Abstract
End-stage kidney disease (ESKD) is a common and morbid disease that affects patients’ quality and length of life, representing a large portion of health care expenditure in the United States. These patients commonly have associated diabetes and cardiovascular disease, with high rates of cardiovascular-related death. Management of ESKD requires renal replacement therapy via dialysis or transplantation. While transplantation provides the greatest improvement in survival and quality of life, the vast majority of patients are treated initially with hemodialysis. However, outcomes differ significantly among patient populations. Barriers in access to care have particularly affected at-risk populations, such as Black and Hispanic patients. These patients receive less pre-ESKD nephrology care, are less likely to initiate dialysis with a fistula, and wait longer for transplants—even in pediatric populations. Priorities for ESKD care moving into the future include increasing access to nephrology care in underprivileged populations, providing patient-centered care based on each patient’s “life plan,” and focusing on team-based approaches to ESKD care. This review explores ESKD from the perspective of epidemiology, costs, vascular access, patient-reported outcomes, racial disparities, and the impact of the COVID-19 crisis.
1. Introduction
End-stage kidney disease (ESKD) is a highly morbid condition that requires costly and time-intensive care. Optimal management of ESKD can improve quality of life and survival significantly; however, barriers, including access to care, reactive rather than proactive treatment, and disparities in minority populations, negatively impact the care of ESKD patients [1].
Kidney failure is a consequence of chronic kidney disease (CKD) progression or acute kidney injury causing rapid deterioration of kidney function. CKD occurs when renal function is diminished for more than 3 months, characterized by either a glomerular filtration rate (GFR) below 60 mL/min/1.73 m2 or the presence of kidney damage markers, such as albuminuria, urine sediment abnormalities, or other electrolyte derangements [1]. In CKD, adaptations occur over time to maintain GFR and allow equilibrium of creatinine and other electrolytes. In acute kidney injury, such adaptations are often unable to occur, resulting in rapid progression to renal failure. In ESKD, defined as GFR < 15 mL/min/1.73 m2, patients are at risk for potentially lethal complications without renal replacement therapy (RRT) [1].
RRT can be completed through hemodialysis, peritoneal dialysis, or transplantation. Dialysis can be used as a short-term temporizing measure if the cause of renal damage is reversible, as a bridge to transplantation, or as ultimate treatment in patients unable to undergo transplantation. Regardless of the treatment modality used, the management of kidney disease can have a significant impact on a patient’s quality of life.
2. Epidemiology of kidney disease
