Abstract
Purpose:
To evaluate the utility of a defecography phase (DP) sequence in dynamic pelvic floor MRI (DPMRI), in comparison to DPMRI utilizing only non-defecography Valsalva maneuvers (VM).
Materials and Methods:
Inclusion criteria identified 237 female patients with symptoms and/or physical exam findings of pelvic floor prolapse. All DPMRI exams were obtained following insertion of ultrasound gel into the rectum and vagina. Steady-state free-precession sequences in sagittal plane were acquired in the resting state, followed by dynamic cine acquisitions during VM and DP. In all phases, two experienced radiologists performed blinded review using the H-line, M-line, Organ prolapse (HMO) system. The presence of a rectocele, enterocele and inferior descent of the anorectal junction, bladder base, and vaginal vault were recorded in all patients using the pubococcygeal line as a fixed landmark.
Results:
DPMRI with DP detected significantly more number of patients than VM (p<0.0001) with vaginal prolapse (231/237, 97.5% vs. 177/237, 74.7%), anorectal prolapse (227/237, 95.8% vs. 197/237, 83.1%), cystocele (197/237, 83.1% vs. 108/237, 45.6%), and rectocele (154/237, 65% vs. 93/237, 39.2%). The median cycstocele (3.2cm vs. 1cm), vaginal prolapse (3cm vs. 1.5cm), anorectal prolapse (5.4cm vs. 4.2cm), H-line (8cm vs. 7.2cm) and M-line (5.3cm vs. 3.9cm) were significantly higher with DP than VM (p<0.0001).
Conclusions:
Addition of DP to DPMRI demonstrates a greater degree of pelvic floor instability as compared to imaging performed during VM alone. Pelvic floor structures may show mild descent or appear normal during VM, with marked prolapse on subsequent DP images.
Introduction
Pelvic organ prolapse afflicts at least 50% of women over age 50, and negatively impacts quality of life.1,2 Historically, pelvic organ prolapse has been evaluated by physical examination alone, but clinical examination may fail to reproduce issues related to defecation, may not reliably differentiate between rectocele and enteroceles, and may misdiagnose the location or severity of prolapse.3 Therefore, imaging has emerged as a complementary method to assess pelvic organ prolapse and direct operative planning.3
Dynamic MR imaging of the pelvic floor is a valuable tool to visualize pelvic organ movement in a real-time fashion. It is a multiphase exam with imaging acquired at rest, during straining/Valsalva, and defecography/evacuation after instillation of vaginal and rectal gel. The defecography or evacuation phase involves expulsion of rectal contrast which simulates physiologic conditions contributing to the pelvic organ prolapse and may visualize prolapse that is not apparent when the external sphincter is closed and the pelvic floor is contracted.4,5 In contrast to conventional fluoroscopic defecography, the superior soft tissue resolution of MR imaging provides precise details of the pelvic floor compartments and the support structures.6 Moreover, while clinical scoring systems such as the Baden-Walker Grading system and the Pelvic Organ Prolapse Quantification system effectively standardize diagnosis, they may be less useful for surgical planning since they do not involve direct assessment of the unique, individual surgical anatomy.
Prior small studies have demonstrated the value of the defecography phase (DP).7,8 We wished to further explore the utility of the DP for prolapse evaluation in a larger patient cohort. Thus, the purpose of our study was to evaluate the utility of a DP sequence in dynamic pelvic floor MRI, in comparison to nondefecography Valsalva maneuvers (VM) in the largest study to date.
Methods
Patient Selection
Our retrospective investigation was approved by our institutional review board and was HIPAA compliant. An electronic search was performed of all patients that presented to our institutional pelvic floor clinic with a clinical suspicion of pelvic floor prolapse. This list was then cross-referenced with a search of the radiology information system for all patients who underwent a pelvic MRI, with the search terms “pelvic floor,” “incontinence” and “prolapse” between March 2012 and August 2015. Patient charts, Dynamic MR images, and MRI reports were reviewed. Patients were excluded from the study if they (1) did not have a pelvic MRI performed, (2) were of male gender, (3) were unable to tolerate the gel administration or (4) did not demonstrate expulsion of rectal gel during defecography maneuvers (Fig 1).
