Supplemental Digital Content is available in the text.
Keywords: biomarkers, chest pain, myocardial infarction, randomized controlled trial, troponin
Abstract
Background:
High-sensitivity cardiac troponin assays enable myocardial infarction to be ruled out earlier, but the safety and efficacy of this approach is uncertain. We investigated whether an early rule-out pathway is safe and effective for patients with suspected acute coronary syndrome.
Methods:
We performed a stepped-wedge cluster randomized controlled trial in the emergency departments of 7 acute care hospitals in Scotland. Consecutive patients presenting with suspected acute coronary syndrome between December 2014 and December 2016 were included. Sites were randomized to implement an early rule-out pathway where myocardial infarction was excluded if high-sensitivity cardiac troponin I concentrations were <5 ng/L at presentation. During a previous validation phase, myocardial infarction was ruled out when troponin concentrations were <99th percentile at 6 to 12 hours after symptom onset. The coprimary outcome was length of stay (efficacy) and myocardial infarction or cardiac death after discharge at 30 days (safety). Patients were followed for 1 year to evaluate safety and other secondary outcomes.
Results:
We enrolled 31 492 patients (59±17 years of age [mean±SD]; 45% women) with troponin concentrations <99th percentile at presentation. Length of stay was reduced from 10.1±4.1 to 6.8±3.9 hours (adjusted geometric mean ratio, 0.78 [95% CI, 0.73–0.83]; P<0.001) after implementation and the proportion of patients discharged increased from 50% to 71% (adjusted odds ratio, 1.59 [95% CI, 1.45–1.75]). Noninferiority was not demonstrated for the 30-day safety outcome (upper limit of 1-sided 95% CI for adjusted risk difference, 0.70% [noninferiority margin 0.50%]; P=0.068), but the observed differences favored the early rule-out pathway (0.4% [57/14 700] versus 0.3% [56/16 792]). At 1 year, the safety outcome occurred in 2.7% (396/14 700) and 1.8% (307/16 792) of patients before and after implementation (adjusted odds ratio, 1.02 [95% CI, 0.74–1.40]; P=0.894), and there were no differences in hospital reattendance or all-cause mortality.
Conclusions:
Implementation of an early rule-out pathway for myocardial infarction reduced length of stay and hospital admission. Although noninferiority for the safety outcome was not demonstrated at 30 days, there was no increase in cardiac events at 1 year. Adoption of this pathway would have major benefits for patients and health care providers.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03005158.
Clinical Perspective.
What Is New?
Patients with suspected acute coronary syndrome frequently attend emergency departments, but the majority do not have myocardial infarction.
Among 31 492 consecutive patients presenting to 7 hospitals, implementation of an early rule-out pathway for patients with suspected acute coronary syndrome reduced length of stay by 3.3 hours and hospital admissions by 59%.
Noninferiority was not demonstrated, but observed differences in myocardial infarction or cardiac death at 30 days and 1 year favored the early rule-out pathway over standard care.
What Are the Clinical Implications?
Existing early rule-out pathways for myocardial infarction are largely based on observational studies or small trials of selected patients.
This trial provides evidence in consecutive patients with suspected acute coronary syndrome that the use of an early rule-out pathway is both safe and effective.
Adoption of an early rule-out pathway for myocardial infarction would have major benefits for both patients and health care providers.
Editorial, see p 2225
There are >20 million presentations of suspected acute coronary syndrome (ACS) each year in the United States alone,1 accounting for up to 10% of hospital visits and 40% of unscheduled admissions.2 Given that most patients do not have myocardial infarction (MI),3 the adoption of effective and safe pathways to rule out MI in the emergency department and avoid hospital admission would have a major effect on patient care and health care provision.
Cardiac troponin testing is an integral component of the assessment of patients with suspected ACS, with guidelines recommending serial testing to rule in and rule out MI.4 The development of high-sensitivity cardiac troponin assays with enhanced precision at very low concentrations permits quantification well below the 99th percentile diagnostic threshold for MI.5 This advance has led to innovative pathways to rule out MI more rapidly, either at presentation or within 3 hours, that have been incorporated into clinical practice guidelines.6–14 However, these studies were observational, and there are few examples where the pathway guided patient care.15,16 The majority were modest in size, or enrolled selected low-risk patients, and therefore the true efficacy and safety of introducing these pathways into clinical practice remains uncertain.
Our aim was to determine the efficacy and safety of implementing an accelerated pathway where high-sensitivity cardiac troponin testing is used to rule out MI at presentation in consecutive patients with suspected ACS.
Methods
Trial Design and Oversight
HiSTORIC (High-Sensitivity Cardiac Troponin on Presentation to Rule Out Myocardial Infarction) is a stepped-wedge cluster randomized controlled trial enrolling consecutive patients with suspected ACS across 7 acute care hospitals in Scotland. In this trial, the hospital site was the unit of randomization and therefore individual patient consent was not sought. Cluster randomization was necessary to avoid the risk of clinical error attributable to simultaneous use of different diagnostic pathways. The trial was approved by the Scotland A Research Ethics Committee and the conduct of the trial was periodically reviewed by an independent trial steering committee. All data were collected from the patient record and national registries, deidentified, and linked in a data repository (DataLoch, Edinburgh, United Kingdom) within secure National Health Service safe havens.17
Trial Population
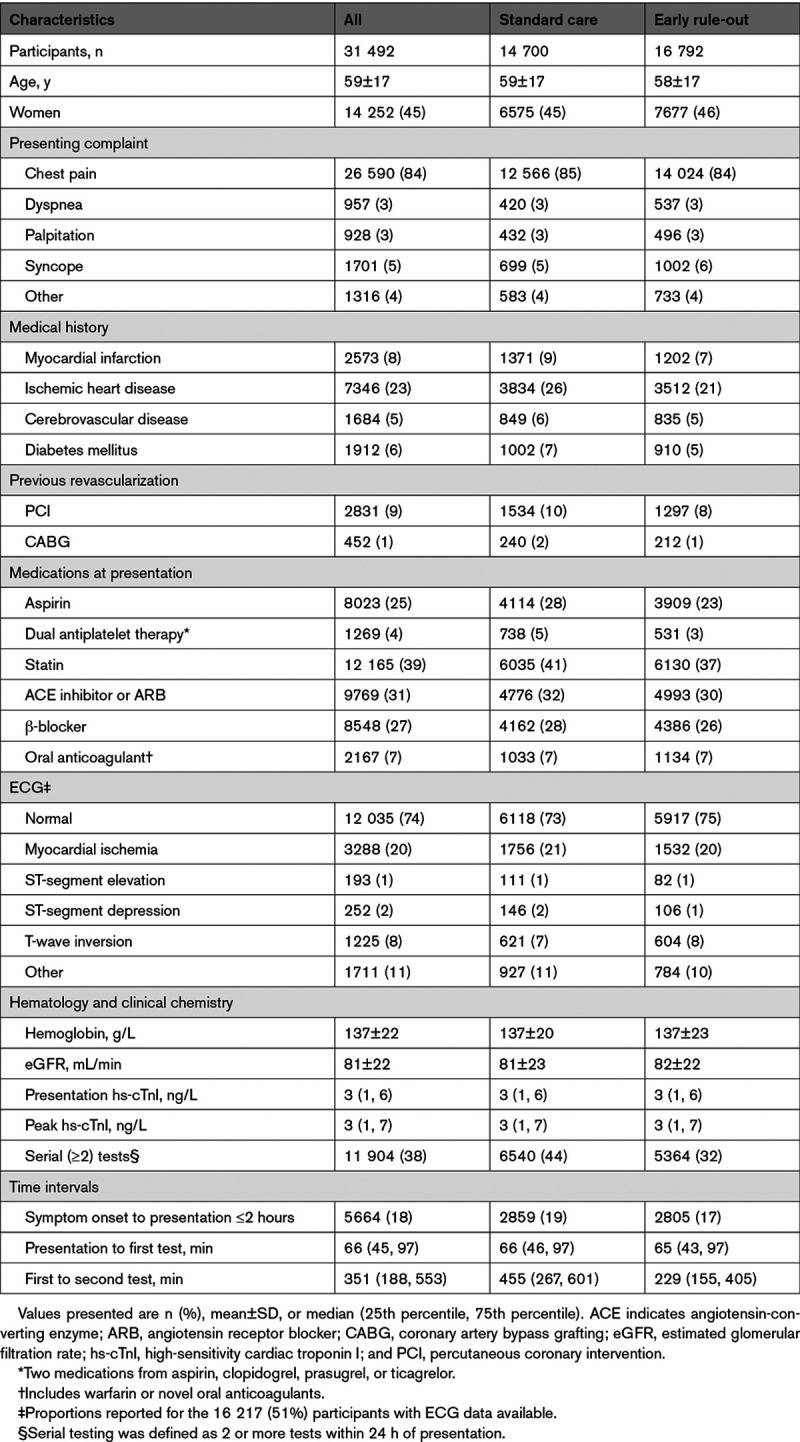
Sites were eligible if they had the capacity to introduce the early rule-out pathway and returned data to the national registry. All patients were identified by the attending clinician using an electronic form integrated into the care pathway at the time troponin was requested. Patients were eligible for inclusion if they presented to the emergency department or acute medical receiving unit with suspected ACS and had a high-sensitivity cardiac troponin I concentration within the normal reference range (less than the sex-specific 99th percentile upper reference limit) at presentation. Patients were excluded if they presented with an out-of-hospital cardiac arrest or ST-segment–elevation MI, had been admitted previously during the trial, or were not resident in Scotland.
