Abstract
Background:
Transplantation of mesenchymal stromal cells (MSC) into cartilage defects led to variable cartilage repair outcomes. Previous in vitro studies showed independently that ascorbic acid and reduced iron can improve chondrogenic differentiation of MSC. However, the combined effect of ascorbic acid and iron supplementation on MSC differentiation has not been investigated.
Purpose:
To investigate combined in vivo effects of ascorbic acid and an FDA-approved iron supplement on MSC-mediated cartilage repair in mature Göttingen minipigs.
Study Design:
Controlled laboratory study.
Methods:
We pretreated bone marrow derived MSC with ascorbic acid and the FDA-approved iron supplement ferumoxytol, followed by transplantation of MSC into full thickness cartilage defects of distal femurs of Göttingen minipigs. Untreated cartilage defects served as negative controls. We evaluated the cartilage repair with magnetic resonance imaging (MRI) at 4 and 12 weeks after MSC implantation, followed by histology and immunofluorescence staining at 12 weeks.
Results:
Ascorbic acid and iron pretreated MSC transplants demonstrated significant cartilage repair with significantly better MOCART scores (73.8±15.5), macroscopic cartilage regeneration score according to the International Cartilage Repair Society (ICRS, 8.6±2.0), Pineda score (2.9±0.8) and significantly larger amount of Collagen II (28469 ±21313) compared to untreated controls (41.3±2.5; 1.8±2.9; 12.8±1.9 and 905 ±1326, respectively). The obtained scores were also better than scores previously reported in the same animal model for MSC implants without ascorbic acid.
Conclusion:
Pretreatment of MSC with ascorbic acid and an FDA-approved iron supplement improve chondrogenesis of MSC and leads to hyaline-like cartilage regeneration in knee joints of minipigs.
Clinical Relevance:
Ascorbic acid and iron supplements are immediately clinically applicable. Thus, results could be in principle translated to clinical applications.
Keywords: ascorbic acid, chondrogenesis, stem cells, cartilage repair, MRI, minipig
INTRODUCTION
Traumatic cartilage defects lead to progressive osteoarthritis.15,19 Surgical repair through microfracture and osteochondral autografts are established in the clinic, but can lead to fibrocartilage formation and donor site morbidity.24,38,45 Autologous chondrocyte transplants have been evaluated as an alternate approach, but require a two step-surgery for cell harvest and implantation.5,49 Mesenchymal stromal cells (MSC) can be more easily obtained from an iliac crest biopsy.10 However, cartilage repair outcomes of matrix associated MSC implants have been highly variable and often lead to fibrocartilage formation.30,47 New approaches for more successful hyaline cartilage repair would address a significant bottleneck in cartilage regeneration research and clinical translation.
Recently, ascorbic acid (Vitamin C) has been suggested as a clinically translatable mediator of hyaline cartilage repair because of three main characteristics:27,43,52 (i). Firstly, ascorbic acid can directly facilitate MSC chondrogenesis by increasing lysine hydroxylase activity and decreasing proline hydroxylase activity,33 which in turn promote the synthesis of hydroxyproline and hydroxylysine,34 facilitate the transcription of collagen genes in MSC12 and stimulate chondrocytes to produce extracellular matrix,36,42 especially collagen II and aggrecan.20 Ascorbic acid also stimulated glycosaminoglycan (GAG) synthesis in cultured human skin fibroblasts.22 (ii). Secondly, ascorbic acid can suppress apoptosis of MSC.26 After transplantation into cartilage defects, more than 50% of the transplanted cells undergo apoptosis.18,37 Ascorbic acid can suppress MSC apoptosis by scavenging mitochondrial superoxide anions,54 suppression of pro-inflammatory cytokines,7,32 reducing pro-inflammatory immune cell responses and increasing expression of wound healing mediators.46 (iii). Thirdly, ascorbic acid can inhibit fibroblast proliferation at concentrations as low as 0.05mM.4 This effect can indirectly support hyaline cartilage formation by inhibiting fibrocartilage formation.
Previous studies have shown that elemental iron plays a complex role in both stem cell growth and inhibition.16,21,35 Low doses of iron ions are essential for cell replication and DNA synthesis.11 However, iron overexposure can lead to DNA damage and impaired chondrogenesis of MSC.25 Iron oxide nanoparticles can provide slow release of iron to implanted cells without a risk of burst release. However, hypertrophied cartilage after an injury stimulates the production of transferrin, which can capture iron.6 Ascorbic acid can reduce iron (Fe3+) to a ferrous state (Fe2+), which leads to iron release from transport proteins and facilitates binding to the ferrous ion receptor on MSC so that the iron can be internalized and metabolized by the cell. In addition, reduced iron can catalyze proline and lysine hydroxylation reactions that are essential to the formation of mature collagen molecules.39
Taking this information together, we hypothesized that co-treatment of MSC with ascorbic acid and the FDA approved iron supplement ferumoxytol will lead to synergistic effects on cartilage repair. To our knowledge, nobody investigated the combined effects of ascorbic acid and ferumoxytol on cartilage repair outcomes of MSC implants thus far. Therefore, the primary objective of our study was to investigate combined in vivo effects of ascorbic acid and ferumoxytol on MSC-mediated cartilage repair in mature Göttingen minipigs. The secondary objective was to evaluate, if ascorbic acid treatment could counteract MSC apoptosis in the cartilage defect. Investigations in a large animal model were necessary to understand the in vivo integration of MSC in cartilage defects of joints with clinically relevant size and biomechanics and to confirm the safety and efficacy of our approach before clinical translation.
METHODS
This controlled laboratory study was approved by the administrative panel on laboratory animal care (APLAC) at our institution and was performed in close collaboration with our veterinary care team and in accordance with the institutional guidelines for the care and use of experimental animals. This study was conducted in five mature Göttingen minipigs (Marshall Farms, North Rose, NY, 1 male and 4 females, age 19.4±9 months, weight 32±5 kg) with 20 cartilage defects in 10 knee (stifle) joints. The overall study procedure and workflow is shown in Figure 1A.
Figure 1.
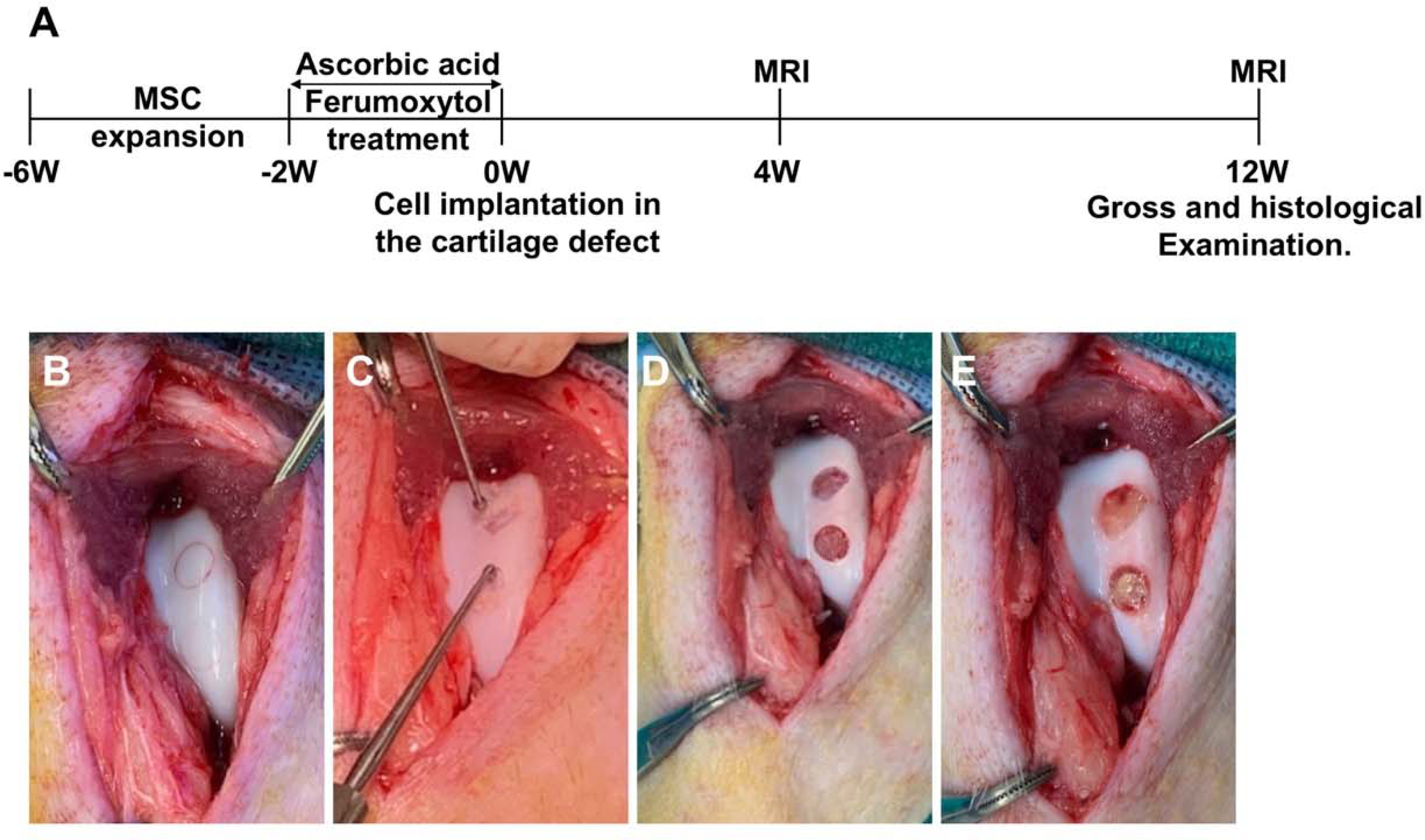
Study design: A. Timeline and procedures of the study, starting with MSC harvest and ending with histopathology. B-E) MSC implantation: Demarcation of 2 cartilage defects with a trochar (B); removal of cartilage within the marked area using a bone curette, carefully preserving the subchondral endplate (C); full thickness cartilage defects with exposure of the subchondral bone (D); and complete filling of the cartilage defects with MSC-derived chondrogenic pellets (E).
