Abstract
This multicenter, open‐label, phase I study assessed the safety and antitumor activity of acalabrutinib in Japanese patients with relapsed/refractory (r/r) B‐cell malignancies. Parts 1 (dose confirmation) and 2 (dose expansion) of this three‐part study are reported. Treatment was a single dose of 100 mg acalabrutinib (day 1), followed by a washout period and then twice daily 100 mg acalabrutinib in part 1, or twice daily 100 mg acalabrutinib in part 2. Patients from parts 1 and 2 with r/r chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL), and r/r mantle cell lymphoma (MCL) were assessed as r/r CLL/SLL and r/r MCL cohorts, respectively. Twenty‐five patients received treatment (part 1, n = 6). Median age was 71.0 years. Nine (one patient from part 1) and 13 (two patients from part 1) patients were included in the r/r CLL/SLL and r/r MCL cohorts, respectively. Treatment‐related adverse events (AEs) occurred in 88% of patients (grade ≥3, 36%); the most common were headache (28%) and purpura (24%), both grade 1/2. No AEs resulted in treatment discontinuation or death. Median duration of treatment was 31, 20, and 7 months for part 1, r/r CLL/SLL cohort, and r/r MCL cohort, respectively. Overall response rate (ORR) was 89% and 62% for the r/r CLL/SLL and r/r MCL cohorts, respectively. The median progression‐free survival (PFS) was not reached for the r/r CLL/SLL cohort and was 7 months for the r/r MCL cohort. Acalabrutinib (100 mg twice daily) was generally safe and well‐tolerated in adult Japanese patients with B‐cell malignancies.
Keywords: Bruton's tyrosine kinase, chronic lymphocytic leukemia, mantle cell lymphoma, pharmacokinetics, small lymphocytic lymphoma
This phase I study assessed the safety and antitumor activity of acalabrutinib in Japanese patients with relapsed/refractory B‐cell malignancies. Twenty‐five patients received treatment, and adverse events occurred in 88% of patients; however, most were grade 1 or 2, and no adverse event resulted in treatment discontinuation. Acalabrutinib was generally safe and well‐tolerated in adult Japanese patients with B‐cell malignancies.

Abbreviations
- AE
adverse event
- AUC
area under the curve
- BID
twice daily
- BTK
Bruton's tyrosine kinase
- CI
confidence interval
- CLL
chronic lymphocytic leukemia
- Cmax
maximum observed plasma concentration
- CR
complete response
- CV
coefficient of variation
- DLBCL
diffuse large B‐cell lymphoma
- DLT
dose‐limiting toxicity
- ECOG
Eastern Cooperative Oncology Group
- ELISA
enzyme‐linked immunosorbent assay
- FL
follicular lymphoma
- MCL
mantle cell lymphoma
- NHL
non‐Hodgkin lymphoma
- ORR
overall response rate
- PD
progressive disease
- PFS
progression‐free survival
- PR
partial response
- PRL
partial response with lymphocytosis
- PS
performance status
- PT
Preferred Term
- r/r
relapsed/refractory
- SAE
serious adverse event
- SLL
small lymphocytic lymphoma
- SOC
System Organ Class
- ULN
upper limit of normal
- WM
Waldenström macroglobulinemia
1. INTRODUCTION
Bruton's tyrosine kinase (BTK) is a cytoplasmic nonreceptor enzyme of the TEC kinase family 1 and is expressed in cells of hematopoietic origin, including B cells, myeloid cells, mast cells, and platelets. 2 , 3 It regulates multiple cellular processes, including proliferation, differentiation, and cell migration. 2 , 3 Functional null mutations in BTK cause X‐linked agammaglobulinemia, an inherited disease that is characterized by a lack of mature peripheral B cells. 4 BTK activation is implicated in the pathogenesis of several B‐cell malignancies, such as B‐cell lymphomas and leukemias, as BTK‐dependent signals are required for their survival. 5 Together, these findings suggest that BTK inhibition may be an attractive strategy for treating B‐cell neoplasms.
Ibrutinib is an oral small‐molecule BTK inhibitor that has been approved for the treatment of chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) and mantle cell lymphoma (MCL). Additionally, it has shown efficacy in other non‐Hodgkin lymphomas (NHLs), including follicular lymphoma (FL), 6 diffuse large B‐cell lymphoma (DLBCL), 7 and Waldenström macroglobulinemia (WM). 8 While ibrutinib is an effective treatment option, adverse events (AEs) owing to off‐target inhibition of other kinases are problematic. 1 As such, there is a need to develop more selective BTK inhibitors with similar efficacy and better safety profiles. Zanubrutinib, another small‐molecule BTK inhibitor, has demonstrated efficacy in patients with MCL, 9 CLL/SLL, 10 , 11 and WM, 12 and was recently approved to treat MCL by the US Food and Drug Administration (FDA). 13 Zanubrutinib is reported to have greater BTK selectivity than ibrutinib, 10 , 14 and in a clinical trial it has demonstrated a trend towards improved safety relative to ibrutinib. 12 However, acalabrutinib has demonstrated higher in vitro kinase selectivity than both ibrutinib and zanubrutinib 15 and therefore may have a more favorable safety profile.
Acalabrutinib is a potent, highly selective orally bioavailable small‐molecule BTK inhibitor. 16 It was first approved by the US FDA in 2017 for the treatment of relapsed/refractory (r/r) MCL 17 based on the phase II ACE‐LY‐004 clinical study results, 18 and in 2019 for the treatment of CLL and SLL based on the results of the ELEVATE‐TN and ASCEND clinical studies. 19 , 20 , 21 , 22 Acalabrutinib was approved in Japan for the treatment of r/r CLL and SLL on January 25, 2021. 23 At the time the present study was initiated, acalabrutinib had not yet been evaluated in the Japanese population and, therefore, we considered that a study to evaluate the safety and efficacy of acalabrutinib in Japanese patients was necessary to facilitate the approval process.
