Abstract
The efficacy of surgical resection in metastatic renal cell carcinoma is an active and important research field in the postcytokine era. Bone metastases, especially in the spine, compromise patient performance status. Metastasectomy is indicated, if feasible, because it helps to achieve the best clinical outcomes possible compared with other treatments. This study examined the postoperative survival and prognostic factors in patients who underwent metastasectomy of spinal lesions. The retrospective study included 65 consecutive patients with metastatic renal cell carcinomas who were operated on by spinal metastasectomy between 1995 and 2017 at our institution. The cancer‐specific survival times from the first spinal metastasectomy to death or the last follow‐up (≥3 years) were determined using Kaplan‐Meier analysis. Potential factors influencing survival were analyzed using Cox proportional hazard models. Planned surgical resection of all the spine tumors was achieved in all patients. Of these, 38 had complete metastasectomy of all visible metastases, including extraspinal lesions. In all patients, the estimated median cancer‐specific survival time was 100 months. The 3‐, 5‐, and 10‐year cancer‐specific survival rates were 77%, 62%, and 48%, respectively. The survival times after spinal metastasectomy were similar in both cytokine and postcytokine groups. In multivariate analyses, postoperative disability, the coexistence of liver metastases, multiple spinal metastases, and incomplete metastasectomy were significant risk factors associated with short‐term survival. Complete metastasectomy, including extraspinal metastases, was associated with improved cancer‐specific survival. Proper patient selection and complete metastasectomy provide a better prognosis in metastatic renal cell carcinoma patients.
Keywords: cancer‐specific survival, metastasectomy, prognostic factor, renal cell carcinoma, spinal metastasis
This study examined the postoperative survival and prognostic factors in 65 metastatic renal cell carcinoma patients who underwent metastasectomy of spinal lesions. In all patients, the 3‐ and 5‐year cancer‐specific survival rates were 77% and 62%, respectively. Postoperative disability, the coexistence of liver metastases, multiple spinal metastases, and incomplete surgical resection with other existing metastases were significant risk factors associated with short‐term survival.

Abbreviations
- CSS
cancer‐specific survival
- ECOG
Eastern Cooperative Oncology Group
- ICI
immune checkpoint inhibitor
- IMDC
International Metastatic Renal Cell Carcinoma Database Consortium
- MSKCC
Memorial Sloan‐Kettering Cancer Center
- mTT
molecular‐targeted therapy
- PS
performance status
- QOL
quality of life
- RCC
renal cell carcinoma
- SRE
skeletal‐related event
- TES
total en bloc spondylectomy
1. INTRODUCTION
Complete surgical resection of renal cell carcinoma (RCC) metastases is associated with improved survival compared with incomplete or no metastasectomy. 1 , 2 , 3 , 4 As per current guidelines, metastasectomy is recommended for patients in whom complete surgical resection is technically feasible or can control symptoms locally. 5 Complete metastasectomy improves survival, and the rate of this procedure has increased in the postcytokine era. 6 , 7 , 8 , 9 However, in previous studies, the distribution of metastatic RCC patients treated with metastasectomy was biased by resectability and not based on the clinical significance of metastasectomy. 6
The bone is the second most common metastatic site of RCC following the lung. 10 RCC bone metastases tend to be highly destructive, easily resulting in pathologic fractures and spinal cord compression from lesions in the spine, which is the most affected bone site. 11 These skeletal‐related events (SREs) severely compromise the patient's performance status (PS) and quality of life (QOL). A lowered PS in patients with metastatic disease affects mortality both directly and indirectly by hindering the delivery of systemic therapies. The presence of bone metastases in patients treated with molecular‐targeted therapy (mTT) has a negative impact on survival. 12 , 13 Bone metastases have the best indication for metastasectomy in patients in whom surgery is feasible. However, there are few comparative studies on the metastasectomy of bone lesions. 14 , 15 Spinal metastasis is considered a negative indicator of overall survival owing to the difficulty of performing surgical resection. 16 The 5‐year survival rate in patients with bone metastasis in the spine is 9%, compared with 30% in the appendicular skeleton. 17 This is because metastasectomy in the spine is more challenging than that in the appendicular skeleton or other organs, even with advancements in surgical techniques and reconstructive materials for spinal disorders. However, there are some institutes well experienced in complete oncological resection of the tumor‐affected vertebra (total en bloc spondylectomy, TES), and spinal metastasectomy has been performed in selected patients. 18 , 19
Our study aimed to examine the survival and potential prognostic factors in RCC patients who underwent spinal metastasectomy. Clinical outcomes were compared between the patients in the cytokine and postcytokine eras, and between those undergoing complete and incomplete metastasectomy.
2. PATIENTS AND METHODS
2.1. Patient selection
After obtaining approval from the Institutional Review Board of the Kanazawa University (IRB number: 2015‐075), a database of RCC patients who underwent spinal metastasectomy at our institution between 1995 and 2017 was retrospectively reviewed at the end of 2020. We obtained the minimum 3‐year follow‐up data of these patients. The study cohort included 65 Asian consecutive patients (53 men and 12 women) with a mean age of 58.8 years. In our institution, spinal metastasectomy was indicated by the following criteria: solitary and removable lesion in the spine (the tumor involving three consecutive vertebrae or less), operability (Eastern Cooperative Oncology Group [ECOG] PS ≤ 3), and stable disease with no other metastasis or a limited number of metastases. However, based on patient request, three patients underwent metastasectomy for symptomatic spinal metastases in coexistence with other small and asymptomatic spinal metastases. Informed consent for surgery was obtained from the patients.
