Abstract
This post hoc analysis of MONARCH 2 and MONARCH 3 assesses the efficacy, safety, and pharmacokinetics (PK) of abemaciclib in combination with endocrine therapy (ET) in East Asian patients with hormone receptor positive (HR+), human epidermal growth factor receptor 2‐negative (HER2−) advanced breast cancer. MONARCH 2 and MONARCH 3 are global, randomized, double‐blind, phase 3 studies of abemaciclib/placebo + fulvestrant and abemaciclib/placebo + nonsteroidal aromatase inhibitor (NSAI, anastrozole or letrozole), respectively. The East Asian population comprised 212 (31.7%) of the 669 intent‐to‐treat (ITT) population in the MONARCH 2 trial and 144 (29.2%) of the 493 ITT patients in the MONARCH 3 trial. In the East Asian population, median progression‐free survival (PFS) was significantly prolonged in the abemaciclib arm compared with placebo in both MONARCH 2 (hazard ratio [HR], 0.520; 95% confidence interval [CI], 0.362 to 0.747; P < .001; median: 21.2 vs 11.6 months) and MONARCH 3 (HR, 0.326; 95% CI, 0.200 to 0.531, P < .001; median: not reached vs 12.82 months). Diarrhea (MONARCH 2: 90%; MONARCH 3: 88%) and neutropenia (MONARCH 2: 68%; MONARCH 3: 58%) were the most frequent adverse events observed in the East Asian populations. Abemaciclib exposures and PK were similar in East Asians and the non‐East Asian populations of both trials. Abemaciclib in combination with ET in the East Asian populations of MONARCH 2 and MONARCH 3 provided consistent results with the ITT populations, demonstrating improvements in efficacy with generally tolerable safety profiles for patients with HR+, HER2− advanced breast cancer.
Keywords: abemaciclib, breast cancer, cyclin‐dependent kinase inhibitor, East Asia, metastatic
Abemaciclib in combination with endocrine therapy (ET) in the East Asian populations of MONARCH 2 and MONARCH 3 improved efficacy for patients with HR+, HER2− advanced breast cancer.
Abbreviations
- ABC
advanced breast cancer
- AE
adverse events
- ALT
alanine aminotransferase
- CBR
clinical benefit rate
- CDK
cyclin‐dependent kinase
- CI
confidence interval
- CR
complete response
- ET
endocrine therapy
- HER2−
human epidermal growth factor receptor 2‐negative
- HR
hazard ratio
- HR+
hormone receptor positive
- ILD
interstitial lung disease
- ITT
intent‐to‐treat
- NSAI
nonsteroidal aromatase inhibitor
- ORR
objective response rate
- OS
overall survival
- PFS
progression‐free survival
- PK
pharmacokinetics
- PR
partial response
- SAE
serious adverse event
- VTE
venous thromboembolic event
1. INTRODUCTION
Recent advances in the treatment of hormone receptor positive (HR+), human epidermal growth factor receptor 2‐negative (HER2−) advanced breast cancer (ABC) have changed the standard of care. 1 Typically these patients have been treated with endocrine therapy (ET), such as an aromatase inhibitor in the first‐line setting and fulvestrant in the second‐line setting. 1 , 2 However, cyclin‐dependent kinase 4 and 6 (CDK4 & 6) inhibitors, such as abemaciclib, are now being combined with ET in these treatment settings and have significantly improved outcomes in terms of progression‐free survival (PFS), objective response rate (ORR), and overall survival (OS). 3 , 4 , 5 , 6 , 7 , 8
Abemaciclib is a CDK4 & 6 inhibitor that targets the cyclin D/CDK/retinoblastoma signaling pathway, creating a G1 cell cycle block resulting in apoptosis and senescence in preclinical models. 9 In enzymatic assays, abemaciclib notably exhibited a 14‐fold higher selectivity in kinases for cyclinD1/CDK4 than cyclinD3/CDK6. 3 , 10 CDK4 & 6 inhibitors are commonly associated with hematological toxicities which have been ascribed to the consequence of CDK6 inhibition, as this kinase plays a crucial role in the proliferation and development of hematopoietic stem cells in the bone marrow. 11 , 12 However, the greater selectivity of abemaciclib toward CDK4 compared with CDK6 may explain why abemaciclib is able to be dosed on a continuous schedule, unlike other CDK4 & 6 inhibitors which require an intermittent schedule due to the dose‐limiting toxicity of neutropenia. 3 , 5 , 13 , 14 , 15 , 16 , 17
Abemaciclib is approved in combination with ET as initial therapy and following progression on ET in the advanced setting based on the MONARCH 3 and MONARCH 2 trials, respectively. 4 , 5 , 8 , 18 In MONARCH 3, abemaciclib in combination with an aromatase inhibitor (anastrozole or letrozole) significantly improved PFS in the intent‐to‐treat (ITT) population (median PFS: 28.18 vs 14.76 months, HR: 0.540; 95% CI: 0.418 to 0.698; P < .001) and ORR in patients with measurable disease (61.0% [95% CI: 55.2% to 66.9%] vs 45.5% [95% CI: 37.0% to 53.9%], P = .003) compared with placebo plus aromatase inhibitor. 18 Likewise, in MONARCH 2, abemaciclib in combination with fulvestrant resulted in significant improvement in PFS and OS compared with placebo plus fulvestrant (median PFS: 16.4 vs 9.3 months, HR: 0.553, 95% CI 0.449‐0.681, P < .001; median OS: 46.7 vs 37.3 months, HR: 0.757; 95% CI, 0.606‐0.945; P = .01). 5 , 8 In patients with measurable disease, the ORR was significantly improved in the abemaciclib arm compared with the placebo arm (ORR: 48.1% [95% CI, 42.6% to 53.6%] vs 21.3% [95% CI, 15.1% to 27.6%], P < .001). 5 The MONARCH trials also reported a consistent and tolerable safety profile of abemaciclib in combination with ET. 5 , 18
The incidence of breast cancer among East Asian women has increased rapidly in recent years and is one of the leading causes of cancer‐related mortality in East Asian women. 19 , 20 , 21 The growing incidence in Asian women may be due to lifestyle changes, including postmenopausal obesity, earlier menarche, later menopause, decreased breastfeeding, and beginning child‐bearing later in life. 22 The age of occurrence varies between regions, with Asian women having a greater incidence in their forties and fifties, while women in the western countries having a greater incidence in their sixties. 23 Response to cancer therapies and the AE profile in the Asian population can also differ from other regions due to geographic variation in clinical practice, racial/ethnic background, genetic variation, and differences in drug metabolism, among others. Therefore, it is important to evaluate the efficacy and safety of cancer therapies within the Asian population. Here, we report a subgroup analysis evaluating the efficacy and safety of abemaciclib in combination with ET in the East Asian patients of the MONARCH 2 and 3 trials.
