Abstract
Levothyroxine is a widely prescribed medication for the treatment of an underactive thyroid. The relationship between levothyroxine use and cancer risk is largely underdetermined. To investigate the magnitude of the possible association between levothyroxine use and cancer risk, this retrospective case‐control study was conducted using Taiwan’s Health and Welfare Data Science Center database. Cases were defined as all patients who were aged ≥20 years and had a first‐time diagnosis for cancer at any site for the period between 2001 and 2011. Multivariable conditional logistic regression models were used to calculate an adjusted odds ratio (AOR) to reduce potential confounding factors. A total of 601 733 cases and 2 406 932 controls were included in the current study. Levothyroxine users showed a 50% higher risk of cancer at any site (AOR: 1.50, 95% CI: 1.46‐1.54; P < .0001) compared with non–users. Significant increased risks were also observed for brain cancer (AOR: 1.90, 95% CI: 1.48‐2.44; P < .0001), skin cancer (AOR: 1.42, 95% CI: 1.17‐1.72; P < .0001), pancreatic cancer (AOR: 1.27, 95% CI: 1.01‐1.60; P = .03), and female breast cancer (AOR: 1.24, 95% CI: 1.15‐1.33; P < .0001). Our study results showed that levothyroxine use was significantly associated with an increased risk of cancer, particularly brain, skin, pancreatic, and female breast cancers. Levothyroxine remains a highly effective therapy for hypothyroidism; therefore, physicians should carefully consider levothyroxine therapy and monitor patients’ condition to avoid negative outcomes. Additional studies are needed to confirm these findings and to evaluate the potential biological mechanisms.
Keywords: association research, cancer risk, case‐control study, levothyroxine, long‐term drug use
This retrospective case‐control study analyzed 23 million patients from Taiwan’s claims data. Levothyroxine use was significantly associated with an increased risk of brain, skin, pancreatic, and female breast cancers.
1. INTRODUCTION
Thyroid disease is a major public health concern worldwide. 1 The number of individuals with thyroid disorders is expected to continue to grow, having reached approximately 750 million globally in 2012. 2 Hypothyroidism is the most common type of thyroid disorder, and women’s risk of hypothyroidism is five to eight times higher than that of men. 3 In the USA, the prevalence of biochemical and clinically evident hypothyroidism is 4.6% and 0.3%, but this can vary considerably by age. 4 Levothyroxine is a first‐line treatment for the hypothyroidism. Levothyroxine therapy is provided to inactivate abnormal thyroid tissue growth or function and to restore the clinical and biochemical euthyroid state in the thyroid gland. 5 , 6 Recently, concerns have been raised regarding the use of levothyroxine medication and its adverse effects, for example, on bone and cardiac function. 7
Despite clinical benefits of levothyroxine, concern about its overall safety has further been raised because of its connection to cancer risk. Several epidemiological studies have attempted to assess the magnitude of the association between levothyroxine use and cancer risk, but their findings have been inconclusive. Cornelli et al 8 reported correlation between levothyroxine and an increased risk of lung cancer; however, long‐term use of levothyroxine was associated with a reduction in risk of colorectal cancer. 9 Biological studies have also analyzed the association between levothyroxine and cancer risk. Some studies have found that such association is linked to increased oxidative stress resulting from overproduction of reactive oxygen species (ROS) in the body, 10 , 11 and oxidative stress is considered the main factor for various chronic conditions such as cancer and autoimmunity.
Owing to insufficient biological evidence and inconclusive results from epidemiological studies, we conducted a nationwide population‐based case‐control study to observe whether long‐term use of levothyroxine is associated with an increased risk of cancer.
2. METHODS
This study was approved by the Taipei Medical University‐Joint Institutional Review Board (TMU‐JIRB), Taiwan (TMU JIRB No.: N201602065; protocol title: to assess the incidence and mortality for long‐term used drugs with cancer; protocol date: 9 March 2016). All subjects in the database used in this study were de‐identified, and, therefore, the requirement for informed consent was waived.
2.1. Data source
This retrospective case‐control study was performed using de‐identified data of a large cohort of patients retrieved from the Health and Welfare Data Science Center (HWDC) database, Ministry of Health and Welfare, Taiwan. This large HWDC database includes data from the National Health Insurance Administration, Ministry of Health and Welfare, Taiwan. The National Health Insurance covers more than 99% of the 23 million inhabitants of Taiwan, who receive all forms of healthcare services, including outpatients, inpatients care, Chinese traditional medicine, dental care, childbirth, physical therapy, preventive health care, home care, and rehabilitation for chronic mental illness. 12 , 13 , 14 , 15 This database also contains information regarding patient demographic characteristics (with encrypted patient identification numbers, birthdates, and sex) and inpatient or outpatient data on prescriptions, diagnoses, and pharmacy records. Furthermore, specific data on the medications prescribed, laboratory and diagnostic tests, dates of visits, lengths of hospitalization, and diagnoses are stored based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM). 16 This study retrieved and analyzed data between 2001 and 2011, which offered a great opportunity to explore the relationship between levothyroxine use and the risk of cancer.
