Abstract
Background
Opioid abuse/dependence is associated with multiple negative outcomes relative to other forms of substance abuse/dependence, including relapse. Research identifying modifiable characteristics associated with opioid dependence and associated negative outcomes may inform the development of targeted interventions for this high-risk population. One factor warranting investigation is low distress tolerance (DT).
Purpose/Objectives
In a sample of patients in substance use disorder (SUD) treatment, the present study examined DT levels among patients with current opioid dependence versus no history of opioid dependence, as well as the moderating role of gender. We predicted that patients with opioid dependence would exhibit lower DT than those without a history of opioid dependence, and that women with opioid dependence would exhibit lower levels of DT than men with opioid dependence.
Methods
A sample of 203 patients in residential SUD treatment were administered a series of diagnostic interviews and a behavioral measure of DT.
Results
DT did not differ significantly as a function of opioid dependence. However, there was a significant opioid dependence by gender interaction, such that men with current opioid dependence exhibited significantly lower levels of DT than women with opioid dependence and men without a history of opioid dependence.
Conclusions/Importance
Findings highlight a modifiable characteristic associated with opioid dependence among men that may be targeted in interventions.
Keywords: emotion regulation, opioid misuse, risk factor, substance use disorder
Introduction
Opioids include both licit and illicit substances, including natural opioid analgesics (e.g., morphine), synthetic (e.g., methadone) and semi-synthetic opioid analgesics (e.g., oxycodone), and heroin. In 2016, an estimated 11.5 million people misused (i.e., non-medical use) prescription opioids, an additional 948,000 used heroin, and 2.1 million people were diagnosed with an opioid use disorder (OUD; Substance Abuse and Mental Health Administration, 2017). Although the misuse of opioids represents a significant public health problem in and of itself, OUDs are associated with worse outcomes. For example, among individuals who misuse prescription opioids, the presence of opioid abuse/dependence is associated with worse health, a greater likelihood of anxiety symptoms, the use of illicit opioids, and the misuse of other prescription medications (Becker, Sullivan, Tetrault, Desai, & Fiellin, 2008). Moreover, relative to other substances, opioid abuse/dependence in particular is associated with worse outcomes following SUD treatment, including greater risk for relapse and a shorter latency until relapse (Chalana, Sachdeva, Kundal, Malhari, & Choudhary, 2015; El, Gaily, & Bashir, 2004; Gossop, Stewart, Browne, & Marsden, 2002; Leukefeld & Tims, 1986). Given the clinical relevance and public health significance of opioid abuse/dependence, research is needed to identify modifiable characteristics that may increase risk for opioid misuse and its associated negative outcomes among high-risk populations, such as patients in residential SUD treatment (Chen et al., 2011).
One factor that warrants investigation in this regard is low distress tolerance (DT), defined as the perceived or actual unwillingness to withstand or persist in the face of aversive internal states, such as emotional distress or physical pain (Simons & Gaher, 2005). Although DT is a relatively stable individual difference characteristic (Cummings et al., 2013; Kiselica, Webber, & Bornovalova, 2014), it has been found to be modifiable through behavioral interventions (Bornovalova, Gratz, Daughters, Hunt, & Lejuez, 2012). DT is typically assessed through self-report (e.g., the Distress Tolerance Scale; Simons & Gaher, 2005) or behavioral (e.g., Paced Auditory Serial Addition Task – Computerized Version [PASAT-C]; Lejuez, Kahler, & Brown, 2003) measures, with self-report measures thought to capture perceived DT and behavioral measures capturing actual DT. Although there is evidence for the construct validity of both self-report and behavioral measures of DT, studies have generally found self-report and behavioral DT measures to be empirically distinct (Anestis et al., 2012; Bernstein, Marshall, & Zvolensky, 2011; McHugh et al., 2011). These findings have led researchers to suggest that these measures may be capturing distinct aspects of the DT construct (Bernstein et al., 2011; McHugh et al., 2011).
Lower self-reported DT has been found to be associated with the presence of SUDs (Allan, Macatee, Norr, Raines, & Schmidt, 2015), alcohol use severity (Kaiser, Milich, Lynam, & Charnigo, 2012), and the use of illicit drugs (Kaiser et al., 2012). Likewise, previous research on patients with substance abuse/dependence has shown that low DT on behavioral measures is associated with negative SUD outcomes, including early treatment dropout (Daughters et al., 2005a; Tull, Gratz, Coffey, Weiss, & McDermott, 2013), shorter periods of abstinence (Daughters, Lejuez, Kahler, Strong, & Brown, 2005b), and substance use problems (Ali, Seitz-Brown, & Daughters, 2015). Likewise, among individuals from the community, low DT on behavioral measures is associated with more frequent and problematic substance use (Gorka, Ali, & Daughters, 2012; Tull, Bardeen, DiLillo, Messman-Moore, & Gratz, 2015). Finally, preliminary evidence supports the relevance of low DT to opioid dependence specifically. For example, Garland, Hanley, Thomas, Knoll, and Ferraro (2015) found that approximately 95% of patients in treatment for prescription opioid dependence reported using opioids to regulate negative affective states, suggesting an unwillingness to experience negative affect. Moreover, low self-reported DT has been found to be associated with opioid misuse among adults prescribed opioid analgesics for chronic pain (McHugh et al., 2016).