2. METHODS
2.1. Study design and population
The study design, procedures, and statistical methods for MONARCH 2 and 3 have been previously published in detail. 4 , 5 MONARCH 2 (NCT02107703) is a randomized, double‐blind, phase 3 study of abemaciclib/placebo + fulvestrant in women with HR+, HER2− ABC whose disease had progressed while receiving neoadjuvant or adjuvant ET, ≤12 months from the end of adjuvant ET, or while receiving first‐line ET for metastatic disease. Patients must not have received more than one ET or any prior chemotherapy in the advanced setting. 5 MONARCH 3 (NCT02246621) is a phase 3, randomized, double‐blind trial of abemaciclib/placebo + NSAI (anastrozole or letrozole per physician's choice) for initial therapy of HR+, HER2− ABC (prior neoadjuvant or adjuvant ET was permitted if the disease‐free interval was >12 months from the completion of prior ET). Patients must not have received systemic therapy for advanced disease. 4 Randomly assigned patients in these studies were stratified by metastatic site: visceral, bone only, or other (MONARCH 2 and 3); ET resistance (MONARCH 2); and prior ET: NSAI, no ET or other (MONARCH 3).
Each center's institutional review board or independent ethics committee approved the trials. The study followed the guiding principles of the Declaration of Helsinki and the Good Clinical Practice Guidelines of the International Conference on Harmonization. All patients provided written informed consent before enrollment.
2.2. Study treatment
Detailed methods for randomization, treatment, dose adjustments, and treatment discontinuation for MONARCH 2 and 3 trials were previously described. 4 , 5 In MONARCH 2, patients were randomized in a 2:1 ratio to receive either abemaciclib + fulvestrant or placebo + fulvestrant. 5 , 24 In MONARCH 3, patients were randomly assigned in a 2:1 ratio to receive either abemaciclib + NSAI or placebo + NSAI. In MONARCH 2, at study initiation, patients in the abemaciclib arm received 200 mg twice daily. Following a review of safety data and dose reduction rates, the protocol was amended to reduce the starting dose to 150 mg for new patients, and all patients who were receiving 200 mg underwent a mandatory dose reduction to 150 mg twice daily. A total of 178 patients (26.6%) were enrolled at the 200 mg starting dose. Patients in the abemaciclib + fulvestrant arm received a median of 34 days of treatment at the 200‐mg starting dose prior to dose reduction or discontinuation. In MONARCH 3, abemaciclib 150 mg was orally administered twice daily.
2.3. Study endpoints
The primary endpoint of MONARCH 2 and 3 was to compare PFS of the experimental arm with that of the control arm. Key secondary endpoints evaluated in both the studies are ORR (percentage of patients with best response of complete [CR] or partial response [PR]), clinical benefit rate (CBR = CR + PR + SD ≥6 months), safety and tolerability, and pharmacokinetics (PK).
2.4. Statistical analysis
Detailed statistical methods of both the studies have been previously published. 4 , 5 , 18 The East Asian population used for this subgroup analysis was defined based on the geographic region in which patients enrolled at study sites in Japan, Korea, and Taiwan. The data are from the final PFS database lock from MONARCH 2 and 3 trials, 14 February, 2017 and 03 November, 2017, respectively. At the time of the final PFS analysis, results for prespecified subgroups including region (North America, Europe, and Asia) were reported. 8 , 18 In the present report, we further described the East Asian subgroup. In both studies, the primary end point, PFS was analyzed using Kaplan‐Meier estimates and an unstratified Cox proportional hazards model.
In MONARCH 2, PK samples were collected cycle (C) 1, day (D) 1: 2 to 4 hours (h) post dose; C1D15: 4 and 7 h post dose; C2D1: prior to dose and 3 h post dose; C3D1; prior to dose. In MONARCH 3, PK samples were collected C1D1: 2 to 4 h post dose; C2D1: 4 and 7 h post dose; C3D1: prior to dose and 3 h post dose; C4D1: 2 to 4 h post dose. A population pharmacokinetic analysis was conducted using NONMEM 7.3.0 to evaluate the contribution of covariates such as race to the interindividual variability in abemaciclib PK. 25 , 26 , 27 In addition, the observed abemaciclib concentrations for East Asians and the non‐East Asian population were plotted versus time from the first dose.
3. RESULTS
3.1. Patients
Of the 669 overall ITT patients in the MONARCH 2 trial, 212 (31.7%) patients were enrolled at sites in the East Asian countries (Japan, Korea, and Taiwan); 147 (69.3%) were randomized to receive abemaciclib + fulvestrant and 65 (30.7%) to receive placebo + fulvestrant. A total of 57 (39%) East Asian patients received 200 mg of abemaciclib as initial dose prior to the mandatory dose reduction amendment in MONARCH 2. In the MONARCH 3 trial, a total of 493 patients were enrolled globally, of which 144 (29.2%) were East Asians; 102 (70.8%) patients were randomized to the abemaciclib + NSAI arm and 42 (29.1%) patients to the placebo + NSAI arm. As the stratification at randomization was applied to the ITT population and not for each region or race, in the East Asian population, the number of patients with visceral disease was higher in the abemaciclib + fulvestrant arm compared with the placebo + fulvestrant arm in the MONARCH 2 trial (61.9% vs 49.2%), and fewer in the abemaciclib + NSAI arm compared with the placebo + NSAI arm in the MONARCH 3 trial (49.0% vs 59.5%). Of note, in MONARCH 2 there were more pre/perimenopausal East Asian patients (35.4%) compared with the ITT (17.0%). In both trials, there were more East Asian patients with an ECOG performance status of 0 (MONARCH 2: 79.2%; MONARCH 3: 74.3%) compared with ITT (MONARCH 2: 59.8%; MONARCH 3: 60.0%). Complete baseline and disease characteristics in the East Asian population are presented in Table 1.
TABLE 1.