2.2. Definition of cases and controls
Eligible cases were defined as all Taiwanese individuals who were ≥20 years with a histologically verified primary cancer diagnosis. The cancer diagnosis was confirmed using the International Classification of Diseases, 9th revision, Clinical Modification (ICD‐9‐CM; ICD‐9‐Code: 140‐208) between 1 January 2001 and 31 December 2011. However, the controls had no previous diagnosis of cancer. For matching, an index date, defined as the date of a cancer diagnosis, was given to each case, who was subsequently matched with four controls 17 for age, sex, and index date. Each control was assigned an index date that was identical to that of the corresponding case. Individuals were eligible to be controls until they were diagnosed with cancer and became cases. We calculated the odds ratio (OR) because it is an unbiased estimate of the incidence rate ratio (IRR) that would have emerged from a cohort study in the source population.
2.3. Exposure definition
Medications in the HWDC database were coded according to the World Health Organization’s Anatomical Therapeutic Chemical (ATC) classification system. 18 Levothyroxine was defined as a prescribed medication with the ATC code H03AA01. Cases and controls were regarded as levothyroxine users if they were prescribed with levothyroxine for at least 2 months within the last 3 years prior to the index date. This 3‐year baseline period was introduced because use of certain drugs is known to increase within the 3 years prior to a cancer diagnosis, most likely because of early symptoms related to a yet undetected cancer. This approach has immense benefits to the drug prevalence among cases and can, therefore, avoid reverse causation bias (ie a spurious association between levothyroxine use and cancer risk). Another reason for adapting this approach was that we considered it very unlikely that recent exposure within 3 years would contribute to cancer risk. After individuals were confirmed to be valid levothyroxine users, their prescription dates, daily doses, and duration of levothyroxine use were extracted from the HWDC database. Each patient who had developed cancer was matched for age, sex, and index date with four subjects who had never been diagnosed with any cancer. 17 The cumulative duration was measured among the levothyroxine users, and it was categorized into the two following groups: (a) short‐term users (2 months to 1 year) and (b) long‐term users (≥1 year).
2.4. Potential confounders
The following potential confounders were included in our model: (a) medications known or suspected to modify the risk of some cancers, including aspirin (ATC code: B01AC06), 19 , 20 , 21 , 22 statins (ATC code: C10AA), 19 , 23 , 24 and angiotensin‐converting enzyme (ACE) inhibitors/angiotensin II receptor blockers (ARB) (ATC codes: C09A and C09C), 19 , 25 , 26 , 27 exposure to any of which was defined as being prescribed within at least 60 days during a 3‐year period prior to the index date; (b) prior diagnoses of diseases known or suspected to modify the risk of some cancers, established within 3 years prior to the index date, including chronic pulmonary disease (composite measure of diagnoses [ICD‐9:490.x‐505.x, 506.4]), myocardial infarction (ICD‐9:410.x, 412.x), congestive heart failure (ICD‐9:428.x), peripheral vascular disease (ICD‐9:443.9, 441.x, 785.4, V43.4), cerebrovascular disease (ICD‐9:430.x‐438.x), dementia (ICD‐9:290.x), rheumatic disease (ICD‐9:710.0, 710.1, 710.4, 714.0‐714.2, 714.81, 725.x), peptic ulcer disease (ICD‐9:531.x‐534.x), liver disease (ICD‐9:571.2, 571.4‐571.6, 456.0‐456.21, 572.2‐572.8), diabetes (ICD‐9:250.x), hemiplegia or paraplegia (ICD‐9:344.1, 342.x), renal disease (ICD‐9:582.x, 583‐583.7, 585.x, 586.x, 588.x), and age‐adjusted Charlson comorbidity index (ACCI) scores. 28
2.5. Statistical analysis
We compared cases and controls with regard to the difference in covariate distribution using the McNemar test for categorical variables and paired t‐test for continuous variables. A conditional logistic regression model was used to estimate the adjusted odds ratios (AOR) with 95% confidence intervals (CI) for quantifying the magnitude of the association between levothyroxine use and cancer risk. The model was adjusted for age, sex, index date, and comorbid condition. An effect of cumulative duration of filled prescriptions (categorized as 60‐364 days and ≥1 year) was evaluated to find the possible magnitude of the association. Finally, we analyzed the effect of daily doses on the association during the 3‐year study period. Doses in this study were standardized by dividing a levothyroxine user’s overall 3‐year dose by 0.15 mg, which was the defined daily dose (DDD) of levothyroxine, either for oral or for parenteral use. 18 A patient’s 3‐year average daily dose (in DDD) was then calculated by dividing the 3‐year total number of DDD by 1095 days. Based on the 3‐year average daily dose (in DDD), the patients were categorized into three groups: <0.34, 0.34‐≤0.67, and >0.67 DDD per day. The SAS statistical package (version 8.02, SAS Institute) was used to analyze the data. All statistical tests were two‐sided. A P‐value ≤.05 was considered statistically significant.
3. RESULTS
A total of 1 434 933 cancer patients and approximately 21.6 million non–cancer patients were identified between 2001 and 2011. We excluded 400 474 cancer patients who were aged <20 years or who had only one cancer diagnosis. In addition, 432 726 patients diagnosed with cancer between 2001 and 2003 were also excluded. The remaining 601 733 were eligible to be the cases and were further matched 1:4 with 2 406 932 controls out of the 2.16 million by age and sex (Figure 1).
FIGURE 1.
