Abstract
Background
COVID-19 is associated with a prothrombotic state leading to adverse clinical outcomes. Whether therapeutic anticoagulation improves outcomes in patients hospitalised with COVID-19 is unknown. We aimed to compare the efficacy and safety of therapeutic versus prophylactic anticoagulation in this population.
Methods
We did a pragmatic, open-label (with blinded adjudication), multicentre, randomised, controlled trial, at 31 sites in Brazil. Patients (aged ≥18 years) hospitalised with COVID-19 and elevated D-dimer concentration, and who had COVID-19 symptoms for up to 14 days before randomisation, were randomly assigned (1:1) to receive either therapeutic or prophylactic anticoagulation. Therapeutic anticoagulation was in-hospital oral rivaroxaban (20 mg or 15 mg daily) for stable patients, or initial subcutaneous enoxaparin (1 mg/kg twice per day) or intravenous unfractionated heparin (to achieve a 0·3–0·7 IU/mL anti-Xa concentration) for clinically unstable patients, followed by rivaroxaban to day 30. Prophylactic anticoagulation was standard in-hospital enoxaparin or unfractionated heparin. The primary efficacy outcome was a hierarchical analysis of time to death, duration of hospitalisation, or duration of supplemental oxygen to day 30, analysed with the win ratio method (a ratio >1 reflects a better outcome in the therapeutic anticoagulation group) in the intention-to-treat population. The primary safety outcome was major or clinically relevant non-major bleeding through 30 days. This study is registered with ClinicalTrials.gov (NCT04394377) and is completed.
Findings
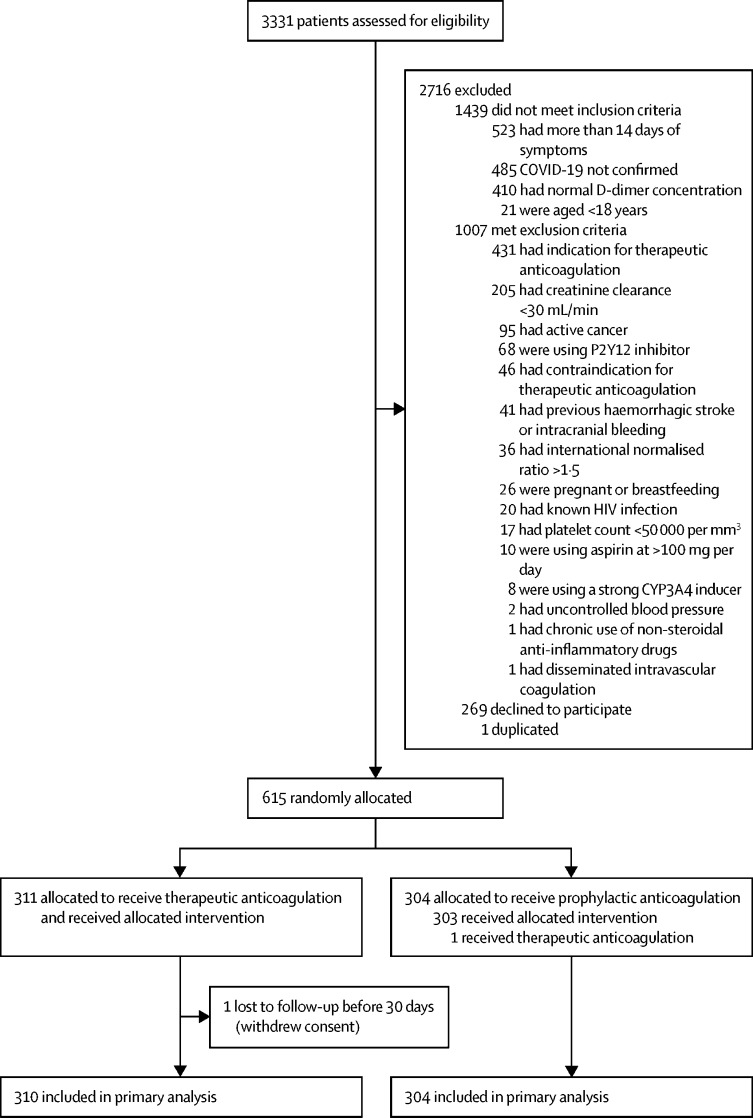
From June 24, 2020, to Feb 26, 2021, 3331 patients were screened and 615 were randomly allocated (311 [50%] to the therapeutic anticoagulation group and 304 [50%] to the prophylactic anticoagulation group). 576 (94%) were clinically stable and 39 (6%) clinically unstable. One patient, in the therapeutic group, was lost to follow-up because of withdrawal of consent and was not included in the primary analysis. The primary efficacy outcome was not different between patients assigned therapeutic or prophylactic anticoagulation, with 28 899 (34·8%) wins in the therapeutic group and 34 288 (41·3%) in the prophylactic group (win ratio 0·86 [95% CI 0·59–1·22], p=0·40). Consistent results were seen in clinically stable and clinically unstable patients. The primary safety outcome of major or clinically relevant non-major bleeding occurred in 26 (8%) patients assigned therapeutic anticoagulation and seven (2%) assigned prophylactic anticoagulation (relative risk 3·64 [95% CI 1·61–8·27], p=0·0010). Allergic reaction to the study medication occurred in two (1%) patients in the therapeutic anticoagulation group and three (1%) in the prophylactic anticoagulation group.
Interpretation
In patients hospitalised with COVID-19 and elevated D-dimer concentration, in-hospital therapeutic anticoagulation with rivaroxaban or enoxaparin followed by rivaroxaban to day 30 did not improve clinical outcomes and increased bleeding compared with prophylactic anticoagulation. Therefore, use of therapeutic-dose rivaroxaban, and other direct oral anticoagulants, should be avoided in these patients in the absence of an evidence-based indication for oral anticoagulation.
Funding
Coalition COVID-19 Brazil, Bayer SA.
Research in context.
Evidence before this study
COVID-19 has been associated with higher incidence and magnitude of thrombotic complications compared with other respiratory infections, and thrombosis has been shown to lead to worse outcomes in these patients. Observational studies have suggested that both therapeutic and prophylactic anticoagulation might be associated with lower in-hospital mortality and less frequent intubation compared with no anticoagulation treatment. Therefore, therapeutic anticoagulation has been considered as a treatment option for patients hospitalised with COVID-19, on the basis of low-quality evidence. We searched MEDLINE, the Cochrane Central register of Controlled Trials (CENTRAL), Web of Science, and Scopus using the terms (“rivaroxaban” OR “apixaban” OR “dabigatran” OR “edoxaban” OR “heparin” OR “enoxaparin”) AND (“SARS-CoV-2” OR “COVID” OR “coronavirus” OR “COVID-19”) AND (“randomised” OR “clinical trials”), with no date or language restrictions. We did not find any published randomised clinical trial assessing the effects of therapeutic anticoagulation on clinical outcomes in COVID-19.
