Abstract
Patients with colon cancer remain largely refractory to current immunotherapeutic strategies. This is, in part, due to the overexpression of the immune checkpoint protein indoleamine 2,3-dioxygenase 1 (IDO). IDO is an important enzyme contributing to tumor-mediated immunosuppression and also correlates with poor prognosis in colon cancer patients. The aim of this study was to assess the therapeutic efficacy of attenuated Salmonella typhimurium delivering an shRNA plasmid targeting IDO (shIDO-ST) in two mouse models of colorectal cancer. In vitro, the CT26 and MC38 murine colon cancer cell lines were shown to upregulate IDO expression following stimulation with interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α). Transfection of both cell lines with shIDO plasmid reduced IDO protein expression and function. In vivo, shIDO-ST treatment significantly delayed CT26 and MC38 tumor progression compared to mice treated with scrambled shRNA control (shScr-ST) or the clinically-tested IDO inhibitor epacadostat. Increased tumor infiltration of neutrophils was found to be the primary immune cell population associated with shIDO-ST treatment, suggesting robust activation of innate immunity. Although increased tumor expression of IDO is associated with resistance to antibody therapy against programed cell death-1 (anti-PD1), co-administration of anti-PD1 with shIDO-ST did not provide additional tumor growth control in either model of colorectal cancer. Altogether, we demonstrate that treatment with shIDO-ST markedly delays tumor growth in two immunocompetent colorectal mouse models and this appears to be a superior therapeutic strategy compared to epacadostat or blocking anti-PD1 antibody therapy in colon cancer.
INTRODUCTION
In 2018, over 140,000 people in the United States will be diagnosed with colorectal cancer and approximately 50,000 will die due to the lack of effective treatment options in the locally advanced or metastatic settings.1, 2 The standard of care for metastatic colorectal cancer (mCRC) remains limited to the use of combination chemotherapy (fluorouracil, leucovorin, and irinotecan (FOLFIRI), infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX), and capecitabine plus oxaliplatin (XELOX), or triplet combination fluorouracil, leucovorin, oxaliplatin and irinotecan (FOLFOXIRI) with angiogenesis-targeting and epidermal growth factor receptor (EGFR)-targeting agents.1 Disease mortality for mCRC remains significant, with a median five-year survival of 13.5% and a median overall survival of three years.2, 3 Immunotherapy has greatly improved outcomes for several solid tumor types; however, in colon cancer, checkpoint inhibitors have thus far only prolonged survival in a small percentage of patients with DNA mismatch repair deficiencies.4 In other solid tumor types, there are additional subsets of patients who benefit from immunotherapy when checkpoint inhibitors are combined with other local or systemic treatments.5 It is clear that tumor-mediated immunosuppression is an important contributor to colon cancer progression and the development of novel immune-mediated approaches will be a critical component to improve patient outcomes.4
The indoleamine 2,3 dioxygenase (IDO) enzyme, which catalyzes the rate-limiting step in tryptophan metabolism to produce kynurenine, is over-expressed in many malignancies, including colorectal cancer.6 Increased expression of IDO correlates with poor prognosis in patients with colorectal cancer.7 IDO overexpression leads to increased concentration of kynurenine metabolites, which induce cell cycle arrest of T cells and increase the frequency of regulatory T cells, making IDO a desirable target for therapy. Several IDO inhibitors, such as the small molecule inhibitor epacadostat, are being actively evaluated in combination with other agents in solid tumors.8 Epacadostat as a single agent or combined with PD1 blockade therapy however, failed to improve clinical outcomes in a clinical trial in metastatic melanoma patients and whether it or other IDO inhibitors adequately inhibited IDO enzymatic activity in solid tumors is unclear.9 IDO expression is up-regulated with exposure to interferon-gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α).10 Gene expression of IDO strongly correlates with expression of PD1 in many solid tumors.11 IDO and PD1 both share an interferon response element that leads to their expression in the tumor immune microenvironment.11 In colon cancer, IDO is up-regulated in tumors that develop in patients with predisposing germ line mutations, somatic mutations or colitis-induced mutations, making it a common target for colon cancer of various etiologies.12 Our laboratory has established that intratumoral IDO levels can be effectively reduced using small hairpin (sh)RNA targeting IDO delivered via an attenuated Salmonella typhimurium (ST).13, 14 This approach elicits a strong innate immune response and may enhance anti-tumor T cell function and improve tumor surveillance for long-term tumor regression.14
ST-based cancer therapies have been repeatedly shown to colonize and regress syngeneic murine tumors and human xenografts.15 Tumor colonization by facultative bacteria is attributable to a hypoxic tumor core (particularly in tumors >2 mm3 in volume) and high availability of nutrients due to the presence of necrosis.16 The ST strain VNP20009 was derived via mutagenesis and selected for hyperinvasion of cancer cells in vitro.17 VNP20009 is modified to depend on purine supplementation (purM-), and its virulence was attenuated 10,000-fold thorough a lipid A modification (msbB-) to avoid LPS-mediated sepsis.18 VNP20009 has been used extensively by our laboratory and others in melanoma, pancreatic cancer and mCRC tumor murine models and has been shown to consistently colonize tumors versus normal tissues, making it an optimal delivery vector for novel genetic intervention.13, 19 VNP20009 safety was established in a Phase I clinical trial of metastatic melanoma and was observed to colonize tumors but did not promote tumor regression alone. VNP20009 can therefore be used as a vector to deliver anticancer enzymes, anticancer agents, tumor specific antigens, and oncogene silencing RNA.15 With the aim of utilizing VNP20009 as a delivery vector and IDO as an ideal target to reverse tumor-mediated immunosuppression, we developed a recombinant ST delivering inhibitory shRNA against IDO (shIDO-ST). This novel therapy utilizing ST as a modified vector for a plasmid harboring a short hairpin RNA targeting IDO (shIDO) was used to demonstrate pancreatic and melanoma tumor regression.14, 20 The aim of this work is to demonstrate that shIDO-ST attenuates colorectal cancer growth and activates innate immunity in an immunocompetent mouse model.
MATERIALS AND METHODS
Tumor cell line and animal model
The CT26 and MC38 murine colon adenocarcinoma cell lines were obtained from the American Type Culture Collection (CRL-2638) and Kerafast (ENH204), respectively. The cells were grown at 37°C, 5% CO2 atmosphere in complete endotoxin-free DMEM media containing high glucose and glutamine supplemented with 10% FBS and 1% penicillin and streptomycin. Female BALB/c mice and C57BL6 (6 weeks old) were purchased from Jackson Lab and maintained under specific pathogen-free conditions. Animals were handled according to Institutional Animal Care and Use Committee (IACUC) guidelines under an approved protocol #16067.