CKD affects 14.9% of the US population overall; the sub-populations with the highest prevalence are females (16%), adults older than 65 years (38.6%), Blacks (17%), and Hispanics (15.3%) [2]. The leading causes of CKD are diabetes, hypertension, and glomerular disease; among patients older than 65 years, CKD is associated with a 2-fold greater prevalence of cardiovascular disease, most commonly in the form of atherosclerotic disease, which is present in 40% of CKD patients [3,4].
In 2018, the incidence of ESKD in the United States was 390.2 per million and prevalence was 242 per million; ESKD is most commonly a result of diabetes, followed by hypertension, glomerulonephritis, and cystic kidney disease [5]. The incidence of ESKD has declined in the United States since 2006, partly due to improved recognition and care of CKD, resulting in decreased progression of CKD to ESKD (Fig. 1). As with CKD, ESKD occurs more commonly in Black and Hispanic patients, with rates 3.4 and 1.5 times higher, respectively, than the general population [5]. Older patients are at the highest risk for developing ESKD. Adults older than 65 years comprise nearly three-fourths of all ESKD cases and are the largest growing segment of patients with ESKD in the United States [5]. Adults older than 75 years had a 4-fold greater incidence of ESKD than the average adult and more than double the incidence of adults in the 45- to 64-year-old age group. Among ESKD patients, 70.7% are treated with hemodialysis (HD) or peritoneal dialysis (PD) and 29.3% have a kidney transplantation [5].
Fig. 1 –
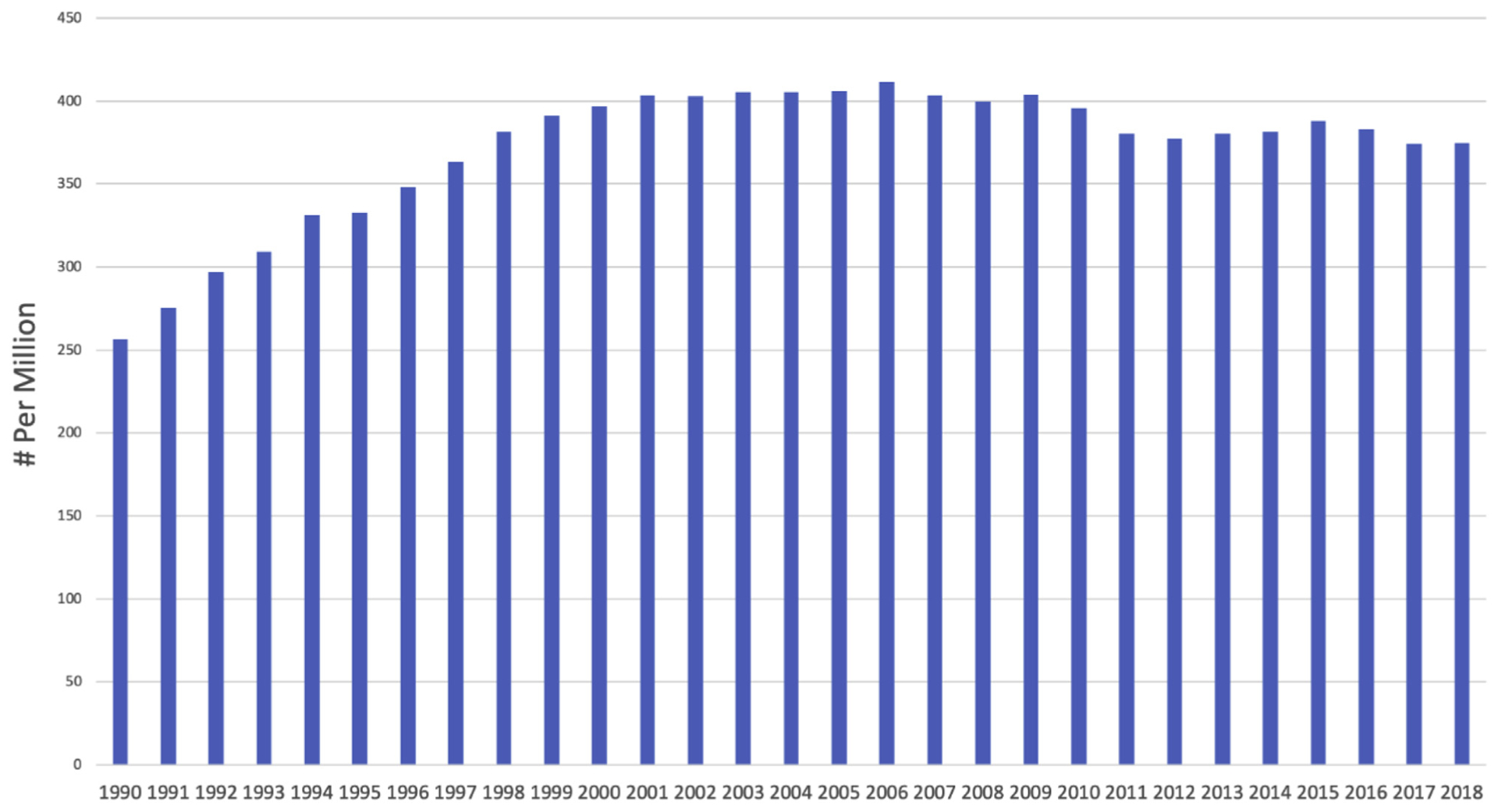
Adjusted end-stage renal disease (ESRD) incidence 1990 to 2018. Data from US Renal Data Service 2020 Annual Report on Chronic Kidney Disease and End Stage Renal Disease [5].
The prevalence of ESKD varies across US geographic regions. Adjusted for demographics, the South (primarily Arkansas, Louisiana, Texas, and Oklahoma) has the highest rates of ESKD with adjusted ESKD incidence of 434 per million. The Northeast (primarily Connecticut, Massachusetts, Maine, Rhode Island, New Hampshire, and Vermont) has the lowest ESKD incidence of 284.6 per million [5].
3. Natural History of ESKD
The onset of CKD is often undetected, with < 10% of patients with stage 3 CKD, or lower, being aware of their condition. The CKD diagnosis is most often made in stage 4, when symptoms become more apparent [6]. CKD progresses to ESKD in approximately 2% of patients overall. The relatively low rate of progression is due in part to high rates of cardiovascular-related death before onset of ESKD [7]. CKD patients are more likely to die (37.6/100 patient-years) or have a myocardial infarction (10.8/100 patient-years) than progress to ESKD requiring RRT (5/100 patient-years) [8]. CKD patients with proteinuria, hypertension, and diabetes, as well as Black and Hispanic CKD patients, all have a 2- to 3-fold greater risk of progression to ESKD than patients without these risk factors [9]. With onset of ESKD, 88% of patients are treated with HD as their initial RRT. Over time, patients may switch to PD or receive a transplant (Fig. 2) [10].
Fig. 2 –
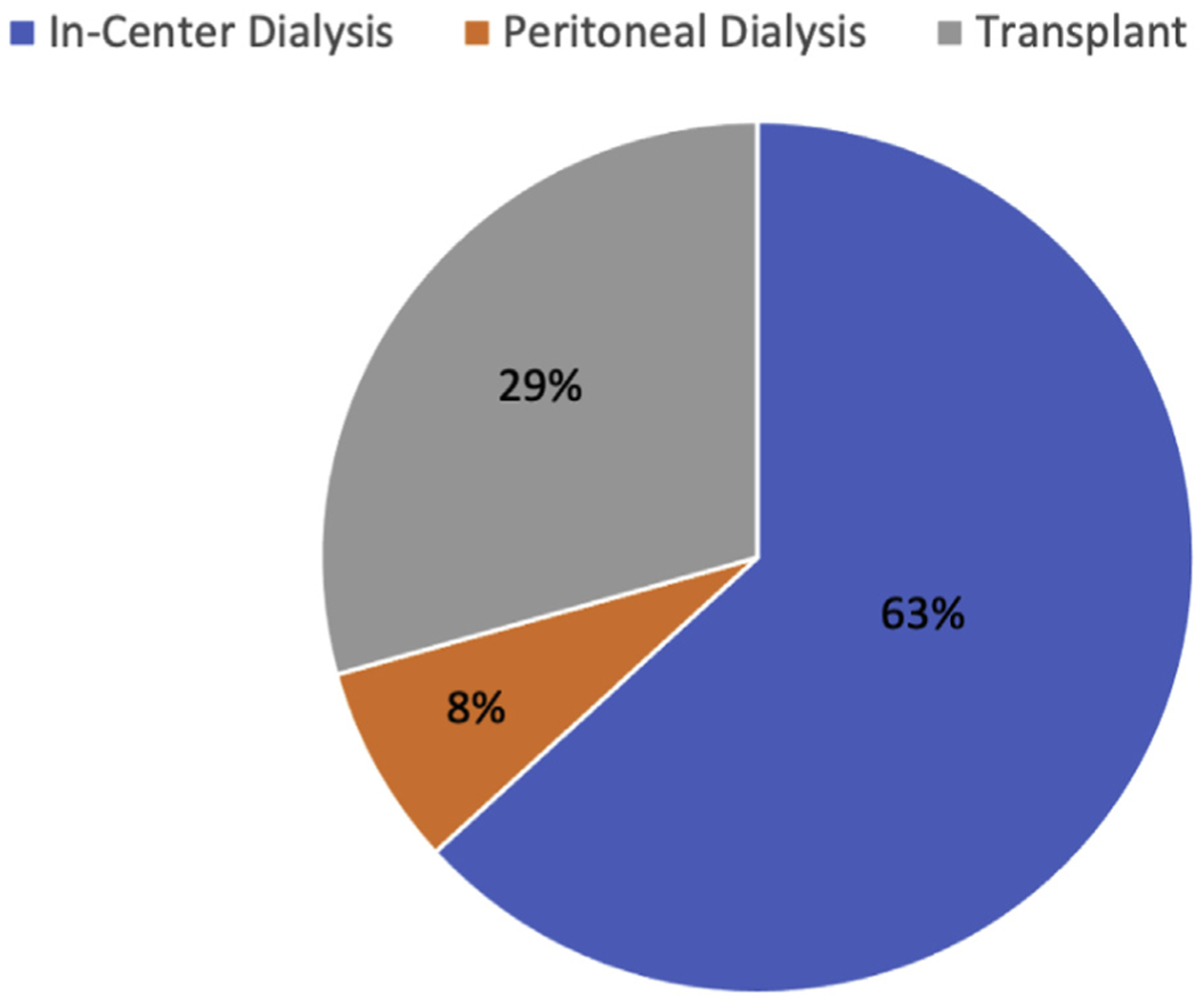
End-stage renal disease (ESRD) treatment by modality in 2018. Data from US Renal Data Service 2020 Annual Report on Chronic Kidney Disease and End Stage Renal Disease [5].
