Abstract
More than one-third of patients with locally advanced cervical cancer do not respond to chemoradiation therapy (CRT). We aimed to characterize the transcriptional landscape of paired human cervical tumors before and during CRT in order to gain insight into the evolution of treatment response and to elucidate mechanisms of treatment resistance. We prospectively collected cervical tumor biopsies from 115 patients both before and 3 weeks into CRT. RNA-sequencing, Gene Set Enrichment Analysis, and HPV gene expression was performed on 20 paired samples that had adequate neoplastic tissue mid-treatment. Tumors from patients with no evidence of disease (NED) at last follow-up had enrichment in pathways related to the immune response both pre- and mid-treatment, while tumors from patients dead of disease (DOD) demonstrated enrichment in biosynthetic and mitotic pathways but not in immune-related pathways. Patients DOD had decreased expression of T-cell and cytolytic genes and increased expression of PD-L2 mid-treatment compared to patients NED. Histological and immunohistochemical analysis revealed a decrease in tumor-associated lymphocytes (TAL) during CRT in all patients but tumors from patients DOD had a significantly more pronounced decrease in TALs and CD8+ cells mid-treatment, which was validated in a larger mid-treatment cohort. Finally, patients DOD retained more HPV E6/E7 gene expression during CRT and this was associated with increased expression of genes driving mitosis, which was corroborated in vitro. Our results suggest that decreased local immune response and retained HPV gene expression may be acting together to promote treatment resistance during CRT in patients with cervical cancer.
Keywords: Cervical cancer, HPV, immune response, radiation therapy, lymphocyte
Introduction
Cervical cancer has a global mortality rate of 50%1 and is second only to brain cancer for the highest average years of life lost.2 Management of locally advanced cervical cancer has not changed in over 20 years and platinum-based chemotherapy concurrent with pelvic radiation and brachytherapy (chemoradiation, CRT) remains the standard of care. It is therefore not surprising that the 5-year survival has remained stagnant at 70% since the 1990’s, demanding improved therapeutic strategies.
Approximately 30% of cervical cancer patients do not respond completely to CRT as evidenced by persistent or new sites of 18F-fluorodeoxyglucose (FDG) uptake on post-therapy positron emission tomography (PET) and these patients have a 5-year cause-specific survival of only 33% and 10% respectively.3 Treatment resistance and tumor recurrence is therefore a significant clinical problem, yet the mechanisms underlying this are largely unknown and it is very challenging to identify which patients will fail therapy.
Transcriptional profiling of tumor tissue is a well-established method to risk stratify patients and has resulted in clinically applicable assays in both breast and prostate cancers4,5 yet similar studies in cervical cancer are lacking. Gene expression profiling of cervical tumors prior to treatment has been reported6–8 but none thus far have been translated to the clinic. We hypothesized that comprehensive gene expression profiling of cervical tumors both before and during CRT would provide novel insight into the mechanisms of treatment resistance.
Approximately 90–99% of cervical cancers are positive for human papillomavirus (HPV), and the viral oncoproteins E6 and E7 are known to contribute to carcinogenesis, cell growth, and immune evasion.9 Very little is known regarding how HPV oncogene expression changes during CRT and whether this affects treatment response. There is ample evidence to suggest that HPV E6/E7 increases radio-resistance in cervical cancer10,11 and siRNA against E6/E7 has been shown to radiosensitize cervical cancer cells in vitro and in vivo.12 Accordingly, E6 mRNA expression is associated with worse survival in cervical cancer13 and our group has shown that high tumoral levels of HPV E6/E7 mRNA predict for distant metastasis and poor survival in patients with HPV-positive oropharyngeal squamous cell carcinoma, despite their generally good prognosis.14 Determining how viral gene expression changes during CRT is likely to provide valuable insight into treatment resistance and may allow for improved prognostication of patients during therapy.
The goal of this study was to characterize the transcriptional landscape of human cervical tumors before and during CRT in order to gain insight into the genes and biological pathways that are associated with treatment resistance. To this end, we prospectively collected cervical tumor tissues before and 3 weeks into CRT and performed RNA sequencing (RNA-seq) to study the changes in both human and viral gene expression during therapy. This unique insight into how individual tumors respond to CRT revealed significant differences in gene expression between patients who died of disease and those who were cured, implicating the immune response and HPV gene expression in this differential outcome.
Materials and Methods
Patients and Treatment
Patients with biopsy proven cervical carcinoma were enrolled with consent into an IRB-approved prospective study in which cervical tumor biopsies were obtained both before and 3 weeks into CRT (mid-treatment). All patients were treated with definitive CRT, which consisted of external beam radiotherapy (EBRT) to the pelvis (50.4 Gy) four days per week using intensity modulated radiation therapy (IMRT) with a split-pelvis technique with six weekly brachytherapy boosts to a dose of 6.5 Gy per fraction to point A, starting at week 1. All patients received concurrent weekly cisplatin (40 mg/m2). Para-aortic lymph node regions were included if involved on pre-treatment FDG-PET and EBRT dose reduction of 10% was employed for elderly patients or those with a body mass index of less than 18.5 kg/m2.15 The mid-treatment sample was collected immediately prior to delivery of the third brachytherapy treatment (Figure 1A). Samples were collected and immediately snap frozen and processed for storage in the Siteman Tissue Bank (STB). Only patients with known outcome due to cervical cancer are included in this analysis.
Figure 1:

(A) Schematic of patient biopsy analysis and experimental cohort. In the treatment paradigm (top), each vertical line represents one week of external beam radiation therapy with concurrent weekly cisplatin. The mid-treatment biopsy was obtained during the third week of CRT, immediately prior to the 3rd brachytherapy treatment. Hematoxylin and Eosin (H&E) stained, and formalin fixed paraffin embedded (FFPE) samples were used to quantify the percentage of tumor associated lymphocytes (TAL) and CD4 and CD8 cells in the biopsy samples, respectively. (B) Example of H&E stained cervical tumor biopsies both pre-treatment and mid-treatment. In the mid-treatment figure, dashed circle indicates area of tumor-associated lymphocytes and solid circle indicates an area containing neoplastic cells. Scale bar, 200 μm.
Tumor processing and RNA isolation
All 115 mid-treatment samples with adequate tissue (defined as having matching pre-treatment tissue and tissue weight > 0.03 mg) available in the STB at the time of initiation of this study were processed as follows: First, frozen sections were stained with Hematoxylin and Eosin (H&E) and reviewed by a board certified pathologist who verified the diagnosis, and determined the percentage of neoplastic cells, lymphocytes, and necrosis. All tumor samples were processed identically, in a blinded fashion, and reviewed (by PFC) with the pathologist and technician performing macrodissection and in general, samples with >50% neoplastic cellularity were selected for further processing. Neoplastic areas as demarcated by the pathologist were macrodissected and in a few select cases, entire sections were used if the neoplastic area represented >70% of the specimen. After the mid-treatment specimen was deemed adequate for further analysis, the matching pre-treatment specimen was processed in a similar manner. RNA was then isolated from macrodissected frozen tissue with TRIzol reagent (Ambion) as per manufacturer instructions. Total RNA was treated with DNase, purified using the RNeasy Micro Kit (Qiagen) and then assessed for concentration and purity. Total RNA was kept frozen at −80 °C for further use.