In all patients, the primary RCC was treated using either radical or partial nephrectomy. Thirty (46%) patients had spinal metastases either when the primary RCC was diagnosed or within 1 year after diagnosis (synchronous metastasis). Seventeen (26%) patients had a history of irradiation of the spinal metastases before metastasectomy. The mean dose of radiation was 43.4 Gy, and the mean duration between irradiation and surgery was 18.6 months. Pre‐ or postoperative systemic therapies were used in 57 (88%) patients, and recently approved systemic therapies, including mTT and/or treatment with immune checkpoint inhibitors (ICIs), were used in 31 (48%) patients. Histological diagnoses of specimens from spinal metastasectomy were clear cell type in 62 patients (95%) and nonclear cell type in three patients (5%). At the time of spinal metastasectomy, 24 patients (37%) had metastases only in the operated spine. Nineteen patients had bone metastases at other sites. Three patients had multiple spinal metastases and underwent metastasectomy only of the symptomatic spinal lesion. Metastases were observed at additional sites, including the lungs (19 patients, 29%), lymph nodes (9, 14%), kidneys (3, 5%), liver (2, 3%), and pancreas (1, 2%). There was no patient with brain metastasis. Patients were followed up mostly every 6 months after spinal metastasectomy. Abdominal and chest computed tomography was performed routinely to check the previous metastases and to detect new metastases.
2.2. Surgical procedure
A detailed description of the surgical techniques for TES has been presented in the literature. 20 , 21 In TES, to salvage the spinal cord, surgeons perform en bloc resection of the posterior element (posterior arch) followed by en bloc resection of the anterior part (vertebral body) via a transpedicular osteotomy using a fine threadwire saw (Figure 1). For TES of the cervical and lower lumbar lesions, a posterior‐anterior approach is usually required. The nerve roots at these levels must be preserved to prevent significant neurological deterioration in the extremities. For TES of other vertebral lesions, a single posterior approach is employed. Transection of the nerve roots at the tumor‐affected level is allowed to salvage the spinal cord during blunt dissection and resection of the vertebral body via a posterior approach. In some patients with enlarged tumors expanding to the anterior paravertebral area, a prior anterior dissection via an anterior approach helps surgeons safely perform the subsequent posterior TES procedure.
FIGURE 1.

Total en bloc spondylectomy. A, Operative schema of transpedicular osteotomy using a fine threadwire saw. B, Operative photograph of the resected specimen (en bloc resection of the anterior and posterior parts)
2.3. Features studied
Since 2008, mTTs for RCC have been available in our country. Patients were divided into two groups: cytokine (23 patients, 35%) and postcytokine (42 patients, 65%) (Table 1). Patients in the cytokine group underwent spinal metastasectomy before 2008, and those in the postcytokine group in 2008 or later. Complete metastasectomy was defined as surgical resection of all visible metastases, including the spine lesion, presented until spinal surgery. In contrast, the patients with incomplete metastasectomy had metastatic lesions remaining at other sites, which presented until spinal surgery and were treated with nonsurgical treatment. The main endpoint of this study was postoperative survival defined as the time from the first spinal metastasectomy to death or last follow‐up of at least 3 years. To identify the predictors of postoperative survival, we analyzed the following clinical parameters and outcome data: age (≥60 years); sex (male); pre‐ and postoperative disability (ECOG PS 3); solitary metastasis only in the operated spine; coexistence of lung, liver, lymph node, and nonspinal bone metastases; multiple spinal metastases; multiorgan metastases; synchronous spinal metastasis with primary RCC (within 1 year after RCC diagnoses); synchronous spinal metastasis with other metastases (within 1 year between the diagnoses); enlarged tumors involving multilevel vertebrae; incomplete metastasectomy with other existing metastases; pre‐ and postoperative systemic therapy; pre‐ and postoperative mTT and/or treatment with ICIs; poor risk group according to the Memorial Sloan‐Kettering Cancer Center (MSKCC) 22 and International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) 23 criteria; nonadaptive characteristics for spinal metastasectomy based on prognostic scoring systems for spinal metastases (Tokuhashi score 24 ≤ 11; Tomita score 25 ≥ 5); and abnormal blood levels of serum c‐reactive protein, hemoglobin, corrected calcium, or albumin (Table 2).
TABLE 1.