In 2018, ESKD patients had 1.58 hospitalizations per person-year compared with 1.82 hospitalizations per person-year in 2009, reflecting improvements in routine outpatient care. Female patients with ESKD were 20% more likely to be hospitalized than male patients [11]. The leading cause of hospitalization among White patients was infection-related, such as sepsis. In contrast, the most common cause of hospitalization for Black patients was cardiovascular or vascular access complications [12]. For ESKD patients requiring hospitalization, 31.1% required 30-day readmission and 10.1% died within 30 days of discharge with or without readmission [11,12].
Cardiovascular disease is the leading cause of death among ESKD patients, and sudden cardiac death from myocardial infarction or arrythmia accounts for 44.2% of the deaths in this population [13]. Cardiovascular disease is amplified by uremia and volume changes; ESKD patients have a 20-fold greater risk of mortality from cardiovascular disease than non-ESKD patients [14]. Common noncardiac causes of death include withdrawal of care (18.7%), infection (6.9%), and stroke (2.6%) [15,16].
ESKD carries a significant decrease in life expectancy regardless of RRT type. The 5-year survival from onset of ESKD was 41.4% for HD patients and 46.9% for PD patients. Five-year survival was highest in patients who received transplants, ranging between 83.4% and 93.8%, depending on the type of renal graft implanted. Younger dialysis patients have the greatest decrease in life expectancy. Patients aged 40 to 44 years have a remaining life expectancy of 10.9 years for males and 10.2 years for females, compared with a remaining life expectancy of 36.5 and 40.3 years for males and females without ESKD, respectively [13]. Patients aged 40 to 44 years who received transplants fared significantly better than dialysis patients in the same age group, with remaining life expectancy of 27.9 and 29.7 years for men and women, respectively (Fig. 3A and B). Even transplant patients with cardiovascular comorbidities such as heart failure and coronary artery disease experienced better survival than dialysis patients who did not have any cardiovascular comorbidities [13]. Survival was also superior in transplant patients after percutaneous coronary intervention, coronary artery bypass grafting, implantable cardioverter-defibrillator placement, and carotid endarterectomy or stenting compared with dialysis patients undergoing the same procedures [13,14].
Fig. 3 –
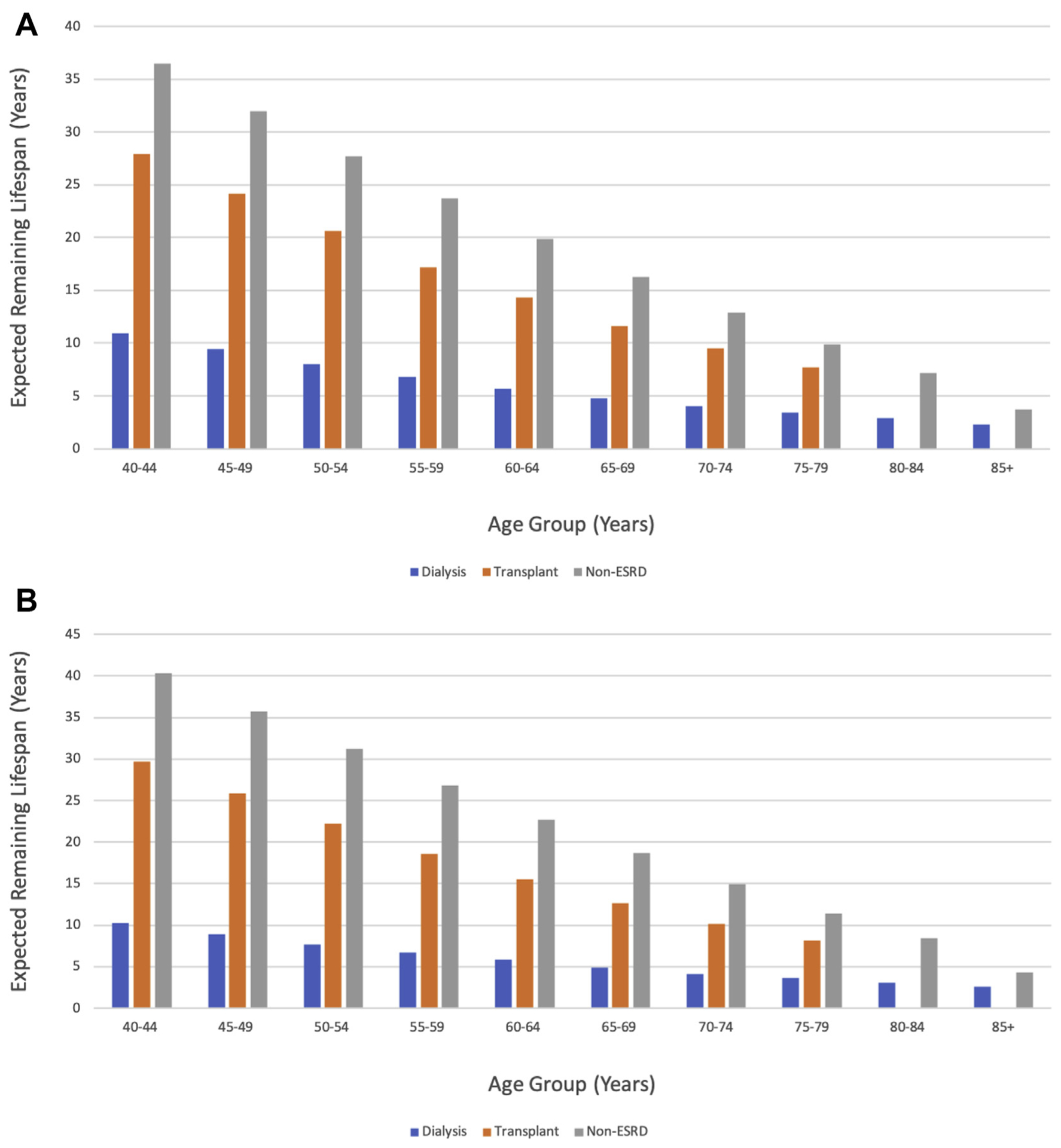
Expected life span in male patients (A) and female patients (B) with end-stage kidney disease (ESKD) by treatment modality compared with non-ESKD patients. Data from US Renal Data Service 2020 Annual Report on Chronic Kidney Disease and End Stage Renal Disease.
4. Pediatric ESKD population
In pediatric patients under 18 years old, ESKD is most commonly due to congenital disorders, glomerular disease, or secondary glomerulonephritis. In 2018, the prevalence of ESKD in the US pediatric population was 5,410 with an incidence of 841. As of December 2020, 1,092 pediatric patients were awaiting kidney transplantation in the United States [17,18]. The youngest ESKD patients have the least favorable outcomes. ESKD patients younger than 1 year have a 5-year survival probability of 76% compared with 83% in patients aged 1 to 5 years and 95% in patients older than 6 years. Pediatric patients undergoing transplantation have the best outcomes with 5-year survival >99% [18].