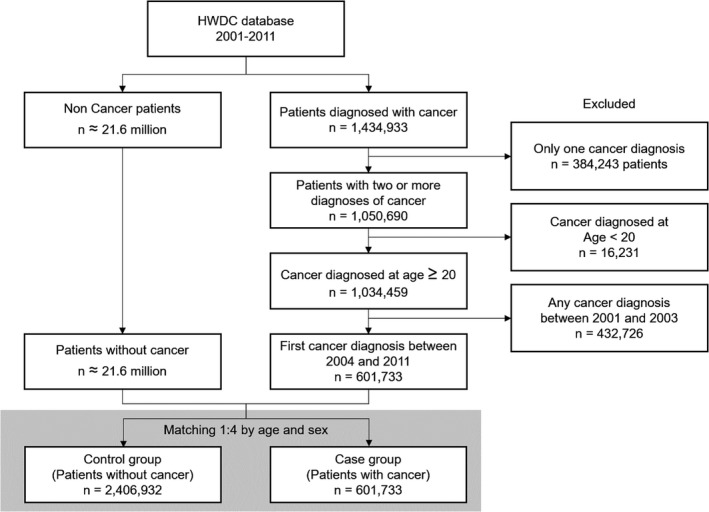
Inclusion and exclusion criteria of cases and controls
Table 1 shows the demographic characteristics of cases and controls. The mean ages of cases and controls were similar (60.47 ± 15.37 years), and more than half of the patients were male (53.96%). Both cases and controls had similar percentages of comorbidities in general, except for liver disease (29.09% vs 20.96%), peptic ulcer (42.71% vs 37.12%), and renal disease (9.20% vs 7.34%). There was no significant difference in the Charlson comorbidity index (P <.0001) for cases and controls.
TABLE 1.
Baseline characteristics of cancer cases and controls
| Characteristics |
Cases (n = 601 733) |
Controls (n = 2 406 932) |
P‐value |
|---|---|---|---|
| Age a | 1 | ||
| Mean (SD) | 60.47 (15.37) | 60.47 (15.37) | |
| Gender b | 1 | ||
| Male (%) | 324 710 (53.96) | 1 298 840 (53.96) | |
| Comorbid conditions, N (%) | |||
| Myocardial infarction | 9920 (1.65) | 37 873 (1.57) | <.0001 |
| Congestive heart failure | 45 691 (7.59) | 157 383 (6.54) | <.0001 |
| Peripheral vascular disease | 21 132 (3.51) | 84 259 (3.50) | .502 |
| Cerebrovascular disease | 99 179 (16.48) | 377 753 (15.69) | <.0001 |
| Dementia | 17 898 (2.97) | 76 309 (3.17) | <.0001 |
| COPD | 148 277 (24.64) | 529 501 (22.00) | <.0001 |
| Rheumatic disease | 24 504 (4.07) | 94 513 (3.93) | <.0001 |
| Peptic ulcer disease | 893 550 (37.12) | <.0001 | |
| Liver disease | 175 036 (29.09) | 504 462 (20.96) | <.0001 |
| Diabetes | 143 890 (23.91) | 514 572 (21.38) | <.0001 |
| Hemiplegia or paraplegia | 6688 (1.11) | 27 754 (1.15) | <.0001 |
| Renal disease | 55 358 (9.20) | 176 645 (7.34) | <.0001 |
| Age‐adjusted CCI scores | <.0001 | ||
| Mean (SD) | 3.58 (2.68) | 3.31 (2.57) | |
| Other drugs, N (%) | |||
| Aspirin | 88 153 (14.65) | 353 001 (14.67) | .599 |
| Statins | 66 975 (11.13) | 264 605 (10.99) | <.0001 |
| ACEI/ARB | 131 636 (21.88) | 513 058 (21.32) | <.0001 |
ACEI/ARB, angiotensin‐converting enzyme inhibitors/angiotensin II receptor blockers; CCI, Charlson comorbidities index; COPD, chronic obstructive pulmonary disease; SD, standard deviation.
The continuous variables were compared using paired t‐tests.
The categorical variables were compared using the McNemar test.
The relationship between levothyroxine use and cancer risk is shown in Table 2. The adjusted odds ratio for levothyroxine use and overall cancer risk was 1.50 (95% CI:1.46‐1.54). The results indicated that levothyroxine use increased the risk of overall cancer when compared with non–users. There were also significantly increased risks of brain (AOR: 1.90, 95% CI: 1.48‐2.44), skin (AOR: 1.42, 95% CI: 1.17‐1.72), pancreatic (AOR: 1.27, 95% CI: 1.01‐1.60), prostate (AOR: 1.26, 95% CI: 1.06‐1.50), female breast (AOR: 1.24, 95% CI: 1.15‐1.33), bladder (AOR: 1.23, 95% CI: 1.02‐1.47), lung (AOR: 1.22, 95% CI: 1.11‐1.35), and colorectal (AOR: 1.14, 95% CI: 1.05‐1.24) cancers with the levothyroxine use in the cases compared to the controls. Levothyroxine use was associated with increased risks of gastric (AOR: 1.19, 95% CI: 1.0‐1.42) and kidney cancers (AOR: 1.15, 95% CI: 0.93‐1.41), but they were not statistically significant. In contrast, decreased risks of esophageal (AOR: 0.80, 95% CI: 0.55‐1.17) and cervical (AOR: 0.75, 95% CI: 0.62‐0.91) cancers were observed in levothyroxine users. Furthermore, no significant association with liver cancer (AOR: 0.98, 95% CI: 0.88‐1.10) was found.
TABLE 2.