Stem Cell Isolation
Animal surgery, biopsy and imaging were performed under general inhalation anesthesia with isoflurane (Fluriso, Vetone, MWI, Boise, Idaho; 1%–3% in oxygen/1–2 L/min) according to institutional veterinary guidelines. To obtain porcine MSC, bone marrow was aspirated from the iliac crest using a Jamshidi needle.48 Bone marrow cells were aspirated into a 20 ml syringe, which was supplemented with heparin (1000 USP Units/ml, APP Pharmaceuticals, LLC, Schaumberg, IL, USA). The cell aspirate was transferred into test tubes, centrifuged at 1500 rpm for 5 minutes, suspended in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% fetal bovine serum (FBS), and 1% penicillin and streptomycin (Life Technologies Corporation, NY, USA), and then cultured on 225cm2 flasks (Corning, NY, USA) in 5% CO2 at 37°C. After 2 days, non-adherent hematopoietic cells were removed with phosphate buffered saline (PBS), adherent MSC were detached with trypsin 0.25% (Invitrogen), resuspended in DMEM media, expanded for 4 weeks and used for further experiments.
Pretreatment of Stem Cells
1.2×106 MSC were plated in 6 well plates and cultured for 14 days with:
(i). AF-MSCs:
Ascorbic acid (0.2mM, Sigma, St. Louis, MO) and ferumoxytol (100 μg Fe/ml, AMAG Pharmaceuticals, Waltham, Mass) in cGMP-compliant MSC expansion media StemPro (Thermo Fisher Scientific, Waltham, Mass).
(ii). AFM-MSCs:
To simulate sequelae of a hostile wound environment, we treated MSCs with 0.2mM ascorbic acid and 100 μg Fe/ml ferumoxytol as described above, followed by incubation with 0.06 mg mitomycin C per ml media for 2 hours to induce apoptosis via the p53-mediated pathway.37 Since ascorbic acid can suppress apoptosis by scavenging mitochondrial superoxide anions,54 we hypothesized that ascorbic acid treatment would counteract apoptotic MSC.
In Vitro Validation Studies
In vitro validation studies are detailed in the Appendix.
Stem Cell Implantation into the Cartilage Defect
A parapatellar medial surgical approach was used with lateral dislocation of the patella to expose the femoral condyles. Two full-thickness, 5 mm wide cartilage defects were created with a bone curette in the medial intercondylar groove of the distal femur of 10 knee (stifle) joints. The resulting 20 cartilage detects were treated as follows: Eight defects each were randomly assigned to implantations of ascorbic acid and ferumoxytol pretreated MSC (4 pellets (1.2×106 MSCs each) of AF-MSCs per defect; primary endpoint) or mitomycin, ascorbic acid and ferumoxytol pretreated MSC (4 pellets, 1.2×106 MSCs each) of AFM-MSCs per defect; secondary endpoint). Four additional defects were left untreated and served as negative controls. Results were compared to our previously published data of cartilage repair outcomes in 10 additional Göttingen minipig joints that had received mitomycin or non-mitomycin challenged iron-labeled MSC implants without ascorbic acid pretreatment (positive controls). 48 The cells were secured in the cartilage defect by fibrin glue (Evicel; Ethicon, Somerville, NJ, USA) (Figure 1, B–E), the patella was repositioned, the joint capsule was closed using absorbable suture materials and the overlying skin was closed with nonabsorbable silk sutures.
Pig Husbandry and Postsurgical Follow-up
We followed guidelines for swine housing established by the National Research Council in the Guide for the Care and Use of Laboratory Animals (NRC 1996). The pigs were housed in individual cages with light/dark cycles, environmental enrichment and visual, olfactory and auditory contact to each other. Following recovery from anesthesia from surgery or MRI, which were performed in the morning after an overnight fast, the pigs were allowed to move freely with weight bearing as tolerated. We evaluated the joint function before and at 1, 2, 4 and 12 weeks post-surgery, using the Feet First® Swine locomotion scoring system.
Evaluation of Cartilage Repair Using MRI
All pigs underwent magnetic resonance imaging (MRI) of both knee joints at 4 and 12 weeks after surgery, using a clinical 3.0-T MRI scanner (Signa HDxt; GE Healthcare, Milwaukee, Wis) and a one-channel receive-only loop coil (GE Healthcare). MRI sequences were composed of a proton density–weighted fat-saturated fast spin-echo (FSE) sequence (repetition time (TR): 3700ms; echo time (TE): 34ms; flip angle (FA): 90°; acquisition time (TA): 16 minutes), a multiecho multislice FSE sequence (TR: 3500ms; TE: 15, 30, 45 and 60ms; FA: 90°; TA: 13 minutes), and a three-dimensional spoiled gradient-echo sequence (TR: 50; TE: 7; FA: 30°; TA: 12 minutes). All images were obtained with a matrix size of 192 × 192 pixels, a slice thickness of 1.3 mm and a field of view (FOV) of 8 cm2. The cartilage of the distal femur in Göttingen minipigs is approximately 7–8 mm deep. We created 5 × 5 × 5 mm full thickness cartilage defects, carefully preserving the subchondral endplate. With a pixel size of 0.41 mm, each defect was covered by 12 × 12 pixels. Two radiologists (HED) and (AJT) assessed cartilage defect repair on MR images according to the MR observation of cartilage repair tissue (MOCART) score, which evaluates nine variables: defect fill, cartilage interface, surface, adhesions, structure, signal intensity, subchondral lamina, subchondral bone, and effusion. The final cartilage repair tissue score ranges from 0 (worst) to 100 points (best).28,54 In addition, one radiologist (AJT) measured T2 relaxation times in each implant and adjacent normal cartilage by drawing operator defined regions of interest using the Osirix Software and the T2 Fit Map plugin (version 10.0, 64bit; Pixmeo, Geneva, Switzerland). The radiologists were blinded to treatment groups.
Macroscopic and Histological Assessment
After completion of the final MRI exam, at 12 weeks post-surgery, the pigs were euthanized with intravenously administered potassium chloride (900 mg/kg) under anesthesia, knee joints were explanted and assessed according to the macroscopic international cartilage repair society (ICRS) score ranging from 0 (worst) to 12 points (best),50 and the macroscopic score by Wayne et al.53 ranging from 0 (worst) to 16 points (best). Tissues were fixed in 10% formalin for 48 hours, decalcified using 0.27M citric acid/0.1 M Ethylenediaminetetraacetic acid/Phosphate-buffered saline (EDTA/PBS) for 8–10 weeks and stained with Hematoxylin and Eosin (H&E) for evaluation of cell morphology, Alcian blue for detection of proteoglycan production, and Collagen type I and II (Collagen I: 1:500, Abcam ab90395; Collagen II: 1:500, Abcam ab34712, USA), followed by incubation with secondary antibody (Alexa Fluor 594 and 488) (1:500, ThermoFisher, USA). Fluorescence images were acquired with a 10× Zeiss Plan Apochromat objective (NA 0.45) on an AxioImager Widefield Fluorescence Microscope (Zeiss) with a Zeiss AxioCam HRm monochrome CCD camera, as described previously.48 Cartilage regeneration was assessed according to the Pineda score ranging from 0 (best) to 14 points (worst)41 and quantification of stained collagen II areas. Images were analyzed using the Immunohistochemistry Image Analysis Toolbox in ImageJ (National Institutes of Health), as described previously.3 Specifically, areas of collagen II in the regenerated cartilage defects, displayed as red were semi automatically detected and quantified by the area in pixels. All analysis was performed with blinding to treatment groups and results from MRI.
Statistical Analysis
The minimal number of MSC implants needed in each experimental group is eight, assuming a two-way analysis of variance (ANOVA) for group comparisons with a 0.05 significance level. A two-way ANOVA will have 91% power to detect differences between viable and apoptotic transplants by means of 25% of a SD, assuming that the common standard deviation is 1.000, when the sample size in each group is eight. For comparisons of cartilage defects with and without MSC implants n=4 per group will have 80% power to detect differences of more than 1.5 SD.
A Mann-Whitney test was used to compare macroscopic scores, Pineda scores, MOCART scores and immunohistochemical data between different experimental groups. Differences between locomotion scores of pigs with MSC implants and untreated controls were analyzed with the Fisher’s exact test. All statistical tests were done by Stata 16.1 (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC). A p value of less than 0.05 indicated significant differences between experimental groups.
RESULTS
Evaluation of Joint Function
All animals showed normal locomotion before their joint surgeries. All animals recovered well from surgery and MRI with no adverse events and were weight bearing as tolerated. During the first week after the surgery, all animals moved around relatively easily, but demonstrated mild signs of lameness of their operated stifle joints. This gradually improved over time. At 4 weeks after the surgery, all pigs had regained pre-operative levels of joint function and mobility. There was no significant difference in stifle joint function of pigs with MSC implants and pigs with untreated cartilage defects at 0, 1, 2, 4, and 12 weeks. (P >0.05; Table 1).
Table 1.
Swine locomotion score before (0) and at different time points after surgery for joints treated with MSC and joints with untreated cartilage defects. A= Ascorbic acid, F= Ferumoxytol, M= Mitomycin.
| Week 0 | Week 1 | Week 2 | Week 4 | Week 12 | |
|---|---|---|---|---|---|
| Treated defects (AF-MSCs & AFM-MSCs groups) | 0±0 | 1.3±0.5 | 0.8±0.5 | 0±0 | 0±0 |
| Empty defects (Untreated group) | 0±0 | 1.0±0.6 | 0.4±0.5 | 0±0 | 0±0 |
| P- values | 1.0 | 1.0 | 0.5 | 1.0 | 1.0 |
Data are means ± SDs of 16 MSC transplants and 4 untreated defects.
0 = Moves easily with little inducement on all feet.
1 = Moves relatively easy, but visible signs of lameness are apparent in at least one leg.
2 = Lameness is involved in one or more limbs.
3 = Reluctance to bear weight and walk on one or more legs.