Added value of this study
Our study is the first randomised clinical trial with a sample size calculated to assess the effect of the therapeutic use of an oral anticoagulant on clinical outcomes in patients hospitalised with COVID-19 and elevated D-dimer concentration in comparison with prophylactic anticoagulation. The results of the current study show that, in patients hospitalised with COVID-19 and elevated D-dimer concentration, therapeutic anticoagulation with rivaroxaban in clinically stable patients and heparin in clinically unstable patients did not improve clinical outcomes or reduce death, and increased bleeding when compared with thromboprophylaxis with heparin. Our protocol and statistical analysis plan were made public and registered before closing the database, and the study was designed to minimise bias by a pragmatic protocol which was strictly followed. All clinical outcomes, including bleeding and thrombotic events, were systematically and centrally adjudicated by a clinical events committee whose members were masked to the treatment assignment. We provided detailed and clear descriptions of clinical parameters and clinical outcomes definitions. Therefore, our study provides high-quality evidence to guide clinical practice in a field where most medical decisions have been made on the basis of low-quality evidence.
Implications of all the available evidence
Contrary to what has been shown in preliminary data with the use of heparins, the current study showed that a strategy primarily using an oral anticoagulant at a therapeutic dose did not result in clinical improvement of patients hospitalised with COVID-19. Because the most favourable anticoagulation approach during hospitalisation is still not defined, and many off-label and non-evidence-based strategies have been used, our results will help physicians in the decision-making process when treating patients in this clinical setting.
Introduction
Initial data have shown that COVID-19 is associated with a higher risk of thrombotic complications, and a greater magnitude of these events, than are other respiratory infections.1, 2 Additionally, thrombosis, disseminated intravascular coagulation, and cytokine storm have been associated with more severe progression and worse outcomes in COVID-19.1, 2, 3 A thromboinflammatory state, associated with endothelial dysfunction, hypercoagulability, and coagulation activation, leads to an increased risk of microvascular and macrovascular thrombosis.1, 2, 3, 4 However, the short-term and long-term clinical effects of thrombotic events in this setting have not yet been elucidated.5
COVID-19 thrombotic complications include arterial and venous events, with microvascular thrombosis possibly contributing to the diffuse lung injury seen in patients with COVID-19.1, 2, 3, 4 D-dimer concentration has also been identified as a marker for both thrombotic and bleeding events in this population, and is thought to identify higher-risk patients.3, 6
Current recommendations for thromboprophylaxis in patients hospitalised with COVID-19 are based on existing evidence in similar medical conditions.7, 8, 9 Observational studies have suggested that, compared with no anticoagulation, both therapeutic and prophylactic anticoagulation might be associated with lower in-hospital mortality and less intubation.10 Based on these observations, therapeutic anticoagulation has been considered as a treatment option for patients hospitalised with COVID-19. However, the optimal strategy, including the type and dose of anticoagulant and the duration of treatment, remains unknown.10, 11
Rivaroxaban is a widely used, direct oral inhibitor of factor Xa that is recommended for thromboprophylaxis in a variety of clinical indications.12, 13 To assess whether therapeutic anticoagulation is effective in preventing complications in patients hospitalised with COVID-19 and elevated D-dimer concentration, we did a randomised controlled trial comparing the efficacy and safety of therapeutic versus prophylactic anticoagulation.
Methods
Study design
The rationale and design for the AntiCoagulaTlon cOroNavirus (ACTION) trial has been published previously.14 In brief, ACTION was a pragmatic, open-label (with blinded adjudication), multicentre, randomised, controlled trial in patients hospitalised with COVID-19 and elevated D-dimer concentration. The study was done at 31 hospitals in Brazil. The aim of the trial was to assess whether in-hospital anticoagulation with rivaroxaban (20 mg once daily) for patients with a stable condition or enoxaparin (1 mg/kg twice daily) for patients with an unstable condition, followed by rivaroxaban for 30 days, compared with mainly in-hospital prophylactic anticoagulation with heparin decreased the time to death, duration of hospitalisation, or duration of supplemental oxygen support.
The study was designed and led by academic executive and steering committees (appendix p 2) whose members, together with operational staff from the Brazilian Clinical Research Institute (BCRI; São Paulo, Brazil), coordinated the medical, scientific, and operational conduct of the study. The BCRI was responsible for data management, site management, clinical events adjudication, safety surveillance, and all statistical analyses. The Academic Research Organization from Hospital Israelita Albert Einstein (São Paulo, Brazil) was responsible for regulatory affairs and did an independent confirmatory statistical analysis. The trial protocol (appendix pp 20–68) was approved by institutional research ethics boards at participating sites. The first and last versions of the protocol and a summary of changes, with their respective dates, are included in the appendix (pp 20–68). An independent data and safety monitoring board reviewed unmasked patient-level data for safety on an ongoing basis during the trial, and did a formal interim analysis with complete 30-day follow-up data for the first 300 patients.
In accordance with local regulatory requirements, informed consent was obtained by written consent or by an approved electronic signature from each patient or from the patient's legal representative.
Participants
We included patients hospitalised with a confirmed diagnosis of COVID-19, with symptoms for up to 14 days before randomisation, and elevated D-dimer concentration (above the upper limit of normal reference range per local laboratory). Study entry required confirmation of COVID-19 based on specific tests used in clinical practice (RT-PCR, antigen test, or IgM test), on samples collected up to 14 days before randomisation, regardless of whether patients were in the hospital or not. Exclusion criteria included a formal indication for therapeutic anticoagulation, contraindications to rivaroxaban or heparin, and conditions placing patients at high risk for bleeding. Patients who had received therapeutic anticoagulation without an indication other than COVID-19 could be included if anticoagulation was used for less than 48 h and could be stopped at study entry. Full eligibility criteria are provided in the appendix (p 6).
Randomisation and masking
Patients were randomly allocated to receive therapeutic anticoagulation for 30 days (rivaroxaban if clinically stable or enoxaparin if clinically unstable) or in-hospital prophylactic anticoagulation (enoxaparin or unfractionated heparin). Randomisation was done in a 1:1 ratio in permuted blocks of variable size, stratified according to clinical condition (stable or unstable), using a central, concealed, web-based, automated randomisation system. There was no masking of patients or investigators to group allocation.
Procedures
Clinically stable patients assigned to receive therapeutic anticoagulation were given oral rivaroxaban at a dose of 20 mg once daily. A reduced dose of 15 mg once daily was used in patients with a creatinine clearance of 30–49 mL/min or those taking azithromycin. Patients were considered to be in a clinically unstable condition if they had COVID-19-related critical illness, a life-threatening condition, a requirement for mechanical ventilation or vasopressors, or were unable (based on investigator assessment) to take oral medication. Those in an unstable condition received subcutaneous enoxaparin at a dose of 1 mg/kg twice per day, or intravenous unfractionated heparin at a dose to achieve a target anti-Xa concentration (0·3–0·7 IU/mL) or a corresponding target activated partial thromboplastin time (1·5–2·5 times the mean normal value). Unfractionated heparin was the preferred option for patients with renal dysfunction or disseminated intravascular coagulation. When these patients became stable, they were transitioned to oral rivaroxaban (20 mg or 15 mg, as described above). All patients in the therapeutic anticoagulation group continued treatment to day 30 with the same dose of rivaroxaban.