Bacteria strains
pLKO.1-puro lentiviral vector containing the 21-mer shRNA sense sequence CGTCTCTCTATTGGTGGAAAT (shIDO), pEQshIDO, or scrambled shRNA (shScr) sequence (Sigma) was transformed into Salmonella typhimurium strain VNP20009 (ATCC#202165) by electroporation as previously described.13 Transformed S. typhimurium (shIDO-ST or shScr-ST) were grown to late log phase [optical density (OD) from 0.7 to 0.8], washed and resuspended in 50μl of sterile PBS immediately before administration to mice. To calculate colony-forming units (CFU)/mL, an OD of 1 equal to 109 CFU/mL was applied in this study.
Tumor challenge and therapy
CT26 or MC38 cells (2.5 × 105) were suspended in PBS and implanted subcutaneously into the right thigh of each mouse (CT26 in Balb/c and MC38 in C57BL6; n=6 for each group). Twelve days after tumor inoculation, when the tumor sizes reached 80-100 mm3, the mice were randomized to their respective groups and received three consecutive daily retro-orbital injections of Salmonella typhimurium VNP2009 shIDO-ST or shScr-ST (5.0 × 106 CFU). The IDO inhibitor Epacadostat or INCB23843 (Incyte Corporation) was dissolved in 3% N, N-Dimethylacetamide and 10% (2-Hydroxypropyl) β-Cyclodextrin and administered by oral gavage at 200 mg/kg every day for one week.
In order to determine the additive effect of anti-mouse-PD1 (Armenian hamster anti-mouse PD1, Clone J 43)21 with shIDO-ST therapy, 12 days after tumor implantation the mice were randomized to their respective groups (n=6 per group) and received two doses (i.p.) of 200 μg/mouse of Armenian hamster IgG isotype control (Molecular Innovations, HT-GF) or anti-PD1with 5 day intervals.
Tumor volume (mm3) was measured every 3 days with a caliper until the tumor volume exceeded 1000mm3 or any experimental endpoint, as pre-determined in the IACUC protocol, was reached (V=1/2 × Length × Width × Depth).
Neutrophil depletion
Monoclonal antibody against Gr1 (RB6-8C5) was purified from the supernatant of hybridoma cultures using HiTrap Protein G HP columns (GE Healthcare). To deplete neutrophils, anti-Gr1 (60 mg/mouse) were administered i.p. 2 days after the third bacterial treatment and then given every 3 days until the end of the experiment (up to 9 doses). Depletion by 80% was confirmed 24 h after anti-Gr1 administration by flow cytometry using peripheral blood mononuclear cells (PBMC) (data not shown).
Detection of IDO expression by Western Blot
CT26 or MC38 were seeded onto 6 well-plates at a density of 5×105 cells/well. The next day, cells were transfected with shIDO or shScr (1μg/well) using Lipofectamine 3000 (Thermofisher). The pLKO.1-puro lentiviral vector containing the 21-mer shRNA sense sequence CGTCTCTCTATTGGTGGAAAT (shIDO#9), pEQshIDO, or scrambled shRNA (shScr) sequence (Sigma) were used. Twenty-four hours after transfection, cells were stimulated with murine IFN-γ (100ng/ml, Peprotech) and/or TNF-α (10ng/ml, Peprotech). Forty-eight hours after IFN-γ treatment, cells were lysed in protein extraction buffer (150 mM NaCl, 10 mM Tris, 1mM EDTA, 1% NP-40, 1mM EGTA, 50 mM NaF containing protease and phosphatase inhibitor cocktail), subjected to SDS-PAGE, and subsequently transferred to PVDF membrane (Invitrogen). The membranes were probed with mouse anti-mouse IDO (05-840, Millipore; 1:500 dilution) or rabbit anti-mouse β-actin (4970, Cell Signaling; 1:1000 dilution) antibodies, followed by goat anti-mouse polyvalent immunoglobulins (IgG, IgA, IgM) peroxidase conjugate (A0412, Sigma; 1:2000 dilution) or goat anti-rabbit IgG (whole molecule) peroxidase conjugate (A6154, Sigma; 1:2000 dilution), respectively. Bioluminescence was catalyzed using a Quick Spray Chemiluminescent HRP Antibody Detection Reagent (Thomas Scientific, E2400), and bands were detected in a luminescent image analyser PXi (Syngene).
IDO activity assay
Cells were seeded in triplicate at a density of 6.25 × 104 cells/well onto 48 well-plates and stimulated with IFN-γ (100ng/ml) and/or TNF-α (10ng/ml) as described above. Twenty-four hours after treatment, the cell supernatants were collected to analyze changes in the tryptophan catabolism to kynurenine. Concentration of tryptophan and kynurenine, the first downstream product resulting from the conversion of tryptophan catalyzed by IDO, were determined by reverse phase high pressure liquid chromatography (HPLC) using previously described method.11 The analysis was performed on Agilent 1260 Infinity instrument (Palo Alto, CA). Briefly, 10 μL of perchloric acid was added to 90 μL of the supernatant to precipitate proteins, and 20 μL of the clear solution was injected onto the column (125 mm× 4.0 mm; Hypersil® C-18; 5 μm particle size, Thermo Fisher Scientific). The mobile phase used was 15 mmol sodium acetate with 6% (v/v) acetonitrile and ran at 1 ml/min. Kynurenine was observed at 360 nm between 0 and 4 minutes and tryptophan at 275 nm between 4 and 6 minutes.
Preparation of single cell suspensions from tumor explants and lymph nodes
Tumors were excised at day 7 or day 14 after the first bacterial injection. Briefly, animals were euthanized and the tumor and adjacent lymph nodes were harvested. The collected tissues were washed with PBS and minced into 2–3 mm3 pieces using sterile blades. The tissue fragments were then incubated with Collagenase IV (1 mg/ml) and DNAse I (50 ug/ml) for 20 minutes at 37°C followed by an incubation on ice for 10 minutes to stop the enzymatic digestion. The digested tissue fragments were gently homogenized using a plunger from a 1 ml syringe, followed by dissociation using a 40 μm cell strainer. The dissociated cells were washed with PBS and re-suspended in complete DMEM or RPMI cell culture media.