Although no research to date has examined the relevance of low DT to the misuse of opioids in particular, relative to other substances, Lejuez, Paulson, Daughters, Bornovalova, and Zvolensky (2006) found that patients in SUD treatment with frequent and primary use of heroin reported significantly higher anxiety sensitivity (i.e., a construct that overlaps with DT and loads onto a higher-order affect tolerance/sensitivity construct; Bernstein, Zvolensky, Vujanovic, & Moos, 2009) than patients who used cocaine or other substances. Moreover, neuroadaptations associated with prolonged opioid use highlight potential pathways through which low DT may be particularly relevant among individuals who misuse opioids (vs. other substances). Specifically, although the experience of negative affect is a common consequence of substance use in general (Brady & Sinha, 2005), opioid use is especially likely to result in an increased sensitivity to painful stimuli or hyperalgesia (Chu, Angst, & Clark, 2008; LeBlanc, McGinn, Itoga, & Edwards, 2015).1 This interplay between increased negative affect and sensitivity to pain may contribute to particularly low DT among individuals who repeatedly misuse opioids. For example, studies show that the experience of negative affect may exacerbate opioid-related hyperalgesia (Carcoba, Contreras, Cepeda-Benito, & Meagher, 2011), and the experience of pain may contribute to negative affect (LeBlanc et al., 2015; Narita et al., 2006). Thus, among individuals who misuse opioids, the experience of negative affect may exacerbate or sensitize the individual to the experience of pain (and vice versa). Over time, this process may result in the experience of increasingly intense unpleasant internal states that exceed the individual’s tolerance. This process may also increase the desire to seek out opioids to obtain relief from these experiences (Garland, Bryan, Nakamura, Froeliger, & Howard, 2017; Garland, Froeliger, Zeidan, Partin, & Howard, 2013) – the repeated use of which may further decrease the tolerance for and ability to regulate these aversive internal states (Trafton & Gifford, 2011). Although negative reinforcement motives are considered to play a role in the use of substances in general (Baker, Piper, McCarthy, Majeskie, & Fiore, 2004), the pain-related neuroadaptations specifically associated with opioid use would be expected to amplify vulnerability for and negative consequences of such motives (i.e., low DT). Thus, low DT may be uniquely related to the misuse of opioids (vs. other substances) and may distinguish between patients with and without opioid dependence.
The present study examined DT (assessed using a laboratory-based behavioral task) among patients in residential SUD treatment as a function of opioid dependence (current versus no history of opioid dependence). We predicted that patients with current opioid dependence would exhibit lower DT than those without a history of opioid dependence. We also explored the moderating role of gender in the relation between DT and opioid dependence. Specifically, given some evidence that women (vs. men) with opioid abuse/dependence are more likely to both use opioids in response to affective distress (Back, Lawson, Singleton, & Brady, 2011; Jamison, Butler, Budman, Edwards, & Wasan, 2010; McHugh et al., 2013) and seek out other medications as a method for coping with anxiety-related sensations (Hearon et al., 2011), we expected that women with current opioid dependence would exhibit lower DT than men with current opioid dependence.
Method
Procedure
All procedures received Institutional Review Board approval. To be eligible for inclusion in the larger study from which these data were drawn, participants were required to: 1) obtain a Mini-Mental Status Exam (Folstein, Folstein, & McHugh, 1975) score of ≥ 24 (indicating the absence of cognitive impairment); and 2) exhibit no current psychotic disorder (as determined by the Structured Clinical Interview for DSM-IV Axis I Disorders [SCID-IV]; First, Spitzer, Gibbon, & Williams, 1996). Eligible participants were recruited for this study no sooner than 72 hours after entry in the facility (to limit the possible interference of withdrawal symptoms on study engagement). To limit selection bias, information on the study was provided to all patients, and recruitment occurred at multiple times throughout the week. Fewer than 1% of participants failed to meet inclusion criteria and fewer than 5% of eligible patients declined to participate. Those who met inclusion criteria and agreed to participate in the study were provided with information about the study procedures and associated risks. Informed consent was then obtained. All participants took part in this study between day 3 and day 14 in residential treatment. The study protocol was conducted across two sessions, with diagnostic interviews administered in the first session and the DT task administered in the second session. Participants were paid $15 per session.
Participants
Data were collected from 250 patients with substance dependence consecutively admitted to a residential SUD treatment program. Twenty-eight participants did not complete the laboratory-based behavioral measure of DT; thus, they were excluded from this study. In addition, to ensure an absence of clinically significant opioid misuse among participants in the comparison group, this group was limited to participants who had never met criteria for opioid dependence. Therefore, participants with past but not current opioid dependence were excluded from analyses (n = 19), resulting in a final sample of 203 participants (35.0% women) who either met criteria for current opioid dependence (n = 33) or had no history of opioid dependence (n = 170). Participants ranged in age from 18 to 56, with an average age of 35.34 (SD = 9.98). As for racial/ethnic background of participants, 52.7% identified as White, 38.4% as African-American, 4.4% as Native American, 2.0% as Latino/a, 2.0% as having a multi-racial/ethnic background, and 0.5% as Asian-American. The majority of participants had an annual income of less than $20,000 per year (64.5%), were unemployed (70.5%), and had a high school education or less (63.1%). Seventy-eight participants (38.4%) reported being court-ordered to treatment. Clinical characteristics for both groups of participants are presented in Table 1.
Table 1.