Patients assigned to receive prophylactic anticoagulation were given standard venous thromboembolism prophylaxis with enoxaparin or unfractionated heparin during hospitalisation and could receive extended prophylaxis at the discretion of the treating physician (appendix p 7). Patients in this group could receive therapeutic anticoagulation if they developed a definitive clinical indication (eg, objectively confirmed deep vein thrombosis) or at the discretion of the investigator if a high clinical suspicion of a thromboembolic event was raised and a confirmatory test was not available. All other clinical care was provided at the discretion of the treating physician based on current guidelines and local standards of care. Because the trial tested two anticoagulation strategies rather than comparing anticoagulants, a crossover was only considered if a patient changed from prophylactic to therapeutic anticoagulation (or vice versa) and not between different drugs within the same study group, which was allowed by the protocol.
Baseline assessment included demographic characteristics, risk factors, medical history, and laboratory data. During hospitalisation, data were collected daily. After discharge, follow-up was done by telephone at 30 days to assess study outcomes and at 60 days for additional safety information.
Adherence to medication was calculated according to the duration of anticoagulation recommended by the protocol for each group: 30 days in the therapeutic group and during the in-hospital period in the prophylaxis group. The analysis of adherence during hospitalisation in both groups was based on daily visits and the analysis of adherence after discharge in the therapeutic group was based on pill count. Days of study treatment interruption due to the occurrence of clinical outcomes or adverse events (eg, bleeding) were not included in the final adherence calculation.
Outcomes
The primary efficacy outcome was a hierarchical composite of time to death, duration of hospitalisation, or duration of supplemental oxygen use through 30 days. We also defined two secondary composite outcomes of venous thromboembolism, acute myocardial infarction, any stroke (ischaemic or haemorrhagic), systemic embolism, and major adverse limb events, with and without all-cause death.14
The primary safety outcome was major or clinically relevant non-major bleeding defined according to the International Society on Thrombosis and Haemostasis (ISTH) criteria.15 Analyses of bleeding events using Bleeding Academic Research Consortium, Thrombolysis in Myocardial Infarction, and Global Use of Strategies to Open Occluded Arteries definitions were also done.16 We also prespecified a composite net benefit outcome composed of the composite efficacy outcome and the primary safety outcomes (see statistical analysis plan in the appendix [pp 72–99]).14
Secondary outcomes were duration of supplemental oxygen or non-invasive or mechanical ventilation support, disease progression (mild, moderate, or severe), rehospitalisation, and World Health Association 8-point ordinal scale (death; invasive mechanical ventilation and support for another organ dysfunction; invasive mechanical ventilation alone; non-invasive ventilation or high-flow oxygen; hospitalised on supplemental oxygen; hospitalised without requirement for supplemental oxygen; not hospitalised, with limitation on activities or requirement for oxygen at home; not hospitalised, without limitations on activities).17, 18 Inflammatory and coagulation biomarkers and troponin were measured on the basis of local practice at each site and analysed in our study as exploratory outcomes (data not shown).
Prespecified subgroup analyses were age; sex; days from symptom onset; D-dimer concentration; baseline use of antiviral drugs, parenteral anticoagulation, or corticosteroids; body-mass index (≤30 kg/m2 or >30 kg/m2); cardiovascular comorbidities; and baseline severity of illness.
An independent clinical events classification committee, whose members were unaware of treatment assignment, adjudicated cause of death and all potential thrombotic and bleeding events. All presumed or suspected thrombotic events were reported for adjudication, regardless of availability of imaging testing. If an imaging test was available and positive, the event was classified as a confirmed pulmonary embolism. If imaging results were not available, but there was a high clinical suspicion of pulmonary embolism (eg, sudden hypoxaemia not explained by worsening of pulmonary infiltrates, or right ventricular dysfunction), the case was classified as probable pulmonary embolism. Finally, patients who died with unknown cause of death were classified as a possible pulmonary embolism according to the clinical events classification charter. Outcome definitions are provided in the appendix (p 5).
Statistical analysis
The statistical analysis plan, including stopping rules for the interim analysis, was completed before the end of the study and unblinding of the study results, and is available in the appendix (pp 72–99). Since the interim analysis used restrictive decision limits (Haybittle-Peto), the final p values were not adjusted in the study results. The primary analyses followed the intention-to-treat principle, including all randomly allocated participants. Results for the primary outcome are reported according to the win ratio method, considering treatment as a fixed effect stratified by clinical condition (stable or unstable) truncated at 30 days.19, 20 Using this method, all patients in the treatment group were compared with all patients in the control group within each strata. Initially, the pairs were compared for time until death, truncated at 30 days. If both patients died, the “winner” of the comparison was the one who had a longer time between the time of randomisation and the date of death (at least 1 day later). If the match was tied (both patients died within the same follow-up time or both remained alive until the 30-day visit), the pair were then compared for the length of hospital stay and the one with the shortest length of stay was declared the “winner” (considering a difference higher than 2 days). Finally, if a second tie occurred, patients were compared for the days of oxygen-free support until the 30-day visit and the one with the longest time without oxygen support was declared the “winner” (considering a difference higher than 2 days). Thus, the win ratio represents the total number of wins divided by the total number of losses between the two study groups (therapeutic vs prophylactic) within each strata, and a value greater than 1 indicates a better outcome in the therapeutic anticoagulation group. The final test used a significance level of 5% (appendix p 11).
Each component of the hierarchical outcome was compared individually using the same win ratio method. Binary secondary outcomes at 30 days were compared using log binomial models and results were expressed as relative risk (RR). Disease progression and 8-point ordinal scale outcomes were compared using cumulative proportional odds ratio models.17, 18 95% CIs were estimated for all effect measures. The widths of the intervals for secondary and other outcomes were not adjusted for multiplicity.
The sample size was calculated on the basis of simulations of scenarios of the win ratio assuming the following outcomes in the control group from the Coalition I trial.21 For a two-sided α of 0·05, 600 patients provide 94% power for the hierarchical analysis by win ratio, considering a mortality of 7%; 6 days SD in number of days alive and out of hospital, and 5 days SD in number of days free of oxygen support; and assuming that therapeutic anticoagulation would reduce by 2% (absolute reduction) all-cause mortality, reduce the mean number of days in hospital by 1·5 days, and reduce the mean number of days of oxygen support by 1·5 days.
Subgroup analyses for the primary outcome by win ratio were done with use of the weighted inverse variance strategy.19, 20
All analyses were done with R software (version 4.0.2).
This study is registered with ClinicalTrials.gov (NCT04394377).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
From June 24, 2020, to Feb 26, 2021, 3331 patients were screened at 31 sites in Brazil and 615 were randomly allocated (311 [50%] to the therapeutic anticoagulation group and 304 [50%] to the prophylactic anticoagulation group; figure 1 ). One (<1%) patient, in the therapeutic group, was lost to follow-up because of withdrawal of consent and was not included in the primary analysis. All patients in the trial were confirmed to be COVID-19 positive, except for one (<1%) patient who tested negative but had a high probability of COVID-19 and was, therefore, included in the primary intention-to-treat analysis. Regarding other protocol deviations, seven (1%) patients (six [2%] in the therapeutic group and one [<1%] in the prophylactic anticoagulation group) had D-dimer concentration assessed more than 72 h before randomisation, and 12 (2%) patients (all in the therapeutic anticoagulation group) received 20 mg rivaroxaban instead of 15 mg, with concomitant use of azithromycin for more than 48 h. The maximum number of days of concomitant use of azithromycin and rivaroxaban 20 mg was 6 days (two patients). No bleeding events were seen in these 12 patients.