Flow cytometry analysis
For surface staining, single cell suspensions (1×107) were prepared as described above, washed with PBS and incubated with 1μl Fixable Viability Dye eFlour 506 for 10 min on ice. Next, cells were washed with PBS and incubated with fluorescence-labeled antibodies against target cell surface molecules for 30 minutes in the dark on ice. Cells were then washed and re-suspended in fixation buffer for 30 minutes. Labeled antibodies to the following were all purchased from eBioscience and used for surface staining: CD45 (30-F11), CD4 (RM4-5), CD8 (53-6.7), B220 (RA3-6B2), F4/80 (BM8), Gr-1 (RB6-8C5), CD11b (M1/70) Ly6G (1A8). At least 10,000 events were analyzed using a FACSCelesta flow cytometer (BD), according to the manufacturer’s instructions. Doublets were excluded and live cells were used for downstream evaluation using FlowJo software (TreeStar). CD4 T cells were gated as CD45+CD4 +, CD8 T cells were gated as CD45+CD8+. B cells were gated as CD45+ B220+. Macrophages were gated as CD45+F4/80+. Neutrophils were gated as CD45+Gr1+CD11b+.
Statistics
Statistical analyses were performed using GraphpPad Prism 3 Software (San Diego, CA). P values were calculated by the students t-test or two-way ANOVA and were considered significant if P<0.05. The mean ± 1 standard deviation (SD) is displayed in the figures.
RESULTS
Stimulation of IDO expression in murine colon cancer cell lines
IDO expression is tightly regulated through IFN-γ.10 In human epithelial cells, IFN-γ-induced IDO expression can be transcriptionally enhanced by TNF-α.10 In order to assess if the murine colon cancer cell lines CT26 and MC38 were capable of expressing IDO, and to determine the optimal IDO stimulatory conditions, cells were cultured in media containing varying amounts of IFN-γ and TNF-α for 48 hours (Fig. S1). The MC38 cell line only required IFN-γ (100 ng/ml) to induce IDO protein expression. In the CT26 cell line, optimal IDO protein expression was achieved using both IFN-γ at 100 ng/ml and TNF-α at 10 ng/ml, indicating CT26 cells require both cytokines for maximal IDO expression.
ShRNA targeting IDO in murine colon adenocarcinoma cell lines decreases protein expression and activity of IDO
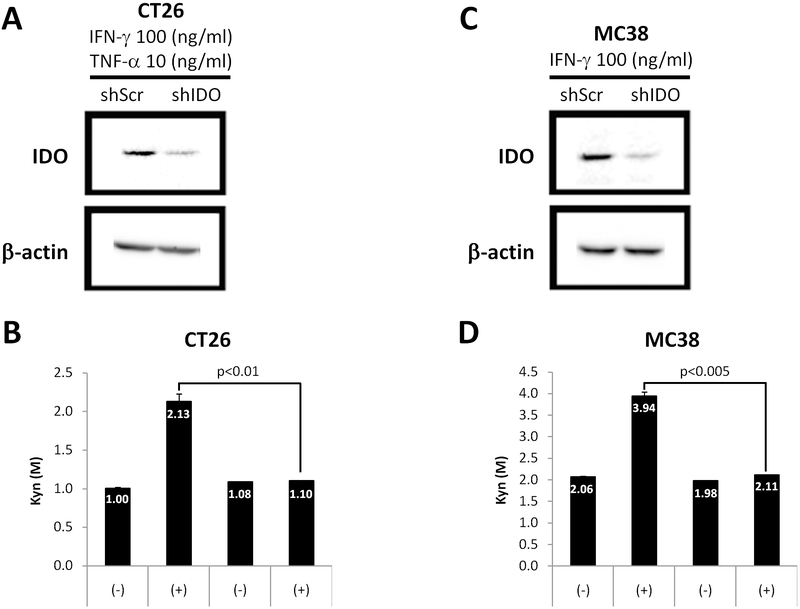
To determine if our shRNA construct targeting IDO (shIDO) is effective in silencing IDO expressed by the CT26 and MC38 cell lines, we assessed IDO expression and activity after lipofectamine transfection of shIDO into cells.14 Cells transfected with shIDO and then stimulated with IFN-γ and TNF-α showed dramatically reduced IDO protein expression compared to scrambled control (shScr) (Fig. 1A, B). Additionally, the concentration of kynurenine, a product of tryptophan catabolism by IDO, in the supernatant remained unchanged in cells transfected with shIDO, whereas those transfected with shScr showed a nearly two-fold increase after cytokine stimulation (Fig. 1C, D). These studies demonstrate that the shIDO construct is capable of inhibiting the expression of IDO, thus preventing the conversion of tryptophan to kynurenine following cytokine stimulation.
Fig. 1.
Silencing IDO expression and activity by shRNA in vitro CT26 (A, B) and MC38 (C, D) cells were transfected with shScr or shIDO plasmids. After 24hrs of transfection, cells were stimulated with 100ng/ml IFN-γ and 10ng/ml TNF-α (CT26) or IFN-γ alone (MC38). For IDO expression, Western blot analysis were performed (A, C). β-actin was used as a loading control. For IDO activity, cell supernatants were collected 16hr post-stimulation and the ratio of Kynurenin/Tryptophan (Kyn/Trp) were measured by high-performance liquid chromatography (HPLC) (B, D). Data are the means of 3 separate experiments ± SD, P values are indicated where appropriate.