RNA-sequencing
200 ng of total RNA was used to construct cDNA libraries as previously described.16 150 rRNA-specific biotinylated DNA oligo probes were used to remove rRNA. This method allows for detection of non-polyadenylated transcripts as found in viruses and humans as well as many non-coding RNAs. rRNA-depleted RNA was then fragmented, reversed transcribed, and ligated to adaptors for PCR amplification of the cDNA libraries using NEB RNA Library Prep kits. Multiplexed sequencing was performed by the Genomics Core at Washington University using the Illumina HiSeq 2500 platform. Raw RNA-seq data was processed to remove low quality reads and were mapped to the human transcriptome and known viral genomes using Bowtie.17 Gene expression analysis was performed using DESeq2.18 RNA-seq was performed in five batches and paired samples were always in the same batch. Pairwise analysis and trimmed mean normalization for all samples was performed to offset the batch effect.
HPV gene expression analysis
HPV type and E6/E7 expression were determined by quantitative real-time polymerase chain reaction (qPCR) using RNA extracted from the macrodissected frozen tissue specimens, with PCR primers specific to E6 and E7 for 13 HPV types as previously described.19 HPV type was determined from the pre-treatment biopsies.
Gene set enrichment analysis (GSEA)
Gene expression values were filtered based on the mean of reads for all samples with mean <50 removed from analysis. Greater than 13,000 genes per study cohort were entered into each GSEA analysis. The C5 Gene Ontology biological processes gene set from the Molecular Signatures Database (MSigDB)20,21 which contains 4,436 gene sets, was used to analyze biological pathways altered during CRT. Per program author guidelines, gene set enrichment with a more stringent cutoff of FDR<5% was used in cases where there were ≤ 7 patients in a group during analysis, whereas phenotype permutation with FDR<25% was used for larger group sizes.
Lymphocyte Analysis and Immunohistochemistry
The percent of tumor-associated lymphocytes (TAL) was determined from H&E stained sections from frozen mid-treatment cervical tumor biopsies with known outcome due to cervical cancer (n=98). The validation cohort was composed of the 78 patients that were not included in the RNA-seq study. Pre-treatment specimens were not analyzed for all of these patients given the scarcity of samples. The first 55 patients of the 98 patients in alphabetical order (including 8 samples from our 20 patient cohort due to the scarcity of mid-treatment samples) along with their matching pre-treatment specimen were selected for immunohistochemistry (IHC). To accomplish this, a small portion of frozen tissue samples was cut and immediately placed into formalin for fixation followed by embedding in paraffin. H&E staining and immunohistochemistry for CD4 and CD8 was then performed on paraffin embedded blocks using procedures validated for diagnostics in a fully automated system (Benchmark XT, Ventana). Antibodies used were Ventana pre-diluted antibodies; CD4 (SP35) and CD8 (SP57). CD4 and CD8 cell counts are given as mean per 10 high-power fields (HPF).
Cell culture
Primary human foreskin keratinocytes (HFK) were prepared from neonatal foreskins obtained from the University of Massachusetts Memorial Hospital as previously described.22 HFKs were infected with E6 or E7 retroviral constructs or a control Babe-puro-based vector as previously described22 and were maintained on mitomycin-C treated J23T3 feeder cells in F medium. Cells within eight passages after drug selection were harvested for RNA sequencing using the methods described above.
Statistics
Statistical analysis for differential gene expression was provided through the DESeq2 package. The Benjamini and Hochberg method was used to adjust the p-values for multiple testing (adjusted p-value < 0.05 regarded as significant). Statistical significance for means for the evaluated clinical parameters was calculated with a 2-sided Student’s t-test and with a Welch’s t-test in the case of tumor lymphocyte counts or Wilcoxon rank-sum test in the case of CD4 and CD8 cells due to the unequal sample sizes and variances.
Results
Clinical Characteristics
At the time of initiation of this study, 115 mid-treatment frozen tissue samples with adequate tissue were available for analysis. H&E staining and pathologic review of the mid-treatment samples revealed that the majority of samples were necrotic or were composed largely of granulation tissue with lymphocytes without neoplastic tissue, as expected after three weeks of CRT. Twenty-two mid-treatment samples had adequate neoplastic tissue for further study. One sample did not have measurable RNA after isolation and one patient was found to have died of other causes. Thus our final cohort on which we report RNA-seq consisted of 20 matched pairs with known outcome due to cervical cancer. With a median follow up of 3.1 years, 7 patients were dead of disease (DOD) while 13 patients had no evidence of disease (NED). Sample processing schema and patient characteristics are shown in Figure 1A and Table 1, respectively. Patients were treated with a median of 5040 cGy to the pelvis with weekly brachytherapy to a total median dose of 3900 cGy. One patient did not complete EBRT (received 18 Gy) but received all prescribed brachytherapy.
Table 1.
Clinical characteristics of RNA-seq cohort
| Clinical Characteristic | Value (%) |
|---|---|
| FIGO stage | |
| IB1 | 1 (5) |
| IB2 | 4 (20) |
| IIB | 11 (55) |
| IIIB | 4 (20) |
| Nodal involvement on PET | |
| Pelvic | 10 (50) |
| Pelvic and Para-aortic | 1 (5) |
| None | 9 (45) |
| Histology | |
| Squamous | 19 (95) |
| Adenocarcinoma | 1 (5) |
| HPV type prior to CRT | |
| HPV 16 | 11 (55) |
| HPV 18 | 3 (15) |
| HPV 33 | 1 (5) |
| HPV 45 | 1 (5) |
| HPV 52 | 1 (5) |
| HPV 68 | 1 (5) |
| HPV negative | 2 (10) |
| Outcome | |
| Dead of disease (DOD) | 7 (35) |
| No Evidence of Disease (NED) | 13 (65) |
Our institution has previously reported the predictive value of 3 month post-treatment PET on disease recurrence3 and this data was available for 19/20 patients. 57% (4/7) of patients DOD had a partial metabolic response (PMR) on post-treatment PET with residual uptake in the cervix. The remaining three patients had a complete metabolic response (CMR) in the cervix but developed new metastatic disease consisting of either pelvic, para-aortic, or supraclavicular lymph nodes. Thus all patients who died of disease either had persistent uptake in the cervix, new sites of metastatic disease or both 3 months after CRT, indicating a resistance to treatment. Conversely, 85% (11/13) of patients NED had a CMR to treatment, one had PMR and one patient did not receive a PET at this time point.
Tumor Characteristics
The majority of tumors (18/20) were HPV positive with HPV16 being the most common subtype, while two tumors were HPV negative (Table 1). This HPV subtype distribution is similar to published series23 implying that the presence of neoplastic tissue after 3 weeks of CRT was not associated with a specific HPV type. HPV types of patients DOD were HPV16+ (n=3), HPV18+ (n=3), and HPV 68+ (n=1), and those of patients NED were HPV16+ (n=8), HPV33, 45 and 52 (n=1 of each) and HPV negative (n=2).
The mean percent neoplastic cells, lymphocytes, and necrosis in biopsy samples of the entire 98 mid-treatment patient cohort were 19%, 6%, and 21% respectively. The same values for our 20 patient cohort were 55%, 7% and 6% respectively. Representative H&E stained samples are shown in Figure 1B. Matching pre-treatment tumor biopsies for each mid-treatment sample in the 20 patient cohort were analyzed in a similar manner. As expected, pre-treatment samples had a higher percentage of neoplastic cells (67% v. 55%, p=0.02) but no significant difference in necrosis (10.5% v. 6.3%, p=0.3) or percent lymphocytes (12.2% v. 6.7%, p=0.1) compared to mid-treatment samples, though it is evident that lymphocyte numbers were nearly halved in all patient tumors during CRT. There were no statistically significant differences in the above values between patients DOD and NED at either time point (Supplementary Table 1), with exception to percentage of lymphocytes, which will be discussed below.
