Abstract
E7777 is a recombinant cytotoxic fusion protein composed of the diphtheria toxin fragments A and B and human interleukin‐2. It shares an amino acid sequence with denileukin diftitox, but has improved purity and an increased percentage of active monomer. We undertook a multicenter, single‐arm phase II study of E7777 in patients with relapsed or refractory peripheral T‐cell lymphoma (PTCL) and cutaneous T‐cell lymphoma (CTCL) to evaluate its efficacy, safety, pharmacokinetics, and immunogenicity. A total of 37 patients were enrolled, of which 17 and 19 patients had PTCL and CTCL, respectively, and one patient with another type of lymphoma (extranodal natural killer/T‐cell lymphoma, nasal type), diagnosed by the Central Pathological Diagnosis Committee. Among the 36 patients with PTCL and CTCL, objective response rate based on the independent review was 36% (41% and 31%, respectively). The median progression‐free survival was 3.1 months (2.1 months in PTCL and 4.2 months in CTCL). The common adverse events (AEs) observed were increased aspartate aminotransferase (AST) / alanine aminotransferase (ALT), hypoalbuminemia, lymphopenia, and pyrexia. Our results indicated that a 9 µg/kg/d dose of E7777 shows efficacy and a manageable safety profile in Japanese patients with relapsed or refractory PTCL and CTCL, with clinical activity observed across the range of CD25 expression. The common AEs were manageable, but increase in ALT / AST, hypoalbuminemia, and capillary leak syndrome should be carefully managed during the treatment.
Keywords: CD25, cutaneous T‐cell lymphoma, E7777, interleukin‐2, peripheral T‐cell lymphoma
Among 36 patients with refractory peripheral T‐cell lymphoma (PTCL) and cutaneous T‐cell lymphoma (CTCL), objective response rate based on the independent review was 36% (41% in PTCL and 31% in CTCL). Our results indicated that a 9 µg/kg/d dose of E7777 shows efficacy and a manageable safety profile in Japanese patients with relapsed or refractory PTCL and CTCL, with clinical activity observed across the range of CD25 expression. The common adverse events were manageable, but increase in alanine aminotransferase / aspartate aminotransferase, hypoalbuminemia, and capillary leak syndrome should be carefully managed during the treatment.

1. INTRODUCTION
Peripheral T‐cell lymphoma (PTCL) and cutaneous T‐cell lymphoma (CTCL) are rare and heterogeneous forms of mature T‐cell neoplasms among the non‐Hodgkin lymphomas (NHLs). 1 Histologically, PTCL are mature T‐cell lymphomas accounting for 12%‐15% of all NHLs and are usually characterized by aggressive clinical types, frequent relapses, and eventual development of refractory disease. 1 The standard first‐line treatment for PTCL is chemotherapy with multiple agents, such as cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP). 2 However, the outcome has been disappointing, with reports of long‐term survival of 20%‐30%. 3 Cutaneous T‐cell lymphoma is generally an indolent and heterogeneous group of NHL with a poor prognosis in advanced‐stage disease. 4 For relapsed or refractory PTCL and/or CTCL, several new agents, such as pralatrexate, romidepsin, brentuximab vedotin, belinostat, chidamide, vorinostat, forodesine, and mogamulizumab were approved and are being developed in several countries, including Japan. However, due to the lack of an exact and optimal regimen for relapsed or refractory T‐cell NHL, new treatment options are required for its management.
E7777 is a recombinant cytotoxic fusion protein composed of the enzymatically active portion of the diphtheria toxin fragments and the receptor‐binding domain of human interleukin‐2 (IL‐2). E7777 shares an amino acid sequence with historical denileukin diftitox (DD), and is manufactured using similar but modified processes. Despite these similarities, E7777 has improved purity and an increased percentage of active protein monomer species and reduced level of misfolded and/or aggregated protein impurities. E7777 and DD directly bind to the IL‐2 receptor (IL‐2R) and are then internalized by receptor‐mediated endocytosis. The diphtheria toxin domain is cleaved and translocated into the cytoplasm, where it catalyzes the covalent linkage of ADP‐ribose to elongation factor‐2, resulting in the inhibition of protein synthesis and cell death. 5 For historical DD, two phase III studies were undertaken in patients with CD25 positive (CD25 in at least 20% of tumor cells) CTCL with objective response rate (ORR) of 30% and 44% in each study. 6 , 7 Moreover, a companion study was undertaken in patients with low CD25 expression (CD25 in less than 20% of tumor cells) CTCL, showing an ORR of 31%, which indicated that low CD25 expression does not preclude a meaningful clinical response by DD. 8 For other relapsed/refractory T‐cell NHL, a phase II study was undertaken and an ORR of 48.1% was observed. 9 For E7777, a phase II study is ongoing in the United States; a phase I study in Japanese patients with PTCL and CTCL was carried out, in which the recommended dose of E7777 was determined to be 9 µg/kg/d for five consecutive days per 21‐day cycle. 10 Preliminary, but clinically meaningful, antitumor activity was also shown in the study. Therefore, we undertook a phase II study to further characterize the efficacy and safety of E7777 in Japanese patients with PTCL and CTCL.
2. METHODS
2.1. Study design and patients
This multicenter, single‐arm, phase II study in Japanese patients with relapsed or refractory PTCL and CTCL aimed to evaluate the efficacy, safety, pharmacokinetics (PK), and immunogenicity of E7777 (ClinicalTrial.gov identifier NCT02676778). The key inclusion criteria included: written informed consent, age 20 years or more, histological diagnosis of PTCL or CTCL, history of previous systemic chemotherapy, an ECOG performance status of 0 or 1, adequate renal, hepatic, and bone marrow function, and a life expectancy of 3 months or more. Histopathological diagnosis of PTCL or CTCL was as follows:
peripheral T‐cell lymphoma, not otherwise specified (PTCL‐NOS)
angioimmunoblastic T‐cell lymphoma
systemic anaplastic large cell lymphoma, anaplastic lymphoma kinase (ALK) positive (ALCL ALK+)
systemic anaplastic large cell lymphoma, ALK negative (ALCL ALK −)
enteropathy‐associated T‐cell lymphoma
hepatosplenic T‐cell lymphoma
mycosis fungoides
Sézary syndrome
primary cutaneous CD30+ T‐cell lymphoproliferative disorders
subcutaneous panniculitis‐like T‐cell lymphoma
primary cutaneous γδT‐cell lymphoma
primary cutaneous CD8+ aggressive epidermotropic cytotoxic T‐cell lymphoma
primary cutaneous CD4+ small/medium T‐cell lymphoma
The key exclusion criteria included: central nervous system invasion, active infection requiring treatment, and prior allogeneic hematopoietic stem cell transplantation (see details in Supporting Information). This study was undertaken in compliance with the Good Clinical Practice guidelines, the Declaration of Helsinki, and local laws. The study protocol was approved by the institutional review board of each institution, and all patients provided informed written consent.