Systemic shIDO-ST treatment inhibits tumor growth in an immunocompetent subcutaneous mouse model of colon cancer
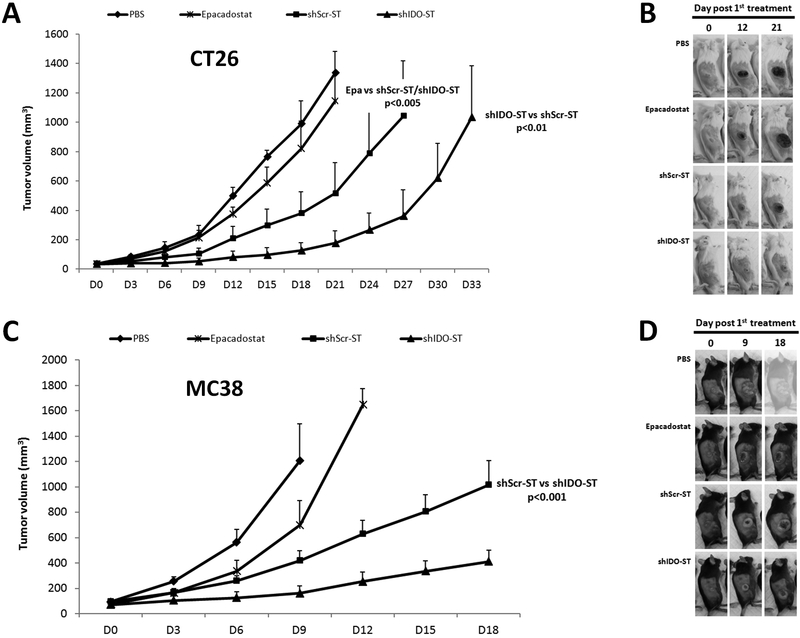
Next, we determined the therapeutic efficacy of shIDO-ST in subcutaneous (s.c.) tumor models of CT26 and MC38. When s.c. tumors reached ≥100 mm3, mice were treated with shIDO-ST, shScr-ST control, epacadostat or PBS (Fig. 2). Although epacadostat treatment marginally delayed tumor growth, statistical significance was not reached in either tumor model when compared to PBS-treated mice (Fig. 2). Similar to early preclinical trials using the unmodified VNP20009 strains, treatment with shScr-ST alone attenuated tumor progression compared to PBS controls in both cell lines.15, 22 However, shIDO-ST was significantly more effective than epacadostat and control treatments in both tumor models in halting tumor progression. In contrast to other treatment groups, shIDO-ST treatment group delayed exponential tumor growth until day 18 or 21 in both mCRC models, resulting in a clear improvement in overall survival. This finding was replicated in over 5 independent experiments using: 1) single flank vs. dual flank tumor models; 2) increasingly larger tumors and 3) lower doses of shIDO-ST treatment (data not shown). These findings demonstrate that further modification of the original VNP20009 strain with shIDO greatly improves its anti-tumor growth capabilities.
Fig. 2.
In vivo assessment of shIDO-ST treatment in CT26 and MC38 tumor-bearing mice. Balb/c or C57BL6 mice (n = 3-4/group) were subcutaneously injected with CT26 cells (A, B) or MC38 (C, D), respectively and treated with PBS shScr-ST, shIDO-ST Epacadostat or vehicles (3% N,N-Dimethylacetamide and 10% (2-Hydroxypropyl) β-Cyclodextrin). (A, C) Tumor volume was measured every 3 days. (B, D) Images of the tumor graft in MC38 and CT26-bearing mice. The shape of the tumor was noted before (0 dpi) and after treatment with PBS or bacteria [(12 and 21 dpi, (B) or 9 and 18 dpi, (D)]. P value at day 21 (A) or at day 18 (C). Data are the means of the experiment ± SD and the P values are indicated above.
Stimulation of programed cell death ligand-1 (PD-L1) expression in murine CT26 and MC38 cells by IFN-γ
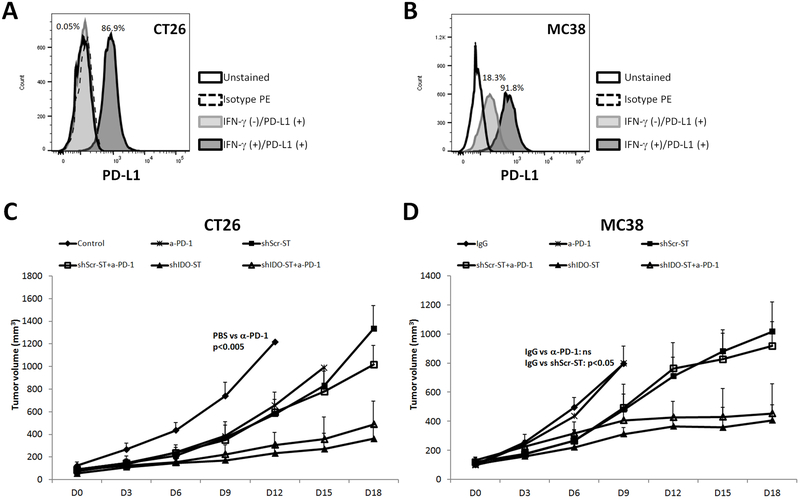
PD-L1 is important for T cell inhibition and is differentially expressed on the surface of endothelial, epithelial and antigen presenting cells (APCs) with immunosuppressive properties.23 PD1 can be found on the surface of activated monocytes, NK cells, T and B lymphocytes. After binding to its ligand B7-H1 or BH-H2/B7-DC (also known as PD-L1 or PD-L2), PD1 can attenuate immune responses resulting in T cell hyporesponsiveness.24, 25 PD-L1 expression has been correlated to response to anti-PD1 in clinical trials.26 Since both IDO and PD1 have interferon response elements and are often up-regulated in the same tumor microenvironment, we sought to evaluate whether IDO inhibition would sensitize the CT26 and MC38 tumors to immune checkpoint blockade therapy using anti-PD1 in vivo.11 In order to evaluate the potential sensitivity of CT26 and MC38 colon cancer cell lines to anti-PD1 blockade therapy, we analyzed the expression of the PD1 ligand biomarker PD-L1 in IFN-γ-stimulated cells growing in culture. In both cell lines, stimulation with IFN-γ resulted in dramatic up-regulation of PD-L1 expression; up to 87% in CT26 cells and up to 92% in MC38 cells (Fig. 3A, B). This demonstrates that both cell lines upregulate expression of both IDO and PD-L1 in response to IFN- γ. Thus, we reasoned that combination of shIDO-ST with anti-PD1 therapy may contribute to increase sensitivity of colon cancer cells to checkpoint inhibition therapy and greater tumor growth control.
Fig. 3.
Addition of Anti-PD-1to shIDO-ST therapy in CT26 and MC38 tumor bearing mice. (A, B) Flow cytometry histograms show PD-L1 expression of CT26 (left plot) or MC38 cells (r) after 24hr of stimulation with 100ng/ml IFN-γ and 10ng/ml TNF-α (CT26) or IFN-γ alone (MC38). Balb/c or C57BL6 mice (n = 4-5/group) were subcutaneously injected with CT26 cells (C) or MC38 (D), respectively. When the tumor volume reached 80 mm3, mice were administered two doses (i.p.) of 200 μg/mouse of hamster IgG isotype control or α-PD-1 with 5-day intervals. 24hrs later, mice received three single daily doses of an retro-orbital injection of PBS, shScr-ST and shIDO-ST (C, D). Data are the means the experiments ± SD, P values are indicated where appropriate.