Evaluation of Cartilage Repair Using MRI
MRI scans of ascorbic acid and ferumoxytol pretreated MSC (AF-MSC; n=8 and AFM-MSC; n=8) demonstrated complete filling of the cartilage defect on MRI scans at 4 weeks (Figure 2A). By comparison, empty defects (untreated controls; n=4) demonstrated incomplete filling of the cartilage defect on 4 week postsurgical MRI scans, with underlying edema of the subchondral bone (Figure 2B). At 12 weeks, ascorbic acid and iron pretreated MSC demonstrated persistent complete filling of the cartilage defect with homogenous internal structure and smooth surface (Figure 2C). By comparison, empty defects demonstrated a persistent cartilage defect and progressive subchondral bone defect (Figure 2D).
Figure 2.
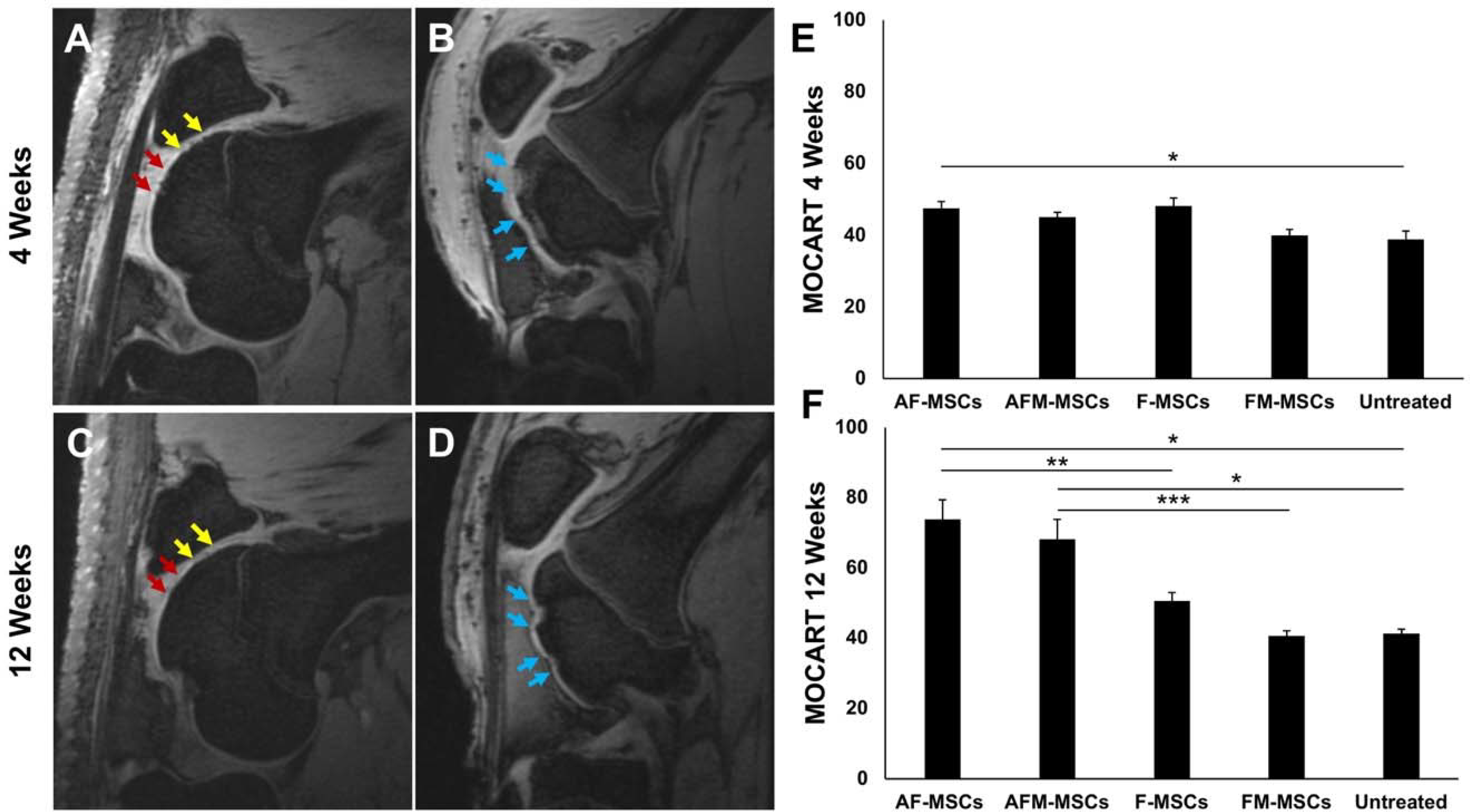
Cartilage repair outcomes of mesenchymal stem cell (MSC) implants, as determined with magnetic resonance imaging (MRI): A) Sagittal MRI scan of the distal femur at 4 weeks after implantation of ascorbic acid and iron treated chondrogenic cell pellets (AF-MSCs, yellow arrows) and mitomycin-challenged AF-MSCs (AFM-MSCs, red arrows). B) Sagittal MRI scan of the distal femur at 4 weeks after cartilage injury, no cell transplant (Untreated group, blue arrows). C) 12 week follow up MRI scan demonstrates complete cartilage defect repair of AF-MSCs (yellow arrows) and AFM-MSCs (red arrows). D) 12 week follow up MRI demonstrates residual cartilage defect and increasing subchondral defect of untreated control (blue arrows). E) MOCART scores at 4 weeks and F) MOCART score at 12 weeks. Data are displayed as means and standard error of the mean of n=8 implants for AF-MSCs, n=8 for AFM-MSCs, n=10 implants for F-MSCs, n=10 implants for FM-MSCs and n=4 for untreated controls. Comparisons between different groups were obtained with Mann-Whitney test. A= Ascorbic acid, F= Ferumoxytol, M= Mitomycin.
AF-MSC demonstrated a MOCART score of 47.5±5.3 at 4 weeks (Figure 2E) and 73.8±15.5 at 12 weeks (Figure 2F) after their implantation. The MOCART score of AF-MSC implants at 12 weeks was significantly higher than the MOCART score reported previously after implantation of MSC without ascorbic acid pretreatment in the same animal model (51.0±7.3; P =0.004, Figure 2F).48 The T2 relaxation time of AF-MSC implants at 12 weeks did not show a significant difference to normal cartilage (32.6±3.7 ms versus 30.2±3.4 ms, respectively; P = 0.08), but was significantly higher than T2 relaxation times of MSC without ascorbic acid (26.2±4.4 ms; P = 0.01, Appendix Figure A1).
To evaluate if ascorbic acid could counteract MSC apoptosis, we next investigated MSC implants, which were treated with ascorbic acid, ferumoxytol and mitomycin (AFM-MSCs). MOCART scores of AFM-MSCs were not significantly different compared to AF-MSCs at 4 weeks (45.0±3.8 versus 47.5±5.3; P =0.30, Figure 2E) and at 12 weeks (68.1±16.0 versus 73.8±15.5; P =0.33, Figure 2F). The MOCART score of AFM-MSCs at 12 weeks was significantly higher than the MOCART score reported previously after implantation of mitomycin-challenged MSC without ascorbic acid in the same animal model (41.0±5.0; P = 0.0008, Figure 2F).48 T2 relaxation time of AFM-MSCs at 12 weeks was not significantly different to normal cartilage (33.6±4.5 ms versus 30.2±3.4 ms, respectively; P = 0.07), but was significantly higher than T2 relaxation times of mitomycin-challenged MSC without ascorbic acid (26.9±3.6 ms; P = 0.008, Appendix Figure A1).
MOCART scores of empty defects (Untreated group) were significantly lower compared to non-mitomycin challenged AF-MSCs at 4 weeks (38.8±4.8 versus 47.5±5.3; P =0.03, Figure 2E), and at 12 weeks for both AF-MSCs and AFM-MSCs (41.3±2.5 versus 73.8±15.5, P =0.02; and 41.3±2.5 versus 68.1±16.0, P =0.02, respectively; Figure 2F) (Table 2). T2 relaxation times of empty defects (24.8±1.8 ms) were significantly lower than adjacent normal cartilage (30.2±3.4 ms; P = 0.004), AF-MSCs (32.6±3.7; P = 0.008) and AFM-MSCs (33.6±4.5; P = 0.008) (Appendix Figure A1).
Table 2.
MOCART scores at 4 and 12 weeks after implantation of ascorbic acid and iron pretreated AF-MSCs, mitomycin-challenged AFM-MSCs and empty defects. Data are displayed as means and standard deviations. A= Ascorbic acid, F= Ferumoxytol, M= Mitomycin.
| MOCART Score | 4 Weeks | 12 weeks | ||||
|---|---|---|---|---|---|---|
| AF-MSCs | AFM-MSCs | Untreated | AF-MSCs | AFM-MSCs | Untreated | |
| Defect fill | 9.4±1.8 | 9.4±1.8 | 7.5±2.9 | 18.1±5.3 | 16.9±6.0 | 10±0 |
| Cartilage interface | 11.3±2.3 | 10.6±1.8 | 6.3±2.5 | 13.1±2.6 | 13.8±2.3 | 6.3±2.5 |
| Surface | 4.4±1.8 | 4.4±1.8 | 5±0 | 6.9±3.7 | 6.3±3.5 | 5±0 |
| Structure | 2.5±2.7 | 1.3±2.3 | 0±0 | 3.8±2.3 | 3.1±2.6 | 0±0 |
| Signal intensity T2 FSE | 5±0 | 5±0 | 5±0 | 10.0±5.3 | 8.8±5.2 | 5±0 |
| Signal intensity 3D SPGR | 5±0 | 4.4±1.8 | 5±0 | 8.8±5.2 | 7.5±4.6 | 5±0 |
| Subchondral lamina | 0±0 | 0±0 | 0±0 | 1.9±2.6 | 1.9±2.6 | 0±0 |
| Subchondral bone | 1.3±2.3 | 1.3±2.3 | 0±0 | 1.3±2.3 | 1.3±2.3 | 0±0 |
| Adhesions | 5±0 | 5±0 | 5±0 | 5±0 | 3.8±2.3 | 5±0 |
| Effusion | 3.8±2.3 | 3.8±2.3 | 5±0 | 5±0 | 5±0 | 5±0 |
| Total score | 47.5±5.3 | 45.0±3.8 | 38.8±4.8 | 73.8±15.5 | 68.1±16.0 | 41.3±2.5 |
Macroscopic and Histological Assessment
On macroscopic examination, joints treated with AF-MSCs (n=8) and AFM-MSCs (n=8) showed complete filling of the cartilage defects with formation of cartilage like tissue, which resembled the surrounding cartilage. Defect margins were still apparent in both groups. There was no evidence of blood vessel formation or granulation tissue (Figure 3A). By contrast, empty defects (n=4) which were not treated with MSC revealed a clearly demarcated defect, absence of repair tissue, and complete exposure of the subchondral bone with evidence of blood vessel formation (Figure 3B). The macroscopic ICRS score and the score by Wayne were not significantly different between AF-MSCs (8.6±2.0 and 13.0±3.7, respectively) and AFM-MSCs (7.9±2.8 and 13.0±4.2, respectively, P =0.687 and P =0.717). By comparison, empty defects demonstrated significantly lower ICRS scores (1.8±2.9, P =0.006, P =0.022) and Wayne scores (2.5±3.8, P =0.006, P =0.006), respectively (Figure 3 C, D).
