Abstract
BACKGROUND:
Patients with sickle cell disease (SCD) often require red blood cell (RBC) transfusions but alloimmunization remains a significant complication. Alloantibodies can lead to delayed hemolytic transfusion reactions (DHTRs) days to weeks after a RBC transfusion, but may be underrecognized in patients with chronic hemolysis.
STUDY DESIGN AND METHODS:
This retrospective study aimed to determine the incidence and severity of DHTRs associated with new antibody detection in a cohort of 624 patients with SCD who received transfusion with C-, E-, and K-matched RBCs from primarily African American donors over a 14-year period. We identified potential DHTRs by the change in hemoglobin (Hb) and % HbS at baseline, before transfusion, and up to 30 days after the transfusion that preceded new antibody identification.
RESULTS:
Laboratory evidence of a DHTR was associated with 54 of 178 evaluable antibodies at first detection (30%), among which less than half were recognized by the patient or provider at the time of the event. A DHTR was associated with 26% of Rh antibodies identified in patients receiving serologic Rh-matched RBCs, and 38% of non-Rh antibodies. Twenty-one of the 54 DHTRs (39%) were associated with a Hb decline greater than 1 g/dL lower than pretransfusion values. Among these 21 severe DHTRs, Rh specificities were identified in 10 of 12 DHTRs in chronically transfused patients, while non-Rh specificities were associated with seven of nine DHTRs in episodically transfused patients.
CONCLUSION:
High clinical suspicion and monitoring for DHTRs is warranted, as they may be more common in patients with SCD than previously appreciated.
Patients with sickle cell disease (SCD) often require red blood cell (RBC) transfusions to manage and prevent complications but alloimmunization remains a significant problem. This patient population is one of the most frequently and heavily alloimmunized, with the prevalence ranging from 7% to 59%,1,2 compared to 2% to 3% of sporadically transfused patients from general hospital populations.3,4 The number of transfusion exposures and RBC antigen differences between donors of primarily European decent and patients of mostly African ancestry contributes to the high rate of alloimmunization. Rh (D, C, c, E, and e) and K antibodies are the most frequent specificities encountered and, therefore, prophylactic C, E, and K (CEK) antigen-matched RBCs are recommended.5,6 While extended matching to also include the Kidd, Duffy, and MNS systems reduces alloimmunization,7 identifying sufficient units in the donor supply is challenging,8,9 and extended typed units are often reserved for individuals who have formed multiple alloantibodies. Inheritance of RH variants that encode partial Rh antigens and result in loss of Rh protein epitopes further contributes to alloimmunization and adds additional complexity to antibody identification and donor Rh antigen matching.9,10
Alloantibodies can shorten the survival of transfused RBCs and lead to delayed hemolytic transfusion reactions (DHTRs) days to weeks after a transfusion. An estimated 1.6% to 11% of transfused patients with SCD develop overt DHTRs with increased fatigue, jaundice, dark urine, fever, and/or pain,11–14 but mild DHTRs are underrecognized.11,15 Since extravascular removal is the primary mechanism of RBC clearance, DHTRs can occur without obvious clinical symptoms. However, laboratory evaluation may demonstrate a decrease in hemoglobin (Hb) or increase in %HbS incongruent with recent transfusion, as well as hyperbilirubinemia above baseline, reticulocytosis, and/or a weakly positive direct antiglobulin test (DAT). DHTRs are also underestimated since new antibody formation is not always detectable at time of symptomatic presentation, or the anemia and hemolysis may precipitate pain and be misdiagnosed as a vaso-occlusive episode.11,15
The terms hyperhemolysis and bystander hemolysis are used to describe DHTRs in patients with SCD when severe hemolysis occurs and the Hb decreases to less than pretransfusion levels.16–18 This suggests hemolysis of the patient’s own RBCs in addition to transfused cells.19 Decreased endogenous erythropoietic drive after transfusion can also exacerbate the anemia associated with a DHTR. Severe hemolysis can occur with no identifiable antibody and a negative DAT. However, recognition is critical since samples should be tested by more sensitive methods and additional transfusions be avoided if possible, as hemolysis may worsen and potentially lead to fatality.11,20
This study aimed to determine the incidence and severity of DHTRs associated with new antibody detection in a cohort of 624 patients with SCD after transfusion with CEK-matched RBCs from primarily African American donors. We demonstrate that 30% of new antibodies were associated with a DHTR, more than half were unrecognized at the time of the event, and the clinical significance varied with antibody specificity and transfusion setting (acute versus chronic).
MATERIALS AND METHODS
Study design
This was a retrospective analysis to determine which alloantibody specificities were associated with DHTRs. Under an institutional review board approved protocol, we reviewed the clinical records of 624 patients with SCD who received at least one RBC transfusion at The Children’s Hospital of Philadelphia between January 1, 2003, and December 31, 2016. We identified all new antibody specificities and recorded the dates detected, number of units received at the time of first detection for each antibody, and complete blood counts and Hb quantifications. We excluded antibodies detected before the first transfusion at our facility and those detected more than 30 days posttransfusion.
Transfusion protocol
Patients were prospectively antigen matched for ABO, D, and CEK; patients who lack the antigen were transfused with antigen-negative units. Units were from the “Blue Tag” program consisting of donors who self-identify as African American, with some exceptions related to need for antigen-negative units. Patients received HbS-negative units less than 21 days old per protocol for patients with hemoglobinopathies. All units were leukoreduced and irradiated per institutional policy.