A multicenter, open‐label, phase I study was conducted to assess the safety, tolerability, pharmacokinetics, pharmacodynamics, and antitumor activity of acalabrutinib in adult Japanese patients with r/r B‐cell malignancies.
2. MATERIALS AND METHODS
2.1. Patients
Japanese patients who were ≥20 years of age at enrollment and who had a measurable lymphadenopathy or extranodal lymphoid malignancy (not applicable for patients with CLL or WM), an Eastern Cooperative Oncology Group (ECOG) performance status (PS) score of ≤2, adequate hematological and organ function, and a serum amylase ≤1.5× the upper limit of normal (ULN) or a serum lipase ≤1.5× ULN were eligible for enrollment in this study. Diagnosis‐related inclusion criteria differed for parts 1 and 2 of the study: Patients with a documented diagnosis of nongerminal center DLBCL, MCL, or indolent NHL (FL, CLL/SLL, WM) were eligible for participation in part 1; and patients with a confirmed diagnosis of CLL/SLL or MCL who had relapsed after or been refractory to ≥1 prior therapies and had either active disease (CLL/SLL) or documented failure to achieve at least a partial response (PR) or had documented disease progression after the most recent treatment regimen (MCL) were eligible to participate in part 2.
Main exclusion criteria were known central nervous system involvement of lymphoma/leukemia, the use of any biologically or immunologically based therapies within 4 weeks of the first dose of acalabrutinib, the time from the last dose of the most recent chemotherapy or experimental therapy to the first dose of study treatment <5 times the half‐life of the previously administered agent(s), prior therapy with B‐cell receptor inhibitors including BTK inhibitors or BCL‐2 inhibitors, and ongoing immunosuppressive therapy. All enrolled patients provided written informed consent.
2.2. Study design and treatments
We conducted a multicenter, open‐label, phase I study of acalabrutinib in adult Japanese patients with r/r B‐cell malignancies. This study was divided into three parts: part 1 was the dose‐confirmation phase, part 2 was the dose‐expansion phase, and part 3 was the dose‐confirmation phase for combination therapy. The results from parts 1 and 2 are described herein, and a schematic showing the study design for parts 1 and 2 is presented in Figure 1.
FIGURE 1.
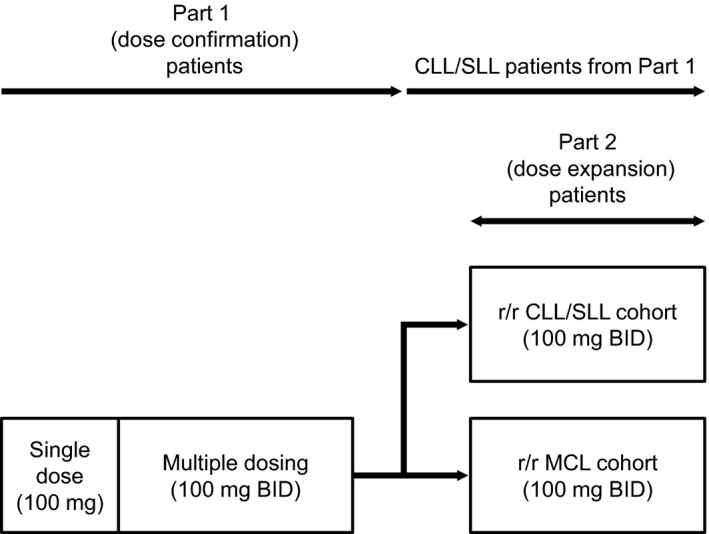
Study design. Abbreviations: BID, twice daily; CLL, chronic lymphocytic leukemia; MCL, mantle cell lymphoma; r/r, relapsed/refractory; SLL, small lymphocytic lymphoma
For part 1, the primary objective was to assess the safety and tolerability of acalabrutinib in Japanese patients with r/r B‐cell malignancies and to define the dose for further clinical evaluation using dose‐limiting toxicity (DLT) data. Specifically, 100 mg twice daily (BID) dosing, which had been established overseas as the dose for clinical development, was assessed. The secondary objectives included evaluation of the antitumor activity, pharmacokinetics, and pharmacodynamics of acalabrutinib. For part 2, the primary objective was to determine the safety profile of acalabrutinib in Japanese patients with r/r CLL/SLL or r/r MCL. The secondary objectives included evaluation of the antitumor activity and pharmacodynamics of acalabrutinib. Patients with r/r CLL/SLL or r/r MCL from part 1 and part 2 were analyzed as “r/r CLL/SLL cohort” and “r/r MCL cohort,” respectively, where appropriate.
In part 1, patients received a single oral dose of acalabrutinib on day 1 (100 mg, morning), followed by a washout period of 2 (minimum) to 7 (maximum) days and then a 28‐day cycle of BID oral acalabrutinib (100 mg, approximately the same time each day in the morning and evening [12 hours apart]). Acalabrutinib BID could be continued from cycle 2 onwards until the patient experienced disease progression or an unacceptable drug‐related toxicity. DLTs were defined as any grade 3 or higher nonhematologic toxicity (except those considered to be a response to supportive therapy, such as alopecia, nausea, vomiting, and diarrhea), grade 4 neutropenia (≥7 days in duration), grade 4 thrombocytopenia or the need for a platelet transfusion, grade ≥3 febrile neutropenia, or a toxicity‐related dosing delay for >7 consecutive days. In part 2, patients received acalabrutinib at a dose of 100 mg BID orally to further explore tolerability at this dose in two disease cohorts, r/r CLL/SLL (n = 6 to 9) and r/r MCL (n = 10 to 13). In each cohort, patients received treatment for a 28‐day cycle (cycle 1), and administration of acalabrutinib continued until the patient experienced disease progression or an unacceptable drug‐related toxicity. Furthermore, bacterial, viral, and/or fungal prophylaxis as per institutional standards was allowed for patients who were at risk of such infections.