Levothyroxine use and risk of cancer
| Cancer types |
Cases (n = 601 733) Exposed/Unexposed |
Control (n = 2 406 932) Exposed/Unexposed |
Unadjusted odds ratio (95% CI) | P‐value | Adjusted odds ratio a (95% CI) | P‐value |
|---|---|---|---|---|---|---|
| Overall | 6535/595 198 | 16 789/2 390 143 | 1.57 (1.52‐1.61) | <.0001 | 1.50 (1.46‐1.54) | <.0001 |
| Esophageal | 34/11 140 | 164/44 532 | 0.83 (0.57‐1.20) | .31 | 0.80 (0.55‐1.17) | .24 |
| Gastric | 180/22 394 | 582/89 714 | 1.24 (1.05‐1.47) | .01 | 1.19 (1.00‐1.42) | .05 |
| Colorectal | 723/85 874 | 2432/343 956 | 1.19 (1.10‐1.30) | .001 | 1.14 (1.05‐1.24) | .001 |
| Liver | 506/80 701 | 1897/322 931 | 1.07 (0.97‐1.18) | .19 | 0.98 (0.88‐1.10) | .76 |
| Pancreatic | 109/10 037 | 311/40 273 | 1.41 (1.13‐1.76) | .002 | 1.27 (1.01‐1.60) | .03 |
| Lung | 581/67 828 | 1822/271 814 | 1.28 (1.16‐1.40) | <.0001 | 1.22 (1.11‐1.35) | <.0001 |
| Skin | 143/15 637 | 393/62 727 | 1.46 (1.21‐1.77) | <.0001 | 1.42 (1.17‐1.72) | <.0001 |
| Female Breast | 916/64 575 | 2991/258 973 | 1.23 (1.14‐1.32) | <.0001 | 1.24 (1.15‐1.33) | <.0001 |
| Cervical | 126/15 010 | 693/59 851 | 0.72 (0.60‐0.88) | .0009 | 0.75 (0.62‐0.91) | .003 |
| Prostate | 168/32 251 | 516/129 160 | 1.30 (1.09‐1.55) | .002 | 1.26 (1.06‐1.50) | .01 |
| Bladder | 161/19 373 | 498/77 638 | 1.30 (1.08‐1.55) | .004 | 1.23 (1.02‐1.47) | .02 |
| Kidney | 134/14 865 | 421/59 575 | 1.28 (1.05‐1.55) | .01 | 1.15 (0.93‐1.41) | .18 |
| Brain | 110/7560 | 225/30 455 | 1.98 (1.57‐2.49) | <.0001 | 1.90 (1.48‐2.44) | <.0001 |
Abbreviation: CI, confidence interval.
Odds ratio adjusted for age, gender, comorbid conditions, age‐adjusted CCI scores, and other drugs in Table 1.
Table 3 shows the risk of cancer in male and female patients. Female patients treated with levothyroxine had a relatively high risk of overall cancer (AOR: 1.56, 95% CI: 1.51‐1.61), as opposed to their male counterparts (AOR: 1.32, 95% CI: 1.24‐1.41). Increased risks of bladder (AOR: 1.32, 95% CI: 1.06‐1.65), lung (AOR: 1.30, 95% CI: 1.16‐1.45), pancreatic (AOR: 1.30, 95% CI: 1.01‐1.68), and esophageal cancers (AOR: 1.23, 95% CI: 0.69‐2.2) were also identified in female patients using levothyroxine.
TABLE 3.
Risk of cancer associated with levothyroxine stratified by gender
| Cancer | Gender | Adjusted odds ratio a (95% CI) | P‐value |
|---|---|---|---|
| Overall | Male | 1.32 (1.24‐1.41) | <.0001** |
| Female | 1.56 (1.51‐1.61) | <.001* | |
| Esophageal | Male | 0.60 (0.36‐1.00) | .04 |
| Female | 1.23 (0.69‐2.20) | .48 | |
| Gastric | Male | 1.49 (1.10‐2.02) | .01 |
| Female | 1.06 (0.85‐1.31) | .61 | |
| Colorectal | Male | 1.20 (1.02‐1.40) | .02 |
| Female | 1.12 (1.01‐1.23) | .02 | |
| Liver | Male | 0.87 (0.71‐1.06) | .16 |
| Female | 1.03 (0.89‐1.18) | .70 | |
| Pancreatic | Male | 1.18 (0.69‐1.99) | .54 |
| Female | 1.30 (1.01‐1.68) | .04 | |
| Lung | Male | 1.05 (0.88‐1.26) | .58 |
| Female | 1.30 (1.16‐1.45) | <.0001** | |
| Skin | Male | 1.53 (1.06‐2.22) | <.001** |
| Female | 1.39 (1.11‐1.74) | .004 | |
| Female Breast | Female | 1.24 (1.15‐1.33) | <.0001** |
| Cervical | Female | 0.75 (0.62‐0.91) | .003 |
| Prostate | Male | 1.26 (1.06‐1.50) | .01 |
| Bladder | Male | 1.04 (0.74‐1.47) | .81 |
| Female | 1.32 (1.06‐1.65) | .012 | |
| Kidney | Male | 1.13 (0.72‐1.78) | .59 |
| Female | 1.14 (0.90‐1.44) | .26 | |
| Brain | Male | 2.71 (1.68‐4.37) | <.0001** |
| Female | 1.64 (1.22‐2.20) | .001** |
CI, confidence interval.
Odds ratio adjusted for age, gender, comorbid conditions, age‐adjusted Charlson comorbidities index scores, and other drugs in Table 1.