Anti-PD1 treatment does not enhance the growth mitigation of shIDO-ST
The findings above demonstrate that both CT26 and MC38 cell lines can express both IDO and PD-L1 following IFN-γ stimulation. Therefore targeting both of these immunosuppressive molecules may generate additive tumor growth attenuation. Mice bearing CT26 or MC38 tumors were treated with anti-PD-1and shIDO-ST in order to assess the durability of tumor growth control. In the CT26 cell lines, treatment with anti-PD1 delayed tumor growth similar to shScr-ST, however, shIDO-ST was more effective than anti-PD1 alone (Fig. 3C). Anti-PD1 with shIDO-ST was not any more effective or durable in controlling CT26 tumors than shIDO-ST alone. In mice bearing MC38 tumors, anti-PD1 treatment did not delay tumor growth compared to IgG controls (Fig. 3D). The addition of anti-PD1 also did not improve tumor growth control compared to shIDO-ST alone. This finding is consistent with clinical results showing that checkpoint inhibition therapy is ineffective in the majority of colon cancer patients with DNA-repair proficient tumors.17 These results indicate that tumor-derived IDO is not a major factor in promoting resistance to checkpoint inhibition therapy in the CT26 and MC38 colon cancer models.
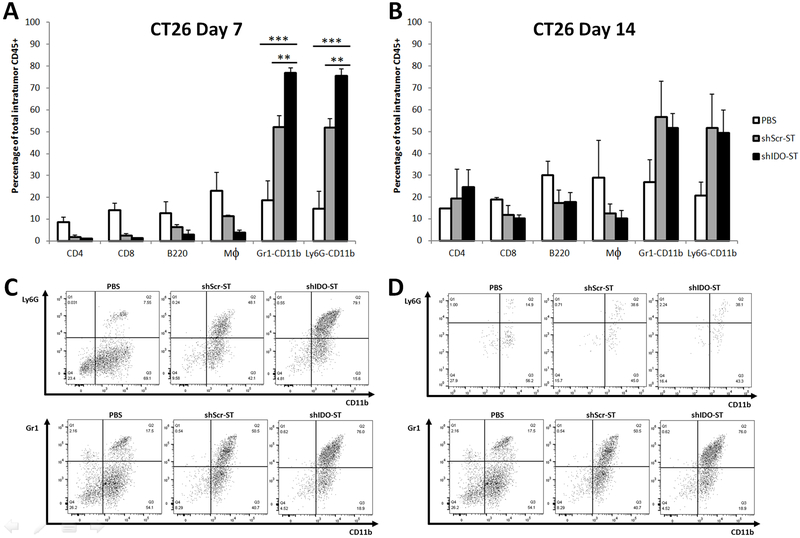
ShIDO-ST treatment induces an influx of neutrophils
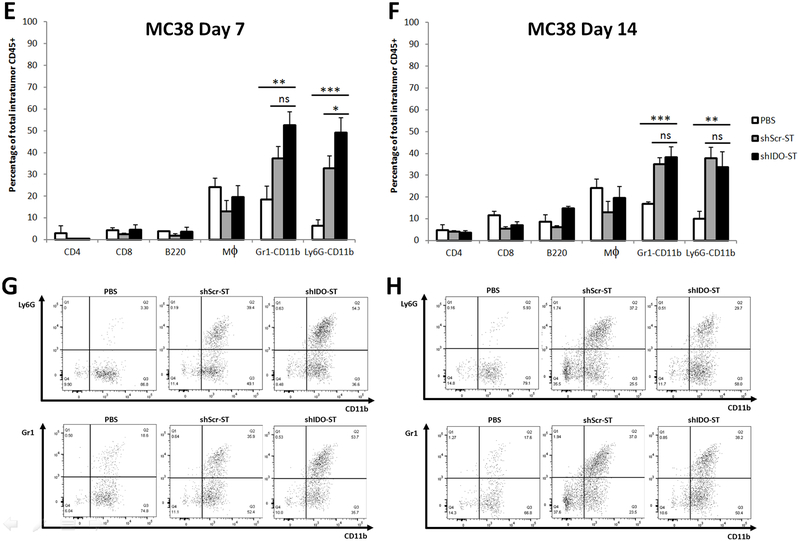
In order to evaluate the role of infiltrating immune cells into tumors after shIDO-ST treatment, tumors were assessed for various immune populations at day 7 after treatment. In the CT26 tumor model, there was a significant increase in the neutrophil population in the shIDO-ST vs. shScr-ST treatment group, as measured by Gr1+CD11b+ (shIDO-ST 77% vs shScr-ST 52%) and Ly6G+CD11b+ cells (shIDO-ST 76% vs shScr-ST 52%) (Fig. 4A, B). We also observed an increase of tumor-infiltrating neutrophils in the shScr-ST group compared to PBS controls, however this percentage was significantly lower than observed in the shIDO-ST group (Fig. 4a-d). Similar to the CT26 tumor model, in the MC38 tumor model, the animals treated with shIDO-ST presented with a higher percentage of tumor-infiltrating neutrophils than any other immune population (Gr1+CD11b: shIDO-ST 53% vs shScr-ST 37%; and Ly6G+CD11b+: shIDO-ST 49% vs shScr-ST 33%) (Fig. 4E-H). Overall, shIDO-ST-treated mice had a significantly greater rise in intratumoral neutrophils, in both tumor models, in comparison to all other treated groups. These findings indicate that the shIDO component greatly enhances the recruitment of neutrophils as soon as 7 days after treatment compared to shScr. The elevated numbers of intratumoral neutrophils in the shIDO-ST vs. shScr-ST-treated group appears to be transient, as by day 14 after treatment, the differential of neutrophil concentrations in the shScr-ST versus the shIDO-ST groups was no longer significantly different (Fig. 4b, f). However, in both cell lines, even though neutrophil populations had declined at day 14 after treatment, overall percentages were still markedly higher than the other immune populations in the ST-treated cohort (Fig. 4B, F).
Fig. 4.
Intratumoral immune cell population assessment after treatment with shIDO-ST. CT26 (A, B, C, D) or MC38 (E, F, G, H) subcutaneous mouse models were treated with shIDO-ST, shScr-ST or PBS as described in the legend of Fig 2. At 7dpi (A, C, E, G) and 14dpi (B, D, F, H) tumors were excised and single-cell suspensions were stained with surface cell markers CD4, CD8, B220, F4/80, Gr-1, Ly6G and CD11b. (A, B, E, F) Percentage of T cells, B cells, macrophages and neutrophils from total intratumoral CD45+ cells using flow cytometry. (C, D, G, H) Percentage of Ly6G+CD11b+ (or Gr1+CD11b+) neutrophils gated from total intratumoral CD45+ cells. Data are the means of the experiment ± SD, *P<0.01, **P<0.001compared to indicated controls.