FIG 1.
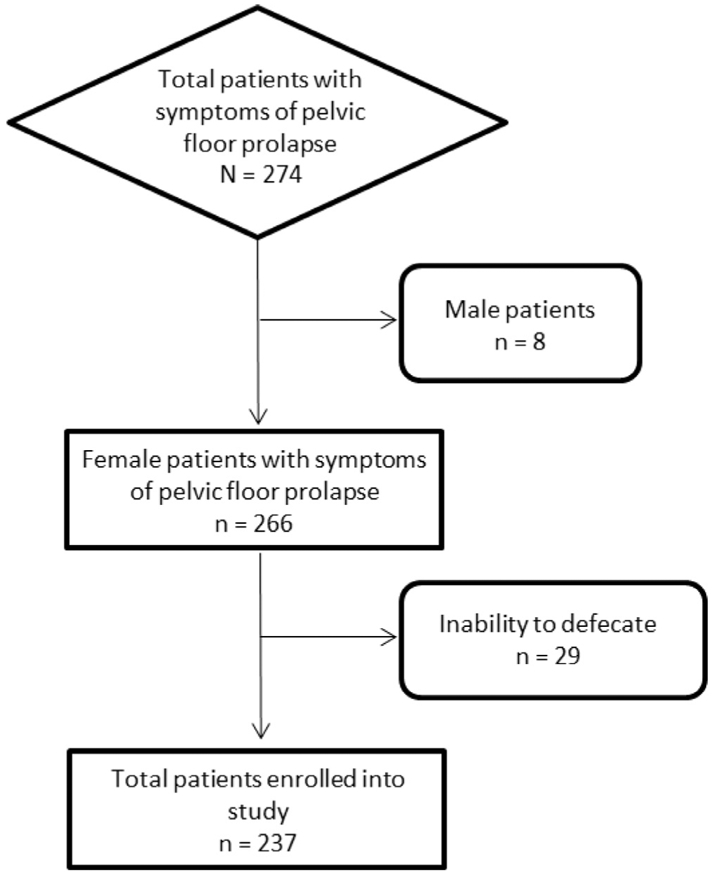
Study accrual flowchart.
MRI Acquisition
Patient Preparation
All patients were prepared prior to MR imaging with the introduction of gel into the patient's vagina and rectum. Ultrasound gel was used for both vaginal and rectal distension. Patients were placed in left lateral decubitus position on the MR scanner table and ~200-300 cc of gel was instilled using plastic enema bottles with cap tips (Medi-Dose EPS, Ivyland, Pennsylvania). A smaller amount (~10-20 cc) of gel was inserted into the vagina via a 20 cc syringe. Patients were asked to refrain from evacuating their bladder for at least one hour prior to imaging to ensure adequate bladder distention.
Patients were verbally instructed regarding the different phases of examination, including rest, nondefecography VM (exerting pressure against a closed anal sphincter) and DP (expulsion of rectal gel).
MR Image Acquisition
All MRI exams were performed on either a 1.5 T or 3.0 T system (Magnetom Aera/Skyra, Siemens Healthineers, Erlangen, Germany), equipped with 45 mT/m gradients operating at a slew rate of 200 T/m/s. An 18 channel torso phased array anterior coil and a 32 channel table-integrated posterior coil were employed for signal reception. A stream-lined imaging protocol was employed for this study population to assess dynamic pelvic floor motion. Balanced steady state free precision sequences were obtained in the midsagittal plane (FOV 400 × 375, 256 matrix, 7 mm slice thickness, TR/TE/flip angle 3.6 ms/1.8 ms/60°, PAT factor = 2), and cine acquisitions were acquired over a 30-second time period during rest, VM, and defecation.
MR Image Analysis
All MRI data sets were collated and anonymized on a workstation with image review software (Intellispace version 4.4, Philips Healthcare, Andover, MA). All MRIs were reviewed in consensus by two attending radiologists (BK and HAT), both fellowship trained in body MRI with 9 and 5 years of experience, respectively.