2.2. Treatments
The E7777 dose of 9 μg/kg/d was i.v. infused for 60 minutes once daily from days 1 through 5 of each 21‐day cycle for a maximum of eight cycles. The dose was decreased to 6 μg/kg/d when the patient could not start the next cycle at a planned schedule in two consequent cycles due to toxicity related to the study drug. The premedication of oral acetaminophen, i.v. injection of H1 antagonists, and dexamethasone, and an oral antiemetic agent with pre/post hydration was required. Premedication after cycle 3 could be discontinued at the investigator’s discretion.
2.3. Efficacy
Antitumor activities were assessed using integrated criteria from “Revised response criteria for malignant lymphoma” 11 for nodal/extranodal lesion and liver/spleen enlargement by computed tomography (CT) or PET/CT assessment, and “Clinical endpoints and response criteria in mycosis fungoides and Sézary syndrome” 12 for cutaneous and peripheral blood assessment, in both PTCL and CTCL. Tumor assessments were carried out before the first dose of study treatment, every 2 months during the treatment, and at the discontinuation of treatment. If applicable, PET scans were carried out before the first dose of treatment, between days 15 and 22 of cycle 4 and 8, and at discontinuation of treatment. The primary objective was to evaluate the ORR based on the independent review. The secondary objectives were to evaluate progression‐free survival (PFS), duration of response (DOR), time to response (TTR), complete response (CR) rate, overall survival (OS), safety, PK, and immunogenicity (assessed using the anti‐E7777 Ab, anti‐IL‐2 Ab, and the neutralizing activity of the anti‐E7777 Ab). The exploratory objectives were to explore CD25 expression in the tumor by immunohistochemistry and other related markers.
2.4. Safety
Safety assessments were adverse event (AE) monitoring, laboratory testing, vital sign measurements, and 12‐lead electrocardiograms. The severity of AEs was classified using the Common Terminology Criteria for Adverse Events (CTCAE) version 4.03. Ophthalmological tests were carried out at screening, on day 15 of odd‐cycles, and if clinically indicated.
2.5. Pharmacokinetics, immunogenicity, and CD25 expression
For PK analysis, patients were recruited until six or more patients continued the treatment for at least five cycles. Blood samples were collected for PK analysis on day 1 of cycles 1, 3, and 5. Samples were collected at predose, 30 minutes after the start of the infusion, the end of infusion, and at 30, 60, 90, and 120 minutes after the end of infusion on day 1 of cycles 1, 3, and 5. In cycle 1, the blood sample at 240 minutes after the end of infusion was also taken. E7777 concentration was measured by a validated electrochemiluminescent method. The lower limit of quantification for E7777 was 10.0 ng/mL. Blood samples were collected before treatment on day 1 of cycles 1, 2, 3, 5, and 8, and at discontinuation for immunogenicity assessments including anti‐E7777 Ab, anti‐IL‐2 Ab, and neutralizing activity of anti‐E7777 Abs. For CD25 expression, tumor samples (formalin‐fixed paraffin‐embedded tissue sample) were collected before drug treatment and measured at the central laboratory.
2.6. Statistical analysis
Efficacy and safety of E7777 were evaluated in patients who received at least one dose of E7777. One subject diagnosed as extranodal natural killer/T‐cell lymphoma of nasal type (ENKL) by the Central Pathological Diagnosis Committee was excluded from the efficacy analysis. The primary endpoint, ORR, was the proportion of patients whose best overall response was CR or PR, and the corresponding two‐sided 95% confidence interval (CI) was calculated using Clopper‐Pearson’s method. Thirty‐five patients were required to detect the lower limit of the 95% CI that exceeded the 5% threshold in ORR, with the expected ORR being 25% with a statistical power of 90%. Progression‐free survival, DOR, TTR, and OS were analyzed by the Kaplan‐Meier method with a 95% CI. Treatment‐emergent AEs, defined as AEs that emerged on or after the first dose of study drug, were analyzed.
3. RESULTS
3.1. Patients
The study was carried out between 28 March 2016 and 26 April 2019 at 20 study sites in Japan. Of the 45 patients screened, 37 were eligible, including 17 PTCL patients, 19 CTCL patients, and 1 patient with ENKL diagnosed by the Central Pathological Diagnosis Committee (Table 1). Among the patients with PTCL and CTCL, the most frequent histopathologic subtypes were PTCL‐NOS (35.1%) and mycosis fungoides (32.4%). The median age was 64.5 years (Table 2). Overall, the median number of prior chemotherapy cycles was two (range, 1‐10) (two in PTCL and one in CTCL). Overall, 20 patients (55.6%) were refractory to the last line of systemic chemotherapy.
TABLE 1.
Histopathologic subtype of relapsed/refractory peripheral T‐cell lymphoma (PTCL) or cutaneous T‐cell lymphoma (CTCL) in 37 Japanese patients
| Characteristic | Patients (N = 37) |
|---|---|
| n (%) | |
| PTCL | 17 (45.9) |
| Peripheral T‐cell lymphoma, NOS | 13 (35.1) |
| Angioimmunoblastic T‐cell lymphoma | 3 (8.1) |
| Anaplastic large cell lymphoma, ALK− | 1 (2.7) |
| CTCL | 19 (51.4) |
| Mycosis fungoides | 12 (32.4) |
| Sézary syndrome | 2 (5.4) |
| Primary cutaneous CD30+ T‐cell lymphoproliferative disorders | 2 (5.4) |
| Primary cutaneous γδ TCL | 1 (2.7) |
| Primary cutaneous aggressive epidermotropic CD8+ CTL | 1 (2.7) |
| PTCL‐NOS | 1 (2.7) |
| Other; extranodal natural killer/T‐cell lymphoma of nasal type | 1 (2.7) |
Abbreviations: ALK, anaplastic lymphoma kinase; NOS, not otherwise specified; TCL, T‐cell lymphoproliferative.