Figure 1.
Trial profile
Baseline characteristics were well balanced between groups (table 1 ; appendix p 8). Overall, the mean age was 56·6 years (SD 14·3), 368 (60%) participants were men, and mean body-mass index was 30·3 kg/m2 (SD 6·0). At baseline, 460 (75%) patients were receiving supplemental oxygen and 39 (6%) were in a clinically unstable condition. Data on medication use at baseline are provided in the appendix (p 9). The median time from symptom onset to hospital admission was 8·0 days (IQR 6·0–10·0) and the median time from hospital admission to randomisation was 2·0 days (1·0–3·0).
Table 1.
Baseline characteristics and medications
| Therapeutic anticoagulation group (n=311) | Prophylactic anticoagulation group (n=304) | ||
|---|---|---|---|
| Age, years | 56·7 (14·1) | 56·5 (14·5) | |
| Sex | |||
| Male | 192 (62%) | 176 (58%) | |
| Female | 119 (38%) | 128 (42%) | |
| Body-mass index, kg/m2 | 30·3 (6·0) | 30·3 (6·1) | |
| Comorbidities | |||
| Asthma | 18 (6%) | 11 (4%) | |
| Chronic lung disease | 7 (2%) | 12 (4%) | |
| Malignant neoplasm | 12 (4%) | 4 (1%) | |
| Diabetes | 83 (27%) | 67 (22%) | |
| Hypertension | 151 (49%) | 151 (50%) | |
| Heart failure | 8 (3%) | 5 (2%) | |
| Coronary disease | 12 (4%) | 16 (5%) | |
| History of thromboembolism | 2 (1%) | 4 (1%) | |
| Smoking habits | |||
| Never smoked | 255 (82%) | 241 (79%) | |
| Current or former smoker | 56 (18%) | 63 (21%) | |
| Clinical condition* | |||
| Unstable | 23 (7%) | 16 (5%) | |
| Stable | 288 (93%) | 288 (95%) | |
| Time from symptom onset to randomisation, days | 10·0 (9·0–12·0) | 10·0 (8·0–12·0) | |
| Time from symptom onset to hospital admission, days | 8·0 (6·0–10·0) | 7·0 (6·0–9·0) | |
| Time from hospital admission to randomisation, days | 2·0 (1·0–3·0) | 2·0 (1·0–3·0) | |
| Oxygen support required | 236 (76%) | 224 (74%) | |
| Catheter or oxygen mask | 185 (59%) | 184 (61%) | |
| High-flow nasal cannula | 26 (8%) | 22 (7%) | |
| Tracheal intubation | 23 (7%) | 15 (5%) | |
| Non-invasive ventilation | 2 (1%) | 3 (1%) | |
| Disease state at baseline† | |||
| Mild | 30 (10%) | 39 (13%) | |
| Moderate | 257 (83%) | 249 (82%) | |
| Severe | 24 (8%) | 16 (5%) | |
| Anticoagulation before randomisation | 285 (92%) | 275 (90%) | |
| Standard prophylactic dose | 175 (56%) | 187 (62%) | |
| Greater than standard prophylactic dose‡ | 110 (35%) | 88 (29%) | |
| Baseline medication | |||
| Antiplatelet | 22 (7%) | 26 (9%) | |
| Vasopressor | 16 (5%) | 8 (3%) | |
| Systemic corticosteroids | 257 (83%) | 253 (83%) | |
| D-dimer concentration | |||
| ≥1 × upper limit of normal | 311 (100%) | 304 (100%) | |
| ≥3 × upper limit of normal | 84 (27%) | 83 (27%) | |
| Creatinine clearance§, mL/min | 106·6 (82·9–143·4) | 105·7 (76·9–145·1) | |
Data are mean (SD), n (%), or median (IQR).
Unstable clinical condition was defined as the presence of a COVID-19-related critical illness with an immediately life-threatening condition that would typically lead to intensive care unit admission.
Mild disease includes cases not meeting the criteria for classification as moderate or severe disease; moderate disease was characterised by an oxygen saturation <94%, pulmonary infiltrates >50%, or a partial pressure of oxygen to fractional concentration of oxygen in inspired air ratio <300; and severe disease was defined as respiratory failure, haemodynamic instability, or multiple organ dysfunction.
Any dose greater than the recommended doses as shown in the appendix (p 7).
Calculated using the Cockcroft-Gault equation.
Overall, all patients in the therapeutic anticoagulation group and 303 (>99%) of 304 in the prophylactic anticoagulation group received anticoagulation according to the study protocol (appendix p 10). In the therapeutic group, 29 (9%) patients initially received enoxaparin (median 11 days [IQR 7–14]) and one (<1%) received unfractionated heparin; these 30 patients included seven who were stable at randomisation but became unstable before their first dose of study medication. The remaining 280 (90%) patients in the therapeutic group received rivaroxaban, of whom 214 (76%) were initially treated with 20 mg once daily and 66 (24%) with 15 mg once daily. Of those in the prophylactic anticoagulation group who received anticoagulation, 256 (84%) patients started on enoxaparin and 47 (16%) on unfractionated heparin. The remaining patient was discharged the same day and, therefore, did not receive any heparin. 38 (13%) were prescribed extended prophylaxis beyond hospital discharge. Mean 30-day adherence to the study intervention was 94·8% (SD 15·2) in patients allocated to therapeutic anticoagulation group and 99·5% (6·2) in the prophylactic group (appendix p 10).