P <.01; **P <.001.
Table 4 shows the effect of cumulative duration of levothyroxine use and cancer risk. Short‐term (60‐<365 days) and long‐term (≥1 year) use of levothyroxine were significantly associated with the overall cancer risk (AOR: 1.46, 95% CI: 1.40‐1.53; AOR: 1.52, 95% CI: 1.4‐1.59, respectively). Patients using levothyroxine for more than 1 year were observed to have higher risks of brain, breast, bladder, lung, and gastric cancers. However, no significant increase was found in risk of esophageal or colorectal cancer with long‐term use of levothyroxine.
TABLE 4.
Risk of cancer according to duration of levothyroxine exposure
| Cancer | Adjusted odds ratio a (95% CI) | P‐trend | |
|---|---|---|---|
| 60‐<365 d | ≥365 d | ||
| Overall | 1.467 (1.405‐1.532) | 1.529 (1.470‐1.590) | <.0001** |
| Esophageal | 0.699 (0.398‐1.229) | 0.898 (0.540‐1.495) | .42 |
| Gastric | 0.971 (0.752‐1.256) | 1.456 (1.141‐1.857) | .01 |
| Colorectal | 1.122 (0.997‐1.262) | 1.165 (1.034‐1.312) | .007* |
| Liver | 0.819 (0.698‐0.962) | 1.187 (1.014‐1.389) | .005* |
| Pancreatic | 1.327 (0.982‐1.793) | 1.210 (0.855‐1.711) | .10 |
| Lung | 1.146 (1.001‐1.311) | 1.307 (1.143‐1.496) | <.0001** |
| Skin | 1.172 (0.878‐1.565) | 1.679 (1.295‐2.176) | <.001** |
| Female breast | 1.219 (1.105‐1.346) | 1.257 (1.122‐1.409) | <.0001** |
| Cervical | 0.724 (0.557‐0.943) | 0.784 (0.594‐1.035) | .01 |
| Prostate | 1.217 (0.944‐1.567) | 1.299 (1.020‐1.655) | .03 |
| Bladder | 1.226 (0.943‐1.593) | 1.228 (0.955‐1.579) | .08 |
| Kidney | 1.071 (0.797‐1.439) | 1.222 (0.923‐1.618) | <.0001** |
| Brain | 1.831 (1.277‐2.625) | 1.965 (1.395‐2.769) | <.0001** |
Abbreviation: CI, confidence interval.
Odds ratio adjusted for age, gender, comorbid conditions, age‐adjusted Charlson comorbidities index scores, and other drugs in Table 1.
P < .01; **P < .001.
Table 5 represents the effects of 3‐year average daily dose of levothyroxine use on cancer risk. Daily doses were categorized into the following three groups: low (<0.34 DDD), medium (0.34‐≤0.67 DDD), and high doses (>0.67 DDD). Low, medium, and high doses of levothyroxine use all showed a higher risk of overall cancer (AOR: 1.49, 95% CI: 1.40‐1.59; AOR: 1.50, 95% CI: 1.45‐1.56; and AOR: 1.47, 95% CI:1.38‐1.58, respectively).
TABLE 5.
Risk of cancer among levothyroxine users according to 3‐year average daily dose
| Cancer | Adjusted odds ratio a (95% CI) | P‐trend | ||
|---|---|---|---|---|
|
<0.34 DDD/day |
0.34‐≤0.67 DDD/day |
>0.67 DDD/day |
||
| Overall | 1.498 (1.406‐1.596) | 1.508 (1.453‐1.564) | 1.479 (1.381‐1.585) | <.0001** |
| Esophageal | 0.823 (0.379‐1.790) | 0.815 (0.502‐1.322) | 0.710 (0.272‐1.853) | .70 |
| Gastric | 0.990 (0.658‐1.489) | 1.263 (1.015‐1.570) | 1.181 (0.764‐1.824) | .17 |
| Colorectal | 1.314 (1.104‐1.564) | 1.120 (1.006‐1.247) | 1.021 (0.831‐1.256) | .003* |
| Liver | 1.185 (0.941‐1.493) | 0.933 (0.807‐1.078) | 0.922 (0.701‐1.214) | .005* |
| Pancreatic | 1.103 (0.665‐1.827) | 1.439 (1.085‐1.910) | 0.947 (0.524‐1.713) | .08 |
| Lung | 1.427 (1.174‐1.734) | 1.155 (1.021‐1.306) | 1.218 (0.965‐1.538) | <.0001** |
| Skin | 1.525 (1.052‐2.210) | 1.444 (1.122‐1.857) | 1.181 (0.715‐1.949) | .03 |
| Female breast | 1.463 (1.247‐1.716) | 1.189 (1.081‐1.308) | 1.157 (0.969‐1.382) | .0001** |
| Cervical | 1.117 (0.767‐1.628) | 0.730 (0.572‐0.930) | 0.445 (0.251‐0.789) | .01 |
| Prostate | 1.656 (1.210‐2.268) | 1.108 (0.867‐1.416) | 1.170 (0.770‐1.779) | .01 |
| Bladder | 1.275 (0.877‐1.853) | 1.084 (0.849‐1.383) | 1.716 (1.141‐2.580) | .03 |
| Kidney | 0.769 (0.476‐1.242) | 1.271 (0.989‐1.632) | 1.248 (0.743‐2.098) | .14 |
| Brain | 1.783 (0.944‐3.366) | 1.794 (1.313‐2.450) | 2.358 (1.392‐3.995) | <.0001** |
CI, confidence interval; DDD, defined daily dose.