Clinical Characteristics of Participants (N = 203).
| Diagnosis | No Opioid Dependence (n = 170) Mean (SD); % Present (n) |
Current Opioid Dependence (n = 33) Mean (SD); % Present (n) |
|---|---|---|
| Gender | 68.8% men (117) | 45.5% men (15) |
| Age | 35.92 (10.10) | 32.36 (8.87) |
| Posttraumatic Stress Disorder | 25.3% (43) | 42.4% (14) |
| Borderline Personality Disorder | 29.4% (50) | 51.5% (17) |
| Major Depressive Disorder | 20.0% (34) | 24.2% (8) |
| Panic Disorder | 14.7% (25) | 45.5% (15) |
| Social Anxiety Disorder | 11.8% (20) | 12.1% (4) |
| Obsessive-Compulsive Disorder | 2.4% (4) | 3.0% (1) |
| Generalized Anxiety Disorder | 27.1% (46) | 51.5% (17) |
| Alcohol Dependence | 38.8% (66) | 27.3% (9) |
| Cocaine Dependence | 36.5% (62) | 21.2% (7) |
| Marijuana Dependence | 19.4% (33) | 18.2% (6) |
| Sedative Dependence | 4.7% (8) | 21.2% (7) |
| Stimulant Dependence | 10.0% (17) | 12.1% (4) |
| Hallucinogen Dependence | 1.2% (2) | 0% (0) |
Note. All diagnoses are current.
Measures
Diagnostic assessment measures
To assess for current and lifetime substance dependence (including opioid dependence) and other psychiatric disorders (except for posttraumatic stress disorder [PTSD] and borderline personality disorder [BPD]), participants were interviewed using the SCID-IV (First, Spitzer, Gibbon, & Williams, 1996). PTSD was assessed with the Clinician-Administered PTSD Scale (CAPS; Blake et al., 1990), and BPD was assessed with the BPD module of the Diagnostic Interview for DSM-IV Personality Disorders (DIPD-IV; Zanarini, Frankenburg, Sickel, & Young, 1996). Interviews were conducted by bachelors- or masters-level clinical assessors trained to reliability (κ ≥ .80) with the principal investigator (first author) and co-investigator (last author). Detailed information on each criterion was collected by interviewers, and all ratings were reviewed by the study investigators. In the case of disagreements, ratings were discussed by the investigators and interviewer until a consensus was reached.
Assessment of DT
The PASAT-C (Lejuez et al., 2003) was used to assess DT. During this task, numbers are sequentially flashed on a computer screen, and participants are instructed to sum the most recent number with the previous number, using the computer mouse to click on the correct answer. After providing each sum, the participant must ignore the sum and add the following number to the most recently presented number. When a correct answer is provided, a point is obtained. If an incorrect answer is provided, or if the participant fails to provide an answer before the next number is presented, an “explosion” sound is played and the score does not change.
The version of the PASAT-C used in this study consisted of 3 levels with increasingly shorter latencies between number presentations. Because the correct answer must be provided before the presentation of the next number to obtain a point, difficulty increases as latencies decrease. Level 1 (low difficulty) had a 3-second latency between number presentations, Level 2 (medium difficulty) had a 2-second latency, and Level 3 (high difficulty) had a 1-second latency. The first level lasted 3 minutes, the second level lasted 5 minutes, and the third level lasted 10 minutes and included an option to terminate the task at any time. However, participants were under the impression that their performance on this task (including the length of time they persisted on it) would determine the amount of money they would receive as reimbursement for their participation (providing an incentive to perform well on the task, although all participants were reimbursed the full amount regardless of their performance). DT was determined by latency to terminate the final level of this task.
As a manipulation check to ensure that the task induced emotional distress, participants rated (0–100) their levels of anxiety, frustration, and irritation both before the task (pre-task) and immediately prior to receiving the option to terminate the task (post-task). Scores were averaged to create a composite variable representing pre- and post-task emotional distress.
The PASAT-C has been used extensively among patients in residential SUD treatment with similar levels of educational achievement as participants in the present study (e.g., Ali et al., 2015; Bornovalova et al., 2012; Daughters et al., 2005a; Daughters, Sargeant, Bornovalova, Gratz, & Lejuez, 2008; Tull et al., 2013). The PASAT-C has been shown to induce emotional distress in the form of anxiety, anger, frustration, and irritability among clinical and nonclinical samples (e.g., Daughters et al., 2005a; Gratz et al., 2011; Lejuez et al., 2003; Tull et al., 2013), and to be significantly correlated with self-report measures of emotion dysregulation and the unwillingness to experience emotional distress (Gratz, Rosenthal, Tull, Lejuez, & Gunderson, 2006). However, the PASAT-C does not demonstrate significant associations with self-report measures of DT (McHugh et al., 2011). Low DT as assessed by the PASAT-C has also been found to predict early SUD treatment dropout (Daughters et al., 2005a; Tull et al., 2013). In addition, performance on the PASAT-C has been found to be stable over time among patients in residential SUD treatment (Bornovalova et al., 2012).
Results
Effect sizes (Cohen’s d and Cohen’s f) are reported for all findings. Suggested benchmarks for Cohen’s d are .20 (small effect), .50 (medium effect), and .80 (large effect; Cohen, 1988). Suggested benchmarks for Cohen’s f are .10 (small effect), .25 (medium effect), and .40 (large effect; Cohen, 1988).