Gene expression changes during CRT
To determine how CRT affects gene expression in human cervical tumors, we performed RNA-seq on the 20 matched pre- and mid-treatment biopsies. First, differential expression analysis was performed using paired pre-versus-mid-treatment values in order to determine global gene expression changes that occur with CRT in all patients. Overall, CRT induced a change in expression of 5,593 genes (>1.5 fold with padj<0.05, Figure 2A). GSEA was performed to determine the biologically relevant pathways represented by these gene expression changes. Pathways enriched pre-treatment with p<0.01 largely represented chromatin regulation and packaging, RNA processing/transcription, DNA repair and cell cycle pathways (Supplementary Table 2). Histone expression was consistently decreased during CRT in all samples which has been previously shown to occur with radiation24,25 largely due to cell cycle check points and inhibition of DNA synthesis following DNA damage. Clustering analysis of the top differentially expressed genes revealed a difference in histone and minichromosome maintenance (MCM) gene expression (downregulated mid-treatment), and genes associated with cellular stress and apoptosis (upregulated mid-treatment) between the two time points (Figure 2B, gene lists Supplementary Table 3). Despite these differences, there were no pathways significantly enriched in the mid-treatment cohort (with FDR<25%), likely due to variation in response to CRT when all patients were analyzed together regardless of clinical outcome. A two-by-two paired differential analysis in DESeq2 revealed changes in only 4 genes with padj <0.05 (CASP14, ELMOD2, UGGT2, and SLC35B3).
Figure 2:
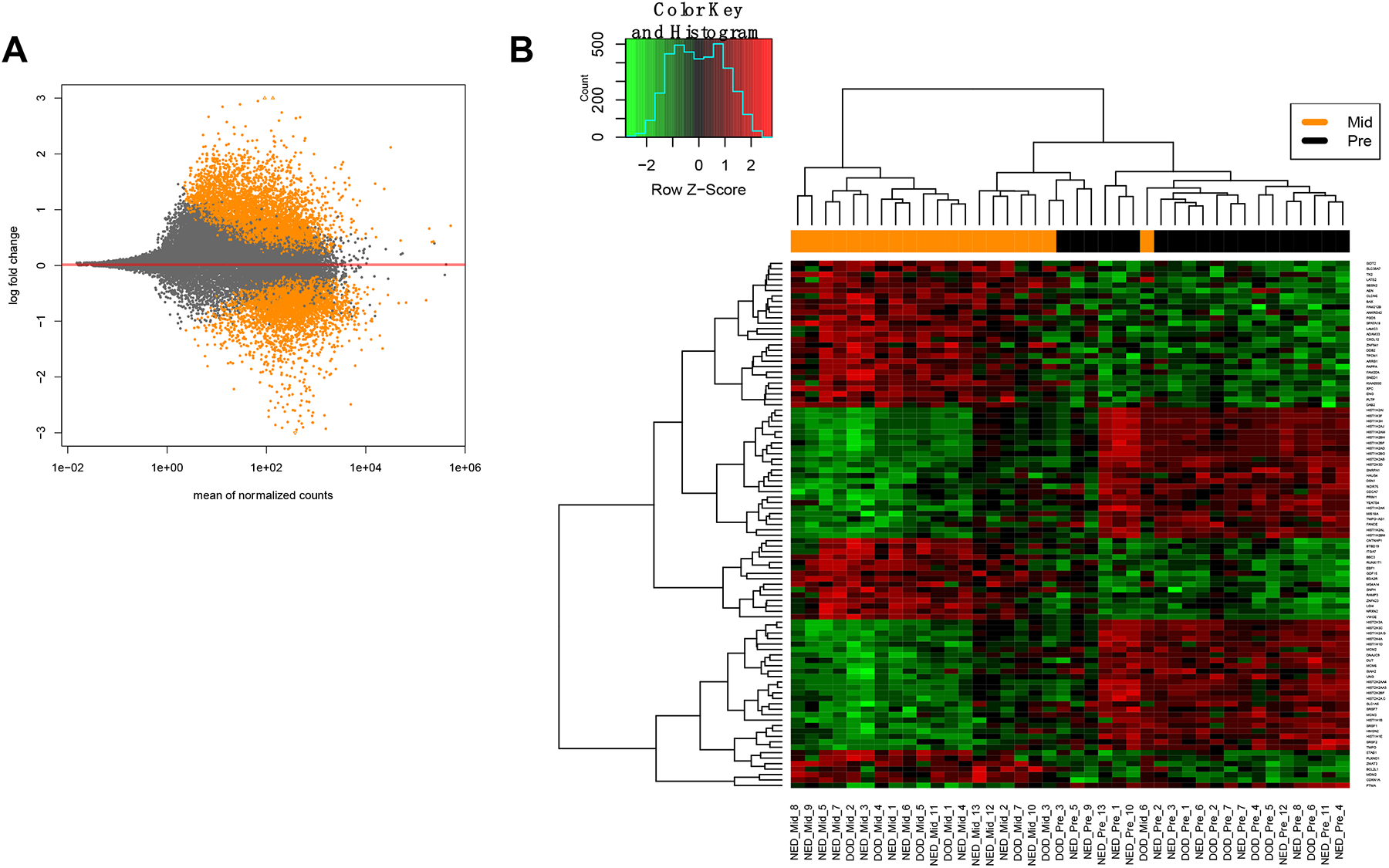
Gene expression changes during CRT in the RNA-seq cohort of 20 matched patient pairs. Mid-treatment time-point represents 3 weeks into CRT. (A) MA plot (mean expression v. log2 fold change) depicting gene expression changes from pre- to mid-treatment. Each orange dot represents one gene with expression fold change>1.5 with padj< 0.05. (B) Heatmap depicting clustered and differentially expressed genes from pre- to mid-treatment in all patients. A list of these genes can be found in Supplementary Table 3.
Gene expression changes during CRT are associated with patient outcome
We then compared gene expression changes during CRT in patients with NED versus those DOD and analyzed paired pre-vs-mid-treatment differential expression to determine genes that may drive or predict patient outcome. As shown in Figure 3A, patients within the DOD cohort exhibited alterations in only 655 genes (362 upregulated and 293 downregulated, fold change (FC)>1.5, padj<0.05), while patients NED had significantly more gene expression changes with 5533 genes altered during treatment (2956 upregulated and 2577 downregulated, FC>1.5, padj<0.05), suggesting significant differences in response to CRT between the two cohorts. Further comparison of the genes altered between each cohort reveal only 138 genes exclusively altered in DOD patients, while NED patients had 5,016 genes exclusively altered (Figure 3A). This result suggests that tumors of patients NED exhibit a coordinated response to therapy with consistent changes in gene expression during CRT while tumors of patients DOD do not, which may explain their resistance to treatment. Additionally, it may explain why there were no pathways enriched mid-treatment in the GSEA analysis when all patients (DOD+NED) were analyzed together, as it implies a heterogeneous response to CRT. Clustering analysis of the top 50 differentially expressed genes in NED v. DOD at each time point are shown in Supplementary Figure 1, with differentially expressed genes provided in Supplementary Tables 4 and 5.