Laboratory testing
A three-cell antibody screen, complete blood count, and Hb quantification was performed before each transfusion or when a clinician suspected new antibody formation or a DHTR. Antibody identification was performed with a gel-based method (Ortho Diagnostics). A warm autoantibody was defined as a positive DAT with a panagglutinin in the plasma or eluate with similar strength of reactivity to all cells tested and no apparent specificity. A cold autoantibody was defined as reactivity with a panagglutinin pattern or no specificity that could be removed by prewarm testing at 37°C. A DAT was performed on samples from patients suspected of having a DHTR and a subsequent eluate with antibody evaluation if the DAT was positive. Antibody identification with a low-frequency antigen RBC panel was performed if a DHTR was suspected but no specificity identified, and 1 or more units demonstrated crossmatch incompatibility with the posttransfusion specimen. RH genotyping was performed with RHD and RHCE BeadChip arrays (Bioarray) and polymerase chain reaction—based assays as described previously for all patients to determine presence of partial Rh antigens.9,10,21
Determination of DHTR by laboratory variables
For each newly identified antibody, we compared the patient’s total Hb or HbA and HbS percentages at time of antibody detection with pretransfusion values. Antibodies were excluded from DHTR analysis if there was insufficient laboratory data; if antibody detection was greater than 31 days posttransfusion; or if the antibody was detected concomitant with acute chest syndrome, splenic sequestration, or sepsis that may have exacerbated the anemia. For antibodies that occurred in chronically transfused individuals (patients transfused at regular intervals, typically every 21–28 days), we calculated the mean pretransfusion Hb and HbA and HbS levels in the 6 to 12 months preceding antibody detection. A DHTR was defined by a Hb decrease and/or HbS% increase greater than 2 standard deviations (SDs) from their individual mean at the time of new antibody detection. For antibodies that occurred in patients who had received an episodic transfusion, the baseline Hb was calculated from outpatient visits in the preceding 12 to 24 months, and the pre- and posttransfusion Hb level and %HbS were recorded. Newly detected antibodies were considered to be associated with a DHTR that was defined by a Hb decrease below the pretransfusion or baseline Hb, and the %HbS values were used as supportive data.
Statistical analysis
Statistical analysis was performed using the t test to compare parametric data between groups, and the Mann-Whitney or Kruskal-Wallis tests for nonparametric data. Fisher’s exact test was used for categorical data. A two-tailed p value of less than 0.05 was considered significant.
RESULTS
Subjects
Among 624 patients with SCD who received at least one RBC transfusion, we identified 124 individuals with one or more newly identified alloantibodies (19.9%). SCD genotypes included 111 SCD-SS (89.5%), four SCD–Sβ0 thalassemia (3.2%), four SCD-Sβ+ thalassemia (3.2%), four SCD-SC (3.2%), and one SCD-SO Arab (0.8%). Ninety-six patients had been on chronic transfusion therapy and 28 patients had only received episodic transfusions. Collectively, 47,601 RBC units were transfused to these 124 patients. For those receiving chronic transfusions, the mean number of exposures per patient was 493 units; median, 417; and range, 8 to 1808. For individuals who received episodic transfusions for acute complications or preoperatively, the lifetime mean was 9 units; median, 8; and range, 1 to 27.
Antibodies identified in patients with SCD transfused with Rh- and K-matched RBCs
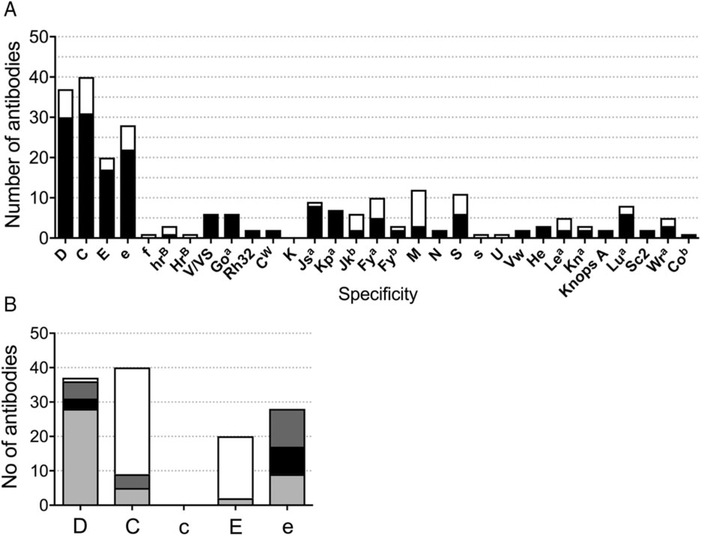
Among 124 patients, 239 specific antibodies were identified (Fig. 1A). A total of 174 antibodies occurred during chronic transfusion therapy (72.8%) and 65 antibodies formed after an episodic transfusion (27.2%). Rh specificities were the most frequent (n = 146, 61%). Despite transfusion with D, CEK-matched RBCs, antibodies against common Rh specificities were the most frequent (n = 125): 37 anti-D, 40 anti-C, 20 anti-E, and 28 anti-e (Figs. 1A and 1B). The RBCs of individuals with RH variant alleles may express partial Rh antigens and lack common Rh epitopes, putting them at risk of immunization when exposed to conventional Rh proteins, and conversely, exposure to variant Rh antigens or epitopes on donor RBCs can stimulate Rh antibodies in the patient.9,10,22 Anti-D was detected in one patient who was D− and in 36 patients with D+ RBCs. Among the D+ individuals with anti-D, five had partial D antigen and no conventional D, three had altered RHD*DAU0, and 28 had one or more conventional RHD (Fig. 1B and Table S2). Anti-C occurred in four C+ individuals with the hybrid RHD*DIIIa-CE(4–7)-D gene, which encodes partial C antigen, but anti-C specificity was also identified in the plasma of five C+ individuals with conventional RHCE*Ce. Anti-C reactivity was also detected in 31 C− patients who had received only C− units. Anti-E was identified in the plasma of two E+ patients with conventional RHCE*cE and 13 patients who were E− and transfused with only E− RBCs. Five anti-E occurred in E− patients: four who had made anti-e and were switched to E+e− units for further transfusion (three chronic, one episodic) and one individual who was transfused non-CEK-matched RBCs when referred to an outside institution for a surgical procedure and presented to our institution with a DHTR. Of 28 e+ patients who formed anti-e, 11 had partial e antigen and no conventional e, eight had altered RHCE*ce (48C), and nine had one or more conventional RHCE*ce.
Fig. 1.
Antibody specificities identified in patients with SCD transfused with prophylactic ABO, D, C, E, and K serologic matched RBCs. (A) A total of 175 antibodies occurred during chronic transfusion therapy (■) and 64 antibodies formed after an episodic transfusion (□). (B) Antibodies detected to common Rh specificities in patients whose RH genotype predicts RBC expression of partial  , altered (■), or conventional
, altered (■), or conventional  antigen or absence of the antigen (□). RHD*DAU0 and RHCE*ce48C, which encode proteins that have not been shown to lack epitopes are described as altered but not partial antigens.
antigen or absence of the antigen (□). RHD*DAU0 and RHCE*ce48C, which encode proteins that have not been shown to lack epitopes are described as altered but not partial antigens.