Manipulation Check
Providing support for the use of latency to terminate the PASAT-C as a measure of DT, results of a 2 (current opioid dependence vs. no history of opioid dependence) × 2 (women vs. men) × 2 (pre- vs. post-task) repeated measures analysis of variance (ANOVA) for emotional distress revealed a significant main effect of time, F (1,199) = 46.82, p < 0.001, f = 0.48, with all participants reporting an increase in distress from pre- to post-task. Further, there was no time × opioid dependence interaction, F (1,199) = 0.002, p = 0.965, f = 0.00, indicating that the task resulted in a comparable increase in distress regardless of opioid dependence status.
Primary Analyses
On average, latency to terminate the PASAT-C was 213.92 (SD = 252.06) seconds (maximum latency is 600 seconds). The latency to termination variable was found to be normally distributed (skew = 0.71, kurtosis = −1.32). Overall, 75.4% (n = 153) of participants terminated the PASAT-C. The percentages of participants terminating the PASAT-C across all groups are as follows: (a) women with no history of opioid dependence = 83.0%; (b) women with opioid dependence = 66.7%; (c) men with no history of opioid dependence = 70.9%; (d) men with opioid dependence = 93.3%. Rates of terminating (vs. persisting) on the PASAT-C did not differ significantly as a function of opioid dependence status, χ2 (1) = 0.25, p = .618, or gender, χ2 (1) = 0.72, p = .395.
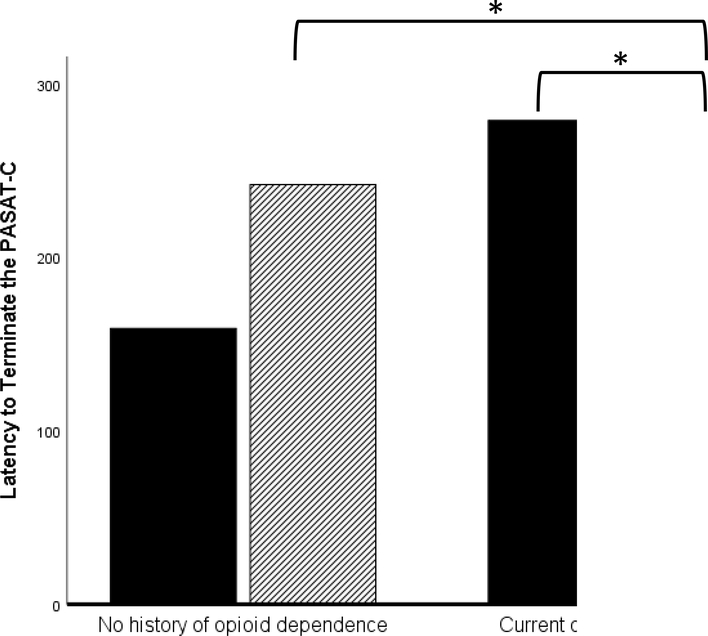
To test hypotheses, we conducted a 2 (current opioid dependence vs. no history of opioid dependence) × 2 (men vs. women) ANOVA, with latency to terminate the PASAT-C serving as the dependent variable. No significant main effects of gender, F (1,199) = 0.77, p = .381, f = 0.00, or opioid dependence status, F (1,199) = 0.12, p = .912, f = 0.00, were found. However, a significant opioid dependence × gender interaction, F (1,199) = 6.75, p = .010, f = 0.17 (see Figure 1) was found. Post-hoc analyses focused on examining (a) differences in DT between men and women with opioid dependence and (b) differences in DT as a function of opioid dependence status (current vs. no history) within each gender. These analyses (using the Welch t statistic [Welch, 1947] when Levene’s test for equality of variances was significant) revealed that men with current opioid dependence exhibited significantly lower DT than both women with current opioid dependence, Welch t (30.26) = 2.13, p = .041, d = 0.72, and men without a history of opioid dependence, Welch t (21.75) = 2.43, p = .024, d = 0.56. Women with current opioid dependence did not differ significantly from women without a history of opioid dependence in DT, t (69) = −1.83, p = .072, d = 0.48.2
Figure 1.
Moderating Role of Gender in the Relation between Opioid Dependence and Distress Tolerance.
Note. Women-no opioid dependence mean latency to terminate = 159.08 (SD = 232.09, n = 53); Men-no opioid dependence mean latency to terminate = 241.92 (SD =260.59, n = 117); Women-opioid dependence mean latency to terminate = 278.89 (SD = 263.97, n = 18); Men-opioid dependence mean latency to terminate = 111.40 (SD = 186.22, n = 15). Bars with an asterisk indicate the presence of a significant difference at p < .05.
To ensure that the observed differences in DT were not due to the presence of other psychiatric disorders associated with low DT, we conducted a 2 (current opioid dependence vs. no history of opioid dependence) × 2 (men vs. women) analysis of covariance (ANCOVA) comparable to the ANOVA reported above but including covariates previously associated with low DT, including current major depressive disorder (Clen, Mennin, & Fresco, 2011), BPD (Gratz et al., 2006), number of current anxiety disorders including PTSD (Schmidt, Mitchell, Keough, & Riccardi, 2011; Vujanovic & Bernstein, 2011), and number of current substances on which participants were dependent (Richards, Daughters, Bornovalova, Brown, & Lejuez, 2011). As before, only a significant opioid dependence × gender interaction was found, F (1,195) = 5.35, p = .022, f = 0.15, with post-hoc analyses again revealing that men with current opioid dependence exhibited significantly lower DT than both women with current opioid dependence, F (1, 27) = 5.09, p = .032, f = .38, and men without a history of opioid dependence, F (1,126) = 4.42, p = .038, f = .16.