Patient demographics and treatment
| Total | Cytokine group | Postcytokine group | P‐value | |
|---|---|---|---|---|
| Number of patients | 65 | 23 | 42 | |
| Clinical features at the time of spinal metastasectomy | ||||
| Age (y), mean (SD) | 58.8 (8.9) | 57.0 (9.8) | 59.8 (8.4) | .24 |
| Male, n (%) | 53 (81.5) | 17 (73.9) | 36 (85.7) | .24 |
| Preoperative ECOG PS, n (%) | ||||
| 0 | 8 (12.3) | 1 (4.3) | 7 (16.7) | .35 |
| 1 | 42 (64.6) | 16 (69.6) | 26 (61.9) | |
| 2 | 3 (3.6) | 2 (8.7) | 1 (2.4) | |
| 3 | 12 (18.5) | 4 (17.4) | 8 (19.0) | |
| Distinct metastatic sites, n (%) | ||||
| One (only the operated spine) | 24 (36.9) | 11 (47.8) | 13 (31.0) | .18 |
| Two or more | 41 (63.1) | 12 (52.2) | 29 (69.0) | |
| Location of other metastases, n (%) | ||||
| Lung | 19 (29.2) | 8 (34.8) | 11 (26.2) | .47 |
| Spine (nonoperated) | 3 (4.6) | 0 (0) | 3 (7.1) | .26 |
| Nonspinal bone | 19 (29.2) | 3 (13.0) | 16 (38.1) | <.05 |
| Lymph nodes | 9 (13.8) | 3 (13.0) | 6 (14.3) | .89 |
| Liver | 2 (3.1) | 1 (4.3) | 1 (2.4) | .59 |
| Other locations | 6 (9.2) | 0 (0) | 6 (14.3) | .06 |
| Synchronous metastasis with primary RCC (<1 y), n (%) | 30 (46.2) | 13 (56.5) | 17 (40.5) | .22 |
| MSKCC risk stratification, n (%) | ||||
| Favorable | 16 (24.6) | 5 (21.7) | 11 (26.2) | .66 |
| Intermediate | 44 (67.7) | 17 (73.9) | 27 (64.3) | |
| Poor | 5 (7.7) | 1 (4.3) | 4 (9.5) | |
| IMDC risk stratification, n (%) | ||||
| Favorable | 15 (23.1) | 5 (21.7) | 10 (23.8) | .97 |
| Intermediate | 44 (67.7) | 16 (69.6) | 28 (66.7) | |
| Poor | 6 (9.2) | 2 (8.7) | 4 (9.5) | |
| Tokuhashi score, median (range) | 12 (7‐13) | 12 (7‐13) | 11 (8‐13) | .72 |
| Tomita score, median (range) | 3 (3‐6) | 3 (3‐6) | 4 (3‐6) | .96 |
| Treatment | ||||
| Complete metastasectomy, n (%) | 38 (58.5) | 17 (73.9) | 21 (50.0) | .06 |
| Preoperative systemic therapy, n (%) | ||||
| Immunotherapy | 23 (35.4) | 11 (47.8) | 12 (28.6) | .12 |
| Molecular targeted therapy | 9 (13.8) | 0 (0) | 10 (23.8) | <.01 |
| ICI treatment | 1 (1.5) | 0 (0) | 1 (2.4) | .65 |
| Postoperative systemic therapy, n (%) | ||||
| Immunotherapy | 24 (36.9) | 16 (69.6) | 8 (19.0) | <.001 |
| Molecular targeted therapy | 25 (38.4) | 2 (8.7) | 24 (57.1) | <.001 |
| ICI treatment | 7 (10.8) | 0 (0) | 7 (16.7) | <.05 |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; ICI, immune checkpoint inhibitor; IMDC, International Metastatic Renal Cell Carcinoma Database Consortium; MSKCC, Memorial Sloan‐Kettering Cancer Center; RCC, renal cell carcinoma; SD, standard deviation.
TABLE 2.
Univariate analysis to search risk factors for prognosis
| Parameter | Patients number (%) | Median survival, y | P‐value |
|---|---|---|---|
| Age (y) | |||
| ≥60 | 34 (52.3) | 5.8 | .82 |
| <60 | 31 (47.7) | 7.8 | |
| Gender | |||
| Male | 53 (81.5) | 7.2 | .86 |
| Female | 12 (18.5) | 8.3 | |
| Preoperative ECOG PS | |||
| 3 | 12 (18.5) | 8.3 | .91 |
| 0‐2 | 53 (81.5) | 7.8 | |
| Postoperative ECOG PS | |||
| 3 | 3 (4.6) | 0.7 | <.001 |
| 0‐2 | 62 (95.4) | 10.4 | |
| Solitary metastasis in the operated spine | |||
| Yes | 24 (36.9) | 10.6 | .32 |
| No | 41 (63.1) | 7.3 | |
| Lung metastasis | |||
| Yes | 19 (29.2) | 5.8 | .25 |
| No | 46 (70.8) | 10.6 | |
| Liver metastasis | |||
| Yes | 2 (3.1) | 0.6 | <.001 |
| No | 63 (96.9) | 8.3 | |
| Lymph node metastasis | |||
| Yes | 9 (13.8) | 3.3 | .07 |
| No | 56 (86.2) | 8.3 | |
| Nonspinal bone metastasis | |||
| Yes | 19 (29.2) | 8.3 | .67 |
| No | 46 (70.8) | 7.8 | |
| Multiple spinal metastases | |||
| Yes | 3 (4.6) | 3.3 | <.01 |
| No | 62 (95.4) | 10.4 | |
| Multiorgan metastases | |||
| Yes | 31 (47.7) | 5.8 | .12 |
| No | 34 (52.3) | 11.1 | |
| Synchronous spinal metastasis with primary RCC | |||
| Yes | 30 (46.2) | 10.4 | .74 |
| No | 35 (53.8) | 7.8 | |
| Synchronous spinal metastasis with other metastases | |||
| Yes | 28 (41.5) | 4.7 | .07 |
| No | 37 (58.5) | 11.1 | |
| Enlarged tumors involving multilevel vertebrae | |||
| Yes | 17 (26.2) | 5.0 | .21 |
| No | 48 (73.8) | 10.4 | |
| C‐reactive protein | |||
| >0.3 mg/dL | 20 (30.8) | 5.8 | .38 |
| 0.3 ≤ mg/dL | 45 (69.2) | 10.4 | |
| Corrected calcium | |||
| ≥10 mg/dL | 6 (9.2) | 5.0 | .92 |
| <10 mg/dL | 59 (90.8) | 8.3 | |
| Hemoglobin | |||
| <13.0 g/dL in male | 22 (33.8) | 4.7 | .16 |
| <11.5 g/dL in female | |||