TABLE 2.
Patient demographics and characteristics of 36 Japanese patients with relapsed/refractory peripheral T‐cell lymphoma (PTCL) and cutaneous T‐cell lymphoma (CTCL)
| Characteristic | PTCL (n = 17) | CTCL (n = 19) | Overall (N = 36) |
|---|---|---|---|
| Age, years | 66 (49‐82) | 52 (27‐81) | 64.5 (27‐82) |
| Age, <65/≥65 years | 6 (35.3)/11 (64.7) | 12 (63.2)/7 (36.8) | 18 (50.0)/18 (50.0) |
| Gender, male/female | 12 (70.6)/5 (29.4) | 13 (68.4)/6 (31.6) | 25 (69.4)/11 (30.6) |
| ECOG PS, 0/1 | 10 (58.8)/7 (41.2) | 13 (68.4)/6 (31.6) | 23 (63.9)/13 (36.1) |
| Number of prior chemotherapy cycles | |||
| 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 1 | 6 (35.3) | 11 (57.9) | 17 (47.2) |
| 2 | 3 (17.6) | 3 (15.8) | 6 (16.7) |
| 3 | 3 (17.6) | 3 (15.8) | 6 (16.7) |
| 4 | 1 (5.9) | 0 (0.0) | 1 (2.8) |
| ≥5 | 4 (23.5) | 2 (10.5) | 6 (16.7) |
| Median (range) | 2.0 (1, 10) | 1.0 (1, 6) | 2 (1, 10) |
| Relapsed/refractory a | 10 (58.8)/7 (41.2) | 5 (26.3)/13 (68.4) | 15 (41.7)/20 (55.6) |
| Unknown 1 (5.3) | Unknown 1 (2.8) | ||
Data are shown as n (%) or median (range).
Abbreviations: PS, performance status; PTCL, peripheral T‐cell lymphoma.
Relapsed, best response was complete response or partial response with last previous systemic chemotherapy. Refractory, best response was stable disease or progressive disease with last previous systemic chemotherapy.
Of the 37 treated patients, seven patients with CTCL completed the study treatment of a maximum of eight cycles, and 30 patients discontinued the study treatment. Three patients (8.3%) had a dose reduction to 6 μg/kg/d. The most common reason for discontinuation was disease progression (Table 3). Overall, patients received a median of four treatment cycles (range, one to eight), and 15 patients (40.5%) received five or more cycles. The median duration of treatment was 68 days (range, 3‐187 days). Overall, all of 37 patients experienced at least 1 TEAE. TEAEs with CTCAE Grade 3 or higher occurred in 35/37 patients (94.6%). Patients with treatment‐emergent adverse events (TEAEs) leading to study drug dose adjustment was shown in Table 4.
TABLE 3.
Patient disposition and reasons for discontinuation of E7777 treatment among 36 Japanese patients with relapsed/refractory peripheral or cutaneous T‐cell lymphoma
| Primary reason for discontinuation | Overall (N = 37) |
|---|---|
| Completed the study a | 7 (18.9) |
| Discontinued from the study | 30 (81.1) |
| Primary reason for discontinuation | |
| Disease progression | 11 (29.7) |
| Could not administer the study drug within 21 d after the scheduled date with adverse reactions | 2 (5.4) |
| Adverse event | 5 (13.5) |
| Patient’s choice | 5 (13.5) |
| Other b | 7 (18.9) |
Number of patients who completed eight cycles.
Clinical progressive disease (PD) (n = 3), PD suspect (n = 1), doctor’s choice (n = 2), and the drug was not sufficient for suppressing the disease severity (n = 1).
TABLE 4.
Patients with treatment‐emergent adverse events (TEAEs) leading to study drug dose adjustment among 37 Japanese patients with relapsed/refractory peripheral or cutaneous T‐cell lymphoma treated with E7777
| Overall (N = 37) | |
|---|---|
| Patients with any TEAEs | 37 (100) |
| Patients with grade 3 or higher TEAEs | 35 (94.6) |
| Patients with any serious TEAE | 17 (45.9) |
| Patients with TEAEs leading to study drug dose adjustment | |
| Study drug withdrawal | 9 (24.3) |
| Study drug dose reduction | 3 (8.1) |
| Study drug interruption | 4 (10.8) |
| Delay of study drug administration | 10 (27.0) |
3.2. Efficacy
The ORR was 36.1% (95% CI, 20.8‐53.8) based on the independent review (Table 5), and the lower limit of 95% CI exceeded the predefined threshold of 5%. The ORR was 41.2% (95% CI, 18.4‐67.1) and 31.6% (95% CI, 12.6‐56.6) in patients with PTCL and CTCL, respectively. Complete response was observed in one patient with PTCL.
TABLE 5.
Summary of tumor response in 36 Japanese patients with relapsed/refractory peripheral T‐cell lymphoma (PTCL) or cutaneous T‐cell lymphoma (CTCL) treated with E7777
| Response | PTCL (N = 17) | CTCL (N = 19) | Overall (N = 36) |
|---|---|---|---|
| ORR | 7 (41.2%) | 6 (31.6%) | 13 (36.1%) |
| ORR 95% CI | 18.4, 67.1% | 12.6, 56.6% | 20.8, 53.8% |
| Best response | |||
| CR | 1 (5.9) | 0 (0) | 1 (2.8) |
| PR | 6 (35.3) | 6 (31.6) | 12 (33.3) |
| SD | 2 (11.8) | 10 (52.6) | 12 (33.3) |
| PD | 7 (41.2) | 1 (5.3) | 8 (22.2) |
| NE | 1 (5.9) | 2 (10.5) | 3 (8.3) |
Abbreviations: CI, confidence interval; CR, complete response; NE, not evaluable; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease.