Discussion
The present study sought to examine whether patients in residential SUD treatment with current opioid dependence exhibit lower DT on a behavioral task than those without any history of opioid dependence, as well as the extent to which gender moderates this relation. Hypotheses were not supported; however, an unexpected finding emerged that warrants attention. Specifically, men with current opioid dependence exhibited significantly lower DT than both women with current opioid dependence and men without any history of opioid dependence. Although this finding was not expected, it is not entirely inconsistent with evidence from nonhuman studies showing that males are more likely to exhibit opioid-induced hyperalgesia than females (although human sex differences in hyperalgesia are less clear; Bodnar & Kest, 2010) – the presence of which could contribute to particularly low DT among males with opioid misuse (Garland et al., 2013). Likewise, given evidence of higher levels of emotional suppression (Gross & John, 2003), emotional inhibition (Matud, 2004), and impulsivity (Cross, Copping, & Campbell, 2011) among men (vs. women) in general, men may be less likely to persist in goal-directed behavior in the context of distress when other risk factors such as prolonged opioid misuse are present. The presence of lower DT among men with opioid dependence is consistent with both theoretical and empirical literature suggesting that opioid misuse is associated with low DT (McHugh et al., 2016) and that patients with substance dependence who use opioids are more likely to exhibit low DT than those without a history of opioid use (Lejuez et al., 2006). However, it is not clear why no differences in DT were found among women as a function of opioid dependence. Future research is needed to explore gender specific factors associated with low DT in the context of prolonged opioid misuse.
When considering the aforementioned findings, it is also important to note that the sample as a whole exhibited relatively low DT. Indeed, 75.4% of the full sample terminated the PASAT-C, consistent with past findings that patients in residential SUD treatment are characterized by low DT (Daughters et al., 2005a). Notably, this rate is much higher than in previous studies examining other psychiatric populations also characterized by low DT (e.g., outpatients with borderline personality disorder, where only 24% of participants terminated the PASAT-C; Gratz et al., 2006). Moreover, approximately 67% of women with current opioid dependence terminated the PASAT-C, and there was no significant difference in DT between women with current opioid dependence and those without any history of opioid dependence. These findings suggest that women with any substance dependence may exhibit lower DT regardless of the presence or absence of opioid dependence in particular.
Although findings highlight one modifiable characteristic associated with opioid dependence among men that could be targeted in interventions, these findings must be evaluated in light of the limitations that are present. First, given suggestions that behavioral and self-report DT measures may be capturing different components of the DT construct (McHugh et al., 2011), future research would benefit from the multimodal assessment of DT. Research that examines the unique relations of both perceived and behaviorally-demonstrated DT to opioid dependence may inform the development of more targeted and effective interventions for these patients. To date, only one study has taken this approach, finding that self-reported low DT, but not low DT on a behavioral measure, was associated with opioid misuse and affective responses to pain among patients with chronic pain (McHugh et al., 2016). Likewise, although the PASAT-C is an empirically supported laboratory measure of DT, it primarily induces distress in the form of anger and anxiety spectrum emotions (Lejuez et al., 2003). As a result, the PASAT-C may not induce the particular aversive emotional states most relevant to opioid misuse. Future research utilizing more personally-relevant or opioid-relevant stressors (e.g., examination of the willingness to tolerate physical pain or emotional distress associated with physical pain) may produce results with greater generalizability and/or clinical utility.
In addition, although our study provides additional support for the relevance of low DT to current opioid dependence in men, this study did not examine the relation of low DT to specific negative outcomes among patients with opioid dependence, such as treatment dropout or relapse. Future prospective studies are needed to evaluate low DT as a risk factor for these outcomes among patients with opioid dependence. In addition, this study focused specifically on the relation of opioid dependence in particular to low DT, given expectations that prolonged and frequent use of opioids would be most likely to contribute to reduced DT. Future research would benefit from examining opioid use dimensionally (i.e., opioid use severity) to better evaluate the role of opioid use severity in the development of low DT. Additionally, future research should examine whether the use of particular types of opioids (heroin vs. prescription opioids) is more likely to contribute to lower levels of DT. Given that this study focused on a specific clinical population – patients in residential SUD treatment – it is also not clear if the results will generalize to other populations shown to be at high risk for opioid misuse, such as patients with chronic pain (Ballantyne & LaForge, 2007) or other psychiatric disorders (Strain, 2002). Generalizability may be further affected by the modest number of men and women meeting criteria for current opioid dependence. Thus, in addition to replicating findings in different populations, future studies involving larger samples of patients with opioid dependence are needed. Finally, it is also worth noting that our data were cross-sectional in nature. Thus, it is not clear if the presence of current opioid dependence among men contributed to the development of low DT, if low DT operates as a risk factor for the development of opioid dependence in men, or if the opioid dependence-DT relation is bidirectional and transactional. Future research examining the complex interrelations of gender, DT, and opioid misuse and dependence over time is needed.