In addition to antibodies directed against the common Rh specificities, three anti-hrB and one anti-HrB were stimulated in individuals who lack these high-prevalence Rh antigens. Conversely, antibodies to Rh antigens that are low prevalence in most populations but frequent in African Americans included six anti-V/VS, six anti-Goa, and two anti-RH32. Anti-CW was also identified in two individuals; CW is a variant Rh antigen that is present in 2% of Caucasians.23
Outside the Rh system, antibodies included six anti-Jkb, 10 anti-Fya, three anti-Fyb, 12 anti-M, two anti-N, 11 anti-S, one anti-s, eight anti-Lua, and one anti-U, which is a high-prevalence antigen absent in 2% of African Americans (Fig. 1A). No anti-K has ever been identified in our prophylactic CEK matching program. Antibodies to low-prevalence antigens included nine anti-Jsa, seven anti-Kpa, two anti-Vw, three anti-He, and five anti-Wra. Jsa and He antigens are low prevalence in most populations (<1%) but have frequencies of 20 and 3%, respectively, on RBCs of African Americans. There were 25 additional specificities among this cohort that were detected prior to the study date and excluded (Table S1). Eighty-four patients (68%) demonstrated a warm autoantibody and eight a cold autoantibody on at least one occasion. Eight individuals had a DHTR reported with no specific antibody identified.
Alloimmunization and number of RBC exposures
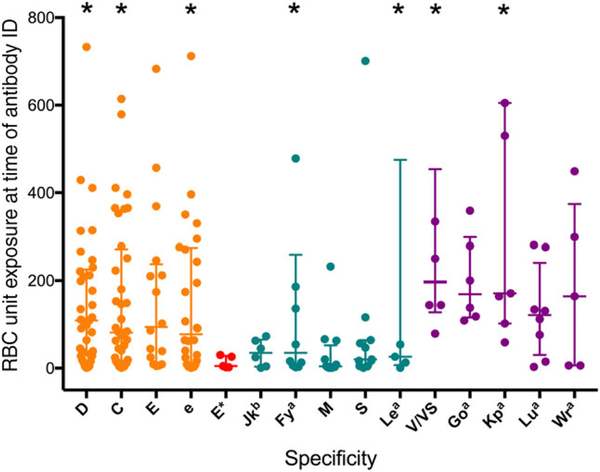
Antibodies occurred after cumulative donor exposures ranging from 1 to 1496 units, with a mean of 173 units and median of 82 units. Antibodies against common Rh antigens, despite CEK-matched transfusions, were detected after a median of 109 units for anti-D (n = 37), 82 for anti-C (n = 40), 94 for anti-E (n = 15), and 78 for anti-e (n = 28) (Fig. 2). Among the five patients who were E− and were exposed to E+ units, the median unit exposure to anti-E formation was 5 units (noted as E* in Fig. 2; range, 2–30 units). The median unit exposure for non-Rh antibodies was lower compared to Rh: 35 for anti-Jkb (n = 6), 35 for anti-Fya (n = 10), 20 for anti-S (n = 11), and 26 for anti-Lea (n = 5). As expected based on prevalence, the median unit exposure at time of detection for antibodies against low-prevalence antigens was higher: 197 for anti-V/VS (n = 6), 169 for anti-Goa (n = 6), 171 for anti-Kpa (n = 7), 122 for anti-Lua (n = 8), and 164 for anti-Wra (n = 5). The median unit exposure was significantly different among Rh, non-Rh, and low-prevalence specificities (p < 0.0001, Kruskal-Wallis test).
Fig. 2.
RBC unit exposure at time of first detection of new antibody. The RBC unit exposure for each antibody at first detection is shown as a circle, along with the median and interquartile range. *Seven specificities were identified with more than 800 unit exposures (882 units for anti-D, 1359 for anti-C, 864 for anti-e, 1496 for anti-Fya, 896 for anti-Lea, 813 for anti-V/VS, 1492 for anti-Kpa).
Laboratory evidence of DHTRs
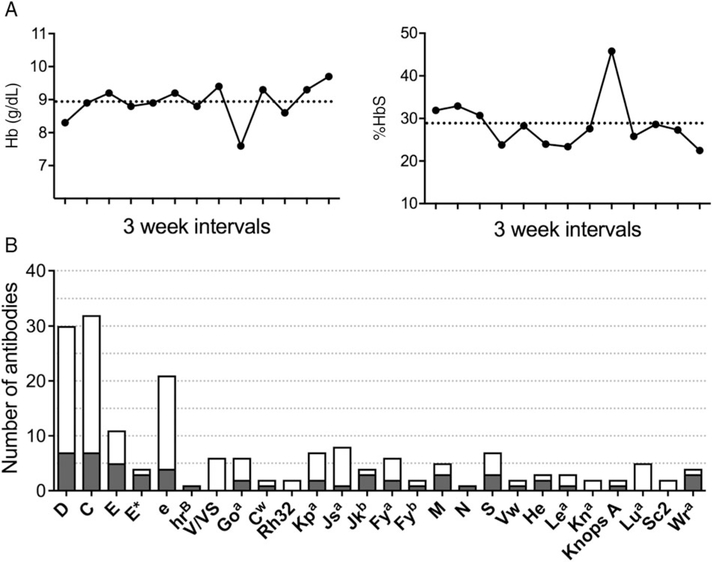
Patients with a DHTR may present with clinical symptoms of anemia, hemolysis, and sometimes pain. DHTRs in this patient population are also typically accompanied by an increased %HbS level and/or a decrease in Hb and may identify DHTRs without overt clinical symptoms. We evaluated whether patients experienced a DHTR at the time of antibody first detection by comparing the Hb and the %HbS with the patient’s baseline and pretransfusion values (Fig. 3A). Among the 239 newly identified antibodies, we excluded 61 antibody events from DHTR analysis for either concomitant acute chest syndrome, splenic sequestration, or sepsis (n = 9); insufficient laboratory data to calculate baseline values (n = 24); or the antibody was detected over 30 days from transfusion (n = 28). There were 178 newly identified antibodies in 89 patients that were evaluable for laboratory evidence of a DHTR.