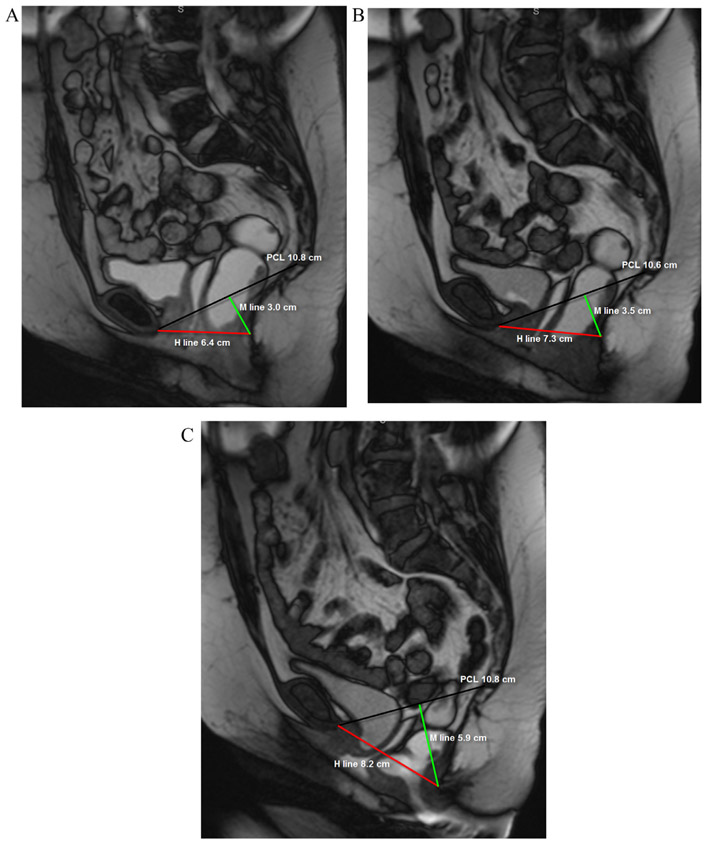
The H-line, M-line, organ prolapse (HMO) grading system for pelvic organ prolapse9 was employed to assess the degree of pelvic floor dysfunction (PFD) for each patient (Fig 2). The pubococcygeal line (PCL), was drawn from the inferior margin of the symphysis pubis to the junction between the first and second coccygeal elements. The H-line (the puborectal line), represents the distance between the inferior margin of the symphysis pubis and the posterior aspect of the puborectalis muscle sling. The M-line is the shortest distance between puborectalis muscle sling and the PCL, drawn as a perpendicular between the PCL and H-lines and is a measure of pelvic floor descent.
FIG 2.
A 49-year-old female with fecal incontinence. Balanced steady state free precession MR images acquired in static (a) and dynamic phases during Valsalva maneuver (VM) (b) and defecography (c) sequences. The patient demonstrates global pelvic floor instability, with progressive prolapse of the pelvic organs on static, VM and defecography sequences. Note that while the PCL (black line) remains unchanged, there is progressive increase in the H line (red line) and M line (green line).
The PCL, H-line and M-line were measured for each patient on each separate image acquisition in conjunction (static, VM, and defecation) as described in previous publications.1,9 In addition, measurements of anorectal junction prolapse, vaginal vault prolapse and the numbers of patients with cystocele and rectocele were recorded for each phase of imaging. A patient was categorized with a cystocele if any portion of the bladder herniated below the PCL (Fig 3) and the degree of bladder herniation below the PCL (in cm) was recorded. The presence of a rectocele was defined as anterior bulging (> 1 cm) of the anterior wall of the rectum compared to static imaging and anorectal junction prolapse was considered when there was a descent of more than 2.5 cm below the PCL, corresponding to the M-line (Fig 4).
FIG 3.