Waterfall plots in Figure 1 indicate the maximum percentage changes in the sum of the product of the diameters of the target lesion from the baseline based on the independent review by the Imaging Review Committee. In 22 patients (16 PTCL and six CTCL) who had pre/post‐baseline scan, 16 patients (10 PTCL and six CTCL) showed tumor shrinkage, and 10 patients (eight PTCL and two CTCL) showed more than 50% shrinkage.
FIGURE 1.

Maximum percentage change from the baseline for the sum of the products of the diameters (SPD) in Japanese patients with relapsed/refractory peripheral T‐cell lymphoma (PTCL) or cutaneous T‐cell lymphoma (CTCL) treated with E7777. The plot represents the percentage changes from the baseline in SPD for each patient relative to the postbaseline nadir. Black and gray bars indicate PTCL and CTCL, respectively
The maximum percentage changes in the modified Severity‐Weighted Assessment Tool (mSWAT) score from the baseline based on investigator review, followed by confirmation in the independent review, are shown in Figure 2. Among 19 patients (two PTCL and 17 CTCL) who had pre/post‐baseline mSWAT score, 16 patients (two PTCL and 14 CTCL) showed a decrease in the mSWAT score, and 10 patients (two PTCL and eight CTCL) showed a more than 50% decrease.
FIGURE 2.

Maximum percentage change from the baseline for the modified Severity‐Weighted Assessment Tool (mSWAT score, independent review) in Japanese patients with relapsed/refractory peripheral T‐cell lymphoma (PTCL) or cutaneous T‐cell lymphoma (CTCL) treated with E7777. The plot represents the percentage change from the baseline in the mSWAT score for each patient relative to the postbaseline nadir. Black and gray bars indicate PTCL and CTCL, respectively
The ORR for patients who had received three or more prior systemic chemotherapies was 38.5% (5/13 patients), and in patients who were refractory to the last prior systemic chemotherapy was 35.0% (7/20 patients) (Table 6). Additionally, the ORR for patients with less than 20% CD25+ cells was 25.0% (3/12 patients) and 41.7% (10/24 patients) in those with 20% or more CD25+ cells (Table 6). The overall ORR for patients who had been treated with newly approved agents in Japan (brentuximab vedotin, forodesine, mogamulizumab, pralatrexate, romidepsin, bexarotene, or vorinostat) was 53.8% (7/13 patients), with three of five patients with PTCL and four of eight patients with CTCL, indicating that E7777 is a useful treatment option for relapsed or refractory patients treated with newly approved agents (Table 6).
TABLE 6.
Subgroup analysis of tumor response in 36 Japanese patients with relapsed/refractory peripheral T‐cell lymphoma (PTCL) or cutaneous T‐cell lymphoma (CTCL) treated with E7777
| Subgroup | Number of responders/number of subjects, objective response rate (%), [95% confidence interval] | ||
|---|---|---|---|
| PTCL (N = 17), n (%) | CTCL (N = 19), n (%) | Overall (N = 36), n (%) | |
| Sex | |||
| Male | 5/12 (41.7) [15.2, 72.3] | 6/13 (46.2) [19.2, 74.9] | 11/25 (44.0) [24.4, 65.1] |
| Female | 2/5 (40.0) [5.3, 85.3] | 0/6 (0.0) [0.0, 45.9] | 2/11 (18.2) [2.3, 51.8] |
| Age group | |||
| ≥65 y | 3/6 (50.0) [11.8, 88.2] | 4/12 (33.3) [9.9, 65.1] | 7/18 (38.9) [17.3, 64.3] |
| <65 y | 4/11 (36.4) [10.9, 69.2] | 2/7 (28.6) [3.7, 71.0] | 6/18 (33.3) [13.3, 59.0] |
| CD25+ cell rate | |||
| <20% | 2/6 (33.3) [4.3, 77.7] | 1/6 (16.7) [0.4, 64.1] | 3/12 (25.0) [5.5, 57.2] |
| ≥20% | 5/11 (45.5) [16.7, 76.6] | 5/13 (38.5) [13.9, 68.4] | 10/24 (41.7) [22.1, 63.4] |
| Relapsed/refractory | |||
| Relapsed | 4/10 (40.0) [12.2, 73.8] | 2/5 (40.0) [5.3, 85.3] | 6/15 (40.0) [16.3, 67.7] |
| Refractory | 3/7 (42.9) [9.9, 81.6] | 4/13 (30.8) [9.1, 61.4] | 7/20 (35.0) [15.4, 59.2] |
| Number of prior systemic chemotherapy cycles | |||
| ≤2 regimens | 4/9 (44.4) [13.7, 78.8] | 4/14 (28.6) [8.4, 58.1] | 8/23 (34.8) [16.4, 57.3] |
| ≥3 regimens | 3/8 (37.5) [8.5, 75.5] | 2/5 (40.0) [5.3, 85.3] | 5/13 (38.5) [13.9, 68.4] |
| Prior use of newly approved agent | |||
| Yes | 3/5 (60.0) [14.7, 94.7] | 4/8 (50.0) [15.7, 84.3] | 7/13 (53.8) [25.1, 80.8] |
| No | 4/12 (33.3) [9.9, 65.1] | 2/11 (18.2) [2.3, 51.8] | 6/23 (26.1) [10.2, 48.4] |
After a median follow‐up of 26.3 months, the median PFS based on the independent review in overall patients with PTCL and CTCL was 3.1 months (95% CI, 1.9‐6.0), with 2.1 months (95% CI, 1.1‐4.5) in PTCL and 4.2 months (95% CI, 2.6‐not evaluable [NE]) in CTCL (Figure 3). The median OS in overall patients with PTCL and CTCL was 19.3 months (95% CI, 13.3‐31.9), with 11.8 months (95% CI, 4.1‐19.3) in PTCL, and 31.9 months (95% CI, 16.9–NE) in CTCL (Table S1). In 13 patients with an objective response, the median DOR was 4.8 months (95% CI, 1.7‐NE); 3.1 (95% CI, 0.9‐4.8) in PTCL, and 4.8 months (95% CI, 2.7‐NE) in CTCL (Table S2). The median TTR was 1.4 months (range, 1.0‐4.6), including 1.3 (range, 1.0‐2.1) and 2.1 months (range, 1.0‐4.6) in patients with PTCL and CTCL, respectively (Table S3).