Figure 3.
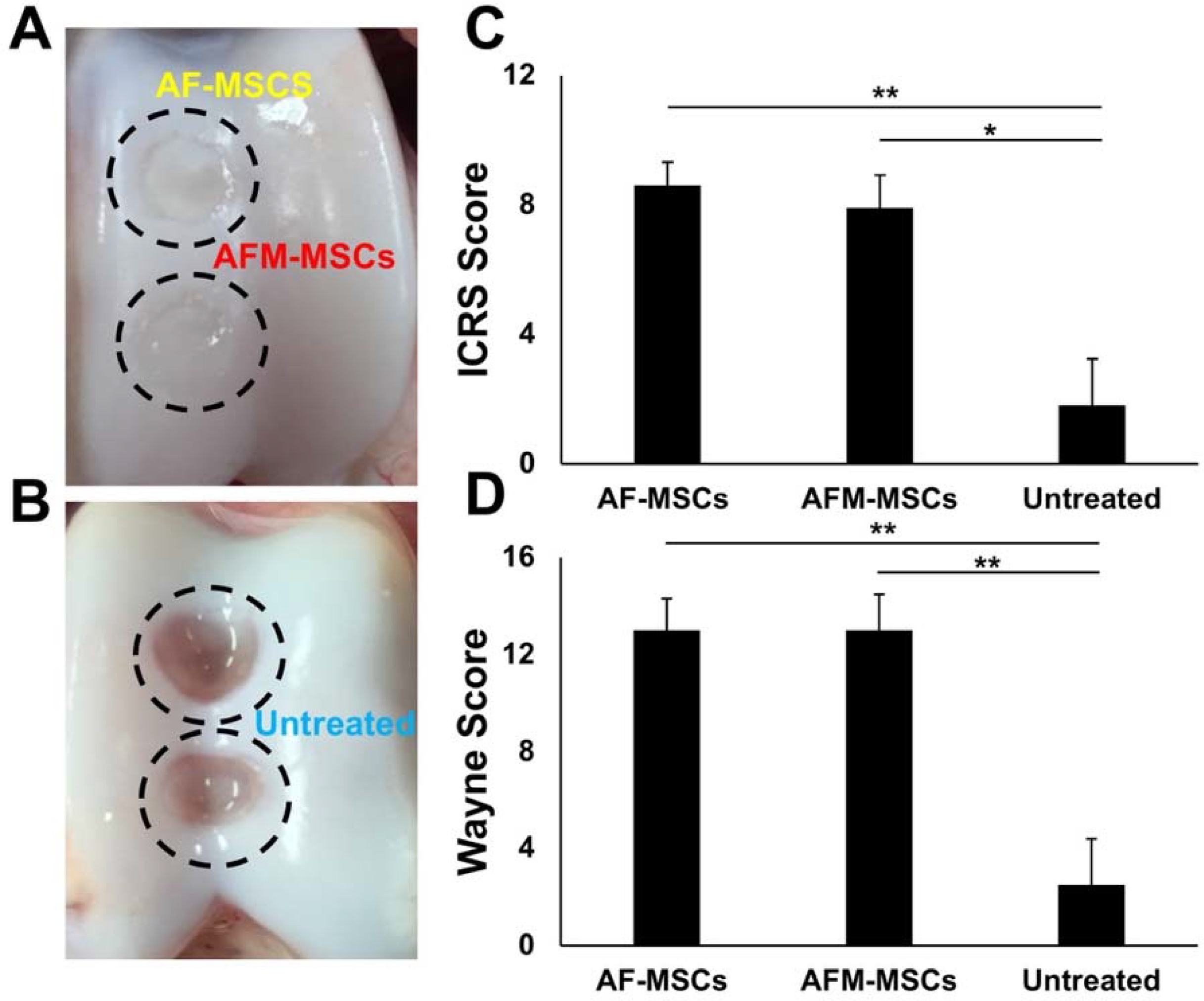
Macroscopic findings and scoring of articular cartilage repair according to the International Cartilage Repair Society (ICRS) and Wayne et al.53: A) Macroscopic specimen at 12 weeks demonstrates complete cartilage defect repair for ascorbic acid and iron treated AF-MSCs (yellow) and ascorbic acid, iron and mitomycin-treated AFM-MSCs (red). B) Macroscopic specimen at 12 weeks after cartilage defect creation and no cell implant demonstrates absent repair (blue). C) Macroscopic ICRS score for AF-MSCs (n=8), AFM-MSCs (n=8) and untreated (n=4) group, displayed as means and standard error of the mean. D) Macroscopic scoring according to Wayne et al.53 Data of different experimental groups were compared with Mann-Whitney test. A= Ascorbic acid, F= Ferumoxytol, M= Mitomycin.
On histological analysis, AF-MSC (n=8) and AFM-MSC-treated defects (n=8) revealed cartilage repair with a hyaline-like matrix, lateral integration into surrounding cartilage, reconstitution of the osteochondral junction, and proteoglycan synthesis. In contrast, empty defects of untreated cartilage defects (n=4) revealed no repair tissue inside the cartilage defect (Figure 4A). The Pineda score of AF-MSCs (2.9±0.8) was better than previously reported for MSC implants without ascorbic acid (3.3±1.3) in the same animal model, albeit not showing significant difference yet (P =0.237; Figure 4B).48 Compared to AF-MSCs, mitomycin-challenged AFM-MSCs showed no significant difference in Pineda score (3.1±0.8, P =0.763, Figure 4B). However, the Pineda score in the AFM-MSCs group was significantly better than previously reported for mitomycin-challenged MSC without ascorbic acid counteraction (5.5±1.4, P =0.001; Figure 4B).48 By comparison, empty defects in the untreated group demonstrated a significantly higher Pineda score compared to mitomycin challenged AFM-MSCs and non-mitomycin challenged AF-MSCs (12.8±1.9 versus 3.1±0.8 and 2.9±0.8, P =0.002, P =0.002, respectively; Figure 4B).
Figure 4.
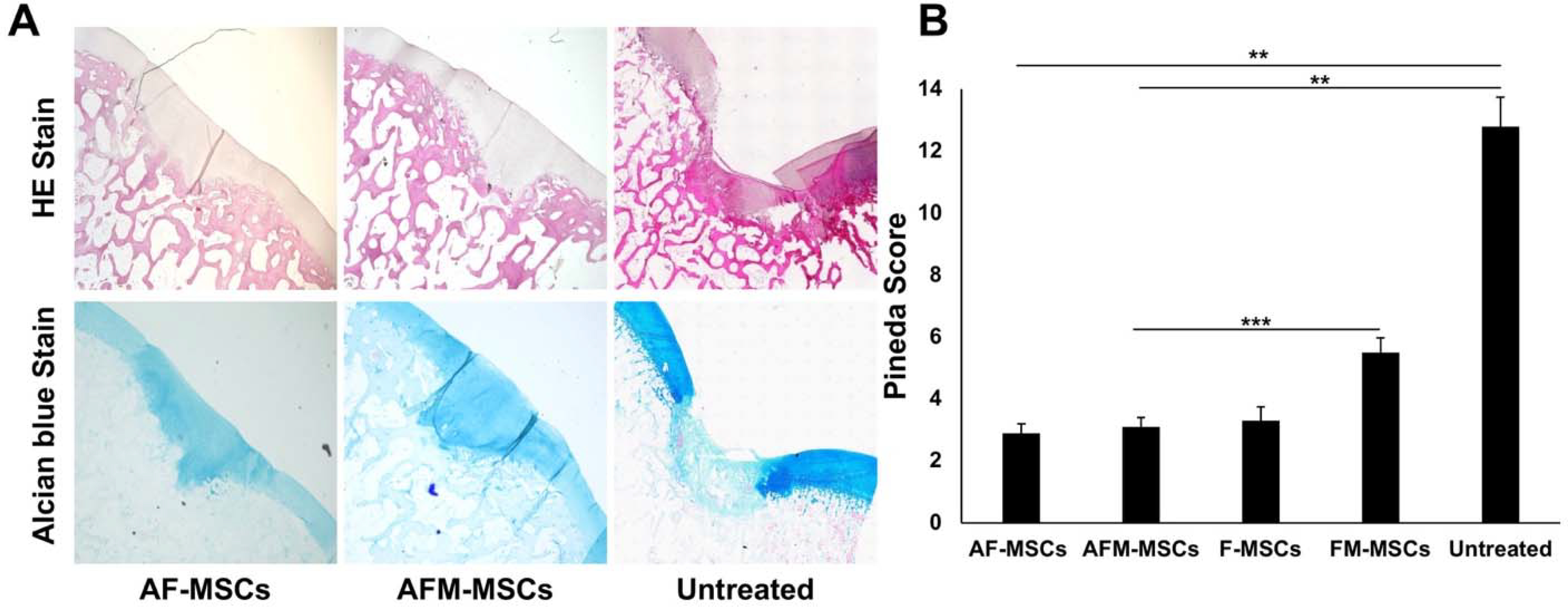
Histological analysis of regenerated articular cartilage. A) Sagittal sections of the articular cartilage stained with Hematoxylin and eosin (HE) (upper) and Alcian blue (lower) demonstrate complete cartilage defect repair for AF-MSCs and AFM-MSCs. Empty defects demonstrate persistent cartilage defects and lack of glycosaminoglycans in the defect. B) Quantification of remaining defect characteristics according to the Pineda score for AF-MSCs (n=8), AFM-MSCs (n=8), F-MSCs (n=10), FM-MSCs (n=10) and untreated controls (n=4). Data are displayed as means and standard error of the mean; Comparisons between different groups were obtained with Mann-Whitney test. A= Ascorbic acid, F= Ferumoxytol, M= Mitomycin.
On immunofluorescence imaging, AF-MSCs (n=8) and AFM-MSCs (n=8) showed marked collagen II formation, while untreated controls (n=4) revealed absence of collagen II (Figure 5A). The amount of collagen type II formation of AF-MSCs (28469±21313 positively stained pixels) was greater than previously reported (23350±16531) for MSC without ascorbic acid in the same animal model, albeit not showing significant difference yet (P =0.317, Figure 5B).48 The collagen type II formation of AF-MSCs and AFM-MSCs (18492±4562) was not significantly different (P =0.574, Figure 5B). However, we found a significantly larger amount of collagen type II in the extracellular matrix of AFM-MSCs group compared to previously reported mitomycin-challenged MSC implants without ascorbic acid (5230±6185, P =0.0007, Figure 5B).48 By contrast, empty defects demonstrated significantly lower collagen II production compared to AF-MSCs and AFM-MSCs (905±1326 versus 28469±21313 and 18492±4562, P =0.004, P =0.004, respectively; Figure 5B).
Figure 5.
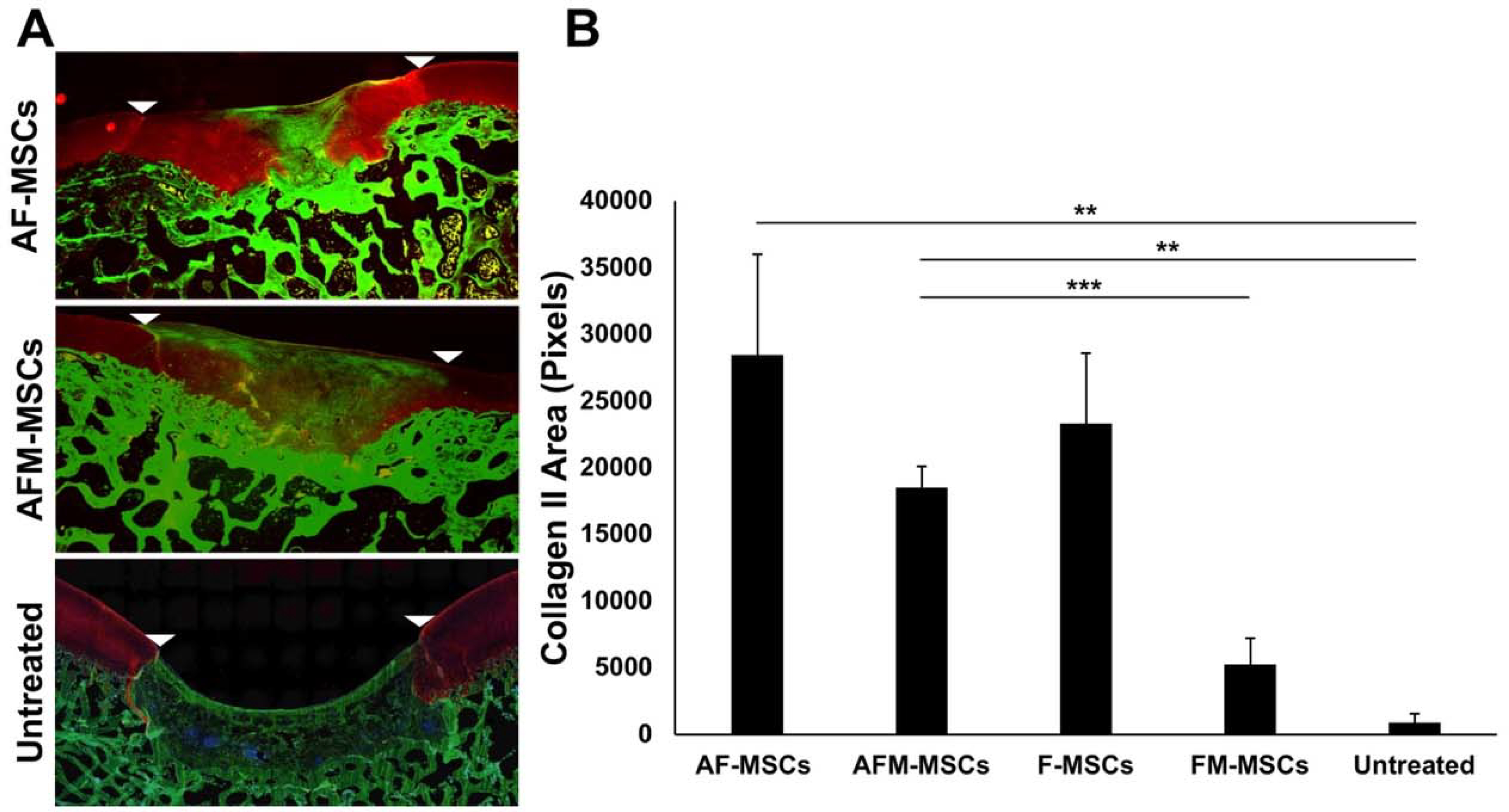
Immunofluorescent staining for collagen II. A) Sagittal sections of the articular cartilage stained with antibodies against collagen II demonstrates marked collagen II production in AF-MSCs and slightly less collagen II in AFM-MSCs. Untreated defects demonstrate lack of collagen II in the defect. White arrow heads indicate defect margins. B) Quantitative analysis of the immunopositive area against collagen II in the repaired cartilage for AF-MSCs (n=8), AFM-MSCs (n=8), F-MSCs (n=10), FM-MSCs (n=10) and Untreated (n=4) group. Data are displayed as means and standard error of the mean. Comparisons between different groups were obtained with Mann-Whitney test. A= Ascorbic acid, F= Ferumoxytol, M= Mitomycin.
Taken together, our results suggest that ascorbic acid and ferumoxytol co-treatment improved cartilage repair outcomes of MSC implants and counteracted MSC apoptosis.
DISCUSSION
Our data showed that ascorbic acid plus iron pretreatment improves chondrogenesis of matrix-associated stem cell implants in knee joints of minipigs compared to previously reported outcomes of iron-labeled MSC implants without ascorbic acid in the same animal model.48 In addition, our data suggested that ascorbic acid pretreatment protected MSC implants against apoptosis: We found improved cartilage defect repair of mitomycin-challenged MSC implants compared to previously reported outcomes of mitomycin-challenged MSC implants without ascorbic acid.48 To the best of our knowledge, this is the first in vivo study to investigate combined effects of ascorbic acid and iron pretreatment on MSC cartilage repair outcomes.
Ascorbic acid has been recognized as a key regulator of chondrogenesis.1,2,9 Ascorbic acid regulates gene expression of chondrocytes via its role as a cofactor of prolyl hydroxylase domain-containing protein 2 (PHD2).28 Hydroxylation of specific proline and lysine residues within the collagen molecule is required for collagen cross-linking, a crucial step in the collagen maturation process which contributes to building bone and cartilage.8,28 In addition, ascorbic acid can stimulate chondrogenesis indirectly by promoting 1,25-dihydroxy vitamin D3 synthesis.13
The effect of ascorbic acid is dependent on the applied dose and cell type: Concentrations of 0.25mM stimulated proliferation of bone marrow-derived MSCs9 and adipose-fat derived stem cells.55 Ascorbic acid concentrations above 1.136mM caused cell death in human chondrocytes.31 Interestingly, fibroblasts are very sensitive to ascorbic acid and demonstrate growth arrest in response to concentrations as low as 0.05mM.4 Thus, the improved cartilage repair outcome of ascorbic acid treated MSC in our study compared to previously reported results with MSC implants only17,44,48 may be due to a combined positive effect on chondrogenesis and negative effect on fibroblast proliferation. This is further supported by the decreased level of type 1 collagen observed in our experimental samples.
Our finding that treatment of ascorbic acid and an FDA approved iron supplement improves chondrogenesis is consistent with previous observations, which described that ascorbic acid reduced metal ions to their biologically active forms and prevented free-radical mediated tissue damage.40 Ascorbic acid reduced iron to the ferrous state, which catalyzed proline and lysine hydroxylation and thereby, supported the formation of mature collagen molecules.39
We previously labeled MSC with ferumoxytol for non-invasive detection with MRI. The administered iron did not impair chondrogenesis and was slowly metabolized over two weeks.23 MSC implants treated with ferumoxytol and ascorbic acid also did not impair chondrogenesis and the iron was metabolized over time, so that it did not affect MOCART assessment at 4 and 12 weeks.
Previous studies showed that implantation of mitomycin-treated MSC into cartilage defects lead to significant MSC apoptosis and inferior cartilage repair compared to MSC implants without mitomycin treatment.48 By comparison, our studies here showed equal cartilage regeneration outcomes of untreated and mitomycin-treated MSCs that had been protected by ascorbic acid. This finding is in accordance with previous studies which showed that ascorbic acid can suppress drug-induced apoptosis by scavenging mitochondrial superoxide anions.54 In addition, ascorbic acid stimulates MSCs to produce extracellular matrix, which further protects them from apoptosis induction.
We have recognized several limitations of our approach: We performed our studies in a large animal model with clinically relevant joint size and biomechanics. We expanded MSC in culture media, which was supplemented with fetal bovine serum (FBS). Future studies have to show, if our results also apply for MSC that are expanded in FBS-free media. Also, a bone marrow aspiration in humans could generate less MSCs than in our study. A bone marrow aspiration from an iliac crest in humans is limited to 15–20 ml/kg or a total volume of 1500 ml, yielding between 100–2300 MSCs/ml.14,29,51 Therefore, a maximum number of only 3.45×106 MSCs can be retrieved in one session. However, with an expansion in FBS-free media 4 cell pellets with 1.2×106 MSCs each could be generated for a treatment of a 5 × 5 × 5 mm cartilage defect. Numbers of animals and MSC implants per experimental group were small, as dictated by power analyses and the goal to use the minimal number of subjects that generate statistically significant results. In order to further minimize the number of animals needed for this study, we compared cartilage repair outcomes of ascorbic acid treated MSC with previously published results of MSC without ascorbic acid in the same animal model48 rather than repeating such experiments. Different locations of different cartilage defects could have affected outcomes of cartilage regeneration. The MOCART score does not provide information regarding the ‘quality’ of the cartilage fill. The T2 mapping data and histologic results supplemented the MOCART score data. We investigated multiple cartilage defects per joint in order to minimize the number of large animals needed for these experiments. In principle, it would be possible that AF-MSCs were releasing extracellular molecules (e.g. exosomes) which modulate differentiation of adjacent AFM-MSCs. However, we did not find differences in cartilage outcomes of joints that were implanted with AF-MSCs and AFM-MSCs versus joints that were implanted with AF-MSCs only and AFM-MSCs only. Thus, the effect of exosomes, if at all present, was minimal. Our studies have been done in relatively young pigs. Thus, results would be most consistent with expected results in young patients with sports-related cartilage injuries rather than older patients with osteoarthritis. Future biomarker analysis of synovial fluid for collagen 2 (Col2) breakdown products could further elucidate, whether cell-based cartilage restoration procedures prevent joint degradation.
In conclusion, our data showed that ascorbic acid and iron pretreated MSC implants improved cartilage repair and led to hyaline-like cartilage regeneration in a large animal model. The applied reagents are in principle clinically translatable to the clinic.
What is known about the subject:
Previous studies showed that ascorbic acid stimulates collagen synthesis and chondrogenesis. In addition, in vitro studies have shown that ascorbic acid reduces iron to the ferrous state, which is necessary for proline and lysine hydroxylation reactions that are essential to the formation of mature collagen molecules.
What this study adds to existing knowledge:
We noted for the first time that co-treatment of mesenchymal stromal cells with an FDA approved iron supplement and ascorbic acid improves stem cell mediated cartilage repair in vivo, in an established large animal model.
Acknowledgments:
We thank all members of the veterinary service center for animal handling, caretaking and valuable teamwork regarding this project. This work was supported by NIH grant R01AR054458 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
APPENDIX
Methods
In Vitro Validation Studies
Pretreatment of stem cells and immunofluorescent imaging
Bone marrow stem cells were grown on glass coverslips in 24-well plates (40,000 cells/well) and treated with ascorbic acid (0.2 mM) and/or ferumoxytol (100 μg Fe/ml) in Stem pro medium (Thermo Fisher, Waltham, MA) for 2 weeks. At the final day, half of the groups were treated with mitomycin (0.06 mg/ml) for 2 hours, then all the cells were fixed for 30 minutes in 4% paraformaldehyde for immunofluorescence imaging with Immunofluorescence Application Solutions Kit (Cell Signaling Technology, Danvers, CA). Briefly, after washing, the cells were blocked in blocking buffer for 1 hour at room temperature before being stained with primary antibody anti-Cleaved Caspase-3 (1:200, ab2302, Abcam, Cambridge, MA) overnight. Subsequently, cells were incubated with anti-Rabbit IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 594 (1:500; A-21442, Thermo Fisher, Waltham, MA) for 1h at room temperature and embedded using Fluoromount-G™ Mounting Medium (with DAPI). The cells were imaged by fluorescence microscopy (Keyence, BZ-X710, Cupertino, CA) and analyzed, using the Immunohistochemistry Image Analysis Toolbox in Image J (National Institutes of Health).
Fluorimetric caspase-3/7 activity assay
Bone marrow stem cells were grown in 24-well plates (40,000 cells/well) and treated with ascorbic acid (0.2 mM) and/or ferumoxytol (100 μg Fe/ml) as described above. After half of the cells were treated with mitomycin (0.06 mg/ml) all the cells were collected by cell scrapers (Fisher Scientific, Waltham, MA). Caspase activity was determined using the SensoLyte® Homogeneous AMC Caspase - 3/7 Assay Kit (Fluorimetric) (Anaspec, Fremont, CA).
In vitro chondrogenic differentiation of labeled stem cells
Bone marrow stem cells were treated with ascorbic acid and/or ferumoxytol in Stem pro medium for 2 weeks and then the cells were harvested and resuspended in chondrogenic differentiation medium (Lonza, Hayward, CA) at 10×106 cells/ml. Droplets (30 μl) were added respectively in each well of the 24-well plate and were grown at 37 °C for 3h for adherence. Afterwards, 500 μl of chondrogenic medium was added to each well and the medium was changed every other day. At day 14, the micromass culture was fixed in 4% paraformaldehyde and stained with a 1% solution of Alcian blue 8-GX (Sigma, St. Louis, MO) in 0.1 N HCI at pH 1 and rinsed twice with 0.1 N HCI to remove any unbound dye. Finally, the cells were air dried for microscopic imaging (Keyence, BZ-X710, Cupertino, CA). Quantification of histochemical staining was performed using ImageJ software (National Institutes of Health).
Results
On immunofluorescent images control and pretreated stem cells incubated with mitomycin showed higher numbers of apoptotic cells than without mitomycin treatment (Appendix Figure A2A). However, treatment with ascorbic acid alone or in combination with ferumoxytol was able to counteract apoptosis. Quantitative immunofluorescent analysis showed significantly lower apoptotic cells in ascorbic acid and mitomycin pretreated mesenchymal stem cells (AM-MSCs, n=3) than in mitomycin only treated stem cells (CM-MSCs, n=3) (18±3 versus 39±2 cells; P <0.0001). Similarly, mitomycin, ascorbic acid and ferumoxytol pretreated MSC (AFM-MSCs, n=3) revealed significantly lower apoptotic cell numbers than mitomycin and ferumoxytol treated cells (FM-MSCS, n=3) (35±2 versus 92±8 cells; P <0.0001) (Appendix Figure A2B). Fluorimetric assay also showed significantly lower caspase 3/7 activity in AM-MSCs (4821±725; n=4) versus CM-MSCs (9323±1414; n=4) and AFM-MSCs (13635±2602; n=4) versus FM-MSCs (21654± 2083; n=4) (P =0.0026; P < 0.0001, respectively) (Appendix Figure A2C).
In vitro chondrogenic differentiation of MSCs with single pretreatment of ascorbic acid (A-MSCs) or ferumoxytol (F-MSCs) did not show any improvement in differentiation capacity on Alcian blue stains compared to controls (Appendix Figure A3A). However, the combination of ascorbic acid and ferumoxytol (AF-MSCs) depicted better chondrogenic differentiation and stronger Alcian blue staining. Quantification of Alcian blue stains revealed significantly higher areas of blue stains on AF-MSCs (19±1%) compared to A-MSCs (8±0.6%), F-MSCs (7±0.2%) and control MSCs (6±0.3%) (each P <0.0001; Appendix Figure A3B).
Appendix Figure A1.

Magnetic Resonance Imaging (MRI) and T2 relaxation times of different experimental groups: A) Sagittal MRI scan with T2 map overlay of the distal femur cartilage at 12 weeks after implantation of ascorbic acid and iron treated chondrogenic pellets (AF-MSCs, yellow arrows) and ascorbic acid, ferumoxytol and mitomycin-treated chondrogenic pellets (AFM-MSCs, red arrows). B) Sagittal MRI scan with T2 map overlay of the distal femur cartilage at 12 weeks after cartilage defect creation without any cell transplant (Untreated group, blue arrows). C) T2 relaxation times of the cartilage of different experimental groups and healthy adjacent cartilage at 12 weeks. All data are means ± SEM. Comparisons between different groups were obtained with Mann-Whitney test. A= Ascorbic acid, F= Ferumoxytol, M= Mitomycin.
Appendix Figure A2.
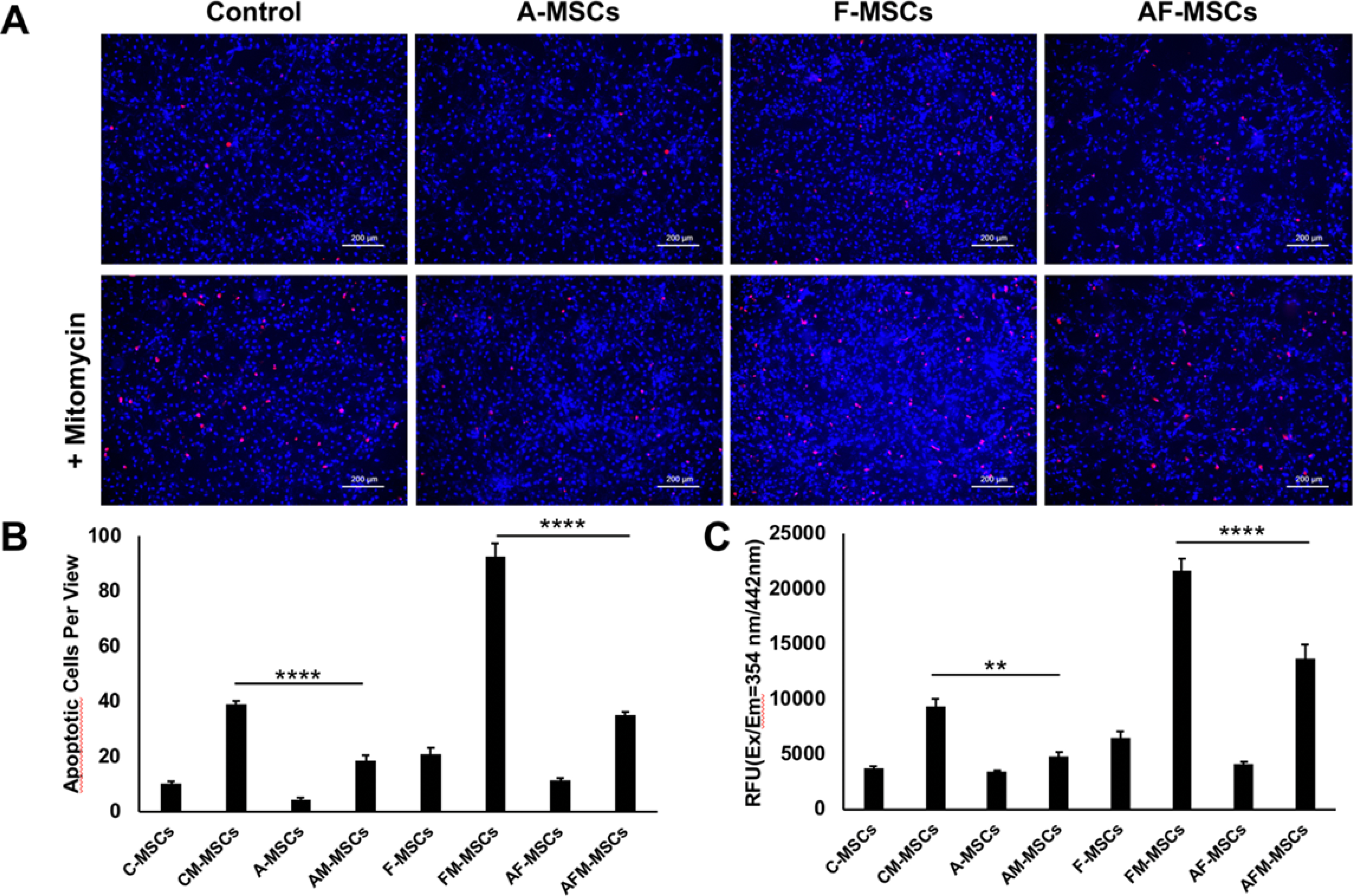
Immunofluorescence microscopy and fluorimetric caspase-3/7 activity assay of different experimental groups. A) Fluorescence microscopy of control and ascorbic acid and/or ferumoxytol pretreated mesenchymal stem cells incubated with and without mitomycin. Treatment with mitomycin shows higher numbers of apoptotic cells than without mitomycin treatment (blue represents nucleus, red represents apoptotic cells). B) Quantification of apoptotic cells per view and C) quantification of caspase 3/7 activity of different experimental groups. All data are means ± SEM; Comparisons between different groups were obtained with one-way analysis of variance. C= Control, A= Ascorbic acid, F= Ferumoxytol, M= Mitomycin.