Randomization
The trial was conducted across 3 phases (Figure 1A). During all phases of the trial, a high-sensitivity cardiac troponin I assay with sex-specific 99th percentile thresholds was used to guide care and rule in MI according to the Universal Definition of Myocardial Infarction.4 During a validation phase of 6 to 9 months, troponin testing was performed at presentation and repeated 6 to 12 hours after the onset of symptoms if indicated (standard care). In accordance with guidelines at the time of enrollment,4,18 MI was ruled out when high-sensitivity cardiac troponin concentrations were <99th percentile at presentation if symptom onset was >6 hours from presentation or after serial testing 6 to 12 hours from symptom onset in those presenting earlier. Sites were paired on the basis of the expected number of patients and randomized to implement the early rule-out pathway (intervention) in 1 of 3 steps during a 6-month randomization phase. All sites completed an implementation phase of 6 to 9 months calendar-matched to the validation phase where care was guided by the early rule-out pathway.
Figure 1.
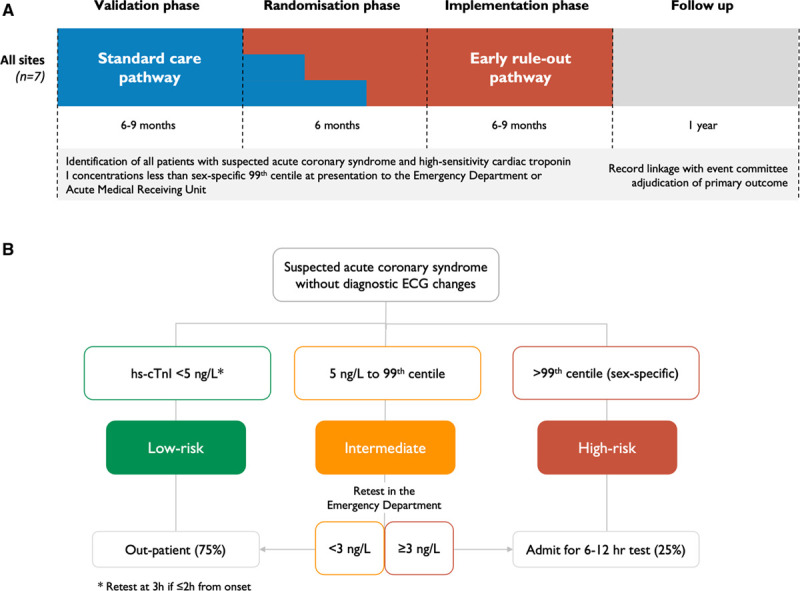
Schematic diagram of the HiSTORIC trial design and the early rule-out pathway. A, A high-sensitivity cardiac troponin I assay with sex-specific 99th percentile thresholds was used to guide care and rule in myocardial infarction during all phases of the trial. During a validation phase of at least 6 months, cardiac troponin testing was performed at presentation and was repeated 6 or 12 hours after the onset of symptoms if indicated. Myocardial infarction was ruled out when high-sensitivity cardiac troponin concentrations were <99th percentile at presentation if symptom onset was >6 hours from presentation or after serial testing 6 to 12 hours from symptom onset in those presenting earlier (standard care). Sites were paired on the basis of the expected number of patients and randomized to implement the early rule-out pathway (intervention) in 1 of 3 steps during a 6-month randomization phase. All sites completed an implementation phase of at least 6 months that was calendar-matched to the validation phase where patient care was guided by the early rule-out pathway. B, The early rule-out pathway rules out myocardial infarction at presentation in patients with cardiac troponin concentrations below a risk stratification threshold of 5 ng/L, unless they presented within 2 hours of symptom onset when testing was repeated 3 hours from presentation. Patients with cardiac troponin concentrations ≥5 ng/L at presentation are retested in the emergency department 3 hours after presentation and myocardial infarction is ruled out if concentrations are unchanged (Δ <3 ng/L) and remain <99th percentile diagnostic threshold. HiSTORIC indicates High-Sensitivity Cardiac Troponin on Presentation to Rule Out Myocardial Infarction.
Intervention
The High-STEACS (High-Sensitivity Troponin in the Evaluation of Patients With Suspected Acute Coronary Syndrome) early rule-out pathway (Figure 1B) has been described previously.19,20 MI is ruled out in patients with troponin concentrations <5 ng/L at presentation unless they present within 2 hours of symptom onset and testing is repeated 3 hours after presentation. Patients with troponin concentrations ≥5 ng/L at presentation are retested 3 hours after presentation and MI is ruled out if concentrations are unchanged (Δ <3 ng/L) and remain <99th percentile. To support implementation, we provided educational material and presentations at each site, a Web app (www.highsteacs.com), and formal training for clinical staff in the emergency department (Supplemental Methods in the Data Supplement). Throughout the trial, all sites used the Abbott Architect STAT high-sensitive troponin I assay to guide clinical decisions. This assay has an interassay coefficient of variation of <10% at 4.7 ng/L8,21 and a 99th percentile of 16 ng/L in women and 34 ng/L in men.22
Trial Outcomes
We used regional and national registries to follow up the trial population.17,23,24 Sequential hypothesis testing evaluated 2 coprimary outcomes in an a priori–defined hierarchical order: the primary efficacy outcome followed by the primary safety outcome. The primary efficacy outcome was length of stay, defined as the length of time from presentation to the emergency department until discharge from hospital. The safety outcome was MI (type 1, type 4b, or type 4c) or cardiac death after discharge, which was evaluated at 30 days (primary) and 1 year (secondary) after presentation. These events were adjudicated by a panel blind to the study phase. All subsequent presentations in which any troponin concentration was >99th percentile were reviewed and adjudicated as described previously (Expanded Methods in the Data Supplement).17,25,26
The secondary efficacy outcome measure was the proportion of patients discharged from the emergency department. Other safety outcome measures included MI, cardiac death, cardiovascular death, all-cause death, unplanned coronary revascularization, and reattendances for any reason after discharge at 1 year. Adherence was evaluated for 3 prespecified components of the early rule-out pathway (Expanded Methods in the Data Supplement).