FIGURE 3.
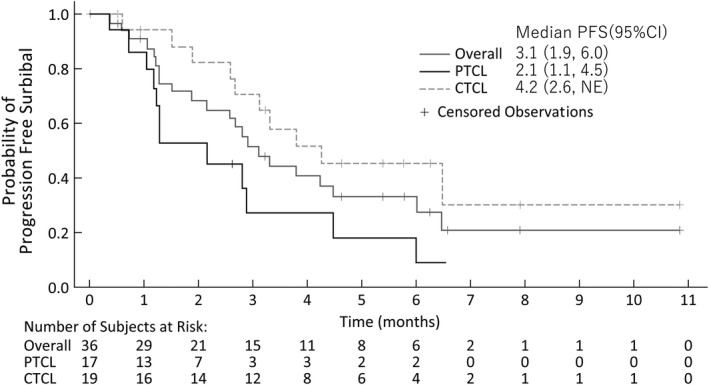
Kaplan‐Meier analysis of progression‐free survival in Japanese patients with relapsed/refractory peripheral T‐cell lymphoma (PTCL) or cutaneous T‐cell lymphoma (CTCL) treated with E7777, per the independent review. Gray line indicates the average result for all patients (n = 36) with censoring events. Black line indicates PTCL (n = 17). Dotted line indicates CTCL (n = 19)
3.3. Safety
Overall, all 37 patients showed at least one AE and treatment‐related AE. Adverse events with CTCAE grade 3 or higher occurred in 94.6% (35/37) patients (Table 7). The most common AEs (50.0% or higher) were increased levels of aspartate aminotransferase (AST) (33 patients, 89.2%) and alanine aminotransferase (ALT) (32 patients, 86.5%), hypoalbuminemia and lymphopenia (26 patients each, 70.3%), and pyrexia (19 patients, 51.4%). Overall, serious AEs (SAEs) were reported in 17 patients (45.9%). Among these, a fatal SAE (rhabdomyolysis) was reported in one patient (2.7%). The SAEs reported in two or more patients showed increased ALT and AST (five patients each, 13.5% cases), capillary leak syndrome (CLS; four patients, 10.8%), pyrexia (three patients, 8.1%), and lymphopenia (two patients, 5.4%).
TABLE 7.
Treatment‐emergent adverse events (TEAEs) occurring in 10% or more of patients with any grade AE or 5% or more of patients with grade 3 or higher AE in 37 Japanese patients with relapsed/refractory peripheral r cutaneous T‐cell lymphoma treated with E7777
| Adverse events, n (%) | Total (N = 37) | |
|---|---|---|
| Any grade, n (%) | Grade 3 or higher, n (%) | |
| Subjects with any TEAE | 37 (100.0) | 35 (94.6) |
| Blood and lymphatic system disorders | 32 (86.5) | 23 (62.2) |
| Lymphopenia | 26 (70.3) | 21 (56.8) |
| Thrombocytopenia | 13 (35.1) | 6 (16.2) |
| Anemia | 5 (13.5) | 2 (5.4) |
| Leukocytosis | 4 (10.8) | 0 (0.0) |
| Leukopenia | 4 (10.8) | 1 (2.7) |
| Neutropenia | 2 (5.4) | 2 (5.4) |
| Gastrointestinal disorders | 29 (78.4) | 1 (2.7) |
| Constipation | 14 (37.8) | 0 (0.0) |
| Nausea | 11 (29.7) | 0 (0.0) |
| Vomiting | 7 (18.9) | 0 (0.0) |
| General disorders and administration site conditions | 31 (83.8) | 1 (2.7) |
| Pyrexia | 19 (51.4) | 0 (0.0) |
| Malaise | 12 (32.4) | 0 (0.0) |
| Oedema peripheral | 8 (21.6) | 0 (0.0) |
| Fatigue | 4 (10.8) | 0 (0.0) |
| Infections and infestations | ||
| Pneumonia | 2 (5.4) | 2 (5.4) |
| Investigations | 34 (91.9) | 28 (75.7) |
| Aspartate aminotransferase increased | 33 (89.2) | 16 (43.2) |
| Alanine aminotransferase increased | 32 (86.5) | 21 (56.8) |
| γ‐Glutamyltransferase increased | 17 (45.9) | 6 (16.2) |
| Lipase increased | 10 (27.0) | 7 (18.9) |
| Weight increased | 7 (18.9) | 0 (0.0) |
| Blood alkaline phosphatase increased | 6 (16.2) | 0 (0.0) |
| Amylase increased | 5 (13.5) | 3 (8.1) |
| Metabolism and nutrition disorders | 30 (81.1) | 13 (35.1) |
| Hypoalbuminemia | 26 (70.3) | 2 (5.4) |
| Decreased appetite | 11 (29.7) | 2 (5.4) |
| Hypertriglyceridemia | 10 (27.0) | 5 (13.5) |
| Hyperglycemia | 4 (10.8) | 3 (8.1) |
| Hyponatremia | 4 (10.8) | 3 (8.1) |
| Hypokalemia | 3(8.1) | 2 (5.4) |
| Musculoskeletal and connective tissue disorders | 10 (27.0) | 1 (2.7) |
| Myalgia | 4 (10.8) | 0 (0.0) |
| Psychiatric disorders | 10 (27.0) | 0 (0.0) |
| Insomnia | 7 (18.9) | 0 (0.0) |
| Renal and urinary disorders | 5 (13.5) | 0 (0.0) |
| Proteinuria | 4 (10.8) | 0 (0.0) |
| Vascular disorders | 14 (37.8) | 5 (13.5) |
| Capillary leak syndrome | 5 (13.5) | 4 (10.8) |
| Hypertension | 5 (13.5) | 1 (2.7) |
Adverse events leading to drug withdrawal were observed in nine patients (24.3%), with fatigue (two patients, 5.4%) being the most common AE. Three (8.1%), four (10.8%), and 10 (27.0%) patients experienced AEs leading to a reduction in drug dose, an interruption of the drug dose (skipping) within a cycle, and a delay in the first treatment in a subsequent cycle, respectively. Capillary leak syndrome and related events were reported in 32 (86.5%) patients, and events of grade 3 or higher were reported in five (13.5%) patients, including CLS (four patients, 10.8%), hypoalbuminemia (two patients, 5.4%), and generalized edema (one patient, 2.7%).
