationale, study design and baseline characteristics. Asian Pac J Cancer Prev. 2015;16(4):1591–7. doi: 10.7314/apjcp.2015.16.4.1591 [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. The Asia-Pacific perspective: redefining obesity and its treatment. 2000. [Google Scholar]
- 24.Bider-Canfield Z, Martinez M, Wang X, Yu W, Bautista M, Brookey J, et al. Maternal obesity, gestational diabetes, breastfeeding and childhood overweight at age 2 years. Pediatric Obesity. 2017;12(2):171–8. doi: 10.1111/ijpo.12125 [DOI] [PubMed] [Google Scholar]
- 25.Torloni M, Betran A, Horta B, Nakamura M, Atallah A, Moron A, et al. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obesity Reviews. 2009;10(2):194–203. doi: 10.1111/j.1467-789X.2008.00541.x [DOI] [PubMed] [Google Scholar]
- 26.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106(4):473–81. Epub 2000/08/23. doi: 10.1172/JCI10842 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reaven GM. Pathophysiology of insulin resistance in human disease. Physiol Rev. 1995;75(3):473–86. Epub 1995/07/01. doi: 10.1152/physrev.1995.75.3.473 . [DOI] [PubMed] [Google Scholar]
- 28.Ryder JW, Gilbert M, Zierath JR. Skeletal muscle and insulin sensitivity: pathophysiological alterations. Front Biosci. 2001;6:D154–63. Epub 2001/02/15. doi: 10.2741/ryder . [DOI] [PubMed] [Google Scholar]
- 29.Pendergrass M, Bertoldo A, Bonadonna R, Nucci G, Mandarino L, Cobelli C, et al. Muscle glucose transport and phosphorylation in type 2 diabetic, obese nondiabetic, and genetically predisposed individuals. Am J Physiol Endocrinol Metab. 2007;292(1):E92–100. Epub 2006/08/10. doi: 10.1152/ajpendo.00617.2005 . [DOI] [PubMed] [Google Scholar]
- 30.Czech MP. Insulin action and resistance in obesity and type 2 diabetes. Nat Med. 2017;23(7):804–14. Epub 2017/07/12. doi: 10.1038/nm.4350 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Day C, Bailey CJ. Obesity in the pathogenesis of type 2 diabetes. The British Journal of Diabetes & Vascular Disease. 2011;11(2):55–61. [Google Scholar]
- 32.Eckel RH, Kahn SE, Ferrannini E, Goldfine AB, Nathan DM, Schwartz MW, et al. Obesity and type 2 diabetes: what can be unified and what needs to be individualized? The Journal of Clinical Endocrinology & Metabolism. 2011;96(6):1654–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meguro S, Kabeya Y, Tanaka K, Kawai T, Tomita M, Katsuki T, et al. Past Obesity as well as Present Body Weight Status Is a Risk Factor for Diabetic Nephropathy. Int J Endocrinol. 2013;2013:590569. Epub 2013/09/26. doi: 10.1155/2013/590569 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ford ES, Williamson DF, Liu S. Weight change and diabetes incidence: findings from a national cohort of US adults. American journal of epidemiology. 1997;146(3):214–22. doi: 10.1093/oxfordjournals.aje.a009256 [DOI] [PubMed] [Google Scholar]
- 35.Park JY, Lee KU, Kim CH, Kim HK, Hong SK, Park KS, et al. Past and current obesity in Koreans with non-insulin-dependent diabetes mellitus. Diabetes Res Clin Pract. 1997;35(1):49–56. Epub 1997/02/01. doi: 10.1016/s0168-8227(96)01363-0 . [DOI] [PubMed] [Google Scholar]
- 36.Shin D, Chung H, Weatherspoon L, Song WO. Validity of prepregnancy weight status estimated from self-reported height and weight. Matern Child Health J. 2014;18(7):1667–74. Epub 2013/12/18. doi: 10.1007/s10995-013-1407-6 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data underlying the results of our study are not publicly available due to KoGES data policy. Data are available from the Division of Genetic Epidemiology and Health Index, NIH, Korea Centers for Disease Control and Prevention (contact via Mi-Jin Cho at whalwls0227@korea.kr) for researchers who meet the criteria for access to confidential data.