Statistical Analysis
The primary efficacy outcome was analyzed using a linear mixed-effects regression model, adjusting for hospital site (random effect), season, time of presentation since the start of the study, and an indicator variable for whether the early rule-out pathway had been implemented. The primary safety outcome was analyzed using a logistic mixed-effects regression model adjusting for the same covariates. For the primary efficacy analysis, length of stay was log-transformed before analysis and results expressed as a geometric mean ratio. If the analysis of the primary efficacy outcome was significant at the 5% level, then we planned to perform a noninferiority analysis of the primary safety outcome reporting a risk difference (intervention – standard care) and a 1-sided 95% CI. If the upper limit of the 1-sided 95% CI was below a 0.5% noninferiority margin, noninferiority was established; if it was below 0%, superiority was established. A sensitivity analysis compared outcomes during the calendar-matched period in the validation and implementation phases using the same regression model as for the primary analysis but without adjustment for time or season. A number of other sensitivity analyses were performed (Expanded Methods in the Data Supplement).
Patient and Public Involvement
A patient review panel was consulted throughout the trial program and provided input on the educational advice provided to clinicians after introduction of the new pathway. Qualitative research capturing the views and experiences of patients treated within these pathways will follow in a separate publication. Patients were not involved in the conception or design of the trial.
Data Sharing
The HiSTORIC trial makes use of multiple routine electronic health care data sources that are linked, deidentified, and held in the National Health Service national safe haven, which is accessible by approved individuals who have undertaken the necessary governance training. Summary data can be made available on request to the corresponding author.
Results
Trial Sites and Population
Seven acute care hospitals were eligible, and all participated (Table I in the Data Supplement). Between December 2014 and December 2016, a total of 31 492 consecutive patients with suspected ACS (59±17 years [mean±SD]; 45% women) met the inclusion criteria (Figure 2). There were 14 700 (47%) and 16 792 (53%) patients assessed before and after implementation of the early rule-out pathway, respectively. Clinical characteristics were similar before and after implementation (Table 1) and across all 3 phases of the trial (Table II in the Data Supplement). The trial concluded in December 2017 with 1 year of follow-up available in 31 428 (99.8%) patients.
Figure 2.
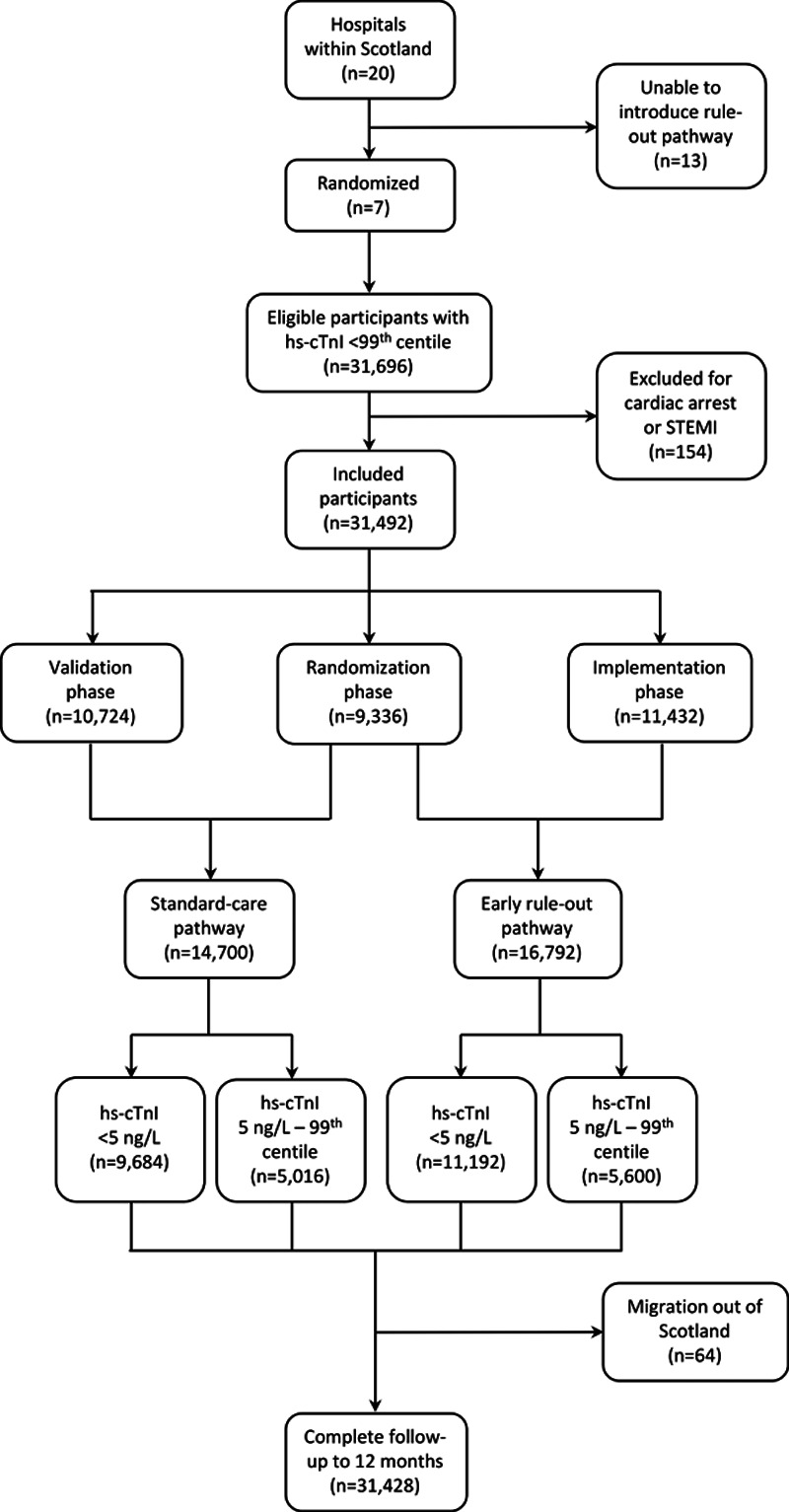
The HiSTORIC trial Consolidated Standards of Reporting Trials diagram. HiSTORIC indicates High-Sensitivity Cardiac Troponin on Presentation to Rule Out Myocardial Infarction; and hs-cTnI, high-sensitivity cardiac troponin I.
Table 1.
Characteristics of the Trial Participants
Primary and Secondary Efficacy Outcomes
Length of stay was reduced from 10.1±4.1 to 6.8±3.9 hours (adjusted geometric mean ratio, 0.78 [95% CI, 0.73–0.83]; P<0.001) after implementation of the early rule-out pathway (Table 2 and Figure 3). The proportion of patients discharged from the emergency department without hospital admission increased from 50% to 71% (adjusted odds ratio, 1.59 [95% CI, 1.45–1.75]). Adherence to all 3 prespecified components of the early rule-out pathway was observed in 11 600/16 792 (69%) patients.
Table 2.