Systemic depletion of neutrophils does not attenuate the anti-tumor effects of shIDO-ST
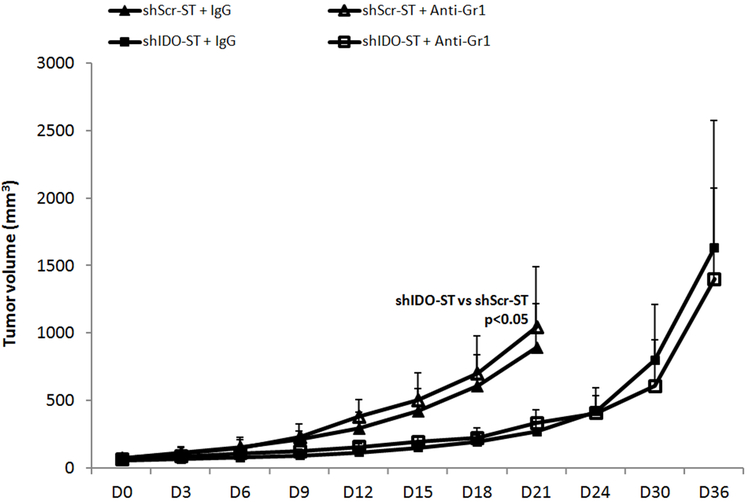
Systemic depletion of neutrophils had no apparent impact on the anti-tumor effect of shIDO-ST therapy. A consistent peripheral neutrophil depletion of at least 80% was feasible in Balb/c mice when they were treated with 60 μg of anti-Gr1 antibody every 3 days (data not shown). Neutrophil depletion had no impact on shIDO-ST mediated growth inhibition in CT26 cells. Both the shIDO-ST and the shIDO-ST/anti-Gr1 have controlled tumor growth till around day 18-21 (Fig. 5) as opposed to day 9-12 in the shScr-ST and shScr-ST/anti-Gr1 group. These results indicate that either: 1. The neutrophils are not the main mediators of the anti-tumor effects of shIDO-ST or; 2. That response to shIDO-ST therapy does not require large numbers of neutrophils, and that activation of a smaller subset of neutrophils are enough to trigger a strong antitumor response and; 3. That most likely activation of additional immune networks contributes to the growth inhibition induced by shIDO-ST treatment.
Fig. 5.
Effect of neutrophil depletion on shIDO-ST tumor growth inhibition in CT26 tumor bearing mice. Balb/c mice (n = 4-5/group) with CT26 cells received retro-orbital injection of PBS, shScr-ST, or shIDO-ST. To deplete neutrophils, anti-Gr1 or hamster IgG isotype control were administered. Tumor volume was measured every 3 days. Data are the means of the experiment ± SD, P values are indicated where appropriate.
Discussion
Colon cancer is the 3rd leading cause of cancer mortality in men and women.2 Novel strategies beyond traditional cytotoxic therapies are needed to prolong survival in metastatic disease that is not amenable to surgical resection. We demonstrate that delivery of an attenuated Salmonella that delivers shIDO-ST mitigates growth of 2 murine colon cancer cell lines in an immunocompetent model. Additionally, shIDO-ST is more effective in slowing tumor growth than Epacadostat, the small molecule inhibitor of IDO that is currently in clinical trials.
Bacteria mediated tumor therapy has long been a cancer fighting strategy in development. The balance between toxicity and anti-tumor efficacy has been in question. The evolution of genetically modified bacteria allows for tumor targeting and reduced toxicity. Unlike bacteria that are obligate anaerobes and thus dependent on necrotic cores of tumors to proliferate, Salmonella typhimurium is a facultative anaerobe with the ability to colonize both the aerobic and anaerobic components of a tumor.27 The Salmonella typhimurium strain VNP20009 is an optimal tumor targeting bacteria, as it is attenuated by the deletion of the purI and msbB genes. The purI deletion results in a need for an external source of adenine and the deletion of the msbB gene reduces the toxicity associated with lipopolysaccharide (LPS).28, 29 The msbB mutation leads to lower toxicity by reducing the induction of pro-inflammatory cytokines such as TNF-α and nitric oxide synthase.29 As it was found to mitigate tumor growth in a murine model, it was tested in a phase I study with intravenous infusion of VNP20009 in 24 patients with metastatic melanoma and one with metastatic renal cell carcinoma.19 Although there was minimal tumor colonization using the unmodified strain, there was no tumor regression. This lead to further studies utilizing VNP20009 as a vector, rather than as a singular therapeutic and to our group utilizing it to deliver an shRNA to IDO.
IDO is a well-described enzyme important in tryptophan metabolism and in the production of the metabolite kynurenine.30 The expression of IDO correlates with tumor-mediated immunosuppression.6 Upregulation of IDO expression leads to both tryptophan depletion and the formation of immunotoxic metabolites, which induces cell cycle arrest of T lymphocytes and makes these cells more sensitive to apoptosis.31, 32 IDO produced metabolites of tryptophan such as kynurenine and several of its downstream metabolites which are highly toxic to lymphocytes.33, 34 They can induce the differentiation of naïve CD4+ T cells into immunosuppressive regulatory T cells (TReg cells).35 Most human tumors constitutively express IDO.6 In colorectal cancer, IDO over-expression correlates with an increased incidence of liver metastases and reduced infiltration of CD3+ lymphocytes further suggesting that IDO can suppress tumor–reactive T cells.7 This conceptual framework led to the idea of delivering an shIDO-ST that would preferentially colonize tumors and thereby down-regulate IDO expression in the tumor microenvironment allowing robust activation of innate immunity to clear the Salmonella and potential, subsequent activation of long-lasting adaptive immunity. What was observed in these experiments is that the shIDO-ST is vastly superior than the IDO enzymatic inhibitor epacadostat and consistently allowed for more delay in tumor growth than the shScr-ST (Fig. 2). Although, the ShScr-ST delayed tumor growth in comparison to PBS controls (consistent to historic preclinical data using the unmodified VNP20009 strain), the shIDO-ST was significantly more effective. Therefore, not only is silencing IDO important in maintaining tumor control, but it also validated the use of the ST VNP20009 strain as a delivery vector.
Work in our lab has established safety and tolerability of shIDO-ST in immune competent animals13, 14. Additionally, in melanoma we have demonstrated tumor regression with shIDO-ST.13 B16 murine melanoma cells were implanted in the flanks of C57BL6 mice and treated with shIDO-ST. These tumors demonstrated growth delay with shIDO-ST treatment. In pancreatic cancer we utilized shIDO-ST in conjunction with PEG-hyaluronidase to overcome the stromal barrier and induce tumor regression in an orthotopic immunocompetent model.14 This report demonstrates response and delayed tumor progression in an immunocompetent model of colorectal cancer without the need of additional stromal modulators. The extent of desmoplasia seen in pancreatic cancer is not seen in colon cancer and therefore this work did not encorporate the use of PEG-hyaluronidase in a colon cancer model.