Baseline characteristics of East Asian patients in the MONARCH 2 and 3 trials
| Characteristics | MONARCH 2 | MONARCH 3 | ||
|---|---|---|---|---|
| Abemaciclib + fulvestrant n = 147 | Placebo + fulvestrant n = 65 | Abemaciclib + NSAI n = 102 | Placebo + NSAI n = 42 | |
| Age, n (%) | ||||
| Median (range) | 55 (32‐76) | 56 (32‐81) | 60 (45‐78) | 62 (46‐85) |
| <65 y | 118 (80.3) | 48 (73.8) | 66 (64.7) | 27 (64.3) |
| ≥65 y | 29 (19.7) | 17 (26.2) | 36 (35.3) | 15 (35.7) |
| Country, n (%) | ||||
| Japan | 64 (43.5) | 31 (47.7) | 38 (37.3) | 15 (35.7) |
| Korea | 58 (39.5) | 20 (30.8) | 41 (40.2) | 18 (42.9) |
| Taiwan | 25 (17.0) | 14 (21.5) | 23 (22.5) | 9 (21.4) |
| Menopausal status | ||||
| Postmenopausal | 96 (65.3) | 41 (63.1) | NA | NA |
| Pre‐ or perimenopausal | 51 (34.7) | 24 (36.9) | ||
| Measurable disease, n (%) | ||||
| Yes | 122 (83.0) | 47 (72.3) | 86 (84.3) | 37 (88.1) |
| No | 25 (17.0) | 18 (27.7) | 16 (15.7) | 5 (11.9) |
| Metastatic site, n (%) | ||||
| Visceral | 91 (61.9) | 32 (49.2) | 50 (49.0) | 25 (59.5) |
| Bone‐only | 28 (19.0) | 20 (30.8) | 21 (20.6) | 7 (16.7) |
| other | 28 (19.0) | 13 (20.0) | 31 (30.4) | 10 (23.8) |
| Disease setting, n (%) a | ||||
| Recurrent locally advanced | 6 (4.1) | 0 | 4 (3.9) | 1 (2.4) |
| Metastatic | 141 (95.9) | 65 (100.0) | 98 (96.1) | 41 (97.6) |
| Prior neoadjuvant systemic therapy, n (%) | ||||
| Endocrine therapy | 4 (2.7) | 2 (3.1) | 0 | 1 (2.4) |
| Chemotherapy | 23 (15.6) | 13 (20.0) | 4 (3.9) | 7 (16.7) |
| Prior adjuvant systemic therapy, n (%) | ||||
| Endocrine | 116 (78.9) | 55 (84.6) | 40 (39.2) | 20 (47.6) |
| Chemotherapy | 73 (49.7) | 37 (56.9) | 36 (35.3) | 14 (33.3) |
| Most recent endocrine therapy | ||||
| Neoadjuvant/adjuvant metastatic | 82 (55.8) | 41 (63.1) | NA | NA |
| 64 (43.5) | 23 (35.4) | |||
| PgR status, n (%)b | ||||
| Positive | 110 (74.8) | 55 (84.6) | 77 (75.5) | 31 (73.8) |
| Negative | 37 (25.2) | 9 (13.8) | 25 (24.5) | 11 (26.2) |
| ECOG PS, n (%) | ||||
| 0 | 114 (77.6) | 54 (83.1) | 74 (72.5) | 33 (78.6) |
| 1 | 33 (22.4) | 11 (16.9) | 28 (27.5) | 9 (21.4) |
| Sensitivity to ET, n (%)d | ||||
| Primary resistance | 35 (23.8) | 19 (29.2) | NA | NA |
| Secondary resistance | 111 (75.5) | 45 (69.2) | ||
Abbreviations: AI, aromatase inhibitor; ET, endocrine therapy; NA, not available; NSAI, nonsteroidal aromatase inhibitor; PgR, progesterone receptor.
Derived based on baseline tumor lesion locations; bOne patient in MONARCH 2 placebo arm had unknown PgR status.
3.2. Efficacy
3.2.1. MONARCH 2
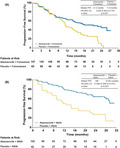
A total of 70 (47.6%) PFS events in the abemaciclib + fulvestrant arm and 51 (78.5%) in the placebo + fulvestrant arm occurred by the data cutoff in East Asian patients. Median PFS was 21.2 months in the abemaciclib + fulvestrant arm and 11.6 months in the placebo + fulvestrant arm (HR, 0.520; 95% CI, 0.362 to 0.747; P < .001) (Figure 1A). Patients with measurable disease achieved an ORR of 47.5% (95% CI, 38.7% to 56.4%) in the abemaciclib + fulvestrant arm and 23.4% (95% CI, 11.3% to 35.5%) in the placebo + fulvestrant arm (Table 2). The CBR for East Asian patients was 76.2% (95% CI, 69.3% to 83.1%) for the abemaciclib + fulvestrant arm and 75.4% (95% CI, 64.9% to 85.9%) for the placebo + fulvestrant arm. For East Asian patients, as observed in the waterfall plot, greater tumor shrinkage was achieved in the abemaciclib + fulvestrant arm compared with the placebo + fulvestrant arm (Figure 2A).
FIGURE 1.

Kaplan‐Meier plot of progression‐free survival for East Asian patients in MONARCH 2 (A) and MONARCH 3 (B) trials. CI, confidence interval; HR, hazard ratio; NR, not reached; NSAI, nonsteroidal aromatase inhibitor; PFS, progression‐free survival
TABLE 2.
Summary of best overall response in the MONARCH 2 and 3 trials (East Asian population)
| Best overall response | MONARCH 2 | MONARCH 3 | ||
|---|---|---|---|---|
| Abemaciclib + fulvestrant n = 147 | Placebo + fulvestrant n = 65 | Abemaciclib + NSAI n = 102 | Placebo + NSAI n =42 | |
| All patients | ||||
| CR, n (%) | 3 (2.0) | 0 | 1 (1.0) | 0 |
| PR, n (%) | 55 (37.4) | 11 (16.9) | 59 (57.8) | 17 (40.5) |
| SD, n (%) | 74 (50.3) | 47 (72.3) | 36 (35.3) | 20 (47.6) |
| ≥6 mo, n (%) | 54 (36.7) | 38 (58.5) | 29 (28.4) | 14 (33.3) |
| PD, n (%) | 9 (6.1) | 6 (9.2) | 1 (1.0) | 4 (9.5) |
| Overall response rate (CR + PR), n (%) 95% CI | 58 (39.5) (31.6‐47.4) | 11 (16.9) (7.8‐26.0) | 60 (58.8) (49.3‐68.4) | 17 (40.5) (25.6‐55.3) |
| Clinical benefit rate (CR + PR + SD ≥6 mo), n (%) 95% CI | 112 (76.2) (69.3‐83.1) | 49 (75.4) (64.9‐85.9) | 89 (87.3) (80.8‐93.7) | 31 (73.8) (60.5‐87.1) |
| Measurable disease population | ||||
| (n) | 122 | 47 | 86 | 37 |
| CR, n (%) | 3 (2.5) | 0 | 1 (1.2) | 0 |
| PR, n (%) | 55 (45.1) | 11 (23.4) | 59 (68.6) | 17 (45.9) |
| SD, n (%) | 50 (41.0) | 31 (66.0) | 22 (25.6) | 15 (40.5) |
| ≥6 mo, n (%) | 32 (26.2) | 24 (51.1) | 16 (18.6) | 9 (24.3) |
| PD, n (%) | 9 (7.4) | 4 (8.5) | 1 (1.2) | 4 (10.8) |
| Overall response rate (CR + PR), n (%) 95% CI | 58 (47.5) (38.7‐56.4) | 11 (23.4) (11.3, 35.5) | 60 (69.8) (60.1‐79.5) | 17 (45.9) (29.9‐62.0) |
| Clinical benefit rate (CR + PR +SD ≥6 mo), n (%) 95% CI | 90 (73.8) (66.0‐81.6) | 35 (74.5) (62.0‐86.9) | 76 (88.4) (81.6‐95.1) | 26 (70.3) (55.5‐85.0) |
Abbreviations: CI, confidence interval; CR, complete response; NSAI, nonsteroidal aromatase inhibitor; PD, progressive disease, PR; partial response, SD; stable disease.