Efficacy and Safety Outcomes at 30 Days and 1 Year
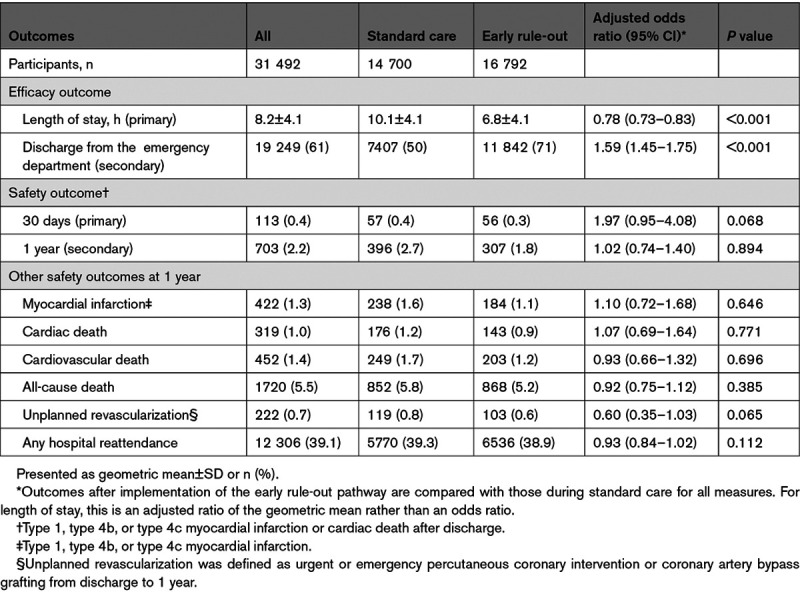
Figure 3.
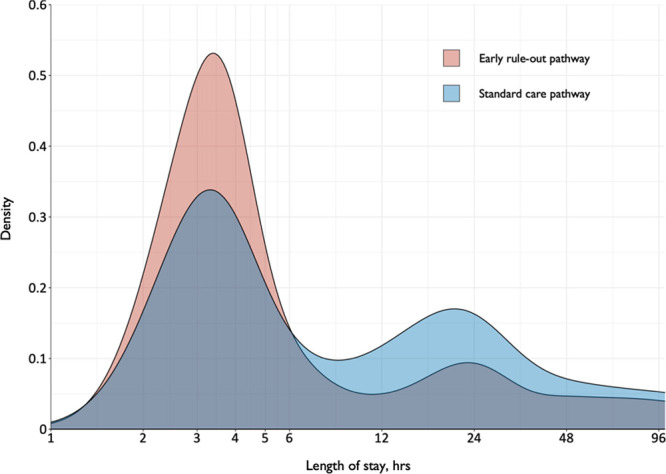
Length of stay before and after implementation of the early rule-out pathway. Shown is a density plot of the length of stay in patients evaluated before (blue) and after (red) implementation of the early rule-out pathway. hs-cTnI indicates high-sensitivity cardiac troponin I; and STEMI, ST-segment–elevation myocardial infarction.
Primary and Secondary Safety Outcomes
Before and after implementation of the early rule-out pathway, the primary safety outcome of MI or cardiac death after discharge at 30 days occurred in 57/14 700 (0.4%) and 56/16 792 (0.3%) patients, respectively (Table 2), with an adjusted odds ratio of 1.97 (95% CI, 0.95–4.08; P=0.068). Comparing the rate of the primary safety outcome after implementation with the rate before implementation, the upper limit of our 1-sided 95% CI for the adjusted risk difference was 0.70%, exceeding our prespecified noninferiority margin of 0.50%. The event rate at 30 days was lower than anticipated and our regression model and prespecified sensitivity analyses gave divergent results (Table III in the Data Supplement). However, there were 703 (2.2%) patients with MI or cardiac death after discharge at 1 year (Figure 4). Before and after implementation, the secondary safety outcome measure occurred in 396/14 700 (2.7%) and 307/16 792 (1.8%) patients, respectively (adjusted odds ratio, 1.02 [95% CI, 0.74–1.40]; P=0.894). This comprised 238 (1.6%) patients with MI and 176 cardiac deaths (1.2%) during standard care, versus 184 (1.1%) patients with MI and 143 (0.9%) cardiac deaths after implementation of the early rule-out pathway. The rate of all other safety outcome measures at 1 year did not differ before and after implementation (Table 2). The findings were consistent in a post hoc analysis of the safety outcome that included type 2 MI events (Table IV and Figure I in the Data Supplement).
Figure 4.
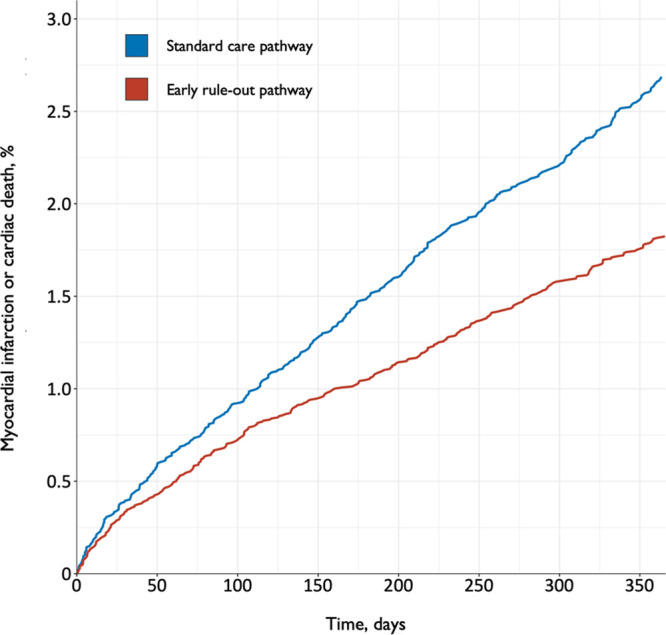
Myocardial infarction or cardiac death after discharge before and after implementation of the early rule-out pathway. Shown are cumulative incidence time-to-event curves for the primary safety outcome of myocardial infarction or cardiac death for patients evaluated before (blue line) and after (red line) implementation of the early rule-out pathway.
Sensitivity Analysis in Calendar-Matched Validation and Implementation Phases
In total, 18 241 (58%) patients attended the calendar-matched phases, with 8673 (48%) and 9568 (52%) evaluated during the validation and implementation phase, respectively (Table III in the Data Supplement). Length of stay was reduced from 10.6±4.1 to 6.8±4.0 hours (adjusted geometric mean ratio, 0.65 [95% CI, 0.62–0.68]) before and after implementation of the early rule-out pathway. The primary safety outcome occurred in 43/8673 (0.5%) and 23/9568 (0.2%) patients at 30 days, with an adjusted odds ratio of 0.48 (95% CI, 0.29–0.80; P=0.005). The upper limit of our 1-sided 95% CI for the adjusted risk difference was –0.13%, which was below our superiority margin of 0%. The secondary safety outcome occurred in 251/8673 (2.9%) and 161/9568 (1.7%) patients at 1 year (adjusted odds ratio, 0.58 [95% CI, 0.47–0.71]; P<0.001).
Discussion
We evaluated the efficacy and safety of implementing an early rule-out pathway in 31 492 consecutive patients presenting with suspected ACS to the emergency department or acute medical receiving unit. Introducing the pathway into clinical practice reduced length of stay by 3.3 hours and increased the odds of patients avoiding hospital admission by 59%. Noninferiority was not formally demonstrated, but the observed differences in MI or cardiac death after discharge favored the early rule-out pathway.