Despite limitations, findings provide further evidence for the relevance of low DT to opioid dependence (among at least some SUD patients) and suggest the utility of interventions that target low DT (e.g., Skills for Improving Distress Intolerance [SIDI]; Bornovalova et al., 2012) in the treatment of opioid dependence. SIDI is a brief (6-session) intervention that was designed to be easily integrated into SUD treatment. Relative to treatment as usual (residential SUD treatment) and supportive counseling, SIDI has been found to be associated with improvements in laboratory-assessed DT (Bornovalova et al., 2012). Given its brevity and demonstrated efficacy within SUD populations, SIDI may be a useful intervention for patients with opioid dependence in residential SUD treatment.
Acknowledgments
This study was supported in part by R21 DA022383 awarded to the first author (MTT) from the National Institute on Drug Abuse of the National Institutes of Health. NIDA had no role in the study design, collection, analysis, or interpretation of the data, writing of the manuscript, or the decision to submit the paper for publication.
Footnotes
Disclosure of Interest
The authors report no conflict of interest.
Hyperalgesia has also been found to occur in the context of alcohol withdrawal (Jochum, Boettger, Burkhardt, Juckel, & Bär, 2010).
Given that conditions (i.e., hyperalgesia) may occur in the context of alcohol abuse/dependence that contribute to low DT and thus could provide an alternative explanation for our findings, we conducted a 2 (alcohol dependence vs. no alcohol dependence) × 2 (men vs. women) ANOVA with latency to terminate the PASAT-C serving as the dependent variable. No significant main effects or interaction were found, Fs (1,199) < 2.21, ps > .138, fs < .08.
In addition, given our hypothesis that prolonged opioid misuse would contribute to the development of low DT, we also examined the effect of lifetime opioid dependence on DT with our full sample of participants with complete PASAT-C data (n = 222). Specifically, we conducted a 2 (lifetime opioid dependence vs. no lifetime opioid dependence) × 2 (men vs. women) ANOVA with latency to terminate the PASAT-C serving as the dependent variable. No significant main effects or interaction emerged. However, the interaction between lifetime opioid dependence and gender was associated with a significance level of .052, F (1, 218) = 3.81, p = .052, f = 0.11. The nature of this interaction was consistent with results obtained in our primary analyses. The non-significance of this finding could be attributed to our lack of data on when participants without current opioid dependence last met criteria for opioid dependence. If opioid dependence had not been present for some time, it would be expected to have less of an influence on DT levels.
References
- Ali B, Seitz-Brown CJ, & Daughters SB (2015). The interacting effect of depressive symptoms, gender, and distress tolerance on substance use problems among residential treatment-seeking substance users. Drug & Alcohol Dependence, 148, 21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan NP, Macatee RJ, Norr AM, Raines AM, & Schmidt NB (2015). Relations between common and specific factors of anxiety sensitivity and distress tolerance and fear, distress, and alcohol and substance use disorders. Journal of Anxiety Disorders, 33, 81–89. [DOI] [PubMed] [Google Scholar]
- Anestis MD, Lavender JM, Marshall-Berenz EC, Gratz KL, Tull MT, & Joiner TE (2012). Evaluating distress tolerance measures: Interrelations and associations with impulsive behaviors. Cognitive Therapy and Research, 36, 593–602. [Google Scholar]
- Back SE, Lawson KM, Singleton LM, & Brady KT (2011). Characteristics and correlates of men and women with prescription opioid dependence. Addictive Behaviors, 36, 829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, & Fiore MC (2004). Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychological Review, 111, 33–51. [DOI] [PubMed] [Google Scholar]
- Ballantyne JC, & LaForge SK (2007). Opioid dependence and addiction during opioid treatment of chronic pain. Pain, 129, 235–255. [DOI] [PubMed] [Google Scholar]
- Becker WC, Sullivan LE, Tetrault JM, Desai RA, & Fiellin DA (2008). Non-medical use, abuse and dependence on prescription opioids among US adults: psychiatric, medical and substance use correlates. Drug and Alcohol Dependence, 94, 38–47. [DOI] [PubMed] [Google Scholar]
- Bernstein A, Marshall EC, & Zvolensky MJ (2011). Multi-method evaluation of distress tolerance measures and construct(s): Concurrent relations to mood and anxiety psychopathology and quality of life. Journal of Experimental Psychopathology, 2, 386–399. [Google Scholar]
- Bernstein A, Zvolensky MJ, Vujanovic AA, & Moos R (2009). Integrating anxiety sensitivity, distress tolerance, and discomfort intolerance: A hierarchical model of affect sensitivity and tolerance. Behavior Therapy, 40, 291–301. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy L, Kaloupek DG, Klauminzer G, Charney DS, & Keane TM (1990). The Clinician Administered PTSD Scale. Boston: National Center for PTSD- Behavioral Science Division. [Google Scholar]
- Bodnar RJ, & Kest B (2010). Sex differences in opioid analgesia, hyperalgesia, tolerance and withdrawal: central mechanisms of action and roles of gonadal hormones. Hormones and Behavior, 58, 72–81. [DOI] [PubMed] [Google Scholar]