FIGURE 2.
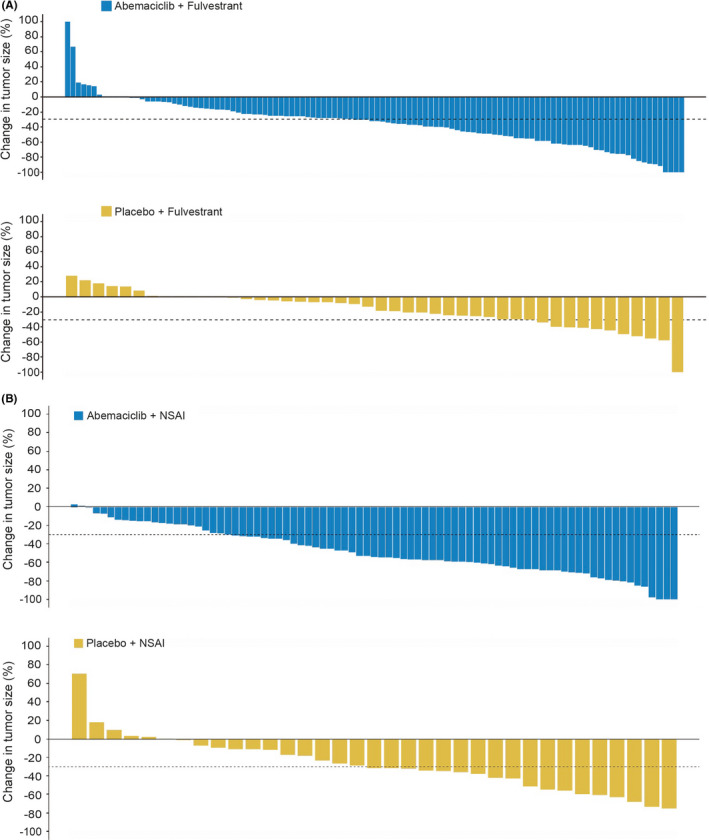
Change in tumor size and response rate for East Asian patients in MONARCH 2 (A) and MONARCH 3 (B) trials. NSAI, nonsteroidal aromatase inhibitor
3.2.2. MONARCH 3
In the East Asian population, 36 (35.3%) PFS events occurred in the abemaciclib + NSAI arm, and 30 (71.4%) occurred in the placebo + NSAI arm. Median PFS was not reached in the abemaciclib + NSAI arm and was 12.82 months in the placebo + NSAI arm (HR, 0.326; 95% CI, 0.200 to 0.531, P < .001) (Figure 1B). Patients with measurable disease achieved an ORR of 69.8% (95% CI, 60.1% to 79.5%) in the abemaciclib + NSAI arm and 45.9% (95% CI, 30% to 62%) in the control arm (Table 2). The CBR for East Asian patients was 87.3% (95% CI, 80.8% to 93.7%) in the abemaciclib + NSAI arm and 73.8% (95% CI, 60.5% to 87.1%) in the placebo + NSAI arm (Table 2). East Asian patients treated with abemaciclib + NSAI experienced greater tumor shrinkage compared with those in the placebo + NSAI arm (Figure 2B).
3.3. Treatment exposure and PK
The median abemaciclib dose intensity of East Asian patients in MONARCH 2 and 3 was 263 and 239 mg/d, respectively. The median duration of abemaciclib treatment in the East Asian population was 65.07 weeks in MONARCH 2 and 96.72 weeks in MONARCH 3. A total of 20 (13.7%) and 30 (29.4%) East Asian patients in the abemaciclib arm discontinued any study drug (discontinuation of one or more study drug) due to an AE in MONARCH 2 and 3, respectively, whereas none of the patients discontinued any study drug in the placebo arms. The dose reduction rate of abemaciclib due to an AE in East Asian patients was 51.4% in MONARCH 2 and 46.1% in MONARCH 3. In population pharmacokinetic analysis, race, including Asian, was not a significant covariate on any of the PK parameters. Furthermore, abemaciclib concentration time profiles for East Asian patients are similar to the global population (Figure 3).
FIGURE 3.
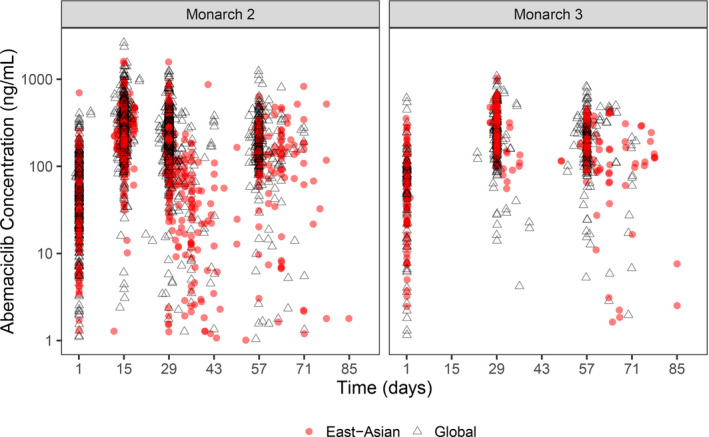
Abemaciclib plasma concentration in MONARCH 2 and MONARCH 3 in East Asian and global populations. Each symbol represents an observation. Red circles indicate the East Asian population, and open triangles represent the global population
In order to investigate the effect of East Asian race on abemaciclib PK or abemaciclib exposure, a population PK analysis was tested. This demonstrated that race was not a significant covariate for any parameter. Therefore, abemaciclib PK or exposure are not expected to be associated with the observations related to East Asian race reported here.