Fig. 3.
Laboratory evidence of DHTRs. (A) Laboratory evidence of a DHTR in a patient chronically receiving RBCs. Arrow denotes time at which a DHTR was suspected. Dotted line indicates the patient’s mean pretransfusion Hb level and %HbS for the 12 months preceding the DHTR. (B) Number of antibodies at first detection that were  or were not (□) associated with a DHTR according to specificity.
or were not (□) associated with a DHTR according to specificity.
Of the 178 evaluable antibodies, 54 were associated with laboratory evidence of a DHTR at time of first detection (30.3%, Fig. 3B). Seven patients had DHTRs associated with two newly identified antibodies simultaneously, of which one or both may have contributed to transfused RBC clearance (Table 1). Among Rh antibodies identified despite receiving serologic Rh-matched RBCs, 27 of 111 (24.3%) were associated with a DHTR, compared to 24 of 63 non-Rh antibodies (38.1%; p = 0.0593, Fisher’s exact test). Seven of 30 anti-D (23.3%), seven of 32 anti-C (21.9%), five of 11 anti-E (45.5%), and four of 21 anti-e (19.0%) were associated with DHTRs. Among four E− individuals exposed to E+ RBCs, three experienced a DHTR. Only one anti-D, one anti-C, and one anti-e associated with a DHTR was correlated with inheritance of partial antigen only. Among patients with a corresponding conventional allele, five of 24 anti-D (21%), two of six anti-C (33%), one of two anti-E (50%), and one of six anti-e (17%) were associated with a DHTR. However, 47% of Rh antibodies associated with a DHTR occurred in individuals with at least one allele encoding any partial Rh antigen (n = 14 of 30). Two of 6 anti-Goa and one of two anti-Cw demonstrated a DHTR, while zero of six anti-V/VS were associated with a DHTR.
TABLE 1.
Individuals with multiple antibodies identified simultaneously with evidence of a DHTR
| UPID | Antibodies identified |
|---|---|
| 77 | Anti-C, anti-D, WAU* |
| 109 | Anti-C, anti-hrB, WAU* |
| 138 | Anti-Cw, anti-D, WAU* |
| 139 | Anti-Jkb, anti-M |
| 180 | Anti-Jsa, anti-Lea, WAU* |
| 326 | Anti-Goa, anti-Kpa |
| 746 | Anti-Wra, anti-M |
Redemonstrating.
Outside the Rh system, three of four anti-Jkb, three of seven anti-S, two of three anti-He, and three of four anti-Wra were associated with a DHTR (Fig. 3B). None of the five anti-Lua and only one of eight anti-Jsa were associated with signs of RBC clearance. Although three anti-M were associated with a DHTR, two were detected simultaneously with another newly identified specificity, Jkb or Wra (Table 1). The third patient with anti-M was receiving monthly erythrocytapheresis and at time of detection the pretransfusion %HbS was 54.9%, compared to the mean pretransfusion level of 44.0% for the prior 6 months (SD, 3.3%). Overall, among the 54 cases with evidence for DHTRs, only 21 (39%) were reported to the blood bank as a suspected transfusion reaction. A DAT was obtained for 33 of the 54 DHTRs, of which 19 were positive (58%) with varying strength (5 = microscopic +, 4 = 1+, 9 = 2+, 1 = 3+).
DHTRs with no antibody specificity identified
Eight individuals had a suspected DHTR reported to the blood bank but no antibody specificity was identified (Table 2). Five cases occurred in patients receiving chronic transfusions and three followed an episodic transfusion. Five patients reported fatigue, dark urine, increased scleral icterus, and/or jaundice and had a decreased Hb or elevated %HbS. Three patients presented for their next scheduled erythrocytapheresis visit without symptoms (unique patient identification numbers [UPIDs], 99, 93, and 119) and a DHTR was suspected due to a decreased Hb and increased %HbS (Table 2). For the chronically transfused patients, the Hb level was 1.3 to 1.6 g/dL lower and HbS was 18% to 35.4% greater than their baseline pretransfusion values. Among the episodic transfused, UPIDs 197 and 292 had 0 and 4.3% HbA remaining at 13 and 11 days after a transfusion, respectively. UPID 447 had a Hb level of 10.2 g/dL with 64.3% HbA after transfusion, which declined to 7.3 g/dL with 31.1% HbA measured 12 days later. Although the gel antibody screen was positive in four of the eight patients, no new specificity was identified. Previously identified anti-E in UPID 447 and anti-S in UPID 197 remained detectable, and antigen-negative units had been transfused to both patients. A warm and a cold autoantibody was detected in UPID 126, who was the only individual to have a positive DAT.
TABLE 2.
DHTRs with no identifiable specificity*
| Hb (g/dL) |
|||||||
|---|---|---|---|---|---|---|---|
| UPID | Transfusion type | Symptoms | % Hb at presentation | % Hb baseline mean (chronic) or post-txn (episodic) | Antibody screen | Antibody identification | DAT |
| 99 | Chronic | None | 7.7 65.0% S |
9.3 29.6% S |
Negative | Negative | Negative |
| 93 | Chronic | None | 8.0 48.1% S |
9.3 29.0% S |
Positive | Negative | Negative |
| 119 | Chronic | None | 6.8 69% S |
8.3 37.5% S |
Negative | Negative | Negative |
| 326 | Chronic | Dark urine, scleral icterus reported at next transfusion | 7.6 45.8% S |
8.9 27.8% S |
Negative | Negative | Negative |
| 126 | Chronic | Jaundice, scleral icterus, dark urine | 6.8 69% S |
8.3 37.5% S |
Positive | WAU Cold antibody |
IgG 2+ C3 micro+ |
| 447 | Episodic† | Jaundice, scleral icterus |
Day 12: 7.3 63.1% S, 31.1% A |
Day 0: 10.2 32.7% S, 64.3% A |
Positive | Anti-E, no new specificity | Negative |
| 197 | Episodic | Jaundice, dark urine | Day 13: 7.1 47.6% S, 44.2% C 0% A |
Day 0: 8.3 8.7% S, 8.4% C 79.5% A |
Positive | Anti-S, no new specificity | Negative |
| 292 | Episodic | Fatigue, dark urine, jaundice |
Day 11: 3.9 89.2% S, 4.3% A |
Day 0: 8.9 62.0% S, 31.4% A |
Negative | Negative | Negative |
Laboratory evidence by Hb and Hb quantification in eight patients for whom a DHTR was suspected by the clinical care team but no new specificity was identified. For chronically transfused, the individuals’ mean pretransfusion values were calculated from the six to 12 prior scheduled transfusion visits.