This study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and that are consistent with the International Council for Harmonisation/Good Clinical Practice, applicable regulatory requirements, and the AstraZeneca policy on bioethics. The study protocol was approved by the institutional review board at each participating center, and the study was registered at ClinicalTrials.gov (identifier: NCT03198650).
2.3. Safety outcomes
AEs and events of clinical interest were summarized using System Organ Class (SOC), Preferred Term (PT), and Common Terminology Criteria for Adverse Events grade according to the Medical Dictionary for Regulatory Activities. Hematologic toxicity in r/r CLL patients (excluding r/r SLL patients) was graded according to the International Workshop on CLL. 24 Events of clinical interest were identified based on preclinical findings, emerging data from clinical studies relating to acalabrutinib, and pharmacological effects of approved BTK inhibitors. Events of clinical interest included cardiac events, cytopenias, hemorrhage, hepatic events, hypertension, infection, interstitial lung disease/pneumonitis, second primary malignancies (excluding skin malignancies), and tumor lysis syndrome.
2.4. Efficacy outcomes
The antitumor activity of acalabrutinib was evaluated by overall response rate (ORR), which was defined as the proportion of patients who achieved a response during the study period as assessed by the investigator; duration of response; and progression‐free survival (PFS). Computed tomography scans were to be performed for tumor assessments at the end of cycle 2, cycle 4, and cycle 6, and then again after every three cycles through to cycle 18, at which point they were performed every 6 cycles or more frequently at the investigator's discretion. Tumor responses were defined using previously published criteria; responses for NHL were assessed using the criteria published in Cheson et al, 25 responses for CLL were assessed using a modified version of the criteria published in Hallek et al (Table S1), 26 and responses for WM were assessed using a modified version of the Third International Workshop on WM Criteria (Table S2). 27
2.5. Pharmacokinetic outcomes
In part 1, pharmacokinetic profiles of acalabrutinib and its active metabolite (ACP‐5862) were assessed under fasting conditions. Single‐dose pharmacokinetics were assessed after a single dose of 100 mg acalabrutinib and multiple‐dose pharmacokinetics were assessed on day 8 during BID dosing (100 mg acalabrutinib).
2.6. Pharmacodynamic outcome
A BTK occupancy enzyme‐linked immunosorbent assay (ELISA) was used to evaluate the pharmacodynamic profile of acalabrutinib (Text S1). In part 1, blood samples were collected pre‐dose and 4 hours post‐dose on day 1 for patients who received a single dose; and pre‐dose and 4 hours post‐dose on days 1 and 8 of cycle 1, and pre‐dose on day 28 of cycles 1 and 2 for patients who received multiple doses. In part 2, blood samples were collected pre‐dose and 4 hours post‐dose on days 1 and 8 of cycle 1, and pre‐dose on day 28 of cycles 1 and 2.
2.7. Statistical methods
The number of patients was set based on the study objectives to obtain adequate tolerability, safety, pharmacokinetics data, and pharmacodynamics data while exposing as few patients as possible to the study treatment and procedures. For part 1, it was determined that six patients would be adequate to evaluate the safety and tolerability of acalabrutinib. Six to nine patients (r/r CLL/SLL cohort) and 10 to 13 patients (r/r MCL cohort) would allow for the evaluation of ORR using an exact test with a one‐sided significance level of 10% and 80% power (assuming a threshold ORR of 50% and an expected ORR of 85% for r/r CLL/SLL, and a threshold ORR of 40% and an expected ORR of 70% for r/r MCL).
All data were summarized by study part (part 1 or part 2) and cohort, unless stated otherwise. Tumor assessment data from part 1 and part 2 were combined for analysis because the assessments were performed using the same criteria. Frequencies were calculated for categorical variables, summary statistics were calculated for continuous data, and 80%, 90%, and 95% confidence intervals (CIs) were calculated for ORR. The average daily dose of acalabrutinib was calculated as total exposure (mg, total amount of study treatment received)/duration of exposure (last dose date − first dose date + 1). No formal statistical hypothesis testing was performed on the data from this study. All statistical analyses were performed using SAS software, version 9.4 (SAS Institute Inc).
3. RESULTS
3.1. Patients
The disposition of patients is shown in Figure 2; in total, 28 patients were enrolled and 25 received treatment at 14 study centers in Japan. The first patient was enrolled on June 27, 2017, and the data cutoff date was March 4, 2020. At the data cutoff, 16 (64%) patients were continuing treatment and nine (36%) patients discontinued the study (two due to death and seven due to disease progression). The two reported deaths were related to progressive/worsening disease.
FIGURE 2.

Patient disposition. aAll patients discontinued treatment because their condition worsened. bStudy termination due to death (n = 2) or progressive disease (PD) (n = 7). cStudy termination due to PD. dStudy termination due to death (n = 2) or PD (n = 6). Abbreviations: CLL, chronic lymphocytic leukemia; MCL, mantle cell lymphoma; SLL, small lymphocytic lymphoma
Twenty‐five patients were included in both the safety analysis set and the tumor response analysis set, six were included in the pharmacokinetics analysis set, and 21 were included in the pharmacodynamic analysis set. Four patients were excluded from the pharmacodynamic analysis set because baseline pharmacodynamic data were not available.
Patient characteristics are shown in Table 1. The median patient age was 71.0 years, the majority (19 [76%]) were male, and the mean body mass index was 21.57 kg/m2. Most patients had an ECOG PS of 0 (19 [76%]) at baseline. The majority of tumor subtypes were MCL, followed by CLL and SLL.
TABLE 1.