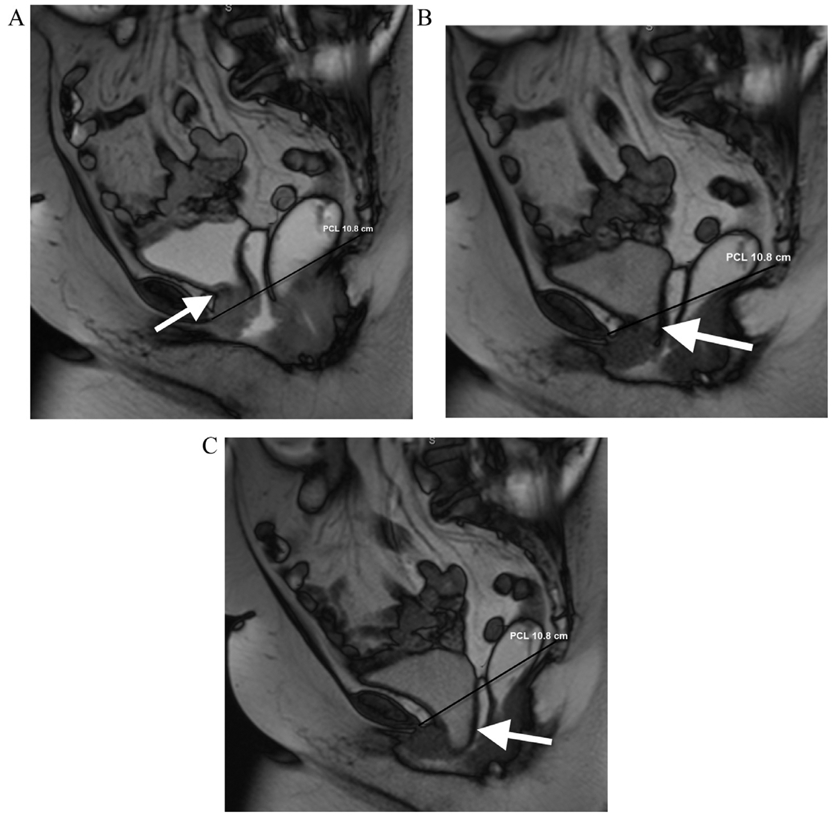
A 70-year-old female with urinary frequency. Balanced steady state free precession MR images acquired in static (a) and dynamic phases during VM (b) and defecography (c) sequences. The patient demonstrates pelvic floor instability that is worst in the anterior compartment. The bladder base remains above the PCL (black line) on static image (arrow, a), which extending slightly below the PCL on VM (arrow, b). On the defecography sequence, there is marked increase in the degree of bladder herniation below the PCL (arrow, c) demonstrating exacerbation of the cystocele.
FIG 4.
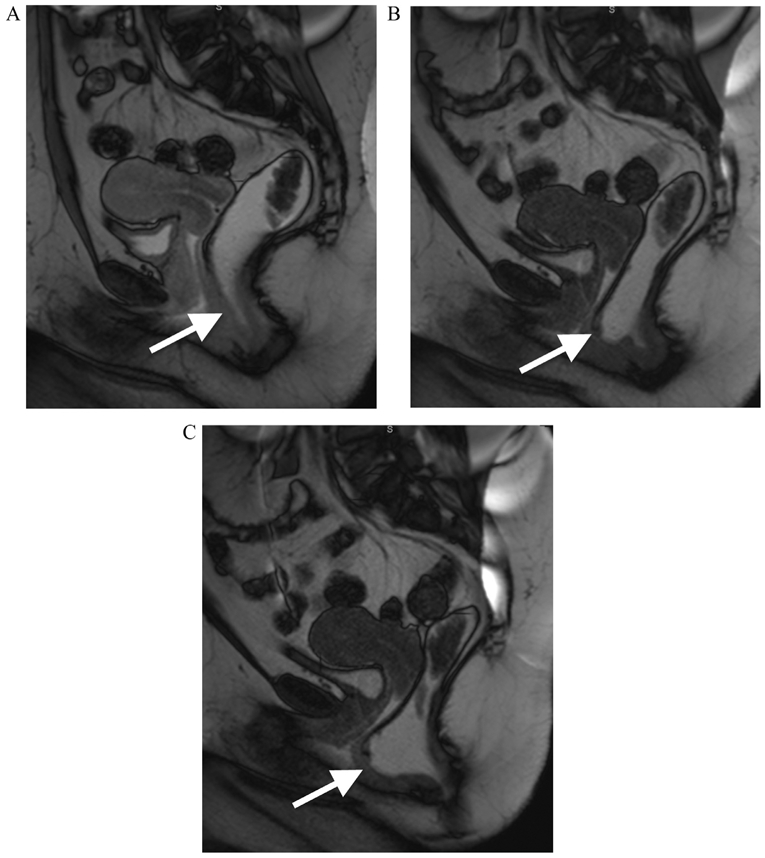
A 34-year-old female with constipation. Balanced steady state free precession MR images acquired in static (a) and dynamic phases during VM (b) and defecography (c) sequences. The patient demonstrates pelvic floor instability that is worst in the posterior compartment. The anterior wall of the rectum shows a normal morphology on static image (arrow, a), however demonstrates focal protrusion on VM (arrow, b). On the defecography sequence, there is marked anterior protrusion of the anterior wall of the rectum, with clear demonstration of a rectocele.