- Bornovalova MA, Gratz KL, Daughters SB, Hunt ED, & Lejuez CW (2012). Initial RCT of a distress tolerance treatment for individuals with substance use disorders. Drug and Alcohol Dependence, 122, 70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KT, & Sinha R (2005). Co-occurring mental and substance use disorders: the neurobiological effects of chronic stress. American Journal of Psychiatry, 162, 1483–1493. [DOI] [PubMed] [Google Scholar]
- Carcoba LM, Contreras AE, Cepeda-Benito A, & Meagher MW (2011). Negative affect heightens opiate withdrawal-induced hyperalgesia in heroin dependent individuals. Journal of Addictive Diseases, 30, 258–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalana H, Sachdeva JK, Kundal T, Malhari AS, & Choudhary R (2015). A double-blind, placebo-controlled, randomized study comparing quetiapine with placebo, along with oral naltrexone, in the treatment of opioid dependent patients. Journal of Evolution of Medical and Dental Sciences, 4, 9158–9167. [Google Scholar]
- Chen KW, Banducci AN, Guller L, Macatee RJ, Lavelle A, Daughters SB, & Lejuez CW (2011). An examination of psychiatric comorbidities as a function of gender and substance type within an inpatient substance use treatment program. Drug & Alcohol Dependence, 118, 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu LF, Angst MS, & Clark D (2008). Opioid-induced hyperalgesia in humans: Molecular mechanisms and clinical considerations. The Clinical Journal of Pain, 24, 479–496. [DOI] [PubMed] [Google Scholar]
- Clen SL, Mennin DS, & Fresco DM (2011). Major depressive disorder. In Zvolensky MJ, Bernstein A, & Vujanovic AA (Eds.), Distress Tolerance: Theory, Research, and Clinical Applications (pp. 149–170). New York, NY: Guilford Press. [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Cross CP, Copping LT, & Campbell A (2011). Sex differences in impulsivity: A meta-analysis. Psychological Bulletin, 137, 97–130. [DOI] [PubMed] [Google Scholar]
- Cummings JR, Bornovalova MA, Ojanen T, Hunt E, MacPherson L, & Lejuez C (2013). Time doesn’t change everything: The longitudinal course of distress tolerance and its relationship with externalizing and internalizing symptoms during early adolescence. Journal of Abnormal Child Psychology, 41, 735–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughters SB, Lejuez CW, Bornovalova MA, Kahler CW, Strong DR, & Brown RA (2005a). Distress tolerance as a predictor of early treatment dropout in a residential substance abuse treatment facility. Journal of Abnormal Psychology, 114, 729–734. [DOI] [PubMed] [Google Scholar]
- Daughters SB, Lejuez CW, Kahler CW, Strong DR, & Brown RA (2005b). Psychological distress tolerance and duration of most recent abstinence attempt among residential treatment-seeking substance abusers. Psychology of Addictive Behaviors, 19, 208–211. [DOI] [PubMed] [Google Scholar]
- Daughters SB, Sargeant MN, Bornovalova MA, Gratz KL, & Lejuez CW (2008). The relationship between distress tolerance and antisocial personality disorder among male inner-city treatment seeking substance users. Journal of Personality Disorders, 22, 509–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El S, Gaily El S, & Bashir TZ (2004). High-risk relapse situations and self-efficacy: Comparison between alcoholics and heroin addicts. Addictive Behaviors, 29, 753–758. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JBW (1996). Structured clinical interview for DSM-IV Axis I Disorders – Patient edition (SCIP-I/P, Version 2.0). New York, NY: New York State Psychiatric Institute. [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189–198. [DOI] [PubMed] [Google Scholar]
- Garland EL, Bryan CJ, Nakamura Y, Froeliger B, & Howard MO (2017). Deficits in autonomic indices of emotion regulation and reward processing associated with prescription opioid use and misuse. Psychopharmacology, 234, 621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Froeliger B, Zeidan F, Partin K, & Howard MO (2013). The downward spiral of chronic pain, prescription opioid misuse, and addiction: Cognitive, affective, and neuropsychopharmacologic pathways. Neuroscience & Biobehavioral Reviews, 37, 2597–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Hanley AW, Thomas EA, Knoll P, & Ferraro J (2015). Low dispositional mindfulness predicts self-medication of negative emotion with prescription opioids. Journal of Addiction Medicine, 9, 61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Ali B, & Daughters SB (2012). The role of distress tolerance in the relationship between depressive symptoms and problematic alcohol use. Psychology of Addictive Behaviors, 26, 621–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossop M, Stewart D, Browne N, & Marsden J (2002). Factors associated with abstinence, lapse or relapse to heroin use after residential treatment: protective effect of coping responses. Addiction, 97, 1259–1267. [DOI] [PubMed] [Google Scholar]
- Gratz KL, Hepworth C, Tull MT, Paulson A, Clarke S, Remington B, & Lejuez CW (2011). An experimental investigation of emotional willingness and physical pain tolerance in deliberate self-harm: The moderating role of interpersonal distress. Comprehensive Psychiatry, 52, 63–74. [DOI] [PubMed] [Google Scholar]
- Gratz KL, Rosenthal MZ, Tull MT, Lejuez CW, & Gunderson JG (2006). An experimental investigation of emotion dysregulation in borderline personality disorder. Journal of Abnormal Psychology, 115, 850–855. [DOI] [PubMed] [Google Scholar]
- Gross JJ, & John OP (2003). Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. Journal of Personality and Social Psychology, 85, 348–362. [DOI] [PubMed] [Google Scholar]