Odds ratio adjusted for age, gender, comorbid conditions, age‐adjusted Charlson comorbidities index scores, and other drugs in Table 1.
P < .01; **P < .001.
4. DISCUSSION
4.1. Main findings
This study showed that levothyroxine use was associated with a significantly increased risk of overall cancer, as well as brain, skin, prostate, pancreatic, female breast, lung, and colorectal cancers, in levothyroxine users when compared to non–users. As a case‐control study, the current study could not determine the causal relationship because of various confounding factors 29 , 30 ; however, our findings of these associations might imply that vigilance is supposed to be exercised when levothyroxine therapy is initiated or ongoing in patients with thyroid disease.
The high proportions of liver disease in both the case (29.09%) and the control (20.96%) groups could be attributed to a high prevalence of hepatitis B viral (HBV) infection. The prevalence of chronic hepatitis B in Taiwan was once estimated to be 15%‐20% in the general population and has plunged to <1% in the younger generation since the launch of the nationwide mass vaccination program against hepatitis B in 1984. 31 Given the mean age of 60.47 years in both groups in this study, the cases and controls, among whom HBV infection was highly prevalent, were predisposed to chronic hepatitis B and liver diseases.
4.2. Biological plausibility
The mechanism of the association between levothyroxine and the risk of cancer remains unclear. This association could possibly be explained by the fact that thyroid medications enhance the activation of the mitochondrial function responsible for overproduction of ROS. The enhanced level of ROS might be linked to increased oxidative stress in the body and further induces cancer. Previous studies have demonstrated that oxidative stress can impair cell function and, consequently, leads to several chronic morbid conditions, including cancer and autoimmunity. 10 , 11 Furthermore, biological mechanisms could also support this association. Two biological studies reported a potential consequence of excessive oxidative stress; they showed a possible biological pathway connecting impairment or destruction of DNA activity due to oxidative stress. Such impairment or destruction could be one of the most important mechanisms contributing to cancer risk. 32 , 33 These studies also demonstrated the role of genetic mutagenesis modification in actively contributing to the progression of cancer. In addition, activation of the PI3K/Akt and nuclear factor κB (NF‐κB) pathways by levothyroxine plays an important role in the development of human tumorigenesis. 34 However, as these studies do not provide solid evidence of mechanisms to explain the association between thyroxine use and cancer risk, a novel approach is needed to further investigate the causality between them. An experimental animal model could be the best option to identify the possible biological mechanism. 35
4.3. Comparison with relevant studies
A Swedish large‐scale cohort study indicated an association between levothyroxine use and increased risks of cancers as follows: the overall cancer risk in both genders, risks of breast, endometrium, genital, bladder, skin cancers, and leukemia in females, and risks of thyroid and other endocrine cancers in males. 36 In the current study, we additionally included risk of brain cancer, which was not analyzed in the Swedish cohort study. However, other studies had dissimilar findings. One case‐control study mentioned that levothyroxine might be an effective agent against colorectal cancer, 9 while another study found a direct correlation with lung cancer but not with breast, colorectal, or gastric cancer. 8 A review article concluded that clinical studies were needed given the limited knowledge and evidence of the causality between levothyroxine and breast cancer risk. 37 Studies on the association between levothyroxine use and cancer risk are to date still inadequate. More relevant studies in diverse populations and countries and meta‐analyses are needed to examine the causality of the association and to clarify its mechanism.
4.4. Public health implications and clinical practice
Cancer is a major public health problem worldwide, and it is the first and second leading cause of mortality in Taiwan and the USA, respectively. 38 , 39 Levothyroxine is generally thought to be a medication with a great safety profile for thyroid disease. However, concerns have been raised in relation to its safety and efficiency. 40 Our findings and previous studies’ results reinforce the suspicion among levothyroxine users regarding cancer risk. Therefore, we recommend monitoring the treatment outcomes and the condition of levothyroxine users, particularly long‐term users, as is generally performed in clinical practice currently. In addition, we suggest considering gender‐based and appropriate dose‐related therapy, so that physicians can provide appropriate drug dosage, efficient treatment, and improved drug safety. To ensure safe and effective treatments, physicians and other healthcare providers should take these findings into consideration while prescribing this medication. Epidemiological studies, to date, have not reached a conclusion on the biological mechanism, and the impact of unmeasured confounding factors remains unclear; 41 , 42 thus, future research, such as randomized control trials, laboratory investigations, and epidemiological studies with a long‐term follow up (at least 20‐25 years), is needed to identify the exact relationship between levothyroxine use and cancer risk. Such research might provide direction for heightening the awareness of safety issues and reshaping clinical decision‐making for levothyroxine‐treated patients.
4.5. Strengths and limitations
Our study has several strengths. First, we retrieved information from a large population‐based database that contains complete high‐quality data on drug prescriptions and cancer diagnoses. Second, our current study showed the high statistical power of the main and the subgroup analyses. We attempted to reduce possible bias by choosing controls who had exposure duration similar to that of the cases. In other words, cases and controls were matched on the index date.
