Abstract
Protein S-palmitoylation, or S-fatty-acylation, regulates many fundamental cellular processes in eukaryotes. Herein, we present a chemical fatty-acylation approach that involves site-specific incorporation of cycloalkyne-containing unnatural amino acids and subsequent bioorthogonal reactions with fatty-acyl tetrazines to install fatty-acylation mimics at target protein sites, allowing gain-of-function analysis of S-palmitoylation in live cells.
Graphical Abstract
Chemically installed fatty-acylation recapitulates the function of S-palmitoylation in regulating protein membrane affinity and signaling in live cells.
S-Palmitoylation, or S-fatty-acylation, is a ubiquitous protein post-translational modification (PTM) in eukaryotes that occurs on cysteines through covalent attachment of palmitic acids.1, 2 Previous proteomic studies have identified hundreds of S-palmitoylated proteins3–7 and suggested that as much as 10% of the human proteome is S-palmitoylated,8 highlighting the essential role of S-palmitoylation in eukaryotic biology. Indeed, S-palmitoylation is widely associated with various fundamental biological processes,9 ranging from cell growth to signaling transduction and immune response. The specific function of S-palmitoylation, however, remains less well understood.
A classical biological approach to study S-palmitoylation involves site-directed mutagenesis of the modification cysteine into serine or alanine to create a “loss-of-function” mutant. This method, however, only allows indirect analysis and cannot distinguish S-palmitoylation from other PTMs on cysteine. Access to protein variants with site-specific S-palmitoylation or mimics can be essential for direct and “gain-of-function” studies. In this regard, strategies have been developed to generate engineered proteins bearing surrogates for S-palmitoylation, as well as for other PTMs.10–12 For example, Ras variants containing S-palmitoylation mimics were semi-synthesized and microinjected into mammalian cells for investigating their intracellular trafficking.13–15 Another approach involves introduction of a unique tag, such as alkene and tetrazole, into the protein and subsequent chemoselective reactions to install S-palmitoylation mimics.16, 17 However, these strategies are both restricted to in vitro purified proteins and require microinjection for protein delivery. Moreover, the latter approach was only demonstrated on model green fluorescent protein (GFP). Therefore, new methods are needed to precisely install S-palmitoylation mimics at target sites of S-palmitoylated proteins in live cells for direct and gain-of-function analysis.
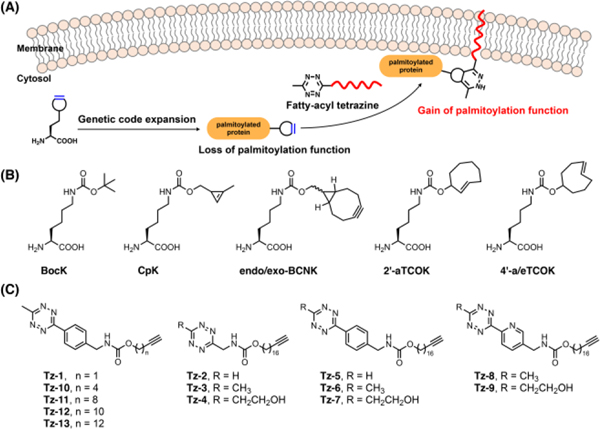
Recently, we and others have demonstrated that site-specific incorporation of strained alkynes or alkenes and inverse–electron–demand Diels–Alder cycloaddition (IEDAC) reactions18, 19 with tetrazine-functionalized fluorophores enable robust fluorescence labeling of proteins in live cells.20–25 We envisioned that the IEDAC reaction, with fast reaction rate (up to 106 M−1 s−1) and excellent biocompatibility in living systems,26 could be harnessed to introduce S-palmitoylation mimics onto proteins in live mammalian cells. For this purpose, a strained alkyne or alkene functionality is site-specifically incorporated into the target S-palmitoylation site via genetic code expansion technology,27, 28 which utilizes orthogonal aminoacyl-tRNA synthetase/tRNA pairs and alterative codons (e.g., TAG) to encode various unnatural amino acids (UAAs). A subsequent bioorthogonal IEDAC reaction of the strained alkyne or alkene with a fatty-acyl-containing tetrazine allows installation of the fatty-acylation mimic in live cells (Fig. 1A). Herein, we present the bioorthogonal chemical fatty-acylation approach to generate proteins with site-specific fatty-acyl groups as S-palmitoylation mimics for gain-of-function studies of S-palmitoylation in live cells.