Transfused in acute setting, but patient on chronic transfusion therapy.
micro = microscopic; txn = transfusion; WAU = warm autoantibody.
DHTRs resulting in Hb below pretransfusion values
Hyperhemolysis is a concern in patients with SCD experiencing a DHTR and is characterized by a Hb decrease to less than pretransfusion levels. Among the 54 antibodies associated with a DHTR, we identified 21 episodes in which the patient’s Hb decreased more than 1 g/dL less than the pretransfusion Hb value after an episodic transfusion (Table 3) or 1 g/dL less than the mean pretransfusion Hb level if chronically transfused (Table 4). After episodic transfusion, nine of these events occurred in seven patients all of whom presented 6 to 30 days later with symptoms including pain, headache, dark urine, fatigue, and/or fever (Table 3). Transfusion indications included preoperative preparation for four patients, chronic pain for two, chronic hypoxia for one, anemia secondary to a prior DHTR for one, and acute chest syndrome for another. Two patients (UPIDs 109 and 292) experienced two sequential DHTRs on initiation of a period of chronic transfusions, halting transfusion therapy. Among 12 antibodies in these seven patients, three had Rh specificity and nine were non-Rh. In three cases, two specificities were detected simultaneously (Table 3). The nadir Hb ranged from 3.6 to 6.8 g/dL and was more than 2 g/dL lower than the pretransfusion level in eight of nine events (median decline, 2.7 g/dL; range, 1.1 to 4.1 g/dL). DHTR management included subsequent transfusion in four events, along with steroids and intravenous immunoglobulin (IVIG) in two cases. Two individuals were managed with steroids and IVIG alone, and the remainder received supportive care only.
TABLE 3.
Severe DHTRs in patients receiving episodic RBC transfusions
| Hb (g/dL) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Post-txn |
||||||||
| UPID | Clinical presentation | RBC indication | New antibody specificity | Pre-txn % Hb | % Hb (days post-RBCs) | Presentation (days post-RBCs) | Nadir Hb quant (%) (days post-RBCs) | DHTR management |
| 109 | HA, dizziness, lower back pain, jaundice | Pain (beginning trial of chronic RBCX) | Jkb | 9.4 7 U (RBCX) |
10.1 (0 days) 74% A (0 days) |
7.8 (+10 days) | 6.1 (+12 days) 62% A (+11 days) |
RBCs |
| 109 | Pain | Anemia due to DHTR (anti-Jkb) | C, hrB | 6.1 62% A |
8.5 (0 days) | 6.9 (+30 days) | 5.0 (+35 days) 6.5% A (+35 days) |
No records |
| 292 | Fatigue and dark urine | Chronic hypoxia, awaiting T+A | D | 6.5 | ND | 5.6 (+10 days) | 4.2 (+10 days) | IVIG, steroids |
| 292 | Dark urine | Preoperative for T+A | Jkb | 7.0 | ND | 6.7 (+6 days) | 4.5 (+6 days) | RBCs IVIG, steroids |
| 139 | BUE and BLE pain | Pain (beginning trial of chronic RBC exchange) | S | 10.9 6 U (RBCX) |
10.1 (0 days) 72.5% A (0 days) |
10.9 (+9 days) | 6.8 (+13 days) 20.6% A (+13 days) |
RBCs |
| 339 | Chest, BUE, BLE pain | Preoperative for hip surgery | S | 8.6 | 11.3 (+4 days) | 6.3 (+15 days) | 5.9 (+16 days) | Supportive |
| 244 | Chest pain and fever | Preoperative for cholecystectomy | Fya | 8.3 | 10.1 (0 days) | 6.5 (+23 days) | 4.5 (+25 days) | IVIG, steroids |
| 180 | BLE pain and fever | Acute chest syndrome | Jsa, Lea | 7.4 | 8.0 (0 days) | 7.2 (+11 days) | 4.8 (+15 days) | Supportive |
| 746 | Back, BUE, BLE pain | Preoperative for dental extraction | Wra, M | 7.7 | 10.8 (+1 day) | 5.7 (+20 days) | 3.6 (+22 days) | RBCs IVIG, steroids |
BLE = bilateral lower extremities; BUE = bilateral upper extremities; HA = headache; quant = quantification; RBCX = RBC exchange; T+A = tonsillectomy and adenoidectomy; txn = transfusion; U = units.
TABLE 4.