Appendix Figure A3.
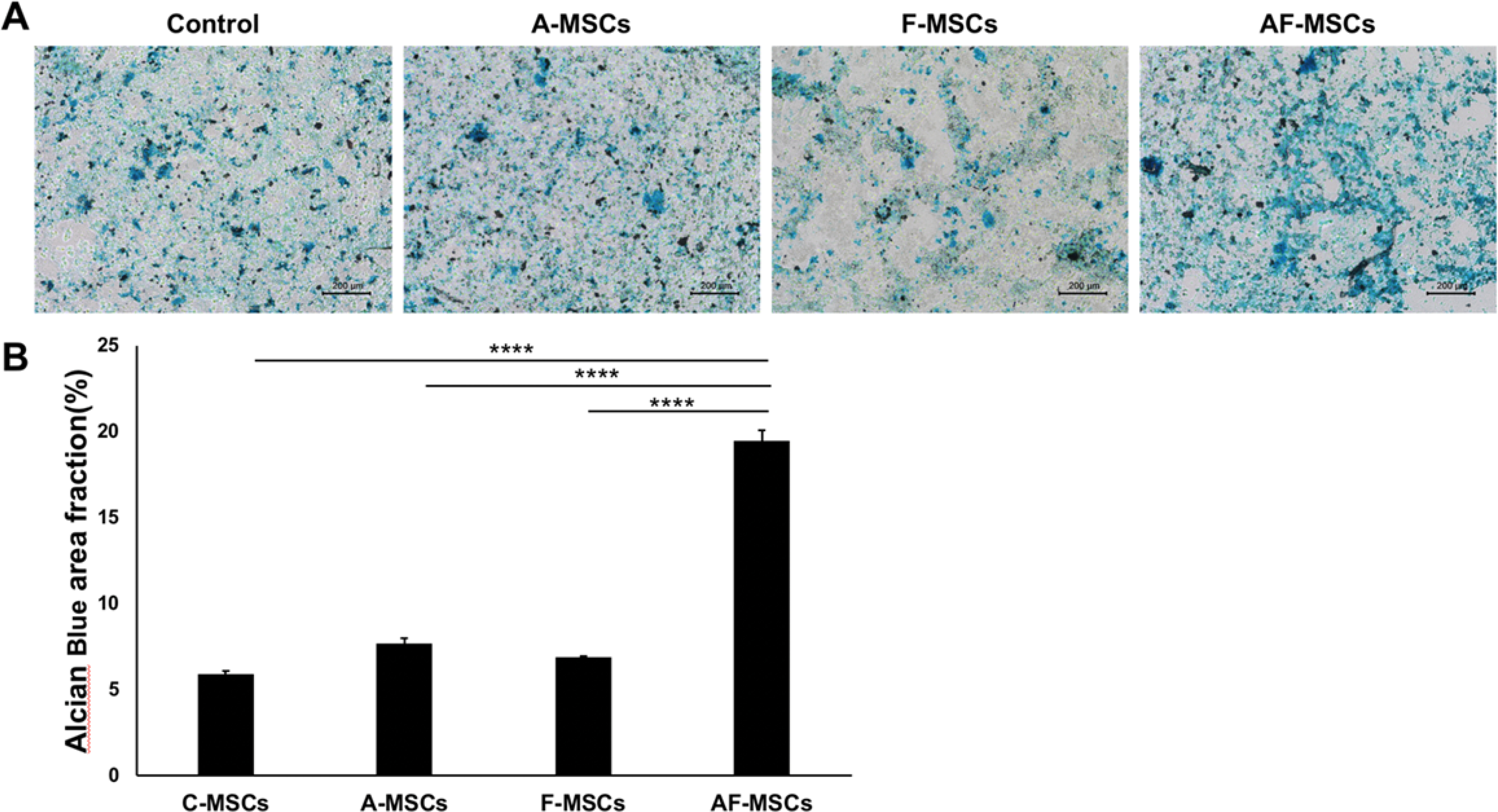
In vitro chondrogenic differentiation of control, ascorbic acid and/or ferumoxytol pretreated mesenchymal stem cells. A) Alcian blue stains of MSCs pretreated with ascorbic acid and ferumoxytol show better chondrogenic differentiation and stronger Alcian blue staining than with no or single pretreatment with ascorbic acid or ferumoxytol. B) Quantification of Alcian blue stains of the different experimental groups. All data are means ± SEM; Comparisons between different groups were obtained with one-way analysis of variance. C= Control, A= Ascorbic acid, F= Ferumoxytol
REFERENCES:
- 1.Altaf FM, Hering TM, Kazmi NH, Yoo JU, Johnstone B. Ascorbate-enhanced chondrogenesis of ATDC5 cells. Eur Cell Mater. 2006;12:64–69; discussion 69–70. [DOI] [PubMed] [Google Scholar]
- 2.Barlian A, Judawisastra H, Alfarafisa NM, Wibowo UA, Rosadi I. Chondrogenic differentiation of adipose-derived mesenchymal stem cells induced by L-ascorbic acid and platelet rich plasma on silk fibroin scaffold. PeerJ. 2018;6:e5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.BaSalamah MA, Abdelghany AH, El-Boshy M, et al. Vitamin D alleviates lead induced renal and testicular injuries by immunomodulatory and antioxidant mechanisms in rats. Sci Rep. 2018;8(1):4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brigelius-Flohe R, Flohe L. Ascorbic acid, cell proliferation, and cell differentiation in culture. Subcell Biochem. 1996;25:83–107. [DOI] [PubMed] [Google Scholar]
- 5.Brittberg M, Lindahl A, Nilsson A, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889–895. [DOI] [PubMed] [Google Scholar]
- 6.Carlevaro MF, Albini A, Ribatti D, et al. Transferrin promotes endothelial cell migration and invasion: implication in cartilage neovascularization. J Cell Biol. 1997;136(6):1375–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Lan J, Liu D, et al. Ascorbic Acid Promotes the Stemness of Corneal Epithelial Stem/Progenitor Cells and Accelerates Epithelial Wound Healing in the Cornea. Stem Cells Transl Med. 2017;6(5):1356–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng S, Aghajanian P, Pourteymoor S, Alarcon C, Mohan S. Prolyl Hydroxylase Domain-Containing Protein 2 (Phd2) Regulates Chondrocyte Differentiation and Secondary Ossification in Mice. Sci Rep. 2016;6:35748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi KM, Seo YK, Yoon HH, et al. Effect of ascorbic acid on bone marrow-derived mesenchymal stem cell proliferation and differentiation. J Biosci Bioeng. 2008;105(6):586–594. [DOI] [PubMed] [Google Scholar]
- 10.Coleman CM, Curtin C, Barry FP, O’Flatharta C, Murphy JM. Mesenchymal stem cells and osteoarthritis: remedy or accomplice? Hum Gene Ther. 2010;21(10):1239–1250. [DOI] [PubMed] [Google Scholar]
- 11.Crowe WE, Maglova LM, Ponka P, Russell JM. Human cytomegalovirus-induced host cell enlargement is iron dependent. American journal of physiology Cell physiology. 2004;287(4):C1023–1030. [DOI] [PubMed] [Google Scholar]
- 12.D’Aniello C, Cermola F, Patriarca EJ, Minchiotti G. Vitamin C in Stem Cell Biology: Impact on Extracellular Matrix Homeostasis and Epigenetics. Stem Cells Int. 2017;2017:8936156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farquharson C, Berry JL, Mawer EB, Seawright E, Whitehead CC. Ascorbic acid-induced chondrocyte terminal differentiation: the role of the extracellular matrix and 1,25-dihydroxyvitamin D. Eur J Cell Biol. 1998;76(2):110–118. [DOI] [PubMed] [Google Scholar]
- 14.Galotto M, Berisso G, Delfino L, et al. Stromal damage as consequence of high-dose chemo/radiotherapy in bone marrow transplant recipients. Exp Hematol. 1999;27(9):1460–1466. [DOI] [PubMed] [Google Scholar]
- 15.Guettler JH, Demetropoulos CK, Yang KH, Jurist KA. Osteochondral defects in the human knee: influence of defect size on cartilage rim stress and load redistribution to surrounding cartilage. Am J Sports Med. 2004;32(6):1451–1458. [DOI] [PubMed] [Google Scholar]
- 16.Han Z, Yu Y, Xu J, et al. Iron Homeostasis Determines Fate of Human Pluripotent Stem Cells Via Glycerophospholipids-Epigenetic Circuit. Stem Cells. 2019;37(4):489–503. [DOI] [PubMed] [Google Scholar]
- 17.Harada Y, Nakasa T, Mahmoud EE, et al. Combination therapy with intra-articular injection of mesenchymal stem cells and articulated joint distraction for repair of a chronic osteochondral defect in the rabbit. J Orthop Res. 2015;33(10):1466–1473. [DOI] [PubMed] [Google Scholar]
- 18.Harrell CR, Markovic BS, Fellabaum C, Arsenijevic A, Volarevic V. Mesenchymal stem cell-based therapy of osteoarthritis: Current knowledge and future perspectives. Biomed Pharmacother. 2019;109:2318–2326. [DOI] [PubMed] [Google Scholar]
- 19.Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58(1):15–25. [DOI] [PubMed] [Google Scholar]
- 20.Hering TM, Kollar J, Huynh TD, Varelas JB, Sandell LJ. Modulation of extracellular matrix gene expression in bovine high-density chondrocyte cultures by ascorbic acid and enzymatic resuspension. Arch Biochem Biophys. 1994;314(1):90–98. [DOI] [PubMed] [Google Scholar]
- 21.Jin X, He X, Cao X, et al. Iron overload impairs normal hematopoietic stem and progenitor cells through reactive oxygen species and shortens survival in myelodysplastic syndrome mice. Haematologica. 2018;103(10):1627–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kao J, Huey G, Kao R, Stern R. Ascorbic acid stimulates production of glycosaminoglycans in cultured fibroblasts. Exp Mol Pathol. 1990;53(1):1–10. [DOI] [PubMed] [Google Scholar]
- 23.Khurana A, Nejadnik H, Chapelin F, et al. Ferumoxytol: a new, clinically applicable label for stem-cell tracking in arthritic joints with MRI. Nanomedicine (Lond). 2013;8(12):1969–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim YS, Park EH, Lee HJ, Koh YG, Lee JW. Clinical comparison of the osteochondral autograft transfer system and subchondral drilling in osteochondral defects of the first metatarsal head. Am J Sports Med. 2012;40(8):1824–1833. [DOI] [PubMed] [Google Scholar]
- 25.Kostura L, Kraitchman DL, Mackay AM, Pittenger MF, Bulte JW. Feridex labeling of mesenchymal stem cells inhibits chondrogenesis but not adipogenesis or osteogenesis. NMR Biomed. 2004;17(7):513–517. [DOI] [PubMed] [Google Scholar]
- 26.Li CJ, Sun LY, Pang CY. Synergistic protection of N-acetylcysteine and ascorbic acid 2-phosphate on human mesenchymal stem cells against mitoptosis, necroptosis and apoptosis. Sci Rep. 2015;5:9819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H, Zeng C, Wei J, et al. Associations between dietary antioxidants intake and radiographic knee osteoarthritis. Clin Rheumatol. 2016;35(6):1585–1592. [DOI] [PubMed] [Google Scholar]