3.4. Safety
The overall abemaciclib safety profile of East Asians in MONARCH 2 and 3 was similar. Rates of grade ≥ 3 AEs in East Asian patients in MONARCH 2 were 67.8% in the abemaciclib + fulvestrant arm versus 21.5% in the placebo + fulvestrant arm; similar rates were observed in MONARCH 3, with 61.8% in the abemaciclib + NSAI arm and 26.2% in the placebo + NSAI arm (Table 3). In the abemaciclib arm, a total of 28 (19.2%) East Asian patients in MONARCH 2 and 22 (21.6%) in MONARCH 3 experienced at least one serious adverse event (SAE), and the most frequently reported SAEs were pyrexia (three [2.1%]) and embolism (three [2.1%]) in MONARCH 2 and lung infection (six [5.9%]) in MONARCH 3. Among the East Asian patients who died in the abemaciclib arms, two (1.4%) patients in MONARCH 2 (cerebral infarction, abnormal hepatic function) and one (1%) patient in MONARCH 3 (lung infection) died due to an AE while on treatment or within 30 days of treatment discontinuation, whereas no deaths occurred during this period in the placebo arms of the East Asian patients in both trials.
TABLE 3.
Most common adverse events observed in the East Asian population in the MONARCH 2 and 3 trials
| MONARCH 2 | ||||||||
|---|---|---|---|---|---|---|---|---|
| ≥20% in either arm | Abemaciclib + fulvestrant (N = 146) | Placebo + fulvestrant (N = 65) | ||||||
| CTCAE Grade | ||||||||
| All n (%) | Grade 2 n (%) | Grade 3 n (%) | Grade 4 n (%) | All | Grade 2 n (%) | Grade 3 n (%) | Grade 4 n (%) | |
| Any | 144 (98.6) | 34 (23.3) | 86 (58.9) | 11 (7.5) | 60 (92.3) | 24 (36.9) | 12 (18.5) | 2 (3.1) |
| Diarrhea | 132 (90.4) | 49 (33.6) | 21 (14.4) | 0 | 13 (20.0) | 2 (3.1) | 1 (1.5) | 0 |
| Neutropenia | 99 (67.8) | 27 (18.5) | 59 (40.4) | 6 (4.1) | 3 (4.6) | 0 | 2 (3.1) | 0 |
| Leukopenia | 60 (41.1) | 33 (22.6) | 19 (13.0) | 1 (0.7) | 2 (3.1) | 1 (1.5) | 0 | 0 |
| Nausea | 54 (37.0) | 12 (8.2) | 3 (2.1) | 0 | 11 (16.9) | 1 (1.5) | 1 (1.5) | 0 |
| Anemia | 54 (37.0) | 31 (21.2) | 16 (11.0) | 1 (0.7) | 3 (4.6) | 0 | 2 (3.1) | 0 |
| Abdominal pain | 45 (30.8) | 8 (5.5) | 1 (0.7) | 0 | 11 (16.9) | 1 (1.5) | 0 | 0 |
| Decreased appetite | 42 (28.8) | 13 (8.9) | 4 (2.7) | 0 | 10 (15.4) | 1 (1.5) | 0 | 0 |
| Thrombocytopenia | 37 (25.3) | 11 (7.5) | 7 (4.8) | 5 (3.4) | 2 (3.1) | 0 | 0 | 1 (1.5) |
| Headache | 36 (24.7) | 9 (6.2) | 1 (0.7) | 0 | 10 (15.4) | 2 (3.1) | 0 | 0 |
| Stomatitis | 35 (24) | 5 (3.4) | 1 (0.7) | 0 | 9 (13.8) | 1 (1.5) | 0 | 0 |
| Vomiting | 35 (24) | 7 (4.8) | 2 (1.4) | 0 | 5 (7.7) | 1 (1.5) | 0 | 0 |
| ALT increased | 34 (23.3) | 12 (8.2) | 9 (6.2) | 1 (0.7) | 2 (3.1) | 1 (1.5) | 0 | 0 |
| Pruritus | 34 (23.3) | 3 (2.1) | 0 | 0 | 5 (7.7) | 0 | 0 | 0 |
| MONARCH 3 | ||||||||
|---|---|---|---|---|---|---|---|---|
| ≥20% in either arm | Abemaciclib + NSAI (N = 102) | Placebo + NSAI (N = 42) | ||||||
| CTCAE Grade | ||||||||
| All n (%) | Grade 2 n (%) | Grade 3 n (%) | Grade 4 n (%) | All n (%) | Grade 2 n (%) | Grade 3 n (%) | Grade 4 n (%) | |
| Any | 102 (100.0) | 31 (30.4) | 56 (54.9) | 6 (5.9) | 39 (92.9) | 16 (38) | 10 (23.8) | 1 (2.4) |
| Diarrhea | 90 (88.2) | 20 (19.6) | 5 (4.9) | 0 | 12 (28.6) | 1 (2.4) | 0 | 0 |
| Neutropenia | 59 (57.8) | 23 (22.5) | 29 (28.4) | 1 (1.0) | 0 | 0 | 0 | 0 |
| Anemia | 36 (35.3) | 16 (15.7) | 13 (12.7) | 0 | 1 (2.4) | 1 (2.4) | 0 | 0 |
| Alopecia | 34 (33.3) | 2 (2) | 0 | 0 | 4 (9.5) | 0 | 0 | 0 |
| Nausea | 34 (33.3) | 4 (3.9) | 0 | 0 | 7 (16.7) | 0 | 0 | 0 |
| ALT increased | 33 (32.4) | 8 (7.8) | 13 (12.7) | 1 (1.0) | 5 (11.9) | 1 (2.4) | 2 (4.8) | 0 |
| AST increased | 33 (32.4) | 7 (6.9) | 9 (8.8) | 0 | 4 (9.5) | 1 (2.4) | 1 (2.4) | 0 |
| Leukopenia | 33 (32.4) | 14 (13.7) | 11 (10.8) | 0 | 3 (7.1) | 1 (2.4) | 0 | 1 (2.4) |
| Decreased appetite | 27 (26.5) | 8 (7.8) | 3 (2.9) | 0 | 2 (4.8) | 0 | 1 (2.4) | 0 |
| Vomiting | 26 (25.5) | 4 (3.9) | 0 | 0 | 7 (16.7) | 0 | 0 | 0 |
| Abdominal pain | 24 (23.5) | 4 (3.9) | 0 | 0 | 2 (4.8) | 0 | 0 | 0 |
| Rash | 22 (21.6) | 5 (4.9) | 0 | 0 | 4 (9.5) | 0 | 0 | 0 |
| Pruritus | 21 (20.6) | 4 (3.9) | 0 | 0 | 2 (4.8) | 0 | 0 | 0 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CTCAE, common terminology criteria for adverse events; n, number of patients in the safety population; N, number of patients in the specified category; NSAI, nonsteroidal aromatase inhibitor.