| ≥13.0 g/dL in male | 43 (66.2) | 10.4 | |
| ≥11.5 g/dL in female | |||
| Albumin | |||
| <4.0 g/dL | 30 (46.2) | 7.8 | .54 |
| ≥4.0 g/dL | 35 (53.8) | 8.3 | |
| Incomplete metastasectomy with existing other metastases | |||
| Yes | 27 (41.5) | 4.7 | <.01 |
| No | 38 (58.5) | 11.1 | |
| Systemic therapy | |||
| Yes | 57 (87.7) | 8.3 | .87 |
| No | 8 (12.3) | NR | |
| Molecular‐targeted therapy or/and treatment with immune checkpoint inhibitor | |||
| Yes | 31 (47.7) | 7.3 | .76 |
| No | 34 (52.3) | 10.6 | |
| Poor risk of MSKCC criteria | |||
| Yes | 5 (9.2) | 0.7 | .21 |
| No | 60 (90.8) | 8.3 | |
| Poor risk of IMDC criteria | |||
| Yes | 6 (86.1) | 3.0 | .88 |
| No | 59 (13.9) | 8.3 | |
| Tokuhashi score | |||
| <12 | 32 (49.2) | 7.3 | .47 |
| ≥12 | 33 (50.8) | 10.6 | |
| Tomita score | |||
| ≤5 | 19 (29.2) | 5.8 | .20 |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; IMDC, International Metastatic Renal Cell Carcinoma Database Consortium; MSKCC, Memorial Sloan‐Kettering Cancer Center; NR, not reached; RCC, renal cell carcinoma.
2.4. Statistical methods
Continuous variables are expressed as means ± standard deviations, and survivals and ordinal variables are expressed as medians. The Shapiro‐Wilk test was used to assess the normality of data distribution. Between‐group differences were examined using Student's t test for parametric data and the Mann‐Whitney U test for nonparametric data. Categorical data were expressed as frequencies and percentages, and comparisons between groups were performed using the chi‐squared test. Three patients who died from other causes were excluded from the cancer‐specific survival (CSS) endpoint. Kaplan‐Meier analysis was used to calculate CSS in all patients, and the log‐rank test was used to compare CSS among patient groups in univariate analyses. Associations with time to death from RCC were evaluated using multivariate Cox proportional hazard regression models and summarized with hazard ratios and 95% confidence intervals.
All statistical analyses were performed using SPSS version 25 (IBM Corp.). P‐values < .05 were considered statistically significant.
3. RESULTS
The mean disease‐free interval between resection of the primary RCC and diagnosis of the first developed metastases, which were mostly spinal lesions, was 38.6 (range, 0‐300) months. The mean interval between resection of the primary RCC and resection of spinal metastases was 47.9 (range, 0‐302) months.
To achieve complete oncological resection of the spinal lesions, total vertebrectomy (complete resection of the diseased vertebra: TES) was performed in 57 patients and hemivertebrectomy (complete resection of the metastatic lesion with preservation of the healthy part of the vertebral body) in eight patients. Forty‐eight, five, and 12 patients underwent a single, two consecutive, and three consecutive vertebral resection(s), respectively. Planned surgical resection of the entire spinal tumor was achieved in all 65 patients. Nine patients experienced major perioperative complications, including intraoperative major vessel injury (one patient), spinal cord infarction associated with preoperative arterial embolization (one patient), transient paraparesis (two patients), and deep infection in the surgical site requiring surgical treatment (five patients). There were no operation‐related deaths. Complete metastasectomy was achieved in 38 patients, including 24 with only one solitary metastasis in the operated spine which was treated by metastasectomy. The remaining 14 patients underwent spinal metastasectomy plus metastasectomy for lesions in other organs, which were mostly in the lung; the patients had no remaining metastatic lesions after the metastasectomies accompanied with or without effective systemic therapy for other metastatic lesions. The proportion of patients with coexisting nonspinal bone metastases at the time of spinal metastasectomy in the postcytokine group was significantly higher than that in the cytokine group. There were significant differences in the pre‐ and postoperative systemic therapies between the two groups (Table 1). The percentages of patients undergoing complete metastasectomy were 73.9% (17/23 patients) and 50.0% (21/42 patients) in the cytokine and postcytokine groups, respectively (near‐significant, P = .06).