Fig. 1.
Site-specific chemical fatty-acylation. (A) Schematic for the chemical fatty-acylation approach based on incorporation of bioorthogonal UAAs and reactions with fatty-acyl tetrazines. (B) Structures of UAAs and (C) fatty-acyl tetrazines used in this study.
To demonstrate the chemical fatty-acylation approach, we explored a series of strained alkyne or alkene-containing UAAs (Fig. 1B), namely cyclopropene-lysine (CpK),29 bicyclo[6.1.0]nonyne-lysine diastereomers (i.e., endo-BCNK and exo-BCNK),21, 30 axial trans-cyclooct-2-ene-lysine (2’-aTCOK),23, 31 and trans-cyclooct-4-ene-lysine axial and equatorial diastereomers (i.e., 4’-aTCOK and 4’-eTCOK),21, 32 all of which can be efficiently incorporated into proteins using variants of the pyrrolysyl-tRNA synthetase (PylRS)/Pyl-tRNA pair. Meanwhile, we synthesized a panel of fatty-acyl tetrazines (e.g., Tz-2–Tz-9; Fig. 1C and Scheme S1, ESI) with varying tetrazine structures and a long-chain fatty-acyl analogous moiety. To detect the fatty-acyl-protein conjugates, a terminal alkynyl group was included in the aliphatic chain for reaction with azide-functionalized rhodamine through Copper(I)-catalyzed Alkyne-Azide Cycloaddition (CuAAC).33 Additionally, a short-chain fatty-acyl analogue (i.e., Tz-1) was also prepared.
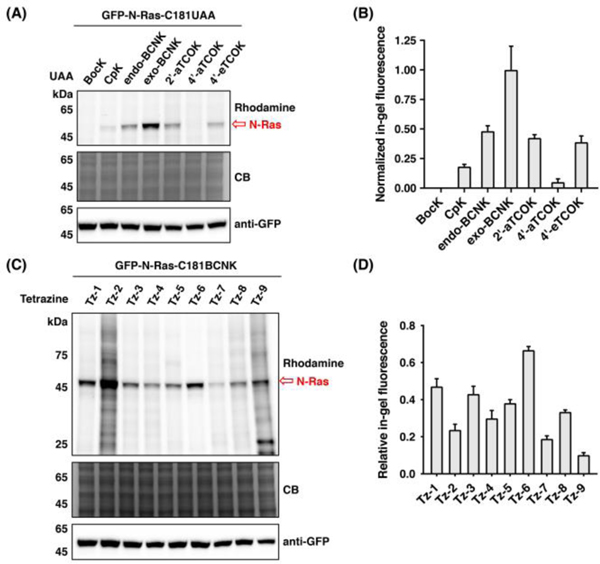
We first evaluated the bioorthogonal UAAs and fatty-acyl tetrazines for protein chemical fatty-acylation in live cells. For this purpose, we focused on N-Ras, which is a prominent S-palmitoylated protein and has only one S-palmitoylation site at cysteine 181, i.e., C181.34 A GFP-tagged N-Ras plasmid was therefore constructed with the C181 residue mutated to TAG. We also modified the previous PylRS/Pyl-tRNA plasmid35 by inserting a mutant of eukaryotic release factor 1 (i.e., RF1-E55D)35 under a CMV constitutive promoter (Fig. S1, ESI), and confirmed that this alteration improves UAA incorporation efficiency (Fig. S2). We then co-transfected HEK293T cells with constructs of GFP-N-Ras-C181TAG and RF1-E55D-PylRS/Pyl-tRNA in the presence of UAA (50 μM), and incubated the cells with fatty-acyl tetrazines (25 μM) in serum-containing media. After extensive washes and cell lysis, the lysates were reacted with azido-rhodamine via CuAAC and analyzed by in-gel fluorescence (Fig. 2). As CuAAC generally proceeds in high efficiency, the fluorescence intensity of N-Ras band is indicative of the efficiency for fatty-acyl-protein conjugation. Indeed, fluorescent bands at ~50 kDa were observed in the gel (Fig. 2A), suggesting that corresponding GFP-N-Ras-C181UAA proteins were modified by Tz-6. Side-by-side comparison of the UAAs demonstrated that exo-BCNK offers optimal efficiency for fatty-acyl-protein conjugation (Fig. 2A and 2B), largely in agreement with previous studies on fluorophore labeling.24, 25 GFP-N-Ras-C181TAG variant bearing an unreactive UAA, i.e., BocK, showed no fluorescence signal, confirming that the bioorthogonal reaction is required for formation of fatty-acyl-protein conjugate. We then proceeded to screen the fatty-acyl tetrazines using GFP-N-Ras with exo-BCNK at the C181 position (i.e., GFP-N-Ras-C181BCNK). In-gel fluorescence analysis showed that Tz-3 and Tz-6 were highly specific for modifying the protein with low to negligible non-specific fluorescence signals (Fig. 2C and 2D). It is worth noting that hydrogen-substituted (e.g., Tz-2 and Tz-5) and pyridyl-derived (e.g., Tz-8 and Tz-9) tetrazines, albeit more reactive,36 are generally less specific and efficient than methyl-substituted (e.g., Tz-3 and Tz-6) and phenyl-derived (e.g., Tz-6 and Tz-7) tetrazines, respectively, probably due to higher non-specific reactivity to proteins and lower stability in media.36 We also examined the fatty-acyl tetrazines using 2’-aTOCK-containing GFP-N-Ras (Fig. S3), and confirmed the high specificity of Tz-3 and Tz-6. Additionally, the concentrations of Tz-3 and Tz-6 were optimized to be 12.5 μM and 50 μM, respectively (Fig. S4). Time-dependent modification of N-Ras with Tz-6 was also investigated (Fig. S5). Together, these studies demonstrate chemical modification of UAA-containing N-Ras by fatty-acyl tetrazines in live cells and identify the optimal conditions.
Fig. 2.
In-gel fluorescence evaluation of UAAs and fatty-acyl tetrazines for chemical fatty-acylation of N-Ras in live cells. (A) Screening of UAAs for chemical fatty-acylation by Tz-6. (B) Quantification of N-Ras fluorescence in (A). Fluorescence intensities of N-Ras bands were quantified and normalized to anti-GFP levels. (C) Screening of fatty-acyl tetrazines for chemical fatty-acylation of GFP-N-Ras-C181BCNK. (D) Quantification of relative N-Ras fluorescence in (C). Fluorescence intensities of N-Ras bands were quantified and normalized to the overall lane intensities. Data are represented as mean ± s.d., n = 3.
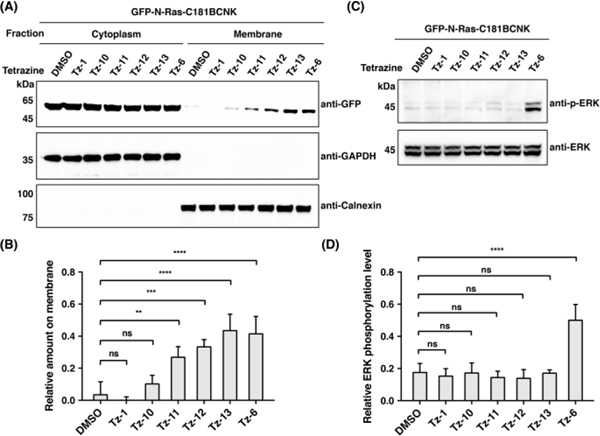
We then moved on to examine whether the chemically introduced fatty-acyl moiety on N-Ras could mimic S- palmitoylation to control protein localization and signaling. To this end, HEK293T cells were transfected to express GFP-N-Ras-C181BCNK and labeled with fatty-acyl tetrazines (i.e., Tz-1, Tz-3, and Tz-6). Cells were lysed and fractionated to isolate membrane and cytoplasm proteins. Western blot and in-gel fluorescence analyses showed that Tz-3 or Tz-6 modified N-Ras was significantly more localized in the membrane fractions, compared to unmodified or Tz-1 modified N-Ras (Fig. 3A, 3B, S6, and S7), indicating that chemical fatty-acylation enhances N-Ras membrane affinity. Time-course analysis suggested that at least 1 h labeling with Tz-6 was required for N-Ras relocation to membrane (Fig. S8). We also performed confocal fluorescence imaging to assess N-Ras localization in live cells, and observed that N-Ras was redistributed from intracellular components to plasma membrane upon modification by Tz-6 (Fig. S9), in line with the fractionation results. In addition, we checked the phosphorylation status of extracellular-signal-regulated kinase (ERK), a known effector regulated by Ras in the classical Ras/ERK signaling pathway,37 upon chemical fatty-acylation of N-Ras. Western blot analysis of the aforementioned HEK293T cell lysates indicated that modification of N-Ras with Tz-3 or Tz-6 can dramatically activate ERK phosphorylation (Fig. 3C, 3D, and S10). Notably, our fractionation and ERK phosphorylation results on chemically fatty-acylated N-Ras are consistent with those on N-Ras wild-type versus C181S mutant (Fig. S11),38 thus validating the chemical fatty-acylation approach. Together, these results demonstrate that chemically installed fatty-acylation on N-Ras is functionally active as an S-palmitoylation mimic to regulate N-Ras localization and signaling.