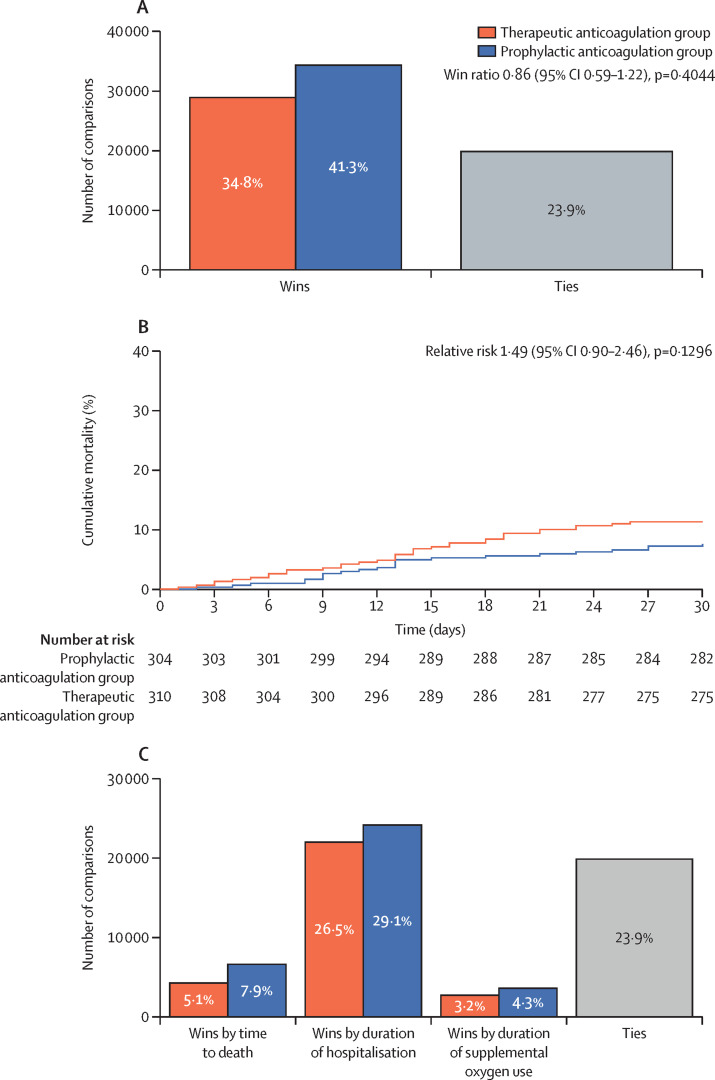
The hierarchical analysis of time to death, duration of hospitalisation, and duration of supplemental oxygen through 30 days was not statistically different between groups. The number of wins was 28 899 (34·8%) in the therapeutic group and 34 288 (41·3%) in the prophylactic group (win ratio 0·86 [95% CI 0·59–1·22], p=0·40; figure 2 , appendix p 11). The total number of ties was 19 837 (23·9%).
Figure 2.
Primary outcome analysis
(A) Hierarchical win ratio analysis of death, duration of hospitalisation, and duration of oxygen use (primary outcome) through 30 days. (B) Cumulative mortality curves through 30 days in the two study groups. (C) Number of wins by group for individual components of the primary outcome.
The total percentages of wins in the therapeutic and prophylactic treatment groups for the individual components of the primary outcome were consistent with the primary analysis, with no differences in time to death, duration of hospitalisation, or duration of supplemental oxygen between the groups (figure 2B, C). The 8-point ordinal scale at day 30 (table 2 ); disease progression at days 7, 15, and 30; and duration of invasive mechanical ventilation at the end of 30 days did not differ between the groups (appendix p 12).
Table 2.
30-day efficacy and safety outcomes
| Therapeutic anticoagulation group (n=310) | Prophylactic anticoagulation group (n=304) | Effect (95% CI) | p value | |||
|---|---|---|---|---|---|---|
| Efficacy outcomes | ||||||
| Composite thrombotic outcome* | 23 (7%) | 30 (10%) | RR 0·75 (0·45–1·26) | 0·32 | ||
| Venous thromboembolism† | 11 (4%) | 18 (6%) | RR 0·60 (0·29–1·25) | 0·19 | ||
| Deep vein thrombosis | 5 (2%) | 5 (2%) | RR 0·98 (0·29–3·35) | 1·00 | ||
| Pulmonary embolism | 7 (2%) | 13 (4%) | RR 0·53 (0·21–1·31) | 0·18 | ||
| Myocardial infarction | 13 (4%) | 14 (5%) | RR 0·91 (0·44–1·91) | 0·85 | ||
| Stroke | 1 (<1%) | 0 | .. | .. | ||
| Major adverse limb event | 0 | 1 (<1%) | .. | .. | ||
| Composite thrombotic outcome* and all-cause death | 46 (15%) | 44 (14%) | RR 1·03 (0·70–1·50) | 0·91 | ||
| Death | 35 (11%) | 23 (8%) | RR 1·49 (0·90–2·46) | 0·13 | ||
| Cardiovascular | 6 (2%) | 0 | .. | .. | ||
| Non-cardiovascular | 29 (9%) | 22 (7%) | .. | .. | ||
| Unknown | 0 | 1 (<1%) | .. | .. | ||
| Rehospitalisation | 2 (1%) | 5 (2%) | RR 0·39 (0·08–2·01) | 0·28 | ||
| World Health Association 8-point ordinal scale at end of 30 days | .. | .. | Proportional OR 1·35 (0·85–2·16) | 0·21 | ||
| Out of hospital with no oxygen therapy | 263 (85%) | 268 (88%) | .. | .. | ||
| Out of hospital with oxygen therapy | 0 | 0 | .. | .. | ||
| Hospitalised with no oxygen therapy | 3 (1%) | 5 (2%) | .. | .. | ||
| Hospitalised with oxygen by mask or nasal catheter | 8 (3%) | 2 (1%) | .. | .. | ||
| Hospitalised with non-invasive ventilation or high-flow oxygen | 0 | 0 | .. | .. | ||
| Hospitalised with invasive mechanical ventilation without additional support | 1 (<1%) | 4 (1%) | .. | .. | ||
| Hospitalised with invasive mechanical ventilation with additional organ support | 0 | 2 (1%) | .. | .. | ||
| Death | 35 (11%) | 23 (8%) | .. | .. | ||
| Safety outcomes | ||||||
| Major bleeding or clinically relevant non-major bleeding (ISTH definitions) | 26 (8%) | 7 (2%) | RR 3·64 (1·61–8·27) | 0·0010 | ||
| Major bleeding | 10 (3%) | 4 (1%) | RR 2·45 (0·78–7·73) | 0·18 | ||
| Clinically relevant non-major bleeding | 16 (5%) | 3 (1%) | RR 5·23 (1·54–17·77) | 0·0039 | ||
| Any bleeding | 36 (12%) | 9 (3%) | RR 3·92 (1·92–8·00) | <0·0001 | ||
| Combined efficacy and safety outcome | ||||||
| Net benefit‡ | 56 (18%) | 47 (15%) | RR 1·17 (0·82–1·66) | 0·45 | ||
Data are n (%) or point estimate (95% CI). RR=relative risk. OR=odds ratio. ISTH=International Society on Thrombosis and Haemostasis.
Defined as any venous thromboembolism, myocardial infarction, stroke, systemic embolism, and major adverse limb events.
One patient had one episode of deep vein thrombosis, followed 6 days later by a pulmonary embolism.
Composite outcome including any composite thrombotic outcome, all-cause death, and ISTH definitions of major or clinically relevant bleeding.
At 30 days, the incidence of individual thrombotic events was not significantly different between groups (table 2; appendix p 12), nor was the composite of venous thromboembolism, myocardial infarction, stroke, systemic embolism, or major adverse limb events (RR 0·75 [95% CI 0·45–1·26], p=0·32).
The primary safety outcome of ISTH-defined major or clinically relevant bleeding occurred in 26 (8%) patients receiving therapeutic anticoagulation and seven (2%) receiving prophylactic anticoagulation (RR 3·64 [95% CI 1·61–8·27], p=0·0010; table 2).