Patient characteristics
| Part 1 | Part 1 + Part 2 | Total | ||
|---|---|---|---|---|
| (N = 6) | r/r CLL/SLL cohort (N = 9; 1 patient from part 1) | r/r MCL cohort (N = 13; 2 patients from part 1) | (N = 2 | |
| Age, median (min, max) | 69.5 (52, 79) | 57.0 (51, 79) | 74.0 (55, 82) | 71.0 (51, 82) |
| Male sex, n (%) | 5 (83.3) | 5 (55.6) | 12 (92.3) | 19 (76.0) |
| Asian race, n (%) | 6 (100.0) | 9 (100.0) | 13 (100.0) | 25 (100.0) |
| BMI (kg/m2) | 21.41 (2.86) | 20.44 (1.51) | 22.36 (2.90) | 21.57 (2.69) |
| Tumor subtype | ||||
| CLL | 1 (16.7) | 5 (55.6) | ‐ | 5 (20.0) |
| SLL | 0 | 4 (44.4) | ‐ | 4 (16.0) |
| MCL | 2 (33.3) | ‐ | 13 (100.0) | 13 (52.0) |
| WM | 1 (16.7) | ‐ | ‐ | 1 (4.0) |
| FL | 2 (33.3) | ‐ | ‐ | 2 (8.0) |
| No. of prior therapies, median (min, max) | 1.5 (1, 2) | 1.0 (1, 4) | 2.0 (1, 7) | 2.0 (1, 7) |
Data are shown as mean (standard deviation) unless otherwise stated.
Abbreviations: BMI, body mass index; CLL, chronic lymphocytic leukemia; FL, follicular lymphoma; MCL, mantle cell lymphoma; r/r, relapsed/refractory; SLL, small lymphocytic lymphoma; WM, Waldenström macroglobulinemia.
3.2. Safety
The duration of exposure is shown in Table 2. The median treatment duration of acalabrutinib was 31 months for part 1, 20 months for the r/r CLL/SLL cohort (parts 1 and 2), and 7 months for the r/r MCL cohort (parts 1 and 2). The mean percentage of the intended dose of acalabrutinib administered to patients was 89% for part 1, and 93% and 97% for the CLL/SLL and MCL cohorts, respectively, in combined parts 1 and 2. No DLTs were observed.
TABLE 2.
Duration of exposure
| Treatment duration (months) | Part 1 | Part 1 + Part 2 | |
|---|---|---|---|
| (N = 6) | r/r CLL/SLL cohort (N = 9; 1 patient from part 1) | r/r MCL cohort (N = 13; 2 patients from part 1) | |
| Time from first dose to end of follow‐up a | |||
| Median (min, max) | 31 (9, 32) | 20 (16, 31) | 9 (1, 32) |
| Total treatment months | 146 | 189 | 169 |
| Total treatment duration of acalabrutinib b | |||
| Median (min, max) | 31 (7, 32) | 20 (14, 31) | 7 (0.1, 32) |
| Mean time from first dose to end of follow‐up a | 24 | 21 | 13 |
| Total treatment months | 142 | 186 | 160 |
| Actual treatment duration of acalabrutinib c | |||
| Median (min, max) | 31 (7, 32) | 18 (14, 31) | 7 (0.1, 31) |
| Total treatment months | 139 | 183 | 156 |
Abbreviations: CLL, chronic lymphocytic leukemia; MCL, mantle cell lymphoma; r/r, relapsed/refractory; SLL, small lymphocytic lymphoma.
Time from first dose to end of follow‐up (end date of follow‐up − first dose date + 1) / (365.25/12).
Total treatment duration (last dose date − first dose date + 1) / (365.25/12).
Actual treatment duration (total treatment duration, excluding dose interruptions).
AEs are listed by number of patients and by number of episodes in Tables S3 and S4, respectively; overall, all 25 patients reported a total of 251 AEs. The most prevalent (n, %) AEs were nasopharyngitis (9, 36%), headache (8, 32%), and rash (7, 28%). AEs considered related to the study treatment were reported by 22 (88%) patients (119 AEs total), the most prevalent (n, %) of which were headache (7, 28%) and purpura (6, 24%).
Grade ≥3 AEs were reported for 14 (56%) patients (35 total events). Nine (36%) patients (16 total events) experienced grade ≥3 AEs that were considered related to treatment. Grade 4 AEs were reported by five (20%) patients and included lymphopenia, neutropenia, thrombocytopenia, hyperuricemia, lymphocyte count decreased, neutrophil count decreased, and white blood cell count decreased. Twenty‐two (88%) patients reported a total of 95 AEs of clinical interest (Table 3). In addition, six (24%) patients reported a total of six serious adverse events (SAEs) and four (16%) patients reported SAEs related to treatment. No AEs with the outcome of death or AEs/SAEs leading to treatment discontinuation were reported. AEs (total) that occurred in ≥10% of patients and those considered possibly related to treatment that occurred in ≥10% of patients are listed by SOC/PT in Tables S5 and S6, respectively.
TABLE 3.