Severe hemolytic transfusion reactions in patients receiving chronic RBC therapy
| Hb (g/dL) |
HbS (%) |
|||||||
|---|---|---|---|---|---|---|---|---|
| UPID | Symptoms on presentation | Transfusion regimen in units, interval | New antibody specificity | Days after transfusion | Pre-txn Hb (g/dL)* | Presentation (difference from mean pre-txn) | Pre-txn HbS (%)* | HbS (%) (difference from mean pre-txn) |
| 50 | Pain | 2 units 21 days |
D | 11 | 8.5 (±0.737) | 6.4 (2.1)† | 44.3 (±9.2) | 58.8 (14.5) |
| 77 | None | 7 unit RBCX 21–28 days |
E | 29 | 9.7 (±0.41) | 5.1 (4.6)† | 26.9 (±3.4) | 52 (25.1)† |
| 78 | None | 8-unit RBCX 25–28 days |
Wra | 28 | 10.6 (±0.31) | 9.1 (1.5)† | 33.2 (±2.3) | 46.9 (13.7)† |
| 95 | HA | 3-unit RBCX 21 days |
C | 18 | 9.3 (±0.65) | 5.0 (4.3)† | 27.0 (±3.6) | 31.2 (4.2) |
| 95 | None | 4-unit RBCX 21 days |
E | 22 | 9.1 (±0.495) | 7.5 (2.6)† | 27.1 (±3.9) | 68.0 (40.9)† |
| 103 | None | 6-unit RBCX 21 days |
E | 20 | 10.0 (±0.36) | 7.6 (2.4)† | 24.3 (±4.7) | 66.1 (41.3)† |
| 108 | None | 2 units 28 days |
e | 25 | 7.6 (±0.32) | 6.3 (1.3)† | 23. (±6.3) | 50.7 (27.6)† |
| 138 | None | 4-unit RBCX 28 days |
CW, D† | 29 | 10.1 (±0.52) | 8.2 (1.9)† | 28.2 (±4.0) | 61.6 (33.4)† |
| 326 | Back pain, HA, and dark urine | 2 units 28 days |
Go(a), Kpa | 17 | 8.3 (±0.60) | 6.0 (2.3)† | 39.1 (±6.3) | |
| 326 | Back pain | 3-unit RBCX 28 days |
He | 20 | 8.6 (±0.36) | 7.5 (1.1) | 30.3 (±3.9) | 48.9 (18.6)† |
| 340 | None | 2 units 21–28 days |
E‡ | 22 | 9.0 (±0.59) | 6.7 (2.3)† | 63.8 (±8.0) | 67.1 (3.3) |
| 348 | No history available | 1 unit 35–42 days |
C | 31 | 9.2 (±0.45) | 4.9 (4.3)† | 42.6 (±7.0) | |
Data are reported as mean (±SD).
Difference >2 SDs from the individual’s mean Hb or HbS.
Anti-Rh specificities identified despite individual carrying at least one conventional corresponding RH gene.
HA = headache; NA = not available; RBCX = red blood cell exchange; txn = transfusion.
In patients receiving chronic transfusion therapy, we identified 12 DHTRs in 10 patients were associated with a Hb level of more than 1 g/dL lower than their mean pretransfusion Hb (Table 4). In eight of 12 events, the Hb decline was greater than 2 g/dL. Only four individuals presented with symptoms including pain, headache, and/or dark urine. Seven patients presented to their next scheduled transfusion visit and were found to have a lower Hb and/or higher %HbS than expected on their chronic transfusion regimen. Compared to baseline pretransfusion values, the Hb decrease ranged from 1.1 to 4.6 g/dL at the time of presentation (median, 2.3 g/dL decrease) and the increase in %HbS ranged from 3.3 to 41.3% at the time of presentation (median, 21.9% increase). Among 14 new antibodies detected in 12 events, 11 had an Rh specificity.
Antibody persistence
We examined whether antibody persistence correlated with clinical significance in patients requiring chronic transfusion (n = 162 antibodies). Antibodies associated with a DHTR were detected for a median of 5.5 months from first detection, compared to 5 months for those without evidence of a DHTR (p = 0.7637, Mann-Whitney test, Fig. Fig. S1A). Antibody detection duration ranged from 0 to 119 and 0 to 179 months for those associated and not associated with a DHTR, respectively. The median duration of antibody detection for Rh antibodies associated with DHTR and those not associated with DHTR was not significant: 4 and 2 months for anti-D (p = 0.5886, Mann-Whitney test), 27 and 1 months for anti-C (p = 0.0646), 1 and 0 months for anti-E (p = 0.5130, excluding E− patients exposed to E+ RBCs), and 8 and 4 months for anti-e (p = 0.234; Fig. Fig. S1B).
DISCUSSION
We report a 14-year experience assessing the clinical significance of alloantibodies formed among patients with SCD transfused with D, CEK-matched RBCs from primarily African American donors. Laboratory evidence of a DHTR at the time of antibody first detection was found in 30% of cases (54 of 178 evaluable antibodies). Among these 54, only 21 (39%) were reported to the blood bank as a suspected DHTR. DHTRs associated with a Hb decrease of more than 1 g/dL below the patient’s pretransfusion value were better recognized, as the majority (76%, n = 16 of 21) of those cases were reported to the transfusion service. These findings suggest that DHTRs are underappreciated and can be missed if the patient lacks overt symptoms. A previous study reported 11 of 23 (48%) DHTR events were misdiagnosed as a painful vaso-occlusive episode at the time of presentation.11 Clinical vigilance for DHTRs in patients with SCD is warranted, as we also report eight additional cases without an antibody identified, but symptoms and laboratory studies were consistent with a DHTR. Specifically, a higher %HbS than expected after transfusion should also raise suspicion for a DHTR, even if the patient appears asymptomatic.
Antibodies directed against the Rh system are the most common specificities among patients with SCD despite providing D, CEK antigen-matched RBCs from primarily African American donors.10 Among alloimmunized patients, more than half of the antibodies had common Rh specificities (52.3%), of which 26.5% were associated with a DHTR. A higher proportion of anti-E were associated with DHTRs compared to the other Rh specificities: five of 11 (46%) anti-E in E− patients transfused with E− units, compared to four of 23 (17%) anti-C that occurred in C− patients transfused with C− units and three of nine (33%) anti-C in C+ individuals, seven of 29 (24%) anti-D in D+ individuals, and four of 21 (19%) anti-e in e+ patients. Among other Rh specificities, zero of six anti-V/VS or two anti-Rh32 were associated with a DHTR, while two of six anti-Goa and the one evaluable anti-hrB demonstrated compromised survival of transfused RBCs. Inheritance of variant RH alleles in patients with SCD explain approximately one-third of cases, while the remainder are likely stimulated by variant Rh on African American donor RBCs.9,10 The clinical significance of these anti-Rh despite serologic Rh matching appears to vary, suggesting that the specific RH variants that an individual inherits or is exposed to from African American donor RBCs may have varying clinical effect. Notably, the median unit exposure at time of detection was significantly different among antibodies directed against Rh (95 units), non-Rh (19 units), and low-prevalence (154 units) antigens, likely related both to immunogenicity and to the likelihood of antigen exposure.