One fatal intracranial bleeding event occurred in a clinically unstable patient in the therapeutic group while receiving enoxaparin (appendix p 13). Any bleeding occurred in 36 (12%) patients treated with therapeutic anticoagulation and nine (3%) who received prophylactic anticoagulation (RR 3·92 [95% CI 1·92–8·00], p<0·0001). Results were consistent when assessed using other bleeding scores (appendix p 13).16 The first bleeding events (any type of bleeding) occurred in hospital for most patients in both groups, with 66% of all first bleeding events occurring in hospital. The median time to first bleeding event in the therapeutic group was 11·0 days (95% CI 5·0–14·5) and in the prophylactic group was 14·0 days (9·0–22·0). 23 clinically unstable patients were allocated to the therapeutic anticoagulation group and started treatment with heparin (22 with enoxaparin and one with unfractionated heparin). Of these, 12 patients died before transitioning to rivaroxaban, ten transitioned to rivaroxaban and were discharged on rivaroxaban, and one transitioned to rivaroxaban but was not discharged on rivaroxaban. The incidence of bleeding in these 23 clinically unstable patients is described in the appendix (p 14). Allergic reactions to the study medication occurred in two (1%) patients assigned to therapeutic anticoagulation and three (1%) assigned to prophylactic anticoagulation.
The net clinical benefit composite of death, thrombotic events, or major or clinically relevant non-major bleeding was not statistically significantly different between groups (RR 1·17 [95% CI 0·82–1·66], p=0·45).
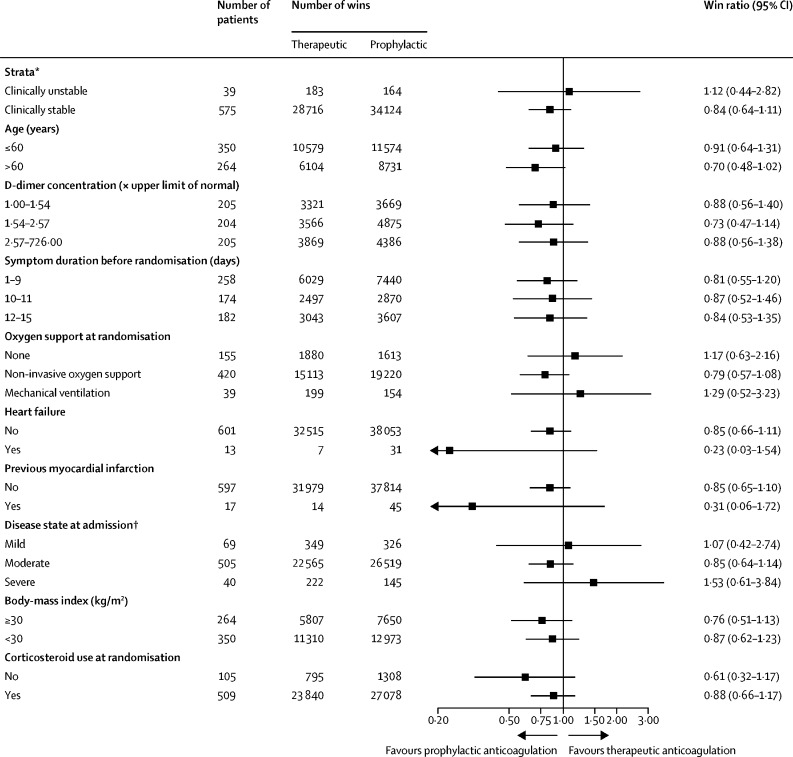
Results for the primary outcome were consistent across subgroups (figure 3 ). Similar results were seen among clinically stable and clinically unstable patients (appendix pp 18–19), in the analysis excluding the patient that did not have a confirmatory COVID-19 test (appendix p 15), and assuming death as a binary endpoint (appendix p 16). Finally, we did not find a difference in study outcomes in patients assigned to the prophylactic group who received either unfractionated heparin or enoxaparin (appendix p 17).
Figure 3.
Subgroup analysis
*Clinically unstable was defined as the presence of a COVID-19-related critical illness with an immediately life-threatening condition that would typically lead to intensive care unit admission. † Mild disease includes cases not meeting the criteria for classification as moderate or severe disease; moderate disease was characterised by an oxygen saturation <94%, pulmonary infiltrates >50%, or a partial pressure of oxygen to fractional concentration of oxygen in inspired air ratio <300; and severe disease was defined as respiratory failure, haemodynamic instability, or multiple organ dysfunction.
Discussion
In this open-label, multicentre, randomised, controlled trial including patients hospitalised with confirmed COVID-19 and elevated D-dimer concentration, a 30-day course of therapeutic anticoagulation with rivaroxaban at 20 mg daily (and enoxaparin 1 mg/kg twice daily for clinically unstable patients) did not result in better clinical outcomes—as measured by a hierarchical composite of time to death, duration of hospitalisation, or duration of supplemental oxygen therapy—when compared with in-hospital prophylactic anticoagulation with heparin. Therapeutic anticoagulation for 30 days with rivaroxaban or enoxaparin led to a higher incidence of major or clinically relevant non-major bleeding than did in-hospital prophylactic anticoagulation.
In light of the COVID-19 pandemic, identifying therapies that improve outcomes is crucial.22, 23 The ACTION trial used a pragmatic approach including common laboratory tests to confirm the diagnosis of COVID-19. The high specificity of these tests, in addition to the high pretest probability in the study population, make it very unlikely that the diagnosis of COVID-19 would be a false positive and also facilitate the external validation of the trial results in clinical practice. Rivaroxaban, the main anticoagulant used in the therapeutic group of the trial, was selected because it has been studied in a comprehensive cardiovascular programme and shown to reduce the risk of venous and arterial thrombotic events in a variety of clinical settings.12, 13, 24, 25, 26 Given its widespread use, favourable toxicity profile, and ease of use, a well designed randomised clinical trial was needed to ascertain whether rivaroxaban mitigates the complications of COVID-19.