As in adults, the leading causes of death in pediatric ESKD patients are cardiovascular disease and infection. Adjusted mortality rates have decreased in pediatric ESKD patients from 43.5 deaths/1,000 patient-years in 2001 to 23.8 deaths/1,000 patient-years in 2018 [18]. Pediatric patients being treated with PD have similar survival but higher hospitalization rates than patients treated with HD (16.1 days per year v 7.9 days) [18]. In 2018, among adults with pediatric onset of ESKD, 22.2% were still receiving HD, 6.1% were receiving PD, 49.5% had a functioning initial kidney transplant graft, and 22.2% had a functioning repeat kidney transplant subsequent to their index graft [17,18].
5. Health care costs of CKD and ESKD
Among Medicare patients, annual costs are $49.2 billion for ESKD-related health care expenses and $50.4 billion for CKD [19]. ESKD cost has continued to rise over the past decade, increasing by 20.3% in inflation-adjusted dollars since 2009 and has consistently comprised 7.1% to 7.3% of all Medicare expenses annually [20]. The annual cost per ESKD patient varies by treatment modality; patients treated with HD have the highest annual maintenance cost ($93,000), followed by patients treated with PD ($78,000) and kidney transplant recipients ($37,000) [20]. Accounting for the initial operation, costs of transplant-associated readmissions and graft failures, maintenance care, and immunosuppression, kidney transplantation is the lowest-cost ESKD treatment in addition to being associated with the highest long-term survival and quality of life [12,21].
6. Vascular access
Nearly 90% of ESKD patients will undergo HD as their initial treatment modality and will continue HD for their lifetime [10]. The effectiveness of HD is directly related to the quality and consistency of the vascular access. More than 80% of patients initiate HD with a central venous catheter (CVC), an unacceptably high rate, given the numerous complications associated with long-term CVC use, including catheter-related infection and death [22,23]. Successful long-term HD access is traditionally dependent on surgical arteriovenous (AV) fistula or graft creation. More recently, in 2018, two devices were approved by the US Food and Drug Administration for percutaneous fistula creation [5].
In 2018, 65.7% of ESKD patients received HD through an AV fistula, 16.7% through an AV graft, and 17.6% through a CVC [5]. The use of an AV fistula or graft instead of CVC increases ESKD survival and decreases infections, hospitalizations, and cost [22]. Prevalence of CVC versus fistula varies significantly among geographic regions (Table 1) [5]. A retrospective review of Medicare data, encompassing more than 400,000 patients, found that initiating HD with a catheter as opposed to an AV fistula was associated with a 51% increase in mortality. Long-term CVC use was associated with a 2.2-fold increase in mortality, and the rates of CVC-related infection and mortality worsen with age [23–25].
Table 1 –
State rankings for central venous catheter and fistula use rates for hemodialysis in all prevalent end-stage kidney disease patients.
| Highest rate of fistula use for HD, % | Lowest rate of fistula use for HD, % | Highest rate of CVC use for HD, % | Lowest rate of CVC use for HD, % |
|---|---|---|---|
| Colorado 73.0 | Alabama 56.7 | West Virginia 27.5 | Alabama 14.7 |
| Rhode Island 72.9 | South Carolina 57.0 | Vermont 27.0 | Kansas 14.8 |
| New Mexico 70.9 | West Virginia 57.7 | Montana 24.5 | Rhode Island 14.9 |
Data from US Renal Data Service 2020 Annual Report on Chronic Kidney Disease and End Stage Renal Disease.