The mean follow‐up time was 75.1 (range, 3‐251) months. Only four patients (6.2%) experienced a local tumor recurrence in the operated spine. The mean interval between spinal metastasectomy and radiographic tumor recurrence was 17.0 (range, 14‐24) months. They had no more tumor recurrences during the follow‐up period after additional excisional surgeries with or without radiotherapy. Fourteen patients died <3 years after surgery (range of survival time after surgery, 3‐31 months), whereas the remaining 51 patients survived ≥3 years after surgery. In all patients, the 3‐, 5‐, and 10‐year CSS rates were 76.9%, 62.3%, and 48.2%, respectively (Figure 2). The estimated median CSS time was 100 months. The 3‐, 5‐, and 10‐year CSS rates in the 23 patients in the cytokine group were 73.9%, 69.6%, and 56.5%, respectively, compared with 78.6%, 56.1%, and 40.1%, respectively, in the 42 patients in the postcytokine group (Figure 3); the CSS rates were not significantly different between the two groups. The patients with complete metastasectomy had better postoperative survival rates compared with those with incomplete metastasectomy (89.5%, 76.7%, and 60.3% vs 59.3%, 42.0%, and 31.5% for the 3‐, 5‐, and 10‐year CSS rates, respectively; Figure 4). For 19 patients with coexisting lung metastases at the time of surgery, the 3‐, 5‐, and 10‐year CSS rates were 68.4%, 56.8%, and 32.5%, respectively, compared with 80.4%, 64.5%, and 54.6%, respectively, for the 46 patients without lung metastases (Figure 5). The CSS rates were not significantly different between the two groups.
FIGURE 2.
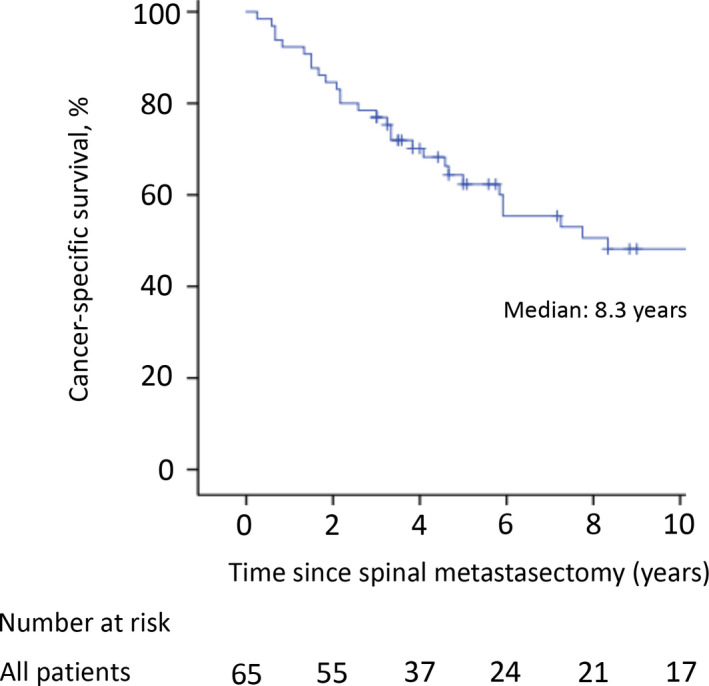
The cancer‐specific survival rates for 3‐, 5‐, and 10 y after surgery were 76.9%, 62.3%, and 48.2%, respectively. The estimated median cancer‐specific survival time was 100 mo. The tick marks indicate the last date of follow‐up
FIGURE 3.
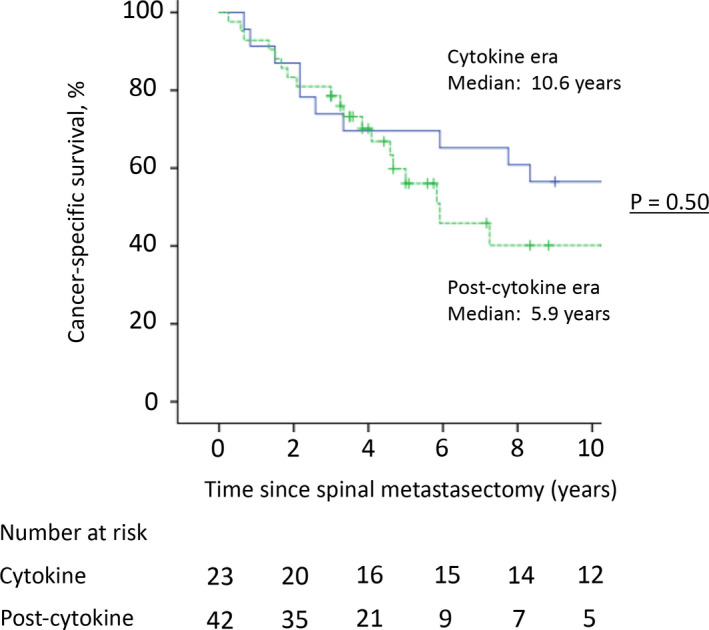
The cancer‐specific survival rates for 3‐, 5‐, and 10 y after surgery in patients undergoing spinal metastasectomy in the cytokine and postcytokine eras were 73.9%, 69.6%, and 56.5%, and 78.6%, 56.1%, and 40.1%, respectively. There was no significant difference between the two groups (P = .50)
FIGURE 4.

Patients who underwent complete metastasectomy, with no remaining metastatic lesions, had better postoperative survival compared with those who underwent incomplete metastasectomy (89.5%, 76.7%, and 60.3% vs 59.3%, 42.0%, and 31.5% for 3‐, 5‐, and 10‐y cancer‐specific survival rates, respectively; P < .01)
FIGURE 5.