Figure 3:

Transcriptomic profiling and histological analysis revealed differences between patients DOD and NED in the local immune response to the tumor during CRT. (A) Summary of gene expression changes revealing genes exclusively altered in NED (blue) and DOD (red), and genes commonly altered in both groups (purple) using matched analysis. Colored dots represent fold expression change > 1.5 with padj< 0.05. (B) Gene Set Enrichment Analysis (GSEA) using C5 biological processes gene set comparing each outcome group both before and during CRT. All pathways were enriched with FDR<25% and p<0.01. Donut charts were created by sorting all enriched pathways (or the top 100 pathways for patients DOD) into biological categories, which are proportionally represented by a section of the chart. The majority of pathways enriched in patients NED were involving the immune response, while patients DOD displayed a more “pro-survival” transcriptome, especially mid-treatment.
To gain insight into biological pathways involved in the differential response to CRT, we again performed a GSEA comparing biologic processes in each outcome group at a specific time point. Patients DOD had enrichment in pathways generally involved in biosynthesis such as ribosome biogenesis, transcription/translation, and cellular metabolism both pre- and mid-treatment, whereas pathways related to DNA replication, cell division and DNA repair were specifically enriched mid-treatment. Interestingly, patients NED displayed strong enrichment in pathways related to the immune system at both time points. The difference was so apparent such that patients NED had 67% (38/56) and 80% (55/69) of pathways (FDR<25%, p<0.01) related to the immune system enriched pre- and mid-treatment respectively, while patients DOD had 0/402 enriched pathways involving the immune response pre-treatment and 0.6% (2/326 pathways, FDR<25% and p<0.01) mid-treatment (Figure 3B). Together, these data indicate a differential immune response to the tumor during CRT that may be contributing to patient outcome.
Tumors from patients DOD have decreased lymphocyte infiltration and expression of T-cell genes during CRT
To determine whether the differential immune response seen in GSEA translates to differences in lymphocyte infiltration, we determined the percentage of TALs by H&E both before and during CRT in our 20 patient cohort. While TALs decreased in all patients during therapy, patients DOD had fewer lymphocytes in their tumor biopsy than patients NED at the mid-treatment time point (2% v. 9%, p=0.01), while there was no significant difference pre-treatment (8% v. 15%, p=0.2) (Figure 4A). To validate these findings, we performed the same analysis in 78 additional mid-treatment cervical biopsies and again found that tumors of patients DOD contained significantly fewer lymphocytes than patients NED mid-treatment (3% v. 8%, p=0.02) (Figure 4B).
Figure 4:
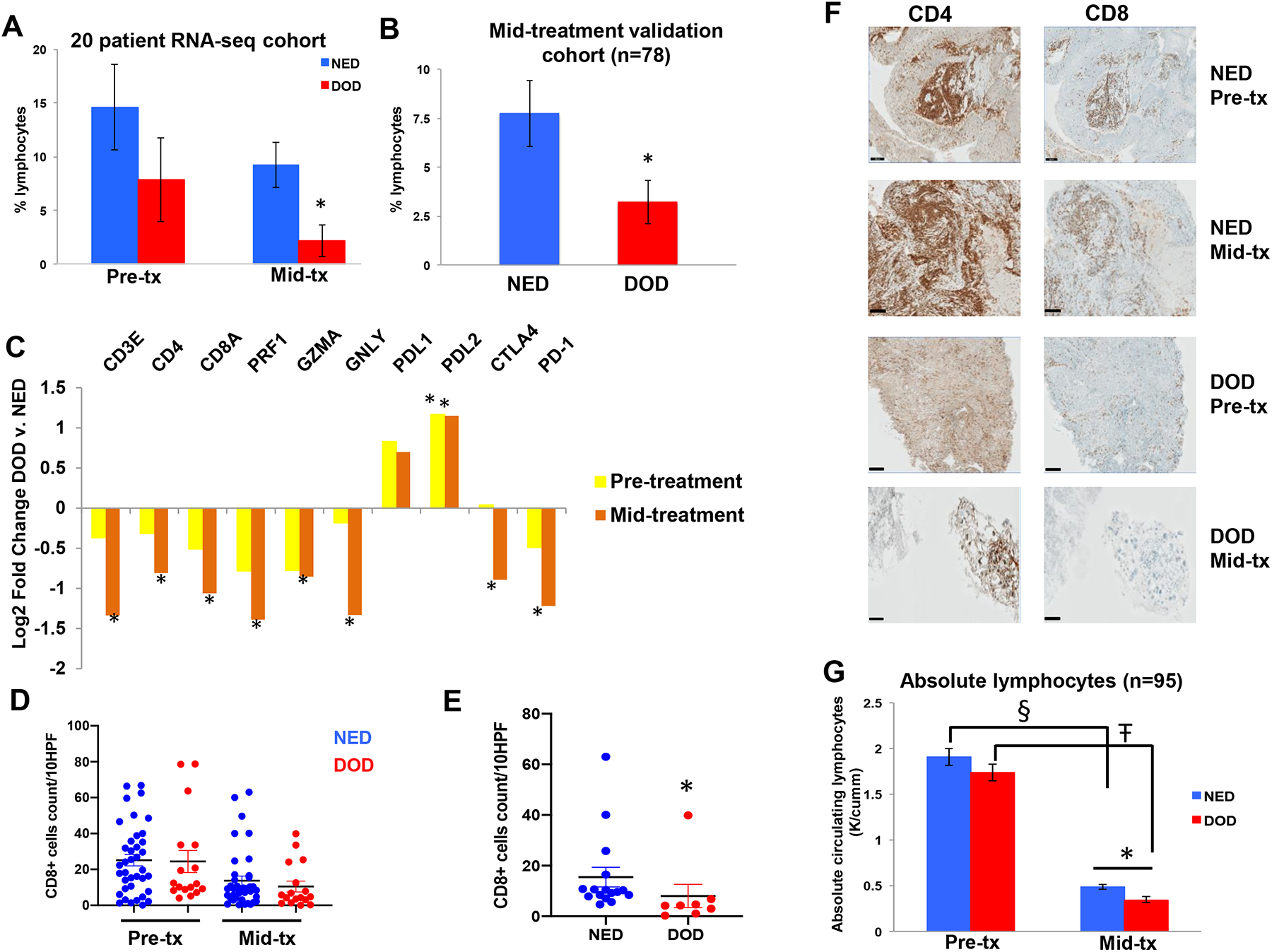
Effect of CRT on tumor-associated lymphocytes and CD4 and CD8 T-cell infiltration. (A) Percent tumor-associated lymphocytes in cervical tumor biopsies in the 20 patient RNA-seq cohort, and (B) in the mid-treatment validation cohort (n=78, (DOD = 25, NED = 53)). (C) Differential expression of immune specific genes at each time point in patients DOD and NED (expressed as fold-change DOD versus NED). (D) Quantification of CD8+ T-cells using IHC both before and during CRT in all 55 patient samples, which did not reveal a difference. (E) Quantification of CD8+ T-cells using IHC in mid-treatment cervical tumor biopsies with > 10% neoplastic cells present in the sample from patients NED and DOD (n=8 DOD, n=16 NED), p=0.004. (F) Representative immunohistochemically stained samples of cervical tumor biopsies pre- and mid-treatment (tx) in patients NED and DOD. Scale bar, 200 μm. (G) Absolute lymphocytes in the blood of cervical cancer patients (n=95) before (mean 15 days prior to CRT), and 3 weeks (mean 21 days ± 0.9 days) into CRT. § p=2.5 × 10−28, Ŧ p=6.7 × 10−21, *p=0.009. For all other panels * significant difference in gene expression between DOD and NED, p < 0.05. Error bars represent SEM.