Delayed hemolytic transfusion reactions occurred more often with non-Rh antibodies (38.1%) compared to Rh antibodies (26.1%) that were encountered despite serologic Rh-matched RBCs. Although the number of evaluable occurrences was limited, anti-Jkb (three of four), anti-S (three of seven), and anti-Wra (three of four) were associated with a higher incidence of a DHTR. Interestingly, among nine cases of severe DHTR after episodic transfusion that resulted in a lower Hb than before transfusion, non-Rh specificities were identified in seven and Rh antibodies in two (Table 3). Conversely, severe DHTRs in chronically transfused patients were associated with a Rh specificity in 10 of 12 events associated with a lower Hb level than before transfusion (Table 4). Risk of alloimmunization in patients with SCD is increased with transfusion in the acute setting and an inflammatory state.24 However, the risk of alloimmunization against particular blood group antigens in a heightened inflammatory state has not been described, and further analysis in larger studies may be warranted.
Among the 178 evaluable antibodies, 14 of 16 antibodies (87.5%) in patients requiring transfusion for an acute complication or preoperatively were associated with a DHTR, compared to 40 of 162 antibodies (24.7%) detected in chronically transfused patients. This appears consistent with a single-institution study of 2158 transfusion episodes, in which 20 of 23 identified DHTR episodes occurred after transfusion in the acute setting.11 However, since we excluded antibodies from the DHTR analysis if detected greater than 1 month after transfusion (n = 26), our results are biased toward antibodies found in episodically transfused patients who returned with symptoms of hemolysis or exacerbated anemia.
Although we found a 30% incidence of DHTRs with new antibody detection, our study may still underestimate both the incidence and the severity of DHTRs. First, we excluded antibodies that were detected at the time of acute chest syndrome, splenic sequestration, or sepsis since the Hb decline may be due to a DHTR or the medical complication. Second, patients who receive episodic transfusions may not return for laboratory evaluation within 1 month of transfusion if the patient does not present with overt complaints. Given that only 40% of DHTRs identified here were reported to the blood bank, mild DHTRs are likely underappreciated. Third, our patient cohort includes many who receive chronic transfusion by erythrocytapheresis, which requires multiple units of RBCs (median, 5 units). Hemolysis after new antibody formation may not be identified if only a fraction of the units transfused were antigen positive, given the relatively strict definition of DHTR requiring a change in Hb or HbS% that was greater than 2 SDs from their baseline pretransfusion values. Regarding severity, most antibodies evaluated in our study occurred in patients on chronic transfusion and were likely primary immune responses since these patients require antibody screens on a regular basis. Thus, these DHTRs may not be as severe as those from a secondary immune response, which are more likely to occur in patients who are episodically transfused given that most antibodies evanesce and, therefore, may go undetected.
Among patients who were chronically transfused for whom antibody screens were obtained regularly, antibody persistence did not correlate with the occurrence of a DHTR. Almost all antibodies became undetectable over time, with a median of 5 months for both antibodies associated with or without a DHTR. This is consistent with a prior study among non-SCD patients in which 49% of antibodies evanesced over time, with half disappearing within 6 months and all by 10 years.25 Among 162 antibodies in the chronically transfused cohort, 40% were nondetectable within 2 months, 56% within 6 months, and 64% within a year. We observed eight antibodies that were detectable or redetected over a 10-year period, including one anti-D in a D− individual requiring chronic transfusion that has been detectable for 14 years, consistent with the persistence of the polyclonal anti-D response seen by others.25 The commonly observed antibody evanescence underscores the importance of communication between transfusion providers to prevent DHTRs when patients receive transfusions at more than one institution.
A specific antibody is not always identified with a DHTR.11,15 A suspected transfusion reaction was reported for eight individuals in whom no antibody specificity was identified. Five patients were symptomatic, but the remaining three presented for their next scheduled transfusion and a DHTR was suspected based on pretransfusion Hb and %HbS measurements. Half had a positive antibody screen, and the DAT was positive in only one case, similar to prior reports of DHTRs with no identifiable antibody in patients with SCD.11,15,18 Remaining vigilant for symptoms or hematologic laboratory evidence of DHTRs despite a negative antibody evaluation is requisite. When no specificity is found during the event, sequential immunohematologic testing over the following weeks may identify the antibody to guide future donor selection.
Delayed hemolytic transfusion reactions are potentially life-threatening complications in patients with SCD.20 Our study demonstrates a high incidence of DHTRs with antibodies at first detection (30%), among which less than half were recognized by the patient or the clinical team at the time of the event. Nearly 40% of DHTRs were associated with a Hb level of more than 1 g/dL lower than pretransfusion values. High clinical suspicion for DHTRs is warranted, as they may be more common in patients with SCD than previously appreciated, and supportive care and medical management can prevent associated morbidity and mortality.
Supplementary Material
Fig. S1. Duration of detection in antibodies associated or not associated with a delayed hemolytic transfusion reaction in patients receiving chronic transfusion. (A) Duration of detection for antibodies that were associated or not associated with a DHTR upon initial detection. (p = 0.7637, Mann-Whitney test). (B) Duration of detection for Rh antibodies that were associated or not associated with a DHTR upon initial detection (p > 0.05, Mann-Whitney test).
TABLE S1. Antibodies detected in 124 patients prior to study period (n = 25)
TABLE S2. RHD* and RHCE* allele status in patient transfused with red cells antigen-matched for CEK but formed Rh antibodies. RHD*DAU0 and RHCE*ce48C which encode proteins that have not been shown to lack epitopes are described as altered but not partial antigens. Ag = antigen; Abs = antibodies.
ACKNOWLEDGMENTS
The authors thank the patients and families who enrolled in the studies and the blood bank staff at the Children’s Hospital of Philadelphia.
This work was supported by the Doris Duke Innovations in Clinical Research Award Grants 2011097 and 2015133 (STC, CMW), National Heart Lung Blood Institute U01 HL134696 (STC, CMW) and by a generous donation from the DiGaetano family.