- Hearon BA, Calkins AW, Halperin DM, McHugh RK, Murray HW, & Otto MW (2011). Anxiety sensitivity and illicit sedative use among opiate-dependent women and men. The American Journal of Drug and Alcohol Abuse, 37, 43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamison RN, Butler SF, Budman SH, Edwards RR, & Wasan AD (2010). Gender differences in risk factors for aberrant prescription opioid use. The Journal of Pain, 11, 312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochum T, Boettger MK, Burkhardt C, Juckel G, & Bär KJ (2010). Increased pain sensitivity in alcohol withdrawal syndrome. European Journal of Pain, 14, 713–718. [DOI] [PubMed] [Google Scholar]
- Kaiser AJ, Milich R, Lynam DR, & Charnigo RJ (2012). Negative urgency, distress tolerance, and substance abuse among college students. Addictive Behaviors, 37, 1075–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselica AM, Webber T, & Bornovalova MA (2014). Stability and change in distress tolerance and its prospective relationship with borderline personality features: A short-term longitudinal study. Personality Disorders: Theory, Research, and Treatment, 5, 247–256 [DOI] [PubMed] [Google Scholar]
- LeBlanc DM, McGinn MA, Itoga CA, & Edwards S (2015). The affective dimension of pain as a risk factor for drug and alcohol addiction. Alcohol, 49, 803–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejuez CW, Kahler CW, & Brown RA (2003). A modified computer version of the Paced Auditory Serial Addition Task (PASAT) as a laboratory-based stressor. The Behavior Therapist, 26, 290–293. [Google Scholar]
- Lejuez CW, Paulson A, Daughters SB, Bornovalova MA, & Zvolensky MJ (2006). The association between heroin use and anxiety sensitivity among inner-city individuals in residential drug use treatment. Behaviour Research and Therapy, 44, 667–677. [DOI] [PubMed] [Google Scholar]
- Leukefeld CG, & Tims FM (1986). Relapse and recovery: Some directions for research and practice. NIDA Monograph: Relapse and Recovery in Drug Abuse, 72, 185–190. [PubMed] [Google Scholar]
- Matud MP (2004). Gender differences in stress and coping styles. Personality and Individual Differences, 37, 1401–1415. [Google Scholar]
- McHugh RK, Daughters SB, Lejuez CW, Murray HW, Hearon BA, Gorka SM, & Otto MW (2011). Shared variance among self-report and behavioral measures of distress intolerance. Cognitive Therapy and Research, 35, 266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RK, DeVito EE, Dodd D, Carroll KM, Potter JS, Greenfield SF, … & Weiss RD (2013). Gender differences in a clinical trial for prescription opioid dependence. Journal of Substance Abuse Treatment, 45, 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RK, Weiss RD, Cornelius M, Martel MO, Jamison RN, & Edwards RR (2016). Distress intolerance and prescription opioid misuse among patients with chronic pain. The Journal of Pain, 17, 806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Kaneko C, Miyoshi K, Nagumo Y, Kuzumaki N, Nakajima M, … & Suzuki T (2006). Chronic pain induces anxiety with concomitant changes in opioidergic function in the amygdala. Neuropsychopharmacology, 31, 739–750. [DOI] [PubMed] [Google Scholar]
- Richards JM, Daughters SB, Bornovalova MA, Brown RA, & Lejuez CW (2011). Substance use disorders. In Zvolensky MJ, Bernstein A, & Vujanovic AA (Eds.), Distress Tolerance: Theory, Research, and Clinical Applications (pp. 171–197). New York, NY: Guilford Press. [Google Scholar]
- Schmidt NB, Mitchell M, Keough M, & Riccardi C (2011). Anxiety and its disorders. In Zvolensky MJ, Bernstein A, & Vujanovic AA (Eds.), Distress Tolerance: Theory, Research, and Clinical Applications (pp. 105–125). New York, NY: Guilford Press. [Google Scholar]
- Simons JS, & Gaher RM (2005). The Distress Tolerance Scale: Development and validation of a self-report measure. Motivation and Emotion, 29, 83–102. [Google Scholar]
- Strain EC (2002). Assessment and treatment of comorbid psychiatric disorders in opioid-dependent patients. The Clinical Journal of Pain, 18, S14–S27. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) (2017). 2016 national survey on drug use and health. Rockville, MD: SAMHSA. [Google Scholar]
- Trafton JA, & Gifford EV (2011). Biological bases of distress tolerance. In Zvolensky MJ, Bernstein A, & Vujanovic AA (Eds.), Distress Tolerance: Theory, Research, and Clinical Applications (pp. 80–102). New York, NY: Guilford Press. [Google Scholar]
- Tull MT, Bardeen JR, DiLillo D, Messman-Moore T, & Gratz KL (2015). A prospective investigation of emotion dysregulation as a moderator of the relation between posttraumatic stress symptoms and substance use severity. Journal of Anxiety Disorders, 29, 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tull MT, Gratz KL, Coffey SF, Weiss NH, & McDermott MJ (2013). Examining the interactive effect of posttraumatic stress disorder, distress tolerance, and gender on residential substance use disorder treatment retention. Psychology of Addictive Behaviors, 27, 763–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vujanovic AA, & Bernstein A (Eds.). (2011). Traumatic stress. In Zvolensky MJ, Bernstein A, & Vujanovic AA (Eds.), Distress Tolerance: Theory, Research, and Clinical Applications (pp. 126–148). New York, NY: Guilford Press. [Google Scholar]
- Welch BL (1947). The generalization of ‘student’s’ problem when several different population variances are involved. Biometrika, 34, 28–35. [DOI] [PubMed] [Google Scholar]
- Zanarini MC, Frankenburg FR, Sickel AE, & Young L (1996). Diagnostic interview for DSM-IV personality disorders. Unpublished measure. Boston, MA: McLean Hospital. [Google Scholar]