Our pragmatic trial design has several strengths. First, we embedded our screening tool into the patient record to ensure that we prospectively enrolled consecutive patients in whom the attending clinician suspected ACS. This minimized the risk of selection bias, ensuring that we did not limit our findings to low-risk patients or those presenting within working hours. Second, because the intervention was implemented at the hospital level, we did not seek individual patient consent. This reduced the risk of a Hawthorne effect, where effectiveness is exaggerated through direct observation of clinical care by researchers. Third, our trial population was larger than the combined number of patients enrolled in 30 previous observational studies.27,28 This ensured we had a greater number of events to evaluate safety. Last, we combined hospital-level data with established registries to ensure follow-up was complete in 99.8% of participants and that our panel was able to adjudicate all safety outcome events.
The High-STEACS early rule-out pathway, which determines whether a patient with suspected ACS requires hospital admission or can be safely discharged, is based on 3 principles. First, patients with very low troponin concentrations are at low risk of cardiac events.6 We defined the optimal risk stratification threshold as the highest concentration that gave a negative predictive value of >99.5% for MI or cardiac death at 30 days8,27 to maximize the effectiveness of this approach while maintaining safety. Second, increasing concentrations above this risk stratification threshold on repeat testing may be important, even if they remain within the normal reference range, and these patients require admission to measure peak troponin concentration.19 We define this using a change in concentration of ≥3 ng/L, based on the lowest measurable change that exceeds biological and analytic variation.29 Third, to ensure our pathway is consistent with international guidelines,4 we applied the sex-specific 99th percentile as the threshold to identify patients who require hospital admission. Adherence was good across all 7 acute care hospitals, which is testament to the simplicity of the pathway and should encourage adoption.
Whereas many pathways that incorporate separate risk stratification and diagnostic thresholds have been described,12,30–32 these have the same limitation as the High-STEACS pathway: no patient was managed according to these pathways during their derivation or validation. Guideline recommendations have therefore been based largely on observational data, with little understanding of the effect of these pathways in clinical practice. Here we evaluated the implementation of an early rule-out pathway in a prospective randomized controlled trial of consecutive patients. We report substantial reductions in length of stay and increases in the proportion of patients avoiding hospital admission. Were these gains to be realized across health care systems, the benefits for both patients and providers would be substantial. In the United States alone, >20 million patients with suspected ACS attend emergency departments every year.1 A reduction in the length of stay of 3 hours could save >$3.6 billion per annum on bed occupancy alone.33
Despite these important reductions in length of stay, during the implementation phase the median stay was 6.8 hours, which is longer than reported in other evaluations of the implementation of early rule-out pathways.15,16,34 This difference likely reflects our enrollment of all consecutive patients rather than selected patients who are less likely to have comorbid conditions requiring hospital admission. We also acknowledge that reductions in length of stay may differ in other health care settings. Although the European Society of Cardiology guidelines have recommended high-sensitivity cardiac troponin testing and a 0/3-hour pathway based on the 99th percentile since 2011,35 we did not adopt this as our standard of care, but instead followed the recommendations of our national guidelines.18 During the design phase of the trial, we prospectively validated the European Society of Cardiology 0/3-hour high-sensitivity cardiac troponin pathway and demonstrated that the diagnostic performance of serial testing at presentation and 3 hours compared with serial testing at presentation and 6 to 12 hours after symptom onset was poor, with a sensitivity and negative predictive value for MI of 89.3% and 97.9%, respectively.19 Our findings were consistent with those from an independent validation in Australia and New Zealand,36 and as a consequence the 2020 European Society of Cardiology guidelines no longer indicate a preference for this approach.37 It is essential that more prospective trials are conducted in which clinical decisions are guided by new diagnostic approaches if we are to ensure our clinical practice guidelines are based on the highest-quality evidence.
Implementation of our early rule-out pathway did not increase the rate of subsequent MI or cardiac death. However, our results were highly sensitive to the model specification. Although noninferiority was not concluded for the primary safety outcome at 30 days, in our prespecified sensitivity analysis restricted to calendar-matched periods, the early rule-out pathway was superior to standard care at 30 days and 1 year. These divergent results may be attributable to the low event rate at 30 days and narrow randomization phase leading to overfitting of the primary analysis model, additional secular changes not accounted for in the sensitivity analysis, or a true exposure–time effect whereby outcomes improved as the intervention became more firmly embedded into practice. We acknowledge that although the number of patients enrolled in each cluster (hospital) was large, the number of clusters was small, which may have made the trial more vulnerable to the effect of confounding bias occurring within individual sites. However, our analyses suggested that including site as a random effect in the model had negligible influence on the overall result. The low event rate for the safety outcome at 30 days and narrow randomization phase made it more likely for chance imbalances to occur between those managed according to the standard care and early rule-out pathway (Figure II in the Data Supplement). This may have produced partial confounding of our estimate of the effect of the intervention because the primary analysis model incorporates both vertical comparisons across sites as well as before-and-after comparisons within sites. We also prespecified a calendar-matched before-and-after sensitivity analysis that did not include a vertical comparison, the results of which favored the early rule-out pathway.
Is it plausible that the introduction of an early rule-out pathway could reduce the risk of subsequent cardiac events? By using a threshold well below the 99th percentile to risk stratify patients and by recognizing that small changes in troponin concentration within the reference range may be important, we may have improved the evaluation of risk compared with using a single higher threshold to rule in and rule out MI. This is supported by recent observational studies, which report that using the 99th percentile to rule out MI at presentation and at 3 hours misses 1 in 10 patients with MI who would have been identified on serial testing 6 to 12 hours after the onset of symptoms.19,36,38 Furthermore, our pathway encourages serial testing to rule out MI in early presenters, which is now recognized by international guidelines.37,39
Our findings add to those from 2 recently published randomized trials. The RAPID-TnT trial (Rapid Assessment of Possible ACS in the Emergency Department With High-Sensitivity Troponin T) compared a 1-hour and 3-hour rule-out pathway in 3378 patients, finding a 1-hour strategy reduced length of stay by 60 minutes and increased discharge rates from 32% to 45%.16 The trial concluded noninferiority for an end point of all-cause mortality or MI within 30 days, although there was an increase in secondary safety outcome events in the 1-hour pathway arm. Because of a perceived lack of equipoise, the monitoring committee recommended the trial stop recruitment with just two-thirds of the target population enrolled, and only 1 patient had a type 1 MI after discharge in each arm. The LoDED trial (Limit of Detection and ECG Discharge) compared standard guideline care with a rule-out pathway using the limit of detection of cardiac troponin in 632 patients.40 The use of a single test approach did not increase the proportion of patients discharged from hospital within 4 hours of presentation. This surprising finding may have been attributable to the small sample size and insufficient power or the enrollment of selected lower risk patients with a normal ECG. It appears that the treating clinician determined the probability of MI to be sufficiently low that admission to hospital was not required in both arms of the trial. However, consistent with our observations, the LoDED investigators report that a single test approach was acceptable to patients and clinicians and that pathway adherence was excellent.