Statistical Methods
In order to determine if the proportion of subjects detected with cystocele, rectocele, vaginal prolapse and anorectal prolapse differed between VM and DP, McNemar's test of correlated proportions that is appropriate for the paired observations was used for analysis. In addition to H-line and M-line measurements, the severity of cystocele, vaginal prolapse or anorectal prolapse among subjects detected either in VM or in DP was analyzed to determine if they differed. For these paired observations in the VM and the DP phases, the differences in continuous variables between the DP and the VM were computed and tested to determine if they satisfied the normality assumption (Shapiro-Wilk's test). Depending upon the results from the normality test, parametric or nonparametric tests for these paired samples were performed to determine if there was a difference between the VM and the DP. Effects associated with P <0.05 were considered statistically significant. All analyses were performed using statistical software (SAS® Version 9.4, SAS Institute Inc., Cary, NC).
Results
Patients
The study cohort consisted of total 274 patients; out of which 8 male patients with symptoms of pelvic floor prolapse were excluded. From the remaining 266 female patients, 29 had difficulty expelling rectal gel during DP and therefore, were excluded. A total of 237 patients that met study inclusion criteria (mean age 63.8 years, range 35-82 years) were used in the final analysis of this study (Table 1).
TABLE 1.
Patient characteristics
| Number of included subjects (all females) |
N = 237 |
|---|---|
| Age (mean, range) | 63.8 years (35-82 years) |
| Defecation abnormalities | 47/237 (19.8%) |
| Subjects seen at pelvic floor clinic | 178/237 (75.1%) |
| Stress urinary incontinence | 104/178 (59.4%); Data unavailable for N = 59 |
| Prior pelvic floor surgery | 80/142 (56.3%); Data unavailable for N = 95 |
| Prior hysterectomies | 128/178 (67.4%) |
| Parity data available | 164/237 (69.2%) |
| Nulliparous | 6/164 (3.7%) |
| Primiparous | 16/164 (9.8%) |
| Multiparous | 142/164(86.6%) |
Patients' chart review revealed urinary and defecation abnormalities. Forty-seven of the 237 (19.8%) underwent dynamic magnetic resonance imaging (dMRI) for constipation, stool trapping, and/or rectal prolapse. Total of 178 patients were seen in the female urology pelvic floor clinic of our institution. A range of symptoms were noted in the chart review of which stress urinary incontinence was positive in (104/178 = 59.4%, 59 patients without stress urinary incontinence data). A significant portion of the patients in our study had undergone prior pelvic floor surgery for prolapse or urinary incontinence (80/142, 56.3%, 95 patients without surgery data). A total of 128 of 178 (67.4%) had prior hysterectomies. One hundred sixty-four of the patients had parity data; 142/164 (79.8%) of the patients were multiparous, 16 were primiparous, 6 were nulliparous, and 73 had no parity data. Of those patients with parity data, the average parity was 3.05 children.