In the 2 cell lines evaluated, the CT26 cell line was stimulated to produce IDO by IFN-γ and TNF-α, whereas the MC38 cell line only needed IFN-γ. As both of these cytokines exist in the tumor microenvironment, this difference in expression may impact the expression of IDO. Although IDO is strictly an IFN-γ induced gene product, TNF-α can synergistically increase the transcriptional activation of the IDO gene in response to IFN-γ.10 This may explain the amplified IDO expression to these 2 cytokines in the CT26 cell line.
Both IDO and PD1 have interferon response elements in the promoter region and are also expressed concurrently11. There are several strategies in clinical trials to combine the IDO inhibitor epacadostat with immune checkpoint inhibition to augment the responses to immunotherapy. As a monotherapy and in combination with pembrolizumab, epacadostat has not succeeded in yielding more durable results than pembrolizumab alone in clinical trials.22 However, there may be a variety of reasons why this combination was not successful, such as: 1. Use of sub-optimal inhibition of IDO activity due to low dosing of epacadostat or; 2: Lack of information regarding patient’s levels of IDO expression.11 In an attempt to augment the durability of response to shIDO-ST, mice were treated with anti-PD1 in combination with shIDO-ST. This combination was not more effective than shIDO-ST alone and was consistent in tumors originated by both cell lines. Perhaps, the infiltration of the Salmonella into tumors did not adequately change the frequency of cells in the tumor microenvironment expressing PD-L1 and therefore this combination was not effective. Even at 14 days there was not an increase in relative CD8+ infiltrating T cell populations indicating perhaps this therapy is centered on engagement of innate rather than adaptive immunity.
Building on our prior work, we evaluated the changes in immune cell composition of tumors treated with PBS, shScr-ST and shIDO-ST at days 7 and 14 as well as the tumor bearing lymph nodes. At day 7 in both cell lines, there was a marked increase in intratumoral neutrophils as determined by antibody for Lys6G+ or Gr-1 in the tumors treated with shIDO-ST (Fig. 4). This is concordant with expectations, as Salmonella should concentrate in the tumor based on the ability to function as facultative anaerobes. Salmonella colonization then generates a trail of recruitment of immune cells and most commonly early on, this is comprised of innate immune populations. This change in immune populations did not persist in the tumor draining lymph nodes. Additionally, the Salmonella was cleared in other areas as would be expected. The Salmonella as previously described, travels through the systemic circulation and through the reticuloendothelial system of the spleen priming neutrophils that subsequently are drawn to the Salmonella rich environment of the tumor by various chemokines. This leads to a bystander effect whereby the PMNs in an attempt to clear the tumor of the bacteria, induce tumor cell lysis. This mechanism is amplified when the Salmonella carry shIDO. Of note, IDO inhibition in neutrophils also enhances their antibacterial properties.36, 37 Therefore silencing IDO not only leads to a larger neutrophil concentration in the tumor, but also results in a more durable reduction of tumor growth.
With the previous findings of increased intratumoral neutrophil concentration with shIDO-ST mediated tumor growth delay in pancreatic tumor models, we wanted to assess if depletion of neutrophils would result in shIDO-ST tumor growth similar to controls of either PBS or the shScr-ST. In this model, neutrophil depletion did not reverse the growth inhibition seen with shIDO-ST. This leads to 2 hypotheses. The first is that the depletion was not done at an early enough time point to impact the tumoricidal impact of intratumoral neutrophil recruitment. The second is that there are additional immune related factors not accounted for, that are responsible for this attenuation of tumor growth. Initially we depleted neutrophils with a Ly6G antibody and this lead to mice overcome with signs and symptoms of bacteremia, sepsis and death. We then experimented with different days of neutrophil depletion and day 4 was the earliest the mice could tolerate and survive for the experiment to be conducted. With this limitation, and at a depletion of 75% of neutrophils we were not able to reverse the tumor mitigation induced by shIDO-ST in CT26 cells implanted in Balb/c mice.
Our results demonstrate that shIDO-ST demonstrates significant tumor growth inhibition that is more effective than epacadostat, an IDO inhibitor in current clinical trials. The immune population that is most concentrated in tumors are neutrophils after shIDO-ST therapy. Effective IDO-targeting is still a highly sought after goal in the immunotherapy field. However, there remains significant work to be done to elucidate the mechanism of this response and to develop this therapy as a durable option for metastatic colorectal cancer.
Supplementary Material
Acknowledgments
Funding: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award number NIH 5K12CA001727-20. The content is solely the responsibility of Laleh Melstrom and does not necessarily represent the official views of the National Institutes of Health. Additionally, the work was also supported by RO1 HL56067 and P01 A134495 (Bruce Blazar). Research reported in this publication also included work performed in the Small Animal Imaging Core and Biostatistics Core supported by the National Cancer Institute of the National Institutes of Health under award number P30CA33572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest: The authors declare that they have no conflict of interest and all experiments were conducted within ethical standards.
Supplementary Information is available at the CGT website.