Initiating HD with an AV fistula or graft requires proper nephrology care and vascular surgery consultation before the onset of ESKD. Despite efforts to improve pre-ESKD surgical evaluation, 80.8% of patients initiated HD via CVC in 2018; representing a reduction of only 1.6% since 2009 [26,27]. Moreover, two-thirds of ESKD patients starting HD through an CVC did not have an immature AV fistula or graft at time of first dialysis. Almost 30% of patients with more than 1 year of nephrology care before ESKD onset started HD with an AV fistula or graft, and only 5% of patients without such care did the same [5].
Most patients will continue to use a catheter for weeks to months after AV fistula or graft creation due to delays in maturation. More than two-thirds of patients will continue using a CVC for 3 months after a vascular access surgery, and the majority of patients require at least 6 months until they are regularly using their surgical access [5]. These findings highlight the importance of pre-ESKD care with coordinated efforts between nephrologists and vascular surgeons to establish permanent vascular access in a timely fashion to minimize CVC dependence.
The National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (KDOQI) 2019 practice guidelines specify a preference for AV fistulas over grafts when both are options. However, multiple factors must be considered when planning vascular access. While grafts mature more quickly and at a higher rate than fistulas, data have shown prosthetic grafts to be associated with worse patency, increased cost, and increased infection compared with autogenous fistula [23,28–30]. Of note, comorbidities including obesity, diabetes, peripheral arterial disease, and heart failure are associated with worse outcomes regardless of whether a fistula or graft is selected [31,32]. KDOQI guidelines outline the limited situations when CVC should be used for HD, namely as a bridge to functional AV fistula or graft, temporary placement in acute or reversible situations, lack of anatomical options for fistula or graft placement, and when aligning with goals of care or limited life expectancy. KDOQI guidelines also recommend placement of vascular access in a more proximal anatomic location in patients with limited life expectancy, evaluation of artery size and flow during surgical planning, postoperative evaluation for complications within 2 weeks, and postoperative evaluation for maturation within 6 weeks.
The role of the vascular surgeon in creating and maintaining AV access is critical. From 2009 to 2015, 69.3% of all AV fistulas were placed by vascular surgeons in the United States, making vascular surgeons the primary source of access creation [33]. Higher surgeon volume of access creation is also associated with greater maturation success [33]. Unfortunately, 1-year AV fistula primary patency rate is only 50% to 60% [34–36] and 77% of AV grafts develop stenosis or thrombosis within the first year [37]. Risk factors such as obesity and peripheral vascular disease worsen fistula durability further [31,38,39]. As such, patients will require interventions to preserve fistula or graft function, and the vascular surgeon plays an important role in maintaining AV access [33]. These interventions include range from endovascular therapies (eg, balloon angioplasty, stent-graft placement, and percutaneous thrombectomy/thrombolysis) to open surgical revision. The vascular surgeons is critical to the decision-making process of when a fistula or graft should be replaced with a new one, as opposed to continuing to intervene. Clearly, the vascular surgeon’s role in ESKD care extends beyond initial access creation and is instead a long-term position on the patient’s care team to maximize the lifespan of their vascular access.
7. Kidney transplantation in ESKD
Kidney transplantation as RRT results in the highest long-term survival, quality of life, and lowest annual maintenance costs compared with HD or PD [40,41]. By replacing native kidney function with a transplant, many of the negative health impacts of ESKD, including uremia, volume over-load, and the inflammatory cascade, are resolved. This allows the patient’s physiology to more closely return to the pre-ESKD state, albeit with the obligation of lifelong immunosuppression. However, kidney graft demand continues to far outweigh supply. In September 2020 in the United States, more than 90,000 patients were awaiting a kidney transplant and an all-time high of 22,393 patients were added to the list in 2018 [42–44]. Meanwhile, only 39,718 kidney transplantations were performed in 2019 [43]. Ongoing efforts to improve donor rates through public information campaigns and legislative efforts to decrease barriers to organ donation are critical to increasing access to kidney transplantation.
8. Patient-reported outcomes and quality of life in ESKD
Generally, transplantation and PD are associated with a higher quality of life than HD, likely because transplantation does not require dialysis and PD allows for more flexibility with treatment at home. However, many patients do not qualify for a transplantation due to medical comorbidities. Others that qualify wait years for a graft or may never receive one [45]. Patients considering PD may not be candidates if they are at high risk of intra-abdominal infection or have anatomic contraindications, such as large hernias or adhesions from previous operations. The multiple limitations of these “preferred” treatment modalities result in HD continuing to be the dominant modality of RRT.
HD in a facility has a significant negative impact on quality of life by altering a patient’s daily schedule and ability to work or attend school. As a result, home HD has emerged over the last decade as an increasingly popular option, demonstrating improved patient satisfaction without affecting safety or efficacy [46–48]. Only 12.5% of patients receiving HD used home therapy in 2018, but this represented an increase of 100% since 2008. Widespread adoption of home HD has been limited by insurance barriers and patient and family training. Yet, as more patients successfully implement home HD into their lives, it represents an opportunity for continued growth as a lifestyle-improving and health-preserving measure.
While the literature has elucidated major ESKD-associated patient concerns, such as schedule disruption, less is known regarding health-related quality of life associated with vascular access. This is due to the limited availability and slow adoption of vascular access-related patient-reported outcomes (PROs). In a review of 168 trials reporting vascular access outcomes, the most commonly included PRO was pain (11% of studies); only 3% reported a surrogate for quality of life [48]. An expanded collection of standardized vascular access PROs has the potential to guide physicians in increasing the value that patients derive from their health care and enhance patients’ ability to participate in the vascular access decision-making process [49]. The use of PROs can also improve outcomes; chemotherapy patients experienced increased survival when periodic assessment of PROs was implemented, alerting physicians when alarming symptoms were registered [50].