Fig. 3.
Effects of chemical fatty-acylation on N-Ras membrane affinity and signaling. (A) HEK293T cells expressing GFP-N-Ras-C181BCNK were incubated with indicated fatty-acyl tetrazines and lysed to separate membrane and cytoplasm fractions. GAPDH and calnexin were probed as cytoplasm and membrane markers, respectively. (B) Quantification of N-Ras on membrane relative to in cytoplasm shown in (A). (C) Same cells as in (A) were lysed and analyzed for phosphorylated ERK. (D) Quantification of ERK phosphorylation relative to total ERK shown in (C). Data are represented as mean ± s.d., n = 3. *** and **** indicate p-values <0.001 and 0.0001, respectively, and ns indicates a p-value >0.05, calculated by One-way ANOVA test.
We next sought to apply the approach to explore how fatty-acyl chain length on N-Ras affects its function, which has not been possible with traditional mutagenesis method. We thus prepared a set of Tz-6 analogues (i.e., Tz-10–Tz-13) with varying fatty-acyl chain lengths (Fig. 1C and Scheme S1). In-gel fluorescence confirmed that they specifically modified GFP-N-Ras-C181BCNK in live cells (Fig. S12). Fractionation experiment indicated that both medium-chain and long-chain fatty-acylation (e.g., Tz-11–Tz-13 and Tz-6) substantially increased membrane affinity of N-Ras, while short-chain fatty-acylation (e.g., Tz-1 and Tz-10) did not (Fig. 4A and 4B), correlating with hydrophobicity of the modification (Fig. S13). Interestingly, confocal fluorescence imaging revealed that Tz-12- or Tz-13-modified N-Ras was not obviously localized to plasma membrane (Fig. S14), indicating that N-Ras protein in the membrane fraction may be due to localization to and/or post-lysis association with internal membranes. Consistent with these results, only chemical fatty-acylation with Tz-6, but not Tz-12 and Tz-13, activated ERK phosphorylation (Fig. 4C and 4D), indicating that chain length of the fatty-acyl group specifically determines N-Ras cellular localization and signaling. In this regard, effector proteins that specifically recognize the fatty-acyl group may be involved. To the best of our knowledge, this is the first example to unambiguously analyze how fatty-acyl composition affects the function of fatty-acylation.
Fig. 4.
Effects of fatty-acyl chain length on N-Ras membrane affinity and signaling. (A) HEK293T cells expressing GFP-N-Ras-C181BCNK were incubated with indicated fatty-acyl tetrazines and lysed to separate membrane and cytoplasm fractions. (B) Quantification of N-Ras on membrane relative to in cytoplasm shown in (A). (C) Same cells as in (A) were lysed and analyzed for phosphorylated ERK. (D) Quantification of ERK phosphorylation relative to total ERK shown in (C). Data are represented as mean ± s.d., n = 3. **, ***, and **** indicate p-values <0.01, 0.001, and 0.0001, respectively, and ns indicates a p-value >0.05, calculated by One-way ANOVA test.
To broaden the applicability of this approach, we pursued our investigation on H-Ras, which is dually S-palmitoylated at cysteine 181 and 184.34 For functional study of individual S-palmitoylation sites, we constructed two HA-tagged H-Ras plasmids with one cysteine mutated to TAG and the other to serine, i.e., HA-H-Ras-C181TAG or C184TAG. Both H-Ras variants expressed in the presence of exo-BCNK were specifically modified by Tz-6, as revealed by in-gel fluorescence (Fig. S15). In the fractionation assay, we observed that chemical fatty-acylation with Tz-6 on H-Ras C181 or C184 increased membrane affinity of the protein (Fig. 5A and 5B). However, only chemical fatty-acylation on C184 triggered detectable ERK phosphorylation (Fig. 5C and 5D), largely consistent with the results on H-Ras Cys mutants (Fig. S16). These data thus demonstrate that the chemical fatty-acylation approach allows site-specific functional analysis of individual S-palmitoylation sites with distinct activities.