HMO Grading Results
There was statistically significant increase (P < 0.0001) in the number of patients with cystoceles detected with DP compared to VM (Table 2). Among 237 subjects, cystocele was detected in 197/237 (83.1%) patients in DP and 108/237 (45.6%) patients in VM. None of the patients with cystocele were detected in VM alone. There was also statistically significant increase in the number of patients with rectoceles detected in the DP compared to the VM (P < 0.0001). Among 237 subjects, rectocele was detected in 154/237 (65%) patients in DP and 93/237 (39.2%) in VM. None of the patients with rectocele was detected in VM alone. The number of patients with vaginal prolapse detected in DP was significantly higher than that in VM (P < 0.0001). Among 237 subjects, vaginal prolapse was detected in 231/237 (97.5%) patients in DP and 177/237 (74.7%) in VM. None of the patients with vaginal prolapse was detected in VM alone. The number of patients with anorectal prolapse detected in DP was also significantly higher than that in VM (P < 0.0001). Anorectal prolapse was detected in 227/237 (95.8%) patients in either DP or VM, of which 197/237 (83.1%) patients were detected in both VM and DP and 30/237 (12.7%) patients in DP but not in VM. None of the patients with anorectal prolapse was detected in VM alone.
TABLE 2.
Proportion of subjects with cystocele, rectocele, vaginal prolapse and anorectal prolapse detected in Valsalva maneuver (VM) and in defecography phase (DP)
| Detected either in VM or DP | Detected both in VM and DP | Detected in DP but not in VM | Detected in VM but not in DP | P value | |
|---|---|---|---|---|---|
| Cystocele | 197/237 (83.1%) | 108/237(45.6%) | 89/237(37.6%) | 0/237 (0%) | P < 0.0001 |
| Rectocele | 154/237 (65%) | 93/237(39.2%) | 61/237 (25.7%) | 0/237 (0%) | P < 0.0001 |
| Vaginal prolapse | 231/237 (97.5%) | 177/237(74.7%) | 54/237 (22.8%) | 0/237 (0%) | P < 0.0001 |
| Anorectal prolapse | 227/237 (95.8%) | 197/237 (83.1%) | 30/237 (12.7%) | 0/237 (0%) | P < 0.0001 |
For the paired observations between the VM and the defecation phase, none of the continuous variables satisfied the normality assumption (P < 0.001, Shapiro-Wilk's test). Hence, Wilcoxon signed ranks test was used for analysis. Both the H-line and M-line showed statistically significant increases (P < 0.0001) when measured on defecography sequences compared to VM sequences (Table 3). The median measurements during VM and DP for the H-line were 7.2 cm and 8.0 cm, respectively and for the M-line were 3.9 cm and 5.3 cm, respectively. The median increase in H-line and M-line in DP compared to VM were 0.9 cm and 1.1 cm, respectively. PCL represented a fixed anatomic landmark; therefore, remained unchanged measuring 10.4 cm on average, on both phases. Among patients with cystocele detected on either DP or VM (n = 197), the median difference between DP and VM was 1.4 cm, indicating that DP quantified disease severity significantly better than VM (P < 0.0001). Among patients diagnosed with vaginal prolapse on either DP or VM (n = 231), the severity of vaginal prolapse quantified on DP was significantly higher (P < 0.0001) with a median difference between DP and VM of 1.2 cm. Also, among patients diagnosed with anorectal prolapse on either DP or VM (n = 227), the severity of anorectal prolapse quantified on DP was significantly higher (P < 0.0001) with a median difference between DP and VM of 1.2 cm.
TABLE 3.
H-line, M-line, and severity of prolapse quantified during Valsalva maneuver (VM) and in defecography phase (DP)
| VM | DP | Difference = DP–VM | |
|---|---|---|---|
| H-line (N = 237) | 7.2 (6.2, 8.1) [1.2, 11.8] | 8.0 (7.1, 9.3) [2.2, 13.4] | 0.9 (0.3, 6.3) [−1.5, 6.3] P < 0.0001 |
| M-line (N = 237) | 3.9 (3, 5.1) [0.8, 8.4] | 5.3 (4.2, 6.3) [1.2, 9.9] | 1.1 (0.5, 1.9) [−1.3, 6.6] P < 0.0001 |
| Cystocele (N = 197) | 1.0 (0, 3.2) [0, 13.5] | 3.2 (2.0, 5.1) [0.6, 9.0] | 1.4 (0.8, 2.5) [−6.2, 6.8] P < 0.0001 |
| Vaginal prolapse (N = 231) | 1.5 (0.8, 2.2) [0, 8.5] | 3.0 (2.0, 4.0) [0.5, 10.5] | 1.2 (0.7, 2.2) [−1.6, 8.0] P < 0.0001 |
| Anorectal prolapse (N = 227) | 4.2 (3.4, 5.3) [2.6, 8.4] | 5.4 (4.4, 6.5) [2.7, 9.9] | 1.2 (0.5, 2.2) [−1.3, 8.1] P < 0.0001 |
Values are reported in cm as median (first quartile, third quartile) [minimum, maximum].