3.4. Pharmacokinetics and immunogenicity
The time profiles for mean serum concentration and PK parameters for E7777 are shown in Figure S1 and Table S4, and confirm those reported in a phase I study. 12 After i.v. administration on day 1 of each cycle, E7777 was rapidly eliminated from the serum after reaching Cmax. As the antidrug Ab interfered with E7777 serum assay, the data with high titer of anti‐E7777 and anti‐IL‐2 Ab were excluded from the analysis. Consequently, one patient was available for further analysis in cycle 3 day 1 and thereafter. As the subject’s E7777 serum concentration in cycle 3 day 1 and cycle 5 day 1 showed negative anti‐E7777 Ab, the relationship between E7777 serum concentration and anti‐E7777 Ab could not be assessed. Posttreatment, 26 of 35 patients (74.3%) had positive anti‐E7777 Abs (Table S5).
4. DISCUSSION
In patients with relapsed or refractory PTCL and CTCL, the ORR of E7777 was 36.1% (95% CI, 20.8‐53.8) (Table 5), and the lower limit of 95% CI exceeded a predefined threshold of 5%, indicating a clinically meaningful efficacy. In patients with PTCL, the ORRs of the other new agents including forodesine, romidepsin, chidamide, and pralatrexate, ranged from 24% to 29%. 13 , 14 , 15 , 16 In the case of CTCL, new agents such as romidepsin and vorinostat showed an ORR ranging from 30% to 34%. 17 , 18 , 19 Brentuximab vedotin, an Ab‐drug conjugate targeting CD30, showed antitumor activities on ALCL and CD30+ PTCL/CTCL and its ORRs were 86%, 41%, and 67%, respectively. 20 , 21 , 22 The ORRs of mogamulizumab, an anti‐CCR4 Ab, were 35% in CCR4+ PTCL/CTCL. 23 Recently, a global phase III study of mogamulizumab indicated prolonged PFS compared with vorinostat, with a reported ORR of 28%. 24 E7777 showed comparable response with these agents in patients, including in those refractory to the last prior systemic chemotherapy and/or in those who had been treated with these newly approved agents. E7777 has a different mode of action compared with cytotoxic agents or other new agents, which could explain its efficacy regardless of prior therapy and number of treatments.
Furthermore, E7777 showed antitumor activity in PTCL and CTCL patients across a wide range of CD25 expression, although the number of patients with low CD25 expression was relatively smaller (Table 6). CD25 is a component of the high‐affinity IL‐2R, which is composed of the IL‐2 alpha (CD25), beta (CD122), and the common gamma (CD132) chains. E7777 binds to all forms of IL‐2R, and high and intermediate affinity receptors commonly found in mature T cells can internalize it by receptor‐mediated endocytosis. 25 , 26 These results suggest that E7777 might have an antitumor activity on T‐cell lymphoma that did not express CD25 but have intermediate affinity IL‐2R. In the previous phase II study in T‐cell non‐Hodgkin lymphoma, historical DD showed an ORR of 62% and 45% for CD25+ (CD25 in 10% or more of tumor cells) and CD25− tumors (CD25 in less than 10% of tumor cells), respectively. 9 CD25 was also expressed on activated T cells, regulatory T cells, and other immune cells; hence, these relationships should be explored in future studies. Grade 3 or higher lymphopenia is frequently observed as an AE (56.8%) on the basis of the antitumor effect of E7777 (Table 7). The median duration was 7 days after the observation of grade 3 or higher lymphopenia.
We observed common hepatobiliary disorders as AEs leading to a drug dose reduction or a delay in drug treatment in several patients, but with no discontinuation. The frequency of hepatobiliary disorders, such as increased ALT/AST, was higher in cycle 1, but lower in the later cycles. Thus, hepatobiliary disorders should be carefully monitored, particularly in the early cycles of E7777 treatment. The historical DD also showed the same AEs, such as hepatic transaminase elevation in 61% and 74.1% and hypoalbuminemia in 79% and 74.1% of patients in CTCL, phase III and T‐NHL, phase II studies, respectively. 6 , 9 Interestingly, most of the common AEs of E7777 were similar to those of tagraxofusp (SL‐401), which is a fusion protein of IL‐3 and a truncated diphtheria toxin, for which increased levels of ALT (64%) and AST (60%), hypoalbuminemia (55%), peripheral edema (51%), and CLS (19%) were reported as the most common AEs. 27 The results of these studies suggest that a truncated diphtheria toxin might be involved in these AEs.
Although one patient discontinued the drug due to thrombocytopenia, hematological toxicities were generally manageable with careful monitoring of E7777 treatment. Capillary leak syndrome and related events were reported in 32 (86.5%) patients, and grade 3 or higher events were reported in five (13.5%) patients, including CLS (four patients, 10.8%), hypoalbuminemia (two patients, 5.4%), and generalized edema (one patient, 2.7%). Adverse events leading to interruption or delay of drug treatment were observed in several patients, but none of the patients discontinued the treatment due to CLS. Thus, CLS was considered as clinically manageable. However, rhabdomyolysis was reported in two patients, leading to the death of one patient. In this fatal case, CLS was observed just after cycle 1. Furosemide was initiated for edema. Prednisolone 60 mg was also given and then the patient was considered to have developed drug‐induced pneumonia and developed rhabdomyolysis. Rhabdomyolysis was also reported in a historical DD‐treated patient after CLS detection. 28 The ensuing muscular swelling caused by CLS might lead to increased intracompartmental pressure and secondary rhabdomyolysis. 29 As both rhabdomyolysis events occurred after grade 3 CLS development in this study, the risk of rhabdomyolysis should be considered if CLS occurs after E7777 treatment. Overall, the safety profile of E7777 was consistent with the known profile of E7777 and/or historical DD. No new safety signals were identified. In conclusion, E7777, given at a dose of 9 μg/kg/d on five consecutive days per the 21‐day cycle, showed promising efficacy and a manageable safety profile in Japanese patients with relapsed or refractory PTCL and CTCL. The common AEs were manageable, but increased ALT/AST, hypoalbuminemia, and CLS should be carefully managed during the treatment. E7777 has the potential to be considered as a new treatment option for such patients in Japan.