Fig. 5.
Site-specific analysis of individual S-palmitoylation sites on H-Ras with chemical fatty-acylation. (A) HEK293T cells expressing HA-H-Ras-C181BCNK or HA-H-Ras-C184BCNK were labeled with Tz-6 and lysed to separate membrane and cytoplasm fractions. (B) Quantification of H-Ras on membrane relative to in cytoplasm shown in (A). (C) Same cells as in (A) were lysed and analyzed for phosphorylated ERK. (D) Quantification of ERK phosphorylation relative to total ERK shown in (C). Data are represented as mean ± s.d., n = 3. ** and *** indicate p-values <0.01 and 0.001, respectively, and ns indicates a p-value >0.05, calculated by Two-way ANOVA test.
In summary, we report a bioorthogonal chemical fatty-acylation approach to site-specifically install S-palmitoylation mimics into a target protein site in live cells. We show that the chemical fatty-acylation recapitulates the function of S-palmitoylation in regulating protein distribution and signaling, and enables direct investigation of the effects of fatty-acyl structures on S-palmitoylation function for the first time, as well as site-specific analysis of individual S-palmitoylation sites. With several unique features, such as gain-of-function and site-specificity, irreversible and variable modification, live-cell compatibility, and no requirement of microinjection, the bioorthogonal chemical fatty-acylation approach should afford new opportunities to precisely manipulate and study S-palmitoylation and other types of fatty-acylation in live cells.
Supplementary Material
Acknowledgments
This work is supported by NIH-NIGMS R01M087544 (to H.C.H.), National Natural Science Foundation of China Grant 21778010 (to T.P.), and Shenzhen Science and Technology Innovation Committee Grant JCYJ20170412150832022 (to T.P.).
Footnotes
Electronic Supplementary Information (ESI) available: Supplemental figures and experimental methods. See DOI: 10.1039/x0xx00000x
Conflicts of interest
The authors declare no conflicts of interests.
Notes and references
- 1.Linder ME and Deschenes RJ, Nat. Rev. Mol. Cell Biol, 2007, 8, 74–84. [DOI] [PubMed] [Google Scholar]
- 2.Zaballa M-E and van der Goot FG, Crit. Rev. Biochem. Mol. Biol, 2018, 53, 420–451. [DOI] [PubMed] [Google Scholar]
- 3.Roth AF, Wan J, Bailey AO, Sun B, Kuchar JA, Green WN, Phinney BS, Yates JR and Davis NG, Cell, 2006, 125, 1003–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang R, Wan J, Arstikaitis P, Takahashi H, Huang K, Bailey AO, Thompson JX, Roth AF, Drisdel RC, Mastro R, Green WN, Yates Iii JR, Davis NG and El-Husseini A, Nature, 2008, 456, 904–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin BR, Wang C, Adibekian A, Tully SE and Cravatt BF, Nat. Methods, 2012, 9, 84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yount JS, Moltedo B, Yang Y-Y, Charron G, Moran TM, López CB and Hang HC, Nat. Chem. Biol, 2010, 6, 610–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng T, Thinon E. and Hang HC, Curr. Opin. Chem. Biol, 2016, 30, 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blanc M, David F, Abrami L, Migliozzi D, Armand F, B¸rgi J. and van der Goot F, F1000Research, 2015, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De I. and Sadhukhan S, Eur. J. Cell Biol, 2018, 97, 319–338. [DOI] [PubMed] [Google Scholar]
- 10.Davis BG, Science, 2004, 303, 480–482. [DOI] [PubMed] [Google Scholar]
- 11.Chalker JM, Bernardes GJL and Davis BG, Acc. Chem. Res, 2011, 44, 730–741. [DOI] [PubMed] [Google Scholar]