We acknowledge several potential study limitations. First, whereas the early rule-out pathway was implemented across 3 steps in the randomization phase, we had to accept flexibility in the date of implementation (Expanded Methods in the Data Supplement). This limited our ability to interpret a planned sensitivity analysis within the randomization phase, when there were sites using both the standard care and early rule-out pathway. Second, we enrolled fewer than the 38 994 patients anticipated in our sample size calculations, and identified fewer safety outcome events at 30 days. This in part contributed to modeling issues when attempting to evaluate the safety outcome at 30 days. However, >700 patients had a MI or cardiac death at 1 year, and the rates of all secondary outcome measures were lower after implementation of the early rule-out pathway. Third, the standard care arm of our trial used a serial testing strategy based on the time of onset of symptoms, rather than a fixed time point 3 to 6 hours from presentation, which is more commonly used in other countries. Despite this limitation, 50% of patients were discharged directly from the emergency department in our standard care arm. Whereas there are differences in the inclusion criteria between trials, the proportion of patients discharged in our standard care arm was already higher than in either arm of the RAPID-TnT trial, which compared a 0/3-hour pathway (32% discharged) with a 0/1-hour pathway (45% discharged).16 Despite the high proportion of patients discharged in our standard care arm, we increased the proportion discharged from 50% to 71% when implementing our early rule-out pathway. Fourth, our early rule-out pathway does not recommend early serial testing in those with elevated cardiac troponin concentrations at presentation. In our previous trial,17 we observed that 2.7% of patients with suspected ACS have evidence of chronic myocardial injury.41 It is possible the effectiveness of our pathway could be further improved if these patients were identified in the emergency department. However, patients with chronic myocardial injury are complex with significant cardiac and noncardiac comorbidities and may benefit from further evaluation before discharge. Additional research is needed to evaluate the effectiveness of approaches to improve the ruling in of MI.12,42 Last, our pathway has been validated for use with 2 troponin I assays and a troponin T assay without modification,19,20,43,44 and although it is likely to perform similarly for other high-sensitivity assays, further research is required to confirm this.
Implementation of an early rule-out pathway for MI substantially reduced length of stay and increased the proportion of patients avoiding hospital admission with no increase in adverse cardiac events. Adoption of this approach would have important benefits for both patients and health care providers.
Acknowledgments
The HiSTORIC trial (High-Sensitivity Cardiac Troponin on Presentation to Rule Out Myocardial Infarction) investigators contributed to the conception or design of the work or the acquisition, analysis, or interpretation of data for the work. They were involved in drafting the article and revising it, and have given final approval of the version to be published. The study sponsor had no role in the study design, collection of data, analysis and interpretation of data, writing of this article, or decision to submit for publication. The corresponding author affirms that the article is an honest, accurate, and transparent account of the study being reported and that no important aspects of the study have been omitted. The authors thank the researchers from the Emergency Medicine Research Group Edinburgh and the Edinburgh Clinical Trials Unit for their support during the conduct of this trial.
Sources of Funding
This trial was funded by the British Heart Foundation (grant PG/15/51/31596) with support from British Heart Foundation Research Excellence Awards (awards RE/18/5/34216 and RE/18/6134217). Dr Anand, Dr Lee, and Dr Chapman are supported by a Clinical Lectureship from the Chief Scientist Office (PCL/18/05), a Clinical Research Training Fellowship from the British Heart Foundation (FS/18/25/33454), and a Clinical Lectureship from the Scottish Clinical Research Excellence Development Scheme, respectively. Dr Newby, Dr Shah, and Dr Mills are supported by the British Heart Foundation through the award of a Chair (chair CH/09/002), an Intermediate Clinical Research Fellowship (fellowship FS/19/17/34172), and the Butler Senior Clinical Research Fellowship and a Program Grant (fellowship FS/16/14/32023 and grant RG/20/10/34966), respectively. Dr Adamson is supported by a National Heart Foundation of New Zealand Senior Fellowship (fellowship 1844). Dr Newby is a recipient of a Wellcome Trust Senior Investigator Award (award WT103782AIA). Dr Weir and R.A. Parker were supported by National Health Service Lothian through the Edinburgh Clinical Trials Unit. The funders played no role in the design, conduct, data collection, analysis, or reporting of the trial.
Disclosures
Drs Anand, Chapman, and Shah have received honoraria from Abbott Diagnostics. Dr Berry reports research grants awarded to the University of Glasgow from Abbott Vascular, AstraZeneca, Boehringer Ingelheim, GSK, HeartFlow, Novartis, and Siemens Healthcare outside the submitted work. Dr Apple reports research grants awarded to the Minneapolis Medical Research Foundation from Abbott Diagnostics, Siemens Diagnostics, Ortho-Clinical Diagnostics, and Beckman Coulter outside the submitted work and personal fees from HyTest Ltd. Dr Mills reports research grants awarded to the University of Edinburgh from Abbott Diagnostics and Siemens Healthineers outside the submitted work and honoraria from Abbott Diagnostics, Siemens Healthineers, Roche Diagnostics, and Singulex. The other authors have no interests to declare.
Supplemental Materials
List of Investigators
Expanded Methods
Data Supplement Tables I–IV
Data Supplement Figures I and II
Supplementary Material
Nonstandard Abbreviations and Acronyms
- ACS
- acute coronary syndrome
- High-STEACS
- High-Sensitivity Troponin in the Evaluation of Patients With Acute Coronary Syndrome
- HiSTORIC
- High-Sensitivity Cardiac Troponin on Presentation to Rule Out Myocardial Infarction
- LoDED
- Limit of Detection and ECG Discharge
- MI
- myocardial infarction
- RAPID-TnT
- Rapid Assessment of Possible ACS in the Emergency Department With High-Sensitivity Troponin T
A. Anand, K.K. Lee, and A.R. Chapman contributed equally.
A list of the HiSTORIC Investigators is provided in the Data Supplement.
This article was sent to Allan Jaffe, Guest Editor, for review by expert referees, editorial decision, and final disposition.
The Data Supplement, podcast, and transcript are available with this article at https://www.ahajournals.org/doi/suppl/10.1161/circulationaha.120.052380.
For Sources of Funding and Disclosures, see page 2223.
Contributor Information
Atul Anand, Email: atul.anand@ed.ac.uk.
Kuan Ken Lee, Email: ken.lee@ed.ac.uk.
Andrew R. Chapman, Email: a.r.chapman@ed.ac.uk.
Amy V. Ferry, Email: Amy.ferry@ed.ac.uk.
Phil D. Adamson, Email: philip.adamson@ed.ac.uk.
Fiona E. Strachan, Email: f.strachan@ed.ac.uk.
Colin Berry, Email: colin.berry@glasgow.ac.uk.
Iain Findlay, Email: iain.findlay@ggc.scot.nhs.uk.
Anne Cruikshank, Email: Anne.Cruickshank@ggc.scot.nhs.uk.
Alan Reid, Email: alan.reid@ggc.scot.nhs.uk.
Paul O. Collinson, Email: paul.collinson@stgeorges.nhs.uk.
Fred S. Apple, Email: apple004@umn.edu.
David A. McAllister, Email: david.mcallister@glasgow.ac.uk.
Donogh Maguire, Email: donogh.maguire@glasgow.ac.uk.
Keith A.A. Fox, Email: k.a.a.fox@ed.ac.uk.
David E. Newby, Email: d.e.newby@ed.ac.uk.
Chris Tuck, Email: chris.tuck@ed.ac.uk.
Ronald Harkess, Email: rharkess@exseed.ed.ac.uk.
Catriona Keerie, Email: Catriona.Keerie@ed.ac.uk.
Christopher J. Weir, Email: christopher.weir@ed.ac.uk.
Richard A. Parker, Email: richard.parker@ed.ac.uk.
Alasdair Gray, Email: ajg4406@gmail.com.
Anoop S.V. Shah, Email: anoopsshah@gmail.com.