Serious adverse events and adverse events of clinical interest (any grade)
| System Organ Class/Preferred Term | Part 1 | Part 1 + Part 2 | Total | |
|---|---|---|---|---|
| (N = 6) | r/r CLL/SLL cohort (N = 9; 1 patient from part 1) | r/r MCL cohort (N = 13; 2 patients from part 1) | (N = 25) | |
| Patients with any SAE | 3 (50.0) | 2 (22.2) | 3 (23.1) | 6 (24.0) |
| Infections and infestations | 1 (16.7) | 1 (11.1) | 2 (15.4) | 3 (12.0 |
| Pneumonia | 1 (16.7) | 1 (11.1) | 2 (15.4) | 3 (12.0) |
| Neoplasms benign, malignant, and unspecified (incl. cysts and polyps) | 1 (16.7) | 1 (11.1) | 0 | 1 (4.0) |
| Colon cancer | 1 (16.7) | 1 (11.1) | 0 | 1 (4.0) |
| Blood and lymphatic system disorders | 1 (16.7) | 0 | 0 | 1 (4.0) |
| Anemia | 1 (16.7) | 0 | 0 | 1 (4.0) |
| Gastrointestinal disorders | 0 | 0 | 1 (7.7) | 1 (4.0) |
| Abdominal pain upper | 0 | 0 | 1 (7.7) | 1 (4.0) |
| Patients with any AE of clinical interest | 6 (100.0) | 8 (88.9) | 11 (84.6) | 22 (88.0) |
| Cardiac events | 0 | 0 | 1 (7.7) | 1 (4.0) |
| Atrial fibrillation | 0 | 0 | 1 (7.7) | 1 (4.0) |
| Anemia | 2 (33.3) | 2 (22.2) | 3 (23.1) | 6 (24.0) |
| Leukopenia | 1 (16.7) | 3 (33.3) | 4 (30.8) | 7 (28.0) |
| Neutropenia | 1 (16.7) | 3 (33.3) | 3 (23.1) | 6 (24.0) |
| Other leukopenia | 0 | 0 | 3 (23.1) | 3 (12.0) |
| Thrombocytopenia | 1 (16.7) | 1 (11.1) | 3 (23.1) | 5 (20.0) |
| Hemorrhage | 0 | 4 (44.4) | 4 (30.8) | 8 (32.0) |
| Other hemorrhage | 0 | 4 (44.4) | 4 (30.8) | 8 (32.0) |
| Hepatotoxicity | 0 | 1 (11.1) | 2 (15.4) | 3 (12.0) |
| Infections | 6 (100.0) | 6 (66.7) | 7 (53.8) | 16 (64.0) |
| Interstitial lung disease/pneumonitis | 1 (16.7) | 0 | 1 (7.7) | 1 (4.0) |
| Second primary malignancy | 1 (16.7) | 1 (11.1) | 0 | 1 (4.0) |
| Second primary malignancies (excl. nonmelanoma skin cancer) | 1 (16.7) | 1 (11.1) | 0 | 1 (4.0) |
| Tumor lysis syndrome | 0 | 0 | 1 (7.7) | 1 (4.0) |
Data are shown as n (%).
Abbreviations: AE, adverse event; CLL, chronic lymphocytic leukemia; MCL, mantle cell lymphoma; r/r, relapsed/refractory; SAE, serious adverse event; SLL, small lymphocytic lymphoma.
One patient experienced a grade 1 AE of interstitial lung disease/pneumonitis that was considered related to the treatment (Table 3). This patient underwent dose interruption and recovered without steroids after being treated with sulfamethoxazole and trimethoprim for interstitial lung disease/pneumonitis. Three patients experienced an SAE of grade 3 pneumonia (Table 3); two of the cases were considered possibly related to the treatment, while the third case was considered not related to the treatment. The pathogen was unknown in all three cases.
3.3. Efficacy
ORR was 89% (8/9 patients; 95% CI: 52, 100) for the r/r CLL/SLL cohort, 100% (9/9 patients; 95% CI: 66, 100) for the r/r CLL/SLL cohort (including PR with lymphocytosis [PRL]), and 62% (8/13 patients; 95% CI: 32, 86) for the r/r MCL cohort (Table 4). The best objective response by tumor subtype is shown in Table 4. For patients with r/r CLL/SLL, two patients achieved complete response (CR) with incomplete bone marrow recovery, and six patients achieved a PR. For patients with r/r MCL, five patients achieved CR, and three patients achieved PR. Two patients with FL achieved PR, and one patient with WM achieved a minor response. For the r/r CLL/SLL cohort, none of the eight patients who responded had progressive disease (PD). For the r/r MCL cohort, three of the eight patients who responded had PD. For both cohorts, the median duration of response could not be estimated. The median PFS for the r/r MCL cohort (combined parts 1 and 2, n = 13; six patients had PD) was 7 months; median PFS was not reached for the r/r CLL/SLL cohort (n = 9; none of the patients had PD). The effect of treatment on tumor response over time for each individual patient in the r/r CLL/SLL and r/r MCL cohorts is illustrated in a swimmer plot (Figure S1A,B).
TABLE 4.
Overall response rate and best objective response rate
| Group | Number (%) of patients with a response | 95% CI |
|---|---|---|
| Part 1 + Part 2 | ||
| CLL/SLL cohort (n = 9) | ||
| ORR | 8 (89) | 52, 100 |
| CR | 0 | |
| CRi | 2 (22) | |
| PR | 6 (67) | |
| Nonresponse (total) | 1 (11) | |
| PRL | 1 (11) | |
| Stable disease | 0 | |
| PD | 0 | |
| Clinical PD | 0 | |
| Not evaluable | 0 | |
| CLL/SLL cohort including PRL (n = 9) | ||
| ORR | 9 (100) | 66, 100 |
| MCL cohort (n = 13) | ||
| ORR | 8 (62) | 32, 86 |
| CR | 5 (38) | |
| PR | 3 (23) | |
| Nonresponse (total) | 5 (38) | |
| Stable disease | 3 (23) | |
| PD | 2 (15) | |
| Not evaluable | 0 | |
Abbreviations: CI, confidence interval; CLL, chronic lymphocytic leukemia; CR, complete response; CRi, complete response with incomplete bone marrow recovery; MCL, mantle cell lymphoma; ORR, overall response rate; PD, progressive disease; PR, partial response; PRL, partial response with lymphocytosis; SLL, small lymphocytic lymphoma.
3.4. Pharmacokinetics
Pharmacokinetic evaluation was performed on patients who participated in part 1 of the study. Individual plasma concentration‐time curves for both acalabrutinib and ACP‐5862 were variable for the single‐dose period (Figure S2A,B) but not for the multiple‐dose period (Figure S2C,D). Data from both the single‐ and multiple‐dose periods demonstrated that acalabrutinib was rapidly absorbed and metabolized into ACP‐5862. Pharmacokinetic parameters for acalabrutinib and ACP‐5862 for the single‐ and multiple‐dose periods are shown in Table 5. The metabolite to parent ratios were 0.53 and 1.23 to 1.40 for the maximum observed plasma concentration (Cmax) and area under the curve (AUC), respectively, after a single dose, and a respective 0.56 and 1.64 to 1.73 after multiple dosing. The coefficient of variation (CV) of acalabrutinib Cmax was 174%, and the elimination half‐life was highly variable after a single dose; interpatient variability was lower after multiple dosing (CVs <30%). Using multiple‐dose data, the elimination half‐life of acalabrutinib was estimated to be 1.8 h and the accumulation ratios of acalabrutinib and ACP‐5862 were 1.69 and 2.72, respectively.