Discussion
Quantifying the degree of prolapse aids in surgical selection and preoperative planning,10 but clinical assessment may be difficult in patients with multi compartment disease which may be masked or even missed at the time of clinical examination. MRI has been utilized as an adjunct to physical examination for preoperative planning in the setting of PFD and prolapse. MR imaging protocols employ VM (exerting pressure against a closed sphincter) during dynamic acquisitions to demonstrate pelvic floor prolapse. However, relying on this phase of dMRI, underestimates the degree of prolapse and potentially impacting presurgical planning. In this study, we found that the addition of a DP sequence to dynamic pelvic MRI showed significantly higher degrees of pelvic floor prolapse, both with quantitative (HMO system) and qualitative assessments (increased detection of cystoceles and rectoceles) as compared to dynamic pelvic MRI utilizing VM alone.
Flushberg et al 7 retrospectively reviewed 85 MR defecography examinations in 83 patients comparing images from four phases (rest, maximal sphincter contraction and squeezing, maximal straining, and defecation). Compared to other phases, defecation phase images showed additional abnormalities similar to our study, including abnormal bladder descent in 43 examinations (50.6%), abnormal vaginal descent in 52 examinations (61.2%), and abnormal rectal descent in 11 examinations (12.9%). Additional abnormalities were seen on defecation phase which were not identified on other phases of the exam (P < 0.005).
Bhan et al8 also showed additional and significantly more pronounced abnormalities on defecation phase in comparison to VM DPMRI in 68 female patients. Posterior compartment abnormalities were also significantly more in defecation phase as compared to with VM (P <0.0001). This study demonstrated the superiority of DP and recommended that the strain phase be eliminated without the loss of diagnostic information and would streamline the examination, simplify patient instructions, and reduce imaging time.
Vanbeckevoort et al.11 found dynamic MRI with VM (without DP) less sensitive than fluoroscopic defecography in a cohort of 35 patients in detection of cystocele, enterocele, vaginal vault prolapse and rectal descent. This is comparable to our findings, where the use of defecography is more effective at detecting cystocele and vaginal prolapse than with VM alone.
The Society of Urogenital Radiology and the European Society of Gastrointestinal and Abdominal Radiology currently advise an evacuation phase in all dMRI studies.12 Released in 2016, this recommendation agrees with prior small studies and is further supported by our results. Defecography is the most crucial phase of dMRI for diagnosis and grading of PFD and VM may be omitted. In situations where the patient is unable or unwilling to perform the defecography/evacuation phase, the valsalva phase may be used in lieu with the understanding that the degree of prolapse may be inadequate.
To the best of our knowledge, this is the largest cohort with 237 patients depicting the comparative usefulness of DP and VM in dMRI exams, utilizing a single standardized dMRI protocol to evaluate all patients at a single institution, and confirmation of similar findings in prior small studies. Our study has some limitations. It was retrospective in nature, and we did not correlate MRI findings with quantitative clinical measures of pelvic floor prolapse, such as Baden-Walker or POP-Q measurements. Some patients were unable to actively defecate during the course of the exam and therefore not included. Patient cooperation is an unpredictable variable in both physical examination and radiologic assessment, and it may impact the accuracy of preoperative DP MRI for assessment of PFD. Finally, we did not directly assess the impact of differences between VM and DP sequences on subsequent surgical planning or surgical outcomes.
Conclusion
This is the largest study comparing dynamic MR valsalva phase to DP in the evaluation of pelvic organ prolapse. The addition of a DP to dynamic pelvic floor MRI increases the detection of pelvic organ prolapse compared to MR imaging with VM alone. Our results confirm that a DP should be included in dynamic pelvic MRI protocol for assessment of pelvic organ prolapse to optimize detection and assessment of the severity of pelvic organ prolapse.