The increased use of PROs in ESKD patients supports the overall movement toward value-based health care. Among studies that have evaluated PROs, differences in defining outcomes and survey methodology have prevented large-scale data aggregation on which to base policy decisions. To address this issue, the International Consortium for Health Outcomes Measurement has been established with the goal of standardizing such measurements for universal use. The CKD Working Group of the Consortium has identified six critical PROs for CKD and ESKD patients: general health care quality of life, pain, fatigue, physician function, depression, and daily activity [51]. Patients surveyed by this group identified health care–related quality of life as the most essential domain of the included outcomes, ranking higher than survival. Importantly, 75% of patients stated this collection of PROs contained all critical measures for ESKD [52]. A standardized set of measures would lead to more uniform data with a broader scope, allowing for larger comparisons of outcomes across multiple areas. This could be applied to better identify critical benchmarks, as well as areas for improvement. Furthermore, PROs could be used to support reimbursement and regulatory action for patient-centered care initiatives, such as home HD, that improve quality of life. Although barriers such as patient participation, physician adoption, electronic medical record tracking, and logistics of incorporation into clinical workflow have prevented standardized PROs from being widely adopted, continued work to this end is necessary to ensure that patients can be stakeholders in their own care [52–54].
9. Disparities in ESKD management
The diagnosis and management of ESKD reflects the socio-demographic disparities underlying the US medical system. Black patients have delayed diagnosis compared with White patients. Although 42% of White patients receive an ESKD diagnosis with a GFR >10 mL/min/1.73 m2, only 28% of Black patients receive an ESKD diagnosis at a GFR above this level. In 2013, Black adults listed for transplant waited on average 1.5 years longer for kidney transplant than White patients. Overall, Blacks and Hispanics were also less likely to receive preemptive transplantation than White and non-Hispanic counterparts. This inequity is attributable in part to the significant difference between pre-ESKD access to care; both Whites and non-Hispanics are more likely to receive nephrology care before ESKD onset than Black and Hispanic patients [41,42,45].
Similarly, according to a review of 74,194 patients, White male patients were most likely to receive HD earlier with autogenous AV fistula and spend the least amount of time receiving HD via CVC. Female patients spent 18 more days on average receiving HD via CVC than male patients; Blacks and Hispanics spent 36 and 18 more days, respectively, on HD through a CVC than Whites. Black female patients fared worst in the study, with an average of 45 extra days of HD via CVC compared with White male patients [55,56]. Black patients are also less likely to undergo in-home dialysis than White patients, although the gap has narrowed over recent years [56].
Disparities are also evident in the pediatric CKD population, where Black children were more likely than White children to be put on dialysis (57.3% v 40.5%) and less likely to receive a kidney transplant (20.2% v 10%). Similarly, Hispanic children were less likely than non-Hispanic children to receive a transplant at or before ESKD onset (12.0% v 20.2%). Overall median waiting times for kidney transplant were shortest for Asian children (20.3 months) followed by White children (23.3 months), other races (34 months), and Blacks (35.2 months). Furthermore, among childhood renal transplant recipients, Black surviving adults had the lowest percentage of life spent with a functioning index graft (57.7%) compared with all other races (75.9% to 79%) [18].
10. Impact of COVID-19 on ESKD patients
The COVID-19 pandemic disproportionately affected ESKD patients, who are at high risk of COVID-related morbidity and mortality due to ESKD-associated cardiovascular disease as well as immunosuppression in transplant recipients. During the first major COVID-19 peak in the United States from March through July 2020, there were 11,200 Medicare patients undergoing dialysis hospitalized with COVID-19. Actual COVD-19–related admissions may be even higher than this number, as there was a 50% increase in upper respiratory infection admissions among ESKD patients during this time period, potentially reflecting patients who were under investigation for COVID-19. Notably, PD patients were hospitalized at a rate 3 to 4 times lower than HD patients, reflecting the social distancing and avoidance of health care facility exposure that is possible with dialysis at home [57].
Geographic distribution of COVID-19 hospitalizations of ESKD patients mirrored national case numbers, with the highest number of hospitalizations during that time in New York, New Jersey, Washington, DC, and Arizona. Similar to national COVID-19 trends, Black and Hispanic patients with ESKD were hospitalized at a rate over two times higher than White patients. During peak periods of viral spread, mortality rates of ESKD dialysis patients increased by 37% compared with the same time period in previous years and mortality increased by 61% for patients who had received transplants [57].
11. Summary of ESKD in 2021 and beyond
CKD and ESKD are life-shortening, life-altering diseases that inflict a great cost to patients and the health care system overall. Yet, a majority of patients still do not receive adequate pre-ESKD care, particularly in minority populations. Treatment improvements have led to decreased rates of CKD progression, hospitalization, and mortality in ESKD patients. However, a sustained effort is required to shift the paradigm from reactive to proactive care. Health care systems must institute protocolized action plans and a team-based approach to ensure that patients with CKD receive optimal care. This includes close primary care follow-up, early nephrology consultation, and ongoing surgical involvement for creation and maintenance of dialysis access, as well as transplant evaluation. This multidisciplinary care team may also include interventional specialties, such as radiology and cardiology, particularly with the introduction of novel percutaneous access technologies [58]. Proactive treatment strategies must also include measures to address the glaring disparities in outcomes for minority populations, which begin with improving access to care.
An ongoing and critical shift in ESKD treatment is toward patient-centered care. The 2019 KDOQI Vascular Access Clinical Practice Guidelines center on the ESKD “Life Plan.” Under this new guidance, patients will develop an individualized RRT and vascular access plan in consultation with family and the multidisciplinary physician team based on his or her health, anticipated quality of life, and goals of care. Emphasis on the “Life Plan,” in addition to increased adoption of PROs will enhance the patient’s role in their care, thereby allowing physicians and patients to better collaborate and further improve ESKD treatment.