TABLE 5.
Pharmacokinetic parameters of acalabrutinib and ACP‐5862 in the single‐ and multiple‐dosing periods (part 1)
| Acalabrutinib (P) single dose | ACP‐5862 (M) single dose | M/P ratio single dose | Acalabrutinib (P) multiple dose | ACP‐5862 (M) multiple dose | M/P ratio multiple dose | |
|---|---|---|---|---|---|---|
| tmax (h), median (min, max) | 0.735 (0.520, 1.020) | 0.965 (0.900, 4.000) | ‐ | 0.615 (0.470, 1.000) | 0.940 (0.750, 1.080) | ‐ |
| Cmax (ng/mL), Gmean (CV) | 601.9 (174%) | 316.5 (260%) | 0.53 | 1120 (30%) | 629.7 (27%) | 0.56 |
| AUC0–12 (ng∙h/mL), Gmean (CV) | 890.7 (95%) | 1092 (124%) | 1.23 | 1208 (25%) | 1987 (8%) | 1.64 |
| AUC0–t (ng∙h/mL), Gmean (CV) | 961.7 (79%) | 1306 (94%) | 1.36 | 1206 (25%) | 1985 (8%) | 1.65 |
| AUC (ng∙h/mL), Gmean (CV) | 1109 (55%) | 1557 (60%) | 1.40 | 1211 (25%) | 2099 (7%) | 1.73 |
| t1/2 (h), Amean ± SD | 9.359 ± 12.46 | 10.18 ± 7.986 | ‐ | 1.795 ± 0.7404 | 3.216 ± 0.3938 | ‐ |
| CL/F (L/h), Amean ± SD | 99.54 ± 44.33 | ‐ | ‐ | 84.89 ± 19.84 | ‐ | ‐ |
| Vz/F (L), Amean ± SD | 1940 ± 2903 | ‐ | ‐ | 219.7 ± 107.1 | ‐ | ‐ |
| RAC, Amean ± SD | ‐ | ‐ | ‐ | 1.694 ± 1.275 | 2.722 ± 2.826 | ‐ |
| TC, Amean ± SD | ‐ | ‐ | ‐ | 1.163 ± 0.4770 | 1.444 ± 0.8136 | ‐ |
Abbreviations: Amean, arithmetic mean; AUC, area under the plasma concentration‐time curve (from zero to infinity); AUC0–12, area under the plasma concentration‐time curve (from zero to 12 h); AUC0–t, area under the plasma concentration‐time curve (from zero to the time of the last measurable concentration); CL/F, oral clearance; Cmax, maximum plasma concentration; CV, coefficient of variation; Gmean, geometric mean; RAC, extent of accumulation on multiple dosing; TC, time dependency of pharmacokinetics; tmax, time to maximum concentration; Vz/F, volume of distribution.
3.5. Pharmacodynamics
Twenty‐one patients were evaluated for BTK target occupancy (six patients from part 1, and eight patients from the r/r CLL/SLL cohort and seven patients from the r/r MCL cohort from part 2). The median BTK occupancy 4 hours after a single dose was >99% for patients with CLL, MCL, FL, or WM in part 1 (Figure 3A). Furthermore, the median steady‐state BTK occupancy was between 97.5% and 99.5% during the BID dosing phase. In part 2, the median steady‐state BTK occupancy ranged from 96.5% to 99.9% and 93.8% to 99.3% in the r/r CLL/SLL and r/r MCL cohorts, respectively (Figure 3B, 3C).
FIGURE 3.

BTK occupancy. BTK occupancy for each individual patient from part 1 (dose confirmation) (A) and part 2 (dose expansion) for the CLL/SLL (B) and MCL cohorts (C); data cutoff: May 9, 2019. Abbreviations: BID, twice daily; BTK, Bruton's tyrosine kinase; CLL, chronic lymphocytic leukemia; FL, follicular lymphoma; MCL, mantle cell lymphoma; SLL, small lymphocytic lymphoma; WM, Waldenström macroglobulinemia
4. DISCUSSION
This is the first clinical study to evaluate the safety, tolerability, pharmacokinetics, pharmacodynamics, and antitumor activity of the BTK inhibitor acalabrutinib in Japanese adults with r/r B‐cell malignancies. We report that acalabrutinib was safe and well‐tolerated, and that treatment resulted in ORRs for r/r CLL and r/r MCL that were similar to those reported for non‐Japanese patient populations with r/r B‐cell malignancies who were treated with acalabrutinib. Acalabrutinib was rapidly absorbed, and there was a high level of BTK receptor occupancy.
Regarding safety, headache was commonly observed in the present study (32%), which is in line with the findings reported for the global phase I/II study of acalabrutinib in r/r CLL and the phase II ACE‐LY‐004 study of acalabrutinib in r/r MCL, in which headache was observed in 43% and 38% of patients, respectively. 16 , 18 In all three studies, all reports of headaches were grade 1 or 2. Importantly, there were no AEs leading to treatment discontinuation or death. Overall, the safety findings in the present study were in line with previously reported safety data for acalabrutinib, and no new safety concerns were identified.
When analyzing efficacy, the ORRs for parts 1 and 2 combined were 89%, and 62% for the r/r CLL/SLL and r/r MCL cohorts, respectively. In acalabrutinib‐treated patients with CLL, an ORR of 81% was reported for the ASCEND study. 21 The ACE‐LY‐004 study reported an ORR of 81% for acalabrutinib‐treated patients with r/r MCL. 18 Overall, the ORRs for the CLL/SLL and MCL cohorts were in line with previous reports, although the results should be interpreted with caution because of the limited number of patients included in the present study.