The cancer‐specific survival rates in patients with or without lung metastases 3‐, 5‐ and 10 y after surgery were 68.4%, 56.8%, and 32.5%, and 80.4%, 64.5%, and 54.6%, respectively. There was no significant difference between the two groups (P = .25)
Based on the MSKCC criteria, 22 16 (24.6%), 44 (67.7%), and 5 (7.7%) patients were classified into the favorable‐, intermediate‐, and poor‐risk categories, respectively. The estimated median CSS times in the intermediate‐ and poor‐risk groups were 100 and 8 months, respectively (median CSS time was not reached in the favorable group). Based on the IMDC criteria, 23 15 (23.1%), 44 (67.7%), and six (9.2%) patients were classified into the favorable‐, intermediate‐, and poor‐risk categories, respectively. The estimated median survival times in the intermediate‐ and poor‐risk groups were 87 and 36 months, respectively (median CSS time was not reached in the favorable group).
Among the variables examined in the univariate analysis, postoperative disability (ECOG PS 3), the coexistence of liver metastases, multiple spinal metastases, and incomplete metastasectomy were significantly associated with short‐term survival after spinal metastasectomy (Table 2). The coexistence of lymph node (P = .07) and synchronous spinal metastasis with other metastases (P = .07) had near‐significant associations with short‐term survival. In the multivariate analysis including the above six factors, postoperative disability (ECOG PS 3), the coexistence of liver metastases, multiple spinal metastases, and incomplete metastasectomy with other existing metastases were significant risk factors associated with short‐term survival after spinal metastasectomy (Table 3).
TABLE 3.
Multivariate analysis to search risk factors for prognosis
| Hazard ratio | 95% CI | P‐value | |
|---|---|---|---|
| Postoperative disability (ECOG PS 3) | 51.4 | 10.7‐245.6 | <.01 |
| Liver metastasis | 89.7 | 5.98‐1344.4 | <.01 |
| Multiple spinal metastases | 5.7 | 1.14‐28.7 | .03 |
| Incomplete metastasectomy with existing other metastases | 3.0 | 1.09‐8.42 | .03 |
| Lymph node metastasis | 1.7 | 0.58‐4.76 | .35 |
| Synchronous spinal metastasis with other metastases | 0.96 | 0.39‐2.37 | .93 |
Abbreviations: CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status.
4. DISCUSSION
Bone metastases were reported as risk factors associated with short‐term survival in patients with metastatic RCC in the cytokine 26 and postcytokine era. 12 , 13 Even recently approved systemic therapies, including mTT and/or treatment with ICIs, have a limited effect on the control of bone metastasis. 27 Metastasectomy of bone lesions, especially in the spine, has a positive impact on prolonged survival, as well as improved PS because it decreases SREs, including intractable pain and paralysis. Our study is unique in terms of reporting postoperative survival after spinal metastasectomy and identifying risk factors for poor prognosis in multivariate analysis, with the largest case series of 65 patients. In the present study, the 3‐, 5‐, and 10‐year CSS rates were 77%, 62%, and 48%, respectively, and the estimated median CSS time was 100 months in patients with RCC metastases after spinal metastasectomy. These results are more favorable than those previously reported in large studies on patients with RCC metastases. 22 , 23 , 28 When patients in this study were categorized using the MSKCC and IMDC criteria to reduce the effect of selection bias in the evaluation of survival, approximately 70% of patients were found to be of intermediate risk. The estimated median survival times in the intermediate‐risk group, defined using the MSKCC and IMDC criteria, were 100 and 87 months, respectively. These survival times were more favorable than the 14 and 27 months in previous studies for patients classified using the MSKCC and IMDC criteria, respectively. 22 , 23
Spinal lesions easily cause severe SREs, compromising PS and QOL, and result in a poor prognosis. 14 , 16 The lowered PS associated with spinal lesions hinders the application of systemic therapy and indirectly leads to poor prognosis. Because of these factors, the indicated treatment with the best outcomes for solitary and removable bone metastases, especially spinal lesions, is metastasectomy. Although the number of studies on the outcomes in metastasectomy of bone lesions is still limited, 29 recent studies reported that radical resection of bone lesions was one of the important prognostic factors for RCC patients with bone metastases, and the authors concluded that the surgery should be considered to achieve local tumor control and increase survival. 14 , 15 Spinal metastasectomy is technically demanding for ordinary spine surgeons. However, it can improve survival in patients with metastatic RCCs. The previous studies with large sample sizes 9 , 28 reported from our country had better prognostic results for patients with metastatic RCC than those from the Western countries. 7 , 22 , 23 The public health insurance system of our country takes care of all citizens and allows them to undergo spinal metastasectomy, as well as standard treatments, including systemic therapies and nephrectomy. Racial differences may be associated with RCC, resulting in differences in the metastasizing mode and prognosis. These extrasurgical factors could have influenced the study results.
Introduction of mTT and ICI treatment has improved prognosis in patients with metastatic RCCs and changed the treatment strategy. 30 In this new era, metastasectomy is still reported to have the potential to prolong survival in metastatic RCC patients. 6 , 7 , 8 , 9 However, although the present study indicated that survival rates after spinal metastasectomy improved in both cytokine and postcytokine groups, there was no significant difference between the two groups. The utilization of metastasectomy has increased in the postcytokine era. 7 In our institution, the surgical indication for spinal metastasectomy became more common in the postcytokine than in the cytokine era, resulting in an increased proportion of the patients with both nonspinal bone metastases and multiple spinal metastases and those undergoing incomplete metastasectomy in the postcytokine era (Table 1). This could have influenced the results of our study.