REFERENCES
- [1].The Kidney Disease: Improving Global Outcomes (KDIGO) 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease (CKD). Available at: https://kdigo.org/wp-content/uploads/2017/02/KDIGO_2012_CKD_GL.pdf. Accessed December 15, 2020.
- [2].US Renal Data System. CKD in the general population. In: 2020 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Volume 1, Chapter 1. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2020. [Google Scholar]
- [3].Said S, Hernandez GT. The link between chronic kidney disease and cardiovascular disease. J Nephropathol 2014;3:99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Centers for Disease Control and Prevention. Age-adjusted prevalence of CKD stages 1–4 by gender 1999–2012. Chronic Kidney Disease (CKD) Surveillance Project. Available at: https://nccd.cdc.gov. Accessed December 15, 2020. [Google Scholar]
- [5].US Renal Data System. End stage renal disease: incidence, prevalence, patient characteristics, and treatment modalities. In: 2020 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Volume 2, Chapter 1. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2020. [Google Scholar]
- [6].Kopyt N. Chronic kidney disease: the new silent killer. J Am Osteopath Assoc 2006;106:133–6. [PubMed] [Google Scholar]
- [7].Anderson S, Halter JB, Hazzard WR, et al. Prediction, progression, and outcomes of chronic kidney disease in older adults. J Am Soc Nephrol 2009;20:1199–209. [DOI] [PubMed] [Google Scholar]
- [8].Foley R, Murray A, Li S, et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol 2005;16:489–95. [DOI] [PubMed] [Google Scholar]
- [9].Xue J, Eggers P, Agodoa LY, et al. Longitudinal study of racial and ethnic differences in developing end-stage renal disease among aged Medicare beneficiaries. J Am Soc Nephrol 2007;18:1299–306. [DOI] [PubMed] [Google Scholar]
- [10].National Institute of Diabetes and Digestive and Kidney Diseases. kidney disease statistics for the United States. Available at: https://www.niddk.nih.gov/health-information/health-statistics/kidney-disease. Accessed December 15, 2020.
- [11].US Renal Data System. End stage renal disease: hospitalization. In: 2020 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Volume 2, Chapter 4. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2020. [Google Scholar]
- [12].Axelrod DA, Schnitzler MA, Xiao H, et al. An economic assessment of contemporary kidney transplant practice. Am J Transplant 2018;18:1168–76. [DOI] [PubMed] [Google Scholar]
- [13].US Renal Data System. End Stage renal disease: cardiovascular disease in patients with ESKD. In: 2020 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Volume 2, Chapter 8. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2020. [Google Scholar]
- [14].Cozzolino M, Mangano M, Stucchi A, et al. Cardiovascular disease in dialysis patients. Nephrol Dial Transplant 2018;33:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Xu JQ, Murphy SL, Kochanek KD, et al. Deaths: final data for 2013. Available at: www.cdc.gov. Published February 16, 2016. Accessed December 15, 2020.
- [16].US Renal Data System. End stage renal disease: mortality. In: 2020 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Volume 2, Chapter 5. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2020. [Google Scholar]
- [17].US Department of Health and Human Services. Organ Procurement and Transplantation Network National Data. Available at: https://optn.transplant.hrsa.gov/data/view-data-reports/national-data. Accessed December 7, 2020.
- [18].US Renal Data System. End stage renal disease: ESKD among children and adolescents. In: 2020 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Volume 2, Chapter 7. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2020. [Google Scholar]
- [19].Golestaneh L, Alvarez PJ, Reaven NL, et al. All-cause costs increase exponentially with increased chronic kidney disease stage. Am J Manag Care 2017;23(suppl):S163–72. [PubMed] [Google Scholar]
- [20].US Renal Data System. End stage renal disease: healthcare expenditures for persons with ESKD. In: 2020 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Volume 2, Chapter 9. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2020. [Google Scholar]
- [21].Voelker R Cost of transplant vs dialysis. JAMA 1999;281:2227. [Google Scholar]
- [22].Vassalotti JA, Jennings WC, Beathard GA, et al. Fistula first breakthrough initiative: targeting catheter last in fistula first. Semin Dial 2012;25:303–10. [DOI] [PubMed] [Google Scholar]
- [23].Arhuidese IJ, Orandi BJ, Nejim B, et al. Utilization, patency, and complications associated with vascular access for hemodialysis in the United States. J Vasc Surg 2018;68: 1166–74. [DOI] [PubMed] [Google Scholar]
- [24].Kim HY, Bae EH, Ma SK, et al. Association between initial vascular access and survival in hemodialysis according to age. Korean J Intern Med 2019;34:867–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Perl J, Wald R, McFarlane P, et al. Hemodialysis vascular access modifies the association between dialysis modality and survival. J Am Soc Nephrol 2011;22:1113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].ESKD National Coordinating Center. Fistula first. Available at: https://esrdncc.org/en/fistula-first-catheter-last. Accessed December 15, 2020.