Pharmacokinetic evaluation revealed that acalabrutinib was rapidly absorbed and had a mean half‐life of 1.8 hours after multiple dosing, and similar results were reported in a phase I/II study of acalabrutinib in patients with CLL. 16 Acalabrutinib was metabolized into ACP‐5862, which has approximately 50% potency for BTK inactivation relative to the parent agent acalabrutinib; however, ACP‐5862 still has a similar kinase selectivity profile. This resulted in metabolite to parent ratios of 0.53 to 0.56 and 1.23 to 1.64 for Cmax and AUC, respectively. These data indicate that ACP‐5862 may also contribute to efficacy and safety after acalabrutinib administration. Meanwhile, pharmacodynamic evaluation showed that there was a high target coverage with near‐complete occupancy, which was similar to the occupancy reported in a previous phase I/II study. 16 , 28
4.1. Limitations
This study had several limitations. First, comparison of efficacy and safety between this study and previous studies of acalabrutinib could not be adequately conducted 29 due to the limited number of patients included in the study. Therefore, current findings should be further investigated in larger studies. Second, efficacy was assessed by study investigators and was not assessed with independent central review. Finally, although all patients enrolled in this study had B‐cell malignancies, the inclusion of patients with pathophysiologically distinct diseases adds complexity to the interpretation of these study results.
5. CONCLUSIONS
This phase I study demonstrated that 100 mg BID acalabrutinib was generally safe and well‐tolerated in adult Japanese patients with r/r B‐cell malignancies. These results were also similar to what has been previously reported in other populations.
DISCLOSURE
Koji Izutsu has received research funds from AstraZeneca. Kiyoshi Ando has received research funds from AstraZeneca. Daisuke Ennishi has no conflict of interest to declare. Hirohiko Shibayama has received honoraria from Takeda, Novartis, Celgene, Janssen, Chugai, and Kyowa Kirin; research funds from Janssen, Ono, Celgene, Novartis, Sanofi, AstraZeneca, AbbVie, and Chugai; and scholarships from Astellas, Teijin, Shionogi, Eisai, Sanofi, Taiho, and Nippon Shinyaku. Junji Suzumiya has received honoraria from AstraZeneca, AbbVie, Bristol Myers Squibb, Celgene, Chugai, Eisai, Janssen, and Takeda; and research funds from AstraZeneca, Bayer, Celgene, Chugai, Eisai, Kyowa Kirin, Ono, SymBio, Takeda, and Yakult. Kazuhito Yamamoto has received honoraria from Chugai, Eisai, HUYA/IQVIA, Mundipharma, and Takeda; and research funds from AbbVie, AstraZeneca, Bayer, Celgene, Chugai, Eisai, Incyte/IQVIA, Mundipharma, Nippon Shinyaku, Novartis, Solasia Pharma, SymBio, Yakult, and Zenyaku Kogyo. Satoshi Ichikawa has received research funds from Chugai. Koji Kato has received honoraria from Novartis, Eisai, Janssen, Celgene, Takeda, MSD, Kyowa Kirin, Janssen, Celgene, Ono, Mundipharma, and Sumitomo Dainippon; and research funds from Chugai, Takeda, Kyowa Kirin, AbbVie, Novartis, Eisai, Janssen, Celgene, and Ono. Kyoya Kumagai has no conflict of interest to declare. Priti Patel is employed by Acerta Pharma. Sakura Iizumi, Nobuya Hayashi, Hisashi Kawasumi, and Kosho Murayama are employed by AstraZeneca. Hirokazu Nagai has received honoraria from Celgene, Takeda, Eisai, Chugai, and Mundipharma; research funds from Bayer, AstraZeneca, Zenyaku Kogyo, Takeda, Mundipharma, SymBio, and Chugai; and scholarships from Chugai.
AUTHORS’ CONTRIBUTION
Koji Izutsu, Kiyoshi Ando, Daisuke Ennishi, Hirohiko Shibayama, Junji Suzumiya, Kazuhito Yamamoto, Satoshi Ichikawa, Koji Kato, Kyoya Kumagai, and Hirokazu Nagai were involved in the collection and assembly of data and in the drafting and final approval of the manuscript. Priti Patel and Hisashi Kawasumi were involved in the study conception, design, and data interpretation, and in the drafting and final approval of the manuscript. Sakura Iizumi, Nobuya Hayashi, and Kosho Murayama were involved in data interpretation and in the drafting and final approval of the manuscript. All authors agree to be accountable for the accuracy and integrity of this work.