Our study also had several limitations. First, some potential factors such as family history, smoking status, body mass index, and alcohol consumption information related to cancer risk were not included in our main and subgroup analyses because the HWDC database does not provide such information. 40 , 43 , 44 Despite the lack of smoking status data in the HWDC database, we included chronic obstructive pulmonary disease, which could be regarded as a surrogate for smoking status, in the confounding comorbidities and adjusted it in the analysis of cancer risk. Second, the current study was conducted in populations in Taiwan, and, hence, our findings might be different from other studies in other populations with different races or ethnicities. Third, a dose‐response association for gender was not included in our analysis, and this may weaken the observed association because male and females respond differently to the same dosage. 45 Fourth, cancer stages were not included in our main analyses due to lack of information. Fifth, we could not obtain information from the HSDC database on whether or not the hypothyroid patients were overtreated with levothyroxine. Finally, the association between levothyroxine exposure and various types of cancer risk was analyzed and identified in this study, yet its biological or metabologic mechanism could not be clarified using our study design.
In conclusion, our study findings showed that levothyroxine use is associated with increased risk of cancer and should be taken into consideration in initiating and renewing levothyroxine therapy for patients with hypothyroidism. However, the findings of the current study should be interpreted with caution. We have to emphasize that this case‐control study describes associations and not causal relationships between levothyroxine and cancer risks. Therefore, levothyroxine is still considered an effective medication, and the current study does not advise against use of this medication. The results of the current study could serve as a reference for interested researchers to conduct further research, and more in‐vivo studies and meta‐analyses are required to examine the causality and to identify the mechanisms of these associations.
DISCLOSURE
All authors declare no conflicts of interest.
ACKNOWLEDGMENTS
Funding/support: This research was sponsored in part by the Ministry of Science and Technology (grant number: MOST 109‐2222‐E‐038‐002‐MY2), the Ministry of Education (grant number: MOE 109‐6604‐001‐400), and Taipei Medical University (grant number: TMU107‐AE1‐B18). Role of the funder/sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Wu C‐C, Islam MM, Nguyen PA, et al. Risk of cancer in long‐term levothyroxine users: Retrospective population‐based study. Cancer Sci. 2021;112:2533–2541. 10.1111/cas.14908
REFERENCES
- 1. Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27:315‐389. [DOI] [PubMed] [Google Scholar]
- 2. Chen T‐Y, Hsu C‐C, Feng I‐J, et al. Higher risk for thyroid diseases in physicians than in the general population: a Taiwan nationwide population‐based secondary analysis study. QJM. 2017;110:163‐168. [DOI] [PubMed] [Google Scholar]
- 3. Tewari N. Prevalence of hypothyroidism in adults: An epidemiological study in eight cities of India. Indian J Endocrinol Metab. 2014;18:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489‐499. [DOI] [PubMed] [Google Scholar]
- 5. Wolter P, Stefan C, Decallonne B, et al. The clinical implications of sunitinib‐induced hypothyroidism: a prospective evaluation. Br J Cancer. 2008;99:448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pinto A, Glick M. Management of patients with thyroid disease: oral health considerations. J Am Dent Assoc. 2002;133:849‐858. [DOI] [PubMed] [Google Scholar]
- 7. Samuels MH, Kolobova I, Smeraglio A, Peters D, Purnell JQ, Schuff KG. Effects of levothyroxine replacement or suppressive therapy on energy expenditure and body composition. Thyroid. 2016;26:347‐355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cornelli U, Belcaro G, Recchia M, Finco A. Levothyroxine and lung cancer in females: the importance of oxidative stress. Reproductive Biology and Endocrinology. 2013;11:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rennert G, Rennert HS, Pinchev M, Gruber SB. A case–control study of levothyroxine and the risk of colorectal cancer. J Natl Cancer Inst. 2010;102:568‐572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lassoued S, Mseddi M, Mnif F, et al. A comparative study of the oxidative profile in Graves’ disease, Hashimoto’s thyroiditis, and papillary thyroid cancer. Biol Trace Elem Res. 2010;138:107‐115. [DOI] [PubMed] [Google Scholar]
- 11. Akinci M, Kosova F, Çetin B, et al. Oxidant/antioxidant balance in patients with thyroid cancer. Acta Cir Bras. 2008;23:551‐554. [DOI] [PubMed] [Google Scholar]
- 12. Chiu H‐F, Ho S‐C, Chang C‐C, Wu T‐N, Yang C‐Y. Statins are associated with a reduced risk of gastric cancer: a population‐based case–control study. Am J Gastroenterol. 2011;106:2098‐2103. [DOI] [PubMed] [Google Scholar]
- 13. Hwang A, Chou L, Islam M, Li Y‐C, Syed‐Abdul S. Risk factors for ectopic pregnancy in the Taiwanese population: a retrospective observational study. Arch Gynecol Obstet. 2016;294:779‐783. [DOI] [PubMed] [Google Scholar]
- 14. Chen K‐C, Iqbal U, Nguyen P‐A, et al. The impact of different surgical procedures on hypoparathyroidism after thyroidectomy: a population‐based study. Medicine. 2017;96;e8245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tseng C‐H. Rosiglitazone reduces breast cancer risk in Taiwanese female patients with type 2 diabetes mellitus. Oncotarget. 2017;8:3042‐3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Centers for Disease Control and Prevention . International classification of diseases, ninth revision, clinical modification (ICD‐9‐CM) 2011. https://www.cdc.gov/nchs/icd/icd9cm.htm. Accessed August 3, 2020.