In the present study, postoperative disability showing ECOG PS 3 (spinal metastasectomy is contraindicated for patients with ECOG PS 4 in our institute), the coexistence of liver metastases or multiple spinal metastases, and incomplete metastasectomy with other existing metastases treated nonsurgically were significant risk factors associated with short‐term survival after spinal metastasectomy. Many previous studies have reported that PS deterioration was associated with the survival of patients with spinal metastases. 24 , 31 The results of our study indicated an association between short‐term survival and postoperative PS deterioration, but not with preoperative PS deterioration, which was reported as a risk factor for poor prognosis. 24 , 31 Spinal metastasectomy has the potential to improve or prevent PS deterioration. However, the surgery cannot improve PS deterioration due to severe paralysis related to spinal disease or other advanced metastatic lesions. Previous studies have reported that liver metastases were associated with poor prognosis due to the advanced stage of the disease. 9 , 13 In contrast, lung metastases were not associated with short‐term survival in the present study (P = .25). This study included several patients with no evidence of disease after metastasectomies of the spine and lung lesions combined with effective systemic therapies. Three patients with multiple spinal lesions who underwent spinal metastasectomy of symptomatic lesions had limited postoperative survival. In contrast, as previously reported, 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 the current study indicated that complete metastasectomy had a clinical benefit, prolonging survival in metastatic RCC patients. The strategy of complete metastasectomy followed by observation has the extra advantage of sparing patients the additional morbidity of systemic agents, while preserving the efficacy of these agents for use later during disease progression. 8 While spinal metastasectomy has the potential to improve PS and survival, patient selection is essential for appropriate surgical indication and favorable results, including those from the potential application of neoadjuvant systemic therapies.
Limitations of the present study include the relatively small cohort in a single center, retrospective data collection, and analyses only of the patients undergoing spinal metastasectomy, which could introduce biases. However, in contrast, this was the largest study to examine the clinical outcomes of spinal metastasectomy. This study does not compare spinal metastasectomy with any controls. Therefore, we compare the efficacy of spinal metastasectomy with the results presented in previous studies. We could obtain neither detailed information about the primary lesion (tumor size, pathologic stage, and tumor nuclear grade) nor the indication of pre‐ and postoperative systemic therapies because nephrectomy and systemic therapies by primary physicians were mostly performed at other hospitals. Despite these limitations, this study provided informative clinical results of spinal metastasectomy and indicated that patients with locally curative and solitary spinal metastases could benefit from complete surgical resection of spinal metastases. Furthermore, for selected patients, this procedure can potentially prolong survival.
In conclusion, in the present study, the 5‐ and 10‐year CSS rates in patients who underwent metastasectomy of RCC‐derived spinal lesions were 62% and 48%, respectively. Although spinal metastasectomy is challenging, proper patient selection and complete metastasectomy provide a better prognosis in metastatic RCC patients.
DISCLOSURE
The authors declare that they have no competing interests.
ACKNOWLEDGEMENTS
We would like to thank Editage (https://www.editage.jp/) for English language editing.
Kato S, Demura S, Murakami H, et al. Clinical outcomes and prognostic factors following the surgical resection of renal cell carcinoma spinal metastases. Cancer Sci. 2021;112:2416–2425. 10.1111/cas.14902
REFERENCES
- 1. Leibovich BC, Cheville JC, Lohse CM, et al. A scoring algorithm to predict survival for patients with metastatic clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. J Urol. 2005;174:1759‐1763. [DOI] [PubMed] [Google Scholar]
- 2. Dabestani S, Marconi L, Hofmann F, et al. Local treatments for metastases of renal cell carcinoma: a systematic review. Lancet Oncol. 2014;15:e549‐e561. [DOI] [PubMed] [Google Scholar]
- 3. Zaid HB, Parker WP, Safdar NS, et al. Outcomes following complete surgical metastasectomy for patients with metastatic renal cell carcinoma: a systematic review and meta‐analysis. J Urol. 2017;197:44‐49. [DOI] [PubMed] [Google Scholar]
- 4. Psutka SP, Master VA. Role of metastasis‐directed treatment in kidney cancer. Cancer. 2018;124:3641‐3655. [DOI] [PubMed] [Google Scholar]
- 5. Motzer RJ, Jonasch E, Agarwal N, et al. Kidney cancer, version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:804‐834. [DOI] [PubMed] [Google Scholar]