Standard abbreviations
- HMO
H-line M-line Organ prolapse classification system. Point landmarks are A (the inferior margin of the symphysis pubis), B (the posterior aspect of the puborectalis muscle sling), and C (the junction between the first and second coccygeal elements). Reference lines are the PCL, which is drawn from A to C and is a fixed anatomic reference line; H (the puborectal line), which represents the anteroposterior hiatal dimension and is drawn from A to B; and M, which is the shortest distance between B and the PCL and is a measure of pelvic floor descent. (Reference: Pannu HK, Kaufman HS, Cundiff GW,. Dynamic MR imaging of pelvic organ prolapse: spectrum of abnormalities. Radiographics. 2000 Nov-Dec;20(6):1567-82.)
- VM
Nondefecography Valsalva maneuvers (VM): Patients are to exert pressure against a closed anal sphincter.
- DP
During the defecography phase (DP) patients were asked to expel out the rectal gel, simulating the physiologic act of defecation.
- MRI
Magnetic resonance Imaging
References
- 1.Pannu HK, Kaufman HS, Cundiff GW. Dynamic MR imaging of pelvic organ prolapse: Spectrum of abnormalities. Radiographics 2000;20:1567–82. [DOI] [PubMed] [Google Scholar]
- 2.Law MY, Fielding JR. MRI of pelvic floor dysfunction: Review. AJR Am J Roentgenol 2008;191:S45–53. [DOI] [PubMed] [Google Scholar]
- 3.Elshazly WG, AelA ElNekady, Hassan H Role of dynamic magnetic resonance imaging in management of obstructed defecation case series. Int J Surg 2010;8:274–82. [DOI] [PubMed] [Google Scholar]
- 4.Mondot L, Novellas S, Senni M, et al. Pelvic prolapse: Static and dynamic MRI. Abdom Imaging 2007;32:775–83, 10.1007/s00261-006-9168-y. [DOI] [PubMed] [Google Scholar]
- 5.De Almeida FG, Rodríguez L V, Raz S. Magnetic resonance imaging in the diagnosis of pelvic floor disorders. Int Braz J Urol 2002;28:553–9 http://www.ncbi.nlm.nih.gov/pubmed/15748406. [PubMed] [Google Scholar]
- 6.Bitti GT, Argiolas GM, Ballicu N. Pelvic floor failure: MR imaging evaluation of anatomic and functional abnormalities. Radiographics 2014;34:429–48. [DOI] [PubMed] [Google Scholar]
- 7.Flusberg M, Sahni VA, Erturk SM. Dynamic MR defecography: Assessment of the usefulness of the defecation phase. AJR Am J Roentgenol 2011;196:W394–9. [DOI] [PubMed] [Google Scholar]
- 8.Bhan SN, Mnatzakanian GN, Nisenbaum R. MRI for pelvic floor dysfunction: Can the strain phase be eliminated? Abdom Radiol (NY). 2016;41:215–20. [DOI] [PubMed] [Google Scholar]
- 9.Comiter CV, Vasavada SP, Barbaric ZL, et al. Grading pelvic prolapse and pelvic floor relaxation using dynamic magnetic resonance imaging. Urology 1999;54:454–7. [DOI] [PubMed] [Google Scholar]
- 10.Hetzer FH, Andreisek G, Tsagari C. MR defecography in patients with fecal incontinence: Imaging findings and their effect on surgical management Radiology 2006;240:449–57. [DOI] [PubMed] [Google Scholar]
- 11.Vanbeckevoort D, Van Hoe L, Oyen R. Pelvic floor descent infemales: Comparative study of colpocystodefecography and dynamic fast MR imaging. J Magn Reson Imaging 1999;9:373–7. [DOI] [PubMed] [Google Scholar]
- 12.El Sayed RF, Alt CD, Maccioni F, et al. Magnetic resonance imaging of pelvic floor dysfunction - joint recommendations of the ESUR and ESGAR Pelvic Floor Working Group. Eur Radiol 2017;27:2067–85. [DOI] [PMC free article] [PubMed] [Google Scholar]