The most common AE of any grade reported among East Asian patients in the abemaciclib arm was diarrhea (MONARCH 2: 90.4%; MONARCH 3: 88.2%), followed by neutropenia (MONARCH 2: 67.8%; MONARCH 3: 57.8%). Neutropenia was the most common grade ≥ 3 AE observed among East Asian patients in both MONARCH 2 and 3 trials (MONARCH 2: 44.5%; MONARCH 3: 29.4. Of note, the incidence of ALT increase (any grade; ≥20% in East Asian patients) was 23.3% and 32.4% (n = 102) in MONARCH 2 and 3, respectively. Aspartate aminotransferase (AST) increase was also observed in 32.4% of East Asian patients in MONARCH 3. Tables comparing AEs among East Asian, non‐East Asian, and the ITT populations are provided in the Supporting Information (Table S1 and S2).
The most frequent AE leading to discontinuation of any study drug was neutropenia (three [2.1%]) in MONARCH 2 and neutropenia (five [4.9%]) and alanine aminotransferase (ALT) increased (five [4.9%]) in MONARCH 3. In MONARCH 2, discontinuations due to ALT increase were infrequent (two [1.4%]). The most frequent AEs leading to dose reduction were diarrhea (MONARCH 2: 19.9%; MONARCH 3: 7.8%) and neutropenia (MONARCH 2: 18.5%; MONARCH 3: 16.7%).
In the abemaciclib arm, there were two (1.4%) East Asian patients in MONARCH 2 and 11 (10.8%) in MONARCH 3 who developed interstitial lung disease (ILD) defined by selected treatment‐emergent AEs with the term of pneumonitis, ILD, organizing pneumonia, pulmonary fibrosis, bronchiolitis obliterans, and obliterative bronchiolitis. Among the 13 East Asian patients with ILD (Grade 1 or 2: n = 11; Grade 3: n = 2), there were no fatal ILD events (Table S3). Due to ILD, five patients received steroid therapy, two patients discontinued study treatment, and two patients had a dose reduction of abemaciclib.
Venous thromboembolic events (VTEs) of any grade occurred in five patients (3.4%) and three patients (2.9%) of abemaciclib‐treated East Asian patients in the MONARCH 2 and 3, respectively. Of these eight patients, four had a Grade 3 event (Table S4). All eight patients received anticoagulation therapy and three discontinued abemaciclib or all study treatment.
4. DISCUSSION
The MONARCH 2 and MONARCH 3 registration trials met their primary PFS endpoints, demonstrating statistically significant improvements in efficacy with a tolerable safety profile in the ITT populations. MONARCH 2 evaluated abemaciclib in combination with fulvestrant in patients who progressed on prior ET, and MONARCH 3 evaluated abemaciclib in combination with NSAI as initial therapy for advanced disease. As part of these pivotal studies, it is important to consider the efficacy and safety of abemaciclib within subgroups, including the East Asian population, which has demonstrated differences in efficacy and safety in response to other cancer therapies. 6 , 28 , 29 Overall, data from the East Asian subpopulation of the MONARCH 2 and 3 trials showed a generally consistent benefit/risk profile with the previously disclosed results of the ITT population.
Baseline demographic and disease characteristics were generally similar between the East Asian and overall ITT populations in both studies, but there were a few notable differences. As noted in the results section, in both trials, there were more East Asian patients with an ECOG performance status of 0 (MONARCH 2: 79.2%; MONARCH 3: 74.3%) compared with the ITT (MONARCH 2: 59.8%; MONARCH 3: 60.0%). Additionally, there was a higher percentage of pre/perimenopausal patients in MONARCH 2 (35.4%) versus the ITT (17.0%), and in both trials, there were more East Asian patients under 65 years of age (MONARCH 2: 78.3%; MONARCH 3: 64.6%) compared with the ITT (MONARCH 2: 63.4%; MONARCH 3: 55%). These findings are consistent with the literature, which reports that Asian patients are being diagnosed with breast cancer at a younger age compared with Western countries. 23
East Asian patients in both trials experienced an improvement in PFS in the abemaciclib arms compared with the placebo arms. We see a numerically longer PFS and higher ORR compared with the ITT population in MONARCH 2 and 3. In MONARCH 3, there was a smaller HR in East Asian patients (HR = 0.326) relative to the ITT population (HR = 0.54); these results should be interpreted with caution due to the differences in important prognostic factors, such as higher performance status observed in east Asian patients in both studies and a greater number of patients in the placebo arm who exhibited visceral disease, which may have contributed to the poor performance of the placebo arm and the larger treatment effect.
The safety profile of abemaciclib for East Asians was generally consistent with the ITT, with diarrhea being the most common AE, which was mostly low grade, manageable, and reversible. However, for patients in the abemaciclib arms, there were certain toxicities, such as neutropenia, leukopenia, and ALT increase that were more frequent in the East Asian patients compared with the ITT population. Although the mechanism is unknown, the increased rates of neutropenia and leukopenia are consistent with data from other CDK4 & 6 inhibitors, where there is a precedent for increased hematological toxicities in Asian patients. 6 Likewise, in a phase 1b study exclusively in Asian patients, high incidences of liver toxicities have been observed in Japanese patients treated with ribociclib. 30 Despite these variations observed in East Asian patients, the dose intensity and the discontinuation rates were similar between East Asians and the ITT of MONARCH 2 and 3, suggesting that the AEs were typically manageable. 18 , 28 Per label recommendations, neutropenia monitoring should include blood counts assessed prior to the start of abemaciclib, every 2 weeks for the first 2 months, and monthly thereafter for 2 months as clinically indicated. It is also suggested that liver function tests should be monitored prior to the start of abemaciclib therapy, every 2 weeks for the first 2 months, monthly for the next two months, and as clinically indicated. In the event of hepatic transaminase elevation, dose modifications, omissions, or discontinuations should be considered as possible management strategies.