References
- 1.Hollander JE, Than M, Mueller C. State-of-the-art evaluation of emergency department patients presenting with potential acute coronary syndromes. Circulation. 2016;134:547–564. doi: 10.1161/CIRCULATIONAHA.116.021886 [DOI] [PubMed] [Google Scholar]
- 2.Goodacre S, Cross E, Arnold J, Angelini K, Capewell S, Nicholl J. The health care burden of acute chest pain. Heart. 2005;91:229–230. doi: 10.1136/hrt.2003.027599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson JL, Morrow DA. Acute myocardial infarction. N Engl J Med. 2017;376:2053–2064. doi: 10.1056/NEJMra1606915 [DOI] [PubMed] [Google Scholar]
- 4.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Katus HA, Lindahl B, Morrow DA, Clemmensen PM, et al. ; Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035. doi: 10.1161/CIR.0b013e31826e105822923432 [Google Scholar]
- 5.Apple FS, Collinson PO; IFCC Task Force on Clinical Applications of Cardiac Biomarkers. Analytical characteristics of high-sensitivity cardiac troponin assays. Clin Chem. 2012;58:54–61. doi: 10.1373/clinchem.2011.165795 [DOI] [PubMed] [Google Scholar]
- 6.Body R, Carley S, McDowell G, Jaffe AS, France M, Cruickshank K, Wibberley C, Nuttall M, Mackway-Jones K. Rapid exclusion of acute myocardial infarction in patients with undetectable troponin using a high-sensitivity assay. J Am Coll Cardiol. 2011;58:1332–1339. doi: 10.1016/j.jacc.2011.06.026 [DOI] [PubMed] [Google Scholar]
- 7.Body R, Mueller C, Giannitsis E, Christ M, Ordonez-Llanos J, de Filippi CR, Nowak R, Panteghini M, Jernberg T, Plebani M, et al. ; TRAPID-AMI Investigators. The use of very low concentrations of high-sensitivity troponin T to rule out acute myocardial infarction using a single blood test. Acad Emerg Med. 2016;23:1004–1013. doi: 10.1111/acem.13012 [DOI] [PubMed] [Google Scholar]
- 8.Shah AS, Anand A, Sandoval Y, Lee KK, Smith SW, Adamson PD, Chapman AR, Langdon T, Sandeman D, Vaswani A, et al. ; High-STEACS Investigators. High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: a cohort study. Lancet. 2015;386:2481–2488. doi: 10.1016/S0140-6736(15)00391-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boeddinghaus J, Nestelberger T, Twerenbold R, Wildi K, Badertscher P, Cupa J, Bürge T, Mächler P, Corbière S, Grimm K, et al. Direct comparison of 4 very early rule-out strategies for acute myocardial infarction using high-sensitivity cardiac troponin I. Circulation. 2017;135:1597–1611. doi: 10.1161/CIRCULATIONAHA.116.025661 [DOI] [PubMed] [Google Scholar]
- 10.Sandoval Y, Smith SW, Love SA, Sexter A, Schulz K, Apple FS. Single high-sensitivity cardiac troponin I to rule out acute myocardial infarction. Am J Med. 2017;130:1076–1083.e1. doi: 10.1016/j.amjmed.2017.02.032 [DOI] [PubMed] [Google Scholar]
- 11.Lindahl B, Jernberg T, Badertscher P, Boeddinghaus J, Eggers KM, Frick M, Rubini Gimenez M, Linder R, Ljung L, Martinsson A, et al. An algorithm for rule-in and rule-out of acute myocardial infarction using a novel troponin I assay. Heart. 2017;103:125–131. doi: 10.1136/heartjnl-2016-309951 [DOI] [PubMed] [Google Scholar]
- 12.Neumann JT, Twerenbold R, Ojeda F, Sörensen NA, Chapman AR, Shah ASV, Anand A, Boeddinghaus J, Nestelberger T, Badertscher P, et al. Application of high-sensitivity troponin in suspected myocardial infarction. N Engl J Med. 2019;380:2529–2540. doi: 10.1056/NEJMoa1803377 [DOI] [PubMed] [Google Scholar]
- 13.Greenslade J, Cho E, Van Hise C, Hawkins T, Parsonage W, Ungerer J, Tate J, Pretorius C, Than M, Cullen L. Evaluating rapid rule-out of acute myocardial infarction using a high-sensitivity cardiac troponin I assay at presentation. Clin Chem. 2018;64:820–829. doi: 10.1373/clinchem.2017.283887 [DOI] [PubMed] [Google Scholar]
- 14.Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, et al. ; ESC Scientific Document Group. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267–315. doi: 10.1093/eurheartj/ehv320 [DOI] [PubMed] [Google Scholar]
- 15.Twerenbold R, Costabel JP, Nestelberger T, Campos R, Wussler D, Arbucci R, Cortes M, Boeddinghaus J, Baumgartner B, Nickel CH, et al. Outcome of applying the ESC 0/1-hour algorithm in patients with suspected myocardial infarction. J Am Coll Cardiol. 2019;74:483–494. doi: 10.1016/j.jacc.2019.05.046 [DOI] [PubMed] [Google Scholar]
- 16.Chew DP, Lambrakis K, Blyth A, Seshadri A, Edmonds MJR, Briffa T, Cullen LA, Quinn S, Karnon J, Chuang A, et al. A randomized trial of a 1-hour troponin T protocol in suspected acute coronary syndromes: the Rapid Assessment of Possible Acute Coronary Syndrome in the Emergency Department With High-Sensitivity Troponin T Study (RAPID-TnT). Circulation. 2019;140:1543–1556. doi: 10.1161/CIRCULATIONAHA.119.042891 [DOI] [PubMed] [Google Scholar]
- 17.Shah ASV, Anand A, Strachan FE, Ferry AV, Lee KK, Chapman AR, Sandeman D, Stables CL, Adamson PD, Andrews JPM, et al. ; High-STEACS Investigators. High-sensitivity troponin in the evaluation of patients with suspected acute coronary syndrome: a stepped-wedge, cluster-randomised controlled trial. Lancet. 2018;392:919–928. doi: 10.1016/S0140-6736(18)31923-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scottish Intercollegiate Guidelines Network (SIGN). Acute Coronary Syndromes (SIGN publication number 93). 2013. Scottish Intercollegiate Guidelines Network [Google Scholar]
- 19.Chapman AR, Anand A, Boeddinghaus J, Ferry AV, Sandeman D, Adamson PD, Andrews J, Tan S, Cheng SF, D’Souza M, et al. Comparison of the efficacy and safety of early rule-out pathways for acute myocardial infarction. Circulation. 2017;135:1586–1596. doi: 10.1161/CIRCULATIONAHA.116.025021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chapman AR, Fujisawa T, Lee KK, Andrews JP, Anand A, Sandeman D, Ferry AV, Stewart S, Marshall L, Strachan FE, et al. Novel high-sensitivity cardiac troponin I assay in patients with suspected acute coronary syndrome. Heart. 2019;105:616–622. doi: 10.1136/heartjnl-2018-314093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chin CW, Shah AS, McAllister DA, Joanna Cowell S, Alam S, Langrish JP, Strachan FE, Hunter AL, Choy AM, Lang CC, et al. High-sensitivity troponin I concentrations are a marker of an advanced hypertrophic response and adverse outcomes in patients with aortic stenosis. Eur Heart J. 2014;35:2312–2321. doi: 10.1093/eurheartj/ehu189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah AS, Griffiths M, Lee KK, McAllister DA, Hunter AL, Ferry AV, Cruikshank A, Reid A, Stoddart M, Strachan F, et al. High sensitivity cardiac troponin and the under-diagnosis of myocardial infarction in women: prospective cohort study. BMJ. 2015;350:g7873. doi: 10.1136/bmj.g7873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The SCOT-HEART Investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet. 2015;385:2383–2391. doi: 10.1016/S0140-6736(15)60291-4 [DOI] [PubMed] [Google Scholar]