DISCLOSURE
Ando reports research funding from Eisai. Maruyama reports honoraria and research funding from Eisai. Yamamoto reports consultancy, honoraria, and research funding from Chugai, Ono, Eisai, Mundipharma, Celgene Corporation, and Takeda, honoraria from Sanofi, Kyowa Kirin, Otsuka, Janssen, Sumitomo Dainippon, and Pfizer, honoraria and research funding from Novartis, research funding from Gilead Sciences, SymBio, Bayer, ARIAD, Solasia Pharma, and Incyte, consultancy and honoraria from Bristol‐Myers Squibb, MSD, HUYA/IQVIA Services Japan, and Meiji Seika Pharma, and consultancy and research funding from AbbVie and Astra‐Zeneca. Kiyohara reports research funding from Eisai. Terui reports research funding from Bristol‐Myers Squibb and honoraria from Bristol‐Myers Squibb, Celgene, Janssen, Takeda, MSD, Eisai, Ono, and Chugai‐Roche Pharmaceuticals Co., Ltd. Fukuhara reports honoraria from Chugai Pharmaceutical Co., Ltd, Kyowa‐Hakko Kirin, Mochida, Mundi, Nippon Shinkyaku, Ono Pharmaceutical Co., Ltd, Zenyaku, and Janssen Pharma, honoraria and research funding from Celgene Corporation, Eisai, and Takeda Pharmaceutical Co., Ltd., and research funding from AbbVie, Bayer, Gilead, and Solasia Pharma. Tokura reports honoraria from Eisai Consultancy. Kuroda reports research funding from Eisai. Uchida reports honoraria from Eisai. Nakanishi, Nakai, and Matsunaga report employment by Eisai. Tobinai reports consultancy and honoraria from HUYA Bioscience, Bristol‐Myers Squibb, Daiichi Sankyo, Verastem, and Zenyaku Kogyo, consultancy, honoraria, and research funding from Takeda Pharmaceutical, Celgene, Mundi Pharma, and Ono Pharmaceutical, honoraria and research funding from Eisai, Kyowa Kirin, Janssen Pharmaceutical, and Chugai Pharmaceutical, research funding from AbbVie, and honoraria from Yakult, Solasia, and Meiji Seika.
Supporting information
Supporting Information
Fig S1
Table S1‐S5
ACKNOWLEDGMENTS
We thank the patients who participated in this study and their families and the investigators, physicians, nurses, and clinical research coordinators who helped this study. We would also like to thank Dr Hirokazu Nagai (Nagoya Medical Center) as the independent efficacy and safety adviser and Dr Akira Tomonari (Eisai Co., Ltd.) as the medical adviser of the sponsor. We thank Dr Kenzo Muramoto and Dr Michiko Sugawara (Eisai Co., Ltd.) for their help in preparing this manuscript. This study was funded and supported by Eisai Co., Ltd.
Kawai H, Ando K, Maruyama D, et al. Phase II study of E7777 in Japanese patients with relapsed/refractory peripheral and cutaneous T‐cell lymphoma. Cancer Sci. 2021;112:2426–2435. 10.1111/cas.14906
ClinicalTrial.gov identifier: NCT02676778
REFERENCES
- 1. Phan A, Veldman R, Lechowicz MJ. T‐cell lymphoma epidemiology: the known and unknown. Curr Hematol Malig Rep. 2016;11(6):492‐503. 10.1007/s11899-016-0353-y [DOI] [PubMed] [Google Scholar]
- 2. Fisher RI, Gaynor ER, Dahlberg S, et al. Comparison of standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non‐Hodgkin’s lymphoma. N Engl J Med. 1993;328(14):1002‐1006. 10.1056/NEJM199304083281404 [DOI] [PubMed] [Google Scholar]
- 3. Hamadani M, Kar SMA, Usmani SZ, Savani BN, Ayala E, Kharfan‐Dabaja MA. Management of relapses after hematopoietic cell transplantation in T‐cell non‐Hodgkin lymphomas. Semin Hematol. 2014;51(1):73‐86. 10.1053/j.seminhematol.2013.11.005 [DOI] [PubMed] [Google Scholar]
- 4. Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28(31):4730‐4739. 10.1200/JCO.2009.27.7665 [DOI] [PubMed] [Google Scholar]
- 5. vanderSpek JC, Mindell JA, Finkelstein A, Murphy JR. Structure/function analysis of the transmembrane domain of DAB389‐interleukin‐2, an interleukin‐2 receptor‐targeted fusion toxin. The amphipathic helical region of the transmembrane domain is essential for the efficient delivery of the catalytic domain to the cytosol of target cells. J Biol Chem. 1993;268(16):12077‐12082. [PubMed] [Google Scholar]
- 6. Olsen E, Duvic M, Frankel A, et al. Pivotal phase III trial of two dose levels of denileukin diftitox for the treatment of cutaneous T‐cell lymphoma. J Clin Oncol. 2001;19(2):376‐388. 10.1200/JCO.2001.19.2.376 [DOI] [PubMed] [Google Scholar]
- 7. Prince HM, Duvic M, Martin A, et al. Phase III placebo‐controlled trial of denileukin diftitox for patients with cutaneous T‐cell lymphoma. J Clin Oncol. 2010;28(11):1870‐1877. 10.1200/JCO.2009.26.2386 [DOI] [PubMed] [Google Scholar]
- 8. Prince HM, Martin AG, Olsen EA, Fivenson DP, Duvic M. Denileukin diftitox for the treatment of CD25 low‐expression mycosis fungoides and Sézary syndrome. Leuk Lymphoma. 2013;54(1):69‐75. 10.3109/10428194.2012.706286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dang NH, Pro B, Hagemeister FB, et al. Phase II trial of denileukin diftitox for relapsed/refractory T‐cell non‐Hodgkin lymphoma. Br J Haematol. 2007;136:439‐447. 10.1111/j.1365-2141.2006.06457.x [DOI] [PubMed] [Google Scholar]