To determine whether this decrease in lymphocytes during CRT in patients DOD correlates with change in expression of lymphocyte specific genes, we queried the RNA-seq data to compare the expression of CD3e, CD4, CD8a and cytotoxic genes expressed by CD8 T-cells (Perforin (PRF1), Granzyme A (GZMA), and Granulysin (GNLY)), which are known markers of immune cytolytic activity26, in each outcome group at each time point and analyzed how the expression of these genes changed with CRT. In general, expression of these genes decreased in all patients undergoing CRT with the exception of CD4, which increased in patients NED, but patients DOD had a significantly greater decrease in all of these genes during treatment. Specifically, tumors from patients DOD had significantly decreased expression of CD3e, CD4, CD8a, PRF1, GZMA and GNLY compared to patients NED at the mid-treatment time point, while the differences were not significant pre-treatment (Figure 4C). This correlates with the differences in TALs noted on H&E-stained slides described above. To determine if expression of immune checkpoint genes may be correlated with a general decreased immune profile, we analyzed the RNA-seq data for the expression of PD-L1, PD-L2, CTLA-4 and PD-1 at both time points. Interestingly we found that patients DOD had increased expression of PD-L1 and PD-L2 at both time points compared to patients NED, though only PD-L2 reached statistical significance. In contrast, patients DOD had decreased expression of CTLA-4 and PD-1 mid-treatment, likely due to the greater decrease in lymphocytes in this cohort during CRT (Figure 4C).
To validate the changes in T-cell genes, we determined the absolute numbers of CD4+ and CD8+ T-cells with IHC in matched pairs before and during CRT in the cervical tumor biopsies of a random sub-cohort of 55 patients out the original 98 patient cohort with known cervical cancer outcome. The number of CD4+ cells in cervical tissue remained stable during CRT (32.5 v. 32.3 counts/10 HPF pre- and mid-treatment respectively) while the number of CD8+ cells decreased significantly during treatment (24.9 v. 12.7 counts/10 HPF pre- and mid-treatment respectively, p=0.0007) when all patients were analyzed together. When CD4+ and CD8+ infiltrate was analyzed according to outcome, there was no difference at either time point (Figure 4D). Given that tumor infiltrating lymphocytes implies the presence of tumor, we limited the analysis to specimens containing >10% neoplastic cells mid-treatment (24 matched pairs including 8 patients from the RNA-seq cohort), and found that tumors of patients DOD had fewer CD8+ cells at the mid-treatment time point (8 v. 15 per 10 HPF, p=0.004 Figure 4E and 4F) than patients NED, but no difference pre-treatment. Patients DOD also had decreased CD4+ cells mid-treatment but this was not statistically significant (25 v. 39 per 10 HPF, p=0.13).
To determine whether patients DOD also have decreased circulating lymphocytes during CRT, complete blood count (CBC) collected as a part of routine care during CRT was evaluated both before and 3 weeks into CRT on the initial 115 patient cohort for which we had analyzed mid-treatment biopsies. After excluding patients who died of other causes or did not have available CBCs, 95 patients remained for analysis. All patients had a significant decrease in absolute lymphocytes during CRT. At an average of 15 days prior to starting CRT, there was no difference in the number of absolute lymphocytes between patients DOD and NED (1.7 v. 1.9 K/ mm3, p=0.26), however 3 weeks into treatment (21 ± 0.9 days) patients DOD had significantly decreased circulating absolute lymphocytes compared to patients NED (0.34 v. 0.48 K/mm3, p = 0.009) (Figure 4G).
Maintenance of HPV gene expression is associated with resistance to CRT and death from cervical cancer
HPV, the most common cause of cervical cancer, is known to evade the immune system27 as well as actively suppress it by decreasing activation and cytotoxic activity of CD8 T-cells.28,29 We therefore hypothesized that the decreased immune response observed in patients DOD is associated with increased HPV gene expression during CRT. Using the 20-patient matched RNA-seq dataset, we determined HPV gene expression (defined as all reads mapped to the HPV genome) in the 18 HPV-positive patients. There was no difference in HPV gene expression between patients NED and DOD when either time point was analyzed alone as there was a large range of gene expression in both patient cohorts. In general, there was a significant decrease in HPV gene expression during CRT in all patients. However, patients NED lost significantly more HPV gene expression during CRT than patients DOD (80% v. 47%, p=0.01) i.e. it was only the change in expression that significantly differed (Figure 5A). The same was true for HPV E6 and E7 specifically (Figure 5B), and this was confirmed by qRT-PCR for the patients (n=12) who had adequate RNA for further validation analysis (E6, p=0.005 and E7, p=0.0008). GSEA using the “Cervical cancer proliferation cluster” which is a published gene set associated with HPV E6/E7 gene expression and unfavorable disease outcome30 revealed significant enrichment (p=0, FDR=0) in patients DOD particularly at the mid-treatment time point (Figure 5C) while it was not enriched in NED patients at any time point despite 85% of NED patients being HPV-positive. The same was true for the “viral life cycle” pathway, which was enriched only in DOD patients mid-treatment (p=0, FDR = 0.007). Additionally, GSEA using the oncogenic signatures gene set revealed that patients DOD had enrichment of pathways related to E2F gene transcription mid-treatment, while NED patients did not, which correlates with the maintenance of E7 expression in this patient population. Together, these data show that oncogenic HPV gene expression is largely retained in tumor cells of patients DOD during CRT and that downstream biological pathways associated with cellular proliferation are activated.
Figure 5:

HPV gene expression during CRT and its downstream biological effects. (A) Percent change in HPV gene expression along the HPV genome in cervical tumors from pre- to mid-treatment during CRT. Expression of HPV-16 and −18 is shown as other HPV subtypes differ in their sequence alignment (DOD, n=6, NED, n=8). (B) Percent change in HPV16 and −18 E6 and E7 gene expression using RNA-seq data in patients DOD and NED (p=0.004 for E6, p=5.8 × 10−9 for E7). Error bars represent SEM. (C) GSEA of patients DOD v. NED at the mid-treatment time point using the cervical cancer proliferation cluster gene set which is associated with HPV E6/E7 gene expression revealed significant enrichment in patients DOD mid-treatment. (D) Venn diagrams displaying overlap in biological pathways (GSEA C5 Biological processes gene set) enriched between patients DOD who maintained HPV expression mid-treatment and HFK cells overexpressing HPV E6 or E7. All pathways were enriched with FDR < 5%, p < 0.01.
HPV is also known to promote cell cycle progression, cellular proliferation, and increase DNA damage repair,9,31,32 all of which were pathways enriched in the DOD cohort mid-treatment. To determine whether enrichment of these pathways is due to the maintenance of HPV oncogene expression specifically, we infected primary human foreskin keratinocytes (HFK) with a retrovirus that stably expressed either HPV E6, E7 or a control virus as previously described22 and cells were then harvested for RNA sequencing. Ectopic expression of E6 or E7 was confirmed by sequencing analysis. GSEA was again performed using the Gene Ontology biological processes gene set comparing gene expression in control versus E6 or E7 infected HFK cells. As expected, E6 and E7 expressing cells had pathways enriched in mitosis/cell division, DNA repair, transcription and cell cycle transition. We then compared the pathways enriched in E6 or E7 expressing cells (n=256 and 234 pathways, respectively with FDR<5% and p<0.01) to those enriched in the DOD cohort (n=360 pathways, FDR<5% and p<0.01) given that these patients lost less, and retained more HPV gene expression during CRT. Interestingly, 192 (75%) and 164 (70%) pathways overlapped between E6 or E7 overexpressing cells, respectively, and DOD patients mid-treatment (Figure 5D). Thus, overexpression of HPV E6 and E7 in vitro leads to alterations of biological pathways similar to that seen in patients DOD mid-treatment. These pathways are mostly involved in cellular replication, mitosis, and DNA damage repair, which may explain their resistance to treatment.