Supporting information
Fig S1‐S2
Text S1,Table S1‐S6
ACKNOWLEDGMENTS
The study was funded by AstraZeneca. The authors would like to thank the patients, their families, and all the investigators involved in this study. We thank Sarah Bubeck, PhD, of Edanz Evidence Generation, for providing medical writing support, which was funded by AstraZeneca through EMC KK in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Izutsu K, Ando K, Ennishi D, et al. Safety and antitumor activity of acalabrutinib for relapsed/refractory B‐cell malignancies: A Japanese phase I study. Cancer Sci. 2021;112:2405–2415. 10.1111/cas.14886
Clinical trial registration: ClinicalTrials.gov Identifier: NCT03198650
DATA AVAILABILITY STATEMENT
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy which is described at http://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure
REFERENCES
- 1. Wu J, Liu C, Tsui ST, Liu D. Second‐generation inhibitors of Bruton tyrosine kinase. J Hematol Oncol. 2016;9:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mohamed AJ, Yu L, Bäckesjö CM, et al. Bruton's tyrosine kinase (Btk): function, regulation, and transformation with special emphasis on the PH domain. Immunol Rev. 2009;228:58‐73. [DOI] [PubMed] [Google Scholar]
- 3. Bradshaw JM. The Src, Syk, and Tec family kinases: distinct types of molecular switches. Cell Signal. 2010;22:1175‐1184. [DOI] [PubMed] [Google Scholar]
- 4. Vihinen M, Mattsson PT, Smith CI. Bruton tyrosine kinase (BTK) in X‐linked agammaglobulinemia (XLA). Front Biosci. 2000;5:D917‐928. [DOI] [PubMed] [Google Scholar]
- 5. Buggy JJ, Elias L. Bruton tyrosine kinase (BTK) and its role in B‐cell malignancy. Int Rev Immunol. 2012;31:119‐132. [DOI] [PubMed] [Google Scholar]
- 6. Advani RH, Buggy JJ, Sharman JP, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI‐32765) has significant activity in patients with relapsed/refractory B‐cell malignancies. J Clin Oncol. 2013;31:88‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Vos S, Wilson W, Gerecitano J, et al. The Bruton's tyrosine kinase (BTK) inhibitor, ibrutinib (PCI‐32765), has preferential activity in the activated B cell‐like (ABC) subtype of relapsed/refractory (R/R) DLBCL: Interim phase 2 results. Haematologica. 2012;S1:490. [Google Scholar]
- 8. Treon SP. Proteasome inhibitors in Waldenström macroglobulinemia. Blood. 2013;122:3243‐3244. [DOI] [PubMed] [Google Scholar]
- 9. Song Y, Zhou K, Zou D, et al. Treatment of patients with relapsed or refractory mantle‐cell lymphoma with zanubrutinib, a selective inhibitor of Bruton's tyrosine kinase. Clin Cancer Res. 2020;26:4216‐4224. [DOI] [PubMed] [Google Scholar]
- 10. Tam CS, Trotman J, Opat S, et al. Phase 1 study of the selective BTK inhibitor zanubrutinib in B‐cell malignancies and safety and efficacy evaluation in CLL. Blood. 2019;134:851‐859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cull G, Simpson D, Opat S, et al. Treatment with the Bruton tyrosine kinase inhibitor zanubrutinib (BGB‐3111) demonstrates high overall response rate and durable responses in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL): updated results from a phase 1/2 trial. Blood. 2019;134(Supplement_1):500.31395583 [Google Scholar]
- 12. Tam CS, Opat S, D'Sa S, et al. A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenstrom macroglobulinemia: the ASPEN study. Blood. 2020;136:2038‐2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. U.S. Food & Drug Administration . FDA grants accelerated approval to zanubrutinib for mantle cell lymphoma. https://www.fda.gov/drugs/resources‐information‐approved‐drugs/fda‐grants‐accelerated‐approval‐zanubrutinib‐mantle‐cell‐lymphoma. Accessed January 14, 2021.
- 14. Honigberg LA, Smith AM, Sirisawad M, et al. The Bruton tyrosine kinase inhibitor PCI‐32765 blocks B‐cell activation and is efficacious in models of autoimmune disease and B‐cell malignancy. Proc Natl Acad Sci USA. 2010;107:13075‐13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kaptein A, de Bruin G, Emmelot‐van Hoek M, et al. Potency and selectivity of BTK inhibitors in clinical development for B‐cell malignancies. Blood. 2018;132:1871.30082493 [Google Scholar]
- 16. Byrd JC, Harrington B, O'Brien S, et al. Acalabrutinib (ACP‐196) in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374:323‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. U.S. Food & Drug Administration . FDA grants accelerated approval to acalabrutinib for mantle cell lymphoma. https://www.fda.gov/drugs/resources‐information‐approved‐drugs/fda‐grants‐accelerated‐approval‐acalabrutinib‐mantle‐cell‐lymphoma. Accessed August 28, 2020.
- 18. Wang M, Rule S, Zinzani PL, et al. Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE‐LY‐004): a single‐arm, multicentre, phase 2 trial. Lancet. 2018;391:659‐667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. U.S. Food and Drug Administration . Project Orbis: FDA approves acalabrutinib for CLL and SLL. https://www.fda.gov/drugs/resources‐information‐approved‐drugs/project‐orbis‐fda‐approves‐acalabrutinib‐cll‐and‐sll. Accessed July 31, 2020.
- 20. Sharman JP, Banerji V, Fogliatto LM, et al. ELEVATE TN: phase 3 study of acalabrutinib combined with obinutuzumab (O) or alone vs O plus chlorambucil (Clb) in patients (Pts) with treatment‐naive chronic lymphocytic leukemia (CLL). Blood. 2019;134:31. [Google Scholar]
- 21. Ghia P, Pluta A, Wach M, et al. ASCEND: Phase III, randomized trial of acalabrutinib versus idelalisib plus rituximab or bendamustine plus rituximab in relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2020;38(25):2849‐2861. [DOI] [PubMed] [Google Scholar]
- 22. Sharman JP, Egyed M, Jurczak W, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment‐naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet. 2020;395:1278‐1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Calquence approved in Japan for CLL . https://news.cision.com/astrazeneca/r/calquence‐approved‐in‐japan‐for‐cll,c3273161. Accessed January 27, 2021.
- 24. Hallek M, Cheson BD, Catovsky D, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131:2745‐2760. [DOI] [PubMed] [Google Scholar]
- 25. Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non‐Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059‐3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute‐Working Group 1996 guidelines. Blood. 2008;111:5446‐5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kimby E, Treon SP, Anagnostopoulos A, et al. Update on recommendations for assessing response from the Third International Workshop on Waldenström's Macroglobulinemia. Clin Lymphoma Myeloma. 2006;6:380‐383. [DOI] [PubMed] [Google Scholar]
- 28. Covey T, Gulranjani M, Cheung J, et al. Pharmacodynamic evaluation of acalabrutinib in relapsed/refractory and treatment‐naive patients with chronic lymphocytic leukemia (CLL) in the phase 1/2 ACE‐CL‐001 study. Blood. 2017;130:1741. [Google Scholar]
- 29. Maruyama D, Nagai H, Fukuhara N, et al. Efficacy and safety of ibrutinib in Japanese patients with relapsed or refractory mantle cell lymphoma. Cancer Sci. 2016;107:1785‐1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐S2
Text S1,Table S1‐S6
Data Availability Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy which is described at http://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure


