Abstract
Small-cell lung cancer (SCLC) represents about 15% of all lung cancers and is marked by an exceptionally high proliferative rate, strong predilection for early metastasis and poor prognosis. SCLC is strongly associated with exposure to tobacco carcinogens. Most patients have metastatic disease at diagnosis, with only one-third having earlier-stage disease that is amenable to potentially curative multimodality therapy. Genomic profiling of SCLC reveals extensive chromosomal rearrangements and a high mutation burden, almost always including functional inactivation of the tumour suppressor genes TP53 and RB1. Analyses of both human SCLC and murine models have defined subtypes of disease based on the relative expression of dominant transcriptional regulators and have also revealed substantial intratumoural heterogeneity. Aspects of this heterogeneity have been implicated in tumour evolution, metastasis and acquired therapeutic resistance. Although clinical progress in SCLC treatment has been notoriously slow, a better understanding of the biology of disease has uncovered novel vulnerabilities that might be amenable to targeted therapeutic approaches. The recent introduction of immune checkpoint blockade into the treatment of patients with SCLC is offering new hope, with a small subset of patients deriving prolonged benefit. Strategies to direct targeted therapies to those patients who are most likely to respond and to extend the durable benefit of effective antitumour immunity to a greater fraction of patients are urgently needed and are now being actively explored.
Small-cell lung cancer (SCLC) is a high-grade neuroendocrine carcinoma arising predominantly in current or former smokers and has an exceptionally poor prognosis1. SCLC makes up about 15% of lung cancer cases. Patients with SCLC typically present with respiratory symptoms, including cough, dyspnoea (laboured breathing) or haemoptysis (coughing up blood), with imaging revealing a centrally located lung mass and often bulky thoracic lymph node involvement; two-thirds of patients have distant metastatic disease at initial diagnosis. The most common sites of metastasis include the contralateral lung, the brain, liver, adrenal glands and bone. Mirroring its high metastatic predilection, the concentration of circulating tumour cells (CTCs) in SCLC is among the highest of any solid tumour2 (FIG. 1). Despite emerging recognition of distinct biological subtypes of SCLC based on the transcription factor expression profile, the current clinical approach to SCLC treatment is consistent irrespective of subtype3. In the rare patients who present with very early-stage disease at diagnosis, treatment can include surgery and adjuvant platinum-based chemotherapy although, more typically, patients with early-stage or locally advanced disease are treated with concurrent radiation and platinum-based chemotherapy. Patients with metastatic disease are treated with systemic chemotherapy with or without immunotherapy. SCLC is initially exceptionally responsive to cytotoxic therapies — up to 25% of patients with early-stage SCLC achieve long-term control of disease with concurrent chemoradiotherapy (CRT) and response rates are consistently over 60%, even in patients with metastatic disease. However, in the vast majority of patients, these responses are transient, resulting in a median survival duration of <2 years for patients with early-stage disease and of ~1 year for patients with metastatic disease. In this Primer, we present an overview of the current understanding of SCLC from both the clinical and biological perspectives and focus in particular on how analyses of both mouse models and human tumours are informing new directions of therapeutic research in SCLC.
Fig. 1 |. Common sites of metastasis in SCLC.

Primary small-cell lung cancer (SCLC) tumours tend to be centrally located and are often bulky at presentation. Common sites of metastatic spread include lymphatic spread to hilar and mediastinal lymph nodes and haematogenous spread to the contralateral lung, the brain, liver, adrenal glands and bone. Circulating tumour cells are common in patients with SCLC and are found as both isolated cells and small clusters. RBC, red blood cell.
Epidemiology
Incidence and prevalence
Lung cancer is the leading cause of cancer mortality worldwide, with an estimated 2.1 million new cases and 1.8 million deaths in 2018 (REF.4). SCLC comprises an estimated 250,000 new cases and at least 200,000 deaths globally each year5. Lung cancer, including all histological subtypes, is more prevalent in high-income countries/regions, reflecting relative levels of tobacco consumption4,6. However, the specific incidence of SCLC in different countries/regions or continents is not well described. As with lung cancer in general, SCLC is most prevalent in men but the proportion of cases in women compared to men has risen worldwide over the past 50 years, again reflecting tobacco consumption trends7. SCLC incidence in the USA has been declining over the past three decades in parallel with the decreasing prevalence of cigarette smoking8. In the USA, the proportion of elderly patients with SCLC (>70 years of age) has increased from 23% in 1975 to 44% in 2010 (REF.9). Despite a higher prevalence of smoking in African-American men and women, SCLC is less prevalent in African-American individuals than in white Americans10,11.
Risk factors
SCLC is among the cancers with the strongest epidemiological link to tobacco, and its prevalence tends to mirror the prevalence of smoking, with a lag time of about 30 years12. SCLC incidence in the USA peaked in men in 1986 and in women in 1991 and has steadily declined since that time (FIG. 2). Only 2% of SCLC cases arise in never-smokers (defined as lifetime smoking of fewer than 100 cigarettes)13. Studies suggest possible links between exposure to air pollution14 and to radon15 and SCLC in never-smokers but evidence for both is limited. Inherited genetic factors are thought to play a minor role in the susceptibility to developing SCLC16. Genetic variation does contribute to risk of nicotine addiction17 and might thereby indirectly influence SCLC risk. In addition to rare cases of de novo SCLC arising in never-smokers, some cases of SCLC in never-smokers arise through histological transformation of EGFR-driven or ALK-driven lung adenocarcinoma to SCLC18, as discussed below.
Fig. 2 |. SCLC incidence and survival statistics.
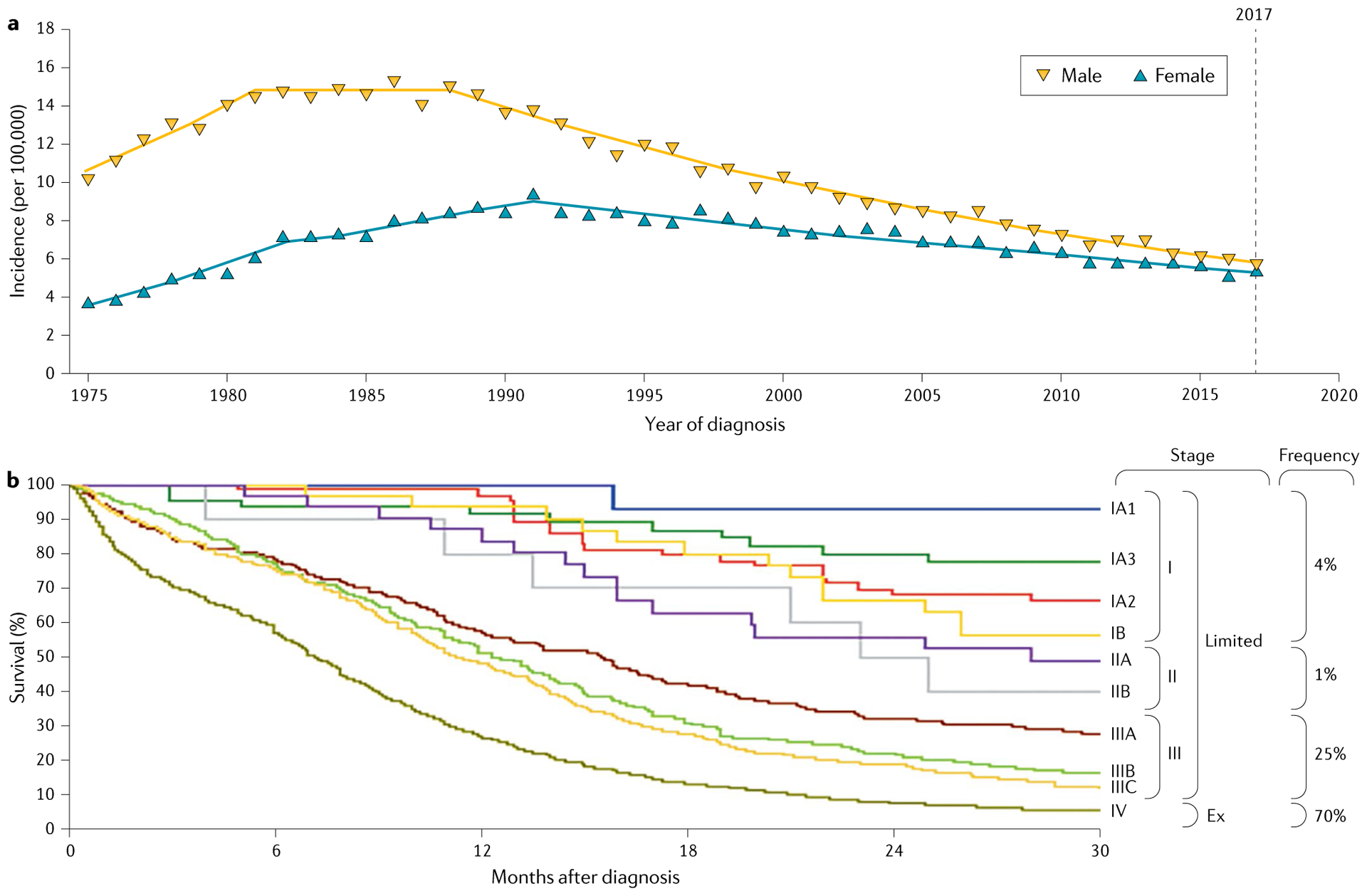
a | Age-adjusted incidence of small-cell lung cancer (SCLC) in the USA for the period 1975–2017. SCLC incidence in the USA has been declining following trends in cigarette use. Although SCLC was substantially more common in men than in women in the 1980s, the difference in incidence between the sexes has narrowed to essentially equal disease incidence by 2017. Data are from the Surveillance, Epidemiology, and End Results (SEER) registry database. b | SCLC survival probability over time by stage at time of diagnosis. SCLC survival according to clinical tumour–node–metastasis (TNM) stage using Union for International Cancer Control eighth edition criteria147. An alternative staging system, that of the Veterans Administration Lung Study Group (VALSG), distinguishes between limited-stage disease (Limited; confined to one hemithorax and a single radiation port) and extensive-stage disease (Ex)148. TNM stages I–III generally correspond to limited-stage disease and TNM stage IV to extensive-stage disease in the VALSG staging system (vertical colour bar). The disease frequency at diagnosis by TNM stage I–IV is indicated. Part b adapted with permission from REF.147, Elsevier.
Chronic obstructive pulmonary disease is a common comorbidity in smokers and an independent risk factor for SCLC12. A Dutch registry study reported that the proportion of patients with SCLC with comorbidities increased between 1995 and 2012 (REF.19). The likelihood of having a comorbidity or comorbidities increased with age and the increase in risk associated with having comorbidities was greater for women than for men. Multimorbidity was associated with a small increase in the risk of death, independent of treatment in patients with limited-stage disease. In a French registry study, the presence of comorbidity was associated with lower 8-year survival rates in patients with SCLC after adjustment for age, sex, stage and diagnostic modality. The hazard ratios were 1.6 (95% CI 1.1–2.3), 1.7 (95% CI 1.1–2.7) and 2.7 (95% CI 1.7–4.4) for Charlson comorbidity index (which predicts the 1-year mortality of a patient with a range of comorbidities) grades 1, 2 and ≥3, respectively20.
SCLC has an exceptionally high mortality rate relative to other common solid tumours. In a US Surveillance, Epidemiology, and End Results (SEER) registry data analysis, although the 5-year survival rate was marginally improving, the median survival for the 1983–2012 period was just 7 months21. Clinical trial data in the past decade (discussed below) suggest that outcomes are improving but entry to these studies is notably delimited by performance status (a patient’s ability to function in daily life) and other criteria that restrict the participation of patients with comorbidities. Current CT screening recommendations that demonstrably reduce overall lung cancer mortality do not seem to improve SCLC outcomes22,23.
Mechanisms/pathophysiology
SCLC tumour mutational profiling reveals a clear smoking signature, providing direct evidence that tobacco carcinogens are responsible for the initiation of SCLC24. Concomitant inactivation of two tumour suppressors, p53 and RB (encoded by TP53 and RB1, respectively), is found in the vast majority of SCLC cases24. This dual inactivation of tumour suppressors is distinct from the primary oncogenic drivers of many other solid tumours, notably non-small-cell lung cancers (NSCLC), in which activating oncogenic mutations seem to be essential for tumorigenesis. Changes in the lung stroma and immune microenvironment also presumably contribute to SCLC tumorigenesis12. However, overall, how these tumour-intrinsic and tumour-extrinsic factors affect the cell type from which SCLC initiates, and how these tumours grow, metastasize and respond to therapy, remains incompletely understood.
Key genetic lesions underlying SCLC
It has been known for several decades that the loss of p53 and RB1 occurs frequently in SCLC25,26. Other early studies described the amplification of MYC family genes (MYC, MYCL and MYCN) in a subset of SCLC tumours27–29. These observations have been validated in DNA and RNA sequencing analyses of larger cohorts of primary tumours and of patient-derived and CTC-derived xenograft models24,30–33. These studies also identified other recurrent alterations (Table 1). Among the few that have been functionally validated in mouse models or cell culture assays are loss-of-function events in RB family members p107 and p130 (encoded by RBL1 and RBL2, respectively)34–36, the tumour suppressor PTEN37,38, NOTCH receptors24,39,40 and the chromatin regulator CREBBP41. In addition to recurrent amplification of MYC family genes42–44, amplification of FGFR1 (encoding fibroblast growth factor receptor 1)45 and GNAS (encoding the α-subunit of the heterotrimeric G protein Gs)46 also occurs. The histone methyltransferase KMT2D (also known as MLL) is mutationally inactivated in 8% of SCLC tumours47. Importantly, primary tumours and patient-derived xenograft models often correspond to early stages of SCLC development, which may introduce a bias in the identification of genetic drivers. However, genetic analysis of more advanced cancers has, thus far, not identified new drivers, except possibly a role for WNT signalling in chemoresistant SCLC48.
Table 1 |.
Frequently altered genes in SCLC
| Gene | Frequency in SCLC (%) | Alteration | Main function |
|---|---|---|---|
| TP53 | 89 | Inactivating mutation; deletion | Tumour suppressor; stress response; transcription regulation |
| RB1 | 64a | Inactivating mutation; deletion | Tumour suppressor; cell cycle regulation; transcription repression |
| KMT2D | 13 | Inactivating mutation; deletion | Tumour suppressor; histone modification; chromatin remodelling |
| PIK3A | 7 | Activating mutation | Oncogene; PTEN–mTOR signalling pathway |
| PTEN | 7 | Inactivating mutation; deletion | Tumour suppressor; PTEN–mTOR signalling pathway |
| NOTCH1 | 6 | Inactivating mutation | Tumour suppressor; cell–cell signalling |
| CREBBP | 5 | Inactivating mutation; deletion | Tumour suppressor; acetyltransferase (histone and non-histone proteins); chromatin remodelling; transcription regulation |
| FAT1 | 4 | Inactivating mutation; deletion | Tumour suppressor; cell–cell signalling |
| NF1 | 4 | Inactivating mutation; deletion | Tumour suppressor; RAS signalling pathway |
| APC | 4 | Inactivating mutation; deletion | Tumour suppressor; WNT signalling pathway |
| EGFR | 4 | Activating mutation | Oncogene; RAS signalling pathway |
| KRAS | 3 | Activating mutation | Oncogene; RAS signalling pathway |
| NOTCH3 | 2.9 | Inactivating mutation; deletion | Tumour suppressor; cell–cell signalling |
| ARID1A | 2.9 | Inactivating mutation; deletion | Tumour suppressor; chromatin remodelling; transcription regulation |
| PTPRD | 2.7 | Inactivating mutation; deletion | Tumour suppressor; chromatin remodelling |
| ATRX | 2.4 | Inactivating mutation; deletion | Tumour suppressor; cell–cell signalling |
| TSC2 | 2.3 | Inactivating mutation; deletion | Tumour suppressor; PTEN–mTOR signalling pathway |
| EP300 | 2.1 | Inactivating mutation; deletion | Tumour suppressor; chromatin remodelling |
Genomic profiling has not identified obvious mutationally defined subtypes of SCLC, but this negative result may be due to the low number of tumour samples that have been analysed. Consistent mutational differences have not been defined based on ethnicity or smoking status, although the prevalence of oncogenic drivers might be anticipated to be higher in the rare never-smokers with SCLC than in tobacco users with SCLC49. A growing number of reports have characterized the histological transformation of lung adenocarcinoma to an aggressive neuroendocrine phenotype resembling SCLC, which is associated with acquired resistance to inhibitors of EGFR or other tyrosine kinase receptors but, again, tumour sample numbers are too small to make strong conclusions regarding specific genetic or epigenetic alterations beyond the ubiquitous loss of p53 and RB in this transition50–52.
A prevalent problem in the SCLC field has been the small amounts of material available for histological diagnosis and subsequent research. The ability to isolate CTCs from the blood of patients with SCLC can alleviate the lack of tumour material53. However, there is a still great need for clinical trials that include the collection of tumour material to identify key genetic drivers of SCLC and accelerate both clinical and basic research.
In addition to the analysis of human material, genetically engineered mouse models have provided an invaluable pre-clinical platform to identify and characterize the molecular and cellular mechanisms of SCLC initiation, progression, metastasis and response to treatment. The requirement for the genetic inactivation of both p53 and RB for the initiation of SCLC was demonstrated in mice54, and mouse tumours acquire genetic alterations similar to those found in human tumours24,37. The histopathological analysis of tumours in these mice shows strong similarities with the range of histological features seen in human tumours55. Mouse models of SCLC were recently reviewed56 and many of the molecular and cellular mechanisms of SCLC development described below have been identified using these mouse models.
Molecular pathways affected in SCLC
Both RB and p53 play key roles in regulating cell cycle progression: RB is a major inhibitor of S phase entry, whereas p53 is integral to multiple cell cycle checkpoints, triggering cell cycle arrest or inducing apoptosis in response to various cellular stresses, for example, aberrant replication. The loss of p107 or p130, amplification of MYC family members, alterations in the PTEN pathway, and a high expression of BCL-2 have all been implicated in promoting cell growth, proliferation and survival in SCLC57–59.
The abrogation of the G1–S cell cycle checkpoint associated with the loss of p53 and RB results in an increased reliance on subsequent cell cycle checkpoints to ensure genome stability and correct chromosomal segregation. Accordingly, the inhibition of kinases that are important for the G2–M transition, such as ATR, WEE1 and CHK1, promotes mitotic catastrophe in SCLC cells, and these kinases are being explored as therapeutic targets44,60–65. Similarly, the dysregulated cell cycle progression in SCLC and the resulting DNA damage may render SCLC vulnerable to multiple strategies that inhibit DNA repair pathways66–68. The activation of the PI3K–AKT–mTOR pathway has been implicated in proliferation and resistance to apoptosis in SCLC69,70.
A number of the alterations found in SCLC cells affect factors involved in stem cell biology, cell fate decisions and lineage plasticity. Both p53 and RB are directly involved in the regulation of these processes in multiple contexts (reviewed elsewhere71,72), including increased lineage plasticity and neuroendocrine differentiation in TP53-deficient and RB1-deficient prostate cancer73,74. High levels of the stem cell transcription factor SOX2 downstream of p53 and RB loss73–75 or as a consequence of genomic amplification31 may further contribute to lineage plasticity in SCLC cells. Mutations in chromatin modifiers are frequent in SCLC, suggesting that alterations in epigenetic regulation may contribute to cell-fate changes24,47,76,77.
The engagement of stem and progenitor pathways in SCLC may facilitate intratumoural plasticity, including through the expression of the REST transcription co-repressor, which may promote the loss of neuroendocrine features in a subset of SCLC cells39. Heterogeneity is prominent in SCLC tumours and may represent a major mechanism by which SCLC tumours evade treatment; additionally, heterogeneity increases in response to treatment78,79. One mechanism might be that the various molecular and cellular fates adopted by SCLC provide reservoirs of cells with intrinsic resistance to treatment, which can then allow for the emergence of acquired resistance mechanisms driven by additional genetic or epigenetic changes over time. The development of strategies to restrict the plasticity of SCLC tumours may restrict the emergence of therapeutic resistance.
In addition to being involved in SCLC evolution and response to therapy, alterations in lineage plasticity and cell fate regulators may also influence the ability of SCLC tumours to develop from diverse cell types (including from lung adenocarcinoma cells)50. Although SCLC has long been assumed to initiate in neuroendocrine lung epithelial cells, observations from mouse models suggest that other lung epithelial cells, in addition to a subset of neuroendocrine cells80, may serve as cells of origin56,81–83. An epigenetic memory of the cell of origin may strongly influence tumour progression, metastasis (see below) and response to therapy.
The extent of genetic heterogeneity within a SCLC tumour seems to be, on average, less than that of NSCLC24. As discussed below, there may still be selection for new genetic drivers during the process of metastasis, but the current observations are consistent with a model in which SCLC tumours have already acquired a set of genetic alterations allowing for rapid growth and rely more on epigenetic mechanisms to generate heterogeneity and respond to their microenvironment.
Cellular pathways affected in SCLC
Although SCLC tumours are highly metastatic, how cell adhesion and cell migration are affected by the genetic and transcriptional changes in SCLC cells is not completely understood. It is possible that the migration potential of SCLC cells is inherently related to the striking migratory phenotypes of neuroendocrine cells during lung development84. Interactions between laminin and fibro nectin in the extracellular matrix and adhesion molecules, such as integrins, have been associated with survival and resistance to therapy85. Similarly, high levels of CXCR4, a receptor for the chemokine stromal cell-derived factor 1 (SDF1; also known as CXCL12), promote the migration and survival of SCLC cells86–88. Interestingly, α3β1 integrin-mediated adhesion stimulates the growth of axon-like protrusions on SCLC cells89, which might promote cell migration by mechanisms similar to those observed in the migration of neuronal progenitors during brain development90. The epithelial-to-mesenchymal transition has been implicated in the resistance to treatment in SCLC77,91,92 but has not been explored as a driver of cell migration. The interplay between adhesion, migration, survival and proliferation may be relevant to the strong metastatic potential of SCLC cells.
SCLC cells have the capacity to communicate with their microenvironment in an autocrine, paracrine and endocrine manner. Several studies suggest that neuropeptides produced by SCLC cells promote tumour cell survival and proliferation by autocrine and paracrine loops93–96. Autocrine KIT, Hedgehog and IGF1 signalling can enhance SCLC cell growth97–99 and paracrine FGF signalling between neuroendocrine and non-neuroendocrine SCLC cells might promote survival and metastasis100. The presence of endocrine paraneoplastic syndromes in patients with SCLC101,102 imply that long-range communication exists between SCLC cells and other cells in the body but it is unclear if these systemic effects have a role in SCLC growth. Overall, the extent of the molecules secreted by SCLC cells, the cell types reached by these molecules, and the effects of these interactions on tumour growth and response to treatment remain largely unknown.
The exceptionally rapid proliferative rate of SCLC cells suggests that they might be selectively dependent on the biosynthetic pathways required for cell replication. Most therapeutic agents commonly used in patients with SCLC, including DNA cross-linkers (such as cisplatin), topoisomerase inhibitors (such as etoposide or topotecan) or γ-radiation, directly or indirectly target DNA synthesis, replication and repair. Additional potential metabolic vulnerabilities in SCLC have begun to be explored, including glycolytic and lipid synthesis pathways103–105. The recurrent mutations in mTOR signalling pathway components24,106 warrant studies of amino acid metabolism in SCLC cells, and a study revealed a key role for arginine in MYC-high SCLC tumours107. Nevertheless, the metabolism of SCLC cells is only beginning to be investigated.
Drivers and trajectories of metastasis
SCLC metastases are rarely resected in patients but insights into the biology of SCLC metastasis have come from both the study of CTCs and the development of mouse models. Patients with SCLC have exceptionally high numbers of CTCs, providing a unique opportunity to investigate possible drivers of metastatic seeding, including genomic alterations, expression changes and heterogeneity2,32,53,108–110. The CTC abundance in SCLC suggests that the circulation is a major route of metastatic transmission, although lymph node metastases are also frequent in patients with SCLC111 and in mouse models of SCLC42,83. Small clusters of malignant cells have been observed in both blood and lymphatic vessels in patients with SCLC: adhesion between CTCs in these small clusters may be an important aspect of cell survival during metastasis2.
SCLC tumours growing in the lungs of genetically engineered mice often metastasize to the pleural space, lymph nodes and distant organs, including the liver, similar to what is observed in patients54. One notable exception is the lack of brain metastasis in SCLC mouse models, which might reflect either biological differences between human and mouse tumours or the relatively rapid death of mice from their primary tumours and liver metastases. The analysis of primary tumours and metastases in mouse models identified the transcription factor NFIB as a major determinant of SCLC metastasis112–114. NFIB levels are also elevated in human SCLC metastases compared with primary tumours112,113. One mechanism underlying the pro-metastatic role of NFIB in SCLC is by the induction of gene expression programmes related to cell adhesion, cell migration and neuronal differentiation90,112. Mechanisms other than NFIB remain poorly understood but factors associated with neuronal differentiation and migration are also implicated in SCLC metastatic potential83,115.
Immune evasion
SCLC cells have a high tumour mutation burden and, on this basis, are predicted to induce strong T cell responses. Indeed, some patients with SCLC with paraneoplastic neurological syndromes exhibit high immune activity and tend to have a better prognosis than patients without these syndromes116. Immunotherapies that enhance the activity of T cells against cancer cells, such as blockade of CTLA4, PD1 or PDL1, have some beneficial effects in patients with SCLC117,118. However, the response to T cell checkpoint blockade is limited to ~15% of patients with SCLC119,120. The limited efficacy of T cell-based immunotherapies against SCLC can be explained by multiple mechanisms, including the low expression of major histocompatibility complex (MHC) class I molecules on the surface of SCLC cells121–124. The presence of immune cells with suppressive properties, such as regulatory T cells, in the SCLC tumour microenvironment may further promote immune evasion125,126. Other mechanisms include the suppression of antigen-presenting cells by neuropeptides secreted by SCLC cells127. It remains unknown whether the interactions between immune cells and SCLC cells are similar in human tumours and genetically engineered mouse models. Of note, mouse tumours have a low tumour mutation burden whereas human SCLC is among the most highly mutated cancers24,37, which could substantially affect T cell responses. The activation of macrophages128 and the development of chimeric antigen receptor-expressing T cells specifically targeting SCLC129 might help to bypass some of the current lack of efficacy of T cells against SCLC.
An emerging molecular classification
While the SCLC tumour mutational landscape does not seem to define subtypes, the expression of specific transcription factors provides a first framework to differentiate biologically distinct SCLC subtypes. Four major subtypes of SCLC are defined based on high levels of ASCL1 (SCLC-A subtype), NEUROD1 (SCLC-N), POU2F3 (SCLC-P) or YAP1 (SCLC-Y)130. Subsequent analyses have suggested a division of SCLC-A into two clusters (SCLC-A and SCLC-A2) differing in their expression of HES1 (REF.131) and a rare subtype demonstrating elevated expression of the transcription factor ATOH1 (REF.79) (FIG. 3). Among other differences, these subtypes tend to reflect the differential expression of MYC family members, with increased MYCL expression being associated with SCLC-A and increased MYC expression occurring in the other subtypes. Data from both mouse models and clinical trials suggest that Aurora kinase inhibitors might be selectively effective in MYC-high SCLC44,132,133. Differences between the transcription programmes of these four subtypes include distinct degrees of neuroendocrine differentiation and differences in metabolism. This emerging molecular classification also serves as a framework within which to further refine additional subtypes131 (FIG. 3). Importantly, single-cell analyses are likely to help define how intratumoural heterogeneity is connected to these and possibly new subtypes109,110. Analogous to the transition of lung adenocarcinoma to SCLC, an important aspect of future studies will be to monitor how SCLC tumours of certain subtypes evolve with time and treatment. Data from mouse SCLC cell lines suggest a possible developmental hierarchy among subtypes, with SCLC-A evolving to SCLC-N and subsequently to SCLC-Y134. Of note, mouse models generated to date only model the SCLC-A and SCLC-N subtypes130, and the development of new models combining various genetic alterations and different putative cell-of-origin types56 will be key to modelling all subtypes, possibly helping to define new subtypes and to investigate intratumoural and intertumoural heterogeneity in SCLC.
Fig. 3 |. Major genetic alterations and molecular subtypes of SCLC.

a | The inactivation of RB1 and TP53 (encoding retinoblastoma-associated protein (RB) and p53, respectively) is a near-ubiquitous event in human small-cell lung cancer (SCLC) tumours. Four major molecular subtypes, SCLC-A, SCLC-N, SCLC-P and SCLC-Y, have been described on the basis of high expression of the transcription factors ASCL1, NEUROD1, POU2F3 and YAP1, respectively130. SCLC-P and SCLC-Y show a less neuroendocrine phenotype than SCLC-A and SCLC-N. Within the less/non-neuroendocrine category, a rare subtype with high expression of the transcription factor ATOH1 has been reported79. SCLC-A tumours have been proposed to comprise two distinct subtypes (SCLC-A and SCLC-A2), with SCLC-A2 distinguished from SCLC-A by its expression of other factors, such as HES1 (REF.131). A few other genetic and epigenetic alterations have been associated with specific subtypes, including the differential expression of MYC family members and mutations in NOTCH family genes, but most recurrent mutations are found in all subtypes. b | Chromosome level copy-number alterations reported by clinical next-generation sequencing of tumours from 409 patients with SCLC. Amplified genes (AMP; red) and homozygous deleted genes (HOMDEL; blue) are plotted for each chromosome. Selected genes of interest, with chromosomal locations and frequency in SCLC tumours (percentage in parentheses) are indicated. Data in part b are from MSK-IMPACT sequencing233.