Discussion
Here, we report both human and viral gene expression changes in cervical tumors during chemoradiation. Analyzing tumor gene expression before and 3 weeks into CRT provides novel insight into treatment response and offers advantages to analyzing one time point alone. To our knowledge, this is the largest, most comprehensive study of its kind in cervical cancer, and analyzes gene expression farther into CRT than prior studies.33–35 Additionally, whole transcriptomic RNA-seq analysis was performed, providing a more robust and in-depth characterization of gene expression compared to targeted microarray analysis. This approach also allowed for analysis of viral gene expression, which is extremely relevant given that HPV is the oncogenic driver in the vast majority of cervical cancer cases.
There was a clear difference in the transcriptional response to therapy between patients. Tumors from patients NED appeared to exhibit a coordinated response to therapy whereas tumors from patients DOD did not, with 36-fold fewer exclusively altered genes during CRT. It is possible that these results represent a consequence, rather than a cause, of treatment response, in that tumor fate had already been decided by the mid-treatment time point and the gene expression is reflective of that fate. Whether the GSEA analysis revealing activation of the immune system in NED patients is a cause or consequence of treatment outcome remains to be determined. It is well established that increased TALs are associated with improved clinical outcome in cervical and other cancers.36–39 While TALs decreased in all patients during therapy, likely due to the short interval between the last radiation treatment and biopsy as well as the use of brachytherapy, patients DOD had a much greater decrease in TALs compared to patients NED (73% v. 37% decrease) during CRT. This finding was validated in an independent cohort and recapitulated in the RNA-seq data by analyzing lymphocyte-specific gene expression. Thus patients NED had a relative enrichment of immune related genes during therapy compared to patients DOD, as indicated by the GSEA analysis. Tumors from patients NED also have higher expression of genes upregulated upon CD8 T-cell activation including granzyme-A, perforin, and granulysin at the mid-treatment time point indicating that in addition to differences in lymphocyte infiltration, there are also potential differences in T-cell function that may be contributing to improved outcomes. Indeed, a comprehensive study using RNA-seq data from thousands of tumor biopsies including cervical cancer found that increased expression of GZMA and PRF1 was associated with improved survival.26 Our data reveals that evaluation of the immune landscape mid-treatment is an important predictive parameter and may provide more prognostic value than pre-treatment data given the differences observed were even greater mid-treatment. However, the feasibility of obtaining mid-treatment samples is severely limited in most other disease sites.
It is currently unclear why patients DOD have a decreased local immune response to the tumor, but maintenance of HPV expression may be contributing. Our group recently reported that increased HPV E6 expression in oropharyngeal cancer is associated with decreased expression of immune-related genes,14 which correlates with our findings. HPV E6 and E7 promote an anti-inflammatory environment by inhibiting IFN-α and IFN-γ production in multiple cell types40,41 and high-risk HPV impairs migration of immune cells.42 Thus tumors that maintain HPV expression during CRT may be creating an immunosuppressive environment, which may explain the decreased infiltration of lymphocytes and decreased expression of immune related genes. Additionally, HPV-positive head and neck cancer cannot be cured with CRT in vivo without a functional immune system,43 reinforcing the importance of the immune system in achieving a complete response to treatment.
Maintenance of HPV E6/E7 expression in cervical tumors during CRT may also be contributing to poor outcomes via established viral mechanisms. HPV E7 is known to interact with the Rb tumor suppressor and target it for degradation, which upregulates the activity of E2F transcription factors. E2F binding sites are found in the promoters of genes involved in DNA synthesis, mitosis, and cell cycle progression, thus E7 activates host DNA replication machinery, promotes G1 to S phase transition and ultimately results in cellular proliferation.9,44 Maintenance of E7 expression is therefore likely contributing to tumor cell survival by increasing cellular proliferation, which was generally enriched in GSEA analysis in patients and in vitro. Additionally, there is evidence that HPV E6 and E7 promote radioresistance in cervical cancer and that radiation resistant clones can express higher levels of oncoproteins after sublethal damage, which could confer a growth advantage.10,11 Increased HPV E6 expression was also recently shown to be associated with cisplatin resistance.45
While this is the first study to our knowledge examining HPV gene expression changes in human tumors during CRT, there have been studies evaluating the effect of persistent HPV infection on outcome following definitive therapy for cervical cancer. Two such studies have reported that patients with persistent HPV infection after radiotherapy have a significantly increased risk of local recurrence.46,47 Similar results have also been reported for HPV-positive head and neck cancer.48 Together with our findings reported here, this suggests that cells harboring HPV may survive CRT, maintain proliferative potential, and result in poor patient outcome.
The main limitation of this study is the small sample size, mostly secondary to the very limited remaining neoplastic tissue after 3 weeks of CRT with high dose rate brachytherapy. Though we likely could have included more patients had we studied an earlier time point, evaluating gene expression 3 weeks into treatment allows for unique insight into a patient’s response to radiation therapy compared to analysis after only a few fractions of radiation, which the existing prior studies have done.34,35 Inclusion in our RNA-seq study mandated presence of neoplastic cells in cervical tissue 3 weeks into CRT, thus a valid concern is that our study is biased towards tumors that are inherently resistant to treatment. However, 65% of the patients in our RNA-seq cohort had no evidence of disease at last follow-up, as did 67% of the 98-patient cohort, which is representative of the overall risk of recurrence for cervical cancer. This suggests that selection bias for resistant cases is less of a concern, allowing for comparison of genes and biological pathways that may be related to treatment failure. Conversely, NED samples did not have a lower percentage of tumor cells than patients DOD as all samples utilized for RNA sequencing had >50% neoplastic cellularity and were macrodissected in an equivalent manner.
The goal of this study was to determine how cervical tumor gene expression changes in response to CRT in order to gain insight into mechanisms of treatment resistance. There was a clear difference in both viral and human gene expression during CRT between patients NED and DOD. It is evident that not all HPV-driven cervical cancers behave similarly and some are able to retain HPV oncogene expression, perhaps enabling them to continue to proliferate and evade the immune system. Further validation and mechanistic studies may uncover biomarkers of early treatment response that can be used to stratify patients for treatment intensification and/or early salvage therapy. Finally, these results may also be applicable to other HPV-driven cancers including head and neck cancer.
Supplementary Material
Novelty and Impact:
Approximately 30% of cervical cancer patients are resistant to chemoradiation therapy (CRT) and have a poor prognosis. Here, we profile the transcriptomes of paired human cervical tumors both before and during CRT, which provides novel insights into the mechanisms of treatment resistance. Patients who were resistant to treatment and died of disease had a decreased local immune response to the tumor and increased expression of biosynthetic and mitotic pathways, which was associated with maintenance of HPV E6/E7 expression during CRT.
Acknowledgments:
The authors would like to thank Dr. Ping Liu for her assistance with RNA sequencing of the HFK cells.
Financial Support:
RSNA Research Resident grant (PFC), NIH R01GM089784 and R01DE026471 (XW). Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number R25 CA190190. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations:
- CRT
chemoradiation therapy
- NED
no evidence of disease
- DOD
dead of disease
- TAL
tumor associated lymphocyte
- HPV
human papilloma virus
- PET
positron emission tomography
- GSEA
gene set enrichment analysis
Bibliography
- 1.Globocan 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012.
- 2.Burnet NG, Jefferies SJ, Benson RJ, Hunt DP, Treasure FP. Years of life lost (YLL) from cancer is an important measure of population burden--and should be considered when allocating research funds. Br J Cancer. 2005;92(2):241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwarz JK, Siegel BA, Dehdashti F, Grigsby PW. Association of posttherapy positron emission tomography with tumor response and survival in cervical carcinoma. JAMA. 2007;298(19):2289–2295. [DOI] [PubMed] [Google Scholar]
- 4.Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T, Hiller W, Fisher ER, Wickerham DL, Bryant J, Wolmark N. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817–2826. [DOI] [PubMed] [Google Scholar]
- 5.Spratt DE, Yousefi K, Deheshi S, Ross AE, Den RB, Schaeffer EM, Trock BJ, Zhang J, Glass AG, Dicker AP, Abdollah F, Zhao SG, Lam LLC, du Plessis M, Choeurng V, Haddad Z, Buerki C, Davicioni E, Weinmann S, Freedland SJ, Klein EA, Karnes RJ, Feng FY. Individual Patient-Level Meta-Analysis of the Performance of the Decipher Genomic Classifier in High-Risk Men After Prostatectomy to Predict Development of Metastatic Disease. J Clin Oncol 2017;35(18):1991–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balacescu O, Balacescu L, Tudoran O, Todor N, Rus M, Buiga R, Susman S, Fetica B, Pop L, Maja L, Visan S, Ordeanu C, Berindan-Neagoe I, Nagy V. Gene expression profiling reveals activation of the FA/BRCA pathway in advanced squamous cervical cancer with intrinsic resistance and therapy failure. BMC Cancer. 2014;14:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grigsby PW, Watson M, Powell MA, Zhang Z, Rader JS. Gene expression patterns in advanced human cervical cancer. Int J Gynecol Cancer. 2006;16(2):562–567. [DOI] [PubMed] [Google Scholar]
- 8.Wong YF, Selvanayagam ZE, Wei N, Porter J, Vittal R, Hu R, Lin Y, Liao J, Shih JW, Cheung TH, Lo KWK, Yim SF, Yip SK, Ngong DT, Siu N, Chan LKY, Chan CS, Kong T, Kutlina E, McKinnon RD, Denhardt DT, Chin K-V, Chung TKH. Expression genomics of cervical cancer: molecular classification and prediction of radiotherapy response by DNA microarray. Clin Cancer Res. 2003;9(15):5486–5492. [PubMed] [Google Scholar]
- 9.Moody CA, Laimins LA. Human papillomavirus oncoproteins: pathways to transformation. Nat Rev Cancer. 2010;10(8):550–560. [DOI] [PubMed] [Google Scholar]
- 10.Santin AD, Hermonat PL, Ravaggi A, Chiriva-Internati M, Pecorelli S, Parham GP. Radiation-enhanced expression of E6/E7 transforming oncogenes of human papillomavirus-16 in human cervical carcinoma. Cancer. 1998;83(11):2346–2352. [DOI] [PubMed] [Google Scholar]
- 11.Hampson L, El Hady ES, Moore JV, Kitchener H, Hampson IN. The HPV16 E6 and E7 proteins and the radiation resistance of cervical carcinoma. FASEB. 2001;15(8):1445–1447. [DOI] [PubMed] [Google Scholar]
- 12.Jung HS, Rajasekaran N, Song SY, Kim YD, Hong S, Choi HJ, Kim YS, Choi J-S, Choi Y-L, Shin YK. Human Papillomavirus E6/E7-Specific siRNA Potentiates the Effect of Radiotherapy for Cervical Cancer in Vitro and in Vivo. Int J Mol Sci. 2015;16(6):12243–12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Boer MA, Jordanova ES, Kenter GG, Peters AA, Corver WE, Trimbos JB, Fleuren GJ. High human papillomavirus oncogene mRNA expression and not viral DNA load is associated with poor prognosis in cervical cancer patients. Clin Cancer Res. 2007;13(1):132–138. [DOI] [PubMed] [Google Scholar]
- 14.Khwaja S, Baker C, Haynes W, Spencer C, Gay H, Thorstad W, Adkins D, Nussenbaum B, Wang X. High E6 Gene Expression Predicts for Distant Metastasis and Poor Survival in Patients with HPV-Positive Oropharyngeal Squamous Cell Carcinoma. Int J Radiat Oncol Biol Phys. 2016; 15;95(4):1132–41 [DOI] [PubMed] [Google Scholar]
- 15.Kizer NT, Thaker PH, Gao F, Zighelboim I, Powell MA, Rader JS, Mutch DG, Grigsby PW. The effects of body mass index on complications and survival outcomes in patients with cervical carcinoma undergoing curative chemoradiation therapy. Cancer. 2011;117(5):948–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang Z, Liu W, Wang Y, Gao Z, Gao G, Wang X. Rational design of microRNA-siRNA chimeras for multifunctional target suppression. RNA. 2013;19(12):1745–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10(3):R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao G, Chernock RD, Gay HA, Thorstad WL, Zhang TR, Wang H, Ma X-J, Luo Y, Lewis JS, Wang X. A novel RT-PCR method for quantification of human papillomavirus transcripts in archived tissues and its application in oropharyngeal cancer prognosis. Int J Cancer. 2013;132(4):882–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liberzon A, Subramanian A, Pinchback R, Thorvaldsdóttir H, Tamayo P, Mesirov JP. Molecular signatures database (MSigDB) 3.0. Bioinforma. 2011;27(12):1739–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci. 2005;102(43):15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Heilman SA, Illanes D, Sluder G, Chen JJ. p53-independent abrogation of a postmitotic checkpoint contributes to human papillomavirus E6-induced polyploidy. Cancer Res. 2007;67(6):2603–2610. [DOI] [PubMed] [Google Scholar]
- 23.Kim J-Y, Park S, Nam B-H, Roh J-W, Lee CH, Kim Y-H, Shin H-J, Lee S-K, Kong S-Y, Seong M-W, Han T-J, Lee M-Y, Cho KH, Park SY. Low initial human papilloma viral load implicates worse prognosis in patients with uterine cervical cancer treated with radiotherapy. J Clin Oncol. 2009;27(30):5088–5093. [DOI] [PubMed] [Google Scholar]
- 24.Su C, Gao G, Schneider S, Helt C, Weiss C, O’Reilly MA, Bohmann D, Zhao J. DNA damage induces downregulation of histone gene expression through the G1 checkpoint pathway. EMBO J. 2004;23(5):1133–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao J. Coordination of DNA synthesis and histone gene expression during normal cell cycle progression and after DNA damage. Cell Cycle. 2004;3(6):695–697. [PubMed] [Google Scholar]
- 26.Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160(1–2):48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tindle RW. Immune evasion in human papillomavirus-associated cervical cancer. Nat Rev Cancer. 2002;2(1):59–65. [DOI] [PubMed] [Google Scholar]