- 12.Wright TH, Vallée MRJ and Davis BG, Angew. Chem., Int. Ed, 2016, 55, 5896–5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bader B, Kuhn K, Owen DJ, Waldmann H, Wittinghofer A. and Kuhlmann J, Nature, 2000, 403, 223–226. [DOI] [PubMed] [Google Scholar]
- 14.Rocks O, Peyker A, Kahms M, Verveer PJ, Koerner C, Lumbierres M, Kuhlmann J, Waldmann H, Wittinghofer A. and Bastiaens PIH, Science, 2005, 307, 1746–1752. [DOI] [PubMed] [Google Scholar]
- 15.Brunsveld L, Kuhlmann J, Alexandrov K, Wittinghofer A, Goody RS and Waldmann H, Angew. Chem., Int. Ed, 2006, 45, 6622–6646. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Schiller SM and Schultz PG, Angew. Chem., Int. Ed, 2007, 46, 6849–6851. [DOI] [PubMed] [Google Scholar]
- 17.Song W, Yu Z, Madden MM and Lin Q, Mol. BioSyst, 2010, 6, 1576–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blackman ML, Royzen M. and Fox JM, J. Am. Chem. Soc, 2008, 130, 13518–13519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devaraj NK, Weissleder R. and Hilderbrand SA, Bioconjug. Chem, 2008, 19, 2297–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lang K, Davis L, Torres-Kolbus J, Chou C, Deiters A. and Chin JW, Nat. Chem, 2012, 4, 298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lang K, Davis L, Wallace S, Mahesh M, Cox DJ, Blackman ML, Fox JM and Chin JW, J. Am. Chem. Soc, 2012, 134, 10317–10320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lang K. and Chin JW, Chem. Rev, 2014, 114, 4764–4806. [DOI] [PubMed] [Google Scholar]
- 23.Nikić I, Plass T, Schraidt O, Szymański J, Briggs JAG, Schultz C. and Lemke EA, Angew. Chem., Int. Ed, 2014, 53, 2245–2249. [DOI] [PubMed] [Google Scholar]
- 24.Nikić I, Kang JH, Girona GE, Aramburu IV and Lemke EA, Nat. Protocols, 2015, 10, 780–791. [DOI] [PubMed] [Google Scholar]
- 25.Peng T. and Hang HC, J. Am. Chem. Soc, 2016, 138, 14423–14433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliveira BL, Guo Z. and Bernardes GJL, Chem. Soc. Rev, 2017, 46, 4895–4950. [DOI] [PubMed] [Google Scholar]
- 27.Chin JW, Nature, 2017, 550, 53. [DOI] [PubMed] [Google Scholar]
- 28.Young DD and Schultz PG, ACS Chem. Biol, 2018, 13, 854–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elliott TS, Townsley FM, Bianco A, Ernst RJ, Sachdeva A, Elsasser SJ, Davis L, Lang K, Pisa R, Greiss S, Lilley KS and Chin JW, Nat. Biotech, 2014, 32, 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borrmann A, Milles S, Plass T, Dommerholt J, Verkade JMM, Wießler M, Schultz C, van Hest JCM, van Delft FL and Lemke EA, ChemBioChem, 2012, 13, 2094–2099. [DOI] [PubMed] [Google Scholar]
- 31.Li J, Jia S. and Chen PR, Nat. Chem. Biol, 2014, 10, 1003–1005. [DOI] [PubMed] [Google Scholar]
- 32.Plass T, Milles S, Koehler C, Szymański J, Mueller R, Wießler M, Schultz C. and Lemke EA, Angew. Chem., Int. Ed, 2012, 51, 4166–4170. [DOI] [PubMed] [Google Scholar]
- 33.Rostovtsev VV, Green LG, Fokin VV and Sharpless KB, Angew. Chem., Int. Ed, 2002, 41, 2596–2599. [DOI] [PubMed] [Google Scholar]
- 34.Ahearn IM, Haigis K, Bar-Sagi D. and Philips MR, Nat. Rev. Mol. Cell Biol, 2012, 13, 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmied WH, Elsässer SJ, Uttamapinant C. and Chin JW, J. Am. Chem. Soc, 2014, 136, 15577–15583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karver MR, Weissleder R. and Hilderbrand SA, Bioconjug. Chem, 2011, 22, 2263–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajalingam K, Schreck R, Rapp UR and Albert Š, Biochim. Biophys. Acta Mol. Cell Res, 2007, 1773, 1177–1195. [DOI] [PubMed] [Google Scholar]
- 38.Xu J, Hedberg C, Dekker FJ, Li Q, Haigis KM, Hwang E, Waldmann H. and Shannon K, Blood, 2012, 119, 1032–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.