The identification of molecular subtypes of SCLC and the association between these molecular subtypes and cellular programmes (such as ‘stem cell’, ‘mesenchymal’ or ‘neuronal’ programmes) may help to focus the development of therapies targeted to subsets of patients that are most likely to benefit from a given therapeutic approach. This personalized approach would of course require the development of a number of new therapeutic approaches and these approaches are likely to have to be combined to combat the plasticity of SCLC cells and the heterogeneity of SCLC tumours. A key aspect of such therapeutic approaches will also be to block the transitions between different states, which might be achieved through the targeting of epigenetic regulators.
Diagnosis, screening and prevention
SCLC is a high-grade malignant epithelial tumour. A confirmed diagnosis relies on characteristic light microscopic features of the tumour with haematoxylin and eosin staining (histopathological features are described below). Immunohistochemistry can be used to exclude other diagnoses. The current WHO classification recognizes only two subtypes: SCLC and combined SCLC135. Combined SCLC has an additional component of non-small-cell carcinoma, which can be of any non-small-cell histological subtype. Cytology is a powerful tool that is sometimes more definitive than histology of small biopsies, which in SCLC often have crush artefacts.
Signs and symptoms
Distinct clinical characteristics of SCLC include the predominantly central location of the primary tumour in the major airways and the often extensive extrapulmonary metastatic spread at presentation. Owing to the rapid tumour growth and widespread metastases, most patients with SCLC are symptomatic at presentation and the duration of symptoms is typically less than 3 months. Bulky mediastinal involvement is common. Intrathoracic local growth explains frequent symptoms at presentation, including cough, wheeze, dyspnoea, haemoptysis, superior vena cava compression resulting in upper body oedema and flushing, oesophageal compression with dysphagia, and recurrent laryngeal nerve compression with left vocal cord paralysis. Fatigue, anorexia, weight loss and neurological complaints are associated with distant spread. The brain, liver, adrenal glands, bone and bone marrow are common sites of metastasis.
SCLC is frequently associated with paraneoplastic syndromes102,136. Common SCLC paraneoplastic endocrinopathies include syndrome of inappropriate anti-diuretic hormone and Cushing syndrome; paraneoplastic neurologic syndromes caused by autoantibodies include Lambert–Eaton syndrome, encephalomyelitis and sensory neuropathy syndromes. Rare manifestations include dermatomyositis, hyperglycaemia, hypoglycaemia, hypercalcaemia and gynecomastia (breast tissue swelling in males). These antibody-dependent syndromes reflect common aberrant activation of humoral (B-cell mediated) immunity in SCLC; interestingly, immune checkpoint blockade, which activates cellular (T cell-mediated) immune responses, showed no apparent increase in paraneoplastic phenomena in patients with SCLC137–139.
Diagnostic work-up
Given the aggressive nature of SCLC, diagnostic and staging work-up should be performed as quickly as possible after presentation. In addition to medical history and physical examination, this assessment includes imaging (typically contrast-enhanced CT or 18F-FDG PET/CT of the chest, abdomen and pelvis, and brain MRI with contrast) to define the extent of disease, blood tests, including cell counts, liver and kidney function and lactate dehydrogenase, and electrocardiography to ensure safety prior to the administration of cytotoxic drugs136. Owing to the usual central location of the tumour, biopsies are often obtained by bronchoscopy with or without endobronchial ultrasonography; alternatives include mediastinoscopy, transthoracic biopsies or thoracoscopy. Depending on accessibility, a preferred option can be the biopsy of a distal metastatic site. The diagnosis is only confirmed by histopathological examination aided by cytology140. A higher CTC count is a negative prognostic factor for patients with SCLC2,141 but is rarely used in practice outside of the clinical trial setting. The analysis of CTCs and/or circulating cell-free DNA is still in the experimental stage but may contri bute to the evaluation of tumour characteristics, including intratumoural heterogeneity142–144.
The radiological findings in SCLC are similar to those of other lung cancers, with a tendency for tumours to be larger, centrally located and at a more advanced stage at presentation145,146. Bulky mediastinal lymph nodes are common. Metastatic spread is often radiologically evident and may include pleural and pericardial effusions. Rare cases (about 5% of patients with SCLC) present with isolated peripheral nodules without lymph node involvement and may be amenable to surgery.
Staging
The tumour–node–metastasis (TNM) classification147 is preferred to the previous staging system of the Veterans Administration Lung Study Group (VALSG), which separates limited-stage disease (tumour confined to one hemi-thorax and one radiation port; no malignant pleural or pericardial effusion) from extensive-stage disease (disease not meeting criteria for limited stage)148. TNM staging provides better anatomic discrimination for the measurement of outcome, prognostic information and more precise lymph nodal staging140,149,150. For example, the use of the VALSG staging system does not differentiate between patients who present with early-stage (T1–T2, N0–N1, M0) SCLC and those who present with locally advanced disease (any T, N2–N3, M0). The use of the TNM classification is therefore beneficial in defining optimal treatment strategies in clinical trials.
Stage for stage, the prognosis of SCLC is consistently poorer than that of NSCLC147. Brain metastases are common in SCLC, with ~10% of patients presenting with brain metastases at the time of diagnosis and an additional 40–50% subsequently developing brain metastases. The majority of patients with brain metastases are symptomatic and ~15% of neurologically asymptomatic patients with SCLC with no evidence of brain involvement by CT have metastases detected by MRI151. Access to optimally accurate staging modalities represents a clear limitation in low-income countries.
Clinicians and cancer registrars are strongly encouraged to use the TNM (eighth edition) staging system147. Nonetheless, the VALSG staging system is still widely used in both designing clinical trials and presenting data from them, as it effectively distinguishes patients treated primarily with CRT (limited-stage disease) from those treated with systemic chemotherapy or chemo-immunotherapy (extensive-stage disease).
Pathology
The WHO pathological classification of SCLC recognizes two subtypes: (pure) SCLC (~80% of cases) and combined SCLC (~20% of cases)135 (FIG. 4). Cardinal histopathological diagnostic criteria include small tumour cells with a round to fusiform shape, scant cytoplasm, finely granular nuclear chromatin, and absent or inconspicuous nucleoli. The mitotic rate is typically high, with >10 mitoses per mm2 and an average of 60 and a median of 80 mitoses per mm2. Apoptotic figures are numerous and necrosis is usually extensive. Nuclear moulding is frequent since cells are situated in close proximity.
Fig. 4 |. Histopathology of SCLC tumours.

a | A prototypical ‘pure’ small-cell lung cancer (SCLC), as defined by the WHO histopathological classification of SCLC. This tumour demonstrates expression of the classic neuroendocrine markers CD56 and chromogranin A (CHGA). INSM1 is a neuroendocrine marker that is positive in two of the four major molecular subtypes of SCLC, SCLC-A and SCLC-N, which are defined by their high expression of the transcription factors ASCL1 and NEUROD1, respectively. In this example, additional staining reveals consistent expression of ASCL1, with scattered NEUROD1-positive cells. b | Combined SCLC. This example demonstrates a predominant area with classic SCLC features, including the expression of CD56 and INSM1, along with a discrete subdomain with contrasting squamous (SQ) cell carcinoma features, including a more abundant cytoplasm and the expression of cytokeratin 5 (CK5), CK6 and p40. H&E, haematoxylin and eosin. Images courtesy of Natasha Rekhtman (Memorial Sloan Kettering Cancer Center, USA).
Densely packed tumour cells typically appear sheet-like with an absence of architectural features. They occasionally show rosettes (rose-shaped collections of cells) and, less commonly, nests (rounded groups of cells separated by stroma), trabeculae (ribbons) and peripheral palisading (parallel arrangement of nuclei at the periphery of nests). Neuroendocrine features may be more evident on surgical specimens than on bronchial biopsy samples152. Crush artefacts are frequent. Occasional large or giant tumour cells can be present but, for a diagnosis of pure SCLC, they must be <10% of the total cell number152.
The most common NSCLC histological subtypes in combined SCLC are large-cell carcinoma or large-cell neuroendocrine carcinoma (LCNEC), which occur in 4–16% of all SCLC tumours; combined tumours with other NSCLC subtypes represent only 1–3% of all SCLC tumours. Because of the histological similarities with SCLC, large-cell carcinoma or LCNEC subtypes must comprise ≥10% of the tumour area for a diagnosis of combined SCLC; there is no percentage requirement for other histological subtypes. Combined SCLC is more frequently recognized in surgical samples than in small biopsy samples, possibly due to increased crush artefact and fewer cells in the latter152,153. The clinical presentation, response to chemotherapy and survival rates of patients with combined SCLC are similar to those of patients with pure SCLC, although the frequency of peripheral and resectable tumours is higher for combined SCLC154. Combined SCLC is notably rare in the SCLC-A and SCLC-N subtypes of disease155. In SCLC combined with adenocarcinoma or in a never smoker, analysis for EGFR mutation or ALK rearrangement should be considered. Following therapy, 13–45% of pure SCLC tumours show morphological changes, including a larger cell size or combined histologies, consistent with an induced lineage plasticity in the context of acquired chemoresistance156,157.
Immunohistochemistry.
In theory, the diagnosis of SCLC relies on light microscopy-based histopathological analysis although, in current practice, immuno histochemistry is commonly performed to differentiate SCLC from other diagnoses. Commonly used neuro endocrine markers include chromogranin, synaptophysin and CD56 (also known as NCAM); CD56 is the most sensitive (positive in 90% of SCLC) but least specific of the three markers158. A new neuroendocrine marker, INSM1, is generally positive in the two most common subtypes of SCLC — SCLC-A and SCLC-N159,160.
Cytology.
Cytological preparations can have diagnostic utility, especially when biopsies are small, crushed or necrotic145. Cytological smears often show isolated tumour cells or loose aggregates with nuclear moulding owing to reciprocal deformation of compressed nuclei. Tumour cell chromatin is hyperchromatic; if well preserved, it is finely or coarsely granulated and evenly distributed, producing a characteristic ‘salt and pepper’ effect. Nucleoli are absent or inconspicuous and the cytoplasm is minimal, resulting in a high nucleus to cytoplasm ratio.
Differential diagnoses.
Primary differential diagnoses in SCLC cases include other neuroendocrine lung tumours, NSCLC and, in particular, basaloid carcinoma, extrapulmonary small-cell tumours and lymphoma. Other neuroendocrine lung tumours (typical and atypical carcinoid and LCNEC) generally share the expression of the same neuroendocrine markers and cytokeratins as SCLC. Both typical and atypical carcinoid tumours differ from SCLC in tumour cell morphology and in mitotic rate as assessed by histopathology, which is exceptionally high in SCLC but low in carcinoids (≤10 mitoses per 2 mm2). The proliferation rate, as reflected by Ki67 nuclear staining, is always >50% in SCLC, often reaching 80–100%, whereas it is <30% in pulmonary carcinoids161. The cytoplasm is more prominent in carcinoid tumour cells than in SCLC tumour cells and necrosis is often extensive in SCLC tumours and absent or focal in carcinoid tumours. Distinguishing SCLC from LCNEC is more challenging but is based on a constellation of morphological features in addition to tumour cell size (>3 lymphocyte diameters in LCNEC). Compared with SCLC, LCNEC tumour cells have a more abundant cytoplasm, a polygonal shape with a distinct cell border, vesicular nuclear chromatin and often visible nucleoli145.
Basaloid carcinoma, a subtype of squamous cell carcinoma, shares the small cell size with SCLC and can be mistaken for SCLC in small or crushed biopsies162. Positive staining for p40 can be used to distinguish basaloid carcinoma from SCLC, as this marker is always negative in SCLC163. Napsin A, a marker of adenocarcinoma, is negative in SCLC164. Cytokeratin staining is useful to distinguish neuroendocrine carcinomas from non-epithelial tumours, such as lymphoma; SCLC tumours typically show positive staining with the wide-spectrum cytokeratin AE1/AE3 antibody cocktail but is always negative for the CK34βE12 antibody165, which recognizes the high-molecular-weight cytokeratins CK1, CK5, CK10 and CK14; lymphomas are negative for cytokeratins and express leukocyte common antigen (also known as CD45)145.
Rarer considerations in the differential diagnosis include metastatic Merkel cell carcinoma, which tends to be positive for CK20 but negative for TTF1 and CK7 (REF.166). Ewing sarcoma (with EWSR1 rearrangement) and other small round-cell sarcomas with rearrangements other than EWSR1 may be considered; compared with SCLC, cells in these tumours are more dyscohesive, the mitotic rate is lower, cytokeratin expression is negative or very focal, and they stain for CD99 (also known as MIC2)167–169. Appropriate fluorescence in situ hybridization (FISH) techniques should be applied in case of doubt. Small undifferentiated SMARCA4-deficient thoracic tumours can be distinguished as epithelial sarcomatoid tumours170.
Screening and prevention
Screening by low-dose CT in patients at risk for lung cancer (smokers and ex-smokers) has detected newly diagnosed SCLC cases. The National Lung Screening Trial (NLST) involved random assignment of over 53,000 individuals at risk for lung cancer (based on age and smoking history) to annual screening for 3 years with either annual low-dose CT or chest X-ray and detected SCLC tumours in 133 individuals22. However, in contrast to the trials of screening for NSCLCs, in which CT screening resulted in a notable shift to earlier stage disease detection at diagnosis, no shift in disease stage at diagnosis was evident for SCLC: 10% of detected tumours were stage I A–B, 6% were stage II A–B, 29% were stage III A–B and 54% were stage IV with metastatic disease; these percentages were identical with CT and chest X-ray screening22. Subsequent analyses of NLST and other similar studies demonstrated that CT screening does not detect SCLC at earlier stages and, thus, does not affect survival in patients with SCLC23,171–173. The NELSON screening trial involved over 15,000 individuals at risk of lung cancer and confirmed an overall reduction in lung cancer mortality with annual low-dose CT screening, but data analyses specific to SCLC have not been reported174. Although multiple protein biomarkers of SCLC can be detected in patient serum175, these have not been translated into an early intervention strategy. To date, there is no approach to early detection that has been shown to be effective for SCLC.
As noted above, SCLC is strongly associated with smoking, with 98% of cases arising in current or former smokers13. Smoking prevention and cessation are the most effective strategies to decrease the societal effect of SCLC, as giving up smoking not only reduces the risk of developing SCLC but also reduces, by almost 50%, the risk of death for patients diagnosed with limited-stage disease (affecting only one side of the chest and encompassed within a single radiation port)176.
Management
The initial approach to SCLC treatment varies substantially by stage (FIG. 5). In non-metastatic SCLC, the goals of treatment include achieving durable control of thoracic disease and reducing the risk of metastatic dissemination. Five-year survival rates of 25–30% can be achieved with combined modality treatments. Local treatment options to control thoracic disease include surgery and radiotherapy. Chemotherapy can both augment the local efficacy of radiation and potentially treat micrometastatic disease. The standard chemotherapy regimen in this setting is cisplatin–etoposide, which has not changed for the past three decades. The advantages of this regimen include that it can be delivered at full dose in patients treated with concurrent CRT and has a well-established toxicity profile. In patients who are not suitable for cisplatin, carboplatin–etoposide can be considered177. Other chemotherapeutic drugs, such as irinotecan or paclitaxel, have activity in these patients but have not shown superiority178. Improved outcomes with immunotherapy in early-stage NSCLC179 and in metastatic SCLC137,138 have led to the investigation of immune checkpoint inhibitors as concurrent primary or adjuvant therapies but these are still considered experimental.
Fig. 5 |. Approaches to SCLC treatment by stage.
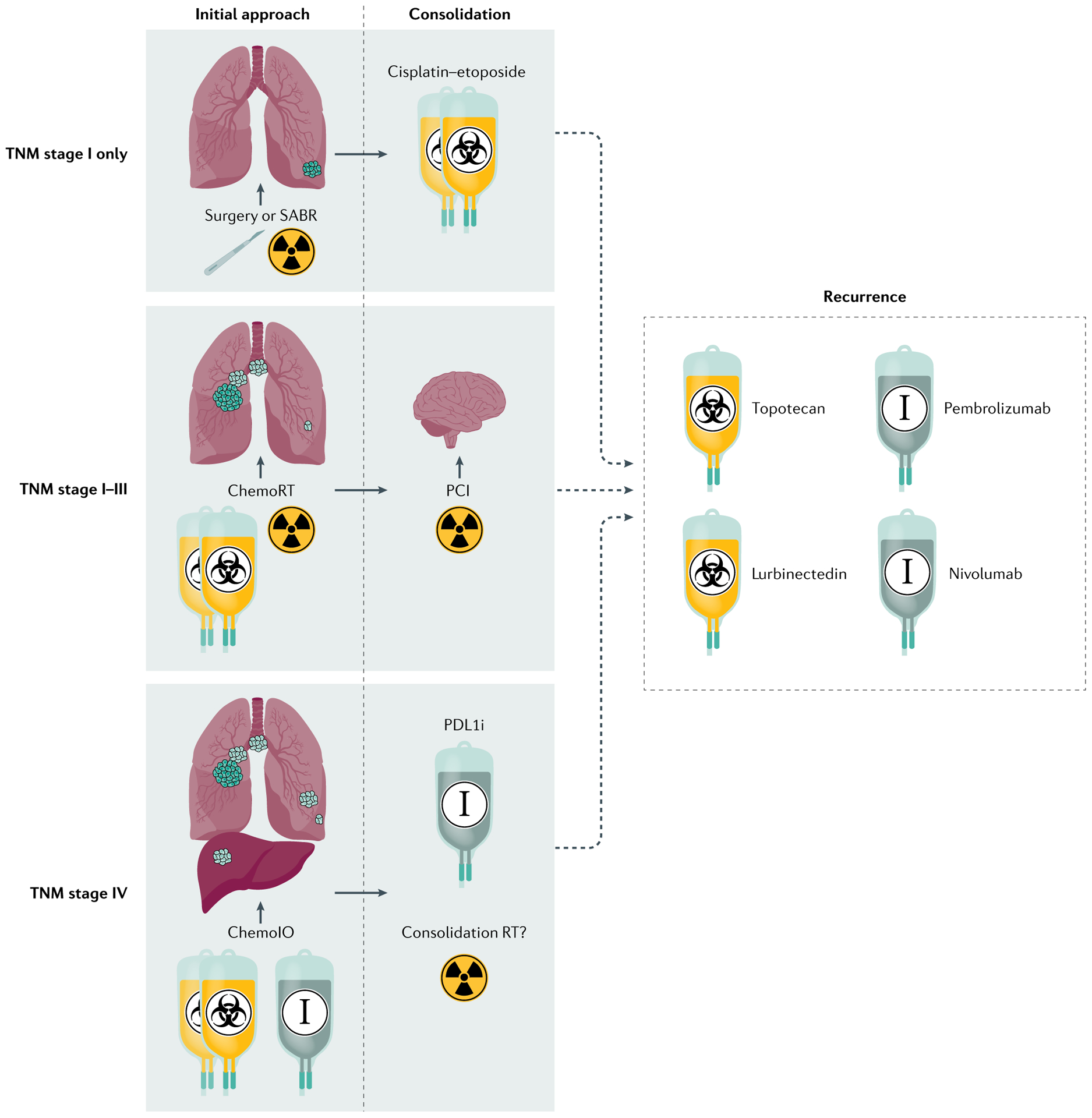
Drugs that received full and accelerated FDA approvals are included. Rare cases of small-cell lung cancer (SCLC) presenting as isolated pulmonary nodules (tumour–node–metastasis (TNM) stage I) may be amenable to surgical resection or treatment with stereotactic ablative radiotherapy (SABR) and adjuvant chemotherapy. More commonly, localized or locally advanced disease (TNM stages I–III) is treated with concomitant chemoradiotherapy (ChemoRT), with consideration of prophylactic cranial irradiation (PCI) in responding patients. Metastatic disease (TNM stage IV) is treated with chemotherapy with or without a PDL1 inhibitor (PDL1i; chemoIO), followed by maintenance PDL1i therapy for up to 1 year. The role of consolidative chest radiotherapy (Consolidation RT) in the context of chemoIO is unclear. For recurrent disease, current approved agents for second-line treatment in the USA include topotecan and lurbinectedin; for third-line and beyond, the indicated anti-PD1 immunotherapy drugs can be considered but their role is also unclear in patients treated with first-line chemoIO.
Prophylactic cranial irradiation (PCI) is also part of the standard management in most patients with non-metastatic SCLC who respond to initial treatment, as it significantly reduces the risk of brain metastases and improves survival3,180.
Early-stage SCLC
A very small proportion of patients with limited-stage SCLC present with early-stage (T1–T2N0–N1M0) SCLC181. The management of these patients is controversial owing to a lack of randomized controlled trials comparing surgical to non-surgical approaches in the era of modern staging and treatments. At least three local treatment options are available for these patients: surgery, fractionated radiotherapy (dividing the total dose of radiation into multiple smaller doses) and stereotactic radiotherapy. The role of PCI is not as well established in these patients as it is in patients with locally advanced SCLC.
Surgery.
Only two phase III trials, one performed in the 1970s and the other in the 1990s182,183, have been reported. In 2017, a systematic review stated that, although currently available randomized controlled trial data do not support a role for surgical resection in the management of SCLC, this conclusion is of limited value owing to a lack of contemporary data and the low quality of available evidence184. This uncertainty has led to inconsistencies in national and international treatment guidelines with regards to the role of surgery in managing SCLC185. Consequently, deciding between surgical and non-surgical approaches is challenging for both clinicians and patients. Primary surgical resection is generally limited to the treatment of patients with clinical stage I or II (cT1–T2N0) disease140,186. The aim of surgical treatment is to achieve a microscopically margin-negative resection (R0 resection)187. Population-based data analyses estimate a 5-year survival rate of ~50% among patients with a complete pathologic R0 resection for pT1–T2N0M0 SCLC188. After surgical resection, adjuvant chemotherapy should be given and there is no role for adjuvant thoracic radiotherapy unless an incomplete resection (R1–R2) was performed or pathology reveals unforeseen mediastinal nodal involvement (N2)189.
Radiation.
The evidence available on fractionated radiotherapy in early-stage SCLC is limited, as the TNM classification was not integrated for the staging of patients in historical clinical trials of CRT. The CONVERT trial showed that concurrent CRT in patients with TNM stage I–II SCLC (representing 15% of the patients enrolled) achieves outcomes comparable to those of surgery, with low rates of acute and late toxicity190. Promising data from small retrospective studies on the role of stereotactic ablative radiotherapy for early-stage SCLC have led to its inclusion as an option in guidelines for the treatment of patients with peripheral T1–T2N0M0 disease191,192. The role of PCI in early-stage SCLC is controversial, particularly in stage I, owing to its lower risk of development of brain metastases compared to locally advanced disease193–195.
Locally advanced SCLC
Most patients with non-metastatic, locally advanced SCLC (any T, N2–N3, M0) present with disease that involves the mediastinal and hilar nodes. Surgery is generally not a treatment option in these patients. The role of radiotherapy with concomitant chemotherapy is well established in the management of locally advanced SCLC196. The standard of care for patients with a performance status of 0–1 (meaning at least ambulatory and able to carry out work of a light or sedentary nature) is twice-daily thoracic radiotherapy (45 Gy in 3 weeks) with concurrent cisplatin–etoposide197,198. If twice-daily radiotherapy cannot be delivered for patient-specific or practical reasons, once-daily radiotherapy is a reasonable alternative197. A 2019 survey of European practice showed that twice-daily radiotherapy is used in only 42% of centres, mostly due to practical reasons199. In the era of modern staging and radiotherapy (that is, 3D conformal radiotherapy or intensity-modulated RT, without elective nodal irradiation), the 5-year survival is expected to be ~30% in patients with a performance status of 0–1 after treatment with concurrent CRT197. Severe treatment-related toxicity has been reduced with contemporary treatment planning: in the CONVERT study, which randomly assigned patients to once-daily or twice-daily radiotherapy, <20% of patients receiving either treatment developed severe oesophagitis compared with >30% in earlier trials using 2D radiotherapy techniques197.
Evidence from randomized controlled trials and meta-analyses favours the initiation of radiotherapy as early as is feasible in the course of CRT, preferably with the first or second cycle of chemotherapy197,198,200–203. In cases of bulky disease at presentation, the dose to the organs at risk may not permit the early administration of thoracic radiotherapy. In such cases, radiotherapy can be postponed until the start of the third cycle of chemotherapy, at which time it is likely that a reduction in disease volume has been achieved204. Another option, particularly in frailer or elderly (≥75 years old) patients, is to consider sequential CRT rather than concurrent CRT205. In the sequential setting, the typical radiotherapy approach is to treat the post-chemotherapy primary tumour volume and the pre-chemotherapy nodal volume206.
PCI significantly decreases the risk of symptomatic brain metastases and increases overall survival in patients with non-metastatic SCLC180,207. PCI is currently offered to patients who respond to initial CRT treatment and have a performance status of 0–1 (REF.3). The evidence supporting PCI is not as clear in patients with a performance status of 2 (meaning ambulatory, capable of selfcare but unable to carry out any work activities; up and about >50% of waking hours) after CRT, in patients >70 years of age and in those with pre-existing neurological conditions, such as stroke or epilepsy. In such patients, a shared decision process should be encouraged208.
Metastatic disease
For over three decades, the first-line chemotherapy for newly diagnosed metastatic SCLC has consisted of a platinum agent (cisplatin or carboplatin) together with etoposide1. A phase III clinical trial reported superiority of cisplatin–irinotecan over cisplatin–etoposide in a Japanese population209, but two subsequent randomized studies in the USA failed to confirm this result210,211. Multiple randomized phase III studies have demonstrated the statistically significant benefits of adding an immune checkpoint inhibitor to first-line chemotherapy in patients with newly diagnosed metastatic SCLC137–139. The addition of either of two anti-PDL1 monoclonal antibodies (atezolizumab or durvalumab) to a standard platinum–etoposide backbone, with continuation of immunotherapy as maintenance, improved both progression-free survival and overall survival137,138. The addition of the anti-PD1 antibody pembrolizumab in the same context resulted in a similar benefit but was only statistically significant for progression-free survival139. In all of these studies, the benefits are less evident in the median (~2-month extension of median survival) than in the tail of the survival curve; together, these studies suggest that immune checkpoint inhibition leads to an approximate doubling of 2-year survival, from 11% to 22%. These observations imply that there is a subset of patients with SCLC who derive durable benefit from immunotherapy, although the majority do not. In contrast to other solid tumours, PDL1 expression does not seem to be a correlate of immunotherapy benefit in SCLC139. The role of tumour mutation burden as a predictive biomarker of SCLC response to immunotherapy is controversial, as the Checkmate-032 analysis suggests a correlation but the IMPOWER133 blood-based analysis showed no evident association137,212. Defining tumour and host characteristics associated with immunotherapy response is an area of active investigation.
After many years with no new therapies for SCLC, in the past 3 years, the FDA has granted accelerated approval to three new drugs. Until 2020, the only standard of care second-line therapy for recurrent meta static SCLC was the topoisomerase I inhibitor topotecan. Lurbinectedin, an alkylating agent that binds to the minor groove of DNA and affects transcription, was granted accelerated approval for second-line use based primarily on lurbinectedin demonstrating a 35% response rate in a single-arm phase II study of 105 patients213. The anti-PD1 monoclonal antibodies nivolumab and pembrolizumab were granted accelerated approval for third-line use119,120, although the role of these agents is unclear in patients whose disease has progressed on first-line immune checkpoint inhibitors. Although not approved for the specific indication by regulatory authorities, many other cytotoxic agents have clinical activity in SCLC and are included as options in treatment guidelines for recurrent SCLC, including the nivolumab–ipilimumab combination, paclitaxel, docetaxel, irinotecan, temozolomide and oral etoposide3. Retreatment with a platinum doublet in patients with response maintained for at least 3 months after first-line treatment is another reasonable choice.
Radiotherapy has traditionally been reserved for the palliation of symptoms in patients with advanced disease, including in those who have poor responses to chemotherapy. The most common site of distant failure in patients with advanced SCLC is the brain, with 40–50% of patients developing brain metastases after completion of palliative chemotherapy. The role of PCI in patients with metastatic disease is controversial, as a European phase III study suggested a survival benefit214. However, a more recent Japanese phase III study found that, with MRI surveillance, PCI did not offer a benefit for patients with metastatic SCLC215. Clinical trials to resolve this controversy are ongoing216. Treatment for leptomeningeal metastases remains a major unmet need.
Given that up to 75% of patients with advanced SCLC have persisting intrathoracic disease after chemotherapy and subsequent intrathoracic disease progression, a rationale exists for considering consolidation thoracic radiotherapy. A European trial that randomly assigned patients to consolidative thoracic radiotherapy or best supportive care found no statistical difference in the primary endpoint of 1-year survival yet a post hoc analysis suggested improvement in 2-year survival with radiotherapy, with a low rate of toxicity217. The benefit of this treatment was more pronounced in patients with residual intrathoracic disease. An important unanswered question in the metastatic setting is the integration of thoracic radiotherapy, PCI and immunotherapy.
Follow-up
Patients with SCLC are at high risk of relapse, with ~75% of patients with locally advanced disease and >90% of patients with metastatic disease progressing within 2 years of treatment137,197. Periodic CT scanning is recommended to identify recurrence as early as possible and to offer salvage treatment if appropriate. However, there is a paucity of data supporting the frequency of imaging and its effect on survival. Including a brain MRI in surveillance is recommended in patients who did not undergo PCI215. A further rationale for regular imaging of patients with SCLC is the high risk of developing second malignancies, which are generally tobacco induced, in the lungs and other organs218,219.
The follow-up of patients with SCLC, particularly those with non-metastatic disease, should also include the management of the multiple comorbidities often associated with this disease (including cardiac and respiratory comorbidities generally caused by smoking)19,220. Patients may also have treatment-related adverse effects, such as pulmonary fibrosis or cardiac complications, which may benefit from specialist input221. The management of these patients by a multidisciplinary team that includes non-oncology specialists is likely to provide better symptom control, improved quality of life and, possibly, improved outcomes.
At the time of diagnosis and during follow up, patients should be actively encouraged to stop smoking. Indeed, continuing smoking after a diagnosis of SCLC is associated with a risk of developing second primary tumours as well as with a risk of cardiovascular, respiratory and cerebrovascular disease, leading to poorer suvival176,222.
Quality of life
Personalized treatment is at the heart of modern oncology and should consider the risk to benefit ratio of therapy for each individual patient. Considering the poor outcome in the majority of patients with SCLC, open and honest discussions that include prognosis, goals of care and supportive care should take place early on in the management of patients. To the greatest extent possible, all patients should be discussed and managed by a multidisciplinary team, including specialist nurses and supportive-care specialists.
When treatment is discussed, patients should be clearly informed about the short-term and long-term adverse effects of treatment and their effect on quality of life. This information is particularly important for patients with metastatic disease who have limited life expectancy and in whom the risk of toxicity should not outweigh the symptomatic benefit of treatment. As SCLC is at diagnosis an exceptionally chemoresponsive disease, patients with poor performance status attributable to disease may substantially improve with the initiation of chemotherapy. In patients with non-metastatic SCLC, improvements in survival rate over the past 20 years have led to an increasing focus on limiting the long-term toxicity of curative-intent treatment, such as CRT and PCI. The use of modern radiotherapy techniques with strict dose limits for in-field organs have led to a reduction in adverse events related to thoracic radiotherapy and chemotherapy toxicity, such as radiation oesophagitis and pneumonitis197. However, data on longer-term toxicities are limited, including the effect of lung fibrosis on respiratory function and quality of life. The rare patients with metastatic SCLC who respond exceptionally well to immunotherapy have further raised the importance of recognizing and minimizing the effect of treatment-related toxicities (box 1).
Box 1 |. A patient’s journey.
Clinician’s note:
Ms. Beaty was initially diagnosed with small-cell lung cancer (SCLC) in January 2014. Her disease recurred and progressed in the chest, abdomen and pelvis after treatment with cisplatin, etoposide and concomitant radiation, prophylactic cranial irradiation (PCI), carboplatin and irinotecan, palliative radiation to the pelvis, and temozolomide. She started participation in an anti-PD1 immunotherapy trial in 2015 and continued on this study for more than 3 years. She has been off all therapy for the past 2 years, with no evident disease. While highly exceptional, her experience serves as a proof of principle that this disease can respond durably to immunotherapy.
Nina Beaty writes:
“Starting with the obvious, what I am not is a ‘regular’ non-small-cell lung cancer [NSCLC] patient. When I joined the outpatient lung cancer support group, I was the sole SCLC-er at the table. Thus, I became quite familiar with what NSCLC-ers had to endure, often including some form of lung surgery that curtailed their activity due to difficulty breathing. Since surgery is not typically an option for SCLC, I never had any, and was able to breathe just fine. Compared to the others, this was a huge plus in my book. The downside was that I felt I was more likely to die within a year if my SCLC treatments stopped being effective. It was a very intense time, like being forced at gunpoint to drive through a tunnel that had an unknown destination. For a normally cheery person like me, I felt uncharacteristically compelled to review the worthiness of my life and figure out what my burial plans could be. SCLC has such a bad reputation that it’s still hard for me to hope I will continue to survive. Since the doctors have called me a “super responder”, I would love to see more research focused on what factors might have contributed to this. Knowing I had those factors might have given me hope earlier in my treatment.”
The improved outcomes, particularly for patients with non-metastatic SCLC, has also prompted increased concern from both clinicians and patients regarding the risk of neurotoxicity associated with PCI223. Memory loss, intellectual deficit, dementia and ataxia have been reported, often in patients with cerebral atrophy and white matter changes on brain imaging. It is recognized that, in addition to PCI, a number of factors can affect neurocognition, including underlying comorbidities caused by smoking, paraneoplastic syndromes, underlying anxiety and depression, chemotherapy, and SCLC itself. This is supported by studies demonstrating impairment in neuropsychological tests in patients with SCLC, even before PCI is given207,224. Minimizing neurotoxicity is an important goal of ongoing clinical trials, including those evaluating the benefit of hippocampus-sparing PCI and comparing PCI to MRI surveillance216.
Outlook
Progress on several fronts is defining new avenues of investigation and providing renewed hope for patients with this recalcitrant cancer. Many new insights regarding SCLC biology have stemmed from the development and analysis of representative genetically engineered mouse models of SCLC and these insights have been complemented and reinforced by parallel analyses of SCLC cell lines, patient-derived in vivo models and primary human tumours225. Analyses of mechanisms of in vivo-acquired therapeutic resistance in SCLC through both transcriptomic77 and proteomic46 approaches have revealed new potential tumour-specific vulnerabilities. The new understanding of key transcriptional drivers of SCLC phenotypes, defining subtypes of disease with distinct dependencies, might help to focus therapeutic clinical research on patient populations that are most likely to respond to particular targeted agents130. Technological improvements in imaging and in the advanced delivery of radiotherapy have increased the survival rates of patients with localized disease, while reducing the short-term and long-term adverse effects197. The introduction of immunotherapy as part of standard treatment for many patients with metastatic SCLC has finally led to improvements in overall survival for this cohort of patients with a particularly poor prognosis137,138. These and other advances underscore tangible progress in the management of SCLC and have defined a number of novel therapeutically tractable targets for this disease (FIG. 6).
Fig. 6 |. Representative therapeutic targets of interest in SCLC.

a | Antitumour immunity. Antibodies disrupting the PD1–PDL1 interaction have demonstrated clinical efficacy in small-cell lung cancer (SCLC). An antibody blocking the T cell-inhibitory receptor TIGIT and a bispecific T cell engager (BiTE) cross-targeting DLL3 on SCLC tumour cells and CD3 on T cells are currently in clinical trials in patients with SCLC (phase III NCT04256421 and phase I NCT03319940, respectively). Antibodies blocking CD47 , the ‘don’t-eat-me’ signal for macrophages, have shown activity in preclinical models128. b | Cell cycle and DNA damage repair pathways. Concomitant loss of TP53 and RB1 in SCLC abrogates multiple cell cycle checkpoints, increasing the dependence on remaining regulators of proliferation and DNA damage repair. Many key targets highlighted are being actively pursued in completed and upcoming clinical trials. The effect of EZH2 is indirect via the modulation of SLFN11 (REF.77). c | Growth and survival signalling pathways. Dependency screens implicate PKA and mTOR as essential kinases in SCLC46,234. BCL-2, a key regulator of apoptosis, is highly expressed in many SCLC tumours, and several studies suggest synergy between the inhibition of PI3K–mTOR and BCL-2 in SCLC69,70,235. This strategy, targeting mTOR and BCL-2, is currently being tested in a phase I/II trial (NCT03366103). d | Epigenetic regulators. The histone acetyltransferases CREBBP and EP300 are frequent and mutually exclusive targets of inactivating mutations in SCLC24. Preclinical data support the increased sensitivity of these CREBBP-inactive or EP300-inactive tumours to histone deacetylase inhibitors41. The inhibition of the histone demethylase LSD1 in SCLC cell lines activates NOTCH signalling, inhibits ASCL1 expression and may have subtype-selective activity in SCLC40 (not shown). The histone methyltransferase EZH2 is highly expressed in SCLC236 and is implicated in both SCLC chemoresistance77 and immune escape237. EZH2 inhibition with chemotherapy is currently being explored in a phase I/II clinical trial of patients with recurrent SCLC (NCT038979798).
Despite these highlights, SCLC remains a largely lethal disease. Several gaps exist in our understanding of the disease, which contribute to the modest effect that current treatments have had on patient survival.
Notably, the societal impact of SCLC could be obviated with effective prevention, especially as the aetiologic agent in oncogenesis is exceptionally clear. SCLC is among the diseases most strongly associated with tobacco carcinogen exposure12. The importance of global and multifaceted public health advocacy and governmental regulatory approaches to reduce the initiation of smoking and increase smoking cessation cannot be overemphasized.
The effective screening for incipient SCLC is a major and entirely unmet need. Highly sensitive, blood-based detection using mutational, proteomic or multiparameter approaches is an area of active investigation. Preclinical studies suggest the detection of neuroendocrine markers through mass spectrometry as one potential approach226. Most patients with SCLC die of metastatic disease. As noted, annual CT screening in a high-risk population fails to detect early-stage SCLC — with or without screening, an identical majority of patients have stage IV disease at diagnosis22. This observation might imply a biological difference between limited-stage SCLCs, which commonly present with a large primary mass and bulky adenopathy, and extensive-stage SCLCs, which often present with widespread metastases at diagnosis. Beyond the role of NFIB in metastasis in some but not all SCLC models83,112, there is a paucity of data on the drivers of haematogenous metastasis in human SCLC.
Studies have uncovered remarkable intertumoural and intratumoural heterogeneity in SCLC. We are only beginning to dissect how this heterogeneity influences the biology of disease. The identification of a distinct subtype of SCLC driven by the transcription factor POU2F3 (REF.227) suggests that different subtypes could reflect different cells of origin in the lung epithelium and distinct pathways of oncogenesis83. We are only beginning to understand the extent to which subtype assignments are mutable and whether tumour evolution between subtypes reflects lineage plasticity or differential selection among pre-existing subclones134. Multiple recurrent mutations affecting epigenetic regulatory pathways in SCLC have been defined24,40,41,77; how these epigenetic pathways could either determine or drive the transition between transcriptional states is unknown. Despite hypothesized subtype-specific vulnerabilities130, the extent to which different predominant subtypes in fact influence clinical prognosis, therapeutic responsiveness and patterns of disease progression has not been defined. Intratumoural hetero geneity, including a mix of interacting neuroendocrine and non-neuroendocrine subpopulations, has been implicated in metastatic potential in mouse models39,228 but has been less extensively characterized in human tumours. Emerging technical advances in single-cell profiling technologies, including single-cell transcriptional profiling, proteomic profiling and spatial multicolour imaging, are ideally suited to begin to study some of these issues.
The advent of chemo-immunotherapy as a new standard of care for the first-line treatment of metastatic disease137,138 is both a remarkable hallmark of progress and a disappointment. The overall improvement in survival in patients with SCLC from the addition of immune checkpoint blockade is modest relative to that seen in many other solid tumours, despite a highly mutated genome in SCLC tumours. These data serve as an important proof of principle — that SCLC can be recog nized by cytotoxic T cells, leading to durable benefit — but also as a challenge: why is this benefit seen in only a small minority of patients with metastatic SCLC and what can be done to activate effective immune responses in the nearly 80% of patients who are still destined to die within 2 years of diagnosis? An intensive focus of clinical research is now on the exploration of complementary pathways to immune activation, including the blockade of alternative immune checkpoints, the use of bispecific T cell engagers or natural killer cell activators, and assessing DNA damage response inhibitors or epigenetically targeted agents as strategies to induce immune-responsiveness in SCLC229.
Similar challenges to improving survival also apply to early-stage and locally advanced SCLC. Building on the successes in treating metastatic disease, multiple trials are in development or in progress to assess the role of PD1–PDL1 checkpoint blockade in patients treated with CRT. There is a strong rationale for combining immunotherapy and radiotherapy: radiation affects the tumour microenvironment through the release of tumour antigens, induction of the cGAS–STING pathway, upregulation of MHC class I expression, stimulation of type I interferons and promotion of CD8+ T cell infiltration230–232. Novel agents targeting DNA damage responses (for example, PARP inhibitors) or epigenetic regulators (for example, LSD1 inhibitors) are being assessed in patients with limited-stage SCLC as well. This context, in which 25–30% of patients treated with combined modality therapy are alive and free of progression at 5 years, but where most patients are destined to develop disease progression within 18 months197, is ideal for the development and testing of biomarkers of residual disease. It would be of substantial interest to know if sensitive methods for the detection of circulating tumour DNA in blood and, possibly, in cerebrospinal fluid could be used to identify those patients who might benefit from additional treatment, such as PCI or additional systemic therapies, versus those who are likely to be cured by thoracic CRT alone.
The advances of the past decade in defining the genetics and biological pathways driving SCLC have identified multiple novel therapeutic strategies. The pace of laboratory research in SCLC has dramatically accelerated, facilitated in large part by an expansion of the number and diversity of representative preclinical models. Many gaps in our characterization of SCLC remain and clinical progress lags behind that seen in NSCLCs. Several novel therapeutic targets are being actively pursued in clinical research today. We believe that continuing to use emerging insights gained from laboratory studies to inform and focus clinical trials is likely to yield clinically meaningful progress for patients with SCLC in the decade ahead.
Acknowledgements
The authors thank Natasha Rekhtman (Memorial Sloan Kettering Cancer Center, USA) for supplying the images for Fig. 4. The authors thank Nina Beaty for her contribution in Box 1. This work is supported by grants from US National Cancer Institute to C.M.R. (R01 CA197936 and U24 CA213274) and to J.S. (U01 CA213273, U01 CA23185, U54 CA2174501 and R35 CA231997). C.F-F. is supported by a grant from the NIHR Manchester Biomedical Research Centre.
Competing interests
C.M.R. has consulted regarding oncology drug development with AbbVie, Amgen, Ascentage, Astra Zeneca, Bicycle, Celgene, Daiichi Sankyo, Genentech/Roche, Ipsen, Jazz, Lilly, Pfizer, PharmaMar, Syros and Vavotek. He serves on the scientific advisory boards of Bridge Medicines and Harpoon Therapeutics. J.S. receives research funding from Stemcentrx/Abbvie, Pfizer, and Revolution Medicines and has licensed a patent to Forty Seven Inc./Gilead on the use of CD47-blocking strategies in SCLC. E.B. and C.F.-F. declare no competing interests.
References
- 1.Hann CL, Wu MA, Rekhtman N & Rudin CM in Cancer Principles & Practice of Oncology Ch. 49 (eds DeVita VT, Lawrence TS & Rosenberg SA) 671–700 (Wolters Kluwer, 2019). [Google Scholar]
- 2.Hou JM et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J. Clin. Oncol 30, 525–532 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Kalemkerian GP et al. NCCN guidelines insights: small cell lung cancer, version 2.2018. J. Natl Compr. Canc Netw 16, 1171–1182 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Bray F et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin 68, 394–424 (2018). [DOI] [PubMed] [Google Scholar]
- 5.International Agency for Research on Cancer. Cancer Incidence in Five Continents Volume X (IARC, 2014). [Google Scholar]
- 6.Francisci S et al. Survival patterns in lung and pleural cancer in Europe 1999–2007: Results from the EUROCARE-5 study. Eur. J. Cancer 51, 2242–2253 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Govindan R et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J. Clin. Oncol 24, 4539–4544 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Breitling LP, Rinke A & Gress TM Recent survival trends in high-grade neuroendocrine neoplasms and lung cancer. Neuroendocrinology 110, 225–233 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Abdel-Rahman O Changing epidemiology of elderly small cell lung cancer patients over the last 40 years; a SEER database analysis. Clin. Respir. J 12, 1093–1099 (2018). [DOI] [PubMed] [Google Scholar]
- 10.American Lung Association. Tobacco use in racial and ethnic populations American Lung Association; https://www.lung.org/stop-smoking/smoking-facts/tobacco-use-racial-and-ethnic.html (2020). [Google Scholar]
- 11.American Cancer Society. Cancer facts & figures 2019 (ACS, 2019). [Google Scholar]
- 12.Huang R et al. Associated links among smoking, chronic obstructive pulmonary disease, and small cell lung cancer: a pooled analysis in the International Lung Cancer Consortium. EBioMedicine 2, 1677–1685 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varghese AM et al. Small-cell lung cancers in patients who never smoked cigarettes. J. Thorac. Oncol 9, 892–896 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamichhane DK et al. Lung cancer risk and residential exposure to air pollution: a korean population-based case-control study. Yonsei Med. J 58, 1111–1118 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez-Martinez A, Torres-Duran M, Barros-Dios JM & Ruano-Ravina A Residential radon and small cell lung cancer. A systematic review. Cancer Lett 426, 57–62 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Wang J et al. Genetic predisposition to lung cancer: comprehensive literature integration, meta-analysis, and multiple evidence assessment of candidate-gene association studies. Sci. Rep 7, 8371 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Improgo MR, Scofield MD, Tapper AR & Gardner PD From smoking to lung cancer: the CHRNA5/A3/B4 connection. Oncogene 29, 4874–4884 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quintanal-Villalonga A et al. Lineage plasticity in cancer: a shared pathway of therapeutic resistance. Nat. Rev. Clin. Oncol 17, 360–371 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aarts MJ et al. Comorbidity in patients with small-cell lung cancer: trends and prognostic impact. Clin. Lung Cancer 16, 282–291 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Seigneurin A et al. Are comorbidities associated with long-term survival of lung cancer? A population-based cohort study from French cancer registries. BMC Cancer 18, 1091 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang S et al. Survival changes in patients with small cell lung cancer and disparities between different sexes, socioeconomic statuses and ages. Sci. Rep 7, 1339 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Lung Screening Trial Research Team. et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med 365, 395–409 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study was the first to demonstrate that CT screening in individuals at risk could decrease lung cancer mortality.
- 23.Thomas A, Pattanayak P, Szabo E & Pinsky P Characteristics and outcomes of small cell lung cancer detected by CT screening. Chest 154, 1284–1290 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.George J et al. Comprehensive genomic profiles of small cell lung cancer. Nature 524, 47–53 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the largest genomic profiling study of SCLC tumours to date.
- 25.Harbour JW et al. Abnormalities in structure and expression of the human retinoblastoma gene in SCLC. Science 241, 353–357 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wistuba II, Gazdar AF & Minna JD Molecular genetics of small cell lung carcinoma. Semin. Oncol 28 (Suppl. 4), 3–13 (2001). [PubMed] [Google Scholar]
- 27.Little CD, Nau MM, Carney DN, Gazdar AF & Minna JD Amplification and expression of the c-myc oncogene in human lung cancer cell lines. Nature 306, 194–196 (1983). [DOI] [PubMed] [Google Scholar]
- 28.Nau MM et al. Amplification, expression and rearrangement of c-myc and N-myc oncogenes in human lung cancer. Curr. Top. Microbiol. Immunol 113, 172–177 (1984). [DOI] [PubMed] [Google Scholar]
- 29.Nau MM et al. L-myc, a new myc-related gene amplified and expressed in human small cell lung cancer. Nature 318, 69–73 (1985). [DOI] [PubMed] [Google Scholar]
- 30.Peifer M et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat. Genet 44, 1104–1110 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rudin CM et al. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat. Genet 44, 1111–1116 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drapkin BJ et al. Genomic and functional fidelity of small cell lung cancer patient-derived xenografts. Cancer Discov 8, 600–615 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Udagawa H et al. Genetic profiling-based prognostic prediction of patients with advanced small-cell lung cancer in large scale analysis. Lung Cancer 126, 182–188 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Schaffer BE et al. Loss of p130 accelerates tumor development in a mouse model for human small-cell lung carcinoma. Cancer Res 70, 3877–3883 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lazaro S et al. Differential development of large-cell neuroendocrine or small-cell lung carcinoma upon inactivation of 4 tumor suppressor genes. Proc. Natl Acad. Sci. USA 116, 22300–22306 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ng SR et al. CRISPR-mediated modeling and functional validation of candidate tumor suppressor genes in small cell lung cancer. Proc. Natl Acad. Sci. USA 117, 513–521 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McFadden DG et al. Genetic and clonal dissection of murine small cell lung carcinoma progression by genome sequencing. Cell 156, 1298–1311 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cui M et al. PTEN is a potent suppressor of small cell lung cancer. Mol. Cancer Res 12, 654–659 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim JS et al. Intratumoural heterogeneity generated by Notch signalling promotes small-cell lung cancer. Nature 545, 360–364 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated heterogeneity in SCLC tumours and cooperativity between neuroendocrine and non-neuroendocrine subsets of cells in SCLC progression.
- 40.Augert A et al. Targeting NOTCH activation in small cell lung cancer through LSD1 inhibition. Sci. Signal 12, eaau2922 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jia D et al. Crebbp loss drives small cell lung cancer and increases sensitivity to HDAC inhibition. Cancer Discov 8, 1422–1437 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bottger F et al. Tumor heterogeneity underlies differential cisplatin sensitivity in mouse models of small-cell lung cancer. Cell Rep 27, 3345–3358.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim DW et al. Genetic requirement for Mycl and efficacy of RNA Pol I inhibition in mouse models of small cell lung cancer. Genes Dev 30, 1289–1299 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mollaoglu G et al. MYC drives progression of small cell lung cancer to a variant neuroendocrine subtype with vulnerability to aurora kinase inhibition. Cancer Cell 31, 270–285 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reported the first genetically engineered mouse model of the SCLC-N subtype.
- 45.Ferone G et al. FGFR1 oncogenic activation reveals an alternative cell of origin of SCLC in Rb1/p53 Mice. Cell Rep 30, 3837–3850.e3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coles GL et al. Unbiased proteomic profiling uncovers a targetable GNAS/PKA/PP2A axis in small cell lung cancer stem cells. Cancer Cell 38, 129–143. e7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Augert A et al. Small cell lung cancer exhibits frequent inactivating mutations in the histone methyltransferase KMT2D/MLL2: CALGB 151111 (Alliance). J. Thorac. Oncol 12, 704–713 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wagner AH et al. Recurrent WNT pathway alterations are frequent in relapsed small cell lung cancer. Nat. Commun 9, 3787 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou F & Zhou C Lung cancer in never smokers — the East Asian experience. Transl. Lung Cancer Res 7, 450 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Niederst MJ et al. RB loss in resistant EGFR mutant lung adenocarcinomas that transform to small-cell lung cancer. Nat. Commun 6, 6377 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Popat S Histologically transformed SCLC from EGFR-mutant NSCLC: understanding the wolf in sheep’s clothing. J. Thorac. Oncol 14, 1689–1691 (2019). [DOI] [PubMed] [Google Scholar]
- 52.Offin M et al. Concurrent RB1 and TP53 alterations define a subset of EGFR-mutant lung cancers at risk for histologic transformation and inferior clinical outcomes. J. Thorac. Oncol 14, 1784–1793 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hodgkinson CL et al. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nat. Med 20, 897–903 (2014). [DOI] [PubMed] [Google Scholar]; This paper was the first to report successful tumour engraftment of CTCs from patients with SCLC in mice.
- 54.Meuwissen R et al. Induction of small cell lung cancer by somatic inactivation of both Trp53 and Rb1 in a conditional mouse model. Cancer Cell 4, 181–189 (2003). [DOI] [PubMed] [Google Scholar]; This paper reported the first genetically engineered mouse model of SCLC.
- 55.Gazdar AF et al. The comparative pathology of genetically engineered mouse models for neuroendocrine carcinomas of the lung. J. Thorac. Oncol 10, 553–564 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferone G, Lee MC, Sage J & Berns A Cells of origin of lung cancers: lessons from mouse studies. Genes Dev 34, 1017–1032 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ben-Ezra JM, Kornstein MJ, Grimes MM & Krystal G Small cell carcinomas of the lung express the Bcl-2 protein. Am. J. Pathol 145, 1036–1040 (1994). [PMC free article] [PubMed] [Google Scholar]
- 58.Ikegaki N, Katsumata M, Minna J & Tsujimoto Y Expression of bcl-2 in small cell lung carcinoma cells. Cancer Res 54, 6–8 (1994). [PubMed] [Google Scholar]
- 59.Lochmann TL et al. Venetoclax is effective in small-cell lung cancers with high BCL-2 expression. Clin. Cancer Res 24, 360–369 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Doerr F et al. Targeting a non-oncogene addiction to the ATR/CHK1 axis for the treatment of small cell lung cancer. Sci. Rep 7, 15511 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sen T et al. CHK1 inhibition in small-cell lung cancer produces single-agent activity in biomarker-defined disease subsets and combination activity with cisplatin or olaparib. Cancer Res 77, 3870–3884 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sen T et al. Targeting AXL and mTOR pathway overcomes primary and acquired resistance to WEE1 inhibition in small-cell lung cancer. Clin. Cancer Res 23, 6239–6253 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hsu WH et al. Checkpoint kinase 1 inhibition enhances cisplatin cytotoxicity and overcomes cisplatin resistance in SCLC by promoting mitotic cell death. J. Thorac. Oncol 14, 1032–1045 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oser MG et al. Cells lacking the RB1 tumor suppressor gene are hyperdependent on aurora B kinase for survival. Cancer Discov 9, 230–247 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Owonikoko TK et al. Randomized phase II study of paclitaxel plus alisertib versus paclitaxel plus placebo as second-line therapy for SCLC: primary and correlative biomarker analyses. J. Thorac. Oncol 15, 274–287 (2020). [DOI] [PubMed] [Google Scholar]
- 66.Byers LA et al. Proteomic profiling identifies dysregulated pathways in small cell lung cancer and novel therapeutic targets including PARP1. Cancer Discov 2, 798–811 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lallo A et al. The combination of the PARP inhibitor olaparib and the WEE1 inhibitor AZD1775 as a new therapeutic option for small cell lung cancer. Clin. Cancer Res 24, 5153–5164 (2018). [DOI] [PubMed] [Google Scholar]
- 68.Sen T et al. Targeting DNA damage response promotes anti-tumor immunity through STING-mediated T-cell activation in small cell lung cancer. Cancer Discov 9, 646–661 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper defined a mechanism of combinatorial synergy between targeting DNA damage repair and immune checkpoints.
- 69.Gardner EE et al. Rapamycin rescues ABT-737 efficacy in small cell lung cancer. Cancer Res 74, 2846–2856 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Faber AC et al. Assessment of ABT-263 activity across a cancer cell line collection leads to a potent combination therapy for small-cell lung cancer. Proc. Natl Acad. Sci. USA 112, E1288–E1296 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dick FA, Goodrich DW, Sage J & Dyson NJ Non-canonical functions of the RB protein in cancer. Nat. Rev. Cancer 18, 442–451 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Levine AJ, Puzio-Kuter AM, Chan CS & Hainaut P The role of the p53 protein in stem-cell biology and epigenetic regulation. Cold Spring Harb. Perspect. Med 6, a026153 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ku SY et al. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science 355, 78–83 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mu P et al. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science 355, 84–88 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kareta MS et al. Inhibition of pluripotency networks by the Rb tumor suppressor restricts reprogramming and tumorigenesis. Cell Stem Cell 16, 39–50 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mohammad HP et al. A DNA hypomethylation signature predicts antitumor activity of LSD1 inhibitors in SCLC. Cancer Cell 28, 57–69 (2015). [DOI] [PubMed] [Google Scholar]
- 77.Gardner EE et al. Chemosensitive relapse in small cell lung cancer proceeds through an EZH2-SLFN11 axis. Cancer Cell 31, 286–299 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper defined the role of epigenetic silencing of SLFN11 as a mechanism of acquired resistance in SCLC.
- 78.Stewart CA et al. Single-cell analyses reveal increased intratumoral heterogeneity after the onset of therapy resistance in small-cell lung cancer. Nat. Cancer 1, 423–436 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Simpson KL et al. A biobank of small cell lung cancer CDX models elucidates inter- and intratumoral phenotypic heterogeneity. Nat. Cancer 1, 437–451 (2020). [DOI] [PubMed] [Google Scholar]
- 80.Ouadah Y et al. Rare pulmonary neuroendocrine cells are stem cells regulated by Rb, p53, and Notch. Cell 179, 403–416.e23 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper describes differentiation pathways of lung neuroendocrine cells and their putative status as a cell of origin for SCLC.
- 81.Park KS et al. Characterization of the cell of origin for small cell lung cancer. Cell Cycle 10, 2806–2815 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sutherland KD et al. Cell of origin of small cell lung cancer: inactivation of Trp53 and Rb1 in distinct cell types of adult mouse lung. Cancer Cell 19, 754–764 (2011). [DOI] [PubMed] [Google Scholar]
- 83.Yang D et al. Intertumoral heterogeneity in SCLC is influenced by the cell type of origin. Cancer Discov 8, 1316–1331 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kuo CS & Krasnow MA Formation of a neurosensory organ by epithelial cell slithering. Cell 163, 394–405 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fridman R et al. Reconstituted basement membrane (matrigel) and laminin can enhance the tumorigenicity and the drug resistance of small cell lung cancer cell lines. Proc. Natl Acad. Sci. USA 87, 6698–6702 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Burger M et al. Functional expression of CXCR4 (CD184) on small-cell lung cancer cells mediates migration, integrin activation, and adhesion to stromal cells. Oncogene 22, 8093–8101 (2003). [DOI] [PubMed] [Google Scholar]
- 87.Hartmann TN, Burger JA, Glodek A, Fujii N & Burger M CXCR4 chemokine receptor and integrin signaling co-operate in mediating adhesion and chemoresistance in small cell lung cancer (SCLC) cells. Oncogene 24, 4462–4471 (2005). [DOI] [PubMed] [Google Scholar]
- 88.Burger JA, Stewart DJ, Wald O & Peled A Potential of CXCR4 antagonists for the treatment of metastatic lung cancer. Expert Rev. Anticancer. Ther 11, 621–630 (2011). [DOI] [PubMed] [Google Scholar]
- 89.Guo N et al. Thrombospondin-1 promotes alpha3beta1 integrin-mediated adhesion and neurite-like outgrowth and inhibits proliferation of small cell lung carcinoma cells. Cancer Res 60, 457–466 (2000). [PubMed] [Google Scholar]
- 90.Yang D et al. Axon-like protrusions promote small cell lung cancer migration and metastasis. eLife 8, e50616 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Allison Stewart C et al. Dynamic variations in epithelial-to-mesenchymal transition (EMT), ATM, and SLFN11 govern response to PARP inhibitors and cisplatin in small cell lung cancer. Oncotarget 8, 28575–28587 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Canadas I et al. Targeting epithelial-to-mesenchymal transition with Met inhibitors reverts chemoresistance in small cell lung cancer. Clin. Cancer Res 20, 938–950 (2014). [DOI] [PubMed] [Google Scholar]
- 93.Cattaneo MG, Codignola A, Vicentini LM, Clementi F & Sher E Nicotine stimulates a serotonergic autocrine loop in human small-cell lung carcinoma. Cancer Res 53, 5566–5568 (1993). [PubMed] [Google Scholar]
- 94.Heasley LE Autocrine and paracrine signaling through neuropeptide receptors in human cancer. Oncogene 20, 1563–1569 (2001). [DOI] [PubMed] [Google Scholar]
- 95.Song P et al. M3 muscarinic receptor antagonists inhibit small cell lung carcinoma growth and mitogen-activated protein kinase phosphorylation induced by acetylcholine secretion. Cancer Res 67, 3936–3944 (2007). [DOI] [PubMed] [Google Scholar]
- 96.Jahchan NS et al. A drug repositioning approach identifies tricyclic antidepressants as inhibitors of small cell lung cancer and other neuroendocrine tumors. Cancer Discov 3, 1364–1377 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Park KS et al. A crucial requirement for Hedgehog signaling in small cell lung cancer. Nat. Med 17, 1504–1508 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Krystal GW, Hines SJ & Organ CP Autocrine growth of small cell lung cancer mediated by coexpression of c-kit and stem cell factor. Cancer Res 56, 370–376 (1996). [PubMed] [Google Scholar]
- 99.Nakanishi Y et al. Insulin-like growth factor-I can mediate autocrine proliferation of human small cell lung cancer cell lines in vitro. J. Clin. Invest 82, 354–359 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kwon MC et al. Paracrine signaling between tumor subclones of mouse SCLC: a critical role of ETS transcription factor Pea3 in facilitating metastasis. Genes Dev 29, 1587–1592 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vanhees SL, Paridaens R & Vansteenkiste JF Syndrome of inappropriate antidiuretic hormone associated with chemotherapy-induced tumour lysis in small-cell lung cancer: case report and literature review. Ann. Oncol 11, 1061–1065 (2000). [DOI] [PubMed] [Google Scholar]
- 102.Kanaji N et al. Paraneoplastic syndromes associated with lung cancer. World J. Clin. Oncol 5, 197–223 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nomura M, Morita M & Tanuma N A metabolic vulnerability of small-cell lung cancer. Oncotarget 9, 32278–32279 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Staal-van den Brekel AJ et al. Metabolism in patients with small cell lung carcinoma compared with patients with non-small cell lung carcinoma and healthy controls. Thorax 52, 338–341 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cristea S et al. The MEK5-ERK5 kinase axis controls lipid metabolism in small cell lung cancer. Cancer Res 80, 1293–1303 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Umemura S et al. Therapeutic priority of the PI3K/AKT/mTOR pathway in small cell lung cancers as revealed by a comprehensive genomic analysis. J. Thorac. Oncol 9, 1324–1331 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chalishazar MD et al. MYC-driven small-cell lung cancer is metabolically distinct and vulnerable to arginine depletion. Clin. Cancer Res 25, 5107–5121 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Carter L et al. Molecular analysis of circulating tumor cells identifies distinct copy-number profiles in patients with chemosensitive and chemorefractory small-cell lung cancer. Nat. Med 23, 114–119 (2017). [DOI] [PubMed] [Google Scholar]
- 109.Simpson KL et al. A biobank of small cell lung cancer CDX models elucidates inter-and intratumoral phenotypic heterogeneity. Nat. Cancer 1, 437–451 (2020). [DOI] [PubMed] [Google Scholar]
- 110.Stewart CA et al. Single-cell analyses reveal increased intratumoral heterogeneity after the onset of therapy resistance in small-cell lung cancer. Nat. Cancer 1, 423–436 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Niho S et al. Detection of unsuspected distant metastases and/or regional nodes by FDG-PET in LD-SCLC scan in apparent limited-disease small-cell lung cancer. Lung Cancer 57, 328–333 (2007). [DOI] [PubMed] [Google Scholar]
- 112.Denny SK et al. Nfib promotes metastasis through a widespread increase in chromatin accessibility. Cell 166, 328–342 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Semenova EA et al. Transcription factor NFIB is a driver of small cell lung cancer progression in mice and marks metastatic disease in patients. Cell Rep 16, 631–643 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; Denny et al. (2016) and Semenova et al. (2016) describe the role of NFIB as a driver of SCLC metastasis.
- 114.Wu N et al. NFIB overexpression cooperates with Rb/p53 deletion to promote small cell lung cancer. Oncotarget 7, 57514–57524 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yang D et al. Axon-like protrusions promote small cell lung cancer migration and metastasis. eLife 8, e50616 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Iams WT et al. Improved prognosis and increased tumor-infiltrating lymphocytes in patients who have SCLC with neurologic paraneoplastic syndromes. J. Thorac. Oncol 14, 1970–1981 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Antonia SJ et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 17, 883–895 (2016). [DOI] [PubMed] [Google Scholar]
- 118.Ready NE et al. Nivolumab monotherapy and nivolumab plus ipilimumab in recurrent small cell lung cancer: results from the checkmate 032 randomized cohort. J. Thorac. Oncol 15, 426–435 (2020). [DOI] [PubMed] [Google Scholar]
- 119.Ready NE et al. Nivolumab monotherapy and nivolumab plus ipilimumab in recurrent small cell lung cancer: results from the checkmate 032 randomized cohort. J. Thorac. Oncol 15, 426–435 (2020). [DOI] [PubMed] [Google Scholar]
- 120.Chung HC et al. Pembrolizumab after two or more lines of previous therapy in patients with recurrent or metastatic SCLC: results from the KEYNOTE-028 and KEYNOTE-158 studies. J. Thorac. Oncol 15, 618–627 (2020). [DOI] [PubMed] [Google Scholar]
- 121.Doyle A et al. Markedly decreased expression of class I histocompatibility antigens, protein, and mRNA in human small-cell lung cancer. J. Exp. Med 161, 1135–1151 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yazawa T et al. Lack of class II transactivator causes severe deficiency of HLA-DR expression in small cell lung cancer. J. Pathol 187, 191–199 (1999). [DOI] [PubMed] [Google Scholar]
- 123.Funa K, Gazdar AF, Minna JD & Linnoila RI Paucity of beta 2-microglobulin expression on small cell lung cancer, bronchial carcinoids and certain other neuroendocrine tumors. Lab. Invest 55, 186–193 (1986). [PubMed] [Google Scholar]
- 124.Traversari C et al. IFN-gamma gene transfer restores HLA-class I expression and MAGE-3 antigen presentation to CTL in HLA-deficient small cell lung cancer. Gene Ther 4, 1029–1035 (1997). [DOI] [PubMed] [Google Scholar]
- 125.Wang W et al. Small cell lung cancer tumour cells induce regulatory T lymphocytes, and patient survival correlates negatively with FOXP3+ cells in tumour infiltrate. Int. J. Cancer 131, E928–E937 (2012). [DOI] [PubMed] [Google Scholar]
- 126.Eerola AK, Soini Y & Paakko P A high number of tumor-infiltrating lymphocytes are associated with a small tumor size, low tumor stage, and a favorable prognosis in operated small cell lung carcinoma. Clin. Cancer Res 6, 1875–1881 (2000). [PubMed] [Google Scholar]
- 127.Makarenkova VP et al. Lung cancer-derived bombesin-like peptides down-regulate the generation and function of human dendritic cells. J. Neuroimmunol 145, 55–67 (2003). [DOI] [PubMed] [Google Scholar]
- 128.Weiskopf K et al. CD47-blocking immunotherapies stimulate macrophage-mediated destruction of small-cell lung cancer. J. Clin. Invest 126, 2610–2620 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Owen DH et al. DLL3: an emerging target in small cell lung cancer. J. Hematol. Oncol 12, 61 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rudin CM et al. Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nat. Rev. Cancer 19, 289–297 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; This consensus report first synthesized the human and mouse data defining SCLC subtypes.
- 131.Wooten DJ et al. Systems-level network modeling of small cell lung cancer subtypes identifies master regulators and destabilizers. PLoS Comput. Biol 15, e1007343 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sos ML et al. A framework for identification of actionable cancer genome dependencies in small cell lung cancer. Proc. Natl Acad. Sci. USA 109, 17034–17039 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Owonikoko TK et al. Randomized phase II study of paclitaxel plus alisertib versus paclitaxel plus placebo as second-line therapy for small-cell lung cancer: primary and correlative biomarker analyses. J. Thorac. Oncol 15, 274–287 (2020). [DOI] [PubMed] [Google Scholar]
- 134.Ireland AS et al. MYC drives temporal evolution of small cell lung cancer subtypes by reprogramming neuroendocrine fate. Cancer Cell 38, 60–78.e12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper describes the role of MYC as a driver of plasticity and transition between SCLC subtypes.
- 135.Travis WD et al. The 2015 World Health Organization Classification of Lung Tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J. Thorac. Oncol 10, 1243–1260 (2015). [DOI] [PubMed] [Google Scholar]
- 136.van Meerbeeck JP, Fennell DA & De Ruysscher DK Small-cell lung cancer. Lancet 378, 1741–1755 (2011). [DOI] [PubMed] [Google Scholar]
- 137.Horn L et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N. Engl. J. Med 379, 2220–2229 (2018). [DOI] [PubMed] [Google Scholar]
- 138.Paz-Ares L et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 394, 1929–1939 (2019). [DOI] [PubMed] [Google Scholar]
- 139.Rudin CM et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, phase III KEYNOTE-604 study. J. Clin. Oncol 38, 2369–2379 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fruh M et al. Small-cell lung cancer (SCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol 24, vi99–vi105 (2013). [DOI] [PubMed] [Google Scholar]
- 141.Tay RY et al. Prognostic value of circulating tumour cells in limited-stage small-cell lung cancer: analysis of the concurrent once-daily versus twice-daily radiotherapy (CONVERT) randomised controlled trial. Ann. Oncol 30, 1114–1120 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Stovold R et al. Biomarkers for small cell lung cancer: neuroendocrine, epithelial and circulating tumour cells. Lung Cancer 76, 263–268 (2012). [DOI] [PubMed] [Google Scholar]
- 143.Fernandez-Cuesta L et al. Identification of circulating tumor DNA for the early detection of small-cell lung cancer. EBioMedicine 10, 117–123 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Almodovar K et al. Longitudinal cell-free DNA analysis in patients with small cell lung cancer reveals dynamic insights into treatment efficacy and disease relapse. J. Thorac. Oncol 13, 112–123 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Travis WD Pathology and diagnosis of neuroendocrine tumors: lung neuroendocrine. Thorac. Surg. Clin 24, 257–266 (2014). [DOI] [PubMed] [Google Scholar]
- 146.Austin JH et al. Small-cell carcinoma of the lung detected by CT screening: stage distribution and curability. Lung Cancer 76, 339–343 (2012). [DOI] [PubMed] [Google Scholar]
- 147.Nicholson AG et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: proposals for the revision of the clinical and pathologic staging of small cell lung cancer in the forthcoming eighth edition of the TNM classification for lung cancer. J. Thorac. Oncol 11, 300–311 (2016). [DOI] [PubMed] [Google Scholar]; This paper describes the most recent clinical staging system for patients with SCLC.
- 148.Davis S, Stanley KE, Yesner R, Kuang DT & Morris JF Small-cell carcinoma of the lung–survival according to histologic subtype: a Veterans Administration Lung Group Study. Cancer 47, 1863–1866 (1981). [DOI] [PubMed] [Google Scholar]
- 149.Shepherd FA et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancer. J. Thorac. Oncol 2, 1067–1077 (2007). [DOI] [PubMed] [Google Scholar]
- 150.Vallieres E et al. The IASLC Lung Cancer Staging Project: proposals regarding the relevance of TNM in the pathologic staging of small cell lung cancer in the forthcoming (seventh) edition of the TNM classification for lung cancer. J. Thorac. Oncol 4, 1049–1059 (2009). [DOI] [PubMed] [Google Scholar]
- 151.Hochstenbag MM, Twijnstra A, Wilmink JT, Wouters EF & ten Velde GP Asymptomatic brain metastases (BM) in small cell lung cancer (SCLC): MR-imaging is useful at initial diagnosis. J. Neurooncol 48, 243–248 (2000). [DOI] [PubMed] [Google Scholar]
- 152.Nicholson SA et al. Small cell lung carcinoma (SCLC): a clinicopathologic study of 100 cases with surgical specimens. Am. J. Surg. Pathol 26, 1184–1197 (2002). [DOI] [PubMed] [Google Scholar]
- 153.Travis WD Update on small cell carcinoma and its differentiation from squamous cell carcinoma and other non-small cell carcinomas. Mod. Pathol 25 (Suppl. 1), S18–S30 (2012). [DOI] [PubMed] [Google Scholar]
- 154.Mangum MD, Greco FA, Hainsworth JD, Hande KR & Johnson DH Combined small-cell and non-small-cell lung cancer. J. Clin. Oncol 7, 607–612 (1989). [DOI] [PubMed] [Google Scholar]
- 155.Baine MK et al. Small cell lung carcinoma subtypes defined by ASCL1, NEUROD1, POU2F3 and YAP1: comprehensive immunohistochemical and histopathologic characterization. J. Thorac. Oncol 15, 1823–1835 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Brambilla E et al. Cytotoxic chemotherapy induces cell differentiation in small-cell lung carcinoma. J. Clin. Oncol 9, 50–61 (1991). [DOI] [PubMed] [Google Scholar]
- 157.Sehested M, Hirsch FR, Osterlind K & Olsen JE Morphologic variations of small cell lung cancer. A histopathologic study of pretreatment and posttreatment specimens in 104 patients. Cancer 57, 804–807 (1986). [DOI] [PubMed] [Google Scholar]
- 158.Pelosi G et al. Classification of pulmonary neuroendocrine tumors: new insights. Transl. Lung Cancer Res 6, 513–529 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kriegsmann K et al. Insulinoma-associated protein 1 (INSM1) in thoracic tumors is less sensitive but more specific compared with synaptophysin, chromogranin A, and CD56. Appl. Immunohistochem. Mol. Morphol 28, 237–242 (2020). [DOI] [PubMed] [Google Scholar]
- 160.Mukhopadhyay S, Dermawan JK, Lanigan CP & Farver CF Insulinoma-associated protein 1 (INSM1) is a sensitive and highly specific marker of neuroendocrine differentiation in primary lung neoplasms: an immunohistochemical study of 345 cases, including 292 whole-tissue sections. Mod. Pathol 32, 100–109 (2019). [DOI] [PubMed] [Google Scholar]
- 161.Pelosi G, Rindi G, Travis WD & Papotti M Ki-67 antigen in lung neuroendocrine tumors: unraveling a role in clinical practice. J. Thorac. Oncol 9, 273–284 (2014). [DOI] [PubMed] [Google Scholar]
- 162.Brambilla E et al. Basal cell (basaloid) carcinoma of the lung: a new morphologic and phenotypic entity with separate prognostic significance. Hum. Pathol 23, 993–1003 (1992). [DOI] [PubMed] [Google Scholar]
- 163.Sturm N et al. Thyroid transcription factor 1 and cytokeratins 1, 5, 10, 14 (34betaE12) expression in basaloid and large-cell neuroendocrine carcinomas of the lung. Hum. Pathol 32, 918–925 (2001). [DOI] [PubMed] [Google Scholar]
- 164.Rekhtman N et al. Pulmonary large cell neuroendocrine carcinoma with adenocarcinoma-like features: napsin A expression and genomic alterations. Mod. Pathol 31, 111–121 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sturm N et al. 34BetaE12 expression along the whole spectrum of neuroendocrine proliferations of the lung, from neuroendocrine cell hyperplasia to small cell carcinoma. Histopathology 42, 156–166 (2003). [DOI] [PubMed] [Google Scholar]
- 166.Cheuk W, Kwan MY, Suster S & Chan JK Immunostaining for thyroid transcription factor 1 and cytokeratin 20 aids the distinction of small cell carcinoma from Merkel cell carcinoma, but not pulmonary from extrapulmonary small cell carcinomas. Arch. Pathol. Lab. Med 125, 228–231 (2001). [DOI] [PubMed] [Google Scholar]
- 167.Devoe K & Weidner N Immunohistochemistry of small round-cell tumors. Semin. Diagn. Pathol 17, 216–224 (2000). [PubMed] [Google Scholar]
- 168.Marino-Enriquez A & Fletcher CD Round cell sarcomas — biologically important refinements in subclassification. Int. J. Biochem. Cell Biol 53, 493–504 (2014). [DOI] [PubMed] [Google Scholar]
- 169.Creytens D SATB2 and TLE1 Expression in BCOR-CCNB3 (Ewing-like) sarcoma, mimicking small cell osteosarcoma and poorly differentiated synovial sarcoma. Appl. Immunohistochem. Mol. Morphol 28, e10–e12 (2020). [DOI] [PubMed] [Google Scholar]
- 170.Rekhtman N et al. SMARCA4-deficient thoracic sarcomatoid tumors represent primarily smoking-related undifferentiated carcinomas rather than primary thoracic sarcomas. J. Thorac. Oncol 15, 231–247 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Aberle DR et al. Results of the two incidence screenings in the National Lung Screening Trial. N. Engl. J. Med 369, 920–931 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Silva M et al. Screening with low-dose computed tomography does not improve survival of small cell lung cancer. J. Thorac. Oncol 11, 187–193 (2016). [DOI] [PubMed] [Google Scholar]
- 173.Cuffe S et al. Characteristics and outcomes of small cell lung cancer patients diagnosed during two lung cancer computed tomographic screening programs in heavy smokers. J. Thorac. Oncol 6, 818–822 (2011). [DOI] [PubMed] [Google Scholar]
- 174.de Koning HJ et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N. Engl. J. Med 382, 503–513 (2020). [DOI] [PubMed] [Google Scholar]
- 175.Harmsma M, Schutte B & Ramaekers FC Serum markers in small cell lung cancer: opportunities for improvement. Biochim. Biophys. Acta 1836, 255–272 (2013). [DOI] [PubMed] [Google Scholar]
- 176.Parsons A, Daley A, Begh R & Aveyard P Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis. BMJ 340, b5569 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Karam I, Jiang SY, Khaira M, Lee CW & Schellenberg D Outcomes of small cell lung cancer patients treated with cisplatin-etoposide versus carboplatin-etoposide. Am. J. Clin. Oncol 38, 51–54 (2015). [DOI] [PubMed] [Google Scholar]
- 178.Kubota K et al. Etoposide and cisplatin versus irinotecan and cisplatin in patients with limited-stage small-cell lung cancer treated with etoposide and cisplatin plus concurrent accelerated hyperfractionated thoracic radiotherapy (JCOG0202): a randomised phase 3 study. Lancet Oncol 15, 106–113 (2014). [DOI] [PubMed] [Google Scholar]
- 179.Antonia SJ et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N. Engl. J. Med 379, 2342–2350 (2018). [DOI] [PubMed] [Google Scholar]
- 180.Auperin A et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic cranial irradiation overview collaborative group. N. Engl. J. Med 341, 476–484 (1999). [DOI] [PubMed] [Google Scholar]
- 181.Wakeam E et al. Surgery versus chemotherapy and radiotherapy for early and locally advanced small cell lung cancer: a propensity-matched analysis of survival. Lung Cancer 109, 78–88 (2017). [DOI] [PubMed] [Google Scholar]
- 182.Lad T et al. A prospective randomized trial to determine the benefit of surgical resection of residual disease following response of small cell lung cancer to combination chemotherapy. Chest 106, 320S–323S (1994). [DOI] [PubMed] [Google Scholar]
- 183.Fox W & Scadding JG Medical research council comparative trial of surgery and radiotherapy for primary treatment of small-celled or oat-celled carcinoma of bronchus. Ten-year follow-up. Lancet 2, 63–65 (1973). [DOI] [PubMed] [Google Scholar]
- 184.Barnes H, See K, Barnett S & Manser R Surgery for limited-stage small-cell lung cancer. Cochrane Database Syst. Rev 4, CD011917 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhao H, Ren D, Liu H & Chen J Comparison and discussion of the treatment guidelines for small cell lung cancer. Thorac. Cancer 9, 769–774 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Quoix E, Fraser R, Wolkove N, Finkelstein H & Kreisman H Small cell lung cancer presenting as a solitary pulmonary nodule. Cancer 66, 577–582 (1990). [DOI] [PubMed] [Google Scholar]
- 187.Rami-Porta R, Wittekind C & Goldstraw P, International Association for the Study of Lung Cancer (IASLC) Staging Committee. Complete resection in lung cancer surgery: proposed definition. Lung Cancer 49, 25–33 (2005). [DOI] [PubMed] [Google Scholar]
- 188.Yang CF et al. Role of adjuvant therapy in a population-based cohort of patients with early-stage small-cell lung cancer. J. Clin. Oncol 34, 1057–1064 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Brock MV et al. Surgical resection of limited disease small cell lung cancer in the new era of platinum chemotherapy: Its time has come. J. Thorac. Cardiovasc. Surg 129, 64–72 (2005). [DOI] [PubMed] [Google Scholar]
- 190.Salem A et al. Association of chemoradiotherapy with outcomes among patients with stage I to II vs stage III small cell lung cancer: secondary analysis of a randomized clinical trial. JAMA Oncol 5, e185335 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Simone CB II et al. Radiation therapy for small cell lung cancer: An ASTRO clinical practice guideline. Pract. Radiat. Oncol 10, 158–173 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Verma V et al. Multi-institutional experience of stereotactic ablative radiation therapy for stage I small cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys 97, 362–371 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wu AJ et al. Patterns of failure in limited-stage small cell lung cancer: Implications of TNM stage for prophylactic cranial irradiation. Radiother. Oncol 125, 130–135 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yang Y et al. Prophylactic cranial irradiation in resected small cell lung cancer: a systematic review with meta-analysis. J. Cancer 9, 433–439 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhu H et al. Prophylactic cranial irradiation improved the overall survival of patients with surgically resected small cell lung cancer, but not for stage I disease. Lung Cancer 86, 334–338 (2014). [DOI] [PubMed] [Google Scholar]
- 196.Pignon JP et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N. Engl. J. Med 327, 1618–1624 (1992). [DOI] [PubMed] [Google Scholar]
- 197.Faivre-Finn C et al. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol 18, 1116–1125 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This large study demonstrated similar survival and toxicity outcomes with once-daily and twice-daily radiation in patients with limited-stage SCLC treated with CRT.
- 198.Turrisi AT III et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N. Engl. J. Med 340, 265–271 (1999). [DOI] [PubMed] [Google Scholar]
- 199.Levy A et al. Current management of limited-stage SCLC and CONVERT trial impact: Results of the EORTC Lung Cancer Group survey. Lung Cancer 136, 145–147 (2019). [DOI] [PubMed] [Google Scholar]
- 200.De Ruysscher D et al. Systematic review and meta-analysis of randomised, controlled trials of the timing of chest radiotherapy in patients with limited-stage, small-cell lung cancer. Ann. Oncol 17, 543–552 (2006). [DOI] [PubMed] [Google Scholar]
- 201.Fried DB et al. Systematic review evaluating the timing of thoracic radiation therapy in combined modality therapy for limited-stage small-cell lung cancer. J. Clin. Oncol 22, 4837–4845 (2004). [DOI] [PubMed] [Google Scholar]
- 202.Huncharek M & McGarry R A meta-analysis of the timing of chest irradiation in the combined modality treatment of limited-stage small cell lung cancer. Oncologist 9, 665–672 (2004). [DOI] [PubMed] [Google Scholar]
- 203.Pijls-Johannesma MC, De Ruysscher D, Lambin P, Rutten I & Vansteenkiste JF Early versus late chest radiotherapy for limited stage small cell lung cancer. Cochrane Database Syst. Rev 1, CD004700 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sun JM et al. Phase III trial of concurrent thoracic radiotherapy with either first- or third-cycle chemotherapy for limited-disease small-cell lung cancer. Ann. Oncol 24, 2088–2092 (2013). [DOI] [PubMed] [Google Scholar]
- 205.Christodoulou M et al. Compliance and outcome of elderly patients treated in the concurrent once-daily versus twice-daily radiotherapy (CONVERT) Trial. J. Thorac. Oncol 14, 63–71 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hu X et al. Final report of a prospective randomized study on thoracic radiotherapy target volume for limited-stage small cell lung cancer with radiation dosimetric analyses. Cancer 126, 840–849 (2020). [DOI] [PubMed] [Google Scholar]
- 207.Le Pechoux C et al. Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99–01, EORTC 22003–08004, RTOG 0212, and IFCT 99–01): a randomised clinical trial. Lancet Oncol 10, 467–474 (2009). [DOI] [PubMed] [Google Scholar]
- 208.Eaton BR et al. Effect of prophylactic cranial irradiation on survival in elderly patients with limited-stage small cell lung cancer. Cancer 119, 3753–3760 (2013). [DOI] [PubMed] [Google Scholar]
- 209.Noda K et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N. Engl. J. Med 346, 85–91 (2002). [DOI] [PubMed] [Google Scholar]
- 210.Lara PN Jr. et al. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomic results from SWOG S0124. J. Clin. Oncol 27, 2530–2535 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hanna N et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J. Clin. Oncol 24, 2038–2043 (2006). [DOI] [PubMed] [Google Scholar]
- 212.Hellmann MD et al. Tumor mutational burden and efficacy of nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer. Cancer Cell 35, 329 (2019). [DOI] [PubMed] [Google Scholar]
- 213.Trigo J et al. Lurbinectedin as second-line treatment for patients with small-cell lung cancer: a single-arm, open-label, phase 2 basket trial. Lancet Oncol 21, 645–654 (2020). [DOI] [PubMed] [Google Scholar]
- 214.Slotman B et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N. Engl. J. Med 357, 664–672 (2007). [DOI] [PubMed] [Google Scholar]
- 215.Takahashi T et al. Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 18, 663–671 (2017). [DOI] [PubMed] [Google Scholar]
- 216.Rusthoven CG & Kavanagh BD Prophylactic cranial irradiation (PCI) versus active MRI surveillance for small cell lung cancer: the case for equipoise. J. Thorac. Oncol 12, 1746–1754 (2017). [DOI] [PubMed] [Google Scholar]
- 217.Slotman BJ et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet 385, 36–42 (2015). [DOI] [PubMed] [Google Scholar]
- 218.Sagman U et al. Second primary malignancies following diagnosis of small-cell lung cancer. J. Clin. Oncol 10, 1525–1533 (1992). [DOI] [PubMed] [Google Scholar]
- 219.Kono M et al. Incidence of second malignancy after successful treatment of limited-stage small-cell lung cancer and its effects on survival. J. Thorac. Oncol 12, 1696–1703 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Verma V et al. Cardiac mortality in limited-stage small cell lung cancer. Radiother. Oncol 128, 492–497 (2018). [DOI] [PubMed] [Google Scholar]
- 221.Ferris MJ et al. Radiation therapy is associated with an increased incidence of cardiac events in patients with small cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys 102, 383–390 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Richardson GE et al. Smoking cessation after successful treatment of small-cell lung cancer is associated with fewer smoking-related second primary cancers. Ann. Intern. Med 119, 383–390 (1993). [DOI] [PubMed] [Google Scholar]
- 223.Giuliani M et al. Utilization of prophylactic cranial irradiation in patients with limited stage small cell lung carcinoma. Cancer 116, 5694–5699 (2010). [DOI] [PubMed] [Google Scholar]
- 224.Grosshans DR, Meyers CA, Allen PK, Davenport SD & Komaki R Neurocognitive function in patients with small cell lung cancer: effect of prophylactic cranial irradiation. Cancer 112, 589–595 (2008). [DOI] [PubMed] [Google Scholar]
- 225.Drapkin BJ & Rudin CM Advances in small-cell lung cancer (SCLC) translational research. Cold Spring Harb. Perspect. Med 10.1101/cshperspect.a038240 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Taguchi A et al. Lung cancer signatures in plasma based on proteome profiling of mouse tumor models. Cancer Cell 20, 289–299 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Huang YH et al. POU2F3 is a master regulator of a tuft cell-like variant of small cell lung cancer. Genes Dev 32, 915–928 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Calbo J et al. A functional role for tumor cell heterogeneity in a mouse model of small cell lung cancer. Cancer Cell 19, 244–256 (2011). [DOI] [PubMed] [Google Scholar]
- 229.Iams WT, Porter J & Horn L Immunotherapeutic approaches for small-cell lung cancer. Nat. Rev. Clin. Oncol 17, 300–312 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Deng L et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity 41, 843–852 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Reits EA et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J. Exp. Med 203, 1259–1271 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Gupta A et al. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J. Immunol 189, 558–566 (2012). [DOI] [PubMed] [Google Scholar]
- 233.Cheng DT et al. Memorial Sloan Kettering-integrated mutation profiling of actionable cancer targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J. Mol. Diagn 17, 251–264 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kern JA et al. Role of mTOR as an essential kinase in SCLC. J. Thorac. Oncol 15, 1522–1534 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Potter DS et al. Inhibition of PI3K/BMX cell survival pathway sensitizes to BH3 mimetics in SCLC. Mol. Cancer Ther 15, 1248–1260 (2016). [DOI] [PubMed] [Google Scholar]
- 236.Poirier JT et al. DNA methylation in small cell lung cancer defines distinct disease subtypes and correlates with high expression of EZH2. Oncogene 34, 5869–5878 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Burr ML et al. An evolutionarily conserved function of polycomb silences the MHC class I antigen presentation pathway and enables immune evasion in cancer. Cancer Cell 36, 385–401.e8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]