Randomised data on the use of therapeutic anticoagulation with heparin in patients hospitalised with COVID-19 have shown divergent results.27, 28 Critically ill patients admitted to intensive care units did not benefit from therapeutic or intermediate doses of anticoagulation compared with prophylactic doses.27, 29 Our study included a small group of clinically unstable patients and our findings were consistent with the previous randomised data in this population. In contrast to these findings, preliminary results from other studies have suggested that patients with moderate COVID-19 could benefit from in-hospital therapeutic anticoagulation with heparin, regardless of D-dimer concentration.28 However, these results are preliminary and not yet published in a peer-reviewed journal, and, by the time they were reported, thrombotic and bleeding outcomes had not been completely adjudicated, making the assessment of risk to benefit balance of this strategy less certain. In one completed and published randomised trial, a strategy of intermediate-dose anticoagulation was not superior to prophylactic anticoagulation, but it remains uncertain whether this absence of benefit could be explained by an anticoagulant dose lower than therapeutic anticoagulation.29 In our study, therapeutic anticoagulation with rivaroxaban did not improve clinical outcomes, but did increase bleeding. The different results between clinically stable patients in ACTION and preliminary reports from other studies might be explained by the type of anticoagulant used in each trial. Nonetheless, the similar rates of thrombotic events and increases in bleeding with therapeutic anticoagulation are important findings for physicians in clinical practice, and might help in the decision-making process when treating patients with COVID-19. The most likely explanation for the lack of effect of rivaroxaban in the ACTION study, compared with heparin and its derivatives in previous studies, is that rivaroxaban, a direct, selective factor Xa inhibitor, does not share heparin's possible pleiotropic effects. Heparins, which inhibit multiple coagulation proteases, might have other anti-inflammatory and antiviral effects, some of which might be specific to COVID-19.30 In addition, the high use of corticosteroids in ACTION might have attenuated the effect of the possible lack of anti-inflammatory effect of rivaroxaban, but does not completely eliminate the potential effect of additional suppression of inflammation with heparin for these patients. Lung microvascular thrombosis contributing to respiratory worsening in COVID-19 might not be primarily preventable by factor Xa inhibition, but possibly mainly by direct thrombin inhibition or antithrombin activation. Additionally, the therapeutic anticoagulation administration route (oral vs subcutaneous) was different between studies and might explain, at least in part, the different results. Patients hospitalised with COVID-19 might have abnormal absorption of oral anticoagulation, leading to erratic and variable effects, which could also contribute to different findings between the studies. We are not aware of other completed randomised clinical trials of therapeutic oral anticoagulants in COVID-19. In this regard, our results are important to the clinical community, suggesting that the routine use of the direct-acting factor Xa inhibitor rivaroxaban at a therapeutic dose does not provide clinical benefit to patients hospitalised with COVID-19 when compared with traditional thromboprophylaxis with heparin.
There are limitations to the ACTION trial. The open-label design has a potential risk of bias, especially with respect to clinical event ascertainment. Adherence to the medication at the end of the study was assessed through pill count done by patients via telephone call and not in an in-person medical evaluation. Nevertheless, the primary composite hierarchical outcome included only objective information: death, days of hospitalisation, and days of oxygen support. There was no routine screening of ischaemic events for asymptomatic patients. However, to further reduce the risk of bias, there was a blinded adjudication process for the secondary outcomes using standard definitions, as well as regular site training and monitoring and sensitive triggers based on laboratory values, reports of adverse events, unknown causes of death, or changes in antithrombotic therapy, to ensure that no relevant events were missed. In addition, for clinical events occurring after hospital discharge, the same source documents were collected as if the patient had the same event during the index hospitalisation. These documents included medical records and results for laboratory tests that were processed and prepared for central and blinded adjudication. Another aspect to be considered is that the primary therapeutic anticoagulation therapy tested in the trial was rivaroxaban and only a small number of patients received therapeutic anticoagulation with enoxaparin, which represented the subgroup of patients who were clinically unstable at randomisation. Thus, regarding external validity, the results of the current study apply mainly to the use of rivaroxaban in clinically stable patients hospitalised with confirmed COVID-19 within 14 days from symptom onset, elevated D-dimer concentration, and without indication for therapeutic anticoagulation. Trials in patients in critical condition have been previously reported,27, 29 and studies of anticoagulation in outpatients with COVID-19 are ongoing.
In conclusion, in patients hospitalised with COVID-19 with elevated D-dimer concentration, initial in-hospital therapeutic anticoagulation with rivaroxaban for stable patients or enoxaparin for unstable patients followed by rivaroxaban through 30 days did not improve clinical outcomes and increased bleeding compared with in-hospital prophylactic anticoagulation. Thus, the use of therapeutic-dose rivaroxaban, and other direct oral anticoagulants, should be avoided in hospitalised patients with COVID-19 who do not have an evidence-based indication for oral anticoagulation. Ongoing clinical trials will address the efficacy and safety of other antithrombotic regimens in patients with COVID-19.
Data sharing
Anonymised participant data can be made available upon requests directed to the corresponding author. Proposals will be reviewed on the basis of scientific merit. After approval of a proposal, data can be shared through a secure online platform after signing a data access agreement.
Declaration of interests
JHA reports grants and personal fees from Bristol-Myers Squibb and CSL Behring; grants from AstraZeneca, CryoLife, US Food & Drug Administration, US National Institutes of Health, Sanofi, VoluMetrix, and Boehringer Ingelheim; personal fees from Pfizer, AbbVie Pharmaceuticals, Portola Pharmaceuticals, Quantum Genetics, Teikoku Pharmaceuticals, VA Cooperative Studies Program, and Zafgen, outside of the submitted work. AA reports consultant and lecture fees from Bayer, NovoNordisk, and LillyBaxter; lecture fees from Daichii-Sankyo; and research grants from Bayer, EMS Pharma, and the Population Health Research Institute, outside of the submitted work. LCPA reports personal fees from Baxter, Pfizer, and Halex-Istar; and grants from Ache Laboratorios Farmaceuticos, outside of the submitted work. OB reports grants from AstraZeneca, Pfizer, Bayer, Boehringer Ingelheim, Servier, and Amgen, and advisory board and personal fees from Novartis, outside of the submitted work. ABC reports grants from Bayer outside of the submitted work. GEC-S reports grants from Novartis and Air Liquide, outside of the submitted work. PGMdBeS reports grants from Bayer, Roche, and Pfizer, outside of the submitted work. MDAD reports personal fees, non-financial support, and other (advisory board participation) from Pfizer; personal fees and non-financial support from Bayer; personal fees and other (advisory board participation) from Servier; and personal fees from Boehringer Ingelheim, Daiichi Sankyo, and AstraZeneca, outside of the submitted work. RHMF reports grants from Bayer during the conduct of the study; and grants and personal fees from AstraZeneca and Servier, personal fees and non-financial support from Bayer, grants and non-financial support from EMS Pharma, and grants from Aché, Health Canada, and the Brazilian Ministry of Health, outside of the submitted work. MBG reports personal fees from COALITION COVID-19 Brazil and Bayer during the conduct of the study. RDL reports grants and personal fees from Bristol-Myers Squibb, Pfizer, GlaxoSmithKline, Medtronic PLC, and Sanofi; and personal fees from Amgen, Bayer, and Boehringer Ingelheim, outside of the submitted work. AVSM reports personal fees, non-financial support, and other (advisory board participation) from Bayer and Pfizer; personal fees and other (advisory board participation) from Novartis; personal fees and non-financial support from Zodiac; and personal fees from Ferring, Janssen, Sanofi, and AstraZeneca, outside of the submitted work. FCN reports grants and personal fees from Boehringer Ingelheim; and personal fees from Bayer and Pfizer, outside of the submitted work. ER reports grants and consulting fees from Bayer and Pfizer; grants from the Brazilian Ministry of Science and Technology; and personal fees from Aspen Pharma, Biomm Pharma, and Daiichi Sankyo, outside of the submitted work. ATR reports personal fees from Sanofi and Bayer, outside of the submitted work. VCV reports grants from Aspen Pharma, Pfizer, and Cristalia, outside of the submitted work. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
This trial was an investigator-initiated study with financial support from Coalition COVD-19 Brazil and Bayer SA, who provided the study drug and partial financial support. We are grateful to all site staff who helped to enrol participants and to all health-care professionals who took care of our patients during this protocol.