Overall, safety findings were consistent across the 200‐mg‐starting‐dose and 150‐mg‐starting‐dose populations. Some differences were observed in toxicities that were expected to occur early in the course of treatment. Incidence of Grade 2 and 3 for gastrointestinal toxicities like nausea, vomiting, or abdominal pain was higher in patients starting at 200 mg than in patients starting at 150 mg. Neutropenia Grade ≥ 3 was also observed in a higher percentage of patients starting at 200 mg than in patients starting at 150 mg, based on TEAE and central laboratory analyses. Overall, based on our review of the safety data, the higher starting dose for the 178 patients who initially received 200 mg in MONARCH 2 contributed to the slightly higher toxicity rates in MONARCH 2. In MONARCH 3, the rate of abemaciclib dose reductions due to an AE in East Asian patients was comparable to the ITT population (46.5%); whereas in MONARCH 2, dose reductions were slightly higher in East Asian patients than in the ITT population (42.9%). The higher dose reduction rate due to an AE in MONARCH 2 may be partially explained by the higher proportion of patients who started abemaciclib with 200 mg twice daily in MONARCH 2 (39% vs 27.4%). 5 However, efficacy was consistent with the overall population. This finding corroborates the results of a previous exploratory analysis demonstrating that PFS was maintained irrespective of dose reductions. 31 Moreover, as previously reported, race was not a significant covariate for abemaciclib PK parameters, 25 , 26 , 27 and in the present study abemaciclib concentrations in East Asians were similar to the ITT populations, indicating that the imbalance in toxicity rate is not attributable to differences in abemaciclib disposition or exposure. The abemaciclib starting dose in combination with fulvestrant or NSAI is 150 mg twice daily, regardless of race or ethnicity.
Interstitial lung disease and VTE were assessed as AEs of special interest. Patients treated with abemaciclib developed serious lung disease in an early postmarketing‐phase pharmacovigilance survey conducted in Japanese patients after its approval in Japan. 32 Due to the small number of ILD and VTE events, it is difficult to assess whether the risk of abemaciclib‐induced ILD or VTE differs with ethnicity or region. Further studies utilizing real‐world data may be needed.
One limitation of this analysis is that it is descriptive in nature and solely focused on the East Asian subgroup. No formal comparisons were planned between East Asian and non‐East Asian or the ITT population due to the relatively small number of patients in this subgroup. However, the PFS results for prespecified subgroups including region (North America, Europe, and Asia) were previously reported. 8 , 18
In conclusion, the East Asian populations of MONARCH 2 and 3 benefited from abemaciclib in combination with ET. As expected, there was a greater occurrence of hematological toxicities in the East Asian subgroup, but the overall safety profile was manageable and generally consistent with the ITT population. These findings suggest that the benefit/risk profile of abemaciclib in combination with NSAIs (letrozole and anastrozole) or with fulvestrant is favorable and remains an effective therapeutic option for East Asian patients with HR+, HER2− ABC.
CONFLICT OF INTEREST
M. T. receives lecture fees, honoraria, or other fees from Pfizer, AstraZeneca, Eli Lilly and Company, Eisai, Chugai, Takeda, Kyowa Kirin, Novartis, and Daiichi Sankyo; research funds by Eli Lilly and Company, Taiho, Shimadzu, Kyowa Kirin, Pfizer, Eisai, Roche, Chugai, AstraZeneca, C&C Res Lab, Nippon Kayaku, Astellas, Bizcom Japan, Terumo, AFI Technologies, Japan Breast Cancer Research Group (JBCRG), Contessa, Daiichi Sankyo, Parexel/Puma Biotechnology, MSD, Bayer, Novartis, Takeda, and GSK; and annual remuneration from Eli Lilly and Company (for travel expenses, gifts, etc. that are not related to research). K. I. receives lecture fees, honoraria, or other fees from Eisai, Chugai, Pfizer, and Eli Lilly and Company; and research funds from Novartis, Pfizer, Chugai, Daiichi‐Sankyo, Parexel/Puma Biotechnology, MSD, Bayer, Eli Lilly and Company, AstraZeneca, Sanofi, Eisai, and Taiho. N. M. receives honoraria (eg, lecture fees) from Chugai, AstraZeneca, Pfizer, Eli Lilly and Company, Eisai, and Takeda; research funding from Chugai, AstraZeneca, Kyowa‐Kirin, MSD, Novartis, Pfizer, Eli Lilly and Company, Eisai, Nippon‐Kayaku, and Daiichi Sankyo; and serves as board of directors for the Japanese Breast Cancer Society (JBCS) and JBCRG. H. I. receives research funds from Daiichi‐Sankyo, Chugai, and Boehringer Ingelheim; J. S.’s wife (K. H. W.) receives profit from shares from Daiichi Sankyo and Celltrione Healthcare. J. S. receives research funds from Roche, Novartis, AstraZeneca, Daiichi Sankyo, MSD, Eli Lilly and Company, and Pfizer. I. H. P. has no conflict of interest to disclose. S. I. reports research funds from Pfizer, AstraZeneca, Roche, Eisai, and Daewong Pharm. S. C. has no conflict of interest to disclose. S. E. is an employee of Eli Lilly and Company and reports profit from shares. P. K. T. is an employee of Eli Lilly and Company. V. A. M. is an employee of Eli Lilly and Company. V. A. M.’s spouse (T. G.) is an employee of Eli Lilly and Company. M. C. H. is a full‐time employee and stock shareholder of Eli Lilly and Company and shares income from Eli Lilly and Company with M. H. (spouse). S. S. is an employee and stock shareholder of Eli Lilly and Company. M. P. G. receives research funds from Eli Lilly and Company and funds for travel expenses from Eli Lilly and Company and the JBCS. G. W. S. receives clinical trial funds from Eli Lilly and Company.
Supporting information
Table S1‐S4
ACKNOWLEDGMENTS
We thank the study investigators and their support staff, as well as the patients and their caregivers for participating in the MONARCH 2 and 3 trials. This study was sponsored by Eli Lilly and Company, the manufacturer of abemaciclib. Sambasiva Kolati and Julie A. Mund from Eli Lilly and Company provided medical writing support.
Toi M, Inoue K, Masuda N, et al. Abemaciclib in combination with endocrine therapy for East Asian patients with HR+, HER2− advanced breast cancer: MONARCH 2 & 3 trials. Cancer Sci. 2021;112:2381–2392. 10.1111/cas.14877
Clinical Trial Register and Clinical Registration number: NCT02107703 and NCT02246621.