- 24.NHS National Services Scotland, Information Services Division. Scottish Heart Disease Statistics: A National Statistics Publication for Scotland. 2018. NHS National Services Scotland; 1–52. [Google Scholar]
- 25.Shah ASV, Sandoval Y, Noaman A, Sexter A, Vaswani A, Smith SW, Gibbins M, Griffiths M, Chapman AR, Strachan FE, et al. Patient selection for high sensitivity cardiac troponin testing and diagnosis of myocardial infarction: prospective cohort study. BMJ. 2017;359:j4788. doi: 10.1136/bmj.j4788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth universal definition of myocardial infarction (2018). Circulation. 2018;138:e618–e651. doi: 10.1161/CIR.0000000000000617 [DOI] [PubMed] [Google Scholar]
- 27.Chapman AR, Lee KK, McAllister DA, Cullen L, Greenslade JH, Parsonage W, Worster A, Kavsak PA, Blankenberg S, Neumann J, et al. Association of high-sensitivity cardiac troponin I concentration with cardiac outcomes in patients with suspected acute coronary syndrome. JAMA. 2017;318:1913–1924. doi: 10.1001/jama.2017.17488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pickering JW, Than MP, Cullen L, Aldous S, Ter Avest E, Body R, Carlton EW, Collinson P, Dupuy AM, Ekelund U, et al. Rapid rule-out of acute myocardial infarction with a single high-sensitivity cardiac troponin T measurement below the limit of detection: a collaborative meta-analysis. Ann Intern Med. 2017;166:715–724. doi: 10.7326/M16-2562 [DOI] [PubMed] [Google Scholar]
- 29.Kavsak PA, Don-Wauchope AC, Hill SA, Worster A. Acceptable analytical variation may exceed high-sensitivity cardiac troponin I cutoffs in early rule-out and rule-in acute myocardial infarction algorithms. Clin Chem. 2016;62:887–889. doi: 10.1373/clinchem.2016.255448 [DOI] [PubMed] [Google Scholar]
- 30.Reichlin T, Schindler C, Drexler B, Twerenbold R, Reiter M, Zellweger C, Moehring B, Ziller R, Hoeller R, Rubini Gimenez M, et al. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med. 2012;172:1211–1218. doi: 10.1001/archinternmed.2012.3698 [DOI] [PubMed] [Google Scholar]
- 31.Than MP, Pickering JW, Aldous SJ, Cullen L, Frampton CM, Peacock WF, Jaffe AS, Goodacre SW, Richards AM, Ardagh MW, et al. Effectiveness of EDACS versus ADAPT accelerated diagnostic pathways for chest pain: a pragmatic randomized controlled trial embedded within practice. Ann Emerg Med. 2016;68:93–102.e1. doi: 10.1016/j.annemergmed.2016.01.001 [DOI] [PubMed] [Google Scholar]
- 32.Möckel M, Searle J, Hamm C, Slagman A, Blankenberg S, Huber K, Katus H, Liebetrau C, Müller C, Muller R, et al. Early discharge using single cardiac troponin and copeptin testing in patients with suspected acute coronary syndrome (ACS): a randomized, controlled clinical process study. Eur Heart J. 2015;36:369–376. doi: 10.1093/eurheartj/ehu178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Probst MA, McConnell JK, Weiss RE, Laurie AL, Yagapen AN, Lin MP, Caterino JM, Shah MN, Sun BC. Estimating the cost of care for emergency department syncope patients: comparison of three models. West J Emerg Med. 2017;18:253–257. doi: 10.5811/westjem.2016.10.31171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ljung L, Lindahl B, Eggers KM, Frick M, Linder R, Löfmark HB, Martinsson A, Melki D, Sarkar N, Svensson P, et al. A rule-out strategy based on high-sensitivity troponin and HEART score reduces hospital admissions. Ann Emerg Med. 2019;73:491–499. doi: 10.1016/j.annemergmed.2018.11.039 [DOI] [PubMed] [Google Scholar]
- 35.Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, Caso P, Dudek D, Gielen S, Huber K, et al. ; ESC Committee for Practice Guidelines. ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the task force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2011;32:2999–3054. doi: 10.1093/eurheartj/ehr236 [DOI] [PubMed] [Google Scholar]
- 36.Pickering JW, Greenslade JH, Cullen L, Flaws D, Parsonage W, George P, Worster A, Kavsak PA, Than MP. Validation of presentation and 3 h high-sensitivity troponin to rule-in and rule-out acute myocardial infarction. Heart. 2016;102:1270–1278. doi: 10.1136/heartjnl-2015-308505 [DOI] [PubMed] [Google Scholar]
- 37.Collet J-P, Thiele H, Barbato E, Barthelemy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, et al. ESC Scientific Document Group. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42:1289–1367. doi: 10.1093/eurheartj/ehaa575 [DOI] [PubMed] [Google Scholar]
- 38.Parsonage WA, Mueller C, Greenslade JH, Wildi K, Pickering J, Than M, Aldous S, Boeddinghaus J, Hammett CJ, Hawkins T, et al. Validation of NICE diagnostic guidance for rule out of myocardial infarction using high-sensitivity troponin tests. Heart. 2016;102:1279–1286. doi: 10.1136/heartjnl-2016-309270 [DOI] [PubMed] [Google Scholar]
- 39.Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Jr, Ganiats TG, Holmes DR, Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, et al. ; ACC/AHA Task Force Members; Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;130:2354–2394. doi: 10.1161/CIR.0000000000000133 [DOI] [PubMed] [Google Scholar]
- 40.Carlton EW, Ingram J, Taylor H, Glynn J, Kandiyali R, Campbell S, Beasant L, Aziz S, Beresford P, Kendall J, et al. Limit of detection of troponin discharge strategy versus usual care: randomised controlled trial. Heart. 2020;106:1586–1594. doi: 10.1136/heartjnl-2020-316692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chapman AR, Adamson PD, Shah ASV, Anand A, Strachan FE, Ferry AV, Lee KK, Berry C, Findlay I, Cruikshank A, et al. ; High-STEACS Investigators. High-sensitivity cardiac troponin and the universal definition of myocardial infarction. Circulation. 2020;141:161–171. doi: 10.1161/CIRCULATIONAHA.119.042960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Than MP, Pickering JW, Sandoval Y, Shah ASV, Tsanas A, Apple FS, Blankenberg S, Cullen L, Mueller C, Neumann JT, et al. ; MI3 collaborative. Machine learning to predict the likelihood of acute myocardial infarction. Circulation. 2019;140:899–909. doi: 10.1161/CIRCULATIONAHA.119.041980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chapman AR, Sandeman D, Ferry AV, Stewart S, Strachan FE, Wereski R, Bularga A, Anand A, Shah ASV, Mills NL. Risk stratification using high-sensitivity cardiac troponin T in patients with suspected acute coronary syndrome. J Am Coll Cardiol. 2020;75:985–987. doi: 10.1016/j.jacc.2019.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bularga A, Lee KK, Stewart S, Ferry AV, Chapman AR, Marshall L, Strachan FE, Cruickshank A, Maguire D, Berry C, et al. High-sensitivity troponin and the application of risk stratification thresholds in patients with suspected acute coronary syndrome. Circulation. 2019;140:1557–1568. doi: 10.1161/CIRCULATIONAHA.119.042866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pedroza C, Truong VT. Performance of models for estimating absolute risk difference in multicenter trials with binary outcome. BMC Med Res Methodol. 2016;16:113. doi: 10.1186/s12874-016-0217-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