Contributors
RDL, PGMdBeS, RHMF, and OB conceived the trial and wrote the initial proposal. All other authors contributed intellectually relevant content. BB and LPD estimated the sample size and drafted the statistical analysis plan. The initial draft of the manuscript was written by RDL and PGMdBeS, who had full access to and verified all the data underlying the study. All authors had access to the data, contributed to the manuscript, agreed to submit for publication, and vouch for the integrity, accuracy, and completeness of the data and for the fidelity of the trial to the protocol.
Supplementary Material
References
- 1.Iba T, Levy JH, Connors JM, et al. Coagulopathy of coronavirus disease 2019. Crit Care Med. 2020;48:1358–1364. doi: 10.1097/CCM.0000000000004458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gungor B, Atici A, Baycan OF, et al. Elevated D-dimer levels on admission are associated with severity and increased risk of mortality in COVID-19: a systematic review and meta-analysis. Am J Emerg Med. 2021;39:173–179. doi: 10.1016/j.ajem.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiménez D, García-Sanchez A, Rali P, et al. Incidence of VTE and bleeding among hospitalized patients with coronavirus disease 2019: a systematic review and meta-analysis. Chest. 2021;159:1182–1196. doi: 10.1016/j.chest.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts LN, Whyte MB, Georgiou L, et al. Postdischarge venous thromboembolism following hospital admission with COVID-19. Blood. 2020;136:1347–1350. doi: 10.1182/blood.2020008086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Samkari H, Karp Leaf RS, Dzik WH, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136:489–500. doi: 10.1182/blood.2020006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moores LK, Tritschler T, Brosnahan S, et al. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: CHEST Guideline and Expert Panel Report. Chest. 2020;158:1143–1163. doi: 10.1016/j.chest.2020.05.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuker A, Tseng EK, Nieuwlaat R, et al. American Society of Hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19. Blood Adv. 2021;5:872–888. doi: 10.1182/bloodadvances.2020003763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spyropoulos AC, Levy JH, Ageno W, et al. Scientific and Standardization Committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1859–1865. doi: 10.1111/jth.14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nadkarni GN, Lala A, Bagiella E, et al. Anticoagulation, bleeding, mortality, and pathology in hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76:1815–1826. doi: 10.1016/j.jacc.2020.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frydman GH, Streiff MB, Connors JM, Piazza G. The potential role of coagulation factor Xa in the pathophysiology of COVID-19: a role for anticoagulants as multimodal therapeutic agents. TH Open. 2020;4:e288–e299. doi: 10.1055/s-0040-1718415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spyropoulos AC, Ageno W, Albers GW, et al. Rivaroxaban for thromboprophylaxis after hospitalization for medical illness. N Engl J Med. 2018;379:1118–1127. doi: 10.1056/NEJMoa1805090. [DOI] [PubMed] [Google Scholar]
- 13.Spyropoulos AC, Ageno W, Albers GW, et al. Post-discharge prophylaxis with rivaroxaban reduces fatal and major thromboembolic events in medically ill patients. J Am Coll Cardiol. 2020;75:3140–3147. doi: 10.1016/j.jacc.2020.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopes RD, de Barros e Silva PGM, Furtado RHM, et al. Randomized clinical trial to evaluate a routine full anticoagulation strategy in patients with coronavirus infection (SARS-CoV-2) admitted to hospital: rationale and design of the ACTION (AntiCoagulaTlon cOroNavirus)–Coalition IV trial. Am Heart J. 2021 doi: 10.1016/j.ahj.2021.04.005. published online April 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaatz S, Ahmad D, Spyropoulos AC, Schulman S. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13:2119–2126. doi: 10.1111/jth.13140. [DOI] [PubMed] [Google Scholar]
- 16.Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–2747. doi: 10.1161/CIRCULATIONAHA.110.009449. [DOI] [PubMed] [Google Scholar]
- 17.WHO COVID-19 therapeutic trial synopsis. Feb 18, 2020. https://www.who.int/publications/i/item/covid-19-therapeutic-trial-synopsis
- 18.US Centers for Disease Control and Prevention Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19) Feb 16, 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html
- 19.Pocock SJ, Ariti CA, Collier TJ, Wang D. The win ratio: a new approach to the analysis of composite endpoints in clinical trials based on clinical priorities. Eur Heart J. 2012;33:176–182. doi: 10.1093/eurheartj/ehr352. [DOI] [PubMed] [Google Scholar]
- 20.Dong G, Qiu J, Wang D, Vandemeulebroecke M. The stratified win ratio. J Biopharm Stat. 2018;28:778–796. doi: 10.1080/10543406.2017.1397007. [DOI] [PubMed] [Google Scholar]
- 21.Cavalcanti AB, Zampieri FG, Rosa RG, et al. Hydroxychloroquine with or without azithromycin in mild-to-moderate COVID-19. N Engl J Med. 2020;383:2041–2052. doi: 10.1056/NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fanaroff AC, Califf RM, Harrington RA, et al. Randomized trials versus common sense and clinical observation: JACC review topic of the week. J Am Coll Cardiol. 2020;76:580–589. doi: 10.1016/j.jacc.2020.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopes RD, Fanaroff AC. Anticoagulation in COVID-19: it is time for high-quality evidence. J Am Coll Cardiol. 2020;76:1827–1829. doi: 10.1016/j.jacc.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 25.Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377:1319–1330. doi: 10.1056/NEJMoa1709118. [DOI] [PubMed] [Google Scholar]
- 26.Guimarães HP, Lopes RD, de Barros E Silva PGM, et al. Rivaroxaban in patients with atrial fibrillation and a bioprosthetic mitral valve. N Engl J Med. 2020;383:2117–2126. doi: 10.1056/NEJMoa2029603. [DOI] [PubMed] [Google Scholar]
- 27.National Institutes of Health NIH ACTIV trial of blood thinners pauses enrollment of critically ill COVID-19 patients. Dec 22, 2020. https://www.nih.gov/news-events/news-releases/nih-activ-trial-blood-thinners-pauses-enrollment-critically-ill-covid-19-patients
- 28.National Heart, Lung, and Blood Institute Full-dose blood thinners decreased need for life support and improved outcome in hospitalized COVID-19 patients. Jan 22, 2021. https://www.nhlbi.nih.gov/news/2021/full-dose-blood-thinners-decreased-need-life-support-and-improved-outcome-hospitalized
- 29.INSPIRATION Investigators Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the INSPIRATION randomized clinical trial. JAMA. 2021;325:1620–1630. doi: 10.1001/jama.2021.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buijsers B, Yanginlar C, Maciej-Hulme ML, de Mast Q, van der Vlag J. Beneficial non-anticoagulant mechanisms underlying heparin treatment of COVID-19 patients. EBioMedicine. 2020;59 doi: 10.1016/j.ebiom.2020.102969. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymised participant data can be made available upon requests directed to the corresponding author. Proposals will be reviewed on the basis of scientific merit. After approval of a proposal, data can be shared through a secure online platform after signing a data access agreement.