REFERENCES
- 1. Milani A, Geuna E, Mittica G, Valabrega G. Overcoming endocrine resistance in metastatic breast cancer: current evidence and future directions. World J Clin Oncol. 2014;5:990‐1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rugo HS, Rumble RB, Macrae E, et al. Endocrine therapy for hormone receptor‐positive metastatic breast cancer: American Society of Clinical Oncology Guideline. J Clin Oncol. 2016;34:3069‐3103. [DOI] [PubMed] [Google Scholar]
- 3. Corona SP, Generali D. Abemaciclib: a CDK4/6 inhibitor for the treatment of HR+/HER2‐ advanced breast cancer. Drug Des Devel Ther. 2018;12:321‐330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goetz MP, Toi M, Campone M, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35:3638‐3646. [DOI] [PubMed] [Google Scholar]
- 5. Sledge GW, Toi M, Neven P, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2‐ advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35:2875‐2884. [DOI] [PubMed] [Google Scholar]
- 6. Iwata H, Im S‐A, Masuda N, et al. PALOMA‐3: Phase III trial of fulvestrant with or without palbociclib in premenopausal and postmenopausal women with hormone receptor‐positive, human epidermal growth factor receptor 2‐negative metastatic breast cancer that progressed on prior endocrine therapy‐safety and efficacy in Asian patients. J Glob Oncol. 2017;3:289‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tripathy D, Im S‐A, Colleoni M, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone‐receptor‐positive, advanced breast cancer (MONALEESA‐7): a randomised phase 3 trial. Lancet Oncol. 2018;19:904‐915. [DOI] [PubMed] [Google Scholar]
- 8. Sledge GW, Toi M, Neven P, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor‐positive, ERBB2‐negative breast cancer that progressed on endocrine therapy‐MONARCH 2: a randomized clinical trial. JAMA Oncol. 2020;6(1):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pernas S, Tolaney SM, Winer EP, Goel S. CDK4/6 inhibition in breast cancer: current practice and future directions. Ther Adv Med Oncol. 2018;10:1758835918786451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Neuman E, Ladha MH, Lin N, et al. Cyclin D1 stimulation of estrogen receptor transcriptional activity independent of cdk4. Mol Cell Biol. 1997;17:5338‐5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sammons SL, Topping DL, Blackwell KL. HR+, HER2− advanced breast cancer and CDK4/6 inhibitors: mode of action, clinical activity, and safety profiles. Curr Cancer Drug Targets. 2017;17:637‐649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Malumbres M, Barbacid M. To cycle or not to cycle: a critical decision in cancer. Nat Rev Cancer. 2001;1:222‐231. [DOI] [PubMed] [Google Scholar]
- 13. Dickler MN, Tolaney SM, Rugo HS, et al. MONARCH 1, a Phase II Study of Abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR(+)/HER2(−) metastatic breast cancer. Clin Cancer Res. 2017;23:5218‐5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Flaherty KT, LoRusso PM, DeMichele A, et al. Phase I, dose‐escalation trial of the oral cyclin‐dependent kinase 4/6 inhibitor PD 0332991, administered using a 21‐day schedule in patients with advanced cancer. Clin Cancer Res. 2012;18:568‐576. [DOI] [PubMed] [Google Scholar]
- 15. Juric D, Hamilton E, Estevez LG, et al. Phase Ib/II study of LEE011 and BYL719 and letrozole in ER+, HER2– breast cancer: safety, preliminary efficacy and molecular analysis. Cancer Res. 2015;75:abstract nr P5‐19‐24. [Google Scholar]
- 16. Martin JM, Goldstein LJ. Profile of abemaciclib and its potential in the treatment of breast cancer. Onco Targets Ther. 2018;11:5253‐5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Patnaik A, Rosen LS, Tolaney SM, et al. Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non‐small cell lung cancer, and other solid tumors. Cancer Discov. 2016;6:740‐753. [DOI] [PubMed] [Google Scholar]
- 18. Johnston S, Martin M, Di Leo A, et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer. 2019;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ghoncheh M, Mahdavifar N, Darvishi E, Salehiniya H. Epidemiology, incidence and mortality of breast cancer in Asia. Asian Pac J Cancer Prev. 2016;17:47‐52. [DOI] [PubMed] [Google Scholar]
- 20. Sung H, Rosenberg PS, Chen W‐Q, et al. Female breast cancer incidence among Asian and Western populations: more similar than expected. J Natl Cancer Inst. 2015;107:djv107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Youlden DR, Cramb SM, Yip CH, Baade PD. Incidence and mortality of female breast cancer in the Asia‐Pacific region. Cancer Biol Med. 2014;11:101‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mizoo T, Taira N, Nishiyama K, et al. Effects of lifestyle and single nucleotide polymorphisms on breast cancer risk: a case‐control study in Japanese women. BMC Cancer. 2013;13:565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Green M, Raina V. Epidemiology, screening and diagnosis of breast cancer in the Asia‐Pacific region: current perspectives and important considerations. Asia‐Pacific J Clin Oncol. 2008;4:S5‐S13. [Google Scholar]
- 24. ESMO Asia 2016 Press Release: Ribociclib Improves Progression‐free Survival in Asian Women with Advanced Breast Cancer. European Society for Medical Oncology, 2016. [Google Scholar]
- 25. Chigutsa E, Kambhampati S, Sykes A, Posada MM, Turner KP. A mechanistic population pharmacokinetic model of abemaciclib and its metabolites and the impact of diarrhea. American Conference on Pharmacometrics. 2017.
- 26. Kambhampati SR, Chigutsa E, Sykes AK, Turner KP. A Simultaneous PK‐Diarrhea Model to Assess the Impact of Diarrhea on Bioavailability of Abemaciclib. American Conference on Pharmacometrics. 2017.
- 27. Chigutsa E, Kambhampati SRP, Sykes AK, Posada MM, van der Walt JS, Turner PK. Development and application of a mechanistic population modeling approach to describe abemaciclib pharmacokinetics. CPT Pharmacometrics Syst Pharmacol. 2020;9(9):523‐533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Toi M, Huang C, Im Y‐H, et al. MONARCH 2: Abemaciclib in combination with fulvestrant in Asian women with HR+, HER2‐ advanced breast cancer who progressed on endocrine therapy. Ann Oncol. 28(suppl_10):x26‐x34. [Google Scholar]
- 29. Masuda N, Nishimura R, Takahashi M, et al. Palbociclib in combination with letrozole as first‐line treatment for advanced breast cancer: a Japanese phase II study. Cancer Sci. 2018;109:803‐813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yap YS, Chiu J, Ito Y, et al. Ribociclib, a CDK 4/6 inhibitor, plus endocrine therapy in Asian women with advanced breast cancer. Cancer Sci. 2020;111:3313‐3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rugo HS, Sledge GW, Johnston SRD, et al. The association of early toxicity and outcomes for patients treated with abemaciclib. J Clin Oncol. 2018;36:1053. [Google Scholar]
- 32. OncLive . Abemaciclib regimens approved in Japan for recurrent HR+/HER2‐. Breast Cancer. 2018. https://www.onclive.com/view/abemaciclib‐regimens‐approved‐in‐japan‐for‐recurrent‐hrher2‐breast‐cancer. Accessed April 08, 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S4