ABBREVIATIONS:
- DHTR(s)
delayed hemolytic transfusion reaction(s)
- SCD
sickle cell disease
- UPID(s)
unique patient identification number(s)
Footnotes
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- 1.Chou ST, Liem RI, Thompson AA. Challenges of alloimmunization in patients with haemoglobinopathies. Br J Haematol 2012;159:394–404. [DOI] [PubMed] [Google Scholar]
- 2.Yazdanbakhsh K, Ware RE, Noizat-Pirenne F. Red blood cell alloimmunization in sickle cell disease: pathophysiology, risk factors, and transfusion management. Blood 2012;120:528–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heddle NM, Soutar RL, O’Hoski PL, et al. A prospective study to determine the frequency and clinical significance of alloimmunization post-transfusion. Br J Haematol 1995;91: 1000–5. [DOI] [PubMed] [Google Scholar]
- 4.Evers D, Middelburg RA, de Haas M, et al. Red-blood-cell alloimmunisation in relation to antigens’ exposure and their immunogenicity: a cohort study. Lancet Haematol 2016;3: e284–92. [DOI] [PubMed] [Google Scholar]
- 5.Vichinsky EP, Luban NL, Wright E, et al. Prospective RBC phenotype matching in a stroke-prevention trial in sickle cell anemia: a multicenter transfusion trial. Transfusion 2001;41: 1086–92. [DOI] [PubMed] [Google Scholar]
- 6.Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA 2014;312:1033–48. [DOI] [PubMed] [Google Scholar]
- 7.Lasalle-Williams M, Nuss R, Le T, et al. Extended red blood cell antigen matching for transfusions in sickle cell disease: a review of a 14-year experience from a single center. Transfusion 2011;51:1732–9. [DOI] [PubMed] [Google Scholar]
- 8.Wilkinson K, Harris S, Gaur P, et al. Molecular blood typing augments serologic testing and allows for enhanced matching of red blood cells for transfusion in patients with sickle cell disease. Transfusion 2012;52:381–8. [DOI] [PubMed] [Google Scholar]
- 9.Chou ST, Evans P, Vege S, et al. RH genotype matching for transfusion support in sickle cell disease. Blood 2018;132: 1198–207. [DOI] [PubMed] [Google Scholar]
- 10.Chou ST, Jackson T, Vege S, et al. High prevalence of red blood alloimmunization in sickle cell disease despite transfusion from Rh-matched minority donors. Blood 2013;122:1062–71. [DOI] [PubMed] [Google Scholar]
- 11.Vidler JB, Gardner K, Amenyah K, et al. Delayed haemolytic transfusion reaction in adults with sickle cell disease: a 5-year experience. Br J Haematol 2015;169:746–53. [DOI] [PubMed] [Google Scholar]
- 12.Aygun B, Padmanabhan S, Paley C, et al. Clinical significance of RBC alloantibodies and autoantibodies in sickle cell patients who received transfusions. Transfusion 2002;42:37–43. [DOI] [PubMed] [Google Scholar]
- 13.Vichinsky EP, Earles A, Johnson RA, et al. Alloimmunization in sickle cell anemia and transfusion of racially unmatched blood. N Engl J Med 1990;322:1617–21. [DOI] [PubMed] [Google Scholar]
- 14.Talano JA, Hillery CA, Gottschall JL, et al. Delayed hemolytic transfusion reaction/hyperhemolysis syndrome in children with sickle cell disease. Pediatrics 2003;111:e661–5. [DOI] [PubMed] [Google Scholar]
- 15.de Montalembert M, Dumont MD, Heilbronner C, et al. Delayed hemolytic transfusion reaction in children with sickle cell disease. Haematologica 2011;96:801–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petz LD. Bystander immune cytolysis. Transfus Med Rev 2006; 20:110–40. [DOI] [PubMed] [Google Scholar]
- 17.Win N, New H, Lee E, et al. Hyperhemolysis syndrome in sickle cell disease: case report (recurrent episode) and literature review. Transfusion 2008;48:1231–8. [DOI] [PubMed] [Google Scholar]
- 18.Gardner K, Hoppe C, Mijovic A, et al. How we treat delayed haemolytic transfusion reactions in patients with sickle cell disease. Br J Haematol 2015;170:745–56. [DOI] [PubMed] [Google Scholar]
- 19.Win N. Hyperhemolysis syndrome in sickle cell disease. Expert Rev Hematol 2009;2:111–5. [DOI] [PubMed] [Google Scholar]
- 20.Nickel RS, Hendrickson JE, Fasano RM, et al. Impact of red blood cell alloimmunization on sickle cell disease mortality: a case series. Transfusion 2016;56:107–14. [DOI] [PubMed] [Google Scholar]
- 21.Chou ST, Flanagan JM, V S, et al. Whole-exome sequencing for RH genotyping and alloimmunization risk in children with sickle cell anemia. Blood Adv 2017;1:1414–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silvy M, Tournamille C, Babinet J, et al. Red blood cell immunization in sickle cell disease: evidence of a large responder group and a low rate of anti-Rh linked to partial Rh phenotype. Haematologica 2014;99:e115–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reid ME, Lomas-Francis C, Olsson ML. The blood group antigen facts book. 3rd ed. San DIego (CA): Elsevier Academic Press; 2012. [Google Scholar]
- 24.Fasano RM, Booth GS, Miles M, et al. Red blood cell alloimmunization is influenced by recipient inflammatory state at time of transfusion in patients with sickle cell disease. Br J Haematol 2015;168:291–300. [DOI] [PubMed] [Google Scholar]
- 25.Tormey CA, Stack G. The persistence and evanescence of blood group alloantibodies in men. Transfusion 2009;49: 505–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Duration of detection in antibodies associated or not associated with a delayed hemolytic transfusion reaction in patients receiving chronic transfusion. (A) Duration of detection for antibodies that were associated or not associated with a DHTR upon initial detection. (p = 0.7637, Mann-Whitney test). (B) Duration of detection for Rh antibodies that were associated or not associated with a DHTR upon initial detection (p > 0.05, Mann-Whitney test).
TABLE S1. Antibodies detected in 124 patients prior to study period (n = 25)
TABLE S2. RHD* and RHCE* allele status in patient transfused with red cells antigen-matched for CEK but formed Rh antibodies. RHD*DAU0 and RHCE*ce48C which encode proteins that have not been shown to lack epitopes are described as altered but not partial antigens. Ag = antigen; Abs = antibodies.