References
- 1.Fakih MG. Metastatic colorectal cancer: current state and future directions. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2015, 33(16): 1809–1824. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: a cancer journal for clinicians 2018, 68(1): 7–30. [DOI] [PubMed] [Google Scholar]
- 3.Cremolini C, Loupakis F, Antoniotti C, Lupi C, Sensi E, Lonardi S, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. The Lancet Oncology 2015, 16(13): 1306–1315. [DOI] [PubMed] [Google Scholar]
- 4.Le DT, Hubbard-Lucey VM, Morse MA, Heery CR, Dwyer A, Marsilje TH, et al. A Blueprint to Advance Colorectal Cancer Immunotherapies. Cancer immunology research 2017, 5(11): 942–949. [DOI] [PubMed] [Google Scholar]
- 5.Kyi C, Postow MA. Immune checkpoint inhibitor combinations in solid tumors: opportunities and challenges. Immunotherapy 2016, 8(7): 821–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nature medicine 2003, 9(10): 1269–1274. [DOI] [PubMed] [Google Scholar]
- 7.Brandacher G, Perathoner A, Ladurner R, Schneeberger S, Obrist P, Winkler C, et al. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clinical cancer research : an official journal of the American Association for Cancer Research 2006, 12(4): 1144–1151. [DOI] [PubMed] [Google Scholar]
- 8.Prendergast GC, Malachowski WP, DuHadaway JB, Muller AJ. Discovery of IDO1 Inhibitors: From Bench to Bedside. Cancer research 2017, 77(24): 6795–6811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beatty GL, O’Dwyer PJ, Clark J, Shi JG, Bowman KJ, Scherle PA, et al. First-in-Human Phase I Study of the Oral Inhibitor of Indoleamine 2,3-Dioxygenase-1 Epacadostat (INCB024360) in Patients with Advanced Solid Malignancies. Clinical cancer research : an official journal of the American Association for Cancer Research 2017, 23(13): 3269–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson CM, Hale PT, Carlin JM. The role of IFN-gamma and TNF-alpha-responsive regulatory elements in the synergistic induction of indoleamine dioxygenase. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research 2005, 25(1): 20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma L, Xu B, Wang W, Deng W, Ding M. Analysis of tryptophan catabolism in HBV patients by HPLC with programmed wavelength ultraviolet detection. Clinica chimica acta; international journal of clinical chemistry 2009, 405(1-2): 94–96. [DOI] [PubMed] [Google Scholar]
- 12.Santhanam S, Alvarado DM, Ciorba MA. Therapeutic targeting of inflammation and tryptophan metabolism in colon and gastrointestinal cancer. Translational research : the journal of laboratory and clinical medicine 2016, 167(1): 67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blache CA, Manuel ER, Kaltcheva TI, Wong AN, Ellenhorn JD, Blazar BR, et al. Systemic delivery of Salmonella typhimurium transformed with IDO shRNA enhances intratumoral vector colonization and suppresses tumor growth. Cancer research 2012, 72(24): 6447–6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manuel ER, Chen J, D’Apuzzo M, Lampa MG, Kaltcheva TI, Thompson CB, et al. Salmonella-Based Therapy Targeting Indoleamine 2,3-Dioxygenase Coupled with Enzymatic Depletion of Tumor Hyaluronan Induces Complete Regression of Aggressive Pancreatic Tumors. Cancer immunology research 2015, 3(9): 1096–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang CZ, Kazmierczak RA, Eisenstark A. Strains, Mechanism, and Perspective: Salmonella-Based Cancer Therapy. International journal of microbiology 2016, 2016: 5678702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Felgner S, Kocijancic D, Frahm M, Weiss S. Bacteria in Cancer Therapy: Renaissance of an Old Concept. International journal of microbiology 2016, 2016: 8451728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clairmont C, Lee KC, Pike J, Ittensohn M, Low KB, Pawelek J, et al. Biodistribution and genetic stability of the novel antitumor agent VNP20009, a genetically modified strain of Salmonella typhimurium. The Journal of infectious diseases 2000, 181(6): 1996–2002. [DOI] [PubMed] [Google Scholar]
- 18.Pawelek JM, Low KB, Bermudes D. Bacteria as tumour-targeting vectors. The Lancet Oncology 2003, 4(9): 548–556. [DOI] [PubMed] [Google Scholar]
- 19.Toso JF, Gill VJ, Hwu P, Marincola FM, Restifo NP, Schwartzentruber DJ, et al. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2002, 20(1): 142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manuel ER, Blache CA, Paquette R, Kaltcheva TI, Ishizaki H, Ellenhorn JD, et al. Enhancement of cancer vaccine therapy by systemic delivery of a tumor-targeting Salmonella-based STAT3 shRNA suppresses the growth of established melanoma tumors. Cancer research 2011, 71(12): 4183–4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. International immunology 1996, 8(5): 765–772. [DOI] [PubMed] [Google Scholar]
- 22.Thamm DH, Kurzman ID, King I, Li Z, Sznol M, Dubielzig RR, et al. Systemic administration of an attenuated, tumor-targeting Salmonella typhimurium to dogs with spontaneous neoplasia: phase I evaluation. Clinical cancer research : an official journal of the American Association for Cancer Research 2005, 11(13): 4827–4834. [DOI] [PubMed] [Google Scholar]
- 23.Zitvogel L, Kroemer G. Targeting PD-1/PD-L1 interactions for cancer immunotherapy. Oncoimmunology 2012, 1(8): 1223–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. The Journal of experimental medicine 2000, 192(7): 1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nature immunology 2001, 2(3): 261–268. [DOI] [PubMed] [Google Scholar]
- 26.Yaghoubi N, Soltani A, Ghazvini K, Hassanian SM, Hashemy SI. PD-1/ PD-L1 blockade as a novel treatment for colorectal cancer. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 2018, 110: 312–318. [DOI] [PubMed] [Google Scholar]
- 27.Ruby T, McLaughlin L, Gopinath S, Monack D. Salmonella’s long-term relationship with its host. FEMS microbiology reviews 2012, 36(3): 600–615. [DOI] [PubMed] [Google Scholar]
- 28.Pawelek JM, Low KB, Bermudes D. Tumor-targeted Salmonella as a novel anticancer vector. Cancer research 1997, 57(20): 4537–4544. [PubMed] [Google Scholar]
- 29.Khan SA, Everest P, Servos S, Foxwell N, Zahringer U, Brade H, et al. A lethal role for lipid A in Salmonella infections. Molecular microbiology 1998, 29(2): 571–579. [DOI] [PubMed] [Google Scholar]
- 30.Lob S, Konigsrainer A, Rammensee HG, Opelz G, Terness P. Inhibitors of indoleamine-2,3-dioxygenase for cancer therapy: can we see the wood for the trees? Nature reviews Cancer 2009, 9(6): 445–452. [DOI] [PubMed] [Google Scholar]
- 31.Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. The Journal of experimental medicine 1999, 189(9): 1363–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee GK, Park HJ, Macleod M, Chandler P, Munn DH, Mellor AL. Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. Immunology 2002, 107(4): 452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. The Journal of experimental medicine 2002, 196(4): 459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Terness P, Bauer TM, Rose L, Dufter C, Watzlik A, Simon H, et al. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. The Journal of experimental medicine 2002, 196(4): 447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen W, Liang X, Peterson AJ, Munn DH, Blazar BR. The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. Journal of immunology 2008, 181(8): 5396–5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El-Zaatari M, Chang YM, Zhang M, Franz M, Shreiner A, McDermott AJ, et al. Tryptophan catabolism restricts IFN-gamma-expressing neutrophils and Clostridium difficile immunopathology. Journal of immunology 2014, 193(2): 807–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loughman JA, Yarbrough ML, Tiemann KM, Hunstad DA. Local Generation of Kynurenines Mediates Inhibition of Neutrophil Chemotaxis by Uropathogenic Escherichia coli. Infection and immunity 2016, 84(4): 1176–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.