- 6. You D, Lee C, Jeong IG, et al. Impact of metastasectomy on prognosis in patients treated with targeted therapy for metastatic renal cell carcinoma. J Cancer Res Clin Oncol. 2016;142:2331‐2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sun M, Meyer CP, Karam JA, et al. Predictors, utilization patterns, and overall survival of patients undergoing metastasectomy for metastatic renal cell carcinoma in the era of targeted therapy. Eur J Surg Oncol. 2018;44:1439‐1445. [DOI] [PubMed] [Google Scholar]
- 8. Lyon TD, Thompson RH, Shah PH, et al. Complete surgical metastasectomy of renal cell carcinoma in the post‐cytokine era. J Urol. 2020;203:275‐282. [DOI] [PubMed] [Google Scholar]
- 9. Ishihara H, Takagi T, Kondo T, et al. Prognostic impact of metastasectomy in renal cell carcinoma in the postcytokine therapy era. Urol Oncol. 2021;39:77.e17‐77.e25. [DOI] [PubMed] [Google Scholar]
- 10. Bianchi M, Sun M, Jeldres C, et al. Distribution of metastatic sites in renal cell carcinoma: a population‐based analysis. Ann Oncol. 2012;23:973‐980. [DOI] [PubMed] [Google Scholar]
- 11. Santini D, Procopio G, Porta C, et al. Natural history of malignant bone disease in renal cancer: final results of an Italian bone metastasis survey. PLoS One. 2013;8:e83026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Beuselinck B, Oudard S, Rixe O, et al. Negative impact of bone metastasis on outcome in clear‐cell renal cell carcinoma treated with sunitinib. Ann Oncol. 2011;22:794‐800. [DOI] [PubMed] [Google Scholar]
- 13. McKay RR, Kroeger N, Xie W, et al. Impact of bone and liver metastases on patients treated with targeted therapy. Eur Urol. 2014;65:577‐584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Du YueJun, Pahernik S, Hadaschik B, et al. Survival and prognostic factors of patients with renal cell cancer with bone metastasis in the era of targeted therapy: a single‐institution analysis. Urol Oncol. 2016;34:433.e1‐8. [DOI] [PubMed] [Google Scholar]
- 15. Ruatta F, Derosa L, Escudier B, et al. Prognosis of renal cell carcinoma with bone metastasis: experience from a large cancer centre. Eur J Cancer. 2019;107:79‐85. [DOI] [PubMed] [Google Scholar]
- 16. Kume H, Kakutani S, Yamada Y, et al. Prognostic factors for renal cell carcinoma with bone metastasis: who are the long‐term survivors? J Urol. 2011;185:1611‐1614. [DOI] [PubMed] [Google Scholar]
- 17. Jung ST, Ghert MA, Harrelson JM, et al. Treatment of osseous metastases in patients with renal cell carcinoma. Clin Orthop Relat Res. 2003;409:223‐231. [DOI] [PubMed] [Google Scholar]
- 18. Kato S, Murakami H, Demura S, et al. More than 10‐year follow‐up after total en bloc spondylectomy for spinal tumors. Ann Surg Oncol. 2014;21:1330‐1336. [DOI] [PubMed] [Google Scholar]
- 19. Xu K, Li J, Hu M, et al. Prognostic significance of preoperative inflammatory biomarkers and traditional clinical parameters in patients with spinal metastasis from clear cell renal cell carcinoma: a retrospective study of 95 patients in a single center. Cancer Manag Res. 2020;12:59‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tomita K, Kawahara N, Murakami H, et al. Total en bloc spondylectomy for spinal tumors: improvement of the technique and its associated basic background. J Orthop Sci. 2006;11:3‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kawahara N, Tomita K, Murakami H, et al. Total en bloc spondylectomy for spinal tumors: surgical techniques and related basic background. Orthop Clin N Am. 2009;40:47‐63. [DOI] [PubMed] [Google Scholar]
- 22. Motzer RJ, Bacik J, Murphy BA, et al. Interferon‐alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20:289‐296. [DOI] [PubMed] [Google Scholar]
- 23. Heng DYC, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor‐targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27:5794‐5799. [DOI] [PubMed] [Google Scholar]
- 24. Tokuhashi Y, Matsuzaki H, Oda H, et al. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976). 2005;30:2186‐2191. [DOI] [PubMed] [Google Scholar]
- 25. Tomita K, Kawahara N, Kobayashi T, et al. Surgical strategy for spinal metastases. Spine (Phila Pa 1976). 2001;26:298‐306. [DOI] [PubMed] [Google Scholar]
- 26. Donskov F, von der Maase H. Impact of immune parameters on long‐term survival in metastatic renal cell carcinoma. J Clin Oncol. 2006;24:1997‐2005. [DOI] [PubMed] [Google Scholar]
- 27. Negishi T, Furubayashi N, Takamatsu D, et al. Radiographical efficacy of systemic treatment for bone metastasis from renal cell carcinoma. Oncol Lett. 2020;20:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Naito S, Yamamoto N, Takayama T, et al. Prognosis of Japanese metastatic renal cell carcinoma patients in the cytokine era: a cooperative group report of 1463 patients. Eur Urol. 2010;57:317‐325. [DOI] [PubMed] [Google Scholar]
- 29. Ouzaid I, Capitanio U, Staehler M, et al. Surgical metastasectomy in renal cell carcinoma: a systematic review. Eur Urol Oncol. 2019;2:141‐149. [DOI] [PubMed] [Google Scholar]
- 30. Lalani A‐K, McGregor BA, Albiges L, et al. Systemic treatment of metastatic clear cell renal cell carcinoma in 2018: current paradigms, use of immunotherapy, and future directions. Eur Urol. 2019;75:100‐110. [DOI] [PubMed] [Google Scholar]
- 31. Massaad E, Hadzipasic M, Alvarez‐Breckenridge C, et al. Predicting tumor‐specific survival in patients with spinal metastatic renal cell carcinoma: which scoring system is most accurate? J Neurosurg Spine. 2020;5:1‐11. [DOI] [PubMed] [Google Scholar]