- 17. Grimes DA, Schulz KF. Compared to what? Finding controls for case‐control studies. Lancet. 2005;365:1429‐1433. [DOI] [PubMed] [Google Scholar]
- 18. WHO Collaborating Centre for Drug Statistics Methodology, ATC/DDD Index 2016. https://www.whocc.no/atc_ddd_index/. Accessed August 3, 2020.
- 19. Yang HC, Islam MM, Nguyen PAA, et al. Development of a Web‐Based System for Exploring Cancer Risk With Long‐term Use of Drugs: Logistic Regression Approach. JMIR Public Health Surveill. 2021;7:e21401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pandeya N, Olsen CM, Thompson BS, et al. Aspirin and nonsteroidal anti‐inflammatory drug use and keratinocyte cancers: a large population‐based cohort study of skin cancer in Australia. Br J Dermatol. 2019;181:749‐760. [DOI] [PubMed] [Google Scholar]
- 21. García Rodríguez LA, Soriano‐Gabarró M, Bromley S, Lanas A, Cea SL. New use of low‐dose aspirin and risk of colorectal cancer by stage at diagnosis: a nested case‐control study in UK general practice. BMC Cancer. 2017;17:637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Simon TG, Ma Y, Ludvigsson JF, et al. Association between aspirin use and risk of hepatocellular carcinoma. JAMA Oncol. 2018;4:1683‐1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta‐analysis. Gastroenterology. 2013;144:323‐332. [DOI] [PubMed] [Google Scholar]
- 24. Choi JH, Lee SH, Huh G, et al. The association between use of statin or aspirin and pancreatic ductal adenocarcinoma: a nested case‐control study in a Korean nationwide cohort. Cancer Med. 2019;8:7419‐7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang W, Liang Z, Li J, Cai S. Angiotensin receptor blockers use and the risk of lung cancer: a meta‐analysis. J Renin Angiotensin Aldosterone Syst. 2015;16(4):768‐773. [DOI] [PubMed] [Google Scholar]
- 26. Dai YN, Wang JH, Zhu JZ, Lin JQ, Yu CH, Li YM. Angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers therapy and colorectal cancer: a systematic review and meta‐analysis. Cancer Causes Control. 2015;26:1245‐1255. [DOI] [PubMed] [Google Scholar]
- 27. Mao Y, Xu X, Wang X, Zheng X, Xie L. Is angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers therapy protective against prostate cancer? Oncotarget. 2016;7:6765‐6773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol. 1992;45:613‐619. [DOI] [PubMed] [Google Scholar]
- 29. Poly TN, Islam MM, Walther BA, et al. Exploring the association between statin use and the risk of Parkinson’s disease: a meta‐analysis of observational studies. Neuroepidemiology. 2017;49:142‐151. [DOI] [PubMed] [Google Scholar]
- 30. Islam MM, Yang H‐C, Nguyen P‐A, et al. Exploring association between statin use and breast cancer risk: an updated meta‐analysis. Arch Gynecol Obstet. 2017;1–11. [DOI] [PubMed] [Google Scholar]
- 31. Wait S, Chen DS. Towards the eradication of hepatitis B in Taiwan. Kaohsiung J Med Sci. 2012;28:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xing M. Oxidative stress: a new risk factor for thyroid cancer. Endocr Relat Cancer. 2012;19:C7‐C11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nichols HB, Anderson C, White AJ, Milne GL, Sandler DP. Oxidative stress and breast cancer risk in premenopausal women. Epidemiology. 2017;28:667‐674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Poli G, Leonarduzzi G, Biasi F, Chiarpotto E. Oxidative stress and cell signalling. Curr Med Chem. 2004;11:1163‐1182. [DOI] [PubMed] [Google Scholar]
- 35. Shanks N, Greek R, Greek J. Are animal models predictive for humans? Philos Ethics Humanit Med. 2009;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wändell P, Carlsson AC, Li X, Sundquist J, Sundquist K. Levothyroxine treatment is associated with an increased relative risk of overall and organ specific incident cancers ‐ a cohort study of the Swedish population. Cancer Epidemiol. 2020;66:101707. [DOI] [PubMed] [Google Scholar]
- 37. Hercbergs A, Mousa SA, Leinung M, Lin HY, Davis PJ. Thyroid hormone in the clinic and breast cancer. Horm Cancer. 2018;9:139‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177‐193. [DOI] [PubMed] [Google Scholar]
- 39. Hsiao A‐J, Chen L‐H, Lu T‐H. Ten leading causes of death in Taiwan: a comparison of two grouping lists. J Formos Med Assoc. 2015;114:679‐680. [DOI] [PubMed] [Google Scholar]
- 40. Tsai C‐Y, Yang H‐C, Islam M, et al. Psychotropic medications prescribing trends in adolescents: a nationwide population‐based study in Taiwan. Int J Qual Health Care. 2017;29:861‐866. [DOI] [PubMed] [Google Scholar]
- 41. de Gage SB, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case‐control study. BMJ. 2014;349:g5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224‐2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tseng C‐H. Sitagliptin use and thyroid cancer risk in patients with type 2 diabetes. Oncotarget. 2016;7:24871–24879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tseng C‐H. Sitagliptin may reduce prostate cancer risk in male patients with type 2 diabetes. Oncotarget. 2017;8:19057–19064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Islam MM, Iqbal U, Walther BA, et al. Gender‐based personalized pharmacotherapy: a systematic review. Arch Gynecol Obstet. 2017;1‐13. [DOI] [PubMed] [Google Scholar]