- [27].Lynch JR, Mohan S, McClellan WM. Achieving the goal: results from the fistula first breakthrough initiative. Curr Opin Nephrol Hypertens 2011;20:583–92. [DOI] [PubMed] [Google Scholar]
- [28].Lee T, Thamer M, Zhang Q, et al. Vascular access type and clinical outcomes among elderly patients on hemodialysis. Clin J Am Soc Nephrol 2017;12:1823–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ravani P, Quinn R, Oliver M, et al. Examining the association between hemodialysis access type and mortality: the role of access complications. Clin J Am Soc Nephrol 2017;12: 955–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Almasri J, Alsawas M, Mainou M, et al. Outcomes of vascular access for hemodialysis: a systematic review and meta-analysis. J Vasc Surg 2016;64:236–43. [DOI] [PubMed] [Google Scholar]
- [31].Chan MR, Young HN, Becker YT, et al. Obesity as a predictor of vascular access outcomes: analysis of the USRDS DMMS Wave II study. Semin Dial 2008;21:274–9. [DOI] [PubMed] [Google Scholar]
- [32].Chan MR, Sanchez RJ, Young HN, et al. Vascular access outcomes in the elderly hemodialysis population: a USRDS study. Semin Dial 2007;20:606–10. [DOI] [PubMed] [Google Scholar]
- [33].Shahinian VB, Zhang X, Tilea AM, et al. Surgeon characteristics and dialysis vascular access outcomes in the united states: a retrospective cohort study. Am J Kidney Dis 2020;75:158–66. [DOI] [PubMed] [Google Scholar]
- [34].Al-Jaishi A, Oliver MJ, Thomas SM, et al. Patency rates of the arteriovenous fistula for hemodialysis: a systematic review and meta-analysis. Am J Kidney Dis 2014;63:464–78. [DOI] [PubMed] [Google Scholar]
- [35].Weale AR, Bevis P, Neary WD, et al. Radiocephalic and brachiocephalic arteriovenous fistula outcomes in the elderly. J Vasc Surg 2008;47:144–50. [DOI] [PubMed] [Google Scholar]
- [36].Al Shakarchi J, Khawaja A, Cassidy D, et al. Efficacy of the ulnar-basilic arteriovenous fistula for hemodialysis: a systematic review. Ann Vasc Surg 2016;32:1–4. [DOI] [PubMed] [Google Scholar]
- [37].Kouvelos GN, Spanos K, Antoniou GA, et al. Balloon angioplasty versus stenting for the treatment of failing arteriovenous grafts: a meta-analysis. Eur J Vasc Endovasc Surg 2018;55:249–56. [DOI] [PubMed] [Google Scholar]
- [38].Woods JD, Turenne MN, Strawderman RL, et al. Vascular access survival among incident hemodialysis patients in the United States. Am J Kidney Dis 1997;30:50–7. [DOI] [PubMed] [Google Scholar]
- [39].Konner K. Primary vascular access in diabetic patients: an audit. Nephrol Dial Transplant 2000;15:1317–25. [DOI] [PubMed] [Google Scholar]
- [40].Abecassis M, Bartlett ST, Collins AJ, et al. Kidney transplantation as primary therapy for end-stage renal disease: a National Kidney Foundation/Kidney Disease Outcomes Quality Initiative conference. Clin J Am Soc Nephrol 2008;3:471–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Milovanov YS, Dobrosmyslov IA, Milovanova SY, et al. Quality of life of chronic kidney disease patients on renal replacement therapy. Ter Arkh 2018;90(6):89–91. [DOI] [PubMed] [Google Scholar]
- [42].Health Resources and Services Administration. Organ Donation Statistics. Available at: https://www.organdonor.gov/statistics-stories/statistics.html. Accessed November 15, 2020.
- [43].US Department of Health and Human Services. Organ Procurement and Transplanation Network data. Available at: https://optn.transplant.hrsa.gov/data/. Accessed November 15, 2020.
- [44].Meier-Kriesche HU, Port FK, Ojo AO, et al. Effect of waiting time on renal transplant outcome. Kidney Int 2000;58:1311–7. [DOI] [PubMed] [Google Scholar]
- [45].US Renal Data System. End stage renal disease: transplantation. In: 2020 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Volume 2, Chapter 6. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2020. [Google Scholar]
- [46].Dąbrowska-Bender M, Dykowska G, Żuk W, et al. The impact on quality of life of dialysis patients with renal insufficiency. Patient Prefer Adherence 2018;12:577–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Al Shakarchi J, Day C, Inston N. Vascular access for home haemodialysis. J Vasc Access 2018;19:593–5. [DOI] [PubMed] [Google Scholar]
- [48].Viecelli AK, O’Lone E, Sautenet B, et al. Vascular access outcomes reported in maintenance hemodialysis trials: a systematic review. Am J Kidney Dis 2018;71:382–91. [DOI] [PubMed] [Google Scholar]
- [49].Porter ME, Larsson S, Lee TH. Standardizing patient outcomes measurement. N Engl J Med 2016;374:504–6. [DOI] [PubMed] [Google Scholar]
- [50].Basch E, Deal AM, Dueck AC, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA 2017;318:197–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Verberne WR, Das-Gupta Z, Allegretti AS, et al. Development of an international standard set of value-based outcome measures for patients with chronic kidney disease: a report of the International Consortium for Health Outcomes Measurement (ICHOM) CKD working group. Am J Kidney Dis 2019;73:372–84. [DOI] [PubMed] [Google Scholar]
- [52].van der Willik EM, Meuleman Y, Prantl K, et al. Patient-re-ported outcome measures: selection of a valid questionnaire for routine symptom assessment in patients with advanced chronic kidney disease—a four-phase mixed methods study. BMC Nephrol 2019;20:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Nair D, Wilson FP. Patient-reported outcome measures for adults with kidney disease: current measures, ongoing initiatives, and future opportunities for incorporation into patient-centered kidney care. Am J Kidney Dis 2019;74:791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Anderson NE, Calvert M, Cockwell P, et al. The use of patient-reported outcomes in patients treated with maintenance hemodialysis: a perspective. Am J Kidney Dis 2019;74:399–406. [DOI] [PubMed] [Google Scholar]
- [55].Shah S, Leonard AC, Meganathan K, et al. Gender and racial disparities in initial hemodialysis access and outcomes in incident end-stage renal disease patients. Am J Nephrol 2018;48:4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Shen JI, Erickson KF, Chen L, et al. Expanded prospective payment system and use of and outcomes with home dialysis by race and ethnicity in the united states. Clin J Am Soc Nephrol 2019;14:1200–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].US Renal Data System. COVID-19 supplement. In: 2020 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Volume 3, Chapter 1. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2020. [Google Scholar]
- [58].Mallios A, Bourquelot P, Franco G, et al. Midterm results of percutaneous arteriovenous fistula creation with the Ellipsys Vascular Access System, technical recommendations, and an algorithm for maintenance. J Vasc Surg 2020;72: 2097–106. [DOI] [PubMed] [Google Scholar]