- 28.Lindsey RC, Cheng S, Mohan S. Vitamin C effects on 5-hydroxymethylcytosine and gene expression in osteoblasts and chondrocytes: Potential involvement of PHD2. PLoS One. 2019;14(8):e0220653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lisenko K, Stadtherr P, Bruckner T, et al. Bone Marrow Harvesting of Allogeneic Donors in an Outpatient Setting: A Single-Center Experience. Biol Blood Marrow Transplant. 2016;22(3):470–474. [DOI] [PubMed] [Google Scholar]
- 30.Mahmoud EE, Kamei N, Kamei G, et al. Role of Mesenchymal Stem Cells Densities When Injected as Suspension in Joints with Osteochondral Defects. Cartilage. 2019;10(1):61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malicev E, Wozniak G, Knezevic M, Radosavljevic D, Jeras M. Vitamin C induced apoptosis in human articular chondrocytes. Pflugers Arch. 2000;440(Suppl 1):R046–R048. [DOI] [PubMed] [Google Scholar]
- 32.Mohammed BM, Fisher BJ, Kraskauskas D, et al. Vitamin C promotes wound healing through novel pleiotropic mechanisms. Int Wound J. 2016;13(4):572–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murad S, Grove D, Lindberg KA, et al. Regulation of collagen synthesis by ascorbic acid. Proc Natl Acad Sci U S A. 1981;78(5):2879–2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murad S, Sivarajah A, Pinnell SR. Regulation of prolyl and lysyl hydroxylase activities in cultured human skin fibroblasts by ascorbic acid. Biochem Biophys Res Commun. 1981;101(3):868–875. [DOI] [PubMed] [Google Scholar]
- 35.Muto Y, Nishiyama M, Nita A, Moroishi T, Nakayama KI. Essential role of FBXL5-mediated cellular iron homeostasis in maintenance of hematopoietic stem cells. Nat Commun. 2017;8:16114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagai T, Sato M, Furukawa KS, et al. Optimization of allograft implantation using scaffold-free chondrocyte plates. Tissue Eng Part A. 2008;14(7):1225–1235. [DOI] [PubMed] [Google Scholar]
- 37.Nejadnik H, Lenkov O, Gassert F, et al. Macrophage phagocytosis alters the MRI signal of ferumoxytol-labeled mesenchymal stromal cells in cartilage defects. Sci Rep. 2016;6:25897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orth P, Goebel L, Wolfram U, et al. Effect of subchondral drilling on the microarchitecture of subchondral bone: analysis in a large animal model at 6 months. Am J Sports Med. 2012;40(4):828–836. [DOI] [PubMed] [Google Scholar]
- 39.Pacifici M, Iozzo RV. Remodeling of the rough endoplasmic reticulum during stimulation of procollagen secretion by ascorbic acid in cultured chondrocytes. A biochemical and morphological study. J Biol Chem. 1988;263(5):2483–2492. [PubMed] [Google Scholar]
- 40.Padh H Vitamin C: newer insights into its biochemical functions. Nutr Rev. 1991;49(3):65–70. [DOI] [PubMed] [Google Scholar]
- 41.Pineda S, Pollack A, Stevenson S, Goldberg V, Caplan A. A semiquantitative scale for histologic grading of articular cartilage repair. Acta Anat (Basel). 1992;143(4):335–340. [DOI] [PubMed] [Google Scholar]
- 42.Pizzute T, Zhang Y, He F, Pei M. Ascorbate-dependent impact on cell-derived matrix in modulation of stiffness and rejuvenation of infrapatellar fat derived stem cells toward chondrogenesis. Biomed Mater. 2016;11(4):045009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Regan EA, Bowler RP, Crapo JD. Joint fluid antioxidants are decreased in osteoarthritic joints compared to joints with macroscopically intact cartilage and subacute injury. Osteoarthritis Cartilage. 2008;16(4):515–521. [DOI] [PubMed] [Google Scholar]
- 44.Samuel S, Ahmad RE, Ramasamy TS, Manan F, Kamarul T. Platelet rich concentrate enhances mesenchymal stem cells capacity to repair focal cartilage injury in rabbits. Injury. 2018;49(4):775–783. [DOI] [PubMed] [Google Scholar]
- 45.Shimozono Y, Coale M, Yasui Y, et al. Subchondral Bone Degradation After Microfracture for Osteochondral Lesions of the Talus: An MRI Analysis. Am J Sports Med. 2018;46(3):642–648. [DOI] [PubMed] [Google Scholar]
- 46.Sun M, Xie Q, Cai X, et al. Preparation and characterization of epigallocatechin gallate, ascorbic acid, gelatin, chitosan nanoparticles and their beneficial effect on wound healing of diabetic mice. Int J Biol Macromol. 2020;148:777–784. [DOI] [PubMed] [Google Scholar]
- 47.Tang C, Jin C, Du X, et al. An autologous bone marrow mesenchymal stem cell-derived extracellular matrix scaffold applied with bone marrow stimulation for cartilage repair. Tissue Eng Part A. 2014;20(17–18):2455–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Theruvath AJ, Nejadnik H, Lenkov O, et al. Tracking Stem Cell Implants in Cartilage Defects of Minipigs by Using Ferumoxytol-enhanced MRI. Radiology. 2019;292(1):129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valderrabano V, Miska M, Leumann A, Wiewiorski M. Reconstruction of osteochondral lesions of the talus with autologous spongiosa grafts and autologous matrix-induced chondrogenesis. Am J Sports Med. 2013;41(3):519–527. [DOI] [PubMed] [Google Scholar]
- 50.van den Borne MP, Raijmakers NJ, Vanlauwe J, et al. International Cartilage Repair Society (ICRS) and Oswestry macroscopic cartilage evaluation scores validated for use in Autologous Chondrocyte Implantation (ACI) and microfracture. Osteoarthritis Cartilage. 2007;15(12):1397–1402. [DOI] [PubMed] [Google Scholar]
- 51.van Walraven SM, Straathof LM, Switzer GE, et al. Immediate and long-term somatic effects, and health-related quality of life of BM donation during early childhood. A single-center report in 210 pediatric donors. Bone Marrow Transplant. 2013;48(1):40–45. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y, Hodge AM, Wluka AE, et al. Effect of antioxidants on knee cartilage and bone in healthy, middle-aged subjects: a cross-sectional study. Arthritis Res Ther. 2007;9(4):R66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wayne JS, McDowell CL, Shields KJ, Tuan RS. In vivo response of polylactic acid-alginate scaffolds and bone marrow-derived cells for cartilage tissue engineering. Tissue Eng. 2005;11(5–6):953–963. [DOI] [PubMed] [Google Scholar]
- 54.Wenzel U, Nickel A, Kuntz S, Daniel H. Ascorbic acid suppresses drug-induced apoptosis in human colon cancer cells by scavenging mitochondrial superoxide anions. Carcinogenesis. 2004;25(5):703–712. [DOI] [PubMed] [Google Scholar]
- 55.Yu J, Tu YK, Tang YB, Cheng NC. Stemness and transdifferentiation of adipose-derived stem cells using L-ascorbic acid 2-phosphate-induced cell sheet formation. Biomaterials. 2014;35(11):3516–3526. [DOI] [PubMed] [Google Scholar]