- 10. Ohmachi K, Ando K, Ogura M, et al. E7777 in Japanese patients with relapsed/refractory peripheral and cutaneous T‐cell lymphoma: a phase I study. Cancer Sci. 2018;109(3):794‐802. 10.1111/cas.13513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579‐586. 10.1200/JCO.2006.09.2403 [DOI] [PubMed] [Google Scholar]
- 12. Olsen EA, Whittaker S, Kim YH, et al. Clinical end points and response criteria in mycosis Fungoides and Sézary Syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29:2598‐2607. 10.1200/JCO.2010.32.0630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maruyama D, Tsukasaki K, Uchida T, et al. Multicenter phase 1/2 study of forodesine in patients with relapsed peripheral T cell lymphoma. Ann Hematol. 2019;98(1):131‐142. 10.1007/s00277-018-3418-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Coiffier B, Pro B, Prince HM, et al. Romidepsin for the treatment of relapsed/refractory peripheral T‐cell lymphoma: pivotal study update demonstrates durable responses. J Hematol Oncol. 2014;7:11. 10.1186/1756-8722-7-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shi Y, Dong M, Hong X, et al. Results from a multicenter, open‐label, pivotal phase II study of chidamide in relapsed or refractory peripheral T‐cell lymphoma. Ann Oncol. 2015;26:1766‐1771. 10.1093/annonc/mdv237 [DOI] [PubMed] [Google Scholar]
- 16. O'Connor OA, Pro B, Pinter‐Brown L, et al. Pralatrexate in patients with relapsed or refractory peripheral T‐cell lymphoma: results from the pivotal PROPEL study. J Clin Oncol. 2011;29:1182‐1189. 10.1200/JCO.2010.29.9024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Piekarz RL, Frye R, Turner M, et al. Phase II multi‐institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T‐cell lymphoma. J Clin Oncol. 2009;27:5410‐5417. 10.1200/JCO.2008.21.6150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Whittaker SJ, Demierre M‐F, Kim EJ, et al. Final results from a multicenter, international, pivotal study of romidepsin in refractory cutaneous T‐cell lymphoma. J Clin Oncol. 2010;28:4485‐4491. 10.1200/JCO.2010.28.9066 [DOI] [PubMed] [Google Scholar]
- 19. Mann BS, Johnson JR, He K, et al. Vorinostat for treatment of cutaneous manifestations of advanced primary cutaneous T‐cell lymphoma. Clin Cancer Res. 2007;13:2318‐2322. 10.1158/1078-0432.CCR-06-2672 [DOI] [PubMed] [Google Scholar]
- 20. Pro B, Advani R, Brice P, et al. Brentuximab vedotin (SGN‐35) in patients with relapsed or refractory systemic anaplastic large‐cell lymphoma: results of a phase II study. J Clin Oncol. 2012;30:2190‐2196. 10.1200/JCO.2011.38.0402 [DOI] [PubMed] [Google Scholar]
- 21. Horwitz SM, Advani RH, Bartlett NL, et al. Objective responses in relapsed T‐cell lymphomas with single‐agent brentuximab vedotin. Blood. 2014;123:3095‐3100. 10.1182/blood-2013-12-542142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Prince HM, Kim YH, Horwitz SM, et al. Brentuximab vedotin or physician’s choice in CD30‐positive cutaneous T‐cell lymphoma (ALCANZA): an international, open‐label, randomised, phase 3, multicenter trial. Lancet. 2017;390:555‐566. 10.1016/S0140-6736(17)31266-7 [DOI] [PubMed] [Google Scholar]
- 23. Ogura M, Ishida T, Hatake K, et al. Multicenter phase II study of mogamulizumab (KW‐0761), a defucosylated anti‐cc chemokine receptor 4 antibody, in patients with relapsed peripheral T‐cell lymphoma and cutaneous T‐cell lymphoma. J Clin Oncol. 2014;32:1157‐1163. 10.1200/JCO.2013.52.0924 [DOI] [PubMed] [Google Scholar]
- 24. Kim YH, Bagot M, Pinter‐Brown L, et al. Mogamulizumab versus vorinostat in previously treated cutaneous T‐cell lymphoma (MAVORIC): an international, open‐label, randomised, controlled phase 3 trial. Lancet Oncol. 2018;19(9):1192‐1204. 10.1016/S1470-2045(18)30379-6 [DOI] [PubMed] [Google Scholar]
- 25. Létourneau S, Krieg C, Pantaleo G, Boyman O. IL‐2‐ and CD25‐dependent immunoregulatory mechanisms in the homeostasis of T‐cell subsets. J Allergy Clin Immunol. 2009;123(4):758‐762. 10.1016/j.jaci.2009.02.011 [DOI] [PubMed] [Google Scholar]
- 26. Morris SC, Gause WC, Finkelman FD. IL‐4 suppression of in vivo T cell activation and antibody production. J Immunol. 2000;164(4):1734‐1740. 10.4049/jimmunol.164.4.1734 [DOI] [PubMed] [Google Scholar]
- 27. Pemmaraju N, Lane AA, Sweet KL, et al. Tagraxofusp in blastic plasmacytoid dendritic‐cell neoplasm. N Engl J Med. 2019;380:1628‐1637. 10.1056/NEJMoa1815105 [DOI] [PubMed] [Google Scholar]
- 28. Avarbock AB, Loren AW, Park JY, et al. Lethal vascular leak syndrome after denileukin diftitox administration to a patient with cutaneous gamma/delta T‐cell lymphoma and occult cirrhosis. Am J Hematol. 2008;83(7):593‐595. 10.1002/ajh.21180 [DOI] [PubMed] [Google Scholar]
- 29. Dolberg‐Stolik OC, Putterman C, Rubinow A, Rivkind AI, Sprung CL. Idiopathic capillary leak syndrome complicated by massive rhabdomyolysis. Chest. 1993;104(1):123‐126. 10.1378/chest.104.1.123 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Fig S1
Table S1‐S5