- 28.Jemon K, Leong C-M, Ly K, Young SL, McLellan AD, Hibma MH. Suppression of the CD8 T cell response by human papillomavirus type 16 E7 occurs in Langerhans cell-depleted mice. Sci Rep. 2016;6:34789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mora-García ML, Ávila-Ibarra LR, García-Rocha R, Weiss-Steider B, Hernández-Montes J, Don-López CA, Gutiérrez-Serrano V, Titla-Vilchis IJ, Fuentes-Castañeda MC, Monroy-Mora A, Jave-Suárez LF, Chacón-Salinas R, Vallejo-Castillo L, Pérez-Tapia SM, Monroy-García A. Cervical cancer cells suppress effector functions of cytotoxic T cells through the adenosinergic pathway. Cell Immunol. 2017;320:46–55. [DOI] [PubMed] [Google Scholar]
- 30.Rosty C, Sheffer M, Tsafrir D, Stransky N, Tsafrir I, Peter M, de Crémoux P, de La Rochefordière A, Salmon R, Dorval T, Thiery JP, Couturier J, Radvanyi F, Domany E, Sastre-Garau X. Identification of a proliferation gene cluster associated with HPV E6/E7 expression level and viral DNA load in invasive cervical carcinoma. Oncogene. 2005;24(47):7094–7104. [DOI] [PubMed] [Google Scholar]
- 31.Bristol ML, Das D, Morgan IM. Why Human Papillomaviruses Activate the DNA Damage Response (DDR) and How Cellular and Viral Replication Persists in the Presence of DDR Signaling. Viruses. 2017;9(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pyeon D, Newton MA, Lambert PF, den Boon JA, Sengupta S, Marsit CJ, Woodworth CD, Connor JP, Haugen TH, Smith EM, Kelsey KT, Turek LP, Ahlquist P. Fundamental differences in cell cycle deregulation in human papillomavirus-positive and human papillomavirus-negative head/neck and cervical cancers. Cancer Res. 2007;67(10):4605–4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwakawa M, Ohno T, Imadome K, Nakawatari M, Ishikawa K, Sakai M, Katoh S, Ishikawa H, Tsujii H, Imai T. The radiation-induced cell-death signaling pathway is activated by concurrent use of cisplatin in sequential biopsy specimens from patients with cervical cancer. Cancer Biol Ther. 2007;6(6):905–911. [DOI] [PubMed] [Google Scholar]
- 34.Klopp AH, Jhingran A, Ramdas L, Story MD, Broadus RR, Lu KH, Eifel PJ, Buchholz TA. Gene expression changes in cervical squamous cell carcinoma after initiation of chemoradiation and correlation with clinical outcome. Int J Radiat Oncol Biol Phys. 2008;71(1):226–236. [DOI] [PubMed] [Google Scholar]
- 35.Weidhaas JB, Li S-X, Winter K, Ryu J, Jhingran A, Miller B, Dicker AP, Gaffney D. Changes in gene expression predicting local control in cervical cancer: results from Radiation Therapy Oncology Group 0128. Clin Cancer Res. 2009;15(12):4199–4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piersma SJ, Jordanova ES, van Poelgeest MIE, Kwappenberg KMC, van der Hulst JM, Drijfhout JW, Melief CJM, Kenter GG, Fleuren GJ, Offringa R, van der Burg SH. High number of intraepithelial CD8+ tumor-infiltrating lymphocytes is associated with the absence of lymph node metastases in patients with large early-stage cervical cancer. Cancer Res. 2007;67(1):354–361. [DOI] [PubMed] [Google Scholar]
- 37.Nedergaard BS, Ladekarl M, Nyengaard JR, Nielsen K. A comparative study of the cellular immune response in patients with stage IB cervical squamous cell carcinoma. Low numbers of several immune cell subtypes are strongly associated with relapse of disease within 5 years. Gynecol Oncol. 2008;108(1):106–111. [DOI] [PubMed] [Google Scholar]
- 38.Balermpas P, Michel Y, Wagenblast J, Seitz O, Weiss C, Rödel F, Rödel C, Fokas E. Tumour-infiltrating lymphocytes predict response to definitive chemoradiotherapy in head and neck cancer. Br J Cancer. 2014;110(2):501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, Budczies J, Huober J, Klauschen F, Furlanetto J, Schmitt WD, Blohmer J-U, Karn T, Pfitzner BM, Kümmel S, Engels K, Schneeweiss A, Hartmann A, Noske A, Fasching PA, Jackisch C, van Mackelenbergh M, Sinn P, Schem C, Hanusch C, Untch M, Loibl S. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19(1):40–50. [DOI] [PubMed] [Google Scholar]
- 40.Barnard P, Payne E, McMillan NA. The human papillomavirus E7 protein is able to inhibit the antiviral and anti-growth functions of interferon-alpha. Virology. 2000;277(2):411–419. [DOI] [PubMed] [Google Scholar]
- 41.Lee SJ, Cho YS, Cho MC, Shim JH, Lee KA, Ko KK, Choe YK, Park SN, Hoshino T, Kim S, Dinarello CA, Yoon DY. Both E6 and E7 oncoproteins of human papillomavirus 16 inhibit IL-18-induced IFN-gamma production in human peripheral blood mononuclear and NK cells. J Immunol. 2001;167(1):497–504. [DOI] [PubMed] [Google Scholar]
- 42.Tummers B, Goedemans R, Pelascini LPL, Jordanova ES, van Esch EMG, Meyers C, Melief CJM, Boer JM, van der Burg SH. The interferon-related developmental regulator 1 is used by human papillomavirus to suppress NFκB activation. Nat Commun. 2015;6:6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spanos WC, Nowicki P, Lee DW, Hoover A, Hostager B, Gupta A, Anderson ME, Lee JH. Immune response during therapy with cisplatin or radiation for human papillomavirus-related head and neck cancer. Arch Otolaryngol Head Neck Surg. 2009;135(11):1137–1146. [DOI] [PubMed] [Google Scholar]
- 44.Cheng S, Schmidt-Grimminger DC, Murant T, Broker TR, Chow LT. Differentiation-dependent up-regulation of the human papillomavirus E7 gene reactivates cellular DNA replication in suprabasal differentiated keratinocytes. Genes Dev. 1995;9(19):2335–2349. [DOI] [PubMed] [Google Scholar]
- 45.Rataj O, Haedicke-Jarboui J, Stubenrauch F, Iftner T. Brd4 inhibition suppresses HPV16 E6 expression and enhances chemoresponse: A potential new target in cervical cancer therapy. Int J Cancer. 2019;144(9):2330–2338. [DOI] [PubMed] [Google Scholar]
- 46.Mahantshetty U, Teni T, Naga P, Hotwani C, Umesh S, Kannan S, Hande V, Pawar S, Engineer R, Chopra S, Deodhar K, Maheshwari A, Gurram L, Gupta S, Shrivastava SK. Impact of HPV 16/18 infection on clinical outcomes in locally advanced cervical cancers treated with radical radio (chemo) therapy - A prospective observational study. Gynecol Oncol. 2018;148(2):299–304. [DOI] [PubMed] [Google Scholar]
- 47.Song YJ, Kim J-Y, Lee S-K, Lim H-S, Lim MC, Seo S-S, Kang S, Lee DO, Park S-Y. Persistent human papillomavirus DNA is associated with local recurrence after radiotherapy of uterine cervical cancer. Int J Cancer. 2011;129(4):896–902. [DOI] [PubMed] [Google Scholar]
- 48.Ahn SM, Chan JYK, Zhang Z, Wang H, Khan Z, Bishop JA, Westra W, Koch WM, Califano JA. Saliva and plasma quantitative polymerase chain reaction-based detection and surveillance of human papillomavirus-related head and neck cancer. JAMA Otolaryngol-- Head Neck Surg. 2014;140